Abstract
A 33-year-old male presenting with subacute abdominal pain was found to have hyperbilirubinaemia, hypokalaemia and hyponatraemia. This was in the setting of transitioning between deferasirox iron chelator formulations, from dispersible tablets to film-coated tablets for ongoing treatment of chronic iron overload secondary to transfusion requirement for beta-thalassemia major. A liver biopsy demonstrated acute cholestasis with patchy confluent hepatocellular necrosis and mild to moderate microvesicular steatosis. Based on the histological, biochemical and clinical findings, the diagnosis of hepatotoxicity and Fanconi-like syndrome was made. The patient improved clinically and biochemically with cessation of the deferasirox film-coated tablets and supportive management. To our knowledge, this is the first case report of hepatotoxicity and Fanconi-like syndrome occurring due to deferasirox film-coated tablets with previous tolerance of dispersible deferasirox tablets. It is important to raise clinical awareness of this potentially severe complication.
Keywords: liver disease, haematology (incl blood transfusion), unwanted effects / adverse reactions, fluid electrolyte and acid-base disturbances
Background
The introduction of oral dispersible deferasirox tablets revolutionised iron overload treatment with the advantage of oral administration over the historical alternative of daily subcutaneous infusions (deferoxamine mesylate). Deferasirox is an iron-chelating agent selective for ferric iron, aiming to prevent iron deposition and end-organ damage by stabilising serum ferritin levels and liver iron concentration.1 It is frequently used in the treatment of chronic iron overload associated with chronic haematological conditions such as beta-thalassemia major.1 2 Deferasirox film-coated tablets have been introduced as a tablet or granule alternative, Pharmaceutical Benefit Scheme (PBS) approved, and therefore dispersible deferasirox tablets’ production has been discontinued since May 2019 in Australia. Dosage adjustment is recommended when transitioning between the two agents; film-coated tablets should be given at a 30% dose reduction from dispersible tablets due to greater bioavailability. Toxicities associated with film-coated tablets are not well described, and no blinded randomised controlled clinical trials have been conducted with film-coated tablets/sprinkle granules.3 4 Described side effects of deferasirox film-coated tablets include diarrhoea, nausea, abdominal pain, headache, dizziness, rash and increased serum creatine.1–3 5 In this report, we describe a rare case of an adult male with beta-thalassemia major developing hepatotoxicity and a Fanconi-like syndrome occurring in close temporal relationship to changing from dispersible deferasirox tablets to deferasirox film-coated tablets.
Case presentation
The patient is a 33-year-old male who had recurrent presentations to a tertiary hospital with left upper quadrant and epigastric pain, weight loss and constipation. This was on a background of beta-thalassemia major with chronic monthly transfusion requirements for 4 units of blood and multiple renal calculi secondary to hypercalciuria (calcium/creatinine ratio >2.0 mmol/mmol creatinine). His ferritin was stable at 600–800 μg/L. His magnetic resonance imaging 2 years prior showed no evidence of significant myocardial or hepatic iron loading. His chronic iron overload had been treated with dispersible deferasirox 2000 mg tablets (40 mg/kg) daily, but 3 months prior to presentation, he had changed to deferasirox film-coated tablets 1440 mg (28 mg/kg) daily due to discontinuation of dispersible deferasirox tablets’ production. His only other medication was hydrochlorothiazide (HCTZ) 25 mg daily, and he had unremarkable electrolytes. The patient had no history of drug allergies or intolerances.
On initial presentations, pathology revealed mild hyponatraemia (134 mmol/L), mild hypokalaemia (3.1 mmol/L), a mild transaminitis (alanine aminotransferase (ALT) 93 U/L, aspartate transaminase (AST) 54 U/L) and ferritin of 981 ng/mL. His computerised tomography of kidney, urethra and bladder identified a left-sided 8 mm intrarenal calculi. Following intravenous fluid administration, laxatives and analgesia, he was discharged with presumed diagnosis of renal colic for consideration of outpatient lithotripsy.
Three weeks after discharge, he represented to the emergency department with ongoing abdominal pain—now right-sided in nature, constipation, loss of weight, malaise and dark orange urine. Observations were within normal limits, he was afebrile, normocardic (pulse rate 98 bpm) and normotensice (blood pressure 105/70 mm Hg). He was of slim build and short stature, jaundiced with scleral icterus, and on abdominal palpation he had epigastric and umbilical tenderness without abdominal guarding.
