Abstract
PURPOSE
Talazoparib has demonstrated efficacy in patients with BRCA-positive metastatic breast cancer. This study evaluated the pathologic response of talazoparib alone for 6 months in patients with a known germline BRCA pathogenic variant (gBRCA-positive) and operable breast cancer.
METHODS
Eligibility included 1 cm or larger invasive tumor and gBRCA-positive disease. Human epidermal growth factor receptor 2–positive tumors were excluded. Twenty patients underwent a pretreatment biopsy, 6 months of once per day oral talazoparib (1 mg), followed by definitive surgery. Patients received adjuvant therapy at physician’s discretion. The primary end point was residual cancer burden (RCB). With 20 patients, the RCB-0 plus RCB-I response rate can be estimated with a 95% CI with half width less than 20%.
RESULTS
Twenty patients were enrolled from August 2016 to September 2017. Median age was 38 years (range, 23 to 58 years); 16 patients were gBRCA1 positive and 4 patients were gBRCA2 positive. Fifteen patients had triple-negative breast cancer (estrogen receptor/progesterone receptor < 10%), and five had hormone receptor-positive disease. Five patients had clinical stage I disease, 12 had stage II, and three had stage III, including one patient with inflammatory breast carcinoma and one with metaplastic chondrosarcomatous carcinoma. One patient chose to receive chemotherapy before surgery and was not included in RCB analyses. RCB-0 (pathologic complete response) rate was 53% and RCB-0/I was 63%. Eight patients (40%) had grade 3 anemia and required a transfusion, three patients had grade 3 neutropenia, and 1 patient had grade 4 thrombocytopenia. Common grade 1 or 2 toxicities were nausea, fatigue, neutropenia, alopecia, dizziness, and dyspnea. Toxicities were managed by dose reduction and transfusions. Nine patients required dose reduction.
CONCLUSION
Neoadjuvant single-agent oral talazoparib once per day for 6 months without chemotherapy produced substantial RCB-0 rate with manageable toxicity. The substantive pathologic response to single-agent talazoparib supports the larger, ongoing neoadjuvant trial (ClinicalTrials.gov identifier: NCT03499353).
INTRODUCTION
Poly-(adenosine diphosphate [ADP]-ribose) polymerase (PARP) is a family of enzymes responsible for cellular activities such as DNA repair via base excision repair pathway and genetic stability.1 The use of PARP inhibitors has been extensively evaluated in patients with multiple metastatic cancers and first achieved US Food and Drug Administration approval for advanced ovarian cancer. Although early studies also included patients with breast cancer, it was not until 2018 that PARP inhibitors were approved by the US Food and Drug Administration for a metastatic/locally advanced breast cancer indication.
Two randomized phase III trials have reported PARP inhibitor efficacy in comparison with physician’s choice of chemotherapy for patients with locally advanced/metastatic breast cancer and a germline BRCA pathogenic variant (gBRCA-positive). The OlympiAD (ClinicalTrials.gov identifier: NCT02000622) trial evaluated olaparib at 300 mg orally twice per day versus capecitabine, eribulin, or vinorelbine. Olaparib improved progression-free survival compared with standard chemotherapy, with a hazard ratio of 0.58 (95% CI, 0.43 to 0.80).2 The EMBRACA trial (ClinicalTrials.gov identifier: NCT01945775) evaluated talazoparib 1 mg orally once per day, also randomized versus physician’s choice of chemotherapy (gemcitabine, eribulin, capecitabine, and vinorelbine), and also showed a significant improvement in progression-free survival, with a hazard ratio of 0.54 (95% CI, 0.42 to 0.71).3 Notably, both of these trials demonstrated improvements in quality of life as well as decrease in time to meaningful deterioration for patients treated with the PARP inhibitors, in comparison with standard chemotherapy.4
To estimate tumor response to single-agent PARP inhibitors and assess drug effect in human tumors, a pilot trial at The University of Texas MD Anderson Cancer Center evaluated the effects of 2 months of neoadjuvant talazoparib before initiating standard neoadjuvant chemotherapy in gBRCA-positive patients with stage I to III breast cancer.5 The primary end point of that pilot study was to determine feasibility of accruing 20 patients over 2 years, thus measuring patient acceptance of delaying chemotherapy by 2 months for single-agent targeted therapy. Within 8 months, 13 patients were accrued to the trial. Two months of treatment with single-agent talazoparib resulted in a median decrease of tumor volume, as measured by breast ultrasound, of 88% (range, 30% to 98%). After additional evaluation of the study accrual rate, response, and lack of grade 4 toxicities, the study was halted early and the results were published. On the basis of these radiographic responses with only 2 months of therapy, this separate study of 20 patients was proposed to evaluate a pathologic response from only talazoparib. The objective of this neoadjuvant trial was to evaluate the pathologic response and toxicity to single-agent talazoparib for 6 months in 20 patients with stage I to III breast cancer and who were gBRCA-positive before definitive surgery.
