Abstract
PURPOSE
Adrenocortical carcinomas (ACC) are rare and aggressive malignancies with limited treatment options. This study was undertaken to evaluate the immunogenicity of ACC.
PATIENTS AND METHODS
Patients with advanced ACC were enrolled in a phase II study to evaluate the clinical activity of pembrolizumab 200 mg every 3 weeks, without restriction on prior therapy. The primary end point was objective response rate. Efficacy was correlated with tumor programmed death-ligand 1 expression, microsatellite-high and/or mismatch repair deficient (MSI-H/MMR-D) status, and somatic and germline genomic correlates.
RESULTS
We enrolled 39 patients with advanced ACC and herein report after a median follow-up of 17.8 months (range, 5.4 months to 34.7 months). The objective response rate to pembrolizumab was 23% (nine patients; 95% CI, 11% to 39%), and the disease control rate was 52% (16 patients; 95% CI, 33% to 69%). The median duration of response was not reached (lower 95% CI, 4.1 months). Two of six patients with MSI-H/MMR-D tumors responded. The other seven patients with objective responses had microsatellite stable tumors. The median progression-free survival was 2.1 months (95% CI, 2.0 months to 10.7 months), and the median overall survival was 24.9 months (95% CI, 4.2 months to not reached). Thirteen percent of patients (n = 5) had treatment-related grade 3 or 4 adverse events. Tumor programmed death-ligand 1 expression and MSI-H/MMR-D status were not associated with objective response.
CONCLUSION
MSI-H/MMR-D tumors, for which pembrolizumab is a standard therapy, are more common in ACC than has been recognized. In advanced ACC that is microsatellite stable, pembrolizumab provided clinically meaningful and durable antitumor activity with a manageable safety profile.
INTRODUCTION
Adrenocortical carcinomas (ACC) are rare tumors with poor prognosis. Most patients present with metastases, and for those with localized disease, recurrences are common.1,2
Mitotane, a derivative of the insecticide dichlorodiphenyltricholorethane, is the only drug approved for ACC by the US Federal Drug Administration; it is marked by low efficacy and a narrow therapeutic window, often resulting in serious toxicity.3-8 Platinum-based chemotherapy is also considered a treatment on the basis of the results of the FIRM-ACT study (ClinicalTrials.gov identifier: NCT00094497); however, progression-free survival (PFS) and overall survival (OS) were short (5.6 months and 14.8 months, respectively), and the rate of serious adverse events (AEs) was 58%.9
Several clinical trials, including those with anti-angiogenic drugs and insulin-like growth factor receptor 1 inhibitors, have failed to provide additional treatments for ACC.10-17 To date, no investigated therapy has offered long-term disease control, and no therapy is standard. Evaluation of immunomodulation in ACC was compelling on the basis of observations of adrenalitis in patients receiving immune checkpoint blockade, the presence of programmed death-ligand 1 (PD-L1) expression in the tumor cell membrane, and tumor-infiltrating mononuclear cells in surgically treated ACC.18,19 In addition, there have been reports of checkpoint inhibitor activity in ACC.20 To evaluate the immunogenicity of ACC, we conducted a phase II study to evaluate pembrolizumab, an anti–PD-1 monoclonal antibody, in patients with advanced ACC.
PATIENTS AND METHODS
Patients
Patients 18 years of age or older with a pathologic diagnosis of unresectable or metastatic ACC considered incurable and an Eastern Cooperative Oncology Group performance status of 0 or 1 (on a 0 to 5 scale, with lower scores indicating less disability) were eligible.21 All patients had adequate organ function and measurable disease. Key exclusion criteria included a history of immunodeficiency or receipt of systemic corticosteroids or immunosuppressive therapy within 7 days of the first dose of pembrolizumab (physiologic replacement of corticosteroids for adrenal and pituitary insufficiency was permitted). Mitotane continuation was not permitted. Complete eligibility criteria are in the Protocol.
Study Oversight
The study was reviewed by the Memorial Sloan Kettering Cancer Center Institutional Review Board and was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. All patients provided written informed consent before study enrollment.
Study Design
This was an investigator-initiated, single-center, phase II study. Enrolled patients were treated intravenously with pembrolizumab 200 mg every 3 weeks (Appendix Fig A1, online only). No dose reduction was permitted, but dose interruption was permitted. Treatment was continued for up to 24 months/35 cycles, or until disease progression, unacceptable AEs or intercurrent illness, investigator decision to withdraw the patient, or patient withdrawal of consent. Patients could continue treatment beyond progression if they were deriving clinical benefit. Additional guidelines for treatment discontinuation and AE management are in the Protocol.
Study Assessments
The primary end point was objective response rate (ORR), according to RECIST v1.1, by means of blinded radiologic review.22 Response was assessed by computed tomography and/or magnetic resonance imaging at baseline and every 9 weeks thereafter. Secondary end points were the duration of response, PFS, OS, and safety. OS was monitored during therapy and every 3 months after discontinuation, until death or consent withdrawal. AEs were monitored throughout treatment and for 30 days after treatment end and were graded in severity according to Common Terminology Criteria for Adverse Events version 4.0.
In patients with available archival tissue, tumor PD-L1 expression was evaluated by immunohistochemistry (IHC; QualTek Molecular Laboratories, Newtown, PA). Positive PD-L1 status was defined as a modified proportion score of 1% or more in the tumor or at tumor stromal interface. The tumor-infiltrating lymphocyte (TIL) score was determined by hematoxylin and eosin stain (0: less than one TIL/high power field [HPF; three to five 20× fields]; 1: one to 10 TIL/HPF; 2: 11 to 20 TIL/HPF; 3: > 20 TIL/HPF).
Mismatch repair (MMR) status was evaluated by IHC; MMR deficiency (MMR-D) was defined as any loss of DNA MMR protein expression. Somatic and germline genetic testing, as well as evaluation of loss of heterozygosity status, were performed using the Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets next-generation sequencing (NGS) platform.23-26 Microsatellite instability (MSI) was assessed from Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets via MSIsensor.27 MSI-high (MSI-H) status was defined as an MSIsensor score ≥ 10 or an MSIsensor score ≥ 3 with tumor mutation burden (TMB) > 10 mutations/megabase (mut/Mb).
Statistical Analysis
A Simon two-stage design was used to test the null hypothesis that the true response rate was ≤ 12% versus the alternative hypothesis that the true response rate was at least 29% (type I/II error rates, 10% each). The null hypothesis was set at a true response rate of ≤ 12% using data from FIRM-ACT, in which the ORR in the control arm was 9% versus 23% in the experimental arm.9 In FIRM-ACT, mitotane combined with chemotherapy was studied as a first-line treatment in advanced ACC; treatment with mitotane, cisplatin, etoposide, and doxorubicin was associated with improved response rate and PFS when compared with mitotane and streptozocin; however, the PFS benefit was marginal (5.6 months v 2.2 months), serious AE rates were high (58% v 42%), and OS was low (14.8 months v 12.0 months) with no significant difference.9 For that reason, in this study, it was decided that an ORR of ≤ 12% would be unworthy of additional investigation. In stage I, 21 patients were enrolled, with the study expanded to stage II to enroll an additional 18 patients if at least three of the 21 patients had a partial response (PR) or a complete response (CR). It was predetermined that additional investigation with pembrolizumab would be worthwhile if eight or more of 39 objective responses were observed. Accrual time was estimated at 24 months, with the probability of early termination 53%. Patients who enrolled and were deemed ineligible before starting treatment could be replaced.
