Abstract
Rationale
Managed withdrawal (i.e., detoxification) from opioid dependence is a widespread clinical procedure that is a necessary step for those pursuing abstinence. Buprenorphine is one effective detoxification treatment, however, consensus regarding effective detoxification procedures is lacking.
Objectives
This study evaluated the efficacy of a buprenorphine transdermal formulation (i.e., patch) in suppressing opioid withdrawal, its safety and tolerability, and its biodelivery when applied for 7 days.
Methods
Physically dependent opioid (heroin) users (n = 12) completed a 10-day opioid detoxification in a residential research unit. Each received a single patch application that remained in place for 7 days. Blood samples were drawn prior to patch application and once daily thereafter. Assessments, four times daily, included: the amount of rescue medications ordered to treat withdrawal discomfort; self-report and observer ratings of opioid withdrawal and agonist effects; and vital sign measures.
Results
Overall, the patch appeared safe and well-tolerated. Buprenorphine plasma levels peaked 48 h after patch application at 0.59 ng/ml. Indices of withdrawal (self-reports, observer ratings, rescue medication) were significantly reduced within 24 h of patch application, continued to decline thereafter, and did not reappear following patch removal.
Conclusions
This study confirms that transdermal buprenorphine is safe and clinically effective, and suggests that a 7-day application may provide an effective and comfortable means of detoxification. This patch formulation would appear to be a useful opioid detoxification treatment by reducing compliance concerns, and administering buprenorphine in a formulation less likely to be diverted to illicit use.
Keywords: Buprenorphine, Detoxification, Patch, Opioid, Transdermal, Withdrawal, Dependence
Introduction
Managed opioid withdrawal (i.e., detoxification) alone is not an effective treatment for the behavioral disorder of opioid dependence. However, it is a necessary step for those pursuing an abstinence goal (Amato et al. 2004; Gowing et al. 2006; Kleber and Riordan 1982). Also, opioid detoxification is a widely-sought and widely-offered clinical service, and minimizing patient discomfort during detoxification is important humanitarian and palliative care (Mattick and Hall 1996). Buprenorphine, a partial agonist at the mu opioid receptor, is as effective in opioid detoxification as moderate doses of methadone (Bickel et al. 1988a; Kosten and Kleber 1988; Lintzeris et al. 2002), and superior to clonidine (Amass et al. 2004; Cheskin et al. 1994; Janiri et al. 1994; Ling et al. 2005; Nigam et al. 1993; O’Connor et al. 1997; Oreskovich et al. 2005). Still, as noted in a Cochrane review by Gowing et al. (2006), data remain limited on buprenorphine’s effectiveness in managed opioid withdrawal, and many aspects of its proper use in detoxification have yet to be resolved, including doses, and frequency and duration of administration.
Due to both cost and regulatory constraints, inpatient detoxification protocols with buprenorphine are often limited to between 3- and 14-day durations (Lintzeris et al. 2003). One protocol commonly used in both inpatient and outpatient settings initially escalates doses to 16 mg sublingual before gradually tapering patients to zero over 12–13 days (Amass et al. 2004; Brigham et al. 2007; Ling et al. 2005). A transdermal buprenorphine formulation (i.e., patch) has been developed that may provide several advantages over the currently marketed sublingual formulation, especially for detoxification use. Notably, with a single administration, it may deliver buprenorphine in a sustained but gradually declining manner over several days, eliminating the fluctuations in concentration associated with daily sublingual administrations. It may be well suited for use either with a brief inpatient stay, or on an outpatient basis.
A previous study assessed the effectiveness of this transdermal buprenorphine formulation when applied for 3 days to opioid-dependent volunteers in a detoxification context (Lanier et al. 2007). Results showed the patch was safe, well-tolerated, and provided sufficient buprenorphine biodelivery to suppress the opioid withdrawal syndrome during the 3 days of application. Blood levels of buprenorphine peaked 48 h after patch application, and volunteers’ self-reports of the presence and severity of withdrawal symptoms were substantially reduced during the 3 days of application. However, following patch removal, plasma levels of buprenorphine declined rapidly, while self-report and observer ratings of withdrawal, and use of non-opioid rescue medications increased; use of opioid rescue medication was abolished during patch application, and returned slightly upon patch removal. The significant biodelivery of buprenorphine and the suppression of the opioid withdrawal syndrome during patch application and its reappearance after patch removal suggested clinically useful pharmacodynamic activity.
Following study completion, analyses of used patches showed that substantial buprenorphine remained. This finding, in addition to evidence of increased withdrawal signs and symptoms following patch removal, suggested that longer-term patch application might provide further therapeutic benefit for opioid detoxification.
The present study evaluated the transdermal buprenorphine formulation for treatment of opioid dependence when applied for 7 days. The primary purposes were to assess the patch’s pharmacodynamics and efficacy in suppressing the opioid withdrawal syndrome for this longer duration, and to assess its likely utility as a detoxification treatment.
Materials and Methods
Study overview
Study methods largely replicate those of our previous buprenorphine patch evaluation (Lanier et al. 2007) except for the number of days the patch was applied (7 in this study, rather than 3). This study was conducted in an opioid-detoxification context. Opioid-dependent volunteers resided on a residential research unit for 10 days, and were assessed four times daily for signs and symptoms of opioid withdrawal. Pre-planned opioid and non-opioid rescue medications were administered as clinically indicated for withdrawal suppression. Each volunteer received a single buprenorphine patch application that remained in place for 7 days. Blood samples were drawn prior to patch application, and once daily thereafter to determine buprenorphine biodelivery.