Investigations
Further investigations revealed marked hypokalaemia (2.4 mmol/L) and hyponatraemia (124 mmol/L), with stable renal function (estimated glomerular filtration rate (eGFR) >90 mL/min/1.73 m2) but elevated creatine (92 mmol/L, baseline 50 mmol/L). His liver function tests were deranged (ALT 212 U/L, AST 161 U/L, alkaline phosphatase (ALP) 100 U/L, gamma-glutamyl transferase (GGT) 62 U/L) with significantly elevated bilirubin (82 μmol/L) and acute rise in ferritin (2077 ng/mL). Haemoglobin level was stable and inflammatory markers were not elevated (white cell count 5.7×109/L, C-reactive protein <2 mg/L). His venous blood gas revealed pH 7.44, pO2 14 mm Hg, pCO2 46 mm Hg, bicarbonate 27.1 mmol/L, his urine pH 6.5, protein 0.54 g/L, sodium 126 mmol/L, protein/creatinine 90 mg/mmol, albumin/creatinine 18.3 mg/mmol and calcium/creatinine 0.58 mmol/mmol. HCTZ-induced hypokalaemia and hyponatraemia were the initial diagnostic concern, however the acute nature of the kidney injury was atypical. He was admitted for further investigation.
Differential diagnosis
On admission, his HCTZ was withheld and he was commenced on intravenous electrolyte replacement and free water fluid restriction of 1 L. He developed a metabolic acidosis (pH 7.31, pCO2 38 mm Hg, bicarbonate 17.7 mmol/L, base excess—7.5 mmol/L) and profound hypophosphatemia (0.27 mmol/L). An abdominal ultrasound excluded obstructing renal calculi, cholelithiasis or sonographic features of cholecystitis. Deferasirox film-coated tablets were withheld 2 days into his admission due to the concern of proximal renal tubulopathy and urinary wastage of compounds usually reabsorbed at the proximal convoluting tubule (Fanconi syndrome) as a known adverse effect of dispersible deferasirox tablets. Confounding the diagnosis of Fanconi syndrome was the recent use of HCTZ, however based on the presentation of hypokalaemia, hypophosphatemia, metabolic acidosis, elevated urinary sodium (urine Na 126 mEq/L), elevated osmolarity (778 mOsm/kg), proteinuria, glucosuria and increased electrolyte replacement requirements, it was felt to be the most likely diagnosis.
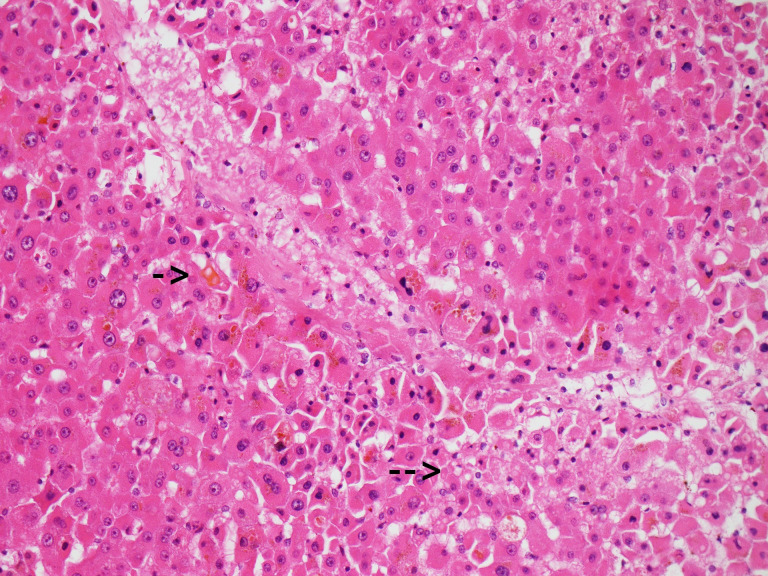
The patient subsequently developed a worsening transaminitis (ALT 819 U/L, AST 670 U/L) and cholestasis (ALP 304 U/L, GGT 301 U/L), with a peak bilirubin of 107 μmol/L. Magnetic resonance cholangiopancreatography found no evidence of obstruction or choledocholithiasis, and no significant results were obtained on haematological viral, autoimmune or heavy metal liver investigations. Nine days into his admission, a transcutaneous liver biopsy was performed with histopathology revealing acute cholestasis, patchy confluent hepatocellular necrosis and mild to moderate microvesicular steatosis (see figure 1). There were no features of significant inflammation or evidence of autoimmune hepatitis. These microscopic findings were highly suggestive of drug-induced liver injury (DILI).
