Abstract
PURPOSE
Phthalate exposure is ubiquitous and especially high among users of drug products formulated with phthalates. Some phthalates mimic estradiol and may promote breast cancer. Existing epidemiologic studies on this topic are small, mostly not prospective, and have given inconsistent results. We estimated associations between longitudinal phthalate exposures and breast cancer risk in a Danish nationwide cohort, using redeemed prescriptions for phthalate-containing drug products to measure exposure.
METHODS
We ascertained the phthalate content of drugs marketed in Denmark using an internal Danish Medicines Agency ingredient database. We enrolled a Danish nationwide cohort of 1.12 million women at risk for a first cancer diagnosis on January 1, 2005. By combining drug ingredient data with the Danish National Prescription registry, we characterized annual, cumulative phthalate exposure through redeemed prescriptions. We then fit multivariable Cox regression models to estimate associations between phthalate exposures and incident invasive breast carcinoma according to tumor estrogen receptor status.
RESULTS
Over 9.99 million woman-years of follow-up, most phthalate exposures were not associated with breast cancer incidence. High-level dibutyl phthalate exposure (≥ 10,000 cumulative mg) was associated with an approximately two-fold increase in the rate of estrogen receptor–positive breast cancer (hazard ratio, 1.9; 95% CI, 1.1 to 3.5), consistent with in vitro evidence for an estrogenic effect of this compound. Lower levels of dibutyl phthalate exposure were not associated with breast cancer incidence.
CONCLUSION
Our results suggest that women should avoid high-level exposure to dibutyl phthalate, such as through long-term treatment with pharmaceuticals formulated with dibutyl phthalate.
INTRODUCTION
Phthalates are used in many consumer goods, including medical supplies, food containers, cosmetics, and toys.1 Phthalates are also used in pharmaceutical capsules to impart delayed- or extended-release properties.2 Phthalates are not covalently bound to other constituents and readily leach out of products, leading to human exposure.3 Studies show that most tested adults in developed regions have measurable urinary phthalate metabolites.1,4-7 Exposure is particularly high among users of phthalate-containing medications.8,9 For example, users of mesalamine had approximately 60-fold higher levels of urinary monobutyl phthalate—a metabolite of dibutyl phthalate (DBP)—than nonusers who were exposed only through environmental sources.8,10 Such widespread exposure11,12 has raised concerns about the health consequences of phthalates.3
Phthalates are potential endocrine disruptors—exogenous compounds that mimic hormones and may therefore affect fertility,13-15 fetal/child development,3,16-18 and some cancers.3,19 Preclinical evidence suggests that some phthalates promote breast tumor growth through estrogen receptor (ER) signaling.20-25 Estrogen-independent breast cancer promotion mechanisms have also been reported for phthalates,21,26-29 as have antiestrogenic effects.23
Epidemiologic evidence is inconsistent regarding the effect of phthalates on breast cancer incidence. Existing studies relied on measurement of urinary metabolites as a proxy for exposure to the parent compounds hypothesized to affect health. A 2010 case-control study measured nine urinary metabolites of six different phthalates in 233 Mexican patients with breast cancer and 221 controls.30 The authors reported a potentially causal association with a diethyl phthalate (DEP) metabolite and potentially protective associations with metabolites of butyl benzyl phthalate (BBP) and dioctyl phthalate (DOP).30 Breast cancer was not notably associated with metabolites of DBP, di-isobutyl phthalate, or diethylhexyl phthalate (DEHP).30 In contrast, a case-control study of Alaskan native women found a potentially causal association for monoethylhexyl phthalate (a DEHP metabolite),31 and a cross-sectional study from the U.S. National Health and Nutrition Examination Survey found no notable breast cancer associations with metabolites representing di-isobutyl phthalate, DEP, DOP, BBP, and DEHP.32 Most recently, a case-control study of postmenopausal Women’s Health Initiative participants measured urinary metabolites at multiple time points in prospectively collected samples. The authors reported no positive associations between phthalates and breast cancer overall.33
Given the ubiquity of phthalate exposure and the inconsistent epidemiologic evidence, we measured associations between prospectively recorded phthalate exposures and breast cancer incidence in a Danish nationwide cohort. To accomplish this, we capitalized on the documented high-level phthalate exposure through use of certain medications. On the basis of preclinical evidence, we hypothesized that exposure to DBP, an estrogenic phthalate that is also a common medication excipient,11,12 would be positively associated with ER-positive breast cancer.