Participants
Applicants were screened by history and physical examination. Volunteers had to be between 18–50 years of age with current opioid physical dependence, seeking or willing to accept opioid detoxification, not pregnant or nursing, and without other significant medical or psychiatric disorders or other physical dependence (except nicotine). The study was approved by the Institutional Review Board (IRB) for human research, and each subject provided written informed consent. Fifteen volunteers received the transdermal buprenorphine formulation. Three left the study early for personal reasons, and twelve completed. Volunteer characteristics are shown in Tables 1 and 2.
Table 1.
Individual characteristics, biodelivery, and pharmacodynamic data
| Subject characteristics | Buprenorphine biodelivery measures | Withdrawal measures sum of days 2–10a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Age (yrs) | Raceb | Sexc | Weight (kg) | Body mass index | AUC [(ng h)/ml]d | Cmax (ng/ml)e | Tmax (h)f | VAS | Adj | Himm |
| 02 | 42 | B | M | 76.4 | 22.2 | 36.96 | 0.37 | 96 | 20 | 20 | 20 |
| 03 | 45 | B | F | 47.3 | 16.3 | 93.36 | 0.76 | 48 | 570 | 197 | 24 |
| 06 | 43 | B | F | 54.1 | 24.1 | 82.92 | 0.65 | 72 | 98 | 41 | 26 |
| 09 | 46 | B | F | 40.0 | 19.1 | 137.28 | 1.13 | 72 | 0 | 24 | 12 |
| 12 | 40 | B | M | 66.8 | 20.5 | 56.40 | 0.42 | 48 | 74 | 101 | 13 |
| 13 | 36 | B | M | 74.5 | 21.7 | 74.64 | 0.70 | 48 | 915 | 482 | 25 |
| 14 | 19 | W | M | 75.9 | 22.7 | 33.12 | 0.64 | 24 | 312 | 172 | 28 |
| 15 | 35 | B | M | 70.5 | 22.3 | 49.68 | 0.59 | 96 | 235 | 424 | 10 |
| 16 | 30 | W | M | 52.7 | 16.2 | 118.56 | 0.96 | 96 | 331 | 359 | 19 |
| 17 | 43 | W | M | 85.0 | 30.3 | 82.32 | 0.64 | 72 | 22 | 25 | 12 |
| 18 | 35 | W | M | 75.5 | 23.9 | 76.08 | 0.66 | 48 | 385 | 230 | 16 |
| 19 | 36 | W | M | 68.6 | 21.4 | 86.40 | 0.70 | 48 | 90 | 93 | 17 |
| Mean | 37.5 | 58.3% | 75.0% | 65.6 | 21.7 | 77.31 | 0.69 | 64.0 | 254.3 | 180.7 | 18.5 |
| SEM | 2.2 | NA | NA | 4.0 | 1.1 | 8.91 | 0.06 | 6.8 | 78.8 | 47.1 | 1.8 |
VAS Withdrawal strength visual analog scale, Adj adjective withdrawal scale, and Himm Himmelsbach observer ratings of withdrawal; values are sums from the first assessment after patch application on Day 2 through the final assessment before discharge on Day 10.
B Black, W white; mean is percent black.
M Male, F female; mean is percent male.
AUC Area under the buprenorphine concentration curve 0–192 h after patch application.
Peak concentration of plasma buprenorphine.
Time to peak concentration of plasma buprenorphine.
Table 2.
Pearson correlation coefficients (r) between variables, with bold values indicating P<0.05; for all non-bold values, P≥0.10
| Weight (kg) | Body mass index | AUC [(ng h)/ml] | Cmax (ng/ml) | Tmax (h) | Withdrawal measures sum of days 2–10a | |||
|---|---|---|---|---|---|---|---|---|
| VAS | Adj | |||||||
| Body mass index | 0.73 | |||||||
| AUC [(ng h)/ml] | -0.72 | −0.37 | ||||||
| Cmax (ng/ml) | -0.69 | −0.42 | 0.89 | |||||
| Tmax (h) | −0.15 | −0.04 | 0.18 | 0.06 | ||||
| Withdrawal measures sum of days 2–10a | VAS | 0.04 | −0.30 | −0.01 | 0.12 | −0.36 | ||
| Adj | 0.09 | −0.31 | −0.06 | 0.12 | 0.04 | 0.78 | ||
| Himm | −0.06 | −0.18 | −0.21 | −0.06 | −0.42 | 0.50 | 0.11 | |
VAS Withdrawal strength visual analog scale, Adj adjective withdrawal scale, and Himm Himmelsbach observer ratings of withdrawal
Participants were not dependent on alcohol or illicit drugs other than heroin, as determined by history and observation. One participant reported 10 days of other opioid use, 7 reported an average of 7.4 ± 3.37 days of cocaine use, 3 reported an average of 1.8 ± 1.20 days of alcohol use, 2 reported an average of 1.3 ± 1.16 days of sedative use, and 4 reported an average of 1.4 ± 0.88 days of marijuana use in the last 30 days.
Physical dependence assessment
At study intake, participants reported using opioids (heroin) an average of 27.8 ± 1.2 days out of the last 30, using an average of 3.0 times per day, and spending an average of US $32 per day on heroin. Urine toxicology specimens collected at screening and at admission tested opioid-positive for all 12 participants. All volunteers required treatment with oral hydromorphone for suppression of withdrawal symptoms prior to receiving the buprenorphine patch. In the 24 h prior to receiving the patch, volunteers received a mean dose of 36.2 mg (range 14–70 mg) of oral hydromorphone. These doses were guided by clinician judgment; they ameliorated but did not abolish opioid withdrawal.