METHODS
This was a pilot study including 20 patients to obtain preliminary data to power a larger trial. Talazoparib was administered as single-agent oral dose of 1 mg per day for six cycles (each cycle was 28 days). The primary objective was pathologic response after 6 months of talazoparib. The secondary objective was evaluation of toxicity. Patients were identified for this trial if they had a germline BRCA1 or BRCA2 pathogenic variant as identified by a Clinical Laboratory Improvement Amendments–certified laboratory and stage I to III breast cancer. The primary tumor had to be 1 cm or larger. Imaging for each tumor included at least a mammogram and ultrasound. Biopsy was performed on suggestive nodes identified on ultrasound to confirm involvement. The tumor could have any hormone receptor (HR) status, but human epidermal growth factor receptor 2 fluorescence in situ hybridization amplified or 3+ by immunohistochemistry (as per ASCO/College of American Pathologists guideline) were excluded.6 Patients also were excluded if they had previous surgery, radiation, or systemic therapy for breast cancer. Exceptions were made for prior surgery for ductal carcinoma in situ or if the patient was at least 5 years from the treatment of a previous nonbreast malignancy. This trial was performed after approval by the institutional review board, and written informed consent was obtained from each participant. This trial was conducted under an institutional review board–approved protocol 2014-0045 and in accordance with relevant guidelines at The University of Texas MD Anderson Cancer Center.
Talazoparib was administered at a starting dose of 1 mg per day. Patients were considered evaluable if they received at least 4 months of talazoparib therapy and then proceeded to surgery within 6 weeks from the date of the last dose of talazoparib. One patient received 5 months of therapy, but with a lymph node enlarging she refused additional biopsy and proceeded instead to systemic chemotherapy before surgery. Her information is included for toxicity but not for the primary end point of pathologic response. Pathologic response was documented using the Residual Cancer Burden (RCB) Calculator (www.mdanderson.org/breastcancer_RCB).7
Toxicities were monitored and recorded per the Common Terminology Criteria for Adverse Events version 4.03. Toxicities were reported with the highest grade observed per individual. Patients could not initiate therapy if hemoglobin was less than 8.0 gm/dL. Dose reductions of 0.25 mg/d were made for grade 3 or 4 toxicity as per protocol.
RESULTS
Nineteen patients completed 6 months of therapy before surgery, and one patient received 5 months of therapy and then received chemotherapy before surgery. Patients participated in this study beginning in August 2016, and the last patient started treatment in September 2017. Patient characteristics are listed in Table 1. All patients were women, although men were eligible to participate in this study.
TABLE 1.
Patient Characteristics

For the 19 patients who had pathologic response outcome data, 10 had RCB-0 (pathologic complete response [pCR]), which correlates to no invasive disease in breast and lymph nodes. Two patients had an RCB-I, five had RCB-II, and three had RCB-III. The RCB-0/pCR rate was 53% (95% CI, 32% to 73%) and RCB-0/I was 63% (95% CI, 41% to 81%). RCB-0 and RCB-I were seen across both BRCA1 and BRCA2 as well as in HR-positive and triple-negative breast cancer (TNBC). In these subgroups, the percentages of RCB-0/I were: TNBC 57% (95% CI, 29% to 82%), HR positive 80% (95% CI, 28% to 99%), T1 tumors 83% (95% CI, 36% to 100%), and T2 or greater 54% (95% CI, 25% to 81%). For patients with BRCA1-positive disease, RCB-0/I disease was 53% (95% CI, 27% to 79%), and all of the BRCA2 patients had RCB-0/I response (95% CI, 40% to 100%). Of note, the one patient with metaplastic chondrosarcomatous carcinoma, one patient with invasive lobular carcinoma, and a third patient with inflammatory breast cancer all had a pCR/RCB-0 response to talazoparib. Table 2 lists the response per patient, with information regarding their clinical stage, HR status, pathologic response, and systemic therapy after surgery.
