Abstract
PURPOSE
Centralization is often proposed as a strategy to improve the quality of certain high-risk health care services. We evaluated the extent to which existing hospital systems centralize high-risk cancer surgery and whether centralization is associated with short-term clinical outcomes.
PATIENTS AND METHODS
We merged data from the American Hospital Association’s annual survey on hospital system affiliation with Medicare claims to identify patients undergoing surgery for pancreatic, esophageal, colon, lung, or rectal cancer between 2005 and 2014. We calculated the degree to which systems centralized each procedure by calculating the annual proportion of surgeries performed at the highest-volume hospital within each system. We then estimated the independent effect of centralization on the incidence of postoperative complications, death, and readmissions after accounting for patient, hospital, and system characteristics.
RESULTS
The average degree of centralization varied from 25.2% (range, 6.6% to 100%) for colectomy to 71.2% (range, 8.3% to 100%) for pancreatectomy. Greater centralization was associated with lower rates of postoperative complications and death for lung resection, esophagectomy, and pancreatectomy. For example, there was a 1.1% (95% CI, 0.8% to 1.4%) absolute reduction in 30-day mortality after pancreatectomy for each 20% increase in the degree of centralization within systems. Independent of volume and hospital quality, postoperative mortality for pancreatectomy was two times higher in the least centralized systems than in the most centralized systems (8.9% v 3.7%, P < .01). Centralization was not associated with better outcomes for colectomy or proctectomy.
CONCLUSION
Greater centralization of complex cancer surgery within existing hospital systems was associated with better outcomes. As hospitals affiliate in response to broader financial and organization pressures, these systems may also present unique opportunities to improve the quality of high-risk cancer care.
INTRODUCTION
The practice of steering patients toward hospitals or providers with the most experience, commonly referred to as centralization, is often proposed as a strategy to improve outcomes and reduce treatment-related adverse events.1 Centralization of high-risk cancer surgery has already received considerable attention because of the established relationship between surgical volume and mortality for operations like pancreatectomy and esophagectomy.2,3 The Leapfrog Group, a national advocate for hospital transparency and patient safety, has supported minimum-volume standards at hospitals that perform high-risk cancer operations for decades. More recently, several academic health systems took a pledge to restrict these operations to hospitals and surgeons meeting predetermined volume thresholds.4
Europe, France and the Netherlands have successfully implemented centralization policies resulting in significant increases in short- and long-term survival for several GI malignancies.5 However, it remains unclear whether this model can work in the United States. In the absence of specific policies or incentives, hospitals may be insufficiently motivated to shift patients and important sources of revenue to other centers. Recent trends in hospital mergers (which have doubled over the past decade) have created new opportunities for hospital systems to voluntarily organize care around the most experienced providers.6 In doing so, systems could improve outcomes through better adherence to volume standards or by developing regional Centers of Excellence for oncologic services.7,8 More than 60% of United States hospitals already participate in a system, but the impact of this national trend on cancer care is not well characterized.
In this study, we assessed the extent and impact of centralization for high-risk cancer surgery in the United States. Using data on Medicare beneficiaries undergoing five common, complex surgical procedures for cancer, we quantified variation in the degree of centralization among hospital systems. We then evaluated the association between centralization and short-term clinical outcomes and health care use.
PATIENTS AND METHODS
Data Source and Study Population
We used data encompassing 100% of Medicare Part A beneficiaries from Medicare Provider Analysis and Review files for the years 2005 to 2014. We collected data on patient age, demographic information, geographic location, and comorbidities. We included only patients from 65 to 99 years of age. We identified patients undergoing colectomy (1731 to 1736, 1739, 4571 to 4576, 4579, 4580 to 4583), proctectomy (4850 to 4852, 4862, 4863, 4869), esophagectomy (4240 to 4242, 4399), pancreatectomy (5251, 5253, 5260, 5270), and lung resection (323, 3230, 3239, 324, 3241, 3249, 325, 3250, 3259) for cancer using and International Classification of Disease, Ninth Revision, Clinical Modification codes. These procedures were chosen because they are common, carry significant risk of morbidity and mortality, and are often discussed within the context of potential centralization policies.5
We linked patient and hospital-level data from the Medicare Provider Analysis and Review files to the American Hospital Association (AHA) Annual Survey for the corresponding years (2005 to 2014) to obtain additional information on hospital characteristics, such as teaching status, urban versus rural location, bed size, and operating business model (eg, not for profit). The AHA database also includes variables (System ID) that permit the identification of discrete hospital systems and all their affiliated acute care hospitals. Although individual systems may have different referral patterns for complex care, there are no data on system behaviors. However, the AHA data are being widely used to study health systems and represent the most comprehensive source of data on hospital affiliations in the United States.