Procedure
Upon admission to the closed residential research unit, volunteers were assessed four times daily (i.e., 0700, 1200, 1700, and 2200) for signs and symptoms of opioid withdrawal, and were administered oral hydromorphone as needed for suppression of withdrawal. On Day 2, oral hydromorphone was withheld until a criterion level of opioid withdrawal signs was observed (observer Himmelsbach withdrawal severity scale score ≥3), at which time volunteers received the buprenorphine patch concurrent with a dose of oral hydromorphone (to accommodate for the slow onset of transdermal drug delivery and effects; mean 13.7 mg, range 4–20 mg). Each volunteer received a single patch application on the upper arm that remained in place for 7 days (168 h). Primary outcome measures collected during the four daily assessments included self-report and observer ratings of opioid withdrawal and agonist effects, physiological measures, and the amount of rescue medications given to treat withdrawal discomfort. Also at each assessment, staff queried volunteers regarding discomfort at the patch site, and examined the site for issues that might affect patch performance or outcomes. After patch removal, staff continued to examine the site for skin irritation or inflammation.
Upon study completion (morning of Day 10), volunteers received one last assessment, and were discharged from the research unit. Volunteers were offered post-study outpatient counseling and assistance with arranging continuing care.
Measures
Self-report ratings of opioid withdrawal and agonist effects were collected by means of visual analog scales (VAS) and by an adjective rating questionnaire sensitive to opioid effects and to opioid withdrawal. Two visual analog scales asked: “Do you feel any withdrawal discomfort?,” and “How strong, or bad, is your withdrawal discomfort?” Using a computer mouse, participants responded by positioning an arrow along a 100 mm line labeled at either end with “Not at all” and “Extremely,” to yield a score between 0 and 100.
Volunteers also completed a 37-item adjective rating scale (Sobel et al. 2004) to assess opioid agonist and antagonist effects. Volunteers rated each item on a 5-point scale from 0 (not at all) to 4 (extremely). Items were scored as two scales: a 21-item Withdrawal Scale (possible score range of 0–84) and a 16-item Agonist Scale (possible score range of 0–64). The Withdrawal scale consisted of items reflecting opioid withdrawal-like effects such as muscle cramps, flushing, watery eyes, and sick to stomach. The Agonist scale consisted of adjectives reflecting morphine-like agonist effects such as nodding, relaxed, friendly, dry mouth, and coasting. Item ratings were summed to produce scale scores.
Observer ratings of opioid withdrawal were made by nursing staff using a modified Himmelsbach (Himmelsbach 1941) withdrawal severity scale (Eissenberg et al. 1996). The observer rated each of six signs of opioid withdrawal (lacrimation, rhinorrhea, yawning, perspiration, piloerection, and restlessness) on a 0–2 scale (none, mild, pronounced); the sum constituted the total score, for a possible score range of 0–12.
Physiological measures included heart rate, blood pressure, skin temperature, respiratory rate and oxygen saturation (by pulse oximetry). Pupil diameter was determined from photographs taken in constant ambient room lighting using 2× magnification.
Drugs
Transdermal buprenorphine formulation
The transdermal buprenorphine (Buprenorphine DermaPatch) was provided by Biotek, Inc. (Wellesley, MA, USA). Each patch contained 46.6 mg buprenorphine in aqueous ethanol and propylene glycol solution, with a small amount of lauric acid as a penetration enhancer, and hydroxypropyl cellulose as a thickener. A macroporous membrane confined the liquid while allowing it to contact the skin. Preclinical laboratory testing showed the formulation to deliver an average flux of 1.9 mg/day of buprenorphine through human skin over 3 days, with mean deliveries of 2.3, 2.2, and 1.1 mg/day for days 1, 2, and 3, respectively. Biodelivery beyond 3 days is unknown.
Hydromorphone (oral treatment)
Oral hydromorphone was administered up to four times (i.e., 0700, 1200, 1700, and 2200) during the day prior to buprenorphine patch application for suppression of opioid withdrawal. Doses ranged from 4 mg to 20 mg per administration, up to an allowable maximum of 80 mg/day. After patch application, volunteers continued to be monitored for signs and symptoms of opioid withdrawal, and oral hydromorphone remained available in the event of insufficient withdrawal suppression by the buprenorphine patch. Throughout the study, doses were selected as clinically indicated to achieve withdrawal suppression without intoxication or sedation. Clinicians received input from study participants and nursing staff, and had access to participants’ withdrawal assessment data.
Other rescue medications
Rescue procedures in the event of insufficient withdrawal relief from the transdermal formulation were to provide pharmacological (opioid and/or non-opioid) treatment as judged clinically appropriate. Available medications included oral hydromorphone as described above, plus oral hydroxyzine for sleep problems, loperamide for diarrhea, and clonidine for other elements of the opioid withdrawal syndrome. These were ordered by medical staff as needed based on their assessment of the presence and severity of withdrawal discomfort, and with the overall clinical detoxification goal of having all medication stopped by study completion. Extent of rescue medication use was an outcome variable.
Blood sample collection and analysis
Blood samples were drawn prior to patch application, and then each 24 h following patch application for the duration of the study. Blood samples (10 ml) were drawn through an intravenous catheter or by needle stick and immediately centrifuged at 3,000 rpm (1,500×g) for 10 min. Plasma was drawn into a glass pipette, transferred to plastic cryotubes, and frozen at −20°F until shipped for analysis. Plasma concentrations of buprenorphine and its major metabolite, norbuprenorphine, were determined by liquid chromatographic-electrospray ionization-tandem mass spectrometry at the Center for Human Toxicology, University of Utah (Moody et al. 2002). The lower limit of detectability for each was 0.10 ng/ml; lower values were treated as zeros.
