Abstract
Dural arteriovenous fistulas are relatively rare. Some cases are difficult to diagnose, leading to unnecessary investigations, treatments and delays, particularly if the presentation is atypical. We report a case of a man who presented with progressive dementia and bulbar symptoms, both under-recognised non-haemorrhagic neurological deficits, caused by cortical venous hypertension. Brain imaging showed unusual bilateral thalamic, tectal plate and midbrain oedema. The patient was investigated and treated for alternative aetiologies, before being correctly diagnosed and managed using angiographic embolisation. His clinical and radiological signs improved significantly following treatment, reducing his risk of neurological morbidity and mortality.
Keywords: neuroimaging; stroke; dementia, vascular; interventional radiology; brain stem / cerebellum
Background
Intracranial dural arteriovenous fistula (dAVF) is a vascular anomaly caused by an abnormal connection between dural arteries and dural veins or venous sinus,1–6 most commonly acquired and present late in life.1 7 They are rare7 and the incidence is thought to be approximately 10%–15% of all intracranial vascular abnormalities.3 4 6 8 They can occur anywhere along the dura with the most common locations being the transverse sinus, cavernous sinus,6 7 tentorium cerebelli and superior sagittal sinus.8 9
The dAVFs can present with a wide range of clinical signs and symptoms.1 8 It is their location and, more importantly, their venous drainage pattern that determines their presentation and potential for serious complications.6–9 Imaging is a key to making the diagnosis and includes the use of CT, CT angiography, MRI and, the gold standard, superselective angiography.3
Unfortunately, due to the diverse clinical8 and imaging features, the diagnosis of dAVF is often delayed or missed, with clinicians working patients up for incorrect diagnoses and giving unnecessary treatments.5 This was the case with our patient. He presented with rapidly progressive dementia and bulbar symptoms and had unusual radiological findings. Because making a timely diagnosis is extremely important in reducing the risk for neurological sequelae or death, it is important that physicians and neuroradiologists have an awareness of this atypical yet distinctive presentation of dAVF.
Case presentation
We present the case of a 79-year-old man with a background history of hypertension, no vascular disease and a high prostate-specific antigen level, for which he was awaiting a prostate biopsy. He denied any previous significant head injury. He had never smoked and only drank alcohol occasionally. He was not admitted on any medications.
His partner reported mild cognitive decline over approximately 1 year. He was occasionally repetitive but was able to have a good conversation, remained independent and continued to drive. He was admitted to hospital with 3 weeks of confusion followed by slurred speech, difficulty swallowing, headache and mobility decline. There was no history of unintentional weight loss, nocturnal sweating, vomiting or diarrhoea. On neurological examination, he had upgaze limitation, dysarthria, hypophonia, mild facial power asymmetry, dysmetria and gait unsteadiness, which required assistance. He scored 4/10 on the Abbreviated Mental Test. His observations, including temperature, and routine blood tests, including inflammatory markers, were normal. Unenhanced axial CT of the brain was unremarkable other than for a subtle, small region of low attenuation in the left thalamus, interpreted at the time as an old infarct, but in retrospect represented oedema.