The Kaplan-Meier method was used to evaluate PFS and OS. For NGS analyses, common recurrent somatic alterations were defined as those occurring in five or more patients. A permutation-based log-rank test was used to examine the association between outcomes and somatic alterations. Allele-specific copy number at HLA class I loci was inferred using Loss Of Heterozygosity of Human Leukocytes Antigen (LOHHLA) software.28 Fisher’s exact test was used to examine the association between somatic alterations, HLA zygosity, TIL score, and tumor MSI, MMR, and PD-L1 status and response. The Mann-Whitney test was used to examine the association between TMB and response. P values in the subgroup analyses were two sided (type I error, 5%); P < .05 was considered statistically significant. Statistical analyses were performed using SAS Version 9.3 (SAS Institute, Cary, NC) or R Version 3.3.2. The data-lock date was January 11, 2019.
RESULTS
Patients
Thirty-nine patients were enrolled between February 10, 2016, and June 20, 2018, and they received at least one dose of pembrolizumab (Table 1). Forty-four patients had undergone eligibility assessment; five were ineligible. In stage I, three patients had objective responses. The study was expanded to stage II with 18 additional patients accrued. Seventy-two percent of the enrolled patients had received prior systemic therapy.
TABLE 1.
Baseline Patient and Disease Characteristics
Radiographic Response
Nine patients of the 39 treated demonstrated objective responses (nine PRs, no CRs; ORR, 23% [95% CI, 11% to 39%]). Seven additional patients (18%) demonstrated stable disease (SD). The disease control rate (percentage of patients who demonstrated objective response or SD) was 52% (95% CI, 33% to 69%).
Median treatment duration (interval from treatment start to end-of-study date) was 2.3 months (range, 0.3-24.3 months). For the nine PR patients, median time to response was 4.1 months (range, 1.7-10.5 months) and median response duration was not reached (lower 95% CI, 4.1 months). Response characteristics to pembrolizumab are listed in Table 2 and Figure 1.
TABLE 2.
Objective Response According to RECIST v1.1 Criteria
FIG 1.
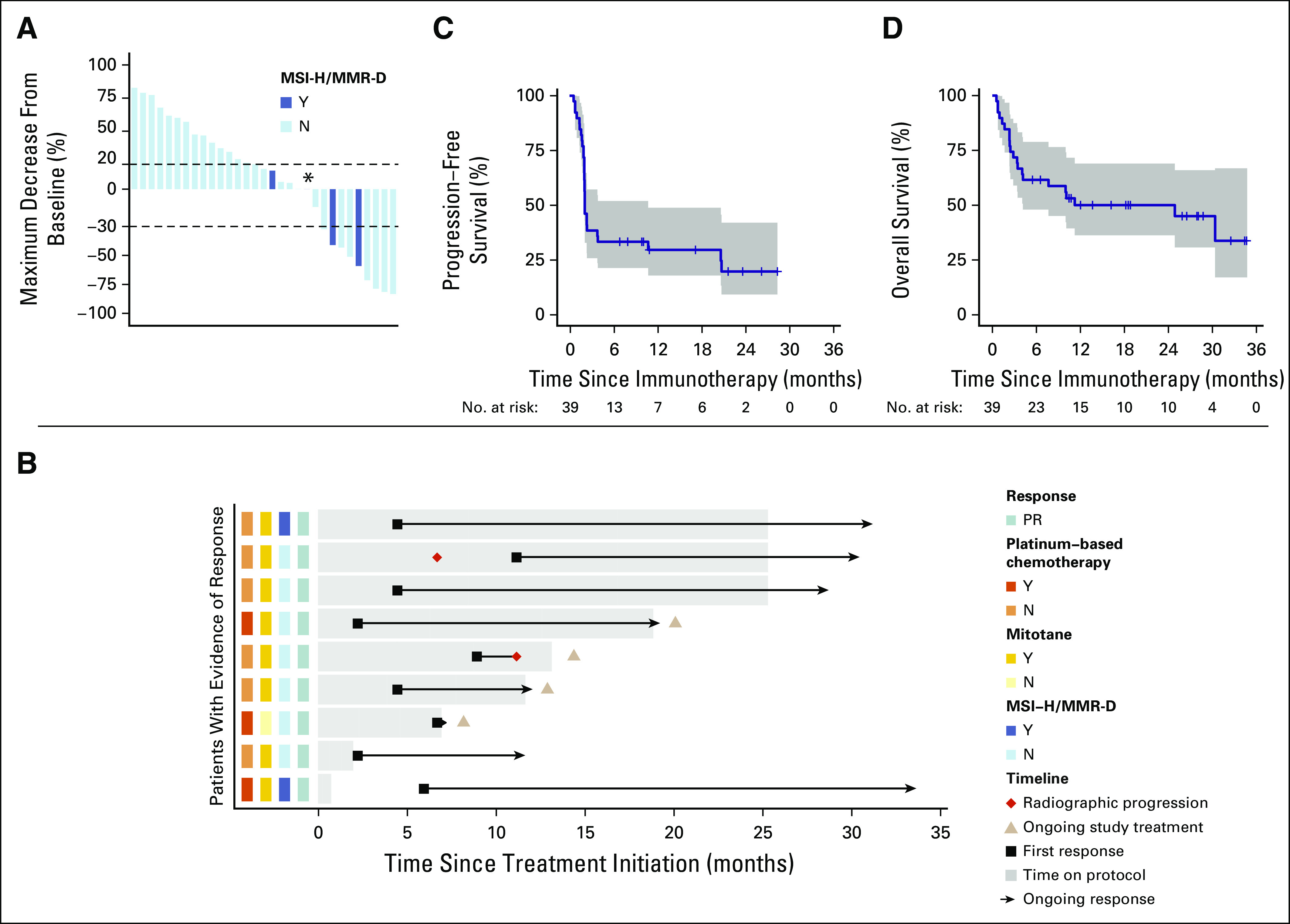
Tumor responses and clinical outcomes. (A) Maximum decrease from baseline in the size of tumors in patients treated with pembrolizumab who underwent blinded radiologic evaluation (n = 31) after initiation of treatment. (B) Response characteristics of patients with an objective response. Each horizontal bar represents one patient. Three patients have completed 24 months of treatment with pembrolizumab and are now on observation (top three bars). One patient experienced pseudoprogression (bar 2). One patient experienced RECIST v1.1–defined progression after objective response (bar 5). Two patients with objective responses discontinued therapy per investigator discretion; both patients are alive with continued response while on observation (bars 8 and 9). (C) Kaplan-Meier curve showing progression-free survival among 39 patients with advanced adrenocortical carcinoma who received pembrolizumab. Progression-free survival was measured from the start of treatment with pembrolizumab until progression of disease or death as a result of any cause, whichever occurred first. For nonevaluable patients by RECIST v1.1 who came off study because of clinical progression (n = 8), the date of progression of disease was noted as the day off study. Median progression-free survival was 2.1 months (95% CI, 2.0 months to 10.7 months). (D) Kaplan-Meier curve showing overall survival among 39 patients with advanced adrenocortical carcinoma who received pembrolizumab. Overall survival was measured as the time from the start of treatment with pembrolizumab until death. For patients alive at the end of study or lost to follow-up, overall survival was censored on the last date when patients were known to be alive. Median overall survival was 24.9 months (95% CI, 4.2 months to not reached). (*) Patient with MSI-H/MMMR-D tumor, maximum decrease from baseline 0%. MSI-H/MMR-D, microsatellite-high/mismatch repair-deficient; PR, partial response by RECIST v1.1.
