Abstract
Wound healing is a complex biological process that requires coordinated cell proliferation, migration, and extracellular matrix production/remodeling, all of which are inhibited/delayed in chronic wounds. In this study, a formulation was developed that marries a fibrin-based, provisional-like matrix with collagen mimetic peptide (CMP)/PDGF gene-modified collagens, leading to the formation of robust gels that supported temporally controlled PDGF expression and facile application within the wound bed. Analysis employing in vitro co-gel scaffolds confirmed sustained and temporally controlled gene release based on matrix metalloproteinase (MMP) activity, with ~30% higher PDGF expression in MMP producing fibroblasts as-compared with non-MMP-expressing cells. The integration of fibrin with the gene-modified collagens resulted in co-gels that strongly supported both fibroblast cell recruitment/invasion as well as multiple aspects of the longer-term healing process. The excisional wound healing studies in mice established faster wound closure using CMP-modified PDGF polyplex-loaded co-gels, which exhibited up to 24% more wound closure (achieved with ~2 orders of magnitude lower growth factor dosing) after 9 days as compared to PDGF-loaded co-gels, and 19% more wound closure after 9 days as compared to CMP-free polyplex loaded co-gels. Moreover, minimal scar formation as well as improved collagen production, myofibroblast activity, and collagen orientation was observed following CMP-modified PDGF polyplex-loaded co-gel application on wounds. Taken together, the combined properties of the co-gels, including their stability and capacity to control both cell recruitment and cell phenotype within the murine wound bed, strongly supports the potential of the co-gel scaffolds for improved treatment of chronic non-healing wounds.
Keywords: Collagen mimetic peptide, gene delivery, MMP, PDGF, temporal control, wound healing
Graphical abstract
1. Introduction
Wound healing is a complex biological process initiated by a breach in tissue integrity. Coordinated cellular processes by fibroblasts, macrophages, endothelial cells, and keratinocytes are required, among other cell types, with the production of intact tissue mediated via clearance of degraded extracellular matrix (ECM) proteins, synthesis of new ECM proteins and growth factors, and cellular responses including migration, proliferation, and differentiation1, 2. Irregularities in these cellular processes result in the development of a chronic wound that does not heal in a well-organized or timely fashion3. The prevalence rate of chronic non-healing wounds is 1–2% of the general population in developed countries with total estimated costs in excess of US$20 billion4.
Chronic wounds tend to show decreased levels of transforming growth factor-β (TGF-β), epidermal growth factor (EGF),, platelet derived growth factor (PDGF), fibroblast growth factor (FGF), and vascular endothelial growth factor (VEGF)5. Accordingly, growth factor treatments have received wide attention because of their potential growth-promoting activity in chronic wounds. However, topically applied growth factors likely have had limited clinical use owing to their low stability/short half-life in vivo, limited absorption through skin in wound lesions, removal by exudation without reaching the wound area, and adverse side effects following topical administration stemming from high local and/or systemic levels6. Even when incorporated into hydrogel matrices the limited stability and off-target effects are still prevalent. The amount of viable growth factor becomes rapidly reduced due to degradation. Accordingly, high topical doses (e.g. Regranex gel is composed of 100 μg PDGF per gram gel) are needed leading to significant off-target effects. The intricacy of the wound healing process requires the coordinated activity of specific growth factors interacting with their receptors, extracellular matrix components, and other growth factors at their target sites7. Therefore, sophisticated delivery methods would offer significant advantages in preventing degradation and allowing spatio-temporally controlled growth factor delivery8.
As an alternative, genes for therapeutic growth factors can be delivered using viral or non-viral vectors for transient expression of the growth factors by endogenous cells, with the aim of improving subsequent tissue recovery8. Growth factor gene delivery has unique advantages including its capacity to induce sustained, localized production of bioactive growth factor; minimization of adverse effects (which include development of ectopic tissue or augmented cancer risk from the administered growth factor via systemic exposure)9; and reduction of cost as compared to topical growth factor delivery10. Moreover, growth factor gene delivery has shown enhanced therapeutic effects even with dose reductions at orders-of-magnitude (≈ 2000-fold) compared to topically administered growth factors11. PDGF represents a particularly appealing target for gene delivery. PDGF is a 30–32 kDa, heat-stable, cationic protein comprised of two-disulfide-linked peptide chains, identified as A and B chains, that combine to form three disulfide-linked dimeric proteins (AA, AB, and BB)12. PDGF plays a pivotal role in multiple important processes necessary for healing including fibroblast proliferation and migration, re-epithelialization, collagen deposition, angiogenesis, and granulation tissue formation within the wound bed13. PDGF is naturally produced by different cell types including fibroblasts, endothelial cells, macrophages, and vascular smooth muscle cells14. It is a potent mitogen for smooth muscle cells, fibroblasts, and glial cells. It also is a chemoattractant for neutrophils and macrophages1. Importantly, a balance in the recruitment of inflammatory cells to normalize the wound bed and promote wound healing is required through the regulation of PDGF delivery/expression within the wounds. Therefore, localized PDGF gene delivery15 and expression in the wound site would be a potential approach for improved chronic wound treatment.
Scaffold-mediated gene transfer is a particularly appealing option for transfection within the topical wound bed due to its capacity to increase the stability of gene cargoes and provide improved control over gene localization and release within the healing site16, 17. Hydrogels have established potential in viral18, 19 and non-viral20 gene delivery and have exhibited successful gene expression and wound healing effects in vivo. Collagen scaffolds represent an especially interesting option because of the wide usage of collagen in the treatment of chronic wounds21. Collagen is the main element of all connective tissues, and it is responsible for their structural integrity and mechanical strength; collagen also serves as a regulator of key processes essential in tissue development and regeneration22. However, the incidence of complete healing of a chronic wound following wound treatment with simple collagen scaffolds remains low; for example, only ~50% of diabetic foot ulcers were shown to fully close after treatments employing artificial skin23. The aforementioned wound healing effects of PDGF could potentially be better attained through localized wound delivery of PDGF genes15. PDGF gene and gene activated matrix strategies exhibit superior healing in experimental chronic wounds24, however, clinical translation is inhibited by construct escape and limited gene transfer24, 25. Improved control over the level, duration, and localization of PDGF gene would greatly advance chronic wound therapies26, 27. Therefore, a combination of biomaterial collagen scaffolds with spatio-temporally controlled PDGF gene delivery could initiate synergistic effects in healing of chronic wounds.
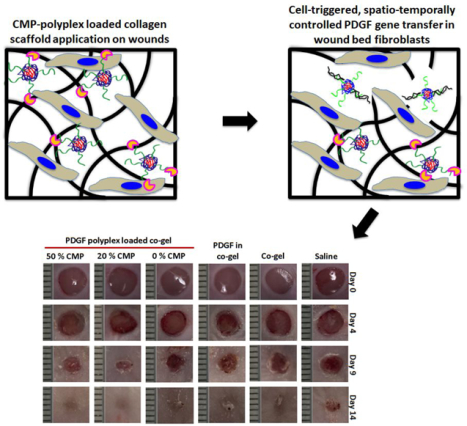
The objective of this study was to develop a biomaterial scaffold able to recruit host fibroblast cells and harness a key hallmark of the early wound environment - cell-mediated collagen-turnover - to trigger PDGF gene delivery and thereby stimulate enhanced skin wound healing. Chronic wounds have several documented complicating factors which include reduction in PDGF expression as well as presence of proteases that can degrade the PDGF28. Therefore, our study aims to induce localized production of PDGF that can be utilized by fibroblasts for further growth, migration, and enhancement in wound healing. Our new formulation fuses a fibrin-based, provisional-like matrix with collagen mimetic peptide (CMP)/PDGF gene-modified collagens, leading to the formation of robust gels that supported temporally controlled PDGF expression and facile application within the wound bed. Scaffolds were formulated comprising a mixture of collagen and fibrin (“co-gels”), as the combination of collagen, thrombin, and fibrinogen mimic the natural healing environment29. On-demand delivery of the PDGF gene was driven by utilizing CMPs linked to plasmid DNA (pDNA) polyplexes. CMPs are short peptides comprising collagen-like (GXY)n motifs that can be incorporated into the native collagen triple helix via thermal annealing and strand exchange methods10. CMPs can dually act as adjustable tethers to regulate the affinity between collagen and polyplexes along with adhesive/endocytic ligands (e.g., incorporating a GEKGER sequence within CMPs was previously shown to engage α2β1 integrins)10, 11. Herein, we demonstrate that CMP-polyplex-modified co-gel-scaffolds permitted highly tunable, sustained release of polyplexes for up to 24 days, with release and expression profiles that could be tailored depending upon the amount of CMP incorporated in the polyplexes. Release was dependent upon matrix metalloproteinase (MMP)-mediated remodeling of the collagen scaffold. In vitro analyses employing 3-D collagen scaffolds loaded with CMP-modified PDGF polyplexes confirmed the temporal control of gene release based on MMP activity, with ~30% higher PDGF expression by MMP producing fibroblasts as compared with non-MMP producing cells. The integration of fibrin with the gene-modified collagens resulted in co-gels that strongly supported both fibroblast cell recruitment/invasion as well as multiple aspects of the longer-term healing response. The in vivo wound healing studies established faster wound closure (~80% wound closure in 9 days) with minimal scar formation following the application of CMP-modified PDGF gene-incorporating collagen scaffolds, with improved collagen production, cell proliferation, cell migration, and myofibroblast activity in vivo (Scheme 1). It is also important to use multiple models to assess the wound repair effect, including chronic wound models such as the db/db mouse. However, db/db wound models are more complicated to implement. It is important to perform the first study on the effect of our materials using a simpler model to confirm the usability and beneficial effects of the CMP-polyplex loaded co-gels in wound healing. Future studies involving chronic wound models (with existing co-morbidities such as diabetes and bacterial colonization in wound site) are thus warranted to further confirm the applicability of co-gels for chronic wound healing. Our recent study regarding infection control in topical wounds by utilizing the vancomycin loaded co-gels30 can be potentially combined with the polyplexes to have dual beneficial effects in wound healing. Further studies involving chronic wound models are warranted to confirm the applicability of co-gels in wound healing.
Scheme 1:

Illustration of the CMP-polyplex-loaded co-gel scaffold and its subsequent fate following wound fibroblast-mediated collagen degradation and polyplex release, leading to cell-driven growth factor expression and enhanced wound healing.
2. Materials and methods
2.1. Materials
H-Rink amide ChemMatrix® resin, O-Benzotriazole-N,N,N’,N’-tetramethyl-uronium-hexafluoro-phosphate (HBTU) and Fmoc-protected amino acids were procured from PCAS Biomatrix (Quebec, Canada), Anaspec (Fremont, CA, USA), and Novabiochem (San Diego, CA, USA), respectively. Trifluoroacetic acid (TFA), N,N dimethyl formamide (DMF), high performance liquid chromatography (HPLC)-grade acetonitrile, and cell culture reagents, including Dulbecco’s phosphate buffered saline (DPBS), Opti-MEM® I Reduced Serum Media (Opti-MEM), trypsin, and Dulbecco’s modified Eagle’s medium (DMEM) were acquired from Fisher Scientific (Fairlawn, NJ, USA). Type I bovine collagen and fetal bovine serum (FBS) were obtained from Advanced BioMatrix (San Diego, CA, USA) and Corning (Manassas, MA, USA), respectively. CellTiter 96® AQueous One Solution [composed of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) and phenazine ethosulfate (PES)] was purchased from Promega (Madison, WI, USA). All cleavage cocktail components, 4-methylmorpholine, piperidine, branched PEI (25 kDa), and syringes were procured from Sigma-Aldrich (St. Louis, MO, USA). The gWIZ-GFP plasmid was procured from Genlantis (San Diego, CA, USA). pCMV-GLuc and pCMV-PDGF-BB plasmid were acquired from New England Biolabs (Ipswich, MA, USA) and Origene Technologies, Inc. (Rockville, MD, USA), respectively. Following the manufacturer’s protocols, the pDNAs were amplified in NEB 5-α electrocompetent E. coli (New England Biolabs, Ipswich, MA, USA) and purified using a Qiagen Megaprep Kit (Valencia, CA, USA). The Mouse/Rat PDGF-BB Quantikine enzyme-linked immunosorbent assay (ELISA) kit was obtained from R&D Systems (Minneapolis, MN, USA). Mouse PDGF-BB was acquired from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Animals
BALB/c mice (8-week-old, male) were acquired from Envigo, Inc. (Indianapolis, IN, USA) and used for in vivo studies. All in vivo experiments were executed as per the protocol approved by an Institutional Animal Care and Use Committee.
