Abstract
Withaferin A (WA) is a promising phytochemical exhibiting in vitro and in vivo anticancer activities against prostate and other cancers, but the mechanism of its action is not fully understood. In this study, we performed RNA-seq analysis using 22Rv1 human prostate cancer cell line to identify mechanistic targets of WA. Kyoto Encyclopedia of Genes and Genomes pathway analysis of the differentially expressed genes showed most significant enrichment of genes associated with metabolism. These results were validated using LNCaP and 22Rv1 human prostate cancer cells and Hi-Myc transgenic mice as models. The intracellular levels of acetyl-CoA, total free fatty acids and neutral lipids were decreased significantly following WA treatment in both cells, which was accompanied by downregulation of mRNA (confirmed by quantitative reverse transcription-polymerase chain reaction) and protein levels of key fatty acid synthesis enzymes, including ATP citrate lyase, acetyl-CoA carboxylase 1, fatty acid synthase and carnitine palmitoyltransferase 1A. Ectopic expression of c-Myc, but not constitutively active Akt, conferred a marked protection against WA-mediated suppression of acetyl-CoA carboxylase 1 and fatty acid synthase protein expression, and clonogenic cell survival. WA was a superior inhibitor of cell proliferation and fatty acid synthesis in comparison with known modulators of fatty acid metabolism including cerulenin and etomoxir. Intraperitoneal WA administration to Hi-Myc transgenic mice (0.1 mg/mouse, three times/week for 5 weeks) also resulted in a significant decrease in circulating levels of total free fatty acids and phospholipids, and expression of ATP citrate lyase, acetyl-CoA carboxylase 1, fatty acid synthase and carnitine palmitoyltransferase 1A proteins in the prostate in vivo.
The results of the present study indicate that WA is a novel inhibitor of fatty acid synthesis in prostate cancer cells in vitro and in vivo.
Introduction
Prostate cancer is a leading cause of cancer-related mortality in American men (1). Chemoprevention of prostate cancer remains appealing for decreasing the morbidity and mortality associated with this disease. Prior chemoprevention efforts with chemical inhibitors of 5α-reductases, which are involved in steroid metabolism but mostly known for their role in conversion of testosterone to more potent dihydrotestosterone, were not fruitful (2,3). Even though administration of 5α-reductase inhibitors (finasteride or dutasteride) caused a ~23–25% decrease in overall relative risk, they were not approved by the United States Food and Drug Administration for chemoprevention of prostate cancer because of prevalence of high-grade tumors in the treatment arm (2–4). Another large clinical trial using selenium and vitamin E combination did not show any preventative benefit for prostate cancer (5,6). These findings underscore the need for a safe and effective intervention for chemoprevention of prostate cancer.
Medicinal plants and/or bioactive phytochemicals derived from them continue to be investigated for therapy and/or chemoprevention of cancer. Withania somnifera (also known as Ashwagandha) is an attractive medicinal plant that is still used heavily in complementary and alternative medicine practices (e.g. Ayurveda, Siddha and Unani medicine practices) in India and bordering countries (7). Some of the pharmacological effects of W.somnifera include management of male reproductive functions, neuroprotective potential and anticancer effects (7–10). The ClinicalTrials.gov lists >15 completed or ongoing clinical trials for W.somnifera extract for different conditions. Some of the published studies have explored clinical effects of W.somnifera extract in patients with anxiety and schizophrenia and on muscle strength and recovery (11–13). Supplements of W.somnifera extract are available over the counter in the United States.
Devi et al. (14) were the first to study in vivo anticancer potential of alcoholic extract of the root of W.somnifera using a transplantable sarcoma 180 model. Complete response was observed in 55% of the mice following daily intraperitoneal administration of 1000 mg/kg extract for 15 days (14). The same group of investigators reported radio-sensitizing effect of the alcoholic extract of W.somnifera in the sarcoma 180 model (15). The phytochemical composition of the W.somnifera root and/or leaf extract is quite complex and includes withanolides (steroidal lactones), alkaloids, and sitoindosides (7). Although the anticancer potency of every phytochemical constituent of W.somnifera extract has not been investigated systematically, this effect is largely attributed to withaferin A (WA). Shohat et al. (16) were the first to demonstrate in vitro anticancer effect of WA. The in vitro and/or in vivo anticancer effect of WA has now been shown for different solid tumors including prostate, breast and non-small cell lung cancer to name a few (17–21). Studies have revealed in vivo growth inhibitory effects of WA against prostate cancer in both therapeutic and chemopreventative settings (17,18,22,23).
Elucidation of the mechanism underlying cancer chemoprevention by WA is important for its clinical development. At the cellular level, anticancer effect of WA in prostate cancer cells is associated with cell cycle arrest and apoptosis induction (18,24). Despite these insights, the mechanism underlying anticancer effect of WA in prostate cancer is not fully appreciated. In this study, we performed RNA-seq analysis using a human prostate cancer cell line to characterize WA-regulated transcriptome and mechanistic targets.
Materials and methods
Ethics statement
Use of the Hi-Myc mice for this study was approved by the Animal Care and Use Committee of the University of Pittsburgh. The Hi-Myc transgenic mice were generated by in-house breeding. Male transgenic FVB-Tg(ARR2/Pbsn-MYC) mice were obtained from the National Institutes of Health Mouse Repository and crossed with wild-type female FVB/N mice to obtain male Hi-Myc transgenic mice.
Reagents and cell lines
WA was purchased from ChromaDex (Irvine, CA), and its stock solution was prepared in dimethyl sulfoxide (DMSO). Antibodies against fatty acid synthase (FASN; for immunoblotting), acetyl-CoA carboxylase 1 (ACC1 or ACACA; for immunoblotting), c-Myc, p-Akt (S473) and total Akt were from Cell Signaling Technology (Danvers, MA), antibodies against ATP citrate lyase (ACLY; for immunoblotting and immunohistochemistry), ACC1 (for immunohistochemistry), FASN (for immunohistochemistry) and carnitine palmitoyltransferase 1A (CPT1A; for immunoblotting and immunohistochemistry) were from Abcam (Cambridge, MA), an antibody against α-smooth muscle actin was from Santa Cruz Biotechnology (Dallas, TX), and anti-β-Actin antibody was from Sigma–Aldrich (St. Louis, MO). Kits for determination of intracellular lactate (K607-100) and acetyl-CoA (K317-100) were from BioVision (Milpitas, CA), whereas kits for the determination of total free fatty acids (MAK044) and total phospholipids (MAK122) were purchased from Sigma–Aldrich.