METHODS
Danish Population-Based Registries
Denmark maintains extensive population-based registries that capture information on health, employment, vital status, and emigration.34 We enumerated a cohort of women at risk for breast cancer using data from several independent registries, all of which were linked at the individual level using the CPR number, a 10-digit identifier assigned to legal residents of Denmark.
Phthalate Content of Medications
The Danish Medicines Agency maintains a database of pharmaceuticals included in the Danish formulary. For each marketed drug, it records the Nordic Article Number (also called a VNR code—a unique identifier assigned to medicinal agents from different manufacturers), the Anatomic Therapeutic Chemical (ATC) code, dates of market entry and removal, and both active and inactive ingredients. Ingredient data include the mass of each component per medication unit (eg, milligrams per capsule). Although the database covers drugs marketed since 1995, excipient data are complete since 2005. We searched ingredient fields for phthalate-related text strings in both Danish and English and prepared a database of phthalate-containing oral medications. Products without an ATC code (ie, herbal products and dietary supplements) were excluded.
We merged the drug database with the Danish National Prescription Registry (DNPR).35 For each redeemed prescription, the DNPR records the patient’s CPR number, transaction date, drug filled (ATC and VNR codes), and quantity dispensed. Information is updated daily and used by the Danish government to partially refund out-of-pocket medication costs.
Source Population and Data Collection
Our source population was all female residents of Denmark who were alive, without a cancer history, and unexposed to phthalate-containing medications between January 1, 1995, and January 1, 2005. We identified this population by linking the Danish Civil Registry, the Danish Cancer Registry, and the augmented DNPR.
Definitions of Analytic Variables
Follow-up began on January 1, 2005. We ascertained incident invasive breast cancer cases from the Danish Cancer Registry.36,37 We classified cases as ER-positive or ER-negative by merging tumor data from the Danish Breast Cancer Group Registry.38,39 Vital status came from the Danish Civil Registry.40,41 We calculated individuals’ person-time as the days elapsed between baseline and the first of breast cancer diagnosis, other cancer diagnosis, death, emigration, or the end of follow-up on December 31, 2015. We calculated the phthalate content of each filled prescription by multiplying the mass of phthalate per capsule by the fill quantity. We characterized phthalate exposures as the cumulative milligrams of cellulose acetate phthalate (CAP), DEP, DBP, hypromellose phthalate (HPMCP), or polyvinyl acetate phthalate (PVAP) contained in all prescriptions filled by a patient during each year of follow-up. Categories for cumulative phthalate exposures were based on the observed distribution of continuous values in the final year of follow-up. Cumulative exposures to DBP, CAP, and HPMCP were categorized as no exposure, 1 to 249 mg, 250 to 999 mg, 1,000 to 9,999 mg, and 10,000 mg or more. Cumulative exposure to DEP was categorized as unexposed, 1 to 9 mg, 10 to 99 mg, and 100 mg or more. PVAP exposure was rare and therefore modeled as a dichotomous variable (unexposed v any exposure [range, 1.3 to 682 cumulative grams]).
We defined the following set of potential confounders. Age was defined at baseline; menopausal status was imputed as pre- or postmenopausal on the basis of age younger than 55 years or age 55 years or older, respectively; other phthalate exposures were classified dichotomously on the basis of any versus no exposure during follow-up (eg, when modeling DBP exposure, we adjusted for exposure to CAP, DEP, PVAP, and HPMCP); medication exposures were classified dichotomously for each year of follow-up on the basis of the fifth level of the ATC code (eg, diclofenac, erythromycin, and mesalamine); exposures to cardiac glycosides,42 hormone therapy,43 aspirin,44 oral contraceptives,45 and statins46 were positive if a patient redeemed one or more relevant prescriptions in the year before follow-up began; and the Charlson comorbidity index (CCI) was calculated for each study participant from baseline diagnoses.47 We could not characterize a complete reproductive history but ascertained parity (nulliparous, one child, two children, or three or more children) from the Danish Medical Birth Register48 for women age 45 years or older in 2005 (ie, women whose reproductive years were expected to be covered by the Register).