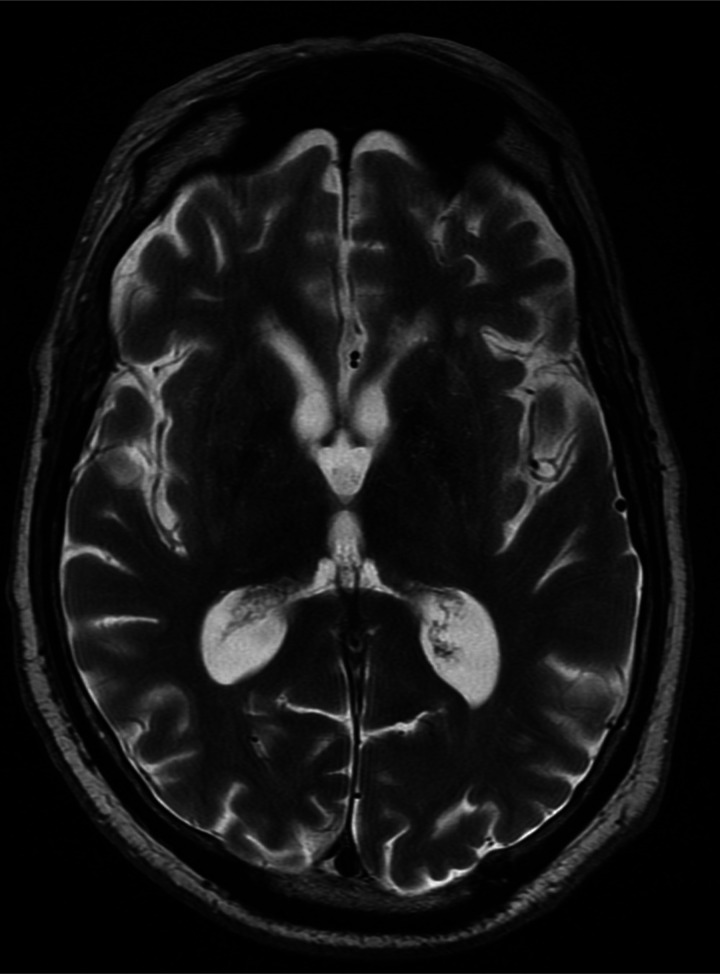
Unenhanced cranial MRI, performed the next day, demonstrated bilateral T2-weight and Fluid-attenuated inversion recovery (FLAIR) hyperintensity in the thalamus, midbrain and tectal plate (figures 1 and 2) without evidence of diffusion restriction or haemorrhage. Radiological differential diagnosis included: Wernicke’s encephalopathy, viral encephalitis, deep cerebral venous thrombosis and glioma. Other causes of bilateral thalamic oedema, such as artery of Percheron infarction, top of the basilar artery infarction and Creutzfeldt-Jakob disease were rejected due to the lack of change on diffusion-weighted sequences. Magnetic resonance venogram (MRV) demonstrated no evidence of cerebral venous thrombosis.
Figure 1.
Axial T2-weighted image at the level of the thalamus demonstrating bilateral thalamic oedema (open arrows).
Figure 2.
Sagittal FLAIR image in the midline demonstrating thalamic (open arrow), midbrain (black arrow) and tectal plate (white arrow) oedema. FLAIR, fluid-attenuated inversion recovery.
It was considered sensible to give him a therapeutic trial of Pabrinex, a relatively innocuous treatment, even though Wernicke’s encephalopathy was thought clinically unlikely, due to the fact that such a diagnosis can be potentially fatal. He was also managed with meropenam, tamiflu, aciclovir and dexamethasone (despite a neurological infection seemed clinically unlikely due to the duration of symptoms and lack of infective markers). The possibility of an ischaemic stroke was excluded due to the subacute progression of the symptoms and the lack of restricted diffusion on MRI. Four days into his admission, he was commenced on levetiracetam because it was felt he may have had a seizure. An infective screen, CT chest/abdomen/pelvis and paraneoplastic antibodies did not reveal any relevant abnormality. Cerebrospinal fluid (CSF) revealed a protein of 1 g/L, red cell count 37×106/L, white cell count 0×109/L, no organisms seen, viral PCR negative and no immunophenotypic evidence of lymphoproliferative disease. Despite the above treatment, he continued to deteriorate, becoming more confused, unable to mobilise and requiring a pureed diet and then a nasogastric tube. The antibiotics, antivirals and dexamethasone were consequently discontinued and the patient was referred to the local neuro-oncology multidisciplinary team meeting.
