Abstract
Introduction:
Excessive alcohol use (EAU), a harmful pattern of drinking that includes binge drinking and heavy use, occurs in 25% (binge) and 6% (heavy use) of the US population, respectively. Little is known about alcohol use in individuals with cystic fibrosis (CF). The objective of this investigation is to examine alcohol consumption patterns in individuals with CF using a health survey administered from a social media platform.
Methods:
Individuals with CF, 18 years of age or older, were recruited for participation through social media and internet-based platforms.
Results:
1135 individuals initially participated in the survey and 84% (n=952) were eligible and completed the survey. Of the respondents, 77% (n=729) currently consume alcohol, 18% (n=171) formerly consumed alcohol, and 5% (n=52) never consumed alcohol. Amongst the people with CF who currently consume alcohol, 54% (N=391) met criteria for EAU. Thirty percent of current drinkers experienced symptoms of harmful alcohol use. Of those who met criteria for EAU, 7% wore oxygen, 6% had a lung transplant, 10% had liver disease and 32% had diabetes. Those with EAU reported more hospitalizations than those without EAU [244 (62%) vs 182 (54%), p=0.034]. Characteristics associated with EAU after multivariable adjustment included younger age, unmarried status, male gender and younger age at initiation of drinking.
Conclusion:
EAU is occurring at a much higher proportion in individuals with CF. A substantial percentage of CF individuals with EAU also have medical co-morbidities. Screening, brief intervention, and referral to treatment for EAU in CF clinics is warranted.
Keywords: Alcohol, Cystic fibrosis, Binge pattern drinking, Excessive alcohol use
INTRODUCTION
Excessive alcohol use (EAU), an unhealthy pattern of drinking which includes both binge drinking and chronic heavy use, affects over a quarter of adults in the United States(US).1,2 Of those with EAU, the majority engage in a binge pattern of alcohol consumption, defined as greater than four drinks for females or five drinks for males in a two-hour period.3 Binge drinking is common in the US, with almost 60 million people ages 12 and older reporting binge drinking, and 37 million adults binge drinking at least once a week.4,5 EAU increases the risk of traumatic injury, violence, and risky sexual behaviors.6–8 Commonly associated health problems with EAU include liver dysfunction, pancreatitis, bone marrow suppression, immune dysfunction, increased cancer risk, development of mood disorders and neurologic consequences.6,9–11 In diabetics, alcohol use is associated with hypoglycemia.12 Less appreciated is alcohol’s impact on the lungs, with EAU doubling the risk of an acute lung injury.13 Individuals with EAU are 10 times more likely to develop pneumonia14,15, and further, the pneumonia is more likely to be protracted, severe, and fatal. 16–20
Cystic fibrosis (CF) is a chronic, life-limiting disease with a reported mean survival of 47 years.21 It is an autosomal recessive genetic disorder which causes altered chloride exchange at the surface of epithelium resulting in multisystem abnormalities, most notoriously characterized by progressive lung disease, exocrine pancreatic insufficiency and liver disease.22 Ultimately, over 90% of people with CF eventually succumb to advanced lung disease with a smaller portion developing CF related liver disease (CFLD) necessitating transplant.22 Additionally, almost 40% of adults with CF have diabetes.12,22 In patients with these serious CF comorbidities, minimizing or abstaining from alcohol use would be advised as it could be assumed that the impact alcohol use has upon the lungs, immune system, diabetes and the liver within the general population would be detrimental to the multisystem dysfunction seen in patients with CF.10,12,23,24
Survival for patients with CF has improved significantly over the previous decades resulting in more adults with CF than pediatric patients with CF.21 Therefore adult concerns, such as EAU, need to be investigated and assessed so that effective interventions can be developed and employed.Overone-third of adults with CF have depression, anxiety, or both, and a large international study of individuals with CF found a 2–3 fold increased rate of symptoms of depression and anxiety compared to the general population. 21,25 Further, the observed prevalence of anxiety and depression increases in adolescents and remains high throughout adulthood in people with CF.21 A potential implication is that this is also the time of life associated with increases in risky behavior, including the initiation and escalation of substance use.26 Anxiety and depression are known risk factors for the development of EAU.27,28 The relationship between mental health and alcohol use in CF patients is unclear. There are only scant investigations into the frequency and quantity of alcohol use in individuals with CF, and therefore this information remains unknown. In one study in the United Kingdom, 94–98% of patients over age 18 with CF had tried alcohol, 83% drank alcohol regularly, and 30% admitted to binge drinking. 29 Information about alcohol use in patients with CF is scarce, and as such, it is unclear if use of alcohol in this population is higher than the general population, and consequently no standardized alcohol interventions have been introduced or implemented within the context of CF care.
Given the many pulmonary, hepatic, metabolic and immune consequences of EAU, and the serious pulmonary, liver, and pancreatic comorbidities present in CF patients, the aim of this investigation is to quantify alcohol use and EAU in individuals with CF to determine the prevalence of EAU in this community. Our secondary aims were to identify risk factors for the development of EAU by examining demographic data and explore the possible impact of EAU on self-reported clinical outcomes. Results from this investigation will guide future investigations into EAU in patients with CF including impact on health outcomes, mental health, and development of screening and effective interventions.
METHODS
Individuals with CF who were at least 18 years of age were recruited for participation in the study through closed membership CF support groups found on social media platforms, CF-specific support groups associated with CF blog postings (www.gunnaresiason.com), as well as by participating Cystic Fibrosis Centers through their webpage and via email (Loyola University Medical Center and Johns Hopkins). Individuals interested in participating in the survey were directed through an introduction and implicit consent into the secure web-based survey system, Opinio (Object Planet, Inc; Oslo, Norway), utilized by Loyola University Chicago. Two screening questions were present at the start of the survey, ‘do you have cystic fibrosis’ and ‘are you 18 years or older’, with a no response immediately terminating the survey to fulfill our inclusion criteria of adult CF patients. A generalized alcohol use survey was developed and included questions from several validated alcohol use questionnaires including the Alcohol Use Disorder and Identification Test (AUDIT) and alcohol use questions which are part of the national survey conducted by the Substance Abuse and Mental Health Service Administration (SAMHSA).2,30 The AUDIT is a screening tool developed by the World Health Organization to assess alcohol consumption, drinking behaviors, and alcohol-related problems.30 It is the most widely tested instrument for screening alcohol use disorders in primary care and has been validated across genders and in a wide range of racial/ethnic groups.30,31 The survey also included questions on basic demographic information and perceived clinical health status and outcomes. There were 36 questions in total included in the survey. To facilitate completion and expedite the participants’ time, question condition settings were included to automatically skip questions that were not relevant to the participant, based on their response patterns. The survey was open and available between November 22, 2017 through December 13th, 2017. This study was reviewed and approved by the IRB at Loyola University Chicago Health Science Division (LU210353).