Statistical Analysis
We tabulated the number of medicinal products contributing to phthalate exposures in the cohort and calculated the median and range of phthalate masses in those products. We compared participant characteristics according to dichotomized phthalate status over follow-up. We then fit cause-specific Cox regression models to estimate associations between phthalate exposures and breast cancer incidence. Cumulative milligrams of phthalate exposure was modeled as a time-varying factor variable (using the categories defined above), updated yearly. We specified a 1-year exposure lag to avoid reverse causation bias and because proximate exposure is unlikely to affect risk. To account for competing risks, we censored follow-up upon diagnosis with a nonbreast malignancy (except nonmelanoma skin cancer), death, emigration from Denmark, or reaching the end of available follow-up on December 31, 2015. We modeled incidence of ER-positive and ER-negative disease by fitting cause-specific Cox regression models in which ER-negative and ER-positive disease, respectively, was included in the set of competing events.49 We evaluated modification of the hazard ratio (HR) by imputed menopausal status by fitting models within strata of women who were premenopausal or postmenopausal throughout their entire follow-up. We visualized heterogeneity of associations between these strata by plotting and visually evaluating CI functions.50 We verified the proportional hazards assumption by evaluating interactions between fixed exposures and the logarithm of person-time.49
Main regression models were adjusted for age (continuous), postmenopausal status (dichotomous, time-varying), exposure to other phthalates (dichotomous), exposure to drug substances contributing to phthalate exposure (dichotomous, time-varying), CCI (factor variable), and baseline use of cardiac glycosides, hormone therapy, oral contraceptives, aspirin, or statins (each dichotomous). The main model of PVAP exposure was not adjusted for drug substance exposures because only one drug contributed to exposure. Models of ER-positive and ER-negative disease could support adjustment only for age, menopausal status, and drug substance exposures. ER-specific models for PVAP exposure could support adjustment only for age. Models stratified by menopausal status were adjusted only for age and drug substance exposures. Analyses were performed with SAS v.9.4 (SAS Institute, Cary, NC).
Sensitivity Analyses
We evaluated the robustness of our findings in the following sensitivity analyses. First, we modeled associations between DBP exposure and breast cancer among users of lithium and mesalamine, the two medications that contributed the most to DBP exposure (Appendix Fig A1, online only; Appendix Tables A1 and A2, online only). Because lithium has been associated with breast cancer growth in vitro,51 and because cumulative lithium doses tended to be higher among DBP-exposed women (Appendix Fig A2, online only), we evaluated the impact of adjustment for time-varying, cumulative moles of lithium ion on the DBP/breast cancer association. Second, we modeled associations without an exposure lag and with a longer lag of 2 years.
RESULTS
Phthalates in Medications
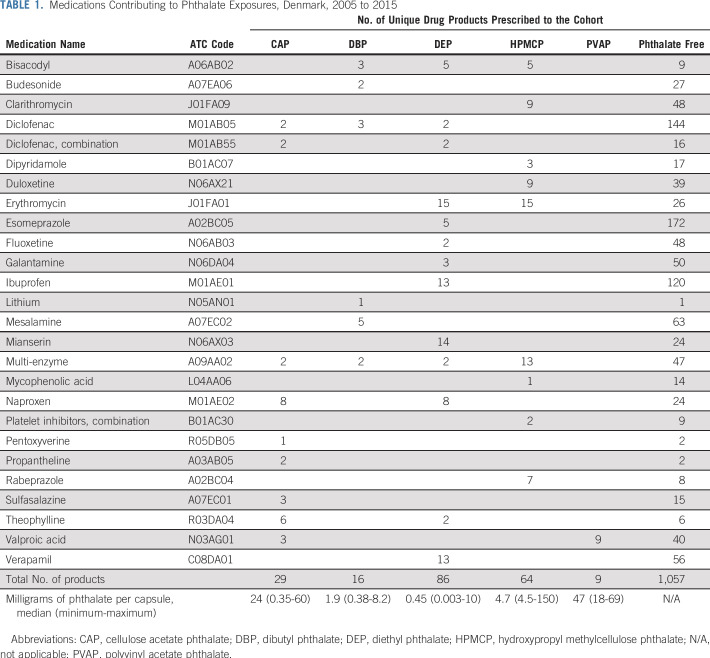
We identified 430 unique drug products from 29 medications in the Danish formulary with one or more phthalates in their formulation. Cohort members filled prescriptions for 204 of these products, representing 26 drug substances (Table 1). PVAP exposure was informed only by valproic acid, but all other phthalate exposures were informed by several drug substances. Phthalate content ranged from 3 µg to 150 mg per capsule. All medications with phthalate-containing products were also represented by one or more products (range, 1 to 172 products) with phthalate-free formulations.