We defined centralization as the proportion of operations performed by the highest-volume center within each system in a given year. Hospital volume was determined as the raw number of each procedure performed annually in the Medicare population. Centralization numbers were calculated separately for each procedure because not all hospitals and systems performed each procedure.
Because Medicare covers only a proportion of patients undergoing these operations at United States hospitals, we used the National Inpatient Sample (NIS) to derive more complete estimates of hospital volume. For each procedure and year, we calculated proportions for each payer using the NIS. We then divided the Medicare-only volume from our primary analytic files by the proportion of patients covered by Medicare in NIS. The resulting number should therefore represent a more accurate estimate of a hospital’s actual volume in a given year.
Outcomes
We used International Classification of Disease, Ninth and Tenth Revisions, Clinical Modification codes to identify 30-day postoperative complications such as pulmonary failure, pneumonia, myocardial infarction, deep venous thrombosis/pulmonary embolism, renal failure, surgical site infection, GI bleeding, and hemorrhage. These complications represent a subset of codes with the highest sensitivity and specificity.9 We identified deaths as those occurring within 30 days of the index operation. We elected to use 30-day instead of 90-day mortality because it is measured more reliably in claims data and has been used most often as an outcome to motivate centralization discussions in the United States.5,10 Readmissions were identified as any claim for a readmission to any hospital within 30 days after discharge.
Statistical Analysis
The purpose of this analysis was to evaluate the association between the extent of system centralization and postoperative complications, mortality, or readmissions. For each procedure and each outcome, we fit mixed-effects logistic regression models accounting for patient age, sex, and 27 Elixhauser comorbidities as fixed effects.11 We further accounted for overall time trends toward better outcomes using the claim year as a categorical dummy variable. We accounted for additional hospital characteristics such as bed size, teaching status, business model, and nursing ratios as fixed effects, while also accounting for hospital-level random effects. We addressed potential confounding from existing hospital relationships (ie, system maturity) in two ways. First, we accounted for the average number of years of participation for all hospitals in a given system. Second, we accounted for the overall number of years for system participation for each hospital in any given year.
We modeled centralization as a continuous variable to derive estimates for the absolute risk difference associated with 20% increases in centralization from the linear regression models. We then used the logistic regression models to derive risk-adjusted outcome rates across systems stratified into quintiles on the basis of their degree of centralization.
We addressed the issue of system and hospital case volume in several ways, treating it as an effect modifier, rather than as a potential confounder. Using established, although arbitrary, annual volume standards from the Leapfrog Group (pancreatectomy, 20; esophagectomy, 20; lung resection, 40; proctectomy, 15), we identified hospitals achieving this benchmark in a given year (high-volume hospitals).12,13 These standards are used to demonstrate volume relationships and not to make a particular comment on their usefulness to inform centralization. Volume standards for colectomy are not reported, but we used a threshold of 15 cases because of its technical similarity to proctectomy, and prior evidence suggests that this would be a clinically reasonable benchmark.14 We also defined low- and high-volume systems as those below or above the median system volume, respectively. We repeated the primary analyses across four discrete groups of systems (low volume, high volume, systems without a high-volume hospital, and systems with a high-volume hospital) to provide estimates that account for differential access to resources across the systems.
All statistical analyses were performed using STATA statistical software version 14 (College Station, Texas). We used a two-sided approach at the 5% significance level for all hypothesis testing. This study was deemed exempt by the institutional review board at the University of Michigan.