Figure 1.
Centrilobular liver parenchyma showing canalicular cholestasis (short arrow) and microvesicular steatosis (bottom right eg, at long arrow) (H&E x200 magnification)
The provision diagnosis of DILI and a Fanconi-like syndrome due to deferasirox film-coated tablets was made.
Treatment
Deferasirox film-coated tablets continued to be withheld with ongoing supportive management and electrolyte replacement. He clinically improved and his transaminitis was downtrending after 8 days of deferasirox film-coated tablet cessation. He was discharged on oral electrolyte replacement therapy, and not recommenced on HCTZ. He resumed his usual blood transfusion regimen following discharge.
Outcome and follow-up
The patient was counselled on the likely role of deferasirox film-coated tablets as the causative agent of the DILI and Fanconi-like syndrome. Liver function normalised within 2 months, and he ceased phosphate and magnesium supplementation after 3 months. Given that subcutaneous iron chelation therapy severely affects patient’s quality of life and the original formulation dispersible deferasirox tablets were not available, the decision about restarting deferasirox film-coated tablets versus continuing the subcutaneous therapy is a very difficult one. Deferiprone was not initiated as the patient had not failed subcutaneous iron chelation therapy with deferoxamine, and deferiprone would be considered as last-line therapy due to the reported toxicities, renal excretion and need for a timely and reliable reduction in ferritin levels. He was subsequently recommenced on subcutaneous deferoxamine mesylate with no further complications to date.
Discussion
We present a rare case of hepatotoxicity and Fanconi-like syndrome occurring in close temporal relationship to changing from iron-chelating agent dispersible deferasirox tablets to deferasirox film-coated tablets. To our knowledge, this is the first case report of hepatotoxicity and Fanconi-like syndrome reported in deferasirox film-coated tablets in a patient previously tolerant to dispersible deferasirox tablets, with normal renal function and previously stable liver function. As this new drug formulation is increasingly being prescribed, it is important that clinicians are aware of this potentially severe adverse effect.
There are insufficient real-world data and long-term safety data conducted with deferasirox film-coated tablets/sprinkle granules,4 but deferasirox in dispersible tablets has several case reports of hepatoxicity6–10 and 8 case reports of Fanconi syndrome.11–15 The articles by Menaker et al and Ramaswami et al describe the recent case reports of acute liver injury in patients on dispersible deferasirox tablets,10 16 however only 2 articles include patients with beta-thalassemia and many reports are in the paediatric population.1 10 Of note, a 3-year-old child with beta-thalassemia on deferasirox tragically died following potential deferasirox-induced acute liver failure and Fanconi syndrome, resulting in respiratory arrest and cerebellar tonsillar herniation.10 Her ferritin had reduced from 1700 μg/L at initiation of deferasirox to 600 μg/L at the time of presentation, Menaker et al16 postulated that there is increased risk of hepatotoxicity using deferasirox with ferratin below 500 ng/mL. As our patient’s ferritin was 981 ng/mL, this is not a possible contributing factor in our case.
The only other case report with likely deferasirox-induced hepatic and renal failure is a paediatric case report of a 12-year-old male with sickle cell anaemia on long-term deferasirox. The 12-year-old male presented with a rapid drop in Glasgow Coma Scale, sudden rise in transaminases, bilirubin and coagulopathy and was found to have hepatic and renal failure.16 Supportive therapy was instituted with rapid recovery on cessation of deferasirox. The 12-year old’s acute kidney injury was exacerbated by rhabdomyolysis with creatine kinase (CK) elevated at 17 205 ng/mL, likely contributing to direct tubular injury. It was unclear if the rhabdomyolysis was secondary to the child’s underlying sickle cell disease or related to deferasirox, of note our patient did not have CK measured.