2.3. Collagen-mimetic peptide (CMP) synthesis
The CMP sequence [(GPP)3GPRGEKGERGPR(GPP)3GPCCG] was synthesized via automated Fmoc solid-phase peptide synthesis (SPPS) on a Tribute™ peptide synthesizer (Protein Technologies Inc., Tucson, AZ). The sequence was synthesized on Rink amide MBHA resin using methods described previously10. Briefly, the activation of amino acid residues for coupling with HBTU in 0.4 M methylmorpholine in DMF was followed by de-protection for 10 mins using 20% piperidine in DMF. A 6 h resin treatment with a cocktail of 94:1:2.5:2.5 TFA/triisopropylsilane (TIS)/water/1,2-ethanedithiol (EDT) was used for the cleavage of CMP sequence. The cocktail was then evaporated, and ethyl ether was used to precipitate the cleaved peptides that were dissolved in water, and lyophilized. Purification of crude peptides was performed by a reversephase high performance liquid chromatography (HPLC) using a Prominence chromatography instrument (Shimadzu, Inc., Columbia, MD, USA) with a Viva C18 (4.2 mm × 50 mm, 5 μm) column (Restek, Lancaster, PA, USA). 0.1% TFA in water (Solvent A) and 0.1% TFA in acetonitrile (Solvent B) were used as HPLC solvents with a gradient flow (25–35% solvent B over 30 minutes). The eluent absorbance was measured at λ = 210 nm and a ~70% purity was determined (Figure S1A). The product was confirmed by matrix-assisted laser desorption/ionization-mass spectroscopy (MALDI-MS) analysis based on observed m/z (≈ 1610) (Figure S1B). Circular dichroism studies were employed, using previously described methods31, to confirm the triple helix and melting temperature of the CMPs (Figure S1C and S1D).
2.4. CMP-polyplex preparation and characterization
CMP was conjugated to PEI using SMCC as a crosslinker (Figure S2A). A solution with an SMCC:PEI molar ratio of 1:1 was prepared by dissolving SMCC in DMSO with PEI in PBS to obtain a 1 mM final concentration of each reagent. The NHS esters of the crosslinking agent react with primary amines of PEI to form stable amide bonds, and subsequently (after an hour), CMP peptides were added to the solution at a CMP:PEI molar ratio of 3:1. The Michael-type reaction was facilitated by incubating the resulting solution for 24 h at room temperature to enable the conjugation between thiol-peptide and the maleimide-functionalized PEI. The obtained product was purified by dialyzing the conjugate mixture using a dialysis bag (MWCO: 10,000 Da), and the PEI-CMP conjugate formation was confirmed with gel permeation chromatography (Figure S2B).
CMP-polyplexes were prepared via self-assembling pDNA (encoding for GLuc or PDGF) with mixtures of non-modified PEI and PEI-CMP conjugate with slight modifications in the established protocols10. Briefly, equal volumes of pDNA and PEI solutions were prepared separately in 20 mM HEPES (pH 6.0). A 20 μg/ml final pDNA concentration was achieved by the drop-wise addition of PEI solution to the pDNA solution under vortexing. Polyplex formation was permitted to occur through incubation for 10 mins at room temperature. The N:P ratio [number of amines (N) in the polymer relative to the number of phosphates (P) in the pDNA] was altered by varying the concentration of PEI. CMP modification was varied by altering the ratio of CMP-PEI to total PEI (from 0%–50%) on a mass basis. Lyophilized polyplexes were prepared by adding a 200 mM sucrose solution to the prepared polyplexes to achieve 20 mM sucrose concentration followed by freeze-drying17. Until further use, the lyophilized polyplexes were stored at −20 °C.
Polyplex diameters, polydispersity index (PDI), and zeta potentials were determined by dynamic light scattering (DLS) using a Nano-S90 ZetaSizer (Malvern, Worcestershire, UK). Following DLS measurements, the hydrodynamic diameters of the polyplexes were calculated by using the Stokes-Einstein equation. Nano DTS software (version 6.34) was used to evaluate the PDI and zeta potential. All measurements were performed in triplicate for each sample at 25 °C with at least 10 runs on each set. pDNA condensation efficiency for polyplexes was determined using agarose gel electrophoresis. Polyplexes were prepared using 0.2 μg pDNA with varying N:P ratios and/or varying CMP-PEI:PEI ratios, and the resulting polyplexes were evaluated in 1% agarose gels comprising ethidium bromide [0.5 μg per mL of tris/borate/ethylenediaminetetraacetic acid (TBE) buffer]. Four microliters of gel loading buffer was mixed with 20 μl of each polyplex solution, and 20 μl of this polyplex mixture was added into each well in the gel. The gels were run for 1.5 h at 100 V and imaged using a BioRad Gel Doc XR (Hercules, CA, USA).
2.5. CMP-polyplex stability studies
The stability of the pDNA and the polyplexes at room temperature and higher temperatures was evaluated using gel electrophoresis to determine whether heating the CMP-polyplexes to a temperature of ~50 °C (necessary to melt the CMP triple helices) affected either the pDNA or the polyplexes. Polyplexes or pDNA were heated to ~50 °C for up to 60 min. Polyplex diameters were determined at different time points (0, 0.5, and 1 h following heating) using the DLS method described previously in section 2.4 to evaluate the effects on polyplex stability. Subsequently, the pDNA from intact polyplexes was released by incubating with 20 mM heparin (a destabilizing agent) for 30 min10. The gel electrophoresis method (as described in Section 2.4) was utilized to assess the pDNA after heating.
2.6. Cell culture and in vitro polyplex transfection in fibroblasts
Fibroblasts (NIH/3T3 cells;ATCC, Manassas, VA) were cultured in complete DMEM [composed of 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (P/S)], and incubated at 37 °C and 5% CO2. For bolus transfection studies, cells were plated at a density of 10,000 cells per cm2, incubated for 24 h, and transfected with polyplexes (encoded for GLuc or PDGF genes) for 90 mins using Opti-MEM. The Opti-MEM was then replaced with complete (e.g., serum-supplemented) DMEM and cells were evaluated for gene expression. GLuc polyplex transfection was examined by measuring luminescence in the conditioned media collected from the culture plates using a BioLux® Gaussia Luciferase Assay (NE Biolab®; Ipswich, MA, USA), as per the the manufacturer’s protocol. The PDGF polyplex bolus transfection was evaluated by quantifying PDGF expression in the collected conditioned media using the Mouse/Rat PDGF-BB Quantikine enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems; Minneapolis, MN, USA).
2.7. Preparation and characterization of co-gels
The co-gels were prepared using collagen, fibrinogen and thrombin. Briefly, 10X PBS and 0.1 N NaOH were mixed in collagen type I (Firbricol®, Advanced BioMatrix, San Diego, CA, USA) to prepare neutralized collagen. Different collagen (2, 3, or 4 mg/ml) concentrations were uniformly mixed with fibrinogen (1.25 mg/ml) and thrombin (0.156 IU/ml) and incubated at 37 °C to allow co-gel formation. The lyophilized polyplexes were mixed in neutralized collagen and incubated at 4 °C for 1 h before adding fibrinogen and thrombin to allow polyplex-loaded co-gels formation via at least 6 h incubation at 37 °C.
An DHR-3 rheometer (TA Instruments, New Castle, DE) was used for oscillatory rheology measurements using a parallel-plate geometry with a 20 mm diameter stainless steel. The above mentioned co-gel components were immediately mixed prior to the placement on rheometer plate. The study was performed using a 500 μm gap distance and 37 °C temperature. Strain sweep (0.110%) and frequency sweep (0.1 to 10 rad s−1) experiments were performed to assess the viscoelastic properties of the co-gels. Time sweep measurements, in the linear viscoelastic regime (performed at 1% strain and 10 rad s−1 angular frequency), were used to monitor the gelation of co-gels. The co-gel’s shear storage moduli (G’) and loss moduli (G”) were recorded. Each experimental analysis included at least three separate samples of co-gels.
2.8. Polyplex retention in co-gels
The co-gels encapsulating CMP-free or CMP-immobilized polyplexes were prepared as described previously (Section 2.4). Following incubation at 37 °C overnight, the co-gels were washed with PBS (5 ml) and the washes were collected for subsequent measurements. Afterwards, the co-gels were incubated with complete DMEM at 37 °C. Another set of co-gels was prepared and treated with conditioned DMEM from NIH/3T3 cells that had been treated with tumor necrosis factor-alpha (TNF-α, 10 ng/ml; TNF-α induces MMP production by the cells as confirmed in the previously published results from our lab using a SensoLyte® 520 Generic MMP Assay Kit *Fluorimetric*10). Collections of conditioned media were performed at days 1, 3, 6, 9, 12, and 15. Disassembly of the polyplexes in the washes as well as in the conditioned media were achieved by 30 min incubation in 20 mM heparin to release pDNA for fluorescence-based analysis. Quantification of the recovered pDNA was performed using a Quant-iT™ PicoGreen® dsDNA Assay Kit.
2.9. In vitro cell proliferation and migration studies
Bolus transfection of fibroblasts was performed as mentioned in section 2.6. NIH/3T3 cells transfected with PDGF polyplexes were evaluated to assess whether the expressed PDGF induced cell proliferation. The transfected cells were trypsinized (cells in 12 well plates were washed with PBS, incubated with trypsin (750 μl/well) at 37 °C for 4 min, and diluted with complete DMEM to 2 mL) and counted at different time points (24 h and 48 h) following transfection to evaluate the changes in cell proliferation as compared to untreated cells. Furthermore, a scratch assay was performed to determine the effects of PDGF transfection on cell migration. The transfected cells were incubated for 24 h and then a scratch (~750 μm wide) was created in the well with a 200 μl pipette tip. Cells were imaged with a bright field microscope at 24 h and 48 h following scratch wounding to evaluate the cell migration effects of the expressed PDGF using the ImageJ software.
2.10. Co-gel transfection studies
The co-gels formulated with immobilized pCMV-GLuc polyplexes (CMP-PEI polyplexes) or encapsulated polyplexes (PEI polyplexes) were washed with 5 ml PBS and incubated in complete DMEM for 3 h prior to seeding the NIH/3T3 cells (15000 cells per cm2) on the co-gel surface. Cells on the co-gel were then incubated either in the presence or absence of 10 ng/mL TNF-α. The approach to stimulate cells with TNF-α was an appropriate simple model of the wound healing environment due to the fact that TNF-α is involved during wound repair cascade with a natural role in stimulating MMP production10, 32. Therefore, the main aim here was to mimic this wound microenvironment condition for MMP production to facilitate transfection through the degradation of co-gels. Gene expression was evaluated over seven days by quantifying the luminescence from the conditioned media collected from the culture plates using a BioLux® Gaussia Luciferase Assay (NE Biolab®; Ipswich, MA, USA), according to the manufacturer’s protocol.
Similarly, the co-gels were formulated with PDGF polyplexes, washed, and seeded with NIH/3T3 cells (incubated with or without TNF-α). PDGF expression by the cells was evaluated using direct ELISA (for the co-gels) or sandwich ELISA (for the conditioned media), using the PDGF-BB Quantikine ELISA Assay kit33. Additionally, NIH/3T3 cell clustering was evaluated in the co-gels through bright field and fluorescence microscopy imaging. The effects of PDGF expression on cluster size were evaluated.