Human prostate cancer cell lines (LNCaP and 22Rv1) were purchased from the American Type Culture Collection (Manassas, VA) and maintained as recommended by the provider. Because more than 90% of castration-resistant prostate cancer exhibits overexpression of androgen receptor and its splice variants, to represent hormone-sensitive and castration-resistant prostate cancer, we selected LNCaP and 22Rv1 cell lines for our studies. Both cell lines were last authenticated by us in March of 2017 by short tandem repeat profiling and found to be of human origin. Details of stable transfection of 22Rv1 cells with pcDNA3 empty vector and the same vector encoding c-Myc and their culture have been described by us previously (25). The 22Rv1 cells were also stably transfected with pcDNA3 empty vector and constitutively active Akt (caAkt) plasmid, and stable clones were selected in the presence of 800 µg/ml G418. The c-Myc and caAkt plasmids were purchased from Addgene (Watertown, MA).
RNA-seq analysis
Cells were plated in 10-cm dishes at a density of 1.5 × 106 cells/dish in triplicate and incubated overnight for attachment. The cells were treated with DMSO (final concentration: 0.01%) or 2 µM of WA for 16 h prior to RNA isolation and RNA-Seq analysis. RNA-seq analysis was outsourced to Novogene (Sacramento, CA). Briefly, the cells were harvested by trypsin treatment and total RNA was extracted using the RNeasy mini kit (Qiagen, Germantown, MD). RNA quality was determined by RNA integrity number and Qubit®. Downstream analysis was performed using a combination of programs including STAR, HTseq, Cufflink and wrapped scripts. Alignments were analyzed with the use of Tophat program, and differential expressions were determined by DESeq2. Reference genome and gene annotation files were downloaded from the NCBI/UCSC/Ensembl. The paired-end clean reads were aligned to the reference genome using STAR (v2.5).
The count/read numbers of the mapped genes were determined using HTSeq v0.6.1. The fragments per kilobase per million mapped (FPKM) was calculated based on the length of the gene as well as read counts mapped to this gene. FPKM accounts for the effect of sequencing depth and gene length for the read count and is currently the most commonly used method for estimating gene expression level (26).
Differential gene expression analysis between control and WA-treated samples was performed using the DESeq2 R package (2_1.6.3), which provides statistical analysis for differential gene expression based on the negative binomial distribution model. The resulting P-values were adjusted using the Benjamini and Hochberg’s approach for controlling the false discovery rate. Genes with an adjusted P-value < 0.05 from DESeq2 were considered significantly different from control. The Venn diagram was created using the Venn Diagram function in R. To determine log adjustment, genes with 0 FPKM were assigned a value of 0.001.
Gene ontology and Kyoto Encyclopedia of Genes and Genomes enrichment analysis of differentially expressed genes
The gene ontology (GO) enrichment analysis of differentially expressed genes was performed using the ClusterProfiler R package in which the gene length bias was corrected. GO terms with corrected P-value less than 0.05 were considered significantly enriched. Kyoto Encyclopedia of Genes and Genomes (KEGG) is a database resource for understanding high-level functions and utilities of the biological system, such as the cell, the organism and the ecosystem, from molecular level information, especially large-scale molecular datasets generated by genome sequencing and other high-through put experimental technologies (http://www.genome.jp/kegg/). We used ClusterProfiler R package to test the statistical enrichment of differentially expressed genes in the KEGG pathways.
Accession number
The RNA-seq data presented in this study have been submitted to the Gene Expression Omnibus of NCBI and can be retrieved by accession number GSE137519.
Determination of intracellular levels of lactate, acetyl-CoA, total free fatty acids, and total phospholipids, and visualization of neutral lipid droplets
Intracellular and circulating (mouse plasma) levels of lactate, total free fatty acids, acetyl-CoA and/or total phospholipids were determined as recommended by the manufacturer of the specific kit. Other details of these assays such as sample preparation have been described by us previously (27,28). Neutral lipid droplets were visualized by BODIPY staining. Cells were plated on coverslips in duplicate, treated with DMSO or WA and incubated with 1 μg/ml BODIPY for 1 h at room temperature. At least five non-overlapping images were captured from each slide, and number of neutral lipid droplet-positive cells was quantified using ImageJ software.
Real-time quantitative RT-PCR
Total RNA from cells was extracted using RNeasy kit from Qiagen (Germantown, MD). Total RNA (1 µg) was used for cDNA synthesis using superscript reverse transcriptase (Life Technologies) and oligo (dT)20 primer. Real-time qRT-PCR was performed using 2× SYBR green qPCR kit (Thermo Fisher Scientific, Waltham, MA) with 95°C (15 s), 60°C (1 min) and 72°C (30 s) for 40 cycles. Primers were as follow: ACLY Forward: 5′- GGTGCTCCGGATTTTGC-3′; Reverse: 5′-ACATGGCTGCAGAGAGACCT-3′; ACC1 (also abbreviated as ACACA) Forward: 5′-AGTGGGTCACCCCATTGTT-3′; Reverse: 5′-TTCTAACAGGAGCTGGAGCC-3′; FASN Forward: 5′-AGTACACACCCAAGGCCAAGT-3′; Reverse: 5′-TCGATGACGTGGACGGATACT-3′; c-Myc Forward: 5′-GCCACGTCTCCACACATCAG-3′; Reverse: 5′-TGGTGCATTTTCGGTTGTTG-3′; glyceraldehyde 3-phosphate dehydrogenase (GAPDH) Forward: 5′-GGACCTGACCTGCCGTCTAGAA-3′; Reverse: 5′-GGTGTCGCTGTTGAAGTCAGAG-3′. Relative gene expression was calculated using the method described by Livak and Schmittgen (29).
Confocal microscopy
Cells were plated on coverslips in 12-well plates, allowed to attach by overnight incubation and then treated with DMSO or WA for 24 h. Other details of immunocytochemistry were essentially the same as described by us previously (30). The primary antibodies used were anti-ACLY (1:2000 dilution), anti-ACC1 (1:1000 dilution) and anti-FASN (1:1000 dilution). Nuclei were stained with DRAQ5. For CPT1A, the cells were treated with 100 nM Mitotracker Red at 37°C for 30 min to label mitochondria followed by incubation with anti-CPT1A antibody (1:3000 dilution). Cells were observed under a confocal microscope. Corrected total cell fluorescence was quantitated using ImageJ software.