TABLE 2.
Tumor Responses per Patient
Toxicity
There were 12 grade 3 toxicities and one grade 4 toxicity. Anemia and nausea were the most common toxicities experienced, and a full list of recorded toxicities can be found in Table 3. Eight patients required transfusions during the course of the therapy. Figure 1 demonstrates hemoglobin levels as trended over time for the eight patients who required transfusions. A total of 29 units were transfused, with 1 to 2 units per transfusion mostly post cycles 2 through 6 of talazoparib. One patient required 1 unit one time, three patients required 2 units one time, two patients required 2 units twice, and two patients required 2 units three times. None of the patients reported a change in their menstrual cycles while taking talazoparib.
TABLE 3.
Toxicity

FIG 1.
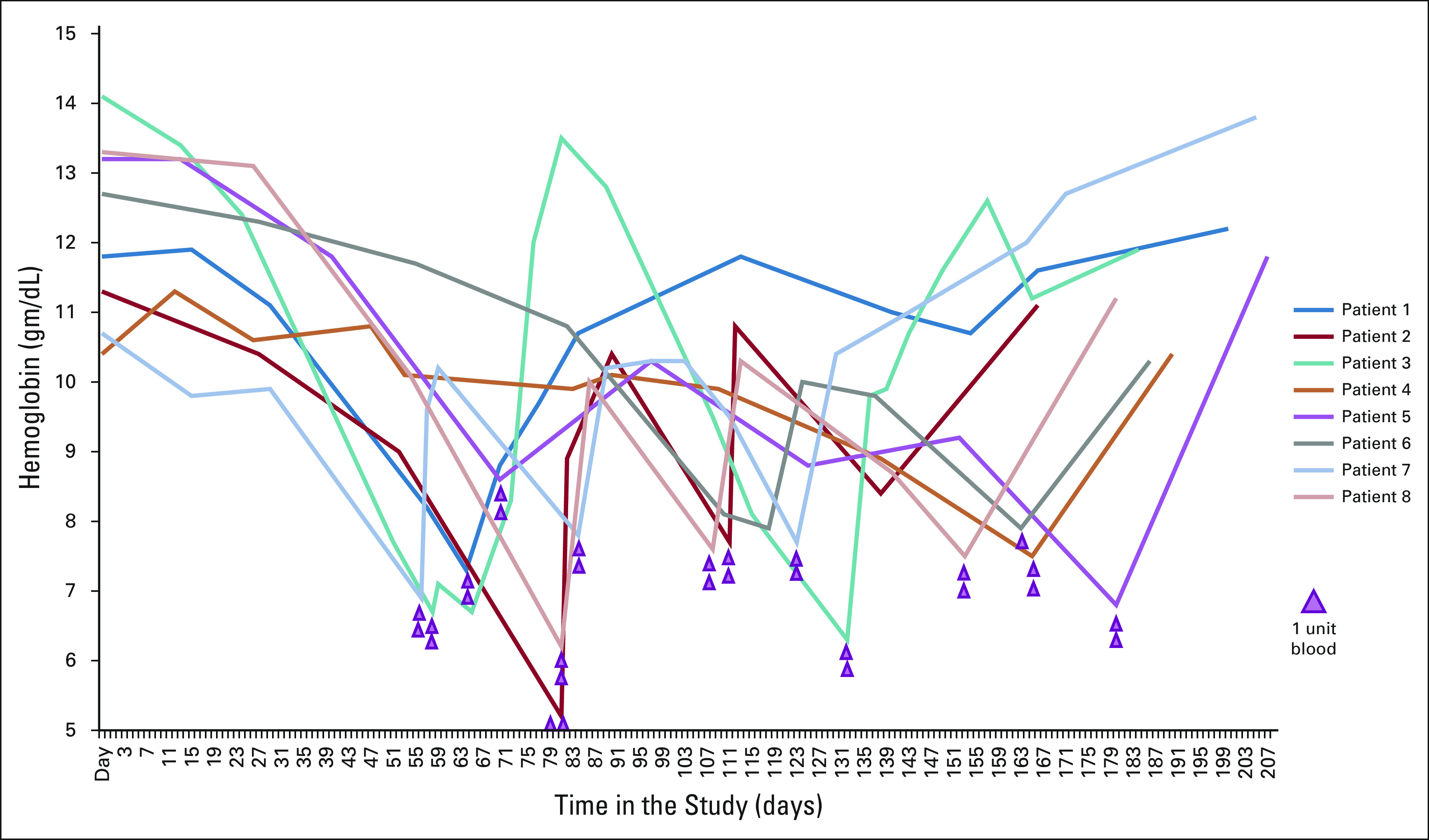
Hemoglobin in the eight patients who required transfusions.