Data analysis
Descriptive analyses were used to characterize the safety, biodelivery, and withdrawal-suppressing effects of transdermal buprenorphine. Data are presented for individual subjects and as group means (SEM). Individual Cmax and Tmax were determined, as were individual areas under the curve (AUC) of plasma buprenorphine concentration for the period from patch application to study end (192 h after patch application). Daily averaged self-report and observer measures (four observations per 24-h period) were analyzed in a one-factor (Day) repeated measures ANOVA. Planned comparisons (mathematical equivalent to paired t tests) were done on specific Day comparisons. No corrections (e.g., Bonferroni) for family wise error were applied to the tests because we did not exceed the degrees of freedom associated with the Day source of variance. All tests were two-sided with p values < 0.05 considered significant. Also, individual sum scores were calculated for each of three pharmacodynamic measures (self-report withdrawal strength VAS, withdrawal adjective rating scale score, and observer Himmelsbach withdrawal sign score). Scores were summed from the first assessment after patch application through the final assessment prior to discharge on Day 10 (8 days × 4 assessments/day = 32 assessments total, for total possible summed scores of 0–3200, 0–2688, and 0–384, respectively). Finally, Pearson correlation coefficients were calculated to examine relationships among buprenorphine biodelivery measures (Cmax, Tmax, and AUC) and weight, body mass index, and pharmacodynamic summed scores.
Results
For comparison purposes, data from this study and selected data from the previously published 3-day application study (Lanier et al. 2007) are presented together.
Buprenorphine plasma concentrations
Most of the rise in mean plasma levels of buprenorphine occurred within the first 24 h following patch application (Fig. 1, top panel), peaking 48 h after application at a mean concentration of 0.59 ng/ml ± 0.06, and remaining at this level at 72 h. Our previously published more detailed time course data show this increase to be quite gradual, with peak levels much delayed and much lower relative to those reported for sublingual buprenorphine administration (Lanier et al. 2007). Levels remained relatively constant at 96 h (0.57 ± 0.07 ng/ml), and declined steadily thereafter. By the time of patch removal (168 h after application), mean buprenorphine concentration was 0.24 ± 0.04 ng/ml. Six volunteers (50%) had detectable levels of buprenorphine at their final blood draw (24 h after patch removal), with an overall group mean of 0.11 ± 0.04 ng/ml.
Fig. 1.

Mean plasma concentrations of buprenorphine (triangles) and norbuprenorphine (circles) are shown for the 3- (unfilled symbols) and 7- (filled symbols) day patch application studies (top panel). Individual plasma concentrations of buprenorphine (ng/ml) for the 12 volunteers who completed the 7-day study are shown (bottom panel)
Mean plasma levels of norbuprenorphine followed a similar time course as buprenorphine, but with a time lag (Fig. 1, top panel). Norbuprenorphine levels peaked 120 h after patch application at a mean concentration of 0.14 ng/ml ± 0.03, remained at this level at 144 h, and then slowly declined. Five volunteers (42%) had detectable levels of norbuprenorphine at their final blood draw, and the overall group mean at this time was 0.07 ng/ml ± 0.03.
Biodelivery data from the individual volunteers indicated variability in the magnitude and time course of buprenorphine levels (Fig. 1, bottom panel). All volunteers had detectable plasma levels by 24 h, though levels ranged from 0.12 to 0.71 ng/ml. Time to peak concentration (Tmax) ranged from 24 to 96 h. Five volunteers (42%) reached peak plasma concentration 48 h after application, one reached peak at 24 h, three at 72 h, and the remaining three at 96 h. There was also considerable variability in the magnitude of peak buprenorphine concentration (Cmax). Cmax ranged from 0.37 to 1.13 ng/ml (three-fold difference), while AUC ranged from 33.12 to 137.28 (ng h)/ml. There were significant inverse relationships between buprenorphine AUC and body weight, and between Cmax and body weight (r = −0.72, p < 0.01, and r = −0.69, p < 0.05, respectively; see Tables 1 and 2), indicating that lower body weight was associated with higher peak and AUC plasma concentrations of buprenorphine. There was no evident relationship of Cmax or AUC to race or sex other than as related to body weight.
Efficacy—withdrawal suppression
Self-reports and observer ratings
Volunteers’ mean self-reports of the presence of any withdrawal symptoms, and the strength (severity) of these symptoms, measured by visual analog scale (VAS) rating, were reduced by more than 50% within 24 h following patch application compared to Day 1 (pre-patch application; see Fig. 2, top panel). These self-report withdrawal ratings continued to decline gradually throughout the study, reaching near-zero levels that were approximately 25 times lower on the last day of patch application (patch Day 7, study Day 8) as compared to pre-patch levels. These low subjective ratings of withdrawal presence and strength continued throughout the final 24 h volunteers remained on the research unit after patch removal (study Days 9–10), indicating that self-reported withdrawal symptoms were virtually nonexistent by the time of discharge. Self-reports of withdrawal on the 21-item Withdrawal Adjective Rating Scale showed a similar pattern (Fig. 2, middle panel).
Fig. 2.

Participants’ mean visual analog scale (VAS) ratings of withdrawal strength are shown for the 3- (unfilled circles) and 7- (filled circles) day patch application studies (top panel). Volunteers also rated “Do you feel any withdrawal discomfort,” which resulted in an almost identical set of values (not shown). Mean participant self-report ratings of withdrawal as measured by a 21-item Withdrawal Adjective Rating Scale are shown for the 3- and 7-day patch application studies (middle panel). Mean observer ratings of withdrawal as measured using a modified Himmelsbach withdrawal severity scale are shown for the 3- and 7-day patch application studies (bottom panel). Vertical bars represent SEM
Observer ratings of opioid withdrawal are shown in Fig. 2, bottom panel. Generally, observer ratings followed a similar pattern as self-report ratings, with scores dropping greatly following patch application on Day 2, and remaining low throughout the remainder of the study. There was no indication of increased withdrawal symptoms following patch removal on the morning of study Day 9. It was also observed by nursing staff and investigators that in addition to withdrawal suppression, patients’ morale and mood improved during the time of patch application. Volunteer comments to investigators and nursing staff indicated that the transdermal patch provided adequate withdrawal relief devoid of intoxication.