RESULTS
Patient, Hospital, and System Characteristics
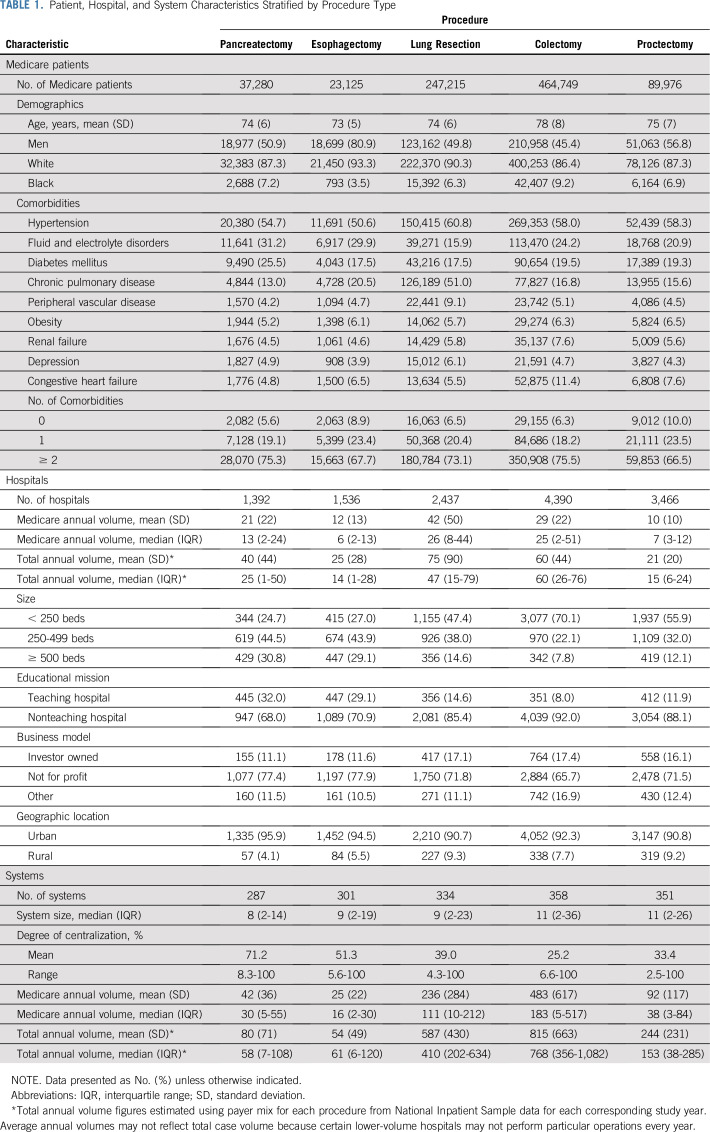
Characteristics of patients, hospitals, and systems are included in Table 1. Patient characteristics were similar overall among procedure groups. The proportion of patients with two or more comorbidities ranged from 66.5% for proctectomy to 75.5% for colectomy. The largest number of hospitals performed colectomy (4,390), whereas fewer performed esophagectomy (1,536) and pancreatectomy (1,392). Median annual hospital volume after applying cohort exclusions ranged from six (interquartile range, two to 13) for esophagectomy to 26 (interquartile range, 8-44) for lung resection. The largest number of systems (358) contained at least one hospital performing colectomy. In contrast, the fewest number of systems (287) contained at least one hospital performing pancreatectomy.
TABLE 1.
Patient, Hospital, and System Characteristics Stratified by Procedure Type
The average degree of centralization varied from 25.2% (range, 6.6% to 100%) for colectomy to 71.2% (range, 8.3% to 100%) for pancreatectomy. The proportion of systems that met annual Leapfrog volume thresholds across all their affiliates combined varied by procedure: pancreatectomy (25.8%), esophagectomy (15.5%), proctectomy (47.1%), lung resection (49.9%), and colectomy (90.4%). A significant proportion of systems did not contain even one hospital that met the annual volume threshold. This ranged from 85% for esophagectomy to 74.2% for pancreatectomy, 52.9% for proctectomy, 50.1% for lung resection, and 9.6% for colectomy.