Western blotting
Lysates from control (DMSO-treated) or WA-treated cells were prepared as described by us previously (31). Western blotting was performed as described by us previously (31). Densitometric quantitation was performed using UN-SCAN-IT software (Silk Scientific, Orem, UT). Blots were stripped and re-probed with anti-β-Actin antibody for protein normalization.
Clonogenic and cell proliferation assays, and quantitation of apoptosis
For clonogenic assay, cells (500 cells) were plated in six-well plates in triplicate. After overnight incubation, cells were treated with DMSO or specified concentrations of WA. The medium containing DMSO or WA was changed every third day. After 12 days, the cells were fixed with methanol and stained with crystal violet for 30 min. Colonies were counted using GelCount (Oxford Optronix, Abingdon, UK). The effect on cell proliferation was determined using a kit from Promega (Madison, WI; G3582), and instructions from the supplier of the kit were followed for this assay. Apoptosis was measured by flow cytometry using Annexin V/propidium iodide kit from BD Biosciences (San Jose, CA; 556547). Briefly, cells (2 × 105 cells/well in six-well plates) were seeded, allowed to attach by overnight incubation and then exposed to DMSO or desired concentrations of WA, cerulenin (Cer) or etomoxir (Eto) for 48 h. The floating and adherent cells were collected and processed for flow cytometry.
In vivo study
After transgene verification, 5 week old male transgenic Hi-Myc mice were divided into two groups. Control group of mice were administered with 100 µl of vehicle consisting of DMSO (10%), ethanol (10%), Cremophor EL (30%) and phosphate-buffered saline (50%), whereas the mice of WA group were administered with 0.1 mg/mouse in the same vehicle. Treatment was done intraperitoneally three times/week for 5 weeks. Body weight was measured weekly. At the end of the study, blood and prostate tissues were collected. Prostate tissues were fixed in 10% neutral-buffered formalin and paraffin-embedded and used for immunohistochemistry.
Immunohistochemistry
Details of immunohistochemistry are described in our prior publications (27,30). Stained sections were examined under Olympus FluoView FV1000 confocal microscope at 40× oil immersion objective magnification. At least 10 non-overlapping and non-necrotic images were captured from each section and analyzed with ImageJ software.
Statistical analysis
Statistical tests were performed using GraphPad Prism (version 7.02). Student’s t-test was performed for statistical comparisons between two groups. One-way analysis of variance (ANOVA) followed by Dunnett’s test was used for dose–response comparisons, whereas ANOVA followed by Bonferroni’s test was used for multiple group comparisons.
Results
RNA-seq analysis
The peak plasma concentration of WA in mice is 1.8 μM following administration of 4 mg/kg that equates to roughly 0.1 mg/mouse (32). Pharmacological effects of WA have been observed after treatment of cancer cells for 8–24 h (17–21). Therefore, 22Rv1 cells were treated with 2 μM WA for 16 h prior to RNA isolation and RNA-seq analysis. The RNA-seq mapping data are summarized in Figure 1A. The total mapping rates for the control and WA-treated samples were about 94 ± 0.1% and 96 ± 0.02%, respectively (Figure 1A).
Figure 1.
Differential expression of genes in response to WA treatment in 22Rv1 cells. (A) Summary of the RNA-seq mapping results. (B) Volcano diagram of the WA-regulated genes. (C) Venn diagram showing unique and overlapping gene expression between control and WA-treated 22Rv1 cells. (D) Heat maps of the differentially expressed genes between control and WA-treated 22Rv1 cells (n = 3).
A Volcano diagram was generated to visualize the distribution of differentially expressed genes between control and WA-treated 22Rv1 cells with an adjusted P-value of <0.05 (Figure 1B). The Venn diagram revealed unique and overlapping gene expression between control and WA treatment groups (Figure 1C). The heatmaps of three replicates of control and WA-treated samples exhibited highly consistent transcriptional changes (Figure 1D).
The KEGG pathway analysis
The KEGG pathway analysis revealed changes in expression of genes linked to cancer, including metabolic pathway, oxidative phosphorylation, cell cycle, ubiquitin-mediated proteolysis and so forth (Figure 2A and B). Top five cancer-relevant pathways with significantly upregulated genes following WA treatment included pathways in cancer (112 genes), mitogen-activated protein kinases signaling (102 genes), focal adhesion (82 genes), endocytosis (86 genes) and protein processing in endoplasmic reticulum (83 genes) as revealed by KEGG analysis (Figure 2A). The KEGG pathway analysis also showed downregulation of expression of genes associated with multiple cancer-relevant pathways in WA-treated cells, including metabolic pathway (476 genes), oxidative phosphorylation (83 genes), tricarboxylic acid cycle (23 genes), cell cycle (82 genes) and base excision repair (24 genes) (Figure 2B). However, the most significant downregulation was observed for genes linked to metabolic pathway (Figure 2B).
Figure 2.
Functional analyses of the differentially expressed genes between control and WA-treated 22Rv1 cells. Scatter plots for Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses (A, B), gene ontology (GO) enrichment analyses (C, D) and Reactome enrichment analyses (E, F) showing upregulation or downregulation of genes following 16 h treatment of 22Rv1 cells with 2 μM WA in comparison with the solvent control. The horizontal axis represents the GeneRatio of the differentially expressed genes in each term. The vertical axis shows description of each term. Count represents the number of differentially expressed genes. Padj defines the adjusted P-value.
The GO enrichment analysis
The GO enrichment analyses including all three ontologies (cellular component, molecular function and biological processes) were performed to identify the biological functions of the genes affected by WA treatment. The WA treatment resulted in upregulation of genes associated with process utilizing autophagic mechanism/macroautophagy, proteasomal protein catabolic process, proteasome-mediated ubiquitin-dependent protein degradation and proteasome complex (Figure 2C). The downregulated genes following WA treatment were associated with GO pathway terms ribonucleoprotein complex biogenesis, mitochondrial matrix/mitochondrial inner membrane, non-coding RNA processing, rRNA processing/rRNA metabolic process and DNA replication (Figure 2D).