Measures
Alcohol use was defined by an affirmative response to the question, ‘Do you presently drink alcohol?’ and the question, ‘In the past 12 months, how many glasses of beer (wine, spirits) on average do you usually have per week’. Response options included 6 choices ranging from 0 to >20. Former drinking was considered with the answer to the following question, ‘If responded no to presently drinking, did you ever drink alcohol?’ Heavy drinking was considered in a series of 3 questions asking about use of wine, beer, and liquor on average glasses consumed per week. For females, 8 or more drinks per week was considered heavy alcohol use and for males, 14 or more drinks per week is considered heavy alcohol use per CDC definition.32 All others were categorized as ‘No Heavy Drinkers’. Binge drinking was measured through two questions worded, ‘How many drinks of alcohol do you have on a typical day when you are drinking?’ with any response of 5 or more qualifying as binge drinking; and the question ‘how often do you have 4 or more drinks (female) or 5 or more drinks (male) containing any kind of alcohol within a 2-hour period’, which is consistent with the CDC definition of binge drinking.32 Response options included seven choices ranging from 0 to everyday. Any response to this question other than “never” qualified as binge drinking. All others were categorized as ‘No Binge Drinking’. EAU was defined as either meeting criteria for a heavy alcohol use or binge pattern drinking or both.
Statistical Analysis
Survey responses were tabulated and reported as counts and percentages. Differences in survey responses by type of alcohol use were assessed for statistical significance with chi-square tests or Fisher’s exact tests as appropriate. A multivariable logistic regression model was developed to calculate adjusted odds ratios for patient characteristics associated with EAU. Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
Once the survey was active, 1135 people initiated the survey, with 84% (n=952) meeting inclusion criteria and completing the survey. Of those included in the sample, n=729 (77%) currently use alcohol, n=171 (18%) had former alcohol use, and n=52 (5%) never used alcohol. Amongst the people with CF who use alcohol, n=174 (24%) answered positively for heavy alcohol use and n=354 (49%) answered positively for binge drinking. Study participation is summarized in Figure 1. Notable differences between the groups include age, with the nondrinkers being younger than those with current or former alcohol use. Current alcohol users were more likely to work full or part-time, and were less likely disabled compared to former or nondrinkers. Respondent demographics by alcohol use status are presented in Table 1.
Figure 1. Study Participants and Alcohol Use.
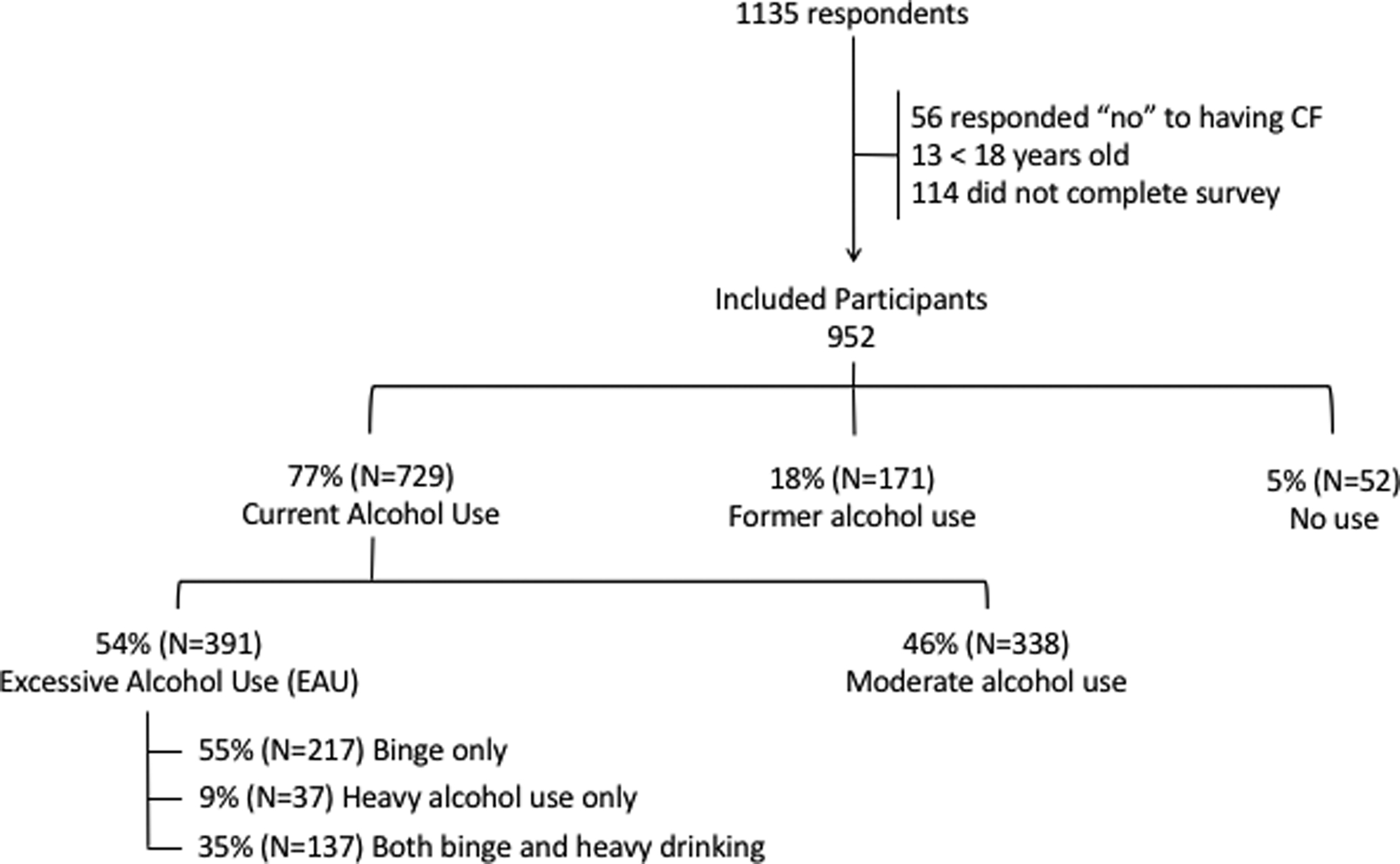
Current alcohol use: Answered affirmatively the question, “Do you drink alcohol”
Former alcohol use: Answered the question, “Do you drink alcohol”, as no; and answered affirmatively the question, “did you ever drink alcohol?”