2.11. In vivo polyplex transfection and luciferase expression studies
Lyophilized pCMV-MetLuc-mem polyplexes encoding a membrane-bound luciferase were prepared as described previously (section 2.4). The lyophilized polyplexes in Matrigel® solution (100 μg pDNA/ml and 5 μg BMP-2/ml) were then vortexed, and incubated on ice for 1.5 h to facilitate strand invasion. BALB/c mice (8 week-old) were anesthetized using isoflurane before shaving and disinfecting the abdomens,and subcutaneously injected with 300 μl Matrigel® solution into defined four different locations to form a visible pellet. Matrigel was employed for the subcutaneous experiments because the co-gel components make them more difficult to inject; and the main objective was to confirm that the polyplexes (with or without CMP modifications) were able to transfect cells in vivo. Each polyplex-containing pellet comprised a consistent composition of Matrigel®, BMP-2, and polyplex as mentioned previously, whereas the control pellet included Matrigel® and BMP-2 only. The MetLuc-mem expression in mice was visualized with a Caliper In vivo Imaging System Lumina (IVIS®) (Perkin Elmer, Waltham, MA, USA) following subcutaneous injection of luciferase substrate (coelenterazine) in the vicinity of each pellet. Images of the mice were obtained 30 mins after coelenterazine injection and the images were analyzed using a Living Image® software region-of-interest (ROI) tool to determine total luminescence. Total luminescence for control pellet (with no polyplex) was deducted as background from each image. All data were normalized to membrane-bound luciferase signal (normalizing factor 0.01). The study was performed in a total of three mice.
2.12. In vivo wound healing studies and histological evaluations
The in vivo wound healing studies were performed in 8-week-old BALB/c mice. Lyophilized PDGF polyplexes and co-gels encapsulating the polyplexes were prepared as described previously (sections 2.4 and 2.8). Mice were anesthetized using isoflurane, hair on the back was shaved, and the skin was disinfected with isopropyl alcohol. Punch biopsy wounds (two wounds per mouse) were created with 5 mm punches34. Fresh wounds were then treated with either saline or various co-gels (containing 20 μg pDNA for each wound; specific samples indicated below) and covered with OpSite wound dressing to protect the wound. The treatment groups included saline, blank co-gel, PDGF-loaded co-gel (1 μg PDGF per co-gel), and PDGF polyplex-loaded co-gels containing polyplexes with different CMP modifications (0%, 20%, or 50% CMP). The gels are quite soft and too fragile to handle as a solid implant therefore, the components were pipetted out and applied onto the anesthesized mouse wounds and covered with OpSite to allow hydrogel formation within the wounds. Photographs of wounds were taken, and body weights were recorded, at day 0, 4, 9, and 14. Wound size reductions were calculated for each group. Animals were euthanized and wound skin samples were collected using 5 mm punches and fixed in 4% paraformaldehyde at day 4, 9, and 14 for all the treatment groups for histological evaluation. Future work is warranted with a splinted wound model to confirm the beneficial effects and potential translation to human wounds. We note that our current study allowed detailed analysis of multiple new aspects of the effect of CMP hydrogels, including their capacity to effect beneficial changes in the wound bed such as increased granulation tissue formation. These aspects of wound healing can be accurately assessed via simpler, non-splinted models studied by Chen et al.35 suggesting that the unsplinted rodent wound model can locate and measure the defined wound bed and re-epithelialization useful for analyzing the early wound healing.
The collected skin samples were paraffin-embedded, cut into 5 μm sections, and stained with hematoxylin and eosin (H&E) or Masson’s Trichome following their deparaffinization and hydration to examine re-epithelialization, granulation tissue formation, and collagen deposition. For immunostaining, antigen retrieval was performed using sodium citrate (pH 6.0), and sections were blocked with 10% normal goat serum at room temperature followed by incubation with primary antibodies for collagen type I, collagen type III, and/or α-smooth muscle actin (α-SMA) overnight at 4 °C. Signal was visualized by subsequent incubation with Alexa Flour 488-tagged secondary antibodies. Coverslips were mounted on a DAPI-based mount for imaging.
The tissue sections were further evaluated for collagen content and orientation using the Zeiss 780 multiphoton confocal microscopy for the detection of second harmonic generation (SHG) in the forward and backward directions using a 40x/1.2 water objective. A drop of water was added on the cover slip placed above the tissue section slide and visualized using the microscope. The forward and backward scattering signals from the tissue sections were recorded, and the ratio of forward/backward signal was determined for all the treatment groups. The orientation index of the collagen was calculated by using the FFT module in Image J36, 37. The binary image obtained was used to determine the 95% confidence ellipse and the orientation index was calculated as the ratio of height/width for the ellipse. Lower orientation index values indicate greater collagen scattering.
2.13. Statistical analysis
The experimental values are expressed as mean ± standard deviation (SD). Student’s t tests and one way ANOVA were utilized to evaluate differences between control and experimental groups. A P < 0.05 was considered statistically significant.
3. Results and discussion
3.1. Preparation, characterization, and stability analysis of CMP-polyplexes
The first part of our study was focused on the preparation, characterization, and stability analysis of pDNA-loaded CMP-polyplexes. The polyplexes were prepared using pDNA and PEI or PEI-CMP at different N:P ratios, as described above. The assembled polyplexes were evaluated for particle diameter, polydispersity, and zeta potential using DLS. The hydrodynamic diameters and polydispersities of all the polyplexes were < 200 nm and < 0.3, respectively (Figure S3 and Figure 1), suitable for efficient endocytosis by fibroblasts38. An increase in polyplex diameter and polydispersity was apparent at higher percentages of CMP modification of the CMP-PEI conjugates, potentially attributable to the formation of less compact polyplexes due to an increase in molecular weight and/or reduction in the charge density of PEI after CMP modification; the reduction in charge and any interaction between CMPs could cause modest polyplex aggregation between CMP-modified polyplexes10, 17. Similarly, slight reductions in the zeta potentials were observed due to the reduction in the charge density of PEI caused by CMP modification. Following lyophilization, there was a further non-significant increase in the polyplex diameter and polydispersity possibly caused either by the interaction between CMPs present in the polyplexes or by the interaction with sucrose on the surface of the polyplexes17.
Figure 1:

Characterization of PDGF polyplexes. (A) Particle diameter measurements (the intensity metric represents the volume averaged scattered light intensity for DLS measurements; experimental values in the table refer to mean ± standard deviation for three separately prepared and analyzed samples) and (B) TEM images for non-lyophilized and lyophilized PDGF-polyplexes (yellow arrows indicate polyplexes).
The CMP-polyplexes for all studies were prepared and incubated at 50 °C for an hour to melt any triple helical CMPs into their monomeric state before lyophilization. Therefore, the stability of the prepared polyplexes and the stability of the encapsulated pDNA were evaluated at this temperature. The particle diameter, PDI, and zeta potential measurements for the PDGF-polyplexes with 20% CMP modification exhibited a slight increase in all of the respective measurements following incubation at 50 °C for up to 1 h, with no significant differences between the non-lyophilized samples (Figure S4A, S4B, S4C, S4D). Furthermore, agarose gel electrophoresis was used to evaluate the stability of the pDNA within the polyplexes (Figure S4E). The pDNA fluorescence emitted by the intercalating dye ethidium bromide39 was reduced in the polyplexes with higher N:P ratios due to complexation of the pDNA by the polymers. Signals were similar for both non-lyophilized and lyophilized polyplexes. The addition of heparin to destabilize the polyplexes led to the release of pDNA that was available for ethidium bromide intercalation and showed comparable fluorescence to that of the pDNA only. Similarly, the pDNA fluorescence signals were evaluated for polyplexes with different CMP modifications (Figure S4F). Polyplexes prepared at all of the CMP ratios (0–50%) successfully encapsulated pDNA that was only apparent in gel electrophoresis experiments when the pDNA was released by heparin treatments to destabilize the polyplexes.
3.2. In vitro cell proliferation and migration studies following bolus PDGF polyplex transfection
PDGF is a potent stimulator of cell proliferation and migration40. Healing of wounds is dependent on the number of fibroblasts within the wound area40, 41 and their subsequent production and remodeling of collagen/extracellular matrix42. Thus, we sought to confirm that bolus polyplex transfection of fibroblasts with the gene for PDGF resulted in sustained production of functional PDGF protein, leading to expected increases in cell proliferation and migration. ELISA assays were performed to evaluate the amount of PDGF expression at day 1 and day 2 following bolus transfection (Figure S5A). As a control, a separate well was treated by adding 10 ng/ml of soluble PDGF protein at day 1 to a well seeded with fibroblasts, and the changes in PDGF available in this well also were evaluated at day 1 and day 2. These experiments demonstrated that the polyplex transfected fibroblasts expressed approximately 150 pg/ml of PDGF at day 1 with similar levels at day 2. By contrast, in the 10 ng/mL PDGF treated group, a drastic reduction in the amount of PDGF (to 180 pg/ml) was observed by day 2, presumably resulting from the uptake of PDGF by the cells and also the limited stability of PDGF in the presence of conditioned media43. In fact, by day 2, the amount of PDGF in the transfected wells was similar to the amount of PDGF remaining in the PDGF-supplemented well. Given that the PDGF-supplemented wells originally contained much higher levels of PDGF, these results suggest that polyplex transfection can provide more consistent PDGF levels over the duration of the study period.
The effect of PDGF on fibroblast cell proliferation and migration also was evaluated (Figure S5B). After 24 h, PDGF significantly enhanced fibroblast proliferation (by ~120% as compared with the original level). In contrast, significant changes in proliferation were not observed in samples treated with PDGF polyplex at 24 h, presumably because the cells required time to produce protein following transfection. By the end of 48 h, both treatment groups of fibroblasts had significantly higher levels of cell proliferation, with a ~135% increase in PDGF-treated cells and a ~130% increase in PDGF polyplex treated cells as compared with untreated cells. The results for the effect of PDGF treatment on cell proliferation were in close agreement with previous reports for experiments conducted at similar PDGF concentrations40, suggesting that PDGF polyplex transfection can be a viable alternative approach to direct growth factor delivery to achieve comparable results with significantly lower local concentrations of growth factor. The effect of PDGF on cell migration was determined using a scratch assay (Figure S5C and S5D). Significantly higher rates of fibroblast migration (~2 fold higher as compared to the control) were observed for both the PDGF and PDGF polyplex groups. Despite the fact that a difference in cell proliferation between the PDGF and PDGF polyplex groups was observed at 24 h, migration rates at this time point were similar, perhaps owing to the concentration dependence of the effects of PDGF on migration. When cells sense PDGF at low concentrations, their phenotypic response consists of cell movement. After reaching a precise threshold PDGF concentration, cells alter from a migrating phenotype to a proliferating one40. Thus, a reasonable interpretation of the data is that the PDGF concentrations in the 0–24 h time frame were sufficient for promoting cell migration in both the PDGF and PDGF polyplex groups; however, the more limited concentration in the PDGF polyplex sample was not high enough to exceed the threshold for cell proliferation until after 24 h. These results indicate the successful applicability of PDGF polyplex transfection in fibroblasts to enhance cell proliferation as well as migration.
3.3. Co-gel preparation and rheological evaluation
Following the successful preparation and evaluation of the polyplexes, co-gels containing encapsulated or CMP-bound polyplex were prepared and characterized. Previous studies from our lab used pure collagen gel scaffolds for CMP-mediated polyplex delivery10, 33. Herein, we prepared co-gels using formulations composed of collagen, fibrinogen, and thrombin proteins that are naturally present in the in vivo wound microenvironment. Different collagen concentrations (2, 3, or 4 mg/ml) were used for co-gel preparation along with fibrinogen (1.25 mg/ml) and thrombin (0.156 IU/ml); co-gels were evaluated for the gel integrity. Among the tested co-gel concentrations, a 4 mg/ml collagen with 1.25 mg/ml fibrinogen and 0.156 IU/ml thrombin exhibited the best integrity as suggested by storage (G’) and loss (G”) modulus analysis (Figure S6). The viscoelastic properties of this co-gel formulation were analyzed by oscillatory shear rheometry (Figure S6B). Frequency sweep and strain sweep experiments on the co-gels showed a constant storage modulus (G’) independent of frequency (0.1 to 10 rad/sec) and shear strain (0.1–10%). The prepared co-gel’s storage modulus (G’) and loss modulus (G”) were 149 Pa and 27 Pa, respectively. These results indicate that the co-gels exhibit rheological properties that should support fibroblast spreading, proliferation, and migration based on matrix remodeling44. The CMP modification in the polyplexes does enable them to form a triple helix with collagen; however, there is a mixture of other components including fibrin in the hydrogel. The mass compositions of hydrogels include collagen: 75.87%, fibrin: 23.71%, and CMP-polyplexes: 0.02%. Therefore, the mechanical properties of hydrogel are mostly affected by the collagen and fibrin interactions and hence negligible effects can be expected with CMP modification.