Dose Reductions
Eleven patients completed therapy at the full dose of 1 mg, two patients completed at a reduced dose of 0.75 mg, six patients completed the study at a dose level of 0.5 mg, and one patient completed the study at a dose level of 0.25 mg. All of the dose reductions were the result of hematologic toxicity.
Compliance
Nine patients experienced dose delays, with a median delay of 17 days (range, 8 to 41 days). Eleven patients received doses as scheduled. Nine patients missed one to three doses that were not planned or protocol mandated during the study, and no patients missed more than three doses that were not planned or protocol mandated.
DISCUSSION
This trial of 20 gBRCA-positive patients who received single-agent oral talazoparib before definitive surgery for early-stage breast cancer demonstrated the ability of a single targeted agent to achieve pCR in a gBRCA-positive patient population. Given the excellent prognosis associated with achieving pCR and RCB-I with standard chemotherapy,8 the 53% rate of pCR and 63% rate of pCR/RCB-I with single-agent talazoparib is encouraging. Additional studies, however, are needed to determine if pCR to PARP inhibitor therapy has the same favorable prognosis as that seen with chemotherapy. Importantly, excellent pathologic responses were seen across BRCA mutation types, in both HR-positive and TNBC tumors, as well as subtypes known for chemotherapy resistance to neoadjuvant therapy, such as inflammatory breast cancer, metaplastic cancer, and invasive lobular carcinoma. Although the numbers are small, the preliminary data suggest that patients with HR-positive and BRCA-positive tumors may respond better, a suggestion also seen in the EMBRACA trial.
The response to neoadjuvant chemotherapy in patients with gBRCA-positive cancer has been described in multiple series. In a cohort of patients with gBRCA-positive breast cancer from The University of Texas MD Anderson Cancer Center, 26 (46%) of 57 patients achieved a pCR with mostly third-generation chemotherapy regimens.9 Silver et al10 described 28 women with TNBC who received four cycles of cisplatin at 75 mg/m2 every 21 days, with a pCR rate of 22%. Both of the gBRCA-positive patients in this cohort had a pCR. Byrski et al11 reported a trial of 107 women with a gBRCA1 mutation who received cisplatin 75 mg/m2 every 3 weeks for four cycles. The overall pCR rate was 61%. The toxicity reported in the article described early discontinuation of therapy in five patients and mostly grade 1 and 2 toxicities, including tinnitus. Interestingly, in a subset of 50 gBRCA-positive patients from the GeparSixto (ClinicalTrials.gov identifier: NCT0146880) trial, pCR was 66.7% and was not improved with the addition of carboplatin.12 The INFORM (ClinicalTrials.gov identifier: NCT01670500) trial has completed accrual, and results are anticipated to directly compare platinum versus doxorubicin and cyclophosphamide (AC) chemotherapy in patients with gBRCA-positive breast cancer.