Heavy alcohol use: For females 8 or more drinks per week; and for males 14 or more drinks per week per CDC definition.
Binge alcohol use: 4 or more drinks (female) or 5 or more drinks (male) containing any kind of alcohol within a 2-hour period per CDC definition
Table 1:
Respondent characteristics
| Current alcohol use n=729 (77%) |
Former alcohol use n=171 (18%) |
No alcohol use n=52 (5%) |
p-value | |
|---|---|---|---|---|
| Age, median (IQR) | 30 (25–38) | 33 (26–42) | 26 (21–38) | <0.001 |
| Gender, n (%) | ||||
| Male | 220 (30) | 48 (28) | 19 (37) | 0.51 |
| Female | 506 (70) | 123 (72) | 33 (64) | |
| BMI, median (IQR) | 21.9 (19.9–24.3) | 21.9 (19.7–25.5) | 21.3 (19.5–24.4) | 0.59 |
| Marital status, n (%) | ||||
| Married | 312 (43) | 76 (45) | 19 (37) | 0.14 |
| Separated or divorced | 60 (8) | 19 (11) | 1 (2) | |
| Single | 356 (49) | 75 (44) | 31 (61) | |
| Education, n (%) | ||||
| Some high school | 25 (3) | 6 (4) | 1 (2) | 0.28 |
| High school/GED | 183 (25) | 44 (26) | 20 (39) | |
| Vocational/trade | 51 (7) | 12 (7) | 3 (6) | |
| Associates | 111 (15) | 38 (23) | 4 (8) | |
| Bachelor’s | 237 (33) | 43 (25) | 15 (29) | |
| Master’s | 91 (13) | 17 (10) | 7 (14) | |
| Professional/doctorate | 29 (4) | 9 (5) | 2 (4) | |
| Employment, n (%) | ||||
| Full-time | 301 (41) | 37 (22) | 15 (29) | <0.001 |
| Part-time | 142 (20) | 31 (18) | 5 (10) | |
| Volunteer | 8 (1) | 3 (2) | 1 (2) | |
| Student | 57 (8) | 14 (8) | 10 (19) | |
| Disabled | 164 (23) | 65 (38) | 16 (31) | |
| Not employed | 55 (8) | 20 (12) | 5 (10) | |
| Age at first drink, n (%) | ||||
| Under 12 | 12 (2) | 3 (2) | 0.99 | |
| 12–15 years old | 122 (17) | 31 (18) | ||
| 16–17 years old | 292 (40) | 68 (40) | ||
| 18–20 years old | 199 (27) | 44 (26) | ||
| ≥ 21 years old | 104 (14) | 25 (15) |
Amongst the people with CF who use alcohol, 49% (n=354) answered positively for binge drinking. Twenty four percent of people with CF who currently use alcohol (n=174) answered positively for heavy alcohol use. Figure 1 contains the percentage and numbers of those responding affirmatively for EAU, either binge drinking, heavy alcohol use, or both. When asked the number of drinks containing alcohol consumed on a typical day of drinking, 38% of females (179/477) responded affirmatively >2 drinks and 55% of males (119/215) responded affirmatively >2 drinks. Of the 38% of females drinking excessively, 24% drank 3–4 drinks/day, 9% drank 5–6 drinks/day, 3% drank 7–9 drinks/day, and 2% drank ≥10 drinks per day. Of the 55% of males drinking excessively, 27% drank 3–4 drinks/day, 14% drank 5–6 drinks/day, 8% drank 7–9 drinks/day, and 6% drank ≥10 drinks per day.
Amongst those who currently drink alcohol, formerly drank alcohol, and never drank alcohol, more people required oxygen in the former alcohol use group compared to the recent alcohol use and no alcohol use groups (p<0.001). Fewer of those currently using alcohol have a lung transplant (10%), compared to those with former alcohol use (16%) and no alcohol use (17%), p=0.032. No differences between alcohol use and no alcohol use groups were noted for those taking pancreatic enzyme replacement therapy (PERT), having cystic fibrosis related diabetes (CFRD) and those with cystic fibrosis related liver disease (CFLD). Of note, 11% of the current alcohol users have known CFLD (Table 2). There were no significant differences in self-reported number of CF exacerbations, hospitalizations and overall health status between those who currently drink alcohol, formerly drank alcohol and never drank alcohol(Figure 2).
Table 2:
Self-reported health status by alcohol use
| Current alcohol use n=729 |
Former alcohol use n=171 |
No alcohol use n=52 |
p-value | EAU n=391 |
No EAU n=338 |
p-value | ||
|---|---|---|---|---|---|---|---|---|
| Does not wear any oxygen in a 24 hour period, n (%) | 651 (89) | 129 (76) | 46 (89) | <0.001 | 362 (93) | 289 (86) | 0.004 | |
| Has a lung transplant, n (%) | 74 (10) | 28 (16) | 9 (17) | 0.032 | 25 (6) | 49 (15) | <0.001 | |
| Takes pancreatic enzymes replacement therapy, n (%) | 631 (87) | 150 (88) | 50 (96) | 0.15 | 343 (88) | 288 (86) | 0.48 | |
| Has CF-related diabetes, n (%) | 262 (36) | 63 (37) | 25 (48) | 0.23 | 123 (32) | 139 (42) | 0.005 | |
| Has CF-related liver disease, n (%) | 80 (11) | 26 (15) | 9 (17) | 0.16 | 38 (10) | 42 (13) | 0.26 |
Figure 2.