3.4. Polyplex retention in co-gels
The lyophilized PDGF polyplexes were loaded into the co-gels and their retention and release from the co-gels were evaluated in the absence (Figure 2A) and presence (Figure 2B) of MMPs using a Quant-iT™ PicoGreen® dsDNA Assay Kit. As presented in Figure 3A, > 8 μg per cm2 of pDNA was initially retained in the co-gels with CMP modification, corresponding to > 2.7 μg pDNA per mg of collagen. The level of pDNA retention by the CMP-modified polyplexes was higher than that of the non-modified polyplexes, with 7–13% higher retention levels by CMP-modified polyplexes. The total amount of pDNA retained was 13% greater in co-gels formed using polyplexes with 50% CMP modification, as compared to non-CMP modified polyplexes. This effect was attributed to the ability of the CMPs to form triple helices with native collagen resulting in stronger interactions between the polyplexes with higher CMP modification levels and the co-gels10. Subsequently, the co-gels were treated with conditioned media from fibroblasts that were cultured in the presence of TNF-α to induce MMP secretion (to mimic the conditions of the wound microenvironment)45. In the absence of MMPs, the pDNA release observed by day 9 for the co-gels containing polyplexes with 0%, 10%, 20%, and 50% CMP modifications were 47%, 39%, 36%, and 30% respectively. In the presence of MMPs, respective increase in pDNA release (61%, 48%, 39%, and 32%) was observed by day 9 for the co-gels containing polyplexes with 0%, 10%, 20%, and 50% CMP modifications. Overall, a reduction in the release of pDNA was correlated with an increase in CMP modification and this trend was similar in co-gels treated with or without MMPs. However, the cumulative pDNA release levels from the MMP treated co-gels were 14%, 9%, 3%, and 2% higher for CMP modifications of 0%, 10%, 20%, and 50%, respectively. The pDNA release was sustained for 24 days when CMP modifications were highest, in agreement with our findings for polyplex-loaded collagen gels10.
Figure 2:
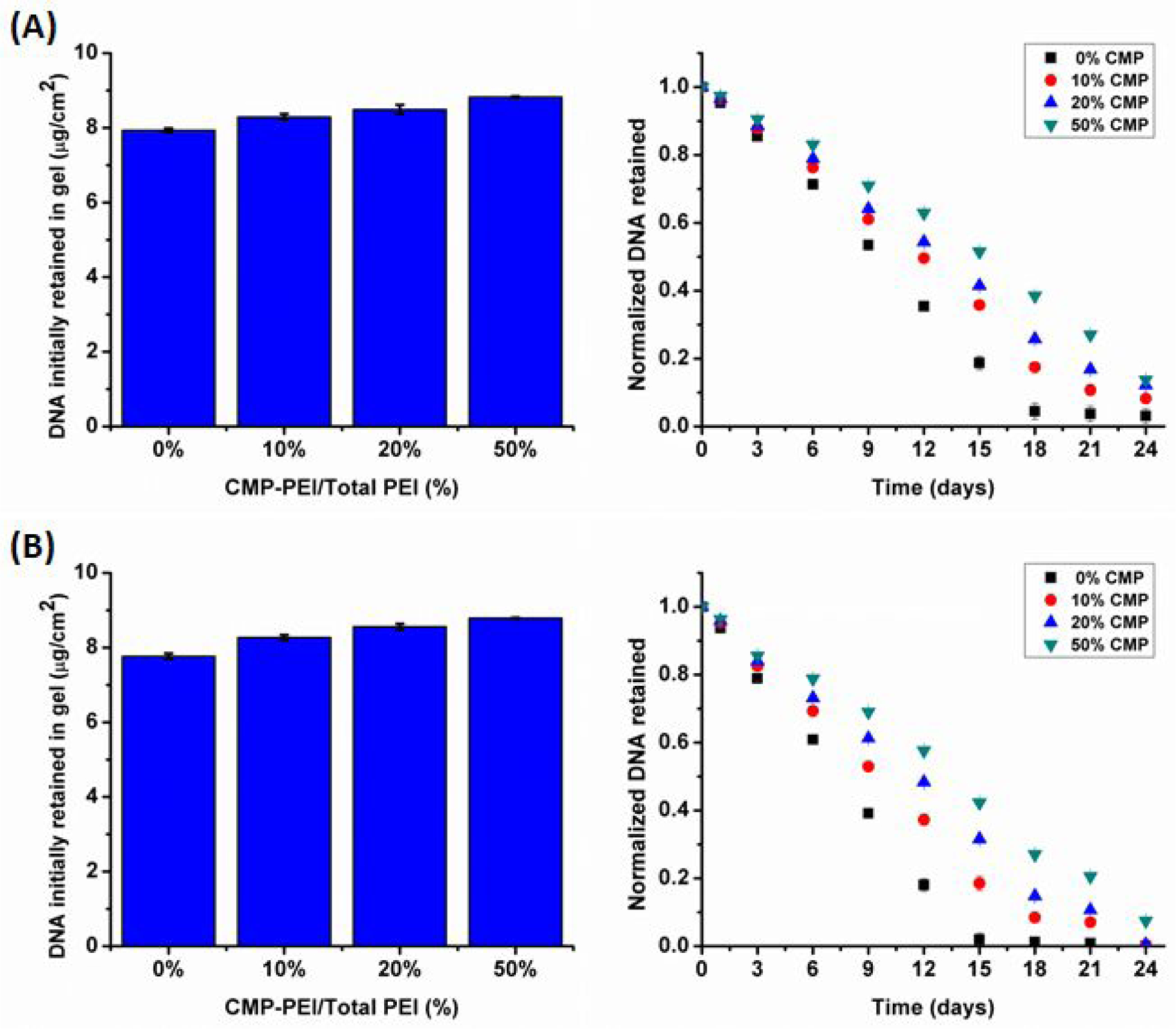
Co-gel polyplex retention studies. Initial pDNA retention and pDNA retention over time were quantified in the co-gels according to the percent CMP-PEI in the polyplexes. (A) Samples analyzed in the absence of TNF-α. (B) Samples analyzed in the presence of TNF-α. Individual data point refer to the mean ± standard deviation for three separately prepared and analyzed samples.
Figure 3:

Evaluation of PDGF expression by fibroblasts. (A) Cellular expression of PDGF following plating of NIH/3T3 cells onto co-gels loaded with polyplexes in the presence or absence of TNF-α (*P<0.05 as compared to GLuc). Individual data point refer to the mean ± standard deviation for three separately prepared and analyzed samples. In the absence of TNF-α treatment, statistical differences (P < 0.05) in PDGF expression were observed for varying CMP modifications at different time points [i.e. (day 5: 0% and 10%, 20%, 50% CMP co-gel; 10% and 50% CMP co-gel, 20% and 50% CMP co-gel), (day 10: 0% and 50% CMP co-gel; 10% and 50% CMP co-gel), (day 15: 0% and 50% CMP co-gel; 10% and 50% CMP co-gel; 20% and 50% CMP co-gel)]. Furthermore, in the presence of TNF-α treatment, statistical differences (P < 0.05) in PDGF expressions were observed for varying CMP modifications at different time points [i.e. (day 5: 0% and 20%, 50% CMP co-gel; 10% and 20%, 50% CMP co-gel; 20% and 50% CMP co-gel), (day 10: 0% and 10%, 20%, 50% CMP co-gel; 10% and 20%, 50% CMP co-gel; 20% and 50% CMP co-gel), (day 15: 0% and 20%, 50% CMP co-gel; 10% and 50% CMP co-gel; 20% and 50% CMP co-gel)]. (B) Evaluation of the effects of expressed PDGF on fibroblast clustering in the PDGF polyplex (20% CMP)-loaded co-gels (*P<0.05 compared to no polyplex without TNF-α; †P<0.05 compared to no polyplex with TNF-α; ‡P<0.05 compared to GLuc polyplex without TNF-α; €P<0.05 compared to GLuc polyplex without TNF-α; ₡P<0.05 compared to PDGF polyplex without TNF-α). Individual data point refer to the mean ± standard deviation for three separate measurements.
3.5. In vitro co-gel transfection studies
The ability of the gene delivery matrices to sustain stable and localized gene structures for longer periods of time is crucial for applicability in the tissue repair microenvironment46, 47. The potential of the polyplex-loaded co-gels to sustain and control polyplex release and transfection over prolonged time periods was thus evaluated. The lyophilized GLuc and PDGF polyplexes (with 0%, 10%, 20%, and 50% CMP modifications) were incorporated into the co-gels through strand invasion with the collagen fibers, and fibroblasts (NIH-3T3 cells) were seeded atop the co-gels to evaluate GLuc and PDGF expression in a format that would mimic invasion into the wound bed. These experiments were performed both in the presence and absence of TNF-α (Figure S7 and Figure 3A). Protein expression was directly correlated with CMP modification. As shown in Figure S7, GLuc expression by fibroblasts grown in polyplex-loaded co-gels without TNF-α treatment resulted in the highest normalized GLuc activity at day 6 in the 0% and 10% CMP modification co-gels; by contrast, the highest normalized GLuc activity occurred at days 9 and 12, respectively, in the 20% and 50% CMP co-gel samples. In the presence of TNF-α, GLuc activity by fibroblasts was increased over initial levels in all samples, with respective activity levels that were 1.3 and 2.3-fold higher for 0% and 10% CMP co-gel at day 6; 3.5-fold higher for 20% CMP co-gel at day 9; and 3.3-fold higher for 50% CMP at day 12. Meanwhile, as shown in Figure 3A, PDGF expression by fibroblasts grown in polyplex-loaded co-gels without TNF-α treatment decreased as a function of CMP modification level when assessed at day 5, with expression ranging from 138 pg/gel in 0% CMP modification co-gels to 85 pg/gel in 50% CMP modification co-gels. By day 10, the trend in PDGF expression as a function of CMP modification was reversed, with expression ranging from 76 pg/gel in the 0% CMP co-gels to 123 pg/gel in the 50% CMP co-gels. With TNF-α treatment, the trends in PDGF expression vs. CMP modification level were maintained, but the overall expression levels were increased; expression levels in the 0%, 10%, 20%, and 50% CMP modification co-gels in the presence of TNF-α treatment were 1.3, 1.4, 1.5, and 1.6-fold higher at day 5 and 1.1, 1.3, 1.4, and 1.5-fold higher at day 10, as compared to non-TNF-α treated samples.