Two patients had mixed responses; both had reduction in lung metastases but progression in hepatic metastases. In one patient, a complete response was noted in the lung metastases after treatment with pembrolizumab; the patient subsequently underwent hepatic arterial bland embolization and currently has no evidence of disease on imaging. Two patients with PRs experienced pseudoprogression (Appendix Fig A2, online only). Data available on seven enrolled patients who went on to receive cytotoxic therapy after treatment with pembrolizumab demonstrated no responses (zero of seven).
Survival Analyses
The median follow-up at the time of data-lock was 17.8 months (range, 5.4-34.7 months). Twenty deaths were observed at analysis. No death was attributed to treatment. Median PFS was 2.1 months (95% CI, 2.0 months to 10.7 months; Fig 1). The 6-month PFS rate was 20% (95% CI, 9% to 42%). Median OS was 24.9 months (95% CI, 4.2 months to not reached; Fig 1). The 2-year OS rate was 50% (95% CI, 36% to 69%).
Three patients had PRs exceeding 24 months of treatment. These patients remain on active surveillance with continued response off therapy (Fig 1).
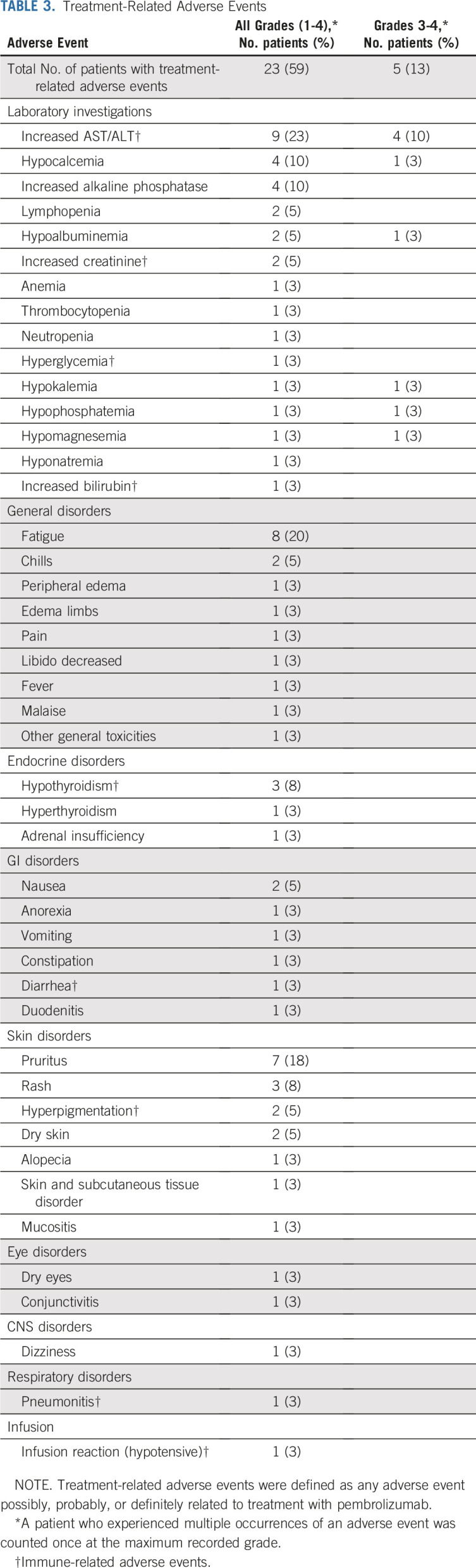
Safety
Treatment-related AEs (TRAEs) are listed in Table 3; TRAEs of any grade occurred in 23 patients (59%). Grade 3/4 TRAEs occurred in five patients (13%). Thirteen patients (33%) experienced immune-mediated AEs; the most common were liver function test (LFT) elevation (23%), hypothyroidism (8%), and kidney dysfunction (5%). Among patients with immune-mediated AEs, 10 (26%) received systemic corticosteroids, seven (18%) experienced treatment interruption, and two (5%) discontinued therapy for LFT elevation per investigator discretion; both patients discontinuing treatment had PRs that continue on observation. All patients with objective responses to pembrolizumab experienced LFT elevation ≥ grade 2.
TABLE 3.
Treatment-Related Adverse Events
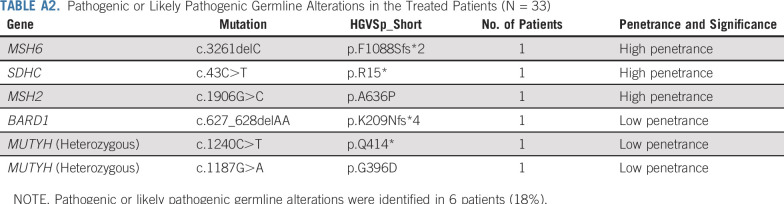
Two patients demonstrating PRs developed skin and tongue hyperpigmentation (Fig 2). Both patients had elevated adrenocorticotropin hormone (ACTH) levels. One patient underwent pituitary magnetic resonance imaging to evaluate for hypophysitis; imaging demonstrated a normal pituitary.