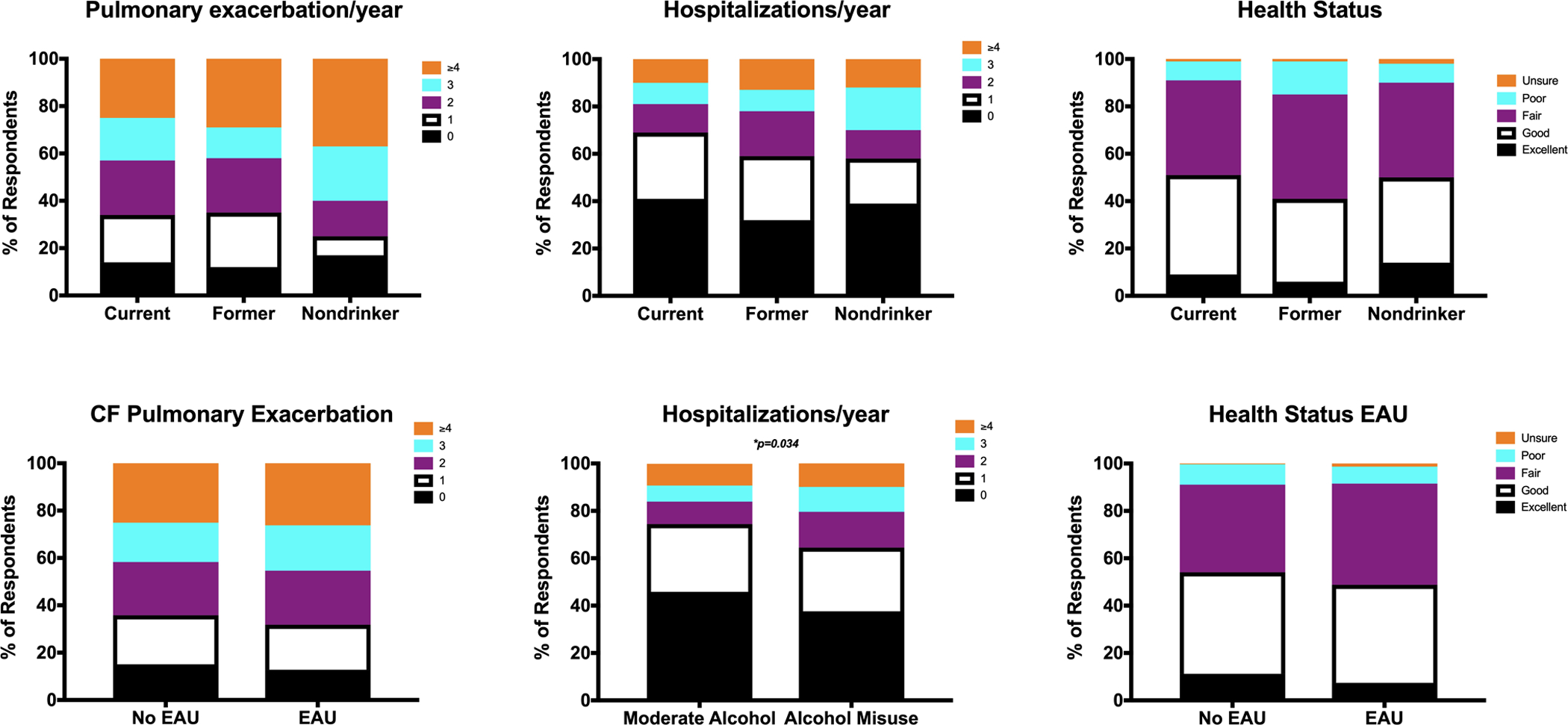
Self-perceived health status varies with different alcohol use exposures. Between current drinkers, previous drinkers and nondrinkers, figure 2A shows the number of CF pulmonary exacerbations per year, p=0.14, figure 2B shows the number of self-reported hospitalizations per year, p=0.08, and figure 2C shows the respondents perceived health status, p=0.13. Between those with no EAU and those with EAU, figure 2D shows the number of reported CF pulmonary exacerbations per year, p=0.78, figure 2E shows the number of self-reported hospitalizations per year, p=0.034, and figure 2F shows the respondents perceived health status, p=0.13.
Amongst those with EAU compared to those without EAU, fewer of those with EAU required oxygen (7% vs. 14%, p=0.004); and fewer of those with EAU had lung transplant (6% vs. 15%, p<0.001). No difference was noted in PERT use or self-reported CFLD between the 2 groups, although almost 10% of the EAU group had known reported CFLD (Table 2). More of the patients with EAU reported at least 1 hospitalization (62%) than reported in those without EAU (54%). Furthermore, 36% of those with EAU reported 2 or more hospitalizations, whereas only 26% of those without EAU reported 2 or more hospitalizations, p=0.034. Figure 2 shows the differences in self-reported health outcomes and the patient’s perception of their overall health (perceived health status) among those with and without EAU.
Amongst those individuals who formerly drank alcohol but stopped, 23% stopped less than 1 year ago, 16% stopped 1–2 years ago and 61% had stopped over 2 years ago. The majority, 65% (n=99) stopped for health reasons. Only 18% (n=28) of those who stopped drinking alcohol did so on the advice of their physician or care team member.
Over 30% (n=222) of individuals currently using alcohol reported experiencing “black outs” within the last year. Twenty-seven percent (n=198) felt guilt or remorse about their drinking. Eleven percent (n=82) had injured someone because of their drinking, and 10% (n=75) identified that a friend or relative was concerned about their drinking habits or suggested cutting down. These questions explore potential alcohol dependence and were adapted from the AUDIT. Among individuals currently using alcohol, 37% (n=268) answered that they would like a CF care team member to discuss alcohol use and the impact on their health with the patient. Even amongst those individuals with no current alcohol use, almost one-third of patients, both former alcohol use (32%) and no alcohol use (29%), answered that they would like a CF care team member to discuss alcohol use and impact on their health.