Taken together, these results demonstrate that in both the GLuc and PDGF polyplex-treated cells, protein expression was dependent on CMP modification, with higher CMP modification levels resulting in later peak expression times. These differences were presumably a result of the altered interactions between the polyplexes and collagen; unmodified polyplexes (0% CMP) had minimal interactions with collagen in the co-gels allowing their easy release even with minimal degradation of collagen within the system. Higher levels of CMP modification allowed a greater number of triple helices to form between CMP-linked polyplexes and collagen resulting in stronger binding that required substantial collagen breakdown for release. Furthermore, the addition of TNF-α enhanced the GLuc and PDGF expression for all of the CMP modified polyplexes. TNF-α is a potent stimulator of MMP secretion, and hence the increased expression levels in the presence of TNF-α showed that MMP-mediated collagen degradation facilitated release of the polyplexes from the co-gels, allowing greater transfection by fibroblasts48. Previous studies from our laboratories illustrated that GLuc and PDGF gene expression levels also were dependent on the degree of CMP modification when fibroblasts were grown on polyplex-loaded collagen-only gels10, 33. In these studies, polyplex-loaded collagen gels were preincubated in complete DMEM for one to two weeks prior to cell plating. The levels of protein expression were dependent on the CMP modification levels in the order: 0% > 10% > 20% > 50% CMP under shorter preincubation times (up to 5 days), whereas the order reversed to 50% > 20% > 10% > 0% CMP at longer preincubation times (> 7 days), highlighting the role of CMP modification in controlling polyplex release for transfection. In our current study, protein expression was determined following fibroblast plating in the freshly prepared polyplex-loaded co-gels with short (~ 3 h) preincubation in complete DMEM. This approach was utilized to mimic real wound effects following in vivo co-gel applications wherein fibroblasts can interact with the co-gel immediately and up to several days to release polyplexes by MMP activity, thus initiating transfection. In the current work, protein expression was sustained for > 2 weeks and was directly correlated to the degree of CMP modification suggesting greater control over polyplex release from co-gels as compared to collagen-only hydrogels (for which greater expression was observed within 4 days of fibroblast plating after preincubation). These findings highlight the potential application of CMP tethering as a mechanism of controlling gene expression in mixed gels (i.e. co-gels) and not only in collagen gels. Furthermore, CMP tethering in mixed gels offers opportunities to control gene expression over longer time scales than in collagen-only gels.
The expression of functional protein (PDGF) by the transfected fibroblasts in the co-gels was further confirmed by imaging the cells under the microscope (Figure 3B). In the absence of PDGF expression, fibroblasts formed clusters in the co-gel. This result is consistent with the expected behavior of fibroblasts in fibrin-containing gels: fibroblasts naturally migrate into fibrin matrices to form an interconnected meshwork with characteristic clusters; this behavior does not occur in collagen matrices lacking fibrin49. Our results suggested that TNF-α-induced MMP production and subsequent collagen degradation minimally assisted cell movement and penetration into gels resulting in non-significant reductions in the clustering effects. Less than 10% reductions in cluster size were evident with TNF-α treatment in both blank co-gels and GLuc polyplex-loaded co-gels, as compared to non-TNF-α treated co-gel samples. However, there were significant reductions in the cluster size for fibroblasts in PDGF polyplex-loaded co-gels; these reductions were particularly significant in the presence of TNF-α, with 85% and 82% reductions in cluster size when cells in PDGF polyplex-loaded co-gels were compared to cells in blank co-gels or GLuc polyplex-loaded co-gels, respectively. Procontractile growth factor conditions (e.g. serum) support fibroblast clustering but not promigratory growth factor conditions (e.g. PDGF)50. Thus, these results indirectly confirmed that PDGF was expressed by the fibroblasts and that higher PDGF expression was evident in the presence of TNF-α treatment.
Both GLuc and PDGF expression studies in co-gels are indirect methods for determination of polyplex transfection and protein expression. An optimal setup is further required to confirm the number of transfected cells that would give an idea of polyplex release and transfection in co-gel. Further studies using fluorescence and flow-based approaches can potentially showcase the number of cells transfected within the co-gel and provide a clearer picture of gene transfection and protein expression.
3.6. In vivo polyplex transfection and luciferase expression studies
Further analysis was performed to determine whether the polyplex-loaded co-gels could support gene expression in vivo (Figure 4 and S8). Polyplexes, with different CMP modifications (0%, 20%, and 50% CMP) and encoding for membrane luciferase pDNA, were prepared as described and incorporated in Matrigel for subcutaneous injection in the dorsa of mice (Figure 4). This simple model for gene expression was used as an initial in vivo test bed for the PDGF expression capacity in the co-gels because of its similarity with the excisional wound environment and wide usage for studies of connective tissue repair in wounds and bone tissues: Matrigel depots induce a minor inflammatory response as part of the foreign body response, resulting in infiltration by mesenchymal cell types found commonly in the wound bed11. Moreover, the high collagen content in Matrigel was previously shown to support CMP hybridization11. In vivo expression of membrane luciferase was evaluated using co-elenterazine, a substrate for membrane luciferase. IVIS imaging of treated animals clearly differentiated distinct patterns of protein expression, depending upon sample composition, at different time points, with CMP-dependent patterns of protein expression that were similar in vivo and in vitro (Figure S8A). An insignificant level of luminescence was observed for blank Matrigel (control gel) at all time points. In contrast, the unmodified (0% CMP) polyplexes exhibited their highest luminescence levels shortly after Matrigel injection. At day 3, the 0% CMP modification co-gels exhibited 23% and 84% higher luminescence than the 20% and 50% CMP modification co-gels, respectively. Meanwhile, by day 5, the 20% CMP modification co-gels presented 23% and 50% higher luminescence levels as compared to 0% and 50% CMP modification co-gels, respectively, and at day 7, the 50% CMP modification co-gels exhibited 47% and 31% higher luminescence levels as compared to the 0% and 20% CMP modification co-gels, respectively. Overall, the 0%, 20%, and 50% CMP modification co-gels produced peak protein expression at different time points [e.g. early (day 3), intermediate (day 5), and late timepoints (day 7), respectively] indicating the in vivo temporal control afforded by CMP modification. Furthermore, there was no significant change in the body weights of mice indicating no direct adverse effects of the components of the polyplexes (Figure S8B). Published reports from our labs and others have confirmed that multiple CMPs incorporation into a nanostructure/polymer significantly affects mobility within collagen-based scaffolds attributed to the CMP-collagen affinity, mainly in remodeled collagen33, 51. Furthermore, a controlled and localized CMP-modified polyplex release and transfection is achieved by the reversible and serum-stable reactions52. Studies involving matrix-assisted gene delivery methods have attained similar expression periods as observed in this work (ranging from few days46 to a month53 to multi-month periods54) within similar subcutaneous animal implants. However, the in vivo results establish the unique advantages of our delivery approach for temporally-tailored gene expression, via varied CMP composition, as well as spatially localized, protease-triggered delivery that is coordinated with collagen remodeling. These innovative features may offer improved healing outcomes with reduced side effects, addressing key challenges that have previously hindered biomaterial efficacy in complex processes like wound repair.
Figure 4:

Evaluation of in vivo transfection efficiency using membrane bound luciferase polyplexes with different CMP modifications encapsulated in Matrigel. Schematic representation and photographs of different groups injected in mice. IVIS Lumina images of mice following transfection with polyplexes encapsulated in Matrigel.
3.7. In vivo wound healing studies and histological evaluations
Wound healing analyses were conducted in an excisional mouse wound model to determine whether polyplex-mediated PDGF expression could support improved wound repair. Mice were divided into six groups and punch biopsy wounds were developed on the dorsa of the animals using a 5 mm punch. Wounds were treated with a series of formulations [saline, blank co-gel, PDGF in co-gel, PDGF polyplex-loaded co-gel (0%, 20%, or 50% CMP modification)] and the wounds were subsequently evaluated for 14 days following the application of the different wound treatments. Representative photographs of the wounds at different time points are presented in Figure 5A. No significant changes in the mice body weights treated with co-gel formulations were observed, indicating no adverse effects (Figure S9). Wound size reductions were further calculated at different time points (Figure 5B). At day 4, the wound size reductions for all groups ranged between 9–16%, with statistically insignificant differences between the groups. Statistically significant differences were observed at day 9 and day 14. At day 9, wounds treated with PDGF polyplex-loaded co-gels (20% CMP) presented significant (79%) wound closure, a level that was 46%, 29%, 24%, 19%, and 18% higher than closure in wounds treated with saline, blank co-gel, PDGF in co-gel, and PDGF polyplex-loaded co-gels (0% CMP or 50% CMP). The blank co-gels exhibited 23% higher wound closure levels as compared to saline treated wounds, presumably because of the collagen and fibrin composition, as these matrix components are involved in various aspects of the wound healing response including fibroblast recruitment through interactions with integrin receptors55. The PDGF protein in the co-gel further enhanced wound closure by 28% and 7%, respectively, as compared to the saline and co-gel treatments, but the PDGF protein sample closed less efficiently than all of the PDGF polyplex-loaded co-gel samples (closure in the PDGF protein-treated sample was 5%, 32%, and 7% lower than in the 0%, 20%, and 50% CMP modification co-gel samples, respectively). These differences likely arose from the limited stability of the PDGF protein within the co-gel, particularly at increasing time points (Figure S10), such that samples with PDGF protein only could not compete with polyplex-loaded co-gels in which transfected cells were able to continuously produce PDGF to enhance cell proliferation and migration. These results provide evidence that 20% CMP-polyplex-mediated PDGF gene delivery induced wound healing effects with much lower concentrations of PDGF than PDGF protein delivery, indicating that the co-gel/CMP transfection approach could be both safer and more cost-effective for clinical use. Furthermore, our preliminary evaluation of the macrophage and fibroblast transfections demonstrated ~10x higher higher transfection in fibroblasts as compared to macrophages suggesting beneficial effects of growth factor gene delivery. Moreover, in a chronic wound microenvironment, that possesses higher MMP levels, the polyplex transfection rates would also differ, with, for example, the 50% CMP modified polyplexes potentially working better. Therefore, future work aiming for assessment in chronic wound models is required to further assess the wound healing properties of co-gels and determine the appropriate CMP modification level.
Figure 5:

In vivo wound healing studies. (A) Photographs of skin wounds and (B) wound size reductions in mice treated with different formulations for up to 14 days [*P<0.05, **P<0.01, ***P<0.001 compared to saline treatment; †P<0.05 compared to co-gel treatment; ‡P<0.05 compared to PDGF-loaded co-gel treatment; €P<0.05 compared to PDGF polyplex-loaded co-gel (0% CMP)]. Individual data point refer to the mean ± standard deviation for three separate measurements.
The wound skin samples were paraffin-embedded and sectioned for histological analyses. The H&E stained sections for the different treatment groups are presented in Figure 6A. At day 4, there were no significant differences between the groups. The wound edges at day 4 were far apart (~ 5 mm) and could not be imaged under the microscope under one frame. So, two images (one for each wound edge) were taken separately as shown in Figure S11. Wound closure was evident in all the treatment groups at day 9; however, there were differences in epidermal layer formation. At day 14, the saline group was still missing the epidermal layer and the blank co-gel group had an incomplete epidermal layer, whereas all of the other groups presented a complete epidermal layer. Granulation tissue formation is another essential hallmark of wound healing56. The thickness of granulation tissue typically increases at earlier time points of wound healing57; however, at later time points, the granulation tissue thickness reduces to promote re-epithelialization and complete wound closure58. At day 9, the average thicknesses of granulation tissue in samples treated with saline, blank co-gel, PDGF-loaded co-gel, and PDGF polyplex-loaded co-gel (0%, 20%, and 50% CMP) were 800, 805, 595, 755, 680, and 675 μm, respectively. Significant differences in the thickness of the granulation tissue became more pronounced for the different treatment groups at day 14 (Figure 6B) due to a continued increase or levelling off in granulation tissue thickness in the saline, blank co-gel, PDGF-loaded co-gel, and 50% CMP PDGF polyplex-loaded co-gel samples, vs. a decrease in granulation tissue thickness in the 0% and 20% CMP PDGF polyplex-loaded co-gel samples. At day 14, the PDGF polyplex-loaded co-gels (0% and 20% CMP) presented significantly lower thicknesses of granulation tissue (approximately 40%, 27%, 21%, and 25% lower for both the 0% and 20% CMP samples as compared to the saline, blank co-gel, PDGF in co-gel, and PDGF polyplex-loaded co-gel (50% CMP) samples, respectively) that were indicative of enhanced wound healing.
Figure 6:

Evaluation of in vivo wound healing. (A) H&E stained tissue sections of murine excisional wounds treated with different formulations (blue arrows indicate epidermal thickness and red arrows indicate distance between wound edges; scale bar: 200 μm). (B) Thickness of granulation tissue, and (C) re-epithelialization in mice wounds treated with different formulations for up to 14 days [*P<0.05, **P<0.01, ***P<0.001 compared to saline treatment; †P<0.05 compared to co-gel treatment; ‡P<0.05 compared to PDGF-loaded co-gel treatment; €P<0.05 compared to PDGF polyplex-loaded co-gel (0% CMP); ₡P<0.05 compared to PDGF polyplex-loaded co-gel (50% CMP)]. Percentage re-epithelialization was calculated as: (distance traveled by migrating keratinocytes/total distance to travel)*100 using ImageJ software (National Institues of Health, Bethesda, MD). Individual data point refer to the mean ± standard deviation for three separate measurements.