Given earlier signs of efficacy for patients with gBRCA-positive metastatic breast cancer in multiple phase I and II trials, PARP inhibitors have also been evaluated in the neoadjuvant setting for treatment of early-stage breast cancer.13 The I-SPY 2 (ClinicalTrials.gov identifier: NCT01042379) trial evaluated the neoadjuvant combination of the PARP inhibitor veliparib with carboplatin and paclitaxel, followed by AC using a Bayesian-based adaptive randomization design to compare response to standard taxane plus anthracycline therapy.13 The estimated pCR rate for the experimental arm was 33% versus 22% (control) in unselected patients and 51% versus 26% in TNBC, respectively. Given these findings, the BrighTNess (ClinicalTrials.gov identifier: NCT02032277) trial evaluated three neoadjuvant therapeutic strategies: paclitaxel alone, paclitaxel and carboplatin, and paclitaxel, carboplatin, and veliparib, followed by AC in each arm.14 Approximately 15% of the patients in BrighTNess also were gBRCA positive. Although the study was not designed to evaluate differences between the three arms, the arms with carboplatin had a higher pCR, which was not further improved with the addition of veliparib. Notably, combining chemotherapies with PARP inhibitors at dosing levels that still inhibit PARP has been challenging because of overlying hematologic toxicities.
Although therapy was administered in a treatment-naïve, newly diagnosed patient population, toxicities were similar to those previously described in patients treated with talazoparib in the metastatic setting. The most common toxicities seen were anemia and nausea. Anemia was manageable by dosing delay, dose reduction, and transfusion, when indicated. Transfusions were administered in eight of the treated patients, because the drug could not be resumed until the hematologic toxicity resolved to a grade 1. Most transfusions were required during cycles 2 through 6; it is important to monitor serial hematologic profiles throughout treatment with talazoparib. Given the relatively substantial number of patients requiring transfusions, it will be critical not only to monitor this in the larger national trial but also to determine if there is an underlying mechanism for patients with little to no anemia versus those requiring multiple transfusions. Most nonhematologic toxicities were grade 1 and manageable with appropriate supportive treatment.
The study has several limitations. First, this was a small, single-institution trial designed to evaluate the ability to accrue and estimate pCR rates to a neoadjuvant trial of talazoparib in a select patient population of gBRCA-positive patients, resulting in wide confidence intervals for pCR prediction. As such, a larger confirmatory trial is needed to more accurately determine single-agent pCR rates. Second, although toxicity was followed and carefully recorded at each study visit, there were no patient-reported outcome (PRO) instruments used for this study. PROs would be of interest in the larger, confirmatory trial given the significant improvements noted in the PROs in the EMBRACA trial.4 In addition, although patients were asked whether they noticed a change in their menstrual cycles at clinic visits, the trial included no objective measurements, such as checking anti-Müllerian hormone levels pre and post therapy. Such measurements will be critical, because many gBRCA-positive patients develop cancer at younger age, and fertility is an important part of the treatment and survivorship plan. Finally, because this is a single-arm study, treatment can be compared only to historic pathologic response rates and hence cannot directly compare single-agent PARP inhibition versus platinum chemotherapy.
Another important question unanswered by this pilot trial is the optimal postsurgical treatment plan for patients. For the six patients with HR-positive disease, five proceeded to adjuvant endocrine therapy only and one to adjuvant docetaxel and cyclophosphamide followed by endocrine therapy. For these younger patients with TNBC, whether to omit chemotherapy after a pCR cannot be answered by this study, because many did receive adjuvant chemotherapy; this question merits additional investigation. Outcomes and long-term symptoms in a larger, multicenter trial are needed to determine if single-agent talazoparib is sustainable as a de-escalation of therapy.
To the best of our knowledge, this study is the first to show a single-agent, targeted therapy achieved pCR in patients with gBRCA-positive breast cancer, including TNBC and HR-positive breast cancer, without the addition of chemotherapy. Other neoadjuvant trials have failed to show significant benefit for PARP inhibition. However, these trials evaluated PARP and chemotherapy combination trials and were not specific to a gBRCA-positive–only population. Also, they may have limited applicability, because the chosen PARP inhibitor, veliparib, is not as strong a PARP inhibitor or a PARP trapper, as is talazoparib.15 In addition, combining PARP inhibition plus chemotherapy has the challenge of overlapping toxicities, requiring consequent dose reductions. There are multiple other ongoing studies evaluating the use of PARP inhibition in combination with chemotherapy, such as the PARTNER (ClinicalTrials.gov identifier: NCT03499353) trial and the GeparOla trial (ClinicalTrials.gov identifier: NCT02789332), or as part of adjuvant therapy for patients with residual disease in the OlympiA trial (ClinicalTrials.gov identifier: NCT02032823). These and other trials are currently accruing and will enhance our understanding of how PARP inhibitors can further affect the treatment of early breast cancer.