Finally, a multivariable logistic regression analysis was performed to identify characteristics associated with EAU. Amongst people with CF and EAU, there were several characteristics associated with problematic drinking including younger age, male gender, unmarried status, high school or lower education, and young age at first alcohol use. After multivariable adjustment, those who were aged 30–39 had a 50% reduction in EAU compared to those who were younger than age 30 (p<0.01) and those who were ≥40 had a 75% reduction in EAU (p<0.01). Males had a 65% increased odds of EAU compared to females (p<0.01). Unmarried patients had a 90% greater odds of EAU compared to married patients (p<0.01). Education level of high-school or less increased the odds of EAU by 56% compared to college or higher education (p<0.05). Age of first alcohol use was the strongest risk factor identified with an almost 2-fold increased odds of EAU if age at first alcohol use was 18–20 years, almost 3-fold increased odds if 16–17 years old at first alcohol use, and an over 5-fold increased odds of EAU if age at first alcohol use was 15 years or less compared to those who started drinking at age 21 or older [18–20 years, OR 1.82 (1.06–3.11), p<0.05; 16–17 years, OR 2.95 (1.77–4.92), p<0.01; and age ≤ 15 years, OR 5.43 (2.97–9.94), p<0.01] BMI and employment status were not associated with EAU after controlling for other covariates (Table 3).
Table 3:
Odds ratios for respondent characteristics associated with excessive alcohol use among current drinkers
| Excessive Alcohol Use | |
|---|---|
| n (%) of respondents | 371/697 (53.2%) |
| Odds ratio (95% Confidence Interval) | |
| Age | |
| < 30 | 1 (Reference) |
| 30–39 | 0.47 (0.32–0.71) ‡ |
| ≥ 40 | 0.26 (0.16–0.42) ‡ |
| Gender | |
| Female | 1 (Reference) |
| Male | 1.65 (1.14–2.41) ‡ |
| BMI | |
| < 18.5 | 0.97 (0.56–1.65) |
| 18.5–25 | 1 (Reference) |
| > 25 | 1.31 (0.86–1.99) |
| Marital status | |
| Not married | 1.90 (1.32–2.75) ‡ |
| Married | 1 (Reference) |
| Education | |
| College | 1 (Reference) |
| High school or less | 1.56 (1.09–2.25) † |
| Employment | |
| Not employed | 1 (Reference) |
| Employed part-time | 1.08 (0.68–1.72) |
| Employed full-time | 1.08 (0.73–1.60) |
| Age at first drink | |
| ≤ 15 years old | 5.43 (2.97–9.94) ‡ |
| 16–17 years old | 2.95 (1.77–4.92) ‡ |
| 18–20 years old | 1.82 (1.06–3.11) † |
| ≥ 21 years old | 1 (Reference) |
p<0.05;
p<0.01
Discussion
In this novel investigation assessing self-report of alcohol use in people with CF, we found that alcohol use is common despite major medical comorbidities such as advanced lung disease, lung transplant, CFLD or CFRD. There is no level of alcohol use which can be deemed safe for one’s health, and globally, alcohol use is a leading risk factor for premature death and disability in people younger than age 50.24 We found a much higher prevalence of EAU, both binge pattern drinking and heavy alcohol use, in people with CF compared to the general US population, with 49% of current alcohol drinkers with CF responding positively for binge drinking and 24% with heavy alcohol use, compared to just 25% binge drinking and 6% having heavy alcohol use amongst current drinkers in the US population.33 People who drink excessively are at risk of developing alcohol use disorders (AUD), which, according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), is defined by problems controlling intake of alcohol, continued use of alcohol despite problems resulting from drinking, development of tolerance or withdrawal symptoms, and drinking that leads to risky situations.34 Most people who drink excessively do not have AUDs, but remain at risk for developing health related consequences.35 Binge drinking in particular is a pattern reported by half of the survey respondents who drink alcohol. Binge pattern drinking is a strong risk factor for developing AUDs.4,32,36 Almost one third of survey respondents in this investigation reported symptoms consistent with alcohol dependence, such as blacking out or guilt about drinking, which could indicate an AUD.30
Contrary to our expectations, patients with CF and EAU had high numbers of comorbidities present including advanced lung disease, transplant, CFLD and CFRD. Given the negative impact and well-established interactions of alcohol, liver disease, diabetes, and lung infection, these subgroups were hypothesized to be lower risk for EAU. These findings add to the complexity of alcohol use in CF. We also observed an increase in self-reported hospitalizations amongst those patients with CF who had EAU. Although in this anonymous self-report of health outcomes it is impossible to determine if the increase in hospitalizations were related to alcohol use, or merely part of progressive CF-lung disease, further attention to the relationship of EAU, hospitalizations and health outcomes in patients with CF is warranted. There are both short-and long-term known health risks in those with EAU. While inebriated, those with EAU are at risk for injuries from trauma such as motor vehicle collisions, drownings and burn injury.37–39 Those with EAU are also vulnerable to violence, suicide, and sexual assault.40 Long-term, EAU can affect health in multiple ways. In addition to the well-documented detrimental impact alcohol has on the liver and pancreas, EAU can lead to high blood pressure, heart disease, stroke, and an increased risk of cancers.23 It has long been noted that individuals with EAU have an increased risk of pneumonia and tuberculosis, as EAU leads to alterations in both the systemic immune response and the innate immune response in the lungs.15,41,42 EAU is associated with learning and memory problems, mental health issues including depression and anxiety, poor adherence and social problems such as unemployment and family difficulties.43,44 Further, a strong association exists with alcohol and poor medical adherence when there is a high treatment burden.45,46 The daily treatment regimen for adults with CF is complex and time consuming. The impact that alcohol use has on adherence to medical treatment regimens in adults with CF who drink alcohol is unclear, but certainly warrants additional investigation as nonadherence could be particularly impactful on health outcomes and could lead to additional hospitalizations in those with CF. Moreover, in those who are adherent, potential drug interactions between alcohol and prescribed medications could impact optimal effectiveness of the treatment regimen, and furthermore, medication interactions with alcohol could increase the risk of medication-induced complications and side effects. It is possible that EAU in those with an increased number of hospitalizations is symptomatic of using alcohol to cope with advancing lung disease and untreated mental health disorders, but given the limited scope of this investigation and reliance on self-reported health outcomes, it is impossible to discern. Further investigation in a prospective manner is necessary to understand the impact of EAU on health-related outcomes in patients with CF.