Granulation tissue offers a matrix for keratinocytes to support migration, resulting in an activation of re-epithelialization from the wound edges59. Re-epithelialization is a crucial component of wound healing referred as an essential parameter of its success. Our re-epithelialization analysis further indicated the beneficial effects of PDGF polyplex-loaded co-gels (Figure 6C). At day 9, the PDGF polyplex-loaded co-gels (20% CMP) presented the highest percentage re-epithelialization among all the treatment groups [re-epithelialization was 46%, 29%, 24%, 19%, and 19% higher as compared to saline, blank co-gel, PDGF in co-gel, and PDGF polyplex-loaded co-gel (0% and 50% CMP), respectively]. By day 14, all the treatment groups showed significantly higher re-epithelialization (8–11% higher) as compared to saline treatment. Cumulatively, the PDGF polyplex-loaded co-gel (20% CMP) presented the highest wound closure rate (e.g. 79% by day 9), the fastest progression in granulation tissue formation/reduction (e.g. the lowest thickness of granulation tissue at day 14), and the highest re-epithelialization rate (e.g. 78% at day 9) to promote effective wound healing, attributable to the incorporation of CMP modification at a level that allowed polyplex release and transfection at favorable time points for wound healing. This rapid wound closure, achieved by proliferation and migration of epithelial cells, is vital for restoration of the barrier function during wound healing60.
Myofibroblasts play a significant role in wound contraction56, and previous studies clearly indicate that PDGF has a role in myofibroblast differentiation61. Therefore, the role of PDGF in myofibroblast differentiation was evaluated (Figure S12). Fibroblasts cultured in 12-well plates were either treated with PDGF (10 ng/mL) or bolus transfected with PDGF polyplex (4 μg/ml of pDNA). In our study, TGF-β treatment (10 ng/mL) in cell culture was used as positive control because it serves as a potent positive regulator of myofibroblast differentiation62, 63. Following treatment, the cells were allowed to grow and differentiate for 5 days prior to antibody staining for α-SMA (expressed by myofibroblasts). As observed by the fluorescence microscopy imaging, TGF-β treatment clearly enhanced myofibroblast differentiation as observed by the increased green fluorescence. Addition of PDGF to the conditioned media also resulted in myofibroblast differentiation. Furthermore, PDGF polyplex transfection in the fibroblasts induced myofibroblast differentiation attributable to the PDGF expression by the fibroblasts. Histology analyses of the tissue sections (retrieved at day 14 following treatments) were performed followed by staining with antibodies for α-SMA (Figure 7). Only wound tissues treated with PDGF polyplex-loaded co-gels (0%, 20%, or 50%) showed the presence of myofibroblasts (stained with α-SMA), which presumably enhanced the wound contraction process and the overall wound healing efficacy56. Few myofibroblasts were present in the 0% CMP treated group whereas slightly higher numbers were present in the 20% CMP group. The higher number of myofibroblasts present in the 50% CMP group could be attributed to delayed polyplex transfection and subsequent late PDGF expression, which resulted in delayed promotion of myofibroblast differentiation. Literature suggests that massive apoptosis of myofibroblasts occurs after wound healing and re-epithelialization to prevent hypertrophic scar formations64. The trend of reduced myofibroblasts in 20% CMP group as opposed to 50% CMP group supports the fact that myofibroblasts activated in the wounds by PDGF polyplexes were disappearing, likely as a part of the normal healing process.
Figure 7:

Evaluation of myofibroblast activation during wound healing using representative fluorescence microscopy images. Tissue sections were treated with Alexa Flour 488 tagged α-SMA antibody to visualize myofibroblasts (scale bar: 250 μm).
Considering the migration of fibroblasts to the wound area, the collagen levels in granulation tissues were tested for comparison between different treatment groups. The efficient wound closure requires collagen and extracellular matrix deposition60. Collagen deposition in the healing wounds (at day 14) was evaluated using Masson’s trichome staining (Figure 8A); healthy skin is composed of high levels of collagen (intense blue stain) distributed in the dermal layer. Wounds treated with saline showed minimal collagen deposition. Co-gel and PDGF in co-gel treatments improved collagen deposition in the wounds. The highest collagen deposition observed in our studies was present for PDGF polyplex-loaded co-gels. Co-gels with PDGF polyplexes (20% CMP modifications) had the highest collagen levels attributable to PDGF polyplex transfection and subsequent fibroblast proliferation and collagen deposition to promote healing. Our results reporting higher collagen deposition in mouse punch biopsy wounds treated with topical co-gel encapsulated PDGF polyplexes were consistent with the results obtained by other studies reporting matrix-enabled PDGF gene delivery in rats following subcutaneous treatment65, in porcine wounds following topical treatment with adenoviral constructs65, and in rats after topical PLGA nanosphere-mediated PDGF gene transfer66. Specifically, as compared to these past works, our polyplex loaded co-gels showed similar activity with an increased safety profile: for example, the polyplex loaded co-gels exhibited enhanced extracellular matrix deposition that was comparable to that obtained following adenoviral PDGF gene delivery from collagen65, with the added benefit of using a non-viral vector with enhanced efficacy. Our study also showed benefits as compared with the PLGA nanosphere/PDGF treatment approach66, as the nanosphere formulations were subcutaneously injected around the wound with more limited control over gene localization.
Figure 8:

Evaluation of in vivo wound healing. (A) Masson’s trichome stained tissue sections of murine wounds treated with different formulations for up to 14 days (nuclei: black; cytoplasm, keratin: red; collagen: blue; scale bar: 200 μm). (B) Epidermal thickness, and (C) distance between wound edges in mice wounds treated with different formulations for up to 14 days [*P<0.05, **P<0.01, ***P<0.001 compared to saline treatment; †P<0.05 compared to co-gel treatment; ‡P<0.05 compared to PDGF-loaded co-gel treatment; €P<0.05 compared to PDGF polyplex-loaded co-gel (0% CMP); ₡P<0.05 compared to PDGF polyplex-loaded co-gel (50% CMP)]. Individual data point refer to the mean ± standard deviation for three separate measurements.
Furthermore, microscopic images were used to evaluate epidermal thicknesses (Figure 8B) and distance between the wound edges (Figure 8C) for different groups. The epidermis for healthy skin was ~15 μm thick. Saline treatment did not support a fully formed epidermis; the reported thickness is the epidermal thickness of the edges, which barely represents the actual thickness in the healed wounds and cannot be compared to measurements of intact epidermis. The co-gel and PDGF in co-gel treatments resulted in formation of fully formed, thicker epidermises of 170 and 145 μm, respectively. However, treatment with PDGF polyplex-loaded co-gels of 0%, 20%, and 50% CMP significantly reduced the epidermal thicknesses to 70, 45, and 75 μm, respectively. The epidermal thickness in wounds treated with polyplexes containing 20% CMP modification was closest to that of heathy skin (~20 μm thickness)67, indicating its effectiveness in promoting healing of wounds. Furthermore, the lack of epidermal hypertrophy in the PDGF polyplex-loaded co-gel samples suggest that these treatments were able to prevent the formation of scars68. The distances between the wound edges was in the order: Saline (2.4 mm) > Co-gel (1.6 mm) > PDGF in co-gel (1.3 mm) > PDGF polyplex-loaded co-gel [50% CMP (1.3 mm) > 0% CMP (1.2 mm) > 20% CMP (0.9 mm)], comparable to previously published reports of growth factor delivery for wound treatment69. Thus, our analyses further support the role of polyplexes for inducing effective wound healing at significantly reduced PDGF dosing.
Collagen deposition in the treated wounds was analyzed using SHG microscopy (Figure 9). Collagen deposition in the different groups (from day 4 to day 14) was enhanced following treatment (Figure 9A). Forward and backward scattering images were taken wherein forward scattering corresponds to mature collagen with thicker collagen fibers whereas backward scattering correspond to immature collagen with thinner collagen fibers37. Forward (Figure 9B) and backward (Figure 9C) scattering intensities were measured for wounds from different treatment groups. The saline group showed a minimal increase in forward and backward scattering at all time points whereas the co-gel and PDGF in co-gel treatments increased backward scattering more than forward scattering even at day 14, indicating the presence of immature collagen in the wounds. Backward scattering was reduced to ~0.36, and forward scattering was increased to ~0.46, for groups treated with PDGF polyplex-loaded co-gels, with comparable patterns as the patterns observed for healthy skin (backward scattering: 0.83 and forward scattering: 0.92); collectively, this result provides evidence for enhanced wound healing. Wounds treated with co-gels carrying PDGF polyplexes with 20% CMP modification, followed by those carrying polyplexes with 50% CMP modification, exhibited forward-to-backward scattering ratios of 2.5 and 2.2, respectively, approaching the value in healthy skin (~2.2) (Figure 9D). Furthermore, the collagen orientation index was calculated from the SHG images for skin sections (Figure 9E). Collagen is randomly oriented in healthy skin as suggested by its lower index value70. The different treatment groups had different collagen orientation indices at different time points. At the end of day 14, the collagen orientation indices for healthy skin, saline, co-gel, PDGF-loaded co-gel, and PDGF polyplex-loaded co-gels (0%, 20%, and 50% CMP) were 0.32, 0.58, 0.47, 0.39, 0.40, 0.31, and 0.38, respectively. PDGF polyplex-loaded co-gels with 20% CMP modification presented orientation index values closest to that of healthy skin.
Figure 9:
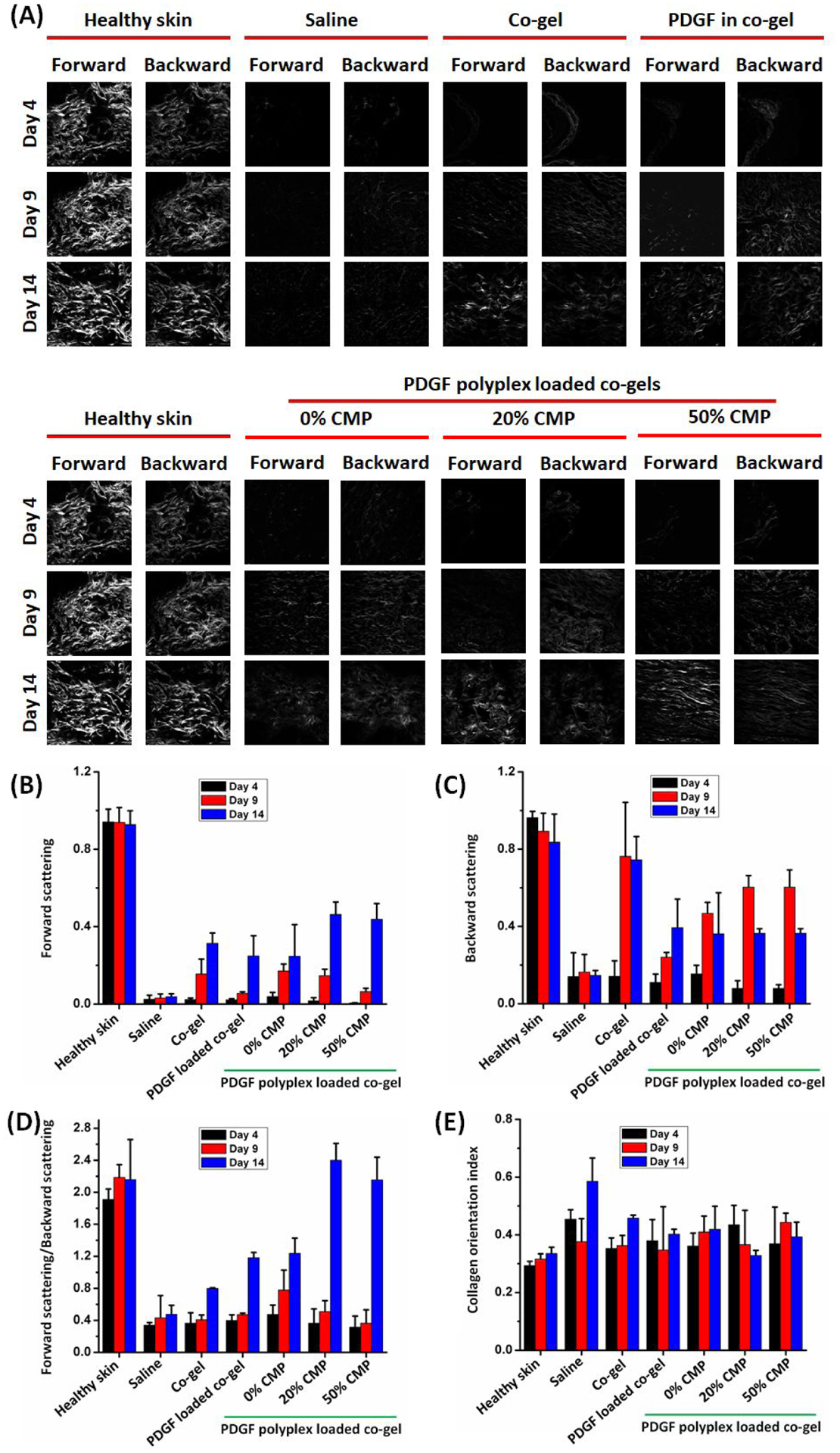
Evaluation of collagen content in treated wounds. (A) Forward and backward scattering images of the wound tissue sections imaged using SHG microscopy. (B) Forward scattering, (C) backward scattering, (D) forward/backward scattering ratio, and (E) collagen orientation index for the wound tissue sections treated with different formulations. Individual data point refer to the mean ± standard deviation for three separate measurements.