In conclusion, this pilot trial of neoadjuvant talazoparib starting at 1 mg orally once per day for 6 months was used before surgery for early breast cancer, resulting in a pCR rate of 53% and RCB-0/I rate of 63%. The toxicities were mostly hematologic and managed by dosing delay, dose reduction, and blood transfusions. In addition, the 2-month window study with a strong biologic rationale, which quickly can lead into a full neoadjuvant therapy, as done in this instance with talazoparib, may be a novel strategy for developing and de-escalating therapy in the neoadjuvant space. This trial is completing the ongoing larger, multicenter trial (ClinicalTrials.gov identifier: NCT03499353).
ACKNOWLEDGMENT
We thank the patients and their families who participated in this trial; Gordon Mills, MD, PhD, and Sanghoon Lee, PhD, for their contributions to the collection of the patient materials for the correlative studies that will be associated with this clinical trial; Delia Stroud for providing patient advocate support and manuscript review; and The Toomim Family Fund for providing support for this trial.
Footnotes
Presented at the ASCO 2018 Annual Meeting, Chicago, IL, June 1-5, 2018.
Supported by the MD Anderson Moonshots Program, the Toomim Family Fund, Biomarin, Medivation/Pfizer, and National Cancer Institute Cancer Center Support Grant No. P30CA016672 (J.K.L.).
AUTHOR CONTRIBUTIONS
Conception and design: Jennifer K. Litton, Marion E. Scoggins, Vicente Valero, Stacy L. Moulder, Banu K. Arun
Administrative support: Thorunn Helgason
Provision of study material or patients: Beatriz E. Adrada, Sarah M. DeSnyder, Vicente Valero, Nuhad Ibrahim, Jill Schwartz-Gomez, Elizabeth A. Mittendorf, Alastair M. Thompson, Thorunn Helgason, Stacy L. Moulder
Collection and assembly of data: Jennifer K. Litton, Marion E. Scoggins, Beatriz E. Adrada, Senthil Damodaran, Sarah M. DeSnyder, Abenaa M. Brewster, Vicente Valero, Gary J. Whitman, Jill Schwartz-Gomez, Elizabeth A. Mittendorf, Thorunn Helgason, Nuhad Ibrahim, Banu K. Arun
Data analysis and interpretation: Marion E. Scoggins, Kenneth R. Hess, Rashmi K. Murthy, Carlos H. Barcenas, Vicente Valero, Alastair M. Thompson, Thorunn Helgason, Helen Piwnica-Worms, Stacy L. Moulder, Banu K. Arun
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Neoadjuvant Talazoparib for Patients With Operable Breast Cancer With a Germline BRCA Pathogenic Variant
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Jennifer K. Litton
Consulting or Advisory Role: Pfizer, AstraZeneca, Medivation/Pfizer
Speakers' Bureau: Physician Education Resource, UpToDate, Med Learning Group, Medscape
Research Funding: Novartis (Inst), Bristol-Myers Squibb (Inst), Genentech (Inst), Pfizer (Inst), EMD Serono (Inst), Jounce Therapeutics (Inst), GlaxoSmithKline (Inst), Medivation/Pfizer (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Travel, Accommodations, Expenses: Physician Education Resource, Med Learning Group, Medscape
Marion E. Scoggins
Research Funding: General Electric (Inst)
Rashmi K. Murthy
Consulting or Advisory Role: Puma Biotechnology, Genentech
Research Funding: Genentech (Inst), Daiichi Sankyo (Inst), Cascadian Therapeutics (Inst), Pfizer (Inst), EMD Serono (Inst), Seattle Genetics (Inst)
Senthil Damodaran
Honoraria: Novartis
Consulting or Advisory Role: Tempus, Taiho Pharmaceutical, Pfizer
Research Funding: EMD Serono, Guardant Health
Travel, Accommodations, Expenses: Phillips Gilmore Oncology Communications
Sarah M. DeSnyder
Research Funding: ImpediMed
Carlos H. Barcenas
Speakers' Bureau: Genomic Health
Research Funding: Puma Biotechnology (Inst)
Vicente Valero
Honoraria: Genentech
Consulting or Advisory Role: Genentech
Travel, Accommodations, Expenses: Genentech
Gary J. Whitman
Stock and Other Ownership Interests: Pfizer
Elizabeth A. Mittendorf
Honoraria: Physician Education Resource