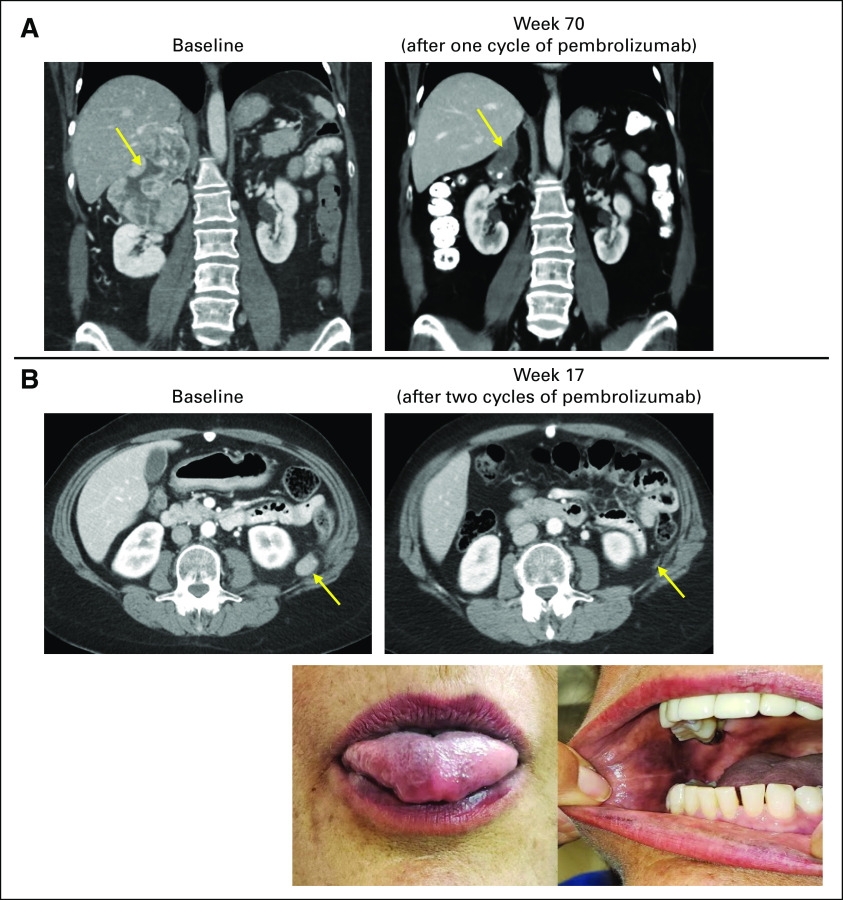
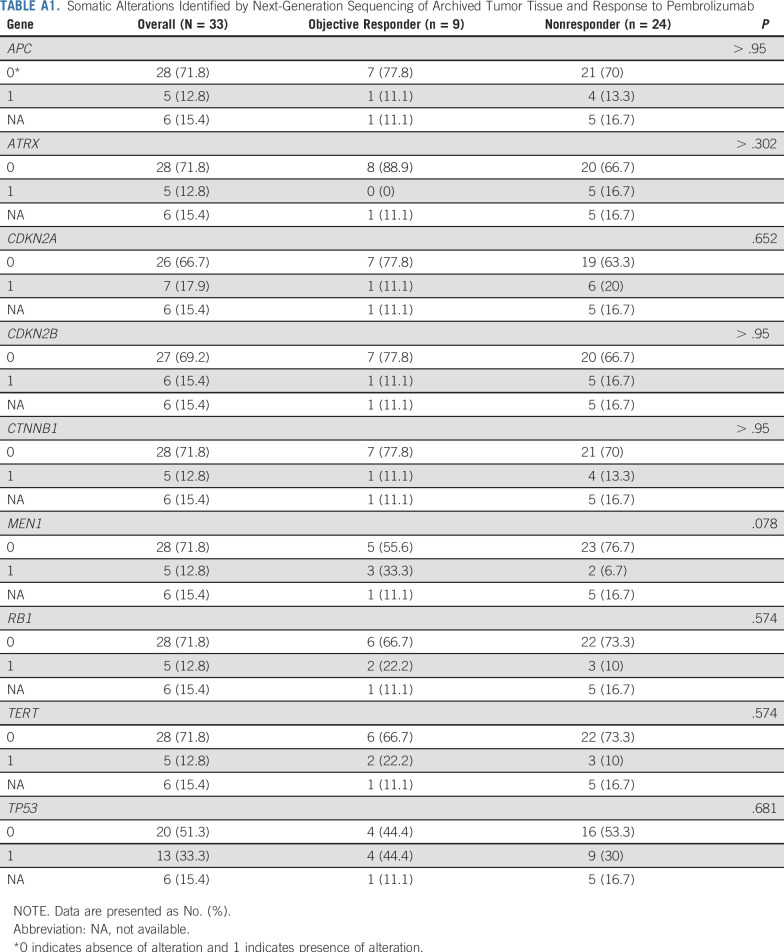
FIG 2.
Exceptional responses to pembrolizumab in advanced adrenocortical carcinoma. (A) Baseline imaging in a patient before initiation of treatment with pembrolizumab, with tumor inferior vena cava invasion with cephalad extension of the tumor into the right atrium. The patient subsequently received a single dose of pembrolizumab, with treatment complicated by immune-mediated elevation of the liver function tests and hospitalization, and additional treatment with pembrolizumab stopped per investigator discretion. Imaging performed at week 70 after the single dose of pembrolizumab demonstrates continued objective response (−45% by RECIST v1.1). In germline genetic testing, this patient was found to have Lynch syndrome (germline MSH6 c.3261delC exon 5 alteration), which was undiagnosed before entry in the study. (B) Baseline imaging in a patient before initiation of treatment with pembrolizumab, with retroperitoneal metastases noted. This patient developed grade 2 immune-mediated elevated liver function tests and stopped additional treatment per investigator discretion after two cycles. At week 17, this patient presented with clinical hyperpigmentation of the lips, tongue, and inner oral mucosal membrane, with imaging at that time demonstrating continued objective response (−58% by RECIST v1.1).
Two patients demonstrating PRs discontinued therapy before 24 months of treatment per investigator discretion. Patient 1 received a single dose of pembrolizumab and experienced grade 3 LFT elevation; germline testing identified an MSH6 deletion consistent with Lynch syndrome (LS). Before treatment, the patient’s disease harbored an aggressive course; imaging 5.5 weeks after receiving pembrolizumab demonstrated response (Fig 2). Because of the early tumor reduction and LFT elevation, the patient did not receive additional pembrolizumab and remains off therapy now, more than 2.5 years after the single treatment dose. Patient 2 discontinued therapy when grade 2 LFT elevation was observed after two treatment cycles. The patient was treated with corticosteroids, and imaging at 9 weeks demonstrated tumor reduction; no additional treatment was administered, and the disease continues to respond off therapy almost 1 year after the second pembrolizumab dose (Fig 2).
Molecular Analyses
Pretreatment, archival tumor tissue and blood normals were collected for correlative testing. Seven of 34 tested tumors (21%) were PD-L1 positive. Response was independent of PD-L1 status (P > .95). ORR in PD-L1–positive tumors was 29% (95% CI, 4% to 71%); ORR in PD-L1–negative tumors was 26% (95% CI, 12% to 47%; Fig 3).
FIG 3.