The case we present differs from previous reports as our patient developed hepatoxicity and a Fanconi-like syndrome despite prior tolerance of dispersible deferasirox tablets. This is of particular significance due to the cessation of dispersible tablets’ production, and the increasing number of beta-thalassemia patients transitioning to the new formulation. Deferasirox film-coated tablets are reported to have 36% greater bioavailability than dispersible tablets,17 18 so the transition from dispersible tablets to film-coated tablets requires a 30% dose reduction, which was appropriately calculated with our patient’s prescription. Though the film-coated tablets contain the same active substance as the dispersible tablets (deferasirox), some of the excipients (lactose and sodium lauryl sulfate) have been removed, so the film-coated tablets may have increased bioavailability if taken with a high-fat meal which may make drug levels less predictable.3 18 Furthermore, film-coated tablets are reported to have increased adherence due to improved palatability and convenience compared with dispersible tablets or subcutaneous injection.18–20 Vigilance with monitoring for adverse events is crucial, as increased adherence and bioavailability may significantly increase exposure to the iron chelator.18 This case highlights the importance of frequent monitoring of liver and renal function in patients in the early stages of transitioning to deferasirox film-coated tablets.
This case was challenging due to the unusual presentation and scarcity of similar case reports in the literature. The diagnosis of Fanconi syndrome was confounded by the concurrent use of HCTZ diuretics and the necessary administration of intravenous electrolyte replacement and fluid restriction. Our patient’s dipstick was positive for blood, but formal urine was negative for red cells. As deferasirox film-coated tablets are mostly excreted in the faeces and not urine, it is unclear whether the dipstick was detecting heme or Fe-deferasirox film-coated complexes, but the acute kidney injury and increased electrolyte requirement were suggestive of a proximal tubule lesion. Histological evidence of acute cholestasis, patchy confluent hepatocellular necrosis and mild to moderate microvesicular steatosis, coupled with the biochemical findings and temporal relationship of events, make the diagnosis of DILI and Fanconi syndrome secondary to deferasirox film-coated tablets most likely.
Patient’s perspective.
After about a month of starting deferasirox film-coated tablets, I started to feel acute pain at my back flank area and also felt nausea. I had initially thought it may have been to do with my kidney stones just passing through as I have had a history of kidney stones. It then started to get worse as weeks went by and I was in and out of emergency for about 3 times until I was told that my liver function was deranged. Therefore, I stayed in hospital for a total of 2 weeks. In the first week, I was experiencing inflammation-like pain around my back flank which had spread around my stomach, I lost significant weight due to loss of appetite, I felt quite weak and I was also experiencing constipation. During this time, I was infused with potassium, magnesium and sodium as I was experiencing low levels.
It has been about 2½ months in total that I was out of the workforce. I had to take some paid leave and some unpaid in order to keep me financially secure.
Currently, I am now back on the desferrioxamine (DBL) via injections in order to manage my ferritin levels. It has been 10 years since the last time I was on desferrioxamine. Now that I am back on, it definitely changed my lifestyle as I now have to be more vigilant of when I need to start injecting and allow the 10 hours infusion to finish (via the infusion pump) on time. For example, allowing it to finish on time overnight before starting my day at work the next morning. I also have increased responsibility to ensure I have enough medical supplies to get me through, especially if I was to go overseas.
I hope this case will raise awareness of the consequences that may happen to a thalassemia patient like me. Since I have been back on the DBL, it feels like I have taken a step backwards in the quality of life.
Learning points.
This case report highlights a potentially serious complication of likely deferasirox film-coated tablet-induced hepatotoxicity.
Some evidence to suggest that deferasirox is known to cause hepatotoxicity and Fanconi syndrome. The aetiology and pathophysiology are not yet completely understood.
Further large population research is required to identify potential complications of deferasirox film-coated tablet therapy. While real-world data on deferasirox film-coated tablet therapy remain limited, clinicians should be vigilant for this potential serious adverse effect.