Mature and immature collagen are known to correspond to collagen type I and type III in the skin, respectively71. Therefore, collagen antibody staining was performed on the tissue sections from the treated wounds (Figure S13) to evaluate the relative amounts of these two collagens in the treated wound beds. The fluorescence microscopy images for collagen type I staining present the fluorescence patterns for the treatment groups as: healthy skin > PDGF polyplex-loaded co-gel (20% CMP > 50% CMP > 0% CMP) > PDGF-loaded co-gel > co-gel > saline. The staining results were in close agreement with the SHG results, with the highest type I collagen levels found in the wounds treated with PDGF polyplex-loaded co-gels with 20% CMP modification, followed by wounds treated with 50% CMP modification. Collagen type III is expressed in early granulation tissue and plays a prominent role in cutaneous tissue repair72. The collagen type III fluorescence patterns for different treatment groups were: healthy skin > saline > co-gel > PDGF polyplex-loaded co-gel (0% CMP > 50% CMP > 20% CMP) > PDGF-loaded co-gel. Both collagen type I and type III were distributed in close proximity to each other because immature collagen is gradually replaced by mature collagen during the course of healing42. Hence, PDGF polyplex-loaded co-gels with 20% CMP modification improved overall collagen deposition, forward-to-backward scattering ratio, and collagen type I/type III ratios to yield tissue comparable to healthy skin, providing clear evidence of enhanced normal wound healing.
4. Conclusions
A PDGF polyplex-loaded collagen-fibrin “co-gel” was successfully prepared that harnessed collagen-mimetic peptides to temporally tune PDGF gene transfer within the wound environment and thereby coordinate PDGF activity with the progression of the healing process. PDGF expression could be controlled for well-defined, multi-day durations by tailoring the CMP composition that was present during co-gel transfection. Co-gel-mediated PDGF expression promoted improved wound healing by stimulating fibroblast proliferation, migration, collagen deposition, and remodeling, with synergistically enhanced efficacy demonstrated due to the combination of collagen, fibrin, and controlled PDGF expression within the same wound bed. PDGF polyplex-loaded co-gels with 20% CMP modification showed the best wound healing activity, attributable to the capacity of these polyplexes to coordinate pDNA release with MMP activity, and potentially promote PDGF expression over an intermediate time period as observed for luciferase expression in vitro. Moreover, the PDGF polyplex-loaded co-gels exhibited enhanced wound healing as compared with PDGF protein application, and polyplex gene transfer induced improved healing while expressing PDGF at levels that were ~2 orders of magnitude lower than the amount of PDGF protein applied to the wound bed. Overall, this study provides a basis for use of CMPs to enable improved growth factor activity in the wound bed by MMP-mediated localized release along with temporally controlled expression of PDGF. The combined properties of the co-gels, including their stability and capacity to control both cell recruitment and cell phenotype within the murine wound bed, strongly supports the potential of the co-gel scaffolds for improved treatment of chronic non-healing wounds. Moreover, the combined effects of matrix CMP-mediated gene transfer and collagen-fibrin biomaterial matrices offer synergistic benefits in wound healing, with reduced growth factor dosing that may address clinical concerns related to wound biomaterials.
Supplementary Material
Acknowledgements:
This work was partially supported by the National Science Foundation (NSF) under Grant nos. 1605130 and 1700980. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily represent the views of the NSF. This work was also partially supported by the National Institutes of Health under Grant no. R01 AR067247.
Footnotes
Supporting Information
Figure S1, characterization of synthesized AMPs; Figure S2, characterization of CMP-PEI conjugate; Figure S3, DLS measurements of GLuc polyplexes; Figure S4, determination of polyplex stability; Figure S5, effect of PDGF on NIH/3T3 cells; Figure S6, characterization of co-gels; Figure S7, determination of GLuc expression by fibroblasts in co-gels; Figure S8, microscopic images of GFP polyplex transfected NIH/3T3 cells; Figure S9, evaluation of in vivo transfection efficiency using membrane bound luciferase polyplexes; Figure S10, stability of PDGF in co-gels; Figure S11, H&E stained section of wound tissues; Figure S12, determination of the effect of PDGF on myofibroblast activation in vitro; Figure S13, evaluation of myofibroblast activation in vivo; Figure S14, visualization of collagen types in co-gel treated in vivo wounds.
Conflict of interest
The authors declare no conflict of interest.
References:
- 1.Bennett NT; Schultz GS, Growth factors and wound healing: Part II. Role in normal and chronic wound healing. The American Journal of Surgery 1993, 166 (1), 74–81. [DOI] [PubMed] [Google Scholar]
- 2.Macri L; Clark RAF, Tissue Engineering for Cutaneous Wounds: Selecting the Proper Time and Space for Growth Factors, Cells and the Extracellular Matrix. Skin Pharmacology and Physiology 2009, 22 (2), 83–93. [DOI] [PubMed] [Google Scholar]
- 3.Eaglstein WH; Falanga V, CHRONIC WOUNDS. Surgical Clinics of North America 1997, 77 (3), 689–700. [DOI] [PubMed] [Google Scholar]
- 4.Nussbaum SR; Carter MJ; Fife CE; DaVanzo J; Haught R; Nusgart M; Cartwright D, An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value in Health 2018, 21 (1), 27–32. [DOI] [PubMed] [Google Scholar]
- 5.Barrientos S; Stojadinovic O; Golinko MS; Brem H; Tomic-Canic M, PERSPECTIVE ARTICLE: Growth factors and cytokines in wound healing. Wound Repair and Regeneration 2008, 16 (5), 585–601. [DOI] [PubMed] [Google Scholar]
- 6.Zhang S; Uludağ H, Nanoparticulate Systems for Growth Factor Delivery. Pharmaceutical Research 2009, 26 (7), 1561. [DOI] [PubMed] [Google Scholar]
- 7.Robson MC; Mustoe TA; Hunt TK, The future of recombinant growth factors in wound healing. The American Journal of Surgery 1998, 176 (2, Supplement 1), 80S–82S. [DOI] [PubMed] [Google Scholar]
- 8.Laiva AL; O’Brien FJ; Keogh MB, Innovations in gene and growth factor delivery systems for diabetic wound healing. Journal of Tissue Engineering and Regenerative Medicine 2018, 12 (1), e296–e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell AC; Briquez PS; Hubbell JA; Cochran JR, Engineering growth factors for regenerative medicine applications. Acta Biomaterialia 2016, 30, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urello MA; Kiick KL; Sullivan MO, A CMP-based method for tunable, cell-mediated gene delivery from collagen scaffolds. Journal of Materials Chemistry B 2014, 2 (46), 8174–8185. [DOI] [PubMed] [Google Scholar]
- 11.Urello MA; Kiick KL; Sullivan MO, ECM turnover-stimulated gene delivery through collagen-mimetic peptide-plasmid integration in collagen. Acta Biomaterialia 2017, 62, 167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ostman A; Thyberg J; Westermark B; Heldin CH, PDGF-AA and PDGF-BB biosynthesis: proprotein processing in the Golgi complex and lysosomal degradation of PDGF-BB retained intracellularly. The Journal of cell biology 1992, 118 (3), 509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGary EC; Weber K; Mills L; Doucet M; Lewis V; Lev DC; Fidler IJ; Bar-Eli M, Inhibition of Platelet-derived Growth Factor-mediated Proliferation of Osteosarcoma Cells by the Novel Tyrosine Kinase Inhibitor STI571. Clinical Cancer Research 2002, 8 (11), 3584. [PubMed] [Google Scholar]
- 14.Heldin C-H; Westermark B, Mechanism of action and in vivo role of platelet-derived growth factor. Physiological Reviews 1999, 79 (4), 1283–1316. [DOI] [PubMed] [Google Scholar]
- 15.Keswani SG; Katz AB; Lim F-Y; Zoltick P; Radu A; Alaee D; Herlyn M; Crombleholme TM, Adenoviral mediated gene transfer of PDGF-B enhances wound healing in type I and type II diabetic wounds. Wound Repair and Regeneration 2004, 12 (5), 497–504. [DOI] [PubMed] [Google Scholar]
- 16.Shepard JA; Wesson PJ; Wang CE; Stevans AC; Holland SJ; Shikanov A; Grzybowski BA; Shea LD, Gene therapy vectors with enhanced transfection based on hydrogels modified with affinity peptides. Biomaterials 2011, 32 (22), 5092–5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lei Y; Huang S; Sharif-Kashani P; Chen Y; Kavehpour P; Segura T, Incorporation of Active DNA/Cationic Polymer Polyplexes into Hydrogel Scaffolds. Biomaterials 2011, 31 (34), 9106–9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin S; Shea LD, Lentivirus immobilization to nanoparticles for enhanced and localized delivery from hydrogels. Molecular Therapy 2010, 18 (4), 700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kidd M; Shin S; Shea LD, Fibrin hydrogel for lentiviral gene delivery in vitro and in vivo. Journal of Controlled Release 2012, 157 (1), 80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tokatlian T; Cam C; Segura T, Porous hyaluronic acid hydrogels for localized non-viral DNA delivery in a diabetic wound healing model. Advanced Healthcare Materials 2015, 4 (7), 1084–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner NJ; Badylak SF, The use of biologic scaffolds in the treatment of chronic nonhealing wounds. Advances in Wound Care (New Rochelle) 2015, 4 (8), 490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stenzel KH; Miyata T; Rubin AL, Collagen as a Biomaterial. Annual Review of Biophysics and Bioengineering 1974, 3 (1), 231–253. [DOI] [PubMed] [Google Scholar]
- 23.Veves A; Falanga V; Armstrong DG; Sabolinski ML, Graftskin, a Human Skin Equivalent, Is Effective in the Management of Noninfected Neuropathic Diabetic Foot Ulcers. Diabetes Care 2001, 24 (2), 290. [DOI] [PubMed] [Google Scholar]
- 24.Gu D-I; Nguyen T; Gonzalez AM; Sonsnowski BA; Phillips ML; Chandler LA, Adenovirus encoding human platelet-derived growth factor delivered in collagen exhibits safety, biodistribution, and immunogenicity profiles favorable for clinical use. Molecular Therapy 2004, 9 (5), 699–711. [DOI] [PubMed] [Google Scholar]
- 25.Blume P; Driver VR; Tallis AJ; Kirsner RS; Kroeker R; Payne WG; Wali S; Marston W; Dove C; Engler RL; Chandler LA; Sosnowski B, Formulated collagen gel accelerates healing rate immediately after application in patients with diabetic neuropathic foot ulcers. Wound Repair and Regeneration 2011, 19 (3), 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schleef M; Blaesen M; Schmeer M; Baier R; Marie C; Dickson G; Scherman D, Production of non viral DNA vectors. Current Gene Therapy 2010, 10 (6), 487–507. [DOI] [PubMed] [Google Scholar]