The cranial images were discussed at a clinicoradiological meeting and it was felt that a CT angiogram and venogram were required to more firmly exclude a venous sinus thrombus. This again demonstrated no evidence of a deep cerebral venous thrombosis. However, there was abnormal angiographic phase opacification of the internal cerebral veins, vein of Galen and straight sinus with multiple engorged collaterals, suggestive of dAVF (figure 3).
Figure 3.
Sagittal maximum intensity projection of a CT angiogram demonstrating abnormal early opacification of the straight sinus (white arrow).
Outcome and follow-up
Our patient was transferred to the local neurosciences unit where he had a cerebral angiogram which confirmed the presence of a torcular dAVF supplied by bilateral occipital arteries and the left middle meningeal artery (figure 4) with retrograde flow in the straight sinus and reflux into cortical veins (Borden II and Cognard type IIa+b).
Figure 4.
Lateral projection digital subtraction angiogram of the left external carotid artery. Left occipital artery (black arrows), dural arteriovenous fistula (white arrow) and abnormal early filling of the straight sinus (red arrow).
In the case of this aggressive presentation, with progressive deterioration during admission, a discussion was held between the neurointerventional and neurosurgical teams regarding treatment options and their associated risks. This included the risk of venous infarction, associated with percutaneous endovascular treatment alone, and the risk of bleeding, if there were to be concomitant drainage through intradiploic collateral vessels during a surgical or combined surgical/endovascular approach.7
Endovascular, either transarterial or transvenous, treatment is often the first line but, as above, there should be a multidisciplinary approach. Surgery is reserved for cases unsuitable for, or that have failed, an endovascular approach or that are anatomically more favourable, such as the floor of the anterior cranial fossa or superior sagittal sinus.6
It was decided to proceed with transarterial Onyx embolisation of the dAVF via the left occipital and middle meningeal arteries (figure 5). The procedure was complicated by the microcatheter becoming stuck in the Onyx cast during right occipital artery embolisation. It was left in situ, requiring the patient to need lifelong antiaggregant treatment. Definitive treatment occurred 29 days following admission.
Figure 5.
Lateral projection digital subtraction angiogram of the left external carotid artery following Onyx embolisation. Left occipital artery (black arrow). The Onyx cast is just appreciable (white arrow). Early filling of the straight sinus is no longer present.
Repeated cranial MRI and MRV showed resolving thalamic oedema 9 days after the procedure, and near total resolution of the thalamic oedema 60 days after the procedure (figure 6). There was evidence of some residual abnormal vessels, mainly within the posterior fossa and brainstem although there was a reduction in flow within the previously demonstrated draining veins.
Figure 6.
Axial T2-weighted image at the level of the thalamus 3 months after presentation and 2 months following embolisation. The previous marked thalamic oedema has resolved.
The patient was transferred to a rehabilitation unit where he showed significant clinical improvement and he was discharged home 72 days after embolisation. On discharge, he was able to eat and drink without any concerns, mobilise unaided and his cognition did not appear to affect his functional ability. One month postembolisation, his Montreal Cognitive Assessment (MoCA) score was 6/30 (deficits highlighted in executive functioning, memory and orientation). Two months postembolisation, his MoCA score had improved to 22/30 (main deficit was highlighted as memory).
Discussion
The dAVFs are classified using two predominant classification systems based on angiographic appearance—the Borden system7 and the Cognard system.8 They stratify lesions according to venous drainage pattern (dural venous sinus vs cortical vein) and the presence of cortical venous drainage (CVD).7–9 This helps to determine whether the natural history of the lesion is likely to be benign or aggressive.9 CVD carries an increased risk of causing intracranial haemorrhage (ICH) and non-haemorrhagic neurological deficits (NHNDs).9 van Dijk et al reported that CVD has an annual risk of ICH of 8.1%, an annual risk of NHND of 6.9% and an annual mortality rate of 10.4%.10
In 2009, Zipfel et al proposed a modification to these classifications by adding the mode of dAVF presentation to angiographic appearance, allowing more accurate risk stratification and assisting with treatment decision-making (table 1).2 The modes of presentation include: (1) incidentally, (2) symptoms, such as pulsatile tinnitus and exopthalmos, resulting from increased blood flow through the dural venous sinuses, (3) ICH resulting from cortical venous hypertension and (4) NHNDs, such as seizures, parkinsonism, cerebellar symptoms and cranial nerve abnormalities, resulting from cortical venous hypertension. Our patient presented with NHND (ie, dementia and bulbar symptoms) and would fit into Zipfel’s classification group 2S, indicating that he had a 7.4%–7.6% risk of ICH and a 3.8% risk of death prior to treatment.