Footnotes
Contributors: JF: author of article and primary researcher. DL: supervisor of research article and primary gastroenterologist. RB: haematology consult registrar and proof reader of article. TH: gastroenterology consult registrar and proof reader of article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Cappellini MD, Cohen A, Piga A, et al. A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with beta-thalassemia. Blood 2006;107:3455–62. 10.1182/blood-2005-08-3430 [DOI] [PubMed] [Google Scholar]
- 2.U.S. National Library of Medicine Deferasirox, 2019. Available: https://livertox.nlm.nih.gov/Deferasirox.htm
- 3.Taher AT, Origa R, Perrotta S, et al. New film-coated tablet formulation of deferasirox is well tolerated in patients with thalassemia or lower-risk MDS: results of the randomized, phase II eclipse study. Am J Hematol 2017;92:420–8. 10.1002/ajh.24668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novartis Pharmaceuticals Jadenu (deferasirox) tablets and Jadenu Sprinkle (deferasirox) granules: US prescribing information, 2018. Available: www.pharma.us.novartis.com/product/pi/pdf/jadenu.pdf
- 5.Exjade (deferasirox) [prescribing information]. Stein, Switz Novartis Pharm Corporation, 2019. Available: https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/exjade.pdf
- 6.Vichinsky E, Torres M, Minniti CP, et al. Efficacy and safety of deferasirox compared with deferoxamine in sickle cell disease: two-year results including pharmacokinetics and concomitant hydroxyurea. Am J Hematol 2013;88:1068–73. 10.1002/ajh.23569 [DOI] [PubMed] [Google Scholar]
- 7.Chaudhary P, Pullarkat V. Deferasirox: appraisal of safety and efficacy in long-term therapy. J Blood Med 2013;4:101–10. 10.2147/JBM.S35478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ling G, Pinsk V, Golan-Tripto I, et al. Acute liver failure in a pediatric patient with congenital dyserythropoietic anemia type I treated with deferasirox. Hematol Rep 2015;7:74–6. 10.4081/hr.2015.5987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aslam N, Mettu P, Marsano-Obando LS, et al. Deferasirox induced liver injury in haemochromatosis. J Coll Physicians Surg Pak 2010;20:551–3. doi:04.2010/JCPSP.551553 [PubMed] [Google Scholar]
- 10.Ramaswami A, Rosen DJ, Chu J, et al. Fulminant liver failure in a child with β-thalassemia on deferasirox. J Pediatr Hematol Oncol 2017;39:235–7. 10.1097/MPH.0000000000000654 [DOI] [PubMed] [Google Scholar]
- 11.Martinelli D, Goffredo BM, Falvella FS, et al. Acute hyperammonemia in children under deferasirox treatment: cutting the Gordian knot. Clin Toxicol 2019;57:375–7. 10.1080/15563650.2018.1523425 [DOI] [PubMed] [Google Scholar]
- 12.Yacobovich J, Stark P, Barzilai-Birenbaum S, et al. Acquired proximal renal tubular dysfunction in β-thalassemia patients treated with deferasirox. J Pediatr Hematol Oncol 2010;32:564–7. 10.1097/MPH.0b013e3181ec0c38 [DOI] [PubMed] [Google Scholar]
- 13.Grangé S, Bertrand DM, Guerrot D, et al. Acute renal failure and Fanconi syndrome due to deferasirox. Nephrol Dial Transplant 2010;25:2376–8. 10.1093/ndt/gfq224 [DOI] [PubMed] [Google Scholar]
- 14.Murphy N, Elramah M, Vats H, et al. A case report of deferasirox-induced kidney injury and Fanconi syndrome. WMJ 2013;112:177–80. [PubMed] [Google Scholar]
- 15.Rheault MN, Bechtel H, Neglia JP, et al. Reversible Fanconi syndrome in a pediatric patient on deferasirox. Pediatr Blood Cancer 2011;56:674–6. 10.1002/pbc.22711 [DOI] [PubMed] [Google Scholar]
- 16.Menaker N, Halligan K, Shur N, et al. Acute liver failure during deferasirox chelation: a toxicity worth considering. J Pediatr Hematol Oncol 2017;39:217–22. 10.1097/MPH.0000000000000786 [DOI] [PubMed] [Google Scholar]
- 17.Yassin MA, Nashwan A, Kassem N, et al. Jadenu substituting Exjade in beta thalassemia major (BTM) patients with iron overload: effect on serum ferritin concentration, liver iron content and biochemical profiles. Blood 2018;132:4905 10.1182/blood-2018-99-113998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kattamis A, Aydinok Y, Taher A. Optimising management of deferasirox therapy for patients with transfusion-dependent thalassaemia and lower-risk myelodysplastic syndromes. Eur J Haematol 2018;101:272–82. 10.1111/ejh.13111 [DOI] [PubMed] [Google Scholar]
- 19.Osman HY, al Qawasmeh K, Hussain S, et al. Iron chelation therapy of thalassemia patients is still a challenge. single centre experience from United Arab Emirates. Blood 2018;132:4908 10.1182/blood-2018-99-114601 [DOI] [Google Scholar]
- 20.Huang V, Luini C, El-Ali A, et al. Iron chelation therapy: a review of the literature on the issues and importance of adherence to treatment in iron overload. Blood 2015;126:4748 10.1182/blood.V126.23.4748.4748 [DOI] [Google Scholar]