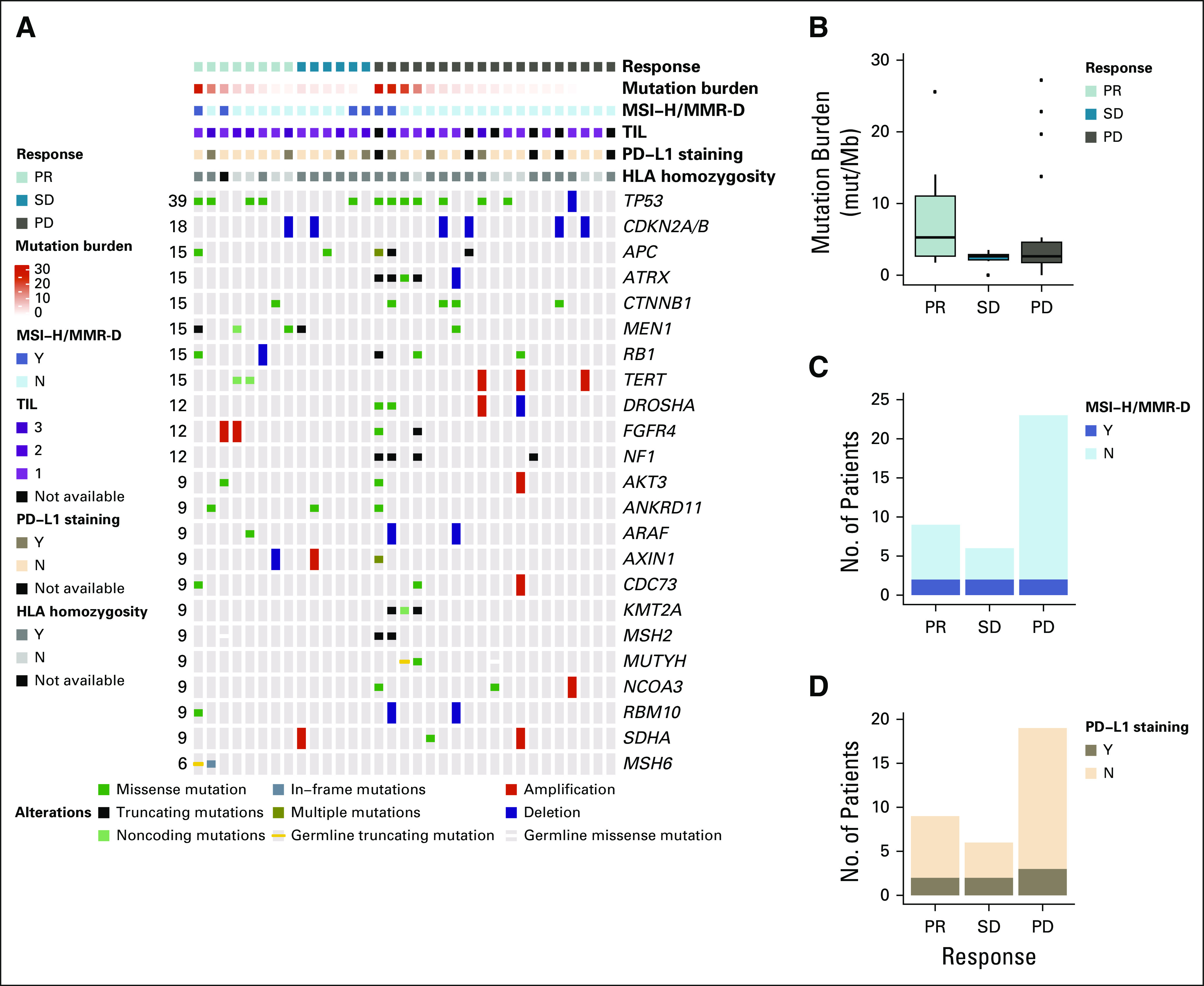
Pathologic and genomic correlates of response. (A) Oncoprint showing the genomic landscape of advanced adrenocortical carcinomas treated with pembrolizumab in this study, as identified by Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT). (B, C, and D) Tumor mutation burden, tumor microsatellite-high/mismatch repair-deficient (MSI-H/MMR-D) status, and tumor programmed death-ligand 1 (PD-L1) status in relation to objective response to pembrolizumab. In correlative analyses, there was no association between genetic alterations, tumor mutation burden, tumor MSI-H/MMR-D status, or tumor PD-L1 status with objective response. Mut/Mb, mutations/megabase; PD, progression of disease by RECIST v1.1; PR, partial response by RECIST v1.1; SD, stable disease by RECIST v1.1; TIL, tumor-infiltrating lymphocyte.
Six of 38 tested tumors (16%) were MSI-H/MMR-D; two patients demonstrated PRs, two demonstrated SD, and two demonstrated rapid progression (prior to radiographic assessment). In the two patients with progression, one was heavily pretreated, presenting after 17 months of therapy. This patient expired after one dose of pembrolizumab (MSIsensor 16.62, MSH2/6 loss by IHC). The second patient’s tumor was MMR proficient by IHC and MSI-H on the basis of MSI-sensor ≥ 3 and TMB > 10. Tumor MSI-H/MMR-D status was not a significant marker predictive of response (P = .61; Fig 3).
NGS results are listed in Figure 3. There was no relationship between any particular somatic alteration and response (Appendix Table A1, online only). All tested tumors had a TIL score of at least 1. There was no relationship between a TIL score of 1 versus TIL score of 2 or 3 and response (P = .22). Median TMB was 2.4 mut/Mb (range, 0-31.5 mut/Mb); no significant relationship was observed between TMB and response (P = .25; Fig 3). A trend of increase in homozygosity was seen in nonresponder patients; of the 32 patients analyzed for HLA zygosity, 76% (19 of 25) nonresponders and 43% (three of seven) responders had germline homozygosity or somatic loss of heterozygosity in at least one HLA locus (P = .17). Germline testing identified six likely pathogenic germline alterations; in the patients who did not have LS, no association was noted between germline status and response (Appendix Table A2, online only).