Reactome analysis
The Reactome database covers curated annotations for diverse set of molecular and cell biological topics, including cell cycle, metabolism, signaling, transport, cell motility, immune function, host–virus interaction and neural function. Genes associated with cellular response to stress/external stimuli, Class I major histocompatibility complex and deubiquitination were significantly upregulated in WA-treated cells compared with control (Figure 2E). Significantly downregulated genes in WA-treated cells in comparison with control were related to translation, tricarboxylic acid cycle, rRNA processing and other pathways (Figure 2F).
WA treatment inhibited fatty acid synthesis
Because downregulation of genes associated with metabolism was the most significant enrichment in the KEGG pathway analysis, we focused on this aspect for validation of the RNA-seq results. Prostate cancer is a metabolically heterogenous disease with enrichment of glycolytic or lipogenic phenotypes (33). Consistent with data in breast cancer animal models (34,35), intracellular level of lactate (a marker of glycolytic phenotype) was decreased in LNCaP cells following WA treatment, and this effect was relatively less pronounced in 22Rv1 cells (Figure 3A). The levels of acetyl-CoA (Figure 3B), which is the building block of fatty acid synthesis (36,37), as well as total free fatty acids (Figure 3C) were significantly lower in WA-treated LNCaP and 22Rv1 cells when compared with corresponding solvent-treated control. Representative microscopic images for BODIPY stain demonstrating accumulation of neutral lipid droplets in LNCaP and 22Rv1 cells after 24 h of treatment with DMSO (control) or WA are shown in Figure 3D. The number of neutral lipid droplet-positive cells was decreased significantly after 24 h exposure of LNCaP and 22Rv1 cells to WA when compared with corresponding DMSO-treated control (Figure 3E). These results indicated suppression of intracellular levels of fatty acids by WA treatment in prostate cancer cells.
Figure 3.
WA treatment suppressed fatty acid levels in human prostate cancer cells. (A) Intracellular lactate levels in LNCaP and 22Rv1 cells after treatment with DMSO or the indicated doses of WA for 24 h. Results are shown as mean ± SD (n = 3). Statistical analysis was performed by one-way ANOVA followed by Dunnetts’s test (*P < 0.05). Levels of (B) acetyl-CoA and (C) total free fatty acids in LNCaP and 22Rv1 cells after 24 h of treatment with DMSO or the indicated doses of WA. Results are shown as mean ± SD (n = 3). *Significantly different (P < 0.05) compared with DMSO-treated control by one-way ANOVA with Dunnett’s adjustment. (D) Representative confocal microscopic images (60× oil objective magnification) showing BODIPY staining in WA-treated LNCaP and 22Rv1 cells (24 h treatment). (E) Quantification of number of neutral lipid droplet positive cells in LNCaP and 22Rv1 cells. Experiment was repeated twice in duplicate and combined results are shown as mean ± SD (n = 4). *Significantly different (P < 0.05) compared with DMSO-treated control by one-way ANOVA with Dunnett’s adjustment. (F) Real-time qRT-PCR analysis for the expression of ACLY, ACC1 (ACACA) and FASN mRNA in LNCaP and 22Rv1 cells after 24 h of treatment with DMSO or the indicated doses of WA. Results shown are mean ± SD (n = 3). Similar results were observed in replicate experiments. *Significantly different (P < 0.05) compared with DMSO-treated control by one-way ANOVA with Dunnett’s adjustment. (G) ACLY, ACC1 (ACACA) and FASN gene expression in control and WA-treated 22Rv1 cells from the RNA-seq analysis. Data shown are mean ± SD (n = 3). Statistical analysis was performed using two-tailed Student’s t-test (*P < 0.05).
WA treatment downregulated expression of key fatty acid synthesis enzymes in vitro
Fatty acid synthesis begins with ACLY-mediated conversion of citrate to acetyl-CoA (36,37). The rate-limiting step of the de novo fatty acid synthesis, i.e., conversion of acetyl-CoA to malonyl-CoA, is catalyzed by ACC (36,37). Finally, one molecule of acetyl-CoA plus seven molecules of malonyl-CoA are utilized to synthesize saturated fatty acids (e.g. 16-carbon palmitate) (36,37). To determine the mechanism underlying fatty acid synthesis inhibition by WA, we determined its effect on mRNA levels of ACLY, ACC1 (ACACA) and FASN. Expression of mRNA of ACLY, ACC1 (ACACA) and FASN was decreased significantly upon treatment of LNCaP and 22Rv1 cells with WA (Figure 3F), and these results were consistent with the RNA-seq data (Figure 3G). Gene expression changes follwing WA treatment for enzyme proteins related to glycolysis and lipid synthesis from the RNA-seq analyses are listed in Supplementary Table S1, available at Carcinogenesis Online.
Confocal microscopy images for ACLY, ACC1, FASN and CPT1A (which plays an important role in mitochondrial transport of fatty acid metabolites) protein expression in DMSO-treated control and WA-treated (24 h treatment) LNCaP and 22Rv1 cells can be visualized in Figure 4A and Supplementary Figure S1, available at Carcinogenesis Online, respectively. As expected, ACLY, ACC1 and FASN proteins were mainly localized in the cytoplasm, whereas CPT1A was associated with the mitochondria (Figure 4A and Supplementary Figure S1, available at Carcinogenesis Online). Quantitation of the immunofluorescence revealed a dose dependent and statistically significant decrease in level of each protein after treatment with WA in both LNCaP and 22Rv1 cells (Figure 4B).
Figure 4.
WA treatment decreased the expression levels of fatty acid metabolism proteins in human prostate cancer cells. (A) Representative confocal microscopic images (60× oil objective magnification) for ACLY, ACC1, FASN and CPT1A protein expression (green fluorescence) in LNCaP cells after 24 h of treatment with DMSO or 2 µM WA. DRAQ5 (blue fluorescence) and Mitotracker (red fluorescence) were used to stain nuclei and mitochondria, respectively. (B) Quantitation of corrected total cell fluorescence (CTCF) for ACLY, ACC1, FASN and CPT1A protein expression in LNCaP and 22Rv1 cells treated with DMSO or the indicated doses of WA for 24 h. Results shown are mean ± SD (n = 6–15). *Significantly different (P < 0.05) compared with DMSO-treated control by one-way ANOVA followed by Dunnett’s adjustment. (C) Western blots for ACLY, ACC1, FASN, and CPT1A proteins using lysates from control and WA-treated LNCaP and 22Rv1 cells. (D) Quantitation of the ACLY, ACC1, FASN and CPT1A protein expression from the western blot experiments. The results shown are mean ± SD from three independent experiments. *Significantly different (P < 0.05) compared with DMSO-treated control by one-way ANOVA followed by Dunnett’s test.