TABLE 1.
Medications Contributing to Phthalate Exposures, Denmark, 2005 to 2015
Characteristics of the Cohort
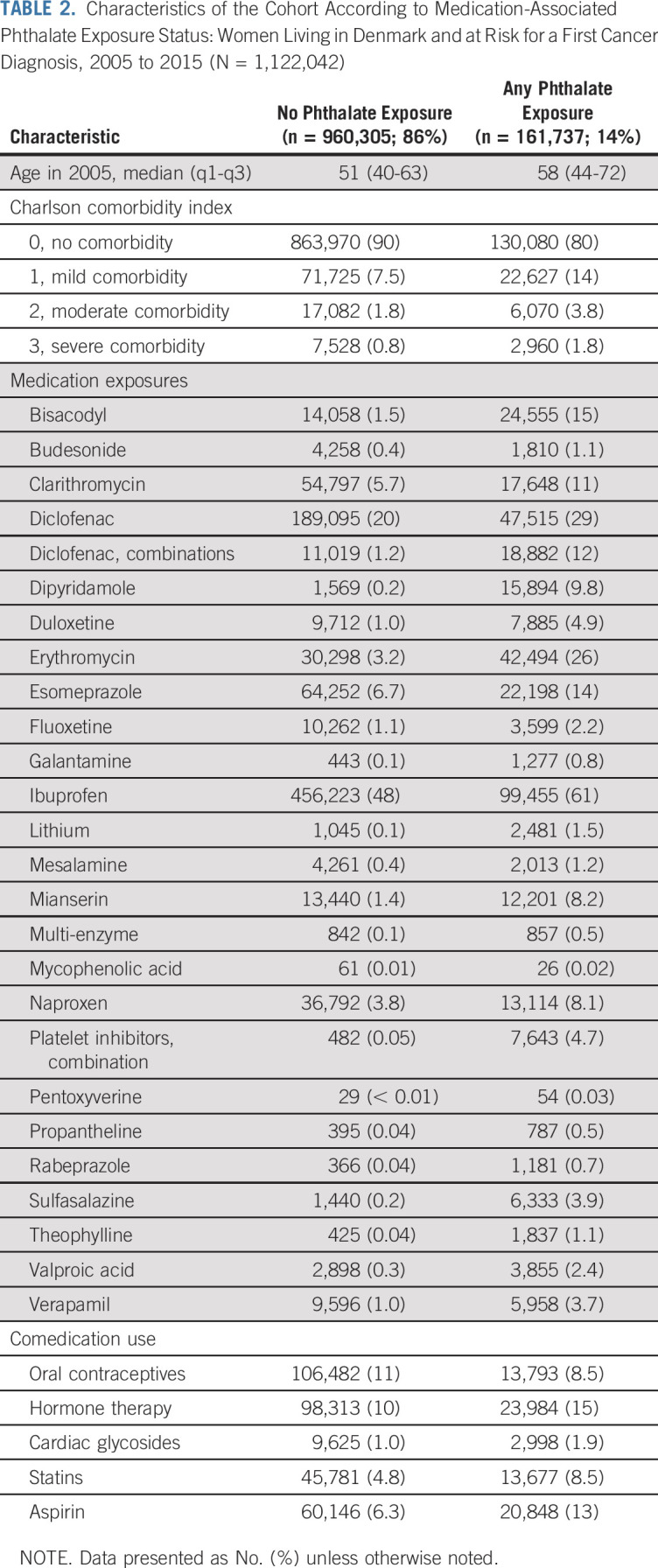
Our cohort included 1,122,042 women. During 9.99 million person-years of follow-up (median, 10 years), 27,111 cases of invasive breast cancer occurred, 84% of which were ER-positive. Approximately 14% of the cohort (n = 161,737) redeemed prescriptions for phthalate-containing medications. Compared with unexposed patients, phthalate-exposed patients were older and more likely to have comorbid disease, be exposed to the drug substances contributing to phthalate exposure, and have taken oral contraceptives, hormone therapy preparations, cardiac glycosides, statins, and aspirin (Table 2).