For the three pharmacodynamic measures shown in Fig. 2, analyses of variance indicated a significant main effect of study day (all p < 0.001), with scores declining over the course of the evaluation. Planned comparisons revealed that pre-patch Day 1 scores were significantly higher than early treatment (Day 2), late treatment (Day 8), and post-patch scores (Days 9–10; all p < 0.001). Self-reported VAS and Adjective Withdrawal scores showed significant reductions between early treatment Day 2 and late treatment Day 8 (both p < 0.01), while observer Himmelsbach withdrawal scores were not significantly different (p = 0.8). Finally, post-patch scores (Days 9–10) declined slightly (non-significantly) from late patch-on scores (Day 8) for all three pharmacodynamic measures.
Individual pharmacodynamic measures summed scores following patch application are shown in Tables 1 and 2. There were no significant relationships between buprenorphine biodelivery measures (AUC, Cmax, and Tmax) and any of the pharmacodynamic measures (all p ≥ 0.10; see Tables 1 and 2). All volunteers appeared to receive adequate withdrawal relief, regardless of buprenorphine plasma levels. Interestingly, VAS and Himmelsbach withdrawal measures summed scores in this study were substantially and significantly lower than in the previous 3-day study (both p < 0.01).
Rescue medication delivered
Following patch application, no oral hydromorphone was required or administered for the remainder of the study. Figure 3 shows and summarizes the temporal pattern of administrations of hydromorphone and of the other pre-planned rescue medications (clonidine, loperamide, and hydroxyzine). These non-opioid rescue medications were administered for withdrawal relief, as necessary, by clinician’s judgment. Generally, these drugs were given to provide symptomatic relief of withdrawal complaints such as subjective distress, muscle and joint discomfort, mild anxiety, insomnia, and gastrointestinal distress. Use of these pre-planned rescue medications remained low throughout the study, with, on average, and for the entire group of medications, less than 0.5 administrations per day over the 10-day study. Additionally, there were sporadic administrations of ibuprofen and gabapentin for general aches and insomnia that may or may not have been related to withdrawal; these administrations are not included in Fig. 3.
Fig. 3.
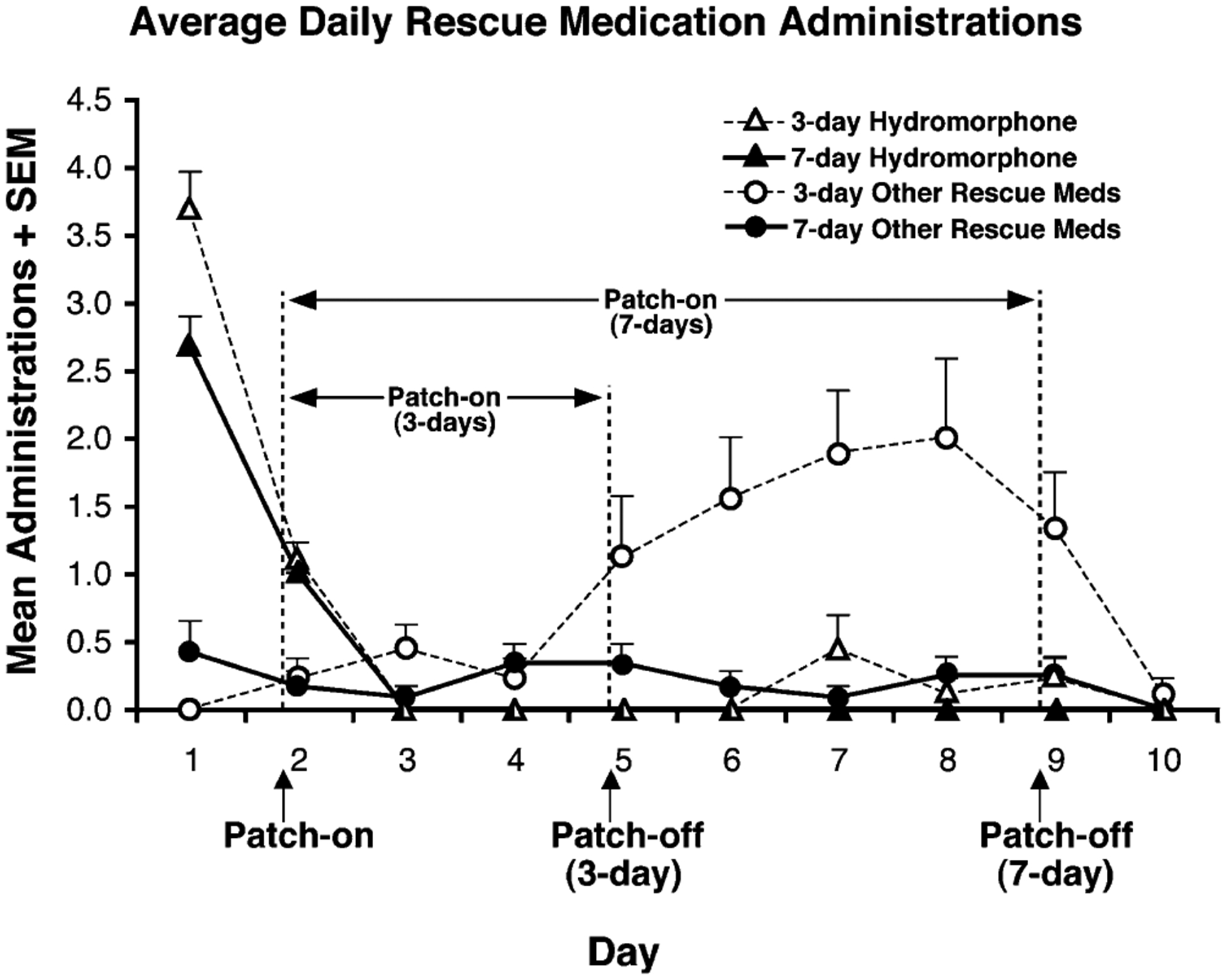
The mean number of times rescue medications were administered per day for the 3- (unfilled symbols) and 7- (filled symbols) day patch application studies are shown. Hydromorphone is represented by the triangles while all other pre-planned rescue medications (hydroxyzine, loperamide, and clonidine) are represented collectively by the circles. Vertical bars represent SEM
Safety and other findings
The patch appeared safe and well-tolerated and without evidence of opioid intoxication or respiratory depression. There were no serious adverse events. During the 7 days of patch application, oxygen saturation never fell below 96% for any individual. Other physiological indices (heart rate, blood pressure, skin temperature, respiration rate, and pupil diameter) also showed no important changes during patch application.