Table 1.
Modified classification of intracranial dAVFs
| Zipfel | Borden type | Cognard type | Venous drainage | CVD | Cortical venous hypertension | Presents with ICH or NHND | ICH risk (%) | Death risk (%) | Treatment |
| 1 | I | I, IIa | Dural sinus | No | No | No | <1 | 0 | Elective for intractable symptoms |
| 2A | II | IIb, IIa+b | Dural sinus | Yes | No | No | 1.4–1.5 | 0 | Elective to prevent ICH/NHND |
| 2S | II | IIb, IIa+IIb | Dural sinus | Yes | Yes | Yes | 7.4–7.6 | 3.8 | Early to prevent ICH/NHND |
| 3A | III | III, IV, V | Cerebral vein | Yes | No | No | 1.4–1.5 | 0 | Elective to prevent ICH/NHND |
| 3S | III | III, IV, V | Cerebral vein | Yes | Yes | Yes | 7.4–7.6 | 3.8 | Early to prevent ICH/NHND |
Source: Zipfel et al.2
CVD, cortical venous drainage; dAVF, dural arteriovenous fistula; ICH, intracranial haemorrhage; NHND, non-haemorrhagic neurological deficit.
The presenting symptom of progressive dementia, as seen with our patient, is a rare feature of dAVF. The cause of this has been described as being from venous hypertension in either the cortex8 or, less commonly, but as in this case, the bilateral thalami.5 The dAVF-induced thalamic dementia is associated with bilateral thalamic T2-weighted/FLAIR (Fluid-attenuated inversion recovery) hyperintensities (oedema), often with patchy gadolinium enhancement, occasionally with petechial haemorrhage, but not with diffusion restriction.5 A thalamic dementia syndrome is more commonly caused by other conditions, such as deep venous sinus thrombosis, malignancy, infection and toxins, thus making a thorough history essential.
It is also extremely unusual for the presence of bulbar symptoms to be related to bilateral thalamic lesions.11 12 However, the presence of abnormal signal in this area, related to hypertensive venous encephalopathy, could explain our patient’s presentation. Nevertheless, a recent article reported a pseudobulbar presentation of a thoracic spinal dAVF that fully resolved with obliteration of the fistula13 resembling the outcome of our patient.
Despite a detailed history, examination and standard imaging, it is advisable to perform a catheter angiogram to firmly obtain the diagnosis because characteristics of differentials can overlap. Treatment of dAVF should be based on patient characteristics, symptom severity and risk of complications, which is largely dependent on the presence of CVD, as mentioned above. Endovascular embolisation is the main management technique used for aggressive dAVF and has good outcome with reversal of symptoms and complication risk, as we saw with our patient. The curability of dAVF makes this diagnosis vital to diagnosis early, even when it presents in rare but distinctive ways.
Learning points.
DAVFs with cortical venous drainage carry an increased risk of complications, such as intracranial haemorrhage and non-haemorrhagic neurological deficits, compared to DAVFs with dural sinus drainage.
DAVF is a rare but reversible cause of dementia and can be caused by cortical or bilateral thalamic venous hypertension.
Medullary and bilateral thalamic oedema should cause consideration for dAVF.