Association Between Centralization and Postoperative Outcomes
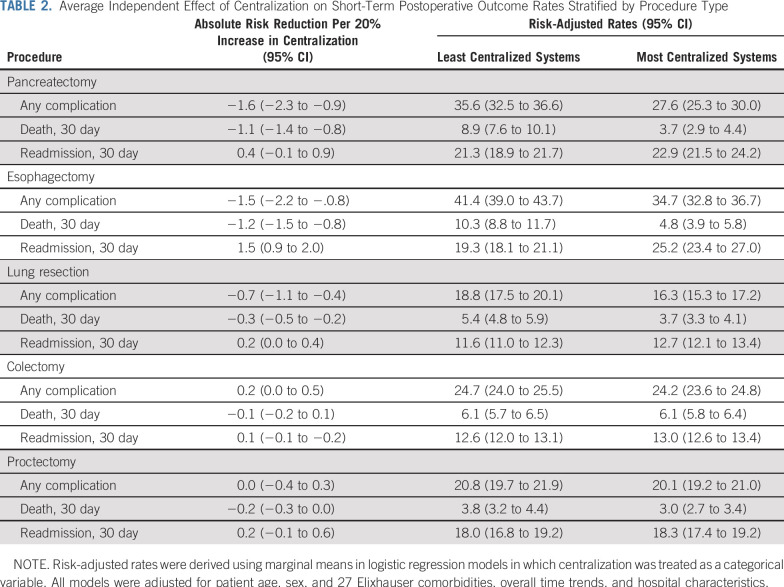
Greater centralization was associated with lower rates of postoperative complications and death for lung resection, esophagectomy, and pancreatectomy (Table 2). For example, there was a 1.1% (95% CI, 1.4% to 0.8%) absolute reduction in 30-day mortality after pancreatectomy for each 20% increase in the degree of centralization within systems. Centralization was associated with changes in readmission rates after esophagectomy only. For esophagectomy, a 20% increase in centralization was associated with a 1.5% (95% CI, 0.9% to 2.0%) absolute increase in readmissions.
TABLE 2.
Average Independent Effect of Centralization on Short-Term Postoperative Outcome Rates Stratified by Procedure Type
Risk-adjusted outcome rates across the continuum of system centralization are illustrated in Fig 1. For example, postoperative mortality for pancreatectomy was two times higher in the least centralized systems than in the most centralized systems (8.9% v 3.7%, P < .01). Similarly, postoperative complications were 22.5% lower in the most centralized systems than in the least centralized systems (27.6% v 35.6%, P < .01) after pancreatectomy. Readmission rates after esophagectomy were 30.1% higher in the most centralized systems than in the least centralized