There was a recurrent problem with inadequate patch adhesion to volunteers’ skin. OpSite Flexigard (Smith & Nephew, Inc., Largo, FL), a flexible, breathable adhesive film was placed around the edge of the patch to keep it in place.
Discussion
This is the second in-human evaluation of this transdermal buprenorphine formulation for treatment of opioid dependence. The primary purpose was to assess the formulation’s pharmacodynamics and efficacy in suppressing the opioid withdrawal syndrome when applied for 7 days in opioid-dependent volunteers. The results show that 7-day application of this transdermal buprenorphine formulation provides a safe, effective opioid detoxification with minimal subjective discomfort. As in our previous study of 3-day patch application (Lanier et al. 2007), this 7-day application resulted in no significant adverse events, no evidence of opioid intoxication or respiratory depression, and no significant local irritation at the sites of transdermal patch application. Also consistent with the previous study, the transdermal formulation was found to be well-accepted by patients, and it appeared to be quite effective in suppressing the opioid withdrawal syndrome throughout the time it was applied. In contrast to the 3-day study, the 7-day application continued to provide withdrawal relief until study completion, and greatly reduced the need for rescue medications to treat opioid withdrawal.
Peak plasma levels of buprenorphine were comparable to trough levels observed with daily 8-mg sublingual buprenorphine tablets (Chiang and Hawks 2003; Schuh and Johanson 1999; Strain et al. 2004). Buprenorphine levels dropped monotonically after the initial 96 h of patch application, with a mean concentration of 0.11 ng/ml, barely above the limit of detectability, at the final blood draw (8 days after application, 24 h after patch removal). Previous studies have reported withdrawal suppression associated with buprenorphine plasma concentrations of 0.70 ng/ml or greater (Kuhlman et al. 1998; Sigmon et al. 2006). With this patch, we have found that buprenorphine plasma concentrations of approximately 0.60 ng/ml and lower can result in significant withdrawal suppression as indicated by substantial reductions in subjective and objective measures of opioid withdrawal, and the near abolition of rescue medication administrations following patch application. These results indicate that for detoxification purposes, lower buprenorphine concentrations can be effective and appropriate, at least when achieved gradually.
Substantial and significant withdrawal suppression was maintained throughout the 7 days of patch application, and in the 24 h following patch removal. In sharp contrast to the 3-day study in which the patch was removed on the morning of study Day 5 resulting in a marginal ‘rebound’ of withdrawal symptoms, there was no increase in withdrawal from Days 5–10 in this study, although buprenorphine plasma concentrations were continually declining. Presumably, the extended period of exposure to declining levels of buprenorphine provided a more gradual transition to a non-opioid state. Global observations suggested that patients were more comfortable in this study as compared to the prior 3-day patch application study, and self-report and observer ratings of withdrawal support this notion. These data and observations suggest that the longer application of the buprenorphine patch may provide a more effective opioid detoxification, and are in agreement with studies indicating that gradual tapering of low to moderate buprenorphine doses results in a comfortable, effective detoxification (Amass et al. 1994; Brigham et al. 2007; Gowing et al. 2006; Ling et al. 2005; Lintzeris et al. 2003; Oreskovich et al. 2005).
As in the 3-day patch application study, there was substantial between-subject variability in both times to reach peak and maximum buprenorphine concentrations. Although the mechanism is not clearly understood, similar large between-subject variability also occurs with sublingual and depot formulations (Chawarski et al. 1999; Chiang and Hawks 2003; Kuhlman et al. 1996; Schuh and Johanson 1999; Sigmon et al. 2006; Strain et al. 2004). Body weight was significantly inversely related to buprenorphine Cmax and AUC, indicating that those weighing less achieved higher peak and AUC levels of buprenorphine in contrast to the 3-day study, in which no significant relationships existed between body weight and Cmax or AUC. Combining data from the two studies (21 volunteers total) affirms the significant inverse relationship between weight and AUC. Data from each study show buprenorphine Cmax and AUC were not related to individual assessments of opioid withdrawal severity based on self-report VAS and withdrawal adjective rating scale, and observer Himmelsbach summed scores. Therefore, although there was variability in the magnitude and time course of buprenorphine levels across individual volunteers, the patch appeared to provide withdrawal relief to all participants.
These data indicate that the transdermal formulation provided pharmacologically significant biodelivery of buprenorphine throughout its 7 days of application, and provided benefits beyond the 3-day duration previously studied, and for which the patch was originally designed. Opioid-dependent participants who received the patch were effectively detoxified after 7 days of use, which may make this formulation ideally suited for clinical use as a short-term inpatient or outpatient palliative treatment for opioid withdrawal. This patch could be especially useful in cases in which multiple-day treatment is needed but the clinician is hesitant to provide patients a supply of multiple doses of sublingual medication, and may reduce adherence or diversion concerns. The patch’s biodelivery characteristics (gradual increase in plasma concentration, long plateau with low peak concentration, followed by gradual decline in plasma concentration) seem ideal for avoiding precipitated withdrawal in heavy opioid abusers, for providing withdrawal relief devoid of intoxication, and for avoiding ‘rebound’ withdrawal from buprenorphine following treatment. Administration of an oral hydromorphone dose concurrent with the patch application may have contributed to the success of the overall procedure. Lintzeris et al. (2003) has suggested that the minimum amount of medication to alleviate discomfort should be used in detoxification to alleviate concerns over ‘rebound’ withdrawal, and this patch formulation appears to do so quite conveniently and effectively. In opioid maintenance treatment of opioid dependence, relatively high doses or drug levels may be needed to attenuate or block exogenous opioid challenge effects, but this is not the goal of detoxification treatment, and withdrawal suppression is achievable with substantially lower doses (Bickel et al. 1988b; Donny et al. 2005; Donny et al. 2002).