DISCUSSION
Our results demonstrate that pembrolizumab provides noteworthy clinical efficacy with dramatically improved safety profile when compared with other therapies.9 We observed a substantial ORR (23%) and disease control rate (52%) during treatment, associated with a median OS of 24.9 months. Our findings are more favorable than those of a phase I study recently published in metastatic ACC, in which a 6% ORR was observed during treatment with avelumab (with 50% of the treated patients also continuing mitotane), with 42% of patients experiencing SD; these patients all previously received platinum-based chemotherapy (average of two lines of prior systemic therapy; 74% received two or more lines).29 Our study included patients in any treatment line (31% with more than one prior therapy line) and required mitotane discontinuation and therefore had fewer AEs (59% v 82%). A reason for our higher response rate may be that many patients had received earlier treatment; although our responders had all had at least one prior therapy line, three (33%) had received platinum-based therapy and eight (89%) had received mitotane (Fig 1). In the phase I cohort, ORR was 15% in patients who had received only one prior therapy line.29
Tumor MSI-H/MMR-D status is a known predictive biomarker of response to immune-based therapies.30,31 Six patients in this study had MSI-H/MMR-D tumors, and two patients, both with LS, demonstrated PRs. Our findings confirm earlier observations of objective response to immune-based therapies in MSI-H/MMR-D tumors and the observation that ACC can arise in conjunction with LS.32-36 In our treated cohort, we observed a notable MSI-H/MMR-D rate (16%), and our LS rate (6%) is similar to that in other tumors, including colorectal and endometrial cancers; separate investigation in patients with ACC through The Cancer Genome Atlas Research Atlas and others, and via MSIsensor, has demonstrated a notable prevalence of both LS and MSI in this disease.37-42 Taken together, the findings illustrate that MSI-H/MMR-D screening should be standard practice for patients with advanced ACC, specifically to evaluate for LS. Four MSI-H/MMR-D ACC tumors did not respond, which may have been in part because of the advanced nature of the disease when pembrolizumab was administered. Notably, most patients with objective responses in this study (78%) did not have LS and had microsatellite-stable (MSS) tumors. Our finding of long-term, highly durable disease control with pembrolizumab is novel and of importance. To date, only one of seven responding patients with MSS tumors later had disease progression. Therefore, the short PFS of the study should be interpreted with caution because those patients who do not respond do so early, affecting the median PFS.
To identify additional biomarkers of response, outcomes were explored by tumor PD-L1 status and genomic correlates. There was no significant association between tumor PD-L1 status, TMB, TIL score, or somatic alterations and response. A trend was noted of an increase in homozygosity in nonresponder patients; this has been previously described and warrants additional investigation.43
This study was based on our understanding that immunomodulatory drug treatment can result in endocrine-related AEs, including adrenalitis.18 Two enrolled patients, both of whom had PRs, experienced notable hyperpigmentation; to our knowledge, this observation has not been described in patients with response to anti–PD-1 therapies. Bloodwork in these patients demonstrated elevated ACTH levels, with hyperpigmentation suspected to be caused by ACTH binding to the melanocortin receptor, triggering melanocyte differentiation.44 All patients with objective responses in this study also had immune-related LFT elevation. These observations highlight that in ACC, host-related factors (PD-L1 inhibition in a presumed immunogenic environment), as described in other cancers, may help inform outcomes during treatment with immunologic drugs.45,46 Some patients in this study received sustained responses with as little as one or two doses of pembrolizumab, suggesting that continuous drug exposure may be unnecessary. All patients with an objective response had low- to moderate-volume disease, suggesting that these responders may have had a more indolent growth pattern.
Study limitations include the heterogeneity of our population, specifically the inclusion of patients in any treatment line, varying degrees of aggressiveness of disease, and the absence of a standard-of-care comparator. However, despite these limitations, response to pembrolizumab was seen. The heterogeneity could also explain the absence of correlates of response. Given the rarity of ACC, these limitations are in line with the challenges of conducting clinical studies in orphan diseases.
Our findings demonstrate that MSI-H/MMR-D screening should be standard practice for patients with advanced ACC and confirm earlier observations of activity for pembrolizumab in MSI-H/MMR-D tumors. Most importantly, we demonstrate the novel finding of clinically meaningful and durable activity in advanced ACC that is MSS. The results demonstrate that pembrolizumab is an effective treatment option for patients with advanced ACC.
APPENDIX
FIG A1.
Study design. (*) All patients who discontinued study treatment proceeded to the follow-up phase. ACC, adrenocortical carcinoma.
FIG A2.
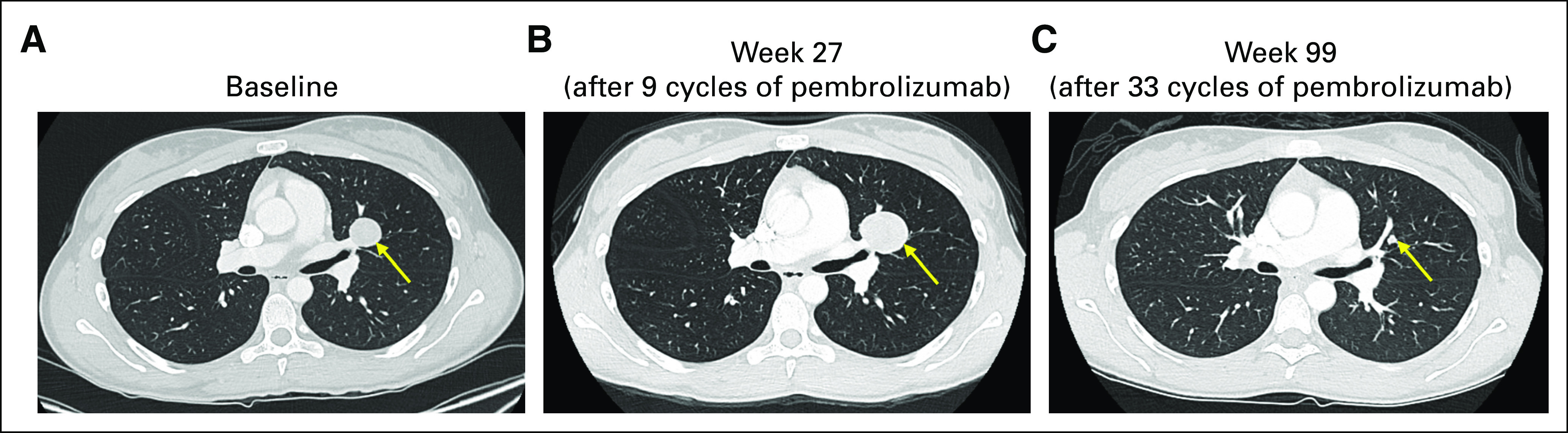
Pseudoprogression in advanced adrenocortical carcinoma during treatment with pembrolizumab. Two patients with objective responses to pembrolizumab experienced growth initially in target and nontarget lesions before response. (A) A left-sided lung metastasis observed on baseline imaging in an enrolled patient prior to initiation of pembrolizumab. (B) RECIST-defined progression (+20%) in this lung metastasis at week 27 on treatment. Objective partial response by RECIST was first noted at week 45 (−42%). (C) The lung metastasis at week 99 on treatment (−73%). A second patient enrolled in the study was also noted to have growth in the disease at week 9 (+19%); at that time, the patient was clinically feeling well, gaining weight, with excellent energy and appetite, and treatment with pembrolizumab continued. In this second patient, imaging at week 18 subsequently demonstrated a partial response by RECIST (−39%).
TABLE A1.
Somatic Alterations Identified by Next-Generation Sequencing of Archived Tumor Tissue and Response to Pembrolizumab
TABLE A2.