systems (25.2 v 19.3%, P < .01). Trends in readmissions to another hospital within the system or overall were similar to our primary analysis. Trends in failure to rescue (death after a complication) were similar to those observed with 30-day mortality.
FIG 1.
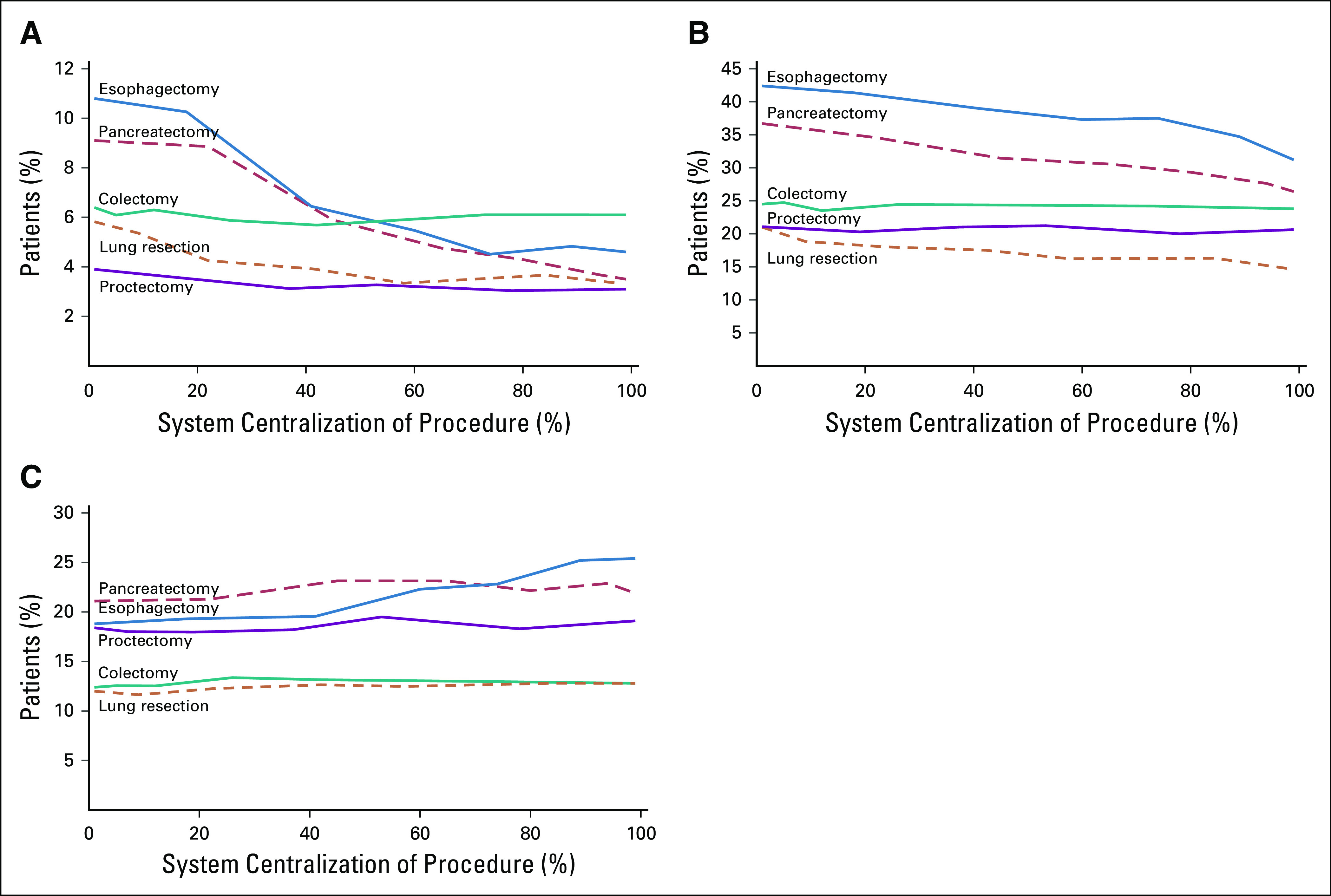
Risk-adjusted rates of postoperative (A) death, (B) complications, or (C) readmissions for each procedure across the continuum of systems’ centralization of each procedure. Estimates reflect the trend in each outcome from models in which centralization was modeled continuously.
Effect of System Versus Hospital Volume
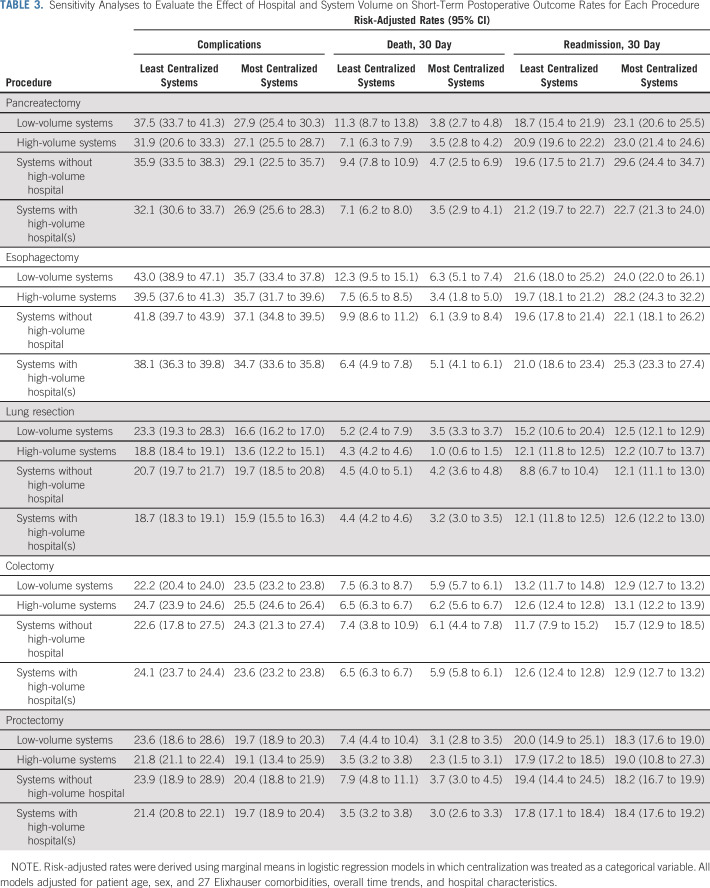
Although hospital systems varied in their overall procedural volume, the association between centralization and short-term outcomes was consistent across the observed range of system volume (Table 3). For example, the most centralized low-volume systems performing esophagectomy (6.3% [95% CI, 5.1% to 7.4%]) had significantly lower mortality than the least centralized systems (12.3% [95% CI, 9.5% to 15.1%], P < .01). Even when restricted to systems without a center meeting the minimum volume standards for safe surgery, mortality rates for the most centralized systems (6.1% [95% CI, 3.9% to 8.4%]) were lower than those at the least centralized systems (9.9% [95% CI, 8.6% to 11.2%], P < .01).