Consulting or Advisory Role: Peregrine Pharmaceuticals, TapImmune, Sellas Life Sciences, Merck, Genomic Health
Research Funding: Galena Biopharma (Inst), Genentech (Inst), AstraZeneca (Inst), EMD Serono (Inst)
Alastair M. Thompson
Honoraria: Novartis (I)
Honoraria: Pfizer
Research Funding: Genentech (Inst)
Travel, Accommodations, Expenses: Novartis (I), Pfizer
Nuhad Ibrahim
Honoraria: Celgene, Roche, Novartis
Consulting or Advisory Role: Immunomedics
Speakers' Bureau: Celgene, Roche, Novartis
Research Funding: Nektar Therapeutics
Travel, Accommodations, Expenses: Roche, Novartis
Stacy L. Moulder
Honoraria: Novartis, Pfizer (I)
Research Funding: Oncothyreon (Inst), Pfizer (Inst), Novartis (Inst), Genentech (Inst), Takeda (Inst), Bayer (Inst), EMD Serono (Inst), Genentech (Inst)
Travel, Accommodations, Expenses: Novartis, Pfizer (I)
Banu K. Arun
Consulting or Advisory Role: Bright Pink, AbbVie
Research Funding: AbbVie (Inst), PharmaMar (Inst), AstraZeneca (Inst), InVitae (Inst)
Travel, Accommodations, Expenses: AstraZeneca
No other potential conflicts of interest were reported.
REFERENCES
- 1.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 2.Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 3.Litton J.K., Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379:753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ettl J, Quek RGW, Lee KH, et al. Quality of life with talazoparib versus physician’s choice of chemotherapy in patients with advanced breast cancer and germline BRCA1/2 mutation: Patient-reported outcomes from the EMBRACA phase III trial. Ann Oncol. 2018;29:1939–1947. doi: 10.1093/annonc/mdy257. [DOI] [PubMed] [Google Scholar]
- 5.Litton J.K., Scoggins M, Ramirez DL, et al. A feasibility study of neoadjuvant talazoparib for operable breast cancer patients with a germline BRCA mutation demonstrates marked activity. NPJ Breast Cancer. 2017;3:49. doi: 10.1038/s41523-017-0052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolff A.C., Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 7.Symmans W.F., Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 8.Symmans W.F., Wei C, Gould R, et al. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol. 2017;35:1049–1060. doi: 10.1200/JCO.2015.63.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arun B, Bayraktar S, Liu DD, et al. Response to neoadjuvant systemic therapy for breast cancer in BRCA mutation carriers and noncarriers: A single-institution experience. J Clin Oncol. 2011;29:3739–3746. doi: 10.1200/JCO.2011.35.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silver D.P., Richardson AL, Eklund AC, et al. Efficacy of neoadjuvant cisplatin in triple-negative breast cancer. J Clin Oncol. 2010;28:1145–1153. doi: 10.1200/JCO.2009.22.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byrski T, Huzarski T, Dent R, et al. Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat. 2014;147:401–405. doi: 10.1007/s10549-014-3100-x. [DOI] [PubMed] [Google Scholar]
- 12.Hahnen E, Lederer B, Hauke J, et al. Germline mutation status, pathological complete response, and disease-free survival in triple-negative breast cancer: Secondary analysis of the GeparSixto randomized clinical trial. JAMA Oncol. 2017;3:1378–1385. doi: 10.1001/jamaoncol.2017.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rugo H.S., Olopade OI, DeMichele A, et al. Adaptive randomization of veliparib-carboplatin treatment in breast cancer. N Engl J Med. 2016;375:23–34. doi: 10.1056/NEJMoa1513749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loibl S, O’Shaughnessy J, Untch M, et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): A randomised, phase 3 trial. Lancet Oncol. 2018;19:497–509. doi: 10.1016/S1470-2045(18)30111-6. [DOI] [PubMed] [Google Scholar]
- 15.Murai J, Huang SY, Das BB, et al. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 2012;72:5588–5599. doi: 10.1158/0008-5472.CAN-12-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]