- 27.Elsabahy M; Nazarali A; Foldvari M, Non-viral nucleic acid delivery: Key challenges and future directions. Current Drug Delivery 2011, 8 (3), 235–244. [DOI] [PubMed] [Google Scholar]
- 28.Beer H-D; Longaker MT; Wener S, Reduced expression of PDGF and PDGF receptors during impaired wound healing. Journal of Investigative Dermatology 1997, 109 (2), 132–138. [DOI] [PubMed] [Google Scholar]
- 29.Shepherd J; Bax D; Best S; Cameron R, Collagen-fibrinogen lyophilised scaffolds for soft tissue regeneration. Materials (Basel) 2017, 10 (6), 568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thapa RK; Kiick KL; Sullivan MO, Encapsulation of collagen mimetic peptide-tethered vancomycin liposomes in collagen-based scaffolds for infection control in wounds. Acta Biomaterialia 2020, 103, 115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krishna OD; Jha AK; Jia X; Kiick KL, Integrin-mediated adhesion and proliferation of human MSCs elicited by a hydroxyproline-lacking, collagen-like peptide. Biomaterials 2011, 32 (27), 6412–6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu X; Liu Q; Wang M; Liang M; Yang X; Xu X; Zou H; Qiu J, Activation of Sirt1 by resveratrol inhibits TNF-α induced inflammation in fibroblasts. PloS one 2011, 6 (11), e27081–e27081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urello MA; Kiick KL; Sullivan MO, Integration of growth factor gene delivery with collagen-triggered wound repair cascades using collagen-mimetic peptides. Bioengineering & Translational Medicine 2016, 1 (2), 207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baba A; Matsushita S; Kitayama K; Asakura T; Sezutsu H; Tanimoto A; Kanekura T, Silk fibrion produced by transgenic silkworms overexpressing the Arg-Gly-Asp motif accelerates cutaneous wound healing in mice. Journal of Biomedical Research Part B: Applied Biomaterials 2019, 107 (1), 97–103. [DOI] [PubMed] [Google Scholar]
- 35.Chen L; Mirza R; Kwon Y; DiPietro L; Koh T, The murine excisional wound model: Contraction revisited. Wound Repair and Regeneration 2015, 23 (6), 874–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu X; Zheng L; Huang X; Wu J; Hong L; Dong J; Zhuo S; Chen J, Nonlinear optical microscopy captures high-resolution images of microstructures within three types of unlabeled rat cartilage. IEEE Photonics Journal 2016, 8 (3), 7100910. [Google Scholar]
- 37.Williams RM; Zipfel WR; Webb WW, Interpreting second-harmonic generation images of collagen I fibrils. Biophysical Journal 2005, 88 (2), 1377–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foroozandeh P; Aziz AA, Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Research Letters 2018, 13, 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sigmon J; Larcom LL, The effect of ethidium bromide on mobility of DNA fragments in agarose gel electrophoresis. Electrophoresis 1996, 17 (10), 1524–1527. [DOI] [PubMed] [Google Scholar]
- 40.De Donatis A; Comito G; Buricchi F; Vinci MC; Parenti A; Caselli A; Camici G; Manao G, Proliferation versus migration in platelet-derived growth factor signaling: the key role of endocytosis. Journal of Biological Chemistry 2008, 283 (29), 19948–19956. [DOI] [PubMed] [Google Scholar]
- 41.Li J; Chen J; Kirsner R, Pathophysiology of acute wound healing. Clinics in Dermatology 2007, 25 (1), 9–18. [DOI] [PubMed] [Google Scholar]
- 42.Xue M; Jackson CJ, Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Advances in Wound Care 2015, 4 (3), 119–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang JS; Huang SS; Kennedy B; Deuel TF, Platelet-derived growth factor. Specific binding to target cells. Journal of Biological Chemistry 1982, 257 (14), 8130–8136. [PubMed] [Google Scholar]
- 44.Xie J; Bao M; Bruekers SMC; Huck WTS, Collagen gels with different fibrillar microarchitectures elicit different cellular responses. ACS Applied Materials & Interfaces 2017, 9, 19630–19637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krishnaswamy VR; Mintz D; Sagi I, Matrix metalloproteinases: The sculptors of chronic cutaneous wounds. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2017, 1864 (11), 2220–2227. [DOI] [PubMed] [Google Scholar]
- 46.Zhang X-Q; Tang H; Hoshi R; De Laporte L; Qiu H; Xu X; Shea LD; Ameer GA, Sustained transgene expression via citric acid-based polyester elastomers. Biomaterials 2009, 30 (13), 2632–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keeney M; Onyiah S; Zhang Z; Tong X; Han L-H; Yang F, Modulating polymer chemistry to enhance non-viral gene delivery inside hydrogels with tunable matrix stiffness. Biomaterials 2013, 34 (37), 9657–9665. [DOI] [PubMed] [Google Scholar]
- 48.Makela M; Slo T; Larjava H, MMP-9 from TNF alpha-stimulated keratinocytes binds to cell membranes and type I collagen: a cause for extended matrix degradation in inflammation? Biochemical and Biophysical Research Communications 1998, 253 (2), 325–335. [DOI] [PubMed] [Google Scholar]
- 49.Miron-Mendoza M; Lin X; Ma L; Ririe P; Petroll WM, Individual versus collective fibroblast spreading and migration: regulation by matrix composition in 3D culture. Experimental eye research 2012, 99, 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.da Rocha-Azevedo B; Grinnell F, Fibroblast morphogenesis on 3D collagen matrices: the balance between cell clustering and cell migration. Experimental cell research 2013, 319 (16), 2440–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.San BH; Li Y; Tarbet EB; Yu SM, Nanoparticle assembly and gelatin binding mediated by triple helical collagen mimetic peptide. ACS Applied Materials and Interfaces 2016, 8 (31), 19907–19915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chattopadhyay S; Guthrie KM; Teixeira L; Murphy CJ; Dubielzig RR; McAnulty JF; Raines RT, Anchoring a cytoactive factor in a wound bed promotes healing. Journal of Tissue Engineering and Regenerative Medicine 2016, 10 (12), 1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meilander-Lin NJ; Cheung PJ; Wilson DL; Bellamkonda RV, Sustained in vivo gene delivery from agarose hydrogel prolongs nonviral gene expression in skin. Tissue Engineering 2005, 11 (3–4), 546–555. [DOI] [PubMed] [Google Scholar]
- 54.Salvay DM; Zelivyanskaya M; Shea LD, Gene delivery by surface immobilization of plasmid to tissue engineering scaffolds. Gene Therapy 2010, 17 (9), 1134–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greiling D; Clark RA, Fibronectin provides a conduit for fibroblast transmigration from collagenous stroma into fibrin clot provisional matrix. Journal of Cell Science 1997, 110, 861–870. [DOI] [PubMed] [Google Scholar]
- 56.Li B; Wang JH-C, Fibroblasts and myofibroblasts in wound healing: Force generation and measurement. Journal of Tissue Viability 2011, 20 (4), 108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Druecke D; Lamme EN, Hermann Sonja; Pieper J; May PS; Steinau H-U; Steinstraesser L, Modulation of scar tissue formation using different dermal regeneration templates in the treatment of experimental full-thickness wounds. Wound Repair and Regeneration 2004, 12 (5), 518–527. [DOI] [PubMed] [Google Scholar]
- 58.Shinozaki M; Okada Y; Ai K; Ikeda K; Saika S; Shinozaki M, Impaired cutaneous wound healing with excess granulation tissue formation in TNFalpha-null mice. Archives of Dermatological Research 2009, 301 (7), 531–537. [DOI] [PubMed] [Google Scholar]
- 59.Evans ND; Oreffo ROC; Healy E; Thurner PJ; Man YH, Epithelial mechanobiology, skin wound healing, and the stem cell niche. Journal of the Mechanical Behavior of Biomedical Materials 2013, 28, 397–409. [DOI] [PubMed] [Google Scholar]
- 60.Devalliere J; Dooley K; Hu Y; Kelangi SS; Uygun BE; Yarmush ML, Co-delivery of a growth factor and a tissue-protective molecule using elastin biopolymers accelerates wound healing in diabetic mice. Biomaterials 2017, 141, 149–160. [DOI] [PubMed] [Google Scholar]
- 61.Singh V; Barbosa FL; Torricelli AAM; Santhiago MR; Wilson SE, Transforming growth factor β and platelet-derived growth factor modulation of myofibroblast development from corneal fibroblasts in vitro. Experimental Eye Research 2014, 120, 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blumbach K; Zweers MC; Brunner G; Peters AS; Schmitz M; Schulz J-N; Schild A; Denton CP; Sakai T; Fassler R; Krieg T; Eckes B, Defective granulation tissue formation in mice with specific ablation of integrin-linked kinase in fibroblasts - role of TGFβ1 levels and RhoA activity. Journal of Cell Science 2010, 123, 3872–3883. [DOI] [PubMed] [Google Scholar]
- 63.Schulz J-N; Cedric Z; Sorensen IW; Barczyk M; Carracedo S; Hallinger R; Niehoff A; Eckes B; Gullberg D, Reduced Granulation Tissue and Wound Strength in the Absence of α11β1 Integrin. Journal of Investigative Dermatology 2015, 135 (5), 1435–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hinz B, Formation and function of the myofibroblast during tissue repair. Journal of Investigative Dermatology 2007, 127 (3), 526–537. [DOI] [PubMed] [Google Scholar]
- 65.Chandler LA; Gu D-L; Ma C; Gonzalez AM; Doukas J; Nguyen T; Pierce GF; Phillips ML, Matrix-enabled gene transfer for cutaneous wound repair. Wound Repair and Regeneration 2000, 8 (6), 473–479. [DOI] [PubMed] [Google Scholar]
- 66.Shi R; Lian W; Han S; Cao C; Jin Y; Yuan Y; Zhao H; Li M, Nanosphere-mediated co-delivery of VEGF-A and PDGF-B genes for accelerating diabetic foot ulcers healing in rats. Gene Therapy 2018, 25, 425–438. [DOI] [PubMed] [Google Scholar]
- 67.Kjaer T; Thorsen K; Jessen N; Stenderup K; Pedersen SB, Resveratrol ameliorates imiquimod-induced psoriasis-like skin inflammation in mice. PLoS One 2015, 10 (5), e0126599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tandara AA; Mustoe TA, The role of the epidermis in the control of scarring: Evidence for mechanism of action for silicone gel. Journal of Plastic, Reconstructive & Aesthetic Surgery 2008, 61 (10), 1219–1225. [DOI] [PubMed] [Google Scholar]
- 69.Ishihara J; Ishihara A; Fukunaga K; Sasaki K; White MJV; Briquez PS; Hubbell JA, Laminin heparin-binding peptides bind to several growth factors and enhance wound healing. Nature Communications 2018, 9, 2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Zuijlen PPM; Ruurda JJB; van Veen HA; van Marle J; van Trier AJM; Groenevelt F; Kreis RW; Middelkoop E, Collagen morphology in human skin and scar tissue: no adaptations in response to mechanical loading at joints. Burns 2003, 29 (5), 423–431. [DOI] [PubMed] [Google Scholar]
- 71.Rosch R; Klinge U; Si Z; Junge K; Klosterhalfen B; Schumpelick V, A role for the collagen I/III and MMP-1/−13 genes in primary inguinal hernia? BMC Medical Genetics 2002, 3, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Volk SW; Wang Y; Mauldin EA; Liechty KW; Adams SL, Diminished type III collagen promotes myofibroblast differentiation and increases scar deposition in cutaneous wound healing. Cell Tissues Organs 2011, 194 (1), 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