We confirmed the results of confocal microscopy by western blotting. As can be seen in Figure 4C, protein levels of ACLY, ACC1 and FASN were decreased in a dose-dependent manner following WA treatment. The inhibitory effect of WA was most pronounced at the 24 h time point but observed even after 16 h of treatment especially at the 2 μM dose (Figure 4D). The results were somewhat different for CPT1A, whose expression was initially increased upon WA treatment followed by a decline in its level at the 24 h time point (Figure 4C and D). These results indicated that WA was an inhibitor of fatty acid synthesis in vitro in prostate cancer cells.
Mechanism underlying WA-mediated inhibition of fatty acid synthesis
Previous studied have implicated c-Myc and Akt in regulation of lipid synthesis (38,39). As can be seen in Figure 5A, the S473 phosphorylation of Akt (a measure of Akt activation) was markedly higher in 22Rv1 cells with stable overexpression of caAkt when compared with the empty vector (Vec) transfected control cells. The level of S473 phosphorylated p-Akt was decreased after treatment with WA (Figure 5A). The level of total Akt protein was also decreased after WA treatment (Figure 5A). These results indicated that WA treatment inhibited activation of Akt by downregulating its protein level. However, overexpression of caAkt had no meaningful influence on WA-mediated downregulation of FASN or ACC1 proteins (Figure 5A). It is puzzling to note that clonogenicity of 22Rv1 cells decreased by overexpression of caAkt in the absence of WA treatment (Figure 5B). The reasons for this discrepancy are not clear. Nevertheless, the WA-mediated downregulation of ACLY protein was partially reversible by overexpression of caAkt (Figure 5A). Overexpression of caAkt did not confer protection against colony formation inhibition by WA treatment (Figure 5B). These results indicated that downregulation of fatty acid synthesis enzyme proteins by WA treatment was probably not mediated by Akt.
Figure 5.
The role of caAkt and c-Myc in WA-mediated suppression of levels of fatty acid metabolism proteins. (A) Immunoblotting for p-Akt (S473), total Akt, ACLY, ACC1, FASN and β-Actin proteins using lysates from 22Rv1 cells stably transfected with the empty vector (Vec) or caAkt, and treated for 24 h with DMSO or the indicated doses of WA. Numbers on top of bands are fold change in protein level relative to corresponding DMSO-treated empty vector transfected Vec cells. (B) Representative images of colony formation for Vec and caAkt cells after treatment with DMSO or the indicated doses of WA. The graphs show quantitation of colony formation from data shown in the upper panel. Results shown as mean ± SD (n = 3). Statistically significant (P < 0.05) compared with corresponding DMSO-treated control (*) or between Vec and caAkt cells at the same dose of WA (#) by one-way ANOVA followed by Bonferroni’s multiple comparisons test. (C) Western blots for c-Myc, ACLY, ACC1, FASN, CPT1A and β-Actin proteins in 22Rv1 cells stably transfected with empty vector (Vec) or c-Myc plasmid (c-Myc) and treated for 24 h with DMSO or the indicated doses of WA. Numbers on top of the bands are fold changes in protein levels relative to DMSO-treated Vec cells. (D) Representative images for colony formation in Vec and c-Myc cells cultured in the absence or presence of different doses of WA for 12 days. Bar graph shows quantitation of colony formation as mean ± SD (n = 3). Statistically significant (P < 0.05) compared with respective DMSO-treated control (*) or between Vec and c-Myc cells at the same dose of WA (#) by one-way ANOVA followed by Bonferroni’s multiple comparisons test. (E) Total free fatty acids in Vec and c-Myc cells after 24 h treatment with DMSO or 2 µM WA. Results are shown as mean ± SD from three experiments (n = 9). Statistically significant (P < 0.05) compared with respective DMSO-treated control (*) or between Vec and c-Myc cells at the same dose of WA (#) by one-way ANOVA followed by Bonferroni’s multiple comparisons test.
Next, we determined the effect of c-Myc overexpression on WA-mediated downregulation of ACLY, ACC1 and FASN proteins by western blotting (Figure 5C). The protein levels of FASN and ACC1, but not ACLY, were also markedly higher in c-Myc overexpressing 22Rv1 cells than in empty vector transfected control Vec cells. Forced expression of c-Myc in 22Rv1 cells conferred marked protection against WA-mediated decrease in expression of FASN and ACC1 proteins (Figure 5C) as well as inhibition of colony formation (Figure 5D) and total free fatty acid level suppression (Figure 5E). The mRNA level of c-Myc was decreased in a dose-dependent manner following WA exposure in both LNCaP and 22Rv1 cells (Supplementary Figure S2A, available at Carcinogenesis Online). We also considered the possibility of post-transcriptional mechanism in WA-mediated downregulation of the c-Myc protein expression. Downregulation of c-Myc protein upon WA treatment was maintained in the presence of a proteasome inhibitor MG132 (Supplementary Figure S2B, available at Carcinogenesis Online). Moreover, RNA-seq analysis also showed downregulation of c-Myc in 22Rv1 cells when compared with control (data not shown). Collectively, these results indicated that c-Myc was involved in WA-mediated downregulation of at least ACC1 and FASN proteins, and WA treatment decreased transcription of c-Myc in both LNCaP and 22Rv1 cells. From the data presented in this study, it is possible that c-Myc is a negative regulator of ACLY but additional experiments are necessary to validate this possibility.
WA was a superior inhibitor of cell proliferation and fatty acid synthesis than Cer and Eto in prostate cancer cells in vitro
We compared efficacies of WA with a few other known inhibitors of fatty acid metabolism, including Cer and Eto for inhibition of cell proliferation and total free fatty acids levels. Same dose and treatment conditions were used for each agent for direct comparison. As can be seen in Supplementary Figure S3A, available at Carcinogenesis Online, WA was superior to Cer and Eto for inhibition of cell proliferation. Because WA and Cer are known to cause apoptotic cell death, their cell death induction potencies were also compared, and the results are shown in Supplementary Figure S3B, available at Carcinogenesis Online. WA treatment resulted in a dose dependent and statistically significant increase in apoptosis in both LNCaP and 22Rv1 cells when compared with the corresponding solvent-treated controls, but no such apoptotic cell death was evident after a similar treatment with Cer or Eto in either cell line (Supplementary Figure S3B, available at Carcinogenesis Online). Finally, suppression of intracellular levels of total free fatty acids was observed after treatment of LNCaP and 22Rv1 cells with WA, but not with Cer or Eto (Supplementary Figure S3C, available at Carcinogenesis Online). There results clearly indicated that WA was superior to Cer and Eto for inhibition of cell proliferation and suppression of fatty acid synthesis in prostate cancer cells.