Over one third of respondents indicated that they would like their CF healthcare team to discuss the impact of alcohol use on their health and well-being. Exploration of social issues and negative health habits, such as alcohol use, is sometimes limited given the time restraints placed on physicians providing care to medically complex patients such as CF patients and therefore, there is no formal screening or intervention currently in the context of CF clinic in the US. Individuals seen in a CF clinic may provide an opportunity to intervene by offering alcohol screening, brief intervention, and referral to treatment (SBIRT), as multidisciplinary staff are commonly available in these settings for social and behavioral interventions, thus providing ideal infrastructure for screening and assessment of mental health issues and substance use and abuse. SBIRT is a validated and recommended process consisting of an early intervention approach that targets individuals with nondependent alcohol use to provide effective strategies for intervention by utilizing motivational interviewing and intervention during the clinic encounter to educate patients.47,48 SBIRT has been successfully investigated and implemented in primary care and emergency care settings49,50, but implementation of SBIRT in the context of specialty care has not yet been investigated. Further study of implementation and utilization of SBIRT in CF clinic is necessary to assess the effectiveness of this intervention in patients with CF who have EAU.
In this investigation, we identified several risk factors for development of EAU in people with CF which may help care providers recognize patients to screen for EAU. Young, unmarried males with CF were more susceptible to developing EAU. Those with lower education levels also had a higher risk of EAU. Male sex, young age and unmarried status are all known risk factors for developing binge drinking.36,51–53 By far, the strongest risk factor we noted for the development of EAU in people with CF was young age at initiation of alcohol use. Age at alcohol initiation has been identified in multiple cross-sectional and longitudinal studies as a risk factor for development of the more severe condition of AUD, which impacts not only health, but overall functioning and wellbeing.51,52,54,55 Compared to adults who initiated drinking at 21 years or older, those who began drinking before age 14 were more likely to experience alcohol dependence.51 A possible explanation for the connection between early age of alcohol use onset and development of AUDs is that early initiation of drinking causes disruption of normal social development, increasing the risk for pathologies such as AUDs; alternatively, early age of alcohol use may be an indicator of other factors such as behavioral issues and poor academic achievement.54,56,57 Regardless of cause, it is clear that counseling and alcohol education need to start in pediatric clinics. By the time patients with CF transition to the adult clinic, harmful alcohol habits may have already developed, placing the patients at risk for an AUD. Therefore, development of effective screening and intervention strategies beginning in adolescence and targeted at those with CF deemed most high-risk for developing EAU is needed.
There is very limited information available about the alcohol consumption patterns of people with CF despite the fact that this population has risk factors for EAU, such as increased rates of anxiety, depression, and social isolation.28,58 In fact, only one investigation in Europe has noted alcohol use in this population previously, but it was not the main focus of the manuscript.29 Therefore our intent was to provide an initial assessment of alcohol use in the CF community. The survey was circulated on closed membership social media groups which limit participation to people with CF, as well as CF blog posts whose intended audience is people with CF. Despite this, 56 people responded to an initial screening question on the survey that they did not have CF, and therefore their data was excluded. Within these groups, there are family members, parents and spouses of individuals with CF who could also be members of these social media support groups who may have gained access to the survey, and were screened out in response to this question. Additionally, the majority of respondents in our investigation were female. This, however, is consistent with many studies which have shown that females are more likely to respond to surveys, particularly online surveys, than males.59–61
In order to limit social desirability bias, a response bias of survey participants to underreport undesirable behavior, we conducted an anonymous survey. There are limitations in the use of an anonymous internet based survey, such as reliance on self-reported data. However, previous investigations into the use of anonymous internet based surveys for reporting substance use has shown participants’ responses to be reliable and valid, with the advantage of limiting social desirability bias.62–65 The identified risk factors for EAU in this population (male, unmarried, early age drink initiation) are consistent with previously identified risk factors for the development of EAU in the general population51,53 and indicate that our survey participants were responding consistently and expectedly in people with EAU. Our purpose was to understand the prevalence of EAU in this community and to inform future investigations which would assess alcohol use and health outcomes in a prospective manner along with developing screening protocols and effective interventions, which is already underway. Therefore, despite the limitations of an anonymous survey, including the inability to associate the alcohol self-report with objective clinical measurements such as lung function, the information obtained in this investigation is an important step in identifying a potential problem which needs further investigation. Lastly, self-report of alcohol use and EAU are most likely to be underestimated in this investigation. As previously noted, the majority of respondents were female, but male gender is a significant risk factor for EAU.51,53 Additionally, heavy drinkers have a high nonparticipation rate in surveys.66 Capturing this data in a face-to-face interview may provide more balanced gender groups and excessive drinkers, but increases the risk of social desirability bias. Therefore, future investigations should consider an anonymous survey of alcohol use as part of clinic, which would allow for more balanced gender groups, but would limit social desirability bias.
In conclusion, EAU in patients with CF, both binge drinking and heavy use, is reported at rates much higher than the general US population. Concerning tendencies seen with alcohol dependence, such as guilt or blackouts with alcohol use were higher than expected. EAU was occurring in patients with medical comorbidities where alcohol abstinence would be recommended, such as in advanced lung disease, transplant, liver dysfunction and diabetes. Overall, CF care providers, mental health coordinators, and social workers embedded in the structure of the multidisciplinary clinic should be aware of risk factors for the development of EAU, such as young age of initiation of alcohol use, and utilize tools such as SBIRT to potentially reduce alcohol consumption in this at-risk population.
Acknowledgements:
Thank you to the people with cystic fibrosis who agreed to respond to the survey. Thank you to The Cystic Fibrosis Foundation, Paula Lomas, Enid Aliaj for editing assistance. Thank you to Sean Sullivan and Gunnar Esiason, and Peter Mogayzel for dissemination of the survey.
Funding Source:
This work was supported by the National Institute of Health NIAAA K23AA022126 (EML), K23AA024503 (MA), R21AA026295 (EJK). The funding source had no involvement in the study design, collection, nor with analysis and interpretation of the data.
Footnotes
Conflict of Interest Statement:
None of the authors of this manuscript have any personal or financial relationships to disclose.