TABLE 2.
Characteristics of the Cohort According to Medication-Associated Phthalate Exposure Status: Women Living in Denmark and at Risk for a First Cancer Diagnosis, 2005 to 2015 (N = 1,122,042)
Phthalate Exposure and Breast Cancer Risk
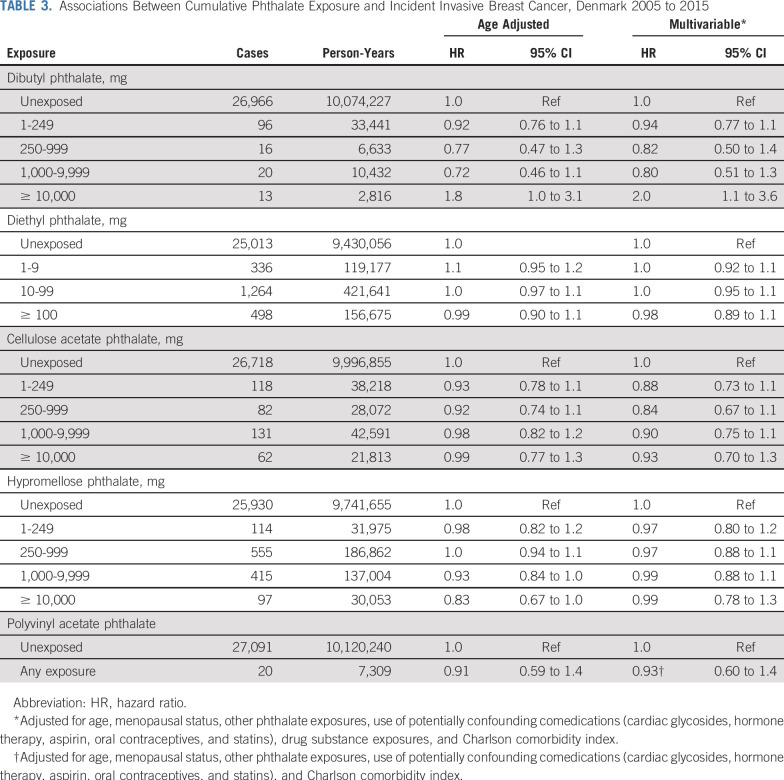
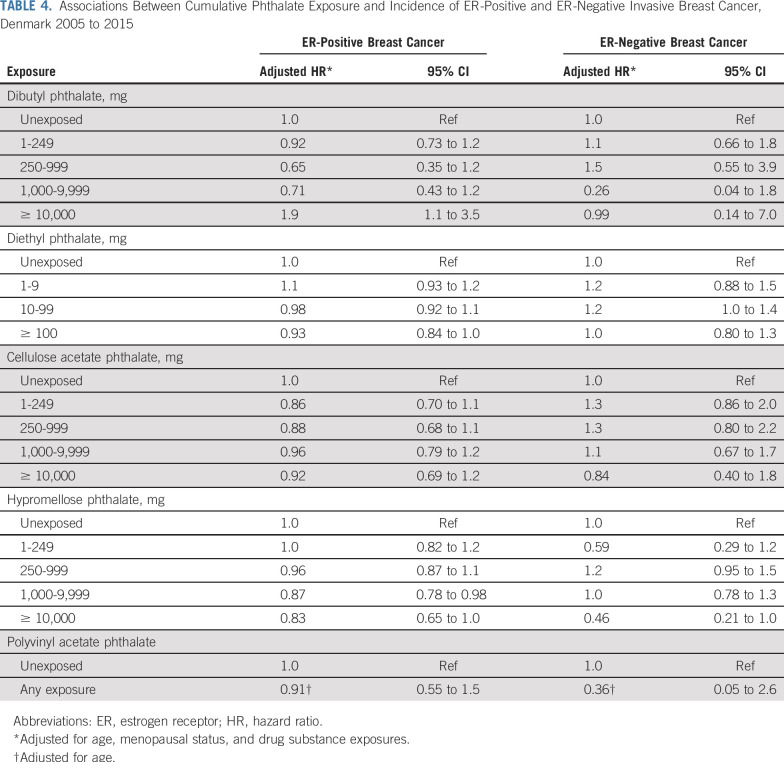
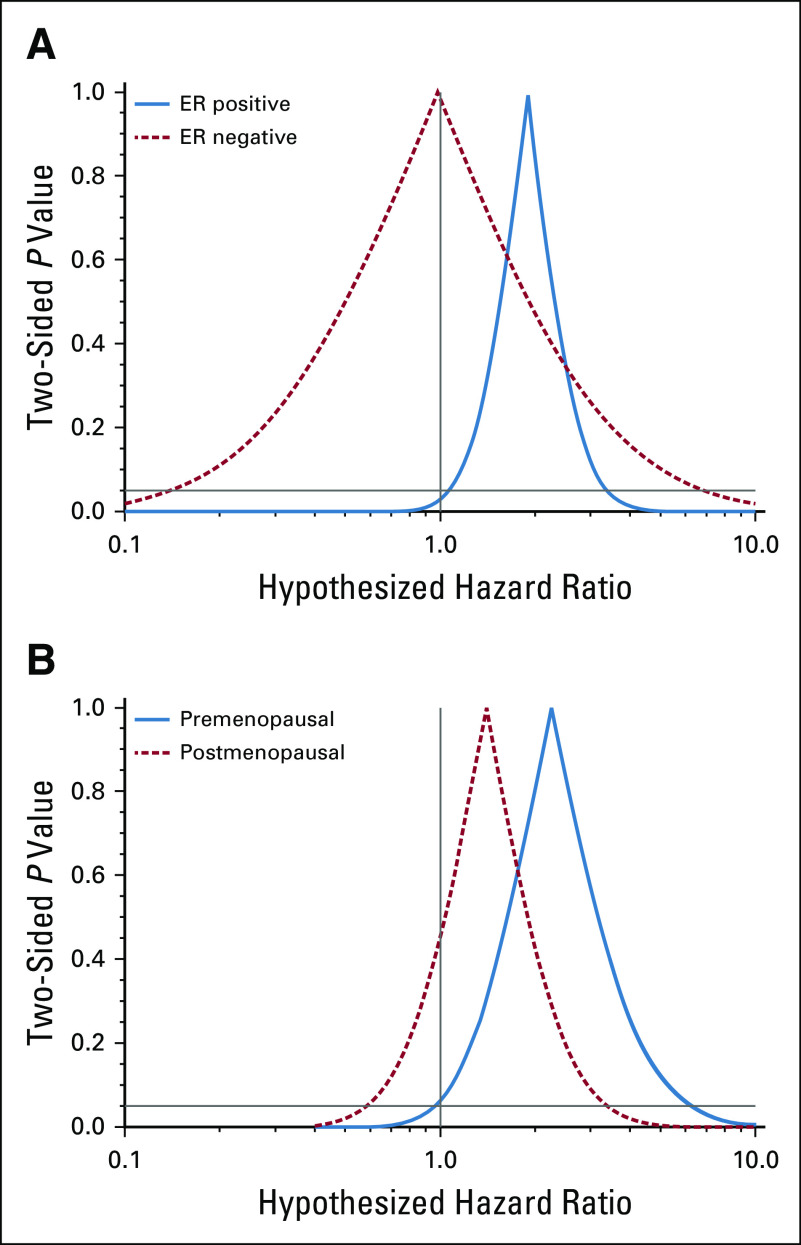
We observed near-null associations between DEP, CAP, HPMCP, and PVAP exposures and invasive breast cancer incidence (Table 3). Associations remained near-null in ER-specific models (Table 4). The highest category of cumulative DBP exposure (≥ 10,000 mg) was associated with a two-fold increase in the breast cancer hazard (adjusted HR [HRadj], 2.0; 95% CI, 1.1 to 3.6). This was entirely driven by an association with ER-positive disease (Fig 1A; ER-positive: HRadj, 1.9; 95% CI, 1.1 to 3.5; ER-negative: HRadj, 0.99; 95% CI, 0.14 to 7.0). The overall DBP association was modestly stronger among premenopausal women (Fig 1B). Lower levels of DBP exposure were not associated with breast cancer incidence. Estimates changed little between age-adjusted models and models additionally adjusted for menopausal status, other phthalate exposures, potentially confounding comedications, drug substance exposures, and CCI. Adjustment for parity had little effect on association estimates (data not shown).