Several further studies would seem desirable for developing this product toward clinical application: (1) randomized, controlled evaluations of clinical effectiveness for opioid detoxification in comparison to other medications or formulations or to placebo; (2) testing whether higher doses or longer durations of patch application might be necessary in certain patients or populations; (3) evaluating effectiveness of the patch in an outpatient cohort; and (4) evaluation in non-drug-abuse medical populations for opioid tapering of physically dependent patients.
In conclusion, evaluation of this transdermal buprenorphine formulation has confirmed the patch to be safe and highly effective in suppressing the opioid withdrawal syndrome for the duration it is applied. Furthermore, the present longer-term application of 7 days appears to provide a substantially more comfortable and successful opioid detoxification than the 3-day application previously tested. The patch provides effective withdrawal relief during opioid detoxification by delivering low, sustained doses of buprenorphine that decline gradually over several days. This buprenorphine patch formulation offers considerable promise as likely to be an effective treatment for opioid dependence, and may be of special utility in situations where multiple-day medication is appropriate, but, due to concerns with adherence or diversion, the clinician is hesitant to provide a multi-dose medication supply for patient self-administration.
Acknowledgements
Supported by research grant R01DA08045 and training grant T32DA07209 to Johns Hopkins University from the National Institute on Drug Abuse (NIDA). Transdermal buprenorphine (Buprenorphine DermaPatch) was provided by Biotek, Inc., Wellesley, MA, USA, through support by Small Business Innovation Research (SBIR) grant R44DA15573 from NIDA. Blood-level analyses were supported by NIDA contract N01DA-3-8829; we thank David Moody, Ph.D. and the staff at the Center for Human Toxicology at the University of Utah, and C. Nora Chiang, Ph.D. at NIDA. We thank John Yingling, Mary Misenhimer, and the nursing staff at the Behavioral Pharmacology Research Unit for their assistance. We also thank Paul Nuzzo for assistance with the statistical analysis. This study complies with current laws of the United States.
Footnotes
Disclosure Bigelow has received, through his university, research contract support from Biotek, Inc., from Titan Pharmaceuticals, and from Purdue Pharma LLP, all of which have been developing buprenorphine products. Nuwayser is an officer of, and has financial interest in Biotek, Inc. Lanier, Umbricht, and Harrison report no conflicting interests.
Contributor Information
Elie S. Nuwayser, Biotek, Inc., 25 Ingraham Road, Wellesley, MA 02482, USA
George E. Bigelow, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, MD, USA
References
- Amass L, Bickel WK, Higgins ST, Hughes JR (1994) A preliminary investigation of outcome following gradual or rapid buprenorphine detoxification. J Addict Dis 13:33–45 [DOI] [PubMed] [Google Scholar]
- Amass L, Ling W, Freese TE, Reiber C, Annon JJ, Cohen AJ, McCarty D, Reid MS, Brown LS, Clark C, Ziedonis DM, Krejci J, Stine S, Winhusen T, Brigham G, Babcock D, Muir JA, Buchan BJ, Horton T (2004) Bringing buprenorphine-naloxone detoxification to community treatment providers: the NIDA Clinical Trials Network field experience. Am J Addict 13(Suppl 1):S42–S66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato L, Davoli M, Ferri M, Gowing L, Perucci CA (2004) Effectiveness of interventions on opiate withdrawal treatment: an overview of systematic reviews. Drug Alcohol Depend 73:219–226 [DOI] [PubMed] [Google Scholar]
- Bickel WK, Stitzer ML, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE (1988a) A clinical trial of buprenorphine: comparison with methadone in the detoxification of heroin addicts. Clin Pharmacol Ther 43:72–78 [DOI] [PubMed] [Google Scholar]
- Bickel WK, Stitzer ML, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE (1988b) Buprenorphine: dose-related blockade of opioid challenge effects in opioid dependent humans. J Pharmacol Exp Ther 247:47–53 [PubMed] [Google Scholar]
- Brigham GS, Amass L, Winhusen T, Harrer JM, Pelt A (2007) Using buprenorphine short-term taper to facilitate early treatment engagement. J Subst Abuse Treat 32:349–356 [DOI] [PubMed] [Google Scholar]
- Chawarski MC, Schottenfeld RS, O’Connor PG, Pakes J (1999) Plasma concentrations of buprenorphine 24 to 72 hours after dosing. Drug Alcohol Depend 55:157–163 [DOI] [PubMed] [Google Scholar]
- Cheskin LJ, Fudala PJ, Johnson RE (1994) A controlled comparison of buprenorphine and clonidine for acute detoxification from opioids. Drug Alcohol Depend 36:115–121 [DOI] [PubMed] [Google Scholar]
- Chiang CN, Hawks RL (2003) Pharmacokinetics of the combination tablet of buprenorphine and naloxone. Drug Alcohol Depend 70: S39–S47 [DOI] [PubMed] [Google Scholar]