TABLE 3.
Sensitivity Analyses to Evaluate the Effect of Hospital and System Volume on Short-Term Postoperative Outcome Rates for Each Procedure
DISCUSSION
Although the volume–outcome benefit for complex cancer surgery is widely recognized, the absence of specific policies and economic incentives has prevented centralization from being effectively studied or used as a strategy to improve the quality of high-risk cancer surgery in the United States. In this study, we capitalized on the longitudinal trend toward hospital consolidation and the natural opportunities that it presents to centralize care within an established system of affiliated hospitals. We found that the degree of centralization varied widely across hospital systems for each procedure, with pancreatectomy and esophagectomy being the most centralized on average. For three procedures (pancreatectomy, esophagectomy, and lung resection) greater centralization was associated with better outcomes. Centralization was not associated with better or worse outcomes after colectomy or proctectomy. We observed that systems varied substantially in their overall volume, such that their opportunity to leverage their collective experience would similarly vary. However, centralization was associated with significant benefits for both low- and high-volume systems, suggesting that this strategy would have broadly applicable benefits.
Several European countries have successfully implemented centralization policies that have proven effective in reducing short-term morbidity from high-risk surgeries in addition to improving long-term oncologic outcomes for gastric, pancreatic, and rectal cancers.15,16 For example, 90-day mortality after gastrectomy decreased from 11% to 7%, whereas 2-year survival increased from 55% 59% after the Netherlands centralized the procedure to select centers in 2012.17 In contrast, Canada centralized lung cancer surgery in 2007, but the policy failed to demonstrate desired improvements in postoperative mortality.18 The extent to which the success of centralization policies is related to the overall population size of the country or the unique design of its health care systems is unclear.
The United States health care system differs from those in Europe and Canada in several important ways relevant to potential policies to centralize care. First, the patient, provider, and hospital populations are much larger. Second, the federal government has little to no control of hospital credentialing. Physicians and hospitals have considerable autonomy in determining where, and by whom, procedures will be performed.19 Third, the payer mix is more diverse, with extensive private markets and no universal public option provided by the government. This study recognizes that ongoing horizontal integration may be a natural framework for studying whether centralization is a tool to improve cancer care in United States markets.6,20,21 Successful mergers should leverage collective assets, reorganize delivery systems, and optimize care around the most experienced providers when necessary.22 That said, the United States is much less centralized at baseline, and approaches to centralize care will likely require greater input from physicians, clinical societies, and policy makers to be most effective. Furthermore, implementing centralization protocols to improve patient safety and overall care should focus on ways to reduce harm. There is a wide body of literature suggesting that failure to rescue is an important driver of postoperative mortality. Centralization of care may help direct patients to the hospitals with not only the most experience treating complex cancers, but also the most experience in managing the complications that are to be expected from major oncologic resections.23,24
The findings in this study should be considered within the context of several important limitations. Each of the cancer-related procedures is more common in aged populations, and our results may not be generalizable to all patients. This study may also be limited by the measurement of only a subset of complications and 30-day mortality. In doing so, we may exclude other clinically meaningful outcomes. However, we used complications that have been shown previously to be measured reliably in claims data, to limit any potential bias from measurement error alone. A key limitation of this study centers on the definition of a hospital system. Although some may interpret the system as a loose affiliation of hospitals, others may be more deliberate in coordinating providers and services. Nonetheless, there are no current standards by which hospital system behavior is categorized. However, in all analyses, we did account for several organizational characteristics that may influence outcomes, such as the hospitals’ time-in-system. We were unable to determine whether centralization occurred randomly or with intention. However, this distinction would not change the underlying premise of our analysis, which explored how centralization overall influences surgical outcomes. Finally, there is an important connection between centralization and hospital volume. Certain procedures may be coded incorrectly in low-volume hospitals. However, we limited this issue by requiring agreement between the procedure and the operative diagnosis. The Leapfrog thresholds are used to demonstrate effect, not to support their use to inform practice changes. Furthermore, simply adjusting for volume as a confounder would not account for the possibility that centralization influences outcomes for other reasons (eg, the coordination of other cancer care specialists). Thus, we presented several secondary analyses that explore how surgical case volumes could influence our primary findings.