References:
- 1.Gonzales K, Roeber J, Kanny D, et al. Alcohol-attributable deaths and years of potential life lost--11 States, 2006–2010. MMWR Morb Mortal Wkly Rep. 2014;63(10):213–216. [PMC free article] [PubMed] [Google Scholar]
- 2.SAMHSA. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration;2011. [Google Scholar]
- 3.NIAAA Council approves binge drinking definition. In. Rockville, MD: National Institute on ALcohol Abuse and Alcoholism; 2004. [Google Scholar]
- 4.Kanny D, Naimi TS, Liu Y, Lu H, Brewer RD. Annual Total Binge Drinks Consumed by U.S. Adults, 2015. Am J Prev Med. 2018;54(4):486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Survey on Drug Use and Health. 2011. http://oas.samhsa.gov/nsduhLatest.htm.
- 6.Organization WH. Global status report on alcohol and health 2018. Geneva: 2018. 2018. [Google Scholar]
- 7.Smith GS, Branas CC, Miller TR. Fatal nontraffic injuries involving alcohol: A metaanalysis. Ann Emerg Med. 1999;33(6):659–668. [PubMed] [Google Scholar]
- 8.Abbey A Alcohol-related sexual assault: a common problem among college students. J Stud Alcohol Suppl. 2002(14):118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osna NA, Donohue TM Jr., Kharbanda KK. Alcoholic Liver Disease: Pathogenesis and Current Management. Alcohol Res. 2017;38(2):147–161. [PMC free article] [PubMed] [Google Scholar]
- 10.Room R, Babor T, Rehm J. Alcohol and public health. Lancet. 2005;365(9458):519–530. [DOI] [PubMed] [Google Scholar]
- 11.Castaneda R, Sussman N, Westreich L, Levy R, O’Malley M. A review of the effects of moderate alcohol intake on the treatment of anxiety and mood disorders. J Clin Psychiatry. 1996;57(5):207–212. [PubMed] [Google Scholar]
- 12.Tetzschner R, Norgaard K, Ranjan A. Effects of alcohol on plasma glucose and prevention of alcohol-induced hypoglycemia in type 1 diabetes-A systematic review with GRADE. Diabetes Metab Res Rev. 2018;34(3). [DOI] [PubMed] [Google Scholar]
- 13.Moss M, Bucher B, Moore FA, Moore EE, Parsons PE. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA. 1996;275(1):50–54. [PubMed] [Google Scholar]
- 14.Lujan M, Gallego M, Belmonte Y, et al. Influence of pneumococcal serotype group on outcome in adults with bacteraemic pneumonia. Eur Respir J. 2010;36(5):1073–1079. [DOI] [PubMed] [Google Scholar]
- 15.Simet SM, Sisson JH. Alcohol’s Effects on Lung Health and Immunity. Alcohol Res. 2015;37(2):199–208. [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta AJ, Guidot DM. Alcohol abuse, the alveolar macrophage and pneumonia. Am J Med Sci. 2012;343(3):244–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jong GM, Hsiue TR, Chen CR, Chang HY, Chen CW. Rapidly fatal outcome of bacteremic Klebsiella pneumoniae pneumonia in alcoholics. Chest. 1995;107(1):214–217. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez-Sola J, Junque A, Estruch R, Monforte R, Torres A, Urbano-Marquez A. High alcohol intake as a risk and prognostic factor for community-acquired pneumonia. Arch Intern Med. 1995;155(15):1649–1654. [DOI] [PubMed] [Google Scholar]
- 19.Bercault N, Boulain T. Mortality rate attributable to ventilator-associated nosocomial pneumonia in an adult intensive care unit: a prospective case-control study. Crit Care Med. 2001;29(12):2303–2309. [DOI] [PubMed] [Google Scholar]
- 20.Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA. 1996;275(2):134–141. [PubMed] [Google Scholar]
- 21.Foundation CF. Cystic Fibrosis Foundation Patient Registry: Annual Data Report. Bethesda, Maryland: 2017. [Google Scholar]
- 22.Elborn JS. Cystic fibrosis. Lancet. 2016;388(10059):2519–2531. [DOI] [PubMed] [Google Scholar]
- 23.Alcohol’s Effects on the Body. National Institute on Alcohol Abuse and Alcoholism. https://www.niaaa.nih.gov/alcohol-health/alcohols-effects-body. Published 2018 Accessed 10-24-2018, 2018.
- 24.Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392(10152):1015–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quittner AL, Goldbeck L, Abbott J, et al. Prevalence of depression and anxiety in patients with cystic fibrosis and parent caregivers: results of The International Depression Epidemiological Study across nine countries. Thorax. 2014;69(12):1090–1097. [DOI] [PubMed] [Google Scholar]
- 26.Johnston LD MR, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME. Monitoring the future: National survey results on drug use, 1975–2017: Overview, key findings on adolescent drug use. Ann Arbor: The University of Michigan;2018. [Google Scholar]
- 27.Grant BF, Harford TC. Comorbidity between DSM-IV alcohol use disorders and major depression: results of a national survey. Drug Alcohol Depend. 1995;39(3):197–206. [DOI] [PubMed] [Google Scholar]
- 28.Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264(19):2511–2518. [PubMed] [Google Scholar]
- 29.Mc Ewan FA, Hodson ME, Simmonds NJ. The prevalence of “risky behaviour” in adults with cystic fibrosis. J Cyst Fibros. 2012;11(1):56–58. [DOI] [PubMed] [Google Scholar]
- 30.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction (Abingdon, England). 1993;88(6):791–804. [DOI] [PubMed] [Google Scholar]
- 31.Allen JP, Litten RZ, Fertig JB, Babor T. A review of research on the Alcohol Use Disorders Identification Test (AUDIT). Alcoholism, clinical and experimental research. 1997;21(4):613–619. [PubMed] [Google Scholar]
- 32.NIAAA Council approves binge drinking definition. Rockville, MD: US Department of Health and Human Services;2004. [Google Scholar]
- 33.Results from the 2017 National Survey on Drug Use and Health. Rockville, MD: Substance Abuse and Mental Health Services Administration (SAMHSA);2018. [Google Scholar]
- 34.Diagnostic and statistical manual of mental disorders. 5th Edition ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 35.Esser MB, Hedden SL, Kanny D, Brewer RD, Gfroerer JC, Naimi TS. Prevalence of alcohol dependence among US adult drinkers, 2009–2011. Prev Chronic Dis. 2014;11:E206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gowin JL, Sloan ME, Stangl BL, Vatsalya V, Ramchandani VA. Vulnerability for Alcohol Use Disorder and Rate of Alcohol Consumption. The American journal of psychiatry. 2017;174(11):1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stubig T, Petri M, Zeckey C, et al. Alcohol intoxication in road traffic accidents leads to higher impact speed difference, higher ISS and MAIS, and higher preclinical mortality. Alcohol. 2012;46(7):681–686. [DOI] [PubMed] [Google Scholar]