- Donny EC, Walsh SL, Bigelow GE, Eissenberg T, Stitzer ML (2002) High-dose methadone produces superior opioid blockade and comparable withdrawal suppression to lower doses in opioid-dependent humans. Psychopharmacology (Berl) 161:202–212 [DOI] [PubMed] [Google Scholar]
- Donny EC, Brasser SM, Bigelow GE, Stitzer ML, Walsh SL (2005) Methadone doses of 100 mg or greater are more effective than lower doses at suppressing heroin self-administration in opioid-dependent volunteers. Addiction 100:1496–1509 [DOI] [PubMed] [Google Scholar]
- Eissenberg T, Greenwald MK, Johnson RE, Liebson IA, Bigelow GE, Stitzer ML (1996) Buprenorphine’s physical dependence potential: antagonist-precipitated withdrawal in humans. J Pharmacol Exp Ther 276:449–459 [PubMed] [Google Scholar]
- Gowing L, Ali R, White J (2006) Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev: CD002025 Himmelsbach CK (1941) The morphine abstinence syndrome, its nature and treatment. Ann Intern Med 15:829–839 [Google Scholar]
- Janiri L, Mannelli P, Persico AM, Serretti A, Tempesta E (1994) Opiate detoxification of methadone maintenance patients using lefetamine, clonidine and buprenorphine. Drug Alcohol Depend 36:139–145 [DOI] [PubMed] [Google Scholar]
- Kleber HD, Riordan CE (1982) The treatment of narcotic withdrawal: a historical review. J Clin Psychiatry 43:30–34 [PubMed] [Google Scholar]
- Kosten TR, Kleber HD (1988) Buprenorphine detoxification from opioid dependence: a pilot study. Life Sci 42:635–641 [DOI] [PubMed] [Google Scholar]
- Kuhlman JJ Jr, Lalani S, Magluilo J Jr, Levine B, Darwin WD (1996) Human pharmacokinetics of intravenous, sublingual, and buccal buprenorphine. J Anal Toxicol 20:369–378 [DOI] [PubMed] [Google Scholar]
- Kuhlman JJ Jr, Levine B, Johnson RE, Fudala PJ, Cone EJ (1998) Relationship of plasma buprenorphine and norbuprenorphine to withdrawal symptoms during dose induction, maintenance and withdrawal from sublingual buprenorphine. Addiction 93:549–559 [DOI] [PubMed] [Google Scholar]
- Lanier RK, Umbricht A, Harrison JA, Nuwayser ES, Bigelow GE (2007) Evaluation of a transdermal buprenorphine formulation in opioid detoxification. Addiction 102:1648–1656 [DOI] [PubMed] [Google Scholar]
- Ling W, Amass L, Shoptaw S, Annon JJ, Hillhouse M, Babcock D, Brigham G, Harrer J, Reid M, Muir J, Buchan B, Orr D, Woody G, Krejci J, Ziedonis D (2005) A multi-center randomized trial of buprenorphine-naloxone versus clonidine for opioid detoxification: findings from the National Institute on Drug Abuse Clinical Trials Network. Addiction 100:1090–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintzeris N, Bell J, Bammer G, Jolley DJ, Rushworth L (2002) A randomized controlled trial of buprenorphine in the management of short-term ambulatory heroin withdrawal. Addiction 97:1395–1404 [DOI] [PubMed] [Google Scholar]
- Lintzeris N, Bammer G, Rushworth L, Jolley DJ, Whelan G (2003) Buprenorphine dosing regime for inpatient heroin withdrawal: a symptom-triggered dose titration study. Drug Alcohol Depend 70:287–294 [DOI] [PubMed] [Google Scholar]
- Mattick RP, Hall W (1996) Are detoxification programmes effective? Lancet 347:97–100 [DOI] [PubMed] [Google Scholar]
- Moody DE, Slawson MH, Strain EC, Laycock JD, Spanbauer AC, Foltz RL (2002) A liquid chromatographic-electrospray ionization-tandem mass spectrometric method for determination of buprenorphine, its metabolite, norbuprenorphine, and a coformulant, naloxone, that is suitable for in vivo and in vitro metabolism studies. Anal Biochem 306:31–39 [DOI] [PubMed] [Google Scholar]
- Nigam AK, Ray R, Tripathi BM (1993) Buprenorphine in opiate withdrawal: a comparison with clonidine. J Subst Abuse Treat 10:391–394 [DOI] [PubMed] [Google Scholar]
- O’Connor PG, Carroll KM, Shi JM, Schottenfeld RS, Kosten TR, Rounsaville BJ (1997) Three methods of opioid detoxification in a primary care setting. A randomized trial. Ann Intern Med 127:526–530 [DOI] [PubMed] [Google Scholar]
- Oreskovich MR, Saxon AJ, Ellis ML, Malte CA, Reoux JP, Knox PC (2005) A double-blind, double-dummy, randomized, prospective pilot study of the partial mu opiate agonist, buprenorphine, for acute detoxification from heroin. Drug Alcohol Depend 77:71–79 [DOI] [PubMed] [Google Scholar]
- Schuh KJ, Johanson CE (1999) Pharmacokinetic comparison of the buprenorphine sublingual liquid and tablet. Drug Alcohol Depend 56:55–60 [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Moody DE, Nuwayser ES, Bigelow GE (2006) An injection depot formulation of buprenorphine: extended biodelivery and effects. Addiction 101:420–432 [DOI] [PubMed] [Google Scholar]
- Sobel BF, Sigmon SC, Walsh SL, Johnson RE, Liebson IA, Nuwayser ES, Kerrigan JH, Bigelow GE (2004) Open-label trial of an injection depot formulation of buprenorphine in opioid detoxification. Drug Alcohol Depend 73:11–22 [DOI] [PubMed] [Google Scholar]
- Strain EC, Moody DE, Stoller KB, Walsh SL, Bigelow GE (2004) Relative bioavailability of different buprenorphine formulations under chronic dosing conditions. Drug Alcohol Depend 74:37–43 [DOI] [PubMed] [Google Scholar]