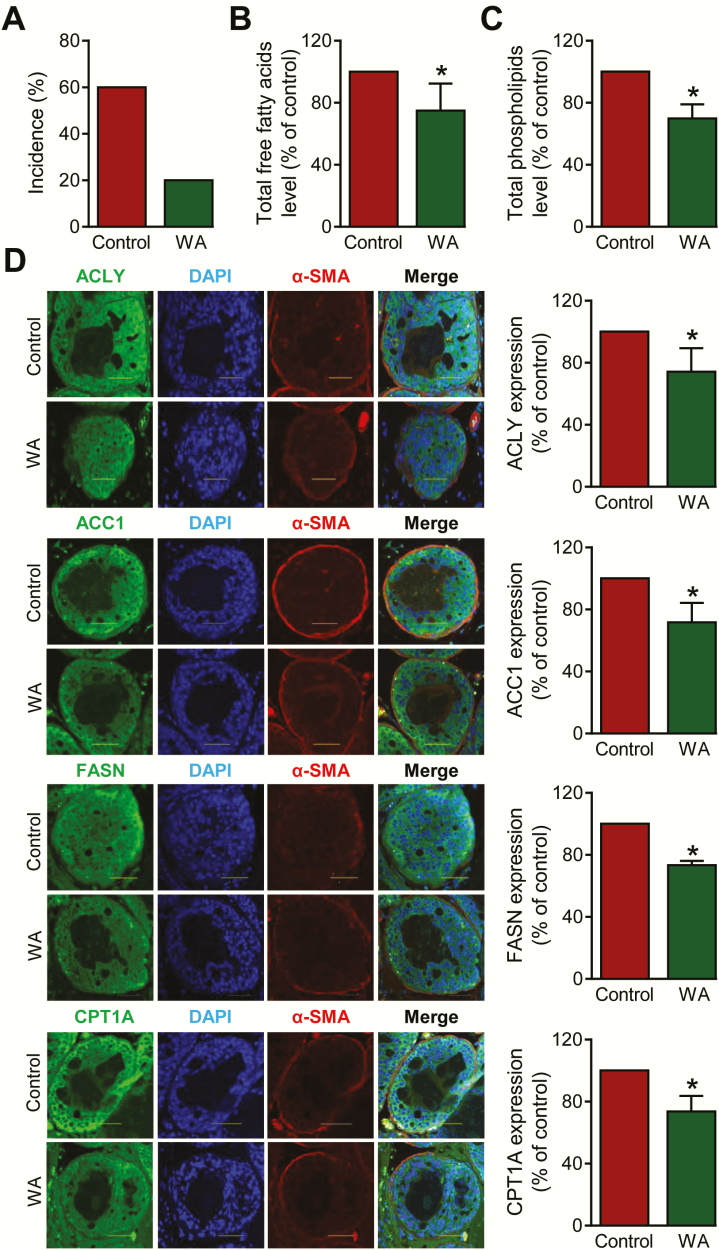
WA administration to Hi-Myc transgenic mice resulted in suppression of circulating levels of total free fatty acids and total phospholipids in vivo
As can be seen in Figure 6A, a mild regimen of WA administration (0.1 mg WA/mouse, three times/week for 5 weeks) to Hi-Myc transgenic mice (n = 5 for both groups) caused ~67% decrease in incidence of carcinoma in situ. The difference was not statistically significant because this study was not powered for cancer incidence, but instead carried out to determine the effect of WA on circulating levels of total free fatty acids. The circulating levels of total free fatty acids (Figure 6B) and total phospholipids (Figure 6C) were decreased significantly by WA administration. The Hi-Myc mice at 10 weeks of age develop microscopic tumors and thus measurements of total free fatty acids or phospholipid was not possible in the prostate tumor tissues. Body weight of the mice was not affected by WA treatment when compared with mice treated with the vehicle (data not shown). We also carried out immunohistochemistry for ACLY, ACC1, FASN and CPT1A protein expression using prostate tissue sections from control- and WA-treated Hi-Myc mice. The α-smooth muscle actin was used to stain stroma. As can be seen in Figure 6D, expression of each protein was significantly lower in the prostate of WA-treated Hi-Myc mice compared with controls. These results provided in vivo evidence for WA-mediated suppression of fatty acid synthesis enzyme proteins in prostate cancer cells.
Figure 6.
WA administration to Hi-Myc transgenic mice resulted in suppression of circulating (plasma) levels of total free fatty acids and total phospholipids. (A) Incidence of carcinoma in situ in Hi-Myc mice of the WA treatment group and the control group. Levels of total free fatty acids (B) and total phospholipids (C) in the plasma samples of control and WA-treated Hi-Myc mice. Results shown are mean ± SD (n = 5 for both group). *Significant compared with control (P < 0.05) by unpaired Student’s t-test. (D) Representative microscopic images for ACLY, ACC1, FASN, CPT1A and α-SMA expression in prostate tissues from Hi-Myc mouse (green or red fluorescence, 40× oil objective magnification, scale bar = 50 µm). Nuclei were visualized by staining with DAPI. Bar graphs show quantitation of ACLY, ACC1, FASN and CPT1A protein expression using ImageJ software. Results shown are mean ± SD (n = 5 for both group). *Statistically significant compared with control (P < 0.05) by Student’s t-test.
Discussion
The present study provides further mechanistic insights into the anticancer activity of WA in prostate cancer cells. Exposure of prostate cancer cells to a pharmacological dose of WA resulted in changes in expression of genes relevant to many cancer-relevant pathways. For example, cell cycle arrest is one of the known consequences of WA exposure in prostate cancer cells (24), and enrichment of cell cycle pathway was observed in the RNA-seq analysis (present study). Moreover, RNA-seq data was consistent with the results of many published studies (17–21). For example, consistent with published results (17–21,24) RNA-seq analyses revealed upregulation of Bax, p27, and Par-4 but downregulation of cyclins A2, E2, and B1, and AKT1 genes in WA-treated 22Rv1 cells compared with control (Supplementary Figure S4, available at Carcinogenesis Online). A decrease in protein level of total Akt was also observed in the present study (Figure 5A).