Any discussion about centralization should consider the structure around which care is to be centralized (ie, geographic, organizational, or through a set of established criteria [eg, volume standards]). Geographic or ad hoc centralization of cancer surgery is well documented. In the decade between the 1990s and the early 2000s, the proportion of esophageal, pancreatic, and colon cancer surgeries performed by the highest-volume hospitals doubled.10 Criteria-based centralization is becoming increasingly common. For example, a recent initiative by the Commission on Cancer and the American College of Surgeons established the National Accreditation Program for Rectal Cancer.25 This initiative uses evidence-based standards to accredit centers with the clinical, research, and outcomes reporting infrastructure to care for patients with rectal cancer. Building on decades of work by the Leapfrog Group, volume criteria have also received considerable attention as certain centers have taken the volume pledge, which restricts complex surgeries to the most experienced providers.4
For each example, however, clinicians and policymakers have raised legitimate concerns that these models for centralization may increase disparities, exacerbate access issues, and even impede the training of future physicians.26 Centralizing cancer care around existing hospital systems may be a strategic alternative that addresses many of these concerns. Hospital systems are oriented regionally and have a clear interest in addressing the clinical needs, and capitalizing on the market demands, of their geographic location.22 Centralization within systems also aligns credentialing entities with the broader strategic planning initiatives for the entire system to improve patient safety. Finally, the system model of centralization preserves existing business relationships and may mitigate some of the concerns that come from imposing external centralization criteria, such as loss of revenue.
In this national, population-based study of Medicare beneficiaries undergoing high-risk cancer surgery, short-term outcomes were better when patients had surgery within the most centralized systems. As hospitals affiliate in response to broader financial and organizational pressures, their systems may present unique opportunities for improving the quality of high-risk cancer care in the United States.
Footnotes
Supported by National Institute of Health Grants No. R01AG039434 (J.BD.) and R01HS023597 (J.B.D).
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Administrative support: Justin B. Dimick, Hari Nathan
Collection and assembly of data: Kyle H. Sheetz, Hari Nathan
Data analysis and interpretation: Kyle H. Sheetz
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST AND ADAT AVAILABILITY STATEMENT
Centralization of High-Risk Cancer Surgery Within Existing Hospital Systems
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Justin B. Dimick
Stock and Other Ownership Interests: ArborMetrix
REFERENCES
- 1.Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301:1364–1369. doi: 10.1056/NEJM197912203012503. [DOI] [PubMed] [Google Scholar]
- 2.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 3.Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–2127. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 4.Urbach DR. Pledging to eliminate low-volume surgery. N Engl J Med. 2015;373:1388–1390. doi: 10.1056/NEJMp1508472. [DOI] [PubMed] [Google Scholar]
- 5.Vonlanthen R, Lodge P, Barkun JS, et al. Toward a consensus on centralization in surgery. Ann Surg. 2018;268:712–724. doi: 10.1097/SLA.0000000000002965. [DOI] [PubMed] [Google Scholar]
- 6.Schmitt M. Do hospital mergers reduce costs? J Health Econ. 2017;52:74–94. doi: 10.1016/j.jhealeco.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Chhabra KR, Dimick JB. Hospital networks and value-based payment: Fertile ground for regionalizing high-risk surgery. JAMA. 2015;314:1335–1336. doi: 10.1001/jama.2015.9803. [DOI] [PubMed] [Google Scholar]