If vascular abnormalities are not found using CTA/MRA, catheter angiography should be performed before more invasive diagnostic investigations (e.g. stereotactic biopsy) or prolonged treatment is given, in order to confirm dAVF and distinguish it from other differential causes.
Footnotes
Contributors: CAH created the initial manuscript of the case. DS selected and edited the MRI and angiographic sequences and created the corresponding headings. He also contributed in the manuscript review. ELT setup the aims and objectives of the first manuscript as well as reviewed and created the final version.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.El Asri AC, El Mostarchid B, Akhaddar A, et al. Factors influencing the prognosis in intracranial dural arteriovenous fistulas with perimedullary drainage. World Neurosurg 2013;79:182–91. 10.1016/j.wneu.2012.09.012 [DOI] [PubMed] [Google Scholar]
- 2.Zipfel GJ, Shah MN, Refai D, et al. Cranial dural arteriovenous fistulas: modification of angiographic classification scales based on new natural history data. Neurosurg Focus 2009;26:E14. 10.3171/2009.2.FOCUS0928 [DOI] [PubMed] [Google Scholar]
- 3.Copelan AZ, Krishnan A, Marin H, et al. Dural arteriovenous fistulas: a characteristic pattern of edema and enhancement of the medulla on MRI. AJNR Am J Neuroradiol 2018;39:238–44. 10.3174/ajnr.A5460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roelz R, Van Velthoven V, Reinacher P, et al. Unilateral contrast-enhancing pontomedullary lesion due to an intracranial dural arteriovenous fistula with perimedullary spinal venous drainage: the exception that proves the rule. J Neurosurg 2015;123:1534–9. 10.3171/2014.11.JNS142278 [DOI] [PubMed] [Google Scholar]
- 5.Holekamp TF, Mollman ME, Murphy RKJ, et al. Dural arteriovenous fistula-induced thalamic dementia: report of 4 cases. J Neurosurg 2016;124:1752–65. 10.3171/2015.5.JNS15473 [DOI] [PubMed] [Google Scholar]
- 6.Gandhi D, Chen J, Pearl M, et al. Intracranial dural arteriovenous fistulas: classification, imaging findings, and treatment. AJNR Am J Neuroradiol 2012;33:1007–13. 10.3174/ajnr.A2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg 1995;82:166–79. 10.3171/jns.1995.82.2.0166 [DOI] [PubMed] [Google Scholar]
- 8.Cognard C, Gobin YP, Pierot L, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology 1995;194:671–80. 10.1148/radiology.194.3.7862961 [DOI] [PubMed] [Google Scholar]
- 9.Davies MA, TerBrugge K, Willinsky R, et al. The validity of classification for the clinical presentation of intracranial dural arteriovenous fistulas. J Neurosurg 1996;85:830–7. 10.3171/jns.1996.85.5.0830 [DOI] [PubMed] [Google Scholar]
- 10.van Dijk JMC, terBrugge KG, Willinsky RA, et al. Clinical course of cranial dural arteriovenous fistulas with long-term persistent cortical venous reflux. Stroke 2002;33:1233–6. 10.1161/01.STR.0000014772.02908.44 [DOI] [PubMed] [Google Scholar]
- 11.Karacostas D, Artemis N, Giannopoulos S, et al. Bilateral thalamic infarcts presenting as acute pseudobulbar palsy. Funct Neurol 1994;9:265–8. [PubMed] [Google Scholar]
- 12.Lee HY, Kim MJ, Kim B-R, et al. Acute pseudobulbar palsy after bilateral paramedian thalamic infarction: a case report. Ann Rehabil Med 2016;40:751–6. 10.5535/arm.2016.40.4.751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki K, Inoue T, Nishijima Y, et al. Thoracic dural arteriovenous fistula presenting with isolated pseudobulbar palsy mimicking brainstem lesion. World Neurosurg 2020;136:157–60. 10.1016/j.wneu.2020.01.074 [DOI] [PubMed] [Google Scholar]