Pathogenic or Likely Pathogenic Germline Alterations in the Treated Patients (N = 33)
Footnotes
Supported by Merck & Co., Cycle for Survival, the Drew O’Donoghue Fund, and the National Cancer Institute MSK Cancer Core Grant (P30-CA008748).Presented in part as a poster presentation at the 2019 ASCO Annual Meeting in Chicago, IL, May 31-June 4, 2019.
Clinical trial information: NCT02673333.
AUTHOR CONTRIBUTIONS
Conception and design: Nitya Raj, Leonard B. Saltz, Charlotte E. Ariyan, Kelly Olino, Neil H. Segal, Diane L. Reidy-Lagunes
Financial support: Diane L. Reidy-Lagunes
Administrative support: Virginia Kelly, Diane L. Reidy-Lagunes
Provision of study material or patients: Nitya Raj, Eileen M. O'Reilly, Diane L. Reidy-Lagunes
Collection and assembly of data: Nitya Raj, Virginia Kelly, Seth S. Katz, Richard K.G. Do, Leonard B. Saltz, Eileen M. O'Reilly, Neil H. Segal, Diane L. Reidy-Lagunes
Data analysis and interpretation: Nitya Raj, Youyun Zheng, Joanne Chou, Marinela Capanu, Dmitriy Zamarin, Leonard B. Saltz, Brian R. Untch, Eileen M. O'Reilly, Anuradha Gopalan, Michael F. Berger, Kelly Olino, Neil H. Segal, Diane L. Reidy-Lagunes
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
PD-1 Blockade in Advanced Adrenocortical Carcinoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Nitya Raj
Research Funding: Novartis (Inst), Xencor (Inst)
Seth S. Katz
Stock and Other Ownership Interests: Edwards LifeSciences, Editas Medicine, Intellia Therapeutics, CRISPR Therapeutics, Sanofi, Quest Diagnostics (I)
Richard K.G. Do
Honoraria: Bayer, ALK (I)
Consulting or Advisory Role: DBV (I)
Patents, Royalties, Other Intellectual Property: UptoDate chapters on Food Allergy (I)
Dmitriy Zamarin
Employment: Acorda Therapeutics (I)
Consulting or Advisory Role: BioMed Valley Discoveries, Merck, PsiOxus Therapeutics, Synlogic, Western Oncolytics, Tesaro, Agenus, Trieza Therapeutics, ACM Biolabs
Research Funding: Merck
Patents, Royalties, Other Intellectual Property: I hold a patent regarding the use of recombinant Newcastle Disease Virus (NDV) for cancer therapy (Inst)
Travel, Accommodations, Expenses: Roche
Leonard B. Saltz
Consulting or Advisory Role: McNeil PPC (I)
Research Funding: Taiho Pharmaceutical
Charlotte E. Ariyan
Employment: Pfizer (I)
Stock and Other Ownership Interests: Pfizer (I)
Speakers' Bureau: Bristol-Myers Squibb
Eileen M. O'Reilly
Consulting or Advisory Role: Ipsen, Merck
Research Funding: AstraZeneca/MedImmune (Inst)
Michael F. Berger
Consulting or Advisory Role: Roche
Research Funding: Illumina
Neil H. Segal
Consulting or Advisory Role: Bristol-Myers Squibb, Pfizer, AstraZeneca/MedImmune, Imugene, Roche/Genentech, Pieris Pharmaceuticals, Synlogic, Aduro Biotech, Kyn Therapeutics, Boehringer Ingelheim, Merck, PureTech, Horizon Pharma, EMD Serono, Gritstone Oncology, Chugai Pharma, TRM Oncology, IFM Therapeutics, PsiOxus Therapeutics, CStone Pharmaceuticals
Research Funding: MedImmune, Bristol-Myers Squibb, Pfizer, Roche/Genentech, Merck, Incyte
Diane L. Reidy-Lagunes
Honoraria: Novartis
Consulting or Advisory Role: Ipsen, Novartis, Lexicon, AAA
Research Funding: Novartis, Ipsen
No other potential conflicts of interest were reported.
REFERENCES
- 1.Lafemina J, Brennan MF. Adrenocortical carcinoma: Past, present, and future. J Surg Oncol. 2012;106:586–594. doi: 10.1002/jso.23112. [DOI] [PubMed] [Google Scholar]
- 2.Schulick RD, Brennan MF. Long-term survival after complete resection and repeat resection in patients with adrenocortical carcinoma. Ann Surg Oncol. 1999;6:719–726. doi: 10.1007/s10434-999-0719-7. [DOI] [PubMed] [Google Scholar]
- 3.Reidy-Lagunes DL, Lung B, Untch BR, et al. Complete responses to mitotane in metastatic adrenocortical carcinoma-a new look at an old drug. Oncologist. 2017;22:1102–1106. doi: 10.1634/theoncologist.2016-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lubitz JA, Freeman L, Okun R. Mitotane use in inoperable adrenal cortical carcinoma. JAMA. 1973;223:1109–1112. [PubMed] [Google Scholar]
- 5.von Slooten H, van Seters AP, Smeenk D, et al. O,p′-DDD (mitotane) levels in plasma and tissues during chemotherapy and at autopsy. Cancer Chemother Pharmacol. 1982;9:85–88. doi: 10.1007/BF00265384. [DOI] [PubMed] [Google Scholar]
- 6.Haak HR, Hermans J, van de Velde CJ, et al. Optimal treatment of adrenocortical carcinoma with mitotane: Results in a consecutive series of 96 patients. Br J Cancer. 1994;69:947–951. doi: 10.1038/bjc.1994.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terzolo M, Pia A, Berruti A, et al. Low-dose monitored mitotane treatment achieves the therapeutic range with manageable side effects in patients with adrenocortical cancer. J Clin Endocrinol Metab. 2000;85:2234–2238. doi: 10.1210/jcem.85.6.6619. [DOI] [PubMed] [Google Scholar]
- 8.Daffara F, De Francia S, Reimondo G, et al. Prospective evaluation of mitotane toxicity in adrenocortical cancer patients treated adjuvantly. Endocr Relat Cancer. 2008;15:1043–1053. doi: 10.1677/ERC-08-0103. [DOI] [PubMed] [Google Scholar]
- 9.Fassnacht M, Terzolo M, Allolio B, et al. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366:2189–2197. doi: 10.1056/NEJMoa1200966. [DOI] [PubMed] [Google Scholar]
- 10.O’Sullivan C, Edgerly M, Velarde M, et al. The VEGF inhibitor axitinib has limited effectiveness as a therapy for adrenocortical cancer. J Clin Endocrinol Metab. 2014;99:1291–1297. doi: 10.1210/jc.2013-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berruti A, Sperone P, Ferrero A, et al. Phase II study of weekly paclitaxel and sorafenib as second/third-line therapy in patients with adrenocortical carcinoma. Eur J Endocrinol. 2012;166:451–458. doi: 10.1530/EJE-11-0918. [DOI] [PubMed] [Google Scholar]
- 12.Kroiss M, Quinkler M, Johanssen S, et al. Sunitinib in refractory adrenocortical carcinoma: A phase II, single-arm, open-label trial. J Clin Endocrinol Metab. 2012;97:3495–3503. doi: 10.1210/jc.2012-1419. [DOI] [PubMed] [Google Scholar]
- 13.Wortmann S, Quinkler M, Ritter C, et al. Bevacizumab plus capecitabine as a salvage therapy in advanced adrenocortical carcinoma. Eur J Endocrinol. 2010;162:349–356. doi: 10.1530/EJE-09-0804. [DOI] [PubMed] [Google Scholar]