- 8.Tsai TC, Jha AK. Hospital consolidation, competition, and quality: Is bigger necessarily better? JAMA. 2014;312:29–30. doi: 10.1001/jama.2014.4692. [DOI] [PubMed] [Google Scholar]
- 9.Iezzoni LI, Daley J, Heeren T, et al. Identifying complications of care using administrative data. Med Care. 1994;32:700–715. doi: 10.1097/00005650-199407000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Stitzenberg KB, Sigurdson ER, Egleston BL, et al. Centralization of cancer surgery: Implications for patient access to optimal care. J Clin Oncol. 2009;27:4671–4678. doi: 10.1200/JCO.2008.20.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Austin JM, D’Andrea G, Birkmeyer JD, et al. Safety in numbers: The development of Leapfrog’s composite patient safety score for U.S. hospitals. J Patient Saf. 2014;10:64–71. doi: 10.1097/PTS.0b013e3182952644. [DOI] [PubMed] [Google Scholar]
- 13. doi: 10.1016/j.jamcollsurg.2010.09.027. Massarweh NN, Flum DR, Symons RG, et al: A critical evaluation of the impact of Leapfrog's evidence-based hospital referral. J Am Coll Surg 212:150-159, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Sheetz KH, Ibrahim AM, Regenbogen SE, et al. Surgeon experience and Medicare expenditures for laparoscopic compared to open colectomy. Ann Surg. 2018;268:1036–1042. doi: 10.1097/SLA.0000000000002312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Leersum NJ, Snijders HS, Henneman D, et al. The Dutch surgical colorectal audit. Eur J Surg Oncol. 2013;39:1063–1070. doi: 10.1016/j.ejso.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Neuzillet C, Gaujoux S, Williet N, et al. Pancreatic cancer: French clinical practice guidelines for diagnosis, treatment and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, ACHBT, AFC) Dig Liver Dis. 2018;50:1257–1271. doi: 10.1016/j.dld.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 17.van Putten M, Nelen SD, Lemmens VEPP, et al. Overall survival before and after centralization of gastric cancer surgery in the Netherlands. Br J Surg. 2018;105:1807–1815. doi: 10.1002/bjs.10931. [DOI] [PubMed] [Google Scholar]
- 18.Bendzsak AM, Baxter NN, Darling GE, et al. Regionalization and outcomes of lung cancer surgery in Ontario, Canada. J Clin Oncol. 2017;35:2772–2780. doi: 10.1200/JCO.2016.69.8076. [DOI] [PubMed] [Google Scholar]
- 19.Teirstein PS, Topol EJ. The role of maintenance of certification programs in governance and professionalism. JAMA. 2015;313:1809–1810. doi: 10.1001/jama.2015.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kocher R, Sahni NR. Hospitals’ race to employ physicians--the logic behind a money-losing proposition. N Engl J Med. 2011;364:1790–1793. doi: 10.1056/NEJMp1101959. [DOI] [PubMed] [Google Scholar]
- 21.Cutler DM, Scott Morton F. Hospitals, market share, and consolidation. JAMA. 2013;310:1964–1970. doi: 10.1001/jama.2013.281675. [DOI] [PubMed] [Google Scholar]
- 22.Dafny LS, Lee TH. The good merger. N Engl J Med. 2015;372:2077–2079. doi: 10.1056/NEJMp1502338. [DOI] [PubMed] [Google Scholar]
- 23.Friese CR, Earle CC, Silber JH, et al. Hospital characteristics, clinical severity, and outcomes for surgical oncology patients. Surgery. 2010;147:602–609. doi: 10.1016/j.surg.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and failure to rescue with high-risk surgery. Med Care. 2011;49:1076–1081. doi: 10.1097/MLR.0b013e3182329b97. [DOI] [PubMed] [Google Scholar]
- 25.Brady JT, Xu Z, Scarberry KB, et al. Evaluating the current status of rectal cancer care in the US: Where we stand at the start of the Commission on Cancer’s National Accreditation Program for Rectal Cancer J Am Coll Surg 226881–890.2018[Erratum: J Am Coll Surg, 2018] [DOI] [PubMed] [Google Scholar]
- 26.Greenberg CC, Ashley SW, Schrag D. Centralization of cancer surgery: What does it mean for surgical training? J Clin Oncol. 2009;27:4637–4639. doi: 10.1200/JCO.2009.23.0052. [DOI] [PubMed] [Google Scholar]