The GO and Reactome pathway analyses of the RNA-seq data also revealed alterations in expression of genes of pathways potentially important in anticancer effect of WA. For example, the GO enrichment analysis revealed upregulation of autophagy-related genes in 22Rv1 cells (Figure 2C). Autophagy has been shown in WA-treated cells (40,41). Autophagy is an evolutionarily conserved process for degradation of macromolecules and different organelles and an established cancer therapeutic target (42). We have reported previously autophagy induction by WA in breast cancer cells using transmission electron microscopy and western blotting for processing of microtubule associated protein 1 light chain 3 isoform B (43). A study in PC-3 prostate cancer cells also demonstrated autophagy induction by WA (40). Further work is necessary to test whether WA treatment causes autophagy in vivo or its anticancer activity in preclinical models is affected by inhibition of autophagy.
Identification of mechanistic pharmacodynamic biomarker(s) and its validation using cellular and in vivo models is an important consideration especially for clinical development of chemopreventive interventions. The present study demonstrates for the first time that WA is a potent inhibitor of fatty acid synthesis in vitro and in vivo. We propose that circulating levels of total free fatty acids and prostate tumor levels of ACLY, ACC1 and FASN represent potential pharmacodynamic biomarkers of WA in future clinical trials. Strikingly, WA appears superior to Cer and Eto with regards to the inhibition of intracellular free fatty acids level as well as cell proliferation.
The β-oxidation of fatty acids is the primary bioenergetics pathway in prostate cancer (44). The CPT1A plays an important role in mitochondrial uptake of fatty acid intermediates and its expression was decreased by WA treatment. The decrease in acetyl-CoA levels resulting from WA treatment could be a consequence of both ACLY and CPT1A suppression because acetyl-CoA is also generated by mitochondrial β-oxidation of fatty acids. The rat fibroblast study also showed a role for c-Myc in regulation of CPT1 expression (39). However, c-Myc overexpression in 22Rv1 cells caused a modest induction of the CPT1A protein. It is possible that WA-mediated suppression of CPT1A protein is regulated by an alternate mechanism. Nevertheless, the results of the present study suggest that WA may inhibit β-oxidation of fatty acids but additional work is necessary to test this possibility.
Studies have shown that a subset of prostate cancer patients exhibit enrichment of Akt-dependent glycolytic phenotype (Warburg effect), whereas Myc overexpression correlated with dysregulated lipid metabolism in a subgroup of patients with prostate cancer (33). c-Myc gene copy number increase or lack of phosphatase and tensin homolog (PTEN) expression, which causes activation of Akt were shown to be prognostic factors in relapse after radiotherapy in prostate cancer patients (45). Moreover, combined MYC activation and PTEN loss were sufficient to create genome instability leading to metastatic prostate cancer in mice (46). The present study shows a role for c-Myc in inhibition of fatty acid synthesis by WA. Interestingly, we have shown previously that prevention of breast cancer by WA administration in a transgenic mouse model and in a chemically-induced rat model is associated with inhibition of glycolysis (34,35). In the present study, glycolysis inhibition by WA was observed in LNCaP and 22Rv1 cells, but this effect was less pronounced in the later cell line. It is important to mention that prevention of prostate cancer by oral WA administration in PTEN-deficient transgenic mouse model was associated with suppression of total Akt and p-Akt (23).
In summary, we show for the first time that WA is a potent inhibitor of fatty acid synthesis in prostate cancer cells that is independent of Akt but mediated by suppression of c-Myc.
Supplementary Material
Acknowledgements
This work was supported by the grants RO1 CA142604-10 and RO1 CA225716-1A1 awarded by the National Cancer Institute (S.V. Singh). This research used the Animal Facility and the Tissue and Research Pathology Facility supported in part by the Cancer Center Support Grant from the National Cancer Institute (P30 CA047904).
Glossary
Abbreviations
- ACC1
acetyl-CoA carboxylase 1
- ACLY
ATP citrate lyase
- ANOVA
one-way analysis of variance
- caAkt
constitutively active Akt
- Cer
cerulenin
- CPT1A
carnitine palmitoyltransferase 1A
- DMSO
dimethyl sulfoxide
- Eto
etomoxir
- FASN
fatty acid synthase
- GO
gene ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- WA
withaferin A
Conflict of Interest Statement: None declared.