- 38.Cummings P, Quan L. Trends in unintentional drowning: the role of alcohol and medical care. JAMA. 1999;281(23):2198–2202. [DOI] [PubMed] [Google Scholar]
- 39.Chen MM, O’Halloran EB, Ippolito JA, Choudhry MA, Kovacs EJ. Alcohol potentiates postburn remote organ damage through shifts in fluid compartments mediated by bradykinin. Shock. 2015;43(1):80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LA G. Alcohol and Crime: An Analysis of National Data on the Prevalence of Alcohol Involvement in Crime. Washington D.C.1998. [Google Scholar]
- 41.Simou E, Britton J, Leonardi-Bee J. Alcohol and the risk of pneumonia: a systematic review and meta-analysis. BMJ open. 2018;8(8):e022344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simou E, Britton J, Leonardi-Bee J. Alcohol consumption and risk of tuberculosis: a systematic review and meta-analysis. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2018;22(11):1277–1285. [DOI] [PubMed] [Google Scholar]
- 43.Alcohol Alert: Alcohol’s Damaging Efects on the Brain. In. Bethesda, Maryland: NIAAA; 2004:1–7. [Google Scholar]
- 44.Forcier MW. Unemployment and alcohol abuse: a review. Journal of occupational medicine : official publication of the Industrial Medical Association. 1988;30(3):246–251. [PubMed] [Google Scholar]
- 45.Braithwaite RS, McGinnis KA, Conigliaro J, et al. A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcoholism, clinical and experimental research. 2005;29(7):1190–1197. [DOI] [PubMed] [Google Scholar]
- 46.Grodensky CA, Golin CE, Ochtera RD, Turner BJ. Systematic review: effect of alcohol intake on adherence to outpatient medication regimens for chronic diseases. J Stud Alcohol Drugs. 2012;73(6):899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Madras BK, Compton WM, Avula D, Stegbauer T, Stein JB, Clark HW. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99(1–3):280–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Babor TF, Del Boca F, Bray JW. Screening, Brief Intervention and Referral to Treatment: implications of SAMHSA’s SBIRT initiative for substance abuse policy and practice. Addiction (Abingdon, England). 2017;112 Suppl 2:110–117. [DOI] [PubMed] [Google Scholar]
- 49.Rahm AK, Boggs JM, Martin C, et al. Facilitators and Barriers to Implementing Screening, Brief Intervention, and Referral to Treatment (SBIRT) in Primary Care in Integrated Health Care Settings. Subst Abus. 2015;36(3):281–288. [DOI] [PubMed] [Google Scholar]
- 50.Moyer VA, Preventive Services Task F. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. preventive services task force recommendation statement. Ann Intern Med. 2013;159(3):210–218. [DOI] [PubMed] [Google Scholar]
- 51.Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Archives of pediatrics & adolescent medicine. 2006;160(7):739–746. [DOI] [PubMed] [Google Scholar]
- 52.Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. Journal of substance abuse. 1997;9:103–110. [DOI] [PubMed] [Google Scholar]
- 53.Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. The American journal of psychiatry. 2000;157(5):745–750. [DOI] [PubMed] [Google Scholar]
- 55.Hawkins JD, Graham JW, Maguin E, Abbott R, Hill KG, Catalano RF. Exploring the effects of age of alcohol use initiation and psychosocial risk factors on subsequent alcohol misuse. Journal of studies on alcohol. 1997;58(3):280–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown SA, McGue M, Maggs J, et al. A developmental perspective on alcohol and youths 16 to 20 years of age. Pediatrics. 2008;121 Suppl 4:S290–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McGue M, Iacono WG, Legrand LN, Malone S, Elkins I. Origins and consequences of age at first drink. I. Associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcoholism, clinical and experimental research. 2001;25(8):1156–1165. [PubMed] [Google Scholar]
- 58.Lemmens PH, Knibbe RA. Seasonal variation in survey and sales estimates of alcohol consumption. J Stud Alcohol. 1993;54(2):157–163. [DOI] [PubMed] [Google Scholar]
- 59.Porter SR, Whitcomb ME. Non-response in student surveys: The role of demographics, engagement and personality. Res High Educ. 2005;46(2):127–152. [Google Scholar]
- 60.Smith G Does gender influence online survey participation?: A record-linkage analysis of university faculty online survey response behavior. ERIC Document Reproduction Service. 2008(ED 501717). [Google Scholar]
- 61.Aerny-Perreten N, Dominguez-Berjon MF, Esteban-Vasallo MD, Garcia-Riolobos C. Participation and factors associated with late or non-response to an online survey in primary care. J Eval Clin Pract. 2015;21(4):688–693. [DOI] [PubMed] [Google Scholar]
- 62.Ramo DE, Liu H, Prochaska JJ. Reliability and validity of young adults’ anonymous online reports of marijuana use and thoughts about use. Psychol Addict Behav. 2012;26(4):801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Richman WL, Kiesler S, Weisband S, Drasgow F. A meta-analytic study of social desirability distortion in computer-administered questionnaires, traditional questionnaires, and interviews. J Appl Psychol. 1999;84(5):754–775. [Google Scholar]
- 64.Durant LE, Carey MP, Schroder KE. Effects of anonymity, gender, and erotophilia on the quality of data obtained from self-reports of socially sensitive behaviors. J Behav Med. 2002;25(5):438–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ong AD, Weiss DJ. The impact of anonymity on responses to sensitive questions. J Appl Soc Psychol. 2000;30(8):1691–1708. [Google Scholar]
- 66.Sobell LC, Sobell MB. Alcohol Consumption Measures In: Assessing Alcohol Problems: A Guide for Clinicians and Researchers. Washington, D.C.: U.S. Department of Health and Human Services; 1995:55–73. [Google Scholar]