- 14.Lerario AM, Worden FP, Ramm CA, et al. The combination of insulin-like growth factor receptor 1 (IGF1R) antibody cixutumumab and mitotane as a first-line therapy for patients with recurrent/metastatic adrenocortical carcinoma: A multi-institutional NCI-sponsored trial Horm Cancer 5232–239.2014[Erratum: Horm Cancer 5:424, 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fassnacht M, Berruti A, Baudin E, et al. Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: A double-blind, randomised, phase 3 study. Lancet Oncol. 2015;16:426–435. doi: 10.1016/S1470-2045(15)70081-1. [DOI] [PubMed] [Google Scholar]
- 16.Naing A, Lorusso P, Fu S, et al. Insulin growth factor receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with metastatic adrenocortical carcinoma. Br J Cancer. 2013;108:826–830. doi: 10.1038/bjc.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jasim S, Habra MA. Management of adrenocortical carcinoma. Curr Oncol Rep. 2019;21:20. doi: 10.1007/s11912-019-0773-7. [DOI] [PubMed] [Google Scholar]
- 18.Ryder M, Callahan M, Postow MA, et al. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: A comprehensive retrospective review from a single institution. Endocr Relat Cancer. 2014;21:371–381. doi: 10.1530/ERC-13-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fay AP, Signoretti S, Callea M, et al. Programmed death ligand-1 expression in adrenocortical carcinoma: An exploratory biomarker study. J Immunother Cancer. 2015;3:3. doi: 10.1186/s40425-015-0047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Habra MA, Campbell M, Jimenez C, et al. Efficacy of pembrolizumab (MK-3475) in patients with adrenocortical carcinoma. J Immunother Cancer. 2017;5:P424. [Google Scholar]
- 21.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 22.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17:251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gnirke A, Melnikov A, Maguire J, et al. Solution hybrid selection with ultra-long oligonucleotides for massively parallel targeted sequencing. Nat Biotechnol. 2009;27:182–189. doi: 10.1038/nbt.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagle N, Emery C, Berger MF, et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J Clin Oncol. 2011;29:3085–3096. doi: 10.1200/JCO.2010.33.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagle N, Berger MF, Davis MJ, et al. High-throughput detection of actionable genomic alterations in clinical tumor samples by targeted, massively parallel sequencing. Cancer Discov. 2012;2:82–93. doi: 10.1158/2159-8290.CD-11-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niu B, Ye K, Zhang Q, et al. MSIsensor: Microsatellite instability detection using paired tumor-normal sequence data. Bioinformatics. 2014;30:1015–1016. doi: 10.1093/bioinformatics/btt755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McGranahan N, Rosenthal R, Hiley CT, et al: Allele-specific HLA loss and immune escape in lung cancer evolution. Cell 171:1259-1271, 2017. [DOI] [PMC free article] [PubMed]
- 29.Le Tourneau C, Hoimes C, Zarwan C, et al. Avelumab in patients with previously treated metastatic adrenocortical carcinoma: Phase 1b results from the JAVELIN solid tumor trial. J Immunother Cancer. 2018;6:111. doi: 10.1186/s40425-018-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lynch HT, Shaw MW, Magnuson CW, et al: Hereditary factors in cancer. Study of two large midwestern kindreds. Arch Intern Med 117:206-212, 1966. [PubMed]
- 33.Berends MJW, Cats A, Hollema H, et al. Adrenocortical adenocarcinoma in an MSH2 carrier: Coincidence or causal relation? Hum Pathol. 2000;31:1522–1527. doi: 10.1053/hupa.2000.20409. [DOI] [PubMed] [Google Scholar]
- 34.Karamurzin Y, Zeng Z, Stadler ZK, et al. Unusual DNA mismatch repair-deficient tumors in Lynch syndrome: A report of new cases and review of the literature. Hum Pathol. 2012;43:1677–1687. doi: 10.1016/j.humpath.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Medina-Arana V, Delgado L, González L, et al. Adrenocortical carcinoma, an unusual extracolonic tumor associated with Lynch II syndrome. Fam Cancer. 2011;10:265–271. doi: 10.1007/s10689-010-9416-8. [DOI] [PubMed] [Google Scholar]
- 36.Broaddus RR, Lynch PM, Lu KH, et al. Unusual tumors associated with the hereditary nonpolyposis colorectal cancer syndrome. Mod Pathol. 2004;17:981–989. doi: 10.1038/modpathol.3800150. [DOI] [PubMed] [Google Scholar]
- 37.Hampel H, Frankel W, Panescu J, et al. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res. 2006;66:7810–7817. doi: 10.1158/0008-5472.CAN-06-1114. [DOI] [PubMed] [Google Scholar]
- 38.Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005;352:1851–1860. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 39.Moreira L, Balaguer F, Lindor N, et al. Identification of Lynch syndrome among patients with colorectal cancer. JAMA. 2012;308:1555–1565. doi: 10.1001/jama.2012.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raymond VM, Everett JN, Furtado LV. Adrenocortical carcinoma is a Lynch syndrome-associated cancer. J Clin Oncol. 2013;31:3012–3018. doi: 10.1200/JCO.2012.48.0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng S, Cherniack AD, Dewal N, et al. Comprehensive pan-genomic characterization of adrenocortical carcinoma Cancer Cell 29723–736.2016[Erratum: Cancer Cell, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Latham A, Srinivasan P, Kemel Y, et al. Microsatellite instability is associated with the presence of Lynch syndrome pan-cancer. J Clin Oncol. 2019;37:286–295. doi: 10.1200/JCO.18.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chowell D, Morris LGT, Grigg CM, et al: Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science 359:582-587, 2018. [DOI] [PMC free article] [PubMed]
- 44.González-Rodríguez E, Rodríguez-Abreu D, Spanish Group for Cancer Immuno-Biotherapy (GETICA) Immune checkpoint inhibitors: Review and management of endocrine adverse events. Oncologist. 2016;21:804–816. doi: 10.1634/theoncologist.2015-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snyder A, Nathanson T, Funt SA, et al. Contribution of systemic and somatic factors to clinical response and resistance to PD-L1 blockade in urothelial cancer: An exploratory multi-omic analysis. PLoS Med. 2017;14:e1002309. doi: 10.1371/journal.pmed.1002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152:45–51. doi: 10.1001/jamadermatol.2015.2707. [DOI] [PubMed] [Google Scholar]