TABLE 3.
Associations Between Cumulative Phthalate Exposure and Incident Invasive Breast Cancer, Denmark 2005 to 2015
TABLE 4.
Associations Between Cumulative Phthalate Exposure and Incidence of ER-Positive and ER-Negative Invasive Breast Cancer, Denmark 2005 to 2015
FIG 1.
Confidence interval functions depicting adjusted associations between cumulative dibutyl phthalate exposure (≥ 10,000 mg v no exposure) and incident breast cancer, (A) according to tumor estrogen receptor (ER) status, and (B) according to menopausal status.
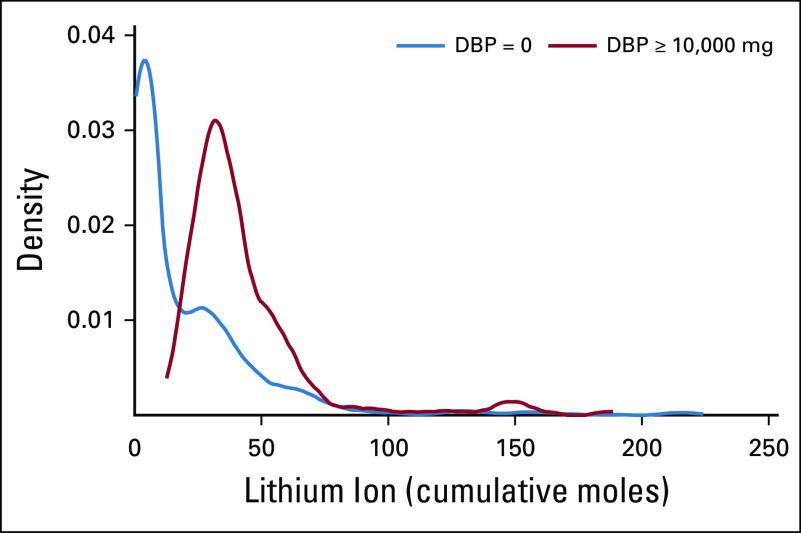
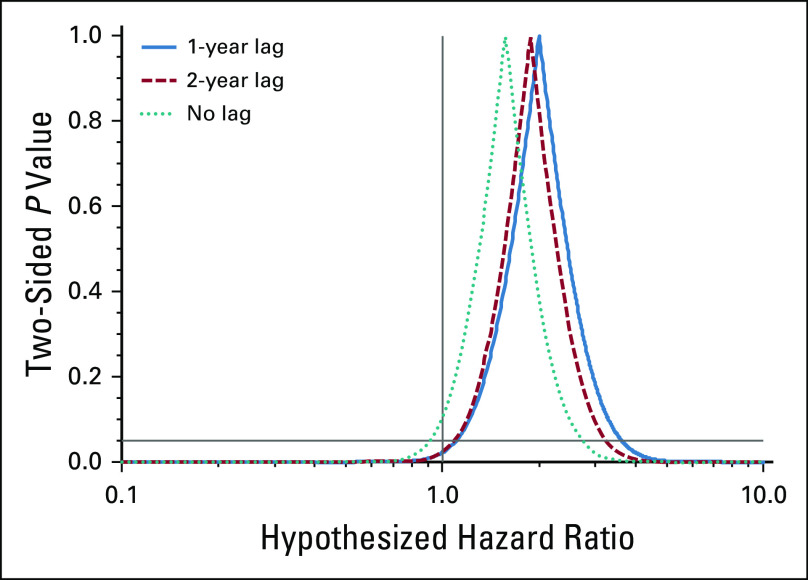