References
- 1. Siegel R.L., et al. (2019) Cancer statistics, 2019. CA. Cancer J. Clin., 69, 7–34. [DOI] [PubMed] [Google Scholar]
- 2. Thompson I.M., et al. (2003) The influence of finasteride on the development of prostate cancer. N. Engl. J. Med., 349, 215–224. [DOI] [PubMed] [Google Scholar]
- 3. Andriole G.L., et al. ; REDUCE Study Group (2010) Effect of dutasteride on the risk of prostate cancer. N. Engl. J. Med., 362, 1192–1202. [DOI] [PubMed] [Google Scholar]
- 4. Thompson I.M., Jr, et al. (2013) Long-term survival of participants in the prostate cancer prevention trial. N. Engl. J. Med., 369, 603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lippman S.M., et al. (2009) Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA, 301, 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klein E.A., et al. (2011) Vitamin E and the risk of prostate cancer: the selenium and vitamin e cancer prevention trial (select). JAMA, 306, 1549–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palliyaguru D.L., et al. (2016) Withania somnifera: from prevention to treatment of cancer. Mol. Nutr. Food Res., 60, 1342–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nasimi D.A.R, et al. (2018) Effects of Withania somnifera on reproductive system: a systematic review of the available evidence. Biomed. Res. Int., 2018, 4076430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sarris J. (2018) Herbal medicines in the treatment of psychiatric disorders: 10-year updated review. Phytother. Res., 32, 1147–1162. [DOI] [PubMed] [Google Scholar]
- 10. Srivastav S., et al. (2017) Important medicinal herbs in Parkinson’s disease pharmacotherapy. Biomed. Pharmacother., 92, 856–863. [DOI] [PubMed] [Google Scholar]
- 11. Chandrasekhar K., et al. (2012) A prospective, randomized double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of ashwagandha root in reducing stress and anxiety in adults. Indian J. Psychol. Med., 34, 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Agnihotri A.P., et al. (2013) Effects of Withania somnifera in patients of schizophrenia: a randomized, double blind, placebo controlled pilot trial study. Indian J. Pharmacol., 45, 417–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wankhede S., et al. (2015) Examining the effect of Withania somnifera supplementation on muscle strength and recovery: a randomized controlled trial. J. Int. Soc. Sports Nutr., 12, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Devi P.U., et al. (1992) In vivo growth inhibitory effect of Withania somnifera (Ashwagandha) on a transplantable mouse tumor, Sarcoma 180. Indian J. Exp. Biol., 30, 169–172. [PubMed] [Google Scholar]
- 15. Devi P.U., et al. (1993) Antitumor and radiosensitizing effects of Withania somnifera (Ashwagandha) on a transplantable mouse tumor, Sarcoma-180. Indian J. Exp. Biol., 31, 607–611. [PubMed] [Google Scholar]
- 16. Shohat B., et al. (1967) Antitumor activity of withaferin A (NSC-101088). Cancer Chemother. Rep., 51, 271–276. [PubMed] [Google Scholar]
- 17. Yang H., et al. (2007) The tumor proteasome is a primary target for the natural anticancer compound withaferin A isolated from “Indian winter cherry”. Mol. Pharmacol., 71, 426–437. [DOI] [PubMed] [Google Scholar]
- 18. Srinivasan S., et al. (2007) Par-4-dependent apoptosis by the dietary compound withaferin A in prostate cancer cells. Cancer Res., 67, 246–253. [DOI] [PubMed] [Google Scholar]
- 19. Stan S.D., et al. (2008) Withaferin A causes FOXO3a- and Bim-dependent apoptosis and inhibits growth of human breast cancer cells in vivo. Cancer Res., 68, 7661–7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vyas A.R., et al. (2014) Molecular targets and mechanisms of cancer prevention and treatment by withaferin a, a naturally occurring steroidal lactone. AAPS J., 16, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cai Y., et al. (2014) Effect of withaferin A on A549 cellular proliferation and apoptosis in non-small cell lung cancer. Asian Pac. J. Cancer Prev., 15, 1711–1714. [DOI] [PubMed] [Google Scholar]
- 22. Suman S., et al. (2016) Oral administration of withaferin A inhibits carcinogenesis of prostate in TRAMP model. Oncotarget, 7, 53751–53761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moselhy J., et al. (2017) Withaferin A inhibits prostate carcinogenesis in a PTEN-deficient mouse model of prostate cancer. Neoplasia, 19, 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roy R.V., et al. (2013) Withaferin A, a steroidal lactone from Withania somnifera, induces mitotic catastrophe and growth arrest in prostate cancer cells. J. Nat. Prod., 76, 1909–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hahm E.R., et al. (2016) c-Myc is a novel target of cell cycle arrest by honokiol in prostate cancer cells. Cell Cycle, 15, 2309–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mortazavi A., et al. (2008) Mapping and quantifying mammalian transcriptomes by RNA-seq. Nat. Methods, 5, 621–628. [DOI] [PubMed] [Google Scholar]
- 27. Singh K.B., et al. (2017) Fatty acid synthesis intermediates represent novel noninvasive biomarkers of prostate cancer chemoprevention by phenethyl isothiocyanate. Cancer Prev. Res. (Phila.), 10, 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singh K.B., et al. (2018) Inhibition of glycolysis in prostate cancer chemoprevention by phenethyl isothiocyanate. Cancer Prev. Res. (Phila.), 11, 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Livak K.J., et al. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- 30. Singh K.B., et al. (2018) Prostate cancer chemoprevention by sulforaphane in a preclinical mouse model is associated with inhibition of fatty acid metabolism. Carcinogenesis, 39, 826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xiao D., et al. (2003) Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis, 24, 891–897. [DOI] [PubMed] [Google Scholar]
- 32. Thaiparambil J.T., et al. (2011) Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. Int. J. Cancer, 129, 2744–2755. [DOI] [PubMed] [Google Scholar]
- 33. Priolo C., et al. (2014) AKT1 and MYC induce distinctive metabolic fingerprints in human prostate cancer. Cancer Res., 74, 7198–7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hahm E.R., et al. (2013) Metabolic alterations in mammary cancer prevention by withaferin A in a clinically relevant mouse model. J. Natl Cancer Inst., 105, 1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Samanta S.K., et al. (2016) Disease subtype-independent biomarkers of breast cancer chemoprevention by the ayurvedic medicine phytochemical withaferin A. J. Natl Cancer Inst., 109, djw293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Suburu J., et al. (2012) Lipids and prostate cancer. Prostaglandins Other Lipid Mediat., 98, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zadra G., et al. (2013) The fat side of prostate cancer. Biochim. Biophys. Acta, 1831, 1518–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yue S., et al. (2014) Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab., 19, 393–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Edmunds L.R., et al. (2014) c-Myc programs fatty acid metabolism and dictates acetyl-CoA abundance and fate. J. Biol. Chem., 289, 25382–25392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nishikawa Y., et al. (2015) Withaferin A induces cell death selectively in androgen-independent prostate cancer cells but not in normal fibroblast cells. PLoS One, 10, e0134137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Piao L., et al. (2017) Lipopolysaccharides-stimulated macrophage products enhance withaferin A-induced apoptosis via activation of caspases and inhibition of NF-κB pathway in human cancer cells. Mol. Immunol., 81, 92–101. [DOI] [PubMed] [Google Scholar]
- 42. Su M., et al. (2013) Role of the crosstalk between autophagy and apoptosis in cancer. J. Oncol., 2013, 102735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hahm E.R., et al. (2013) Autophagy fails to alter withaferin A-mediated lethality in human breast cancer cells. Curr. Cancer Drug Targets, 13, 640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu Y. (2006) Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic Dis., 9, 230–234. [DOI] [PubMed] [Google Scholar]
- 45. Zafarana G., et al. (2012) Copy number alterations of c-MYC and PTEN are prognostic factors for relapse after prostate cancer radiotherapy. Cancer, 118, 4053–4062. [DOI] [PubMed] [Google Scholar]
- 46. Hubbard G.K., et al. (2016) Combined MYC activation and PTEN loss are sufficient to create genomic instability and lethal metastatic prostate cancer. Cancer Res., 76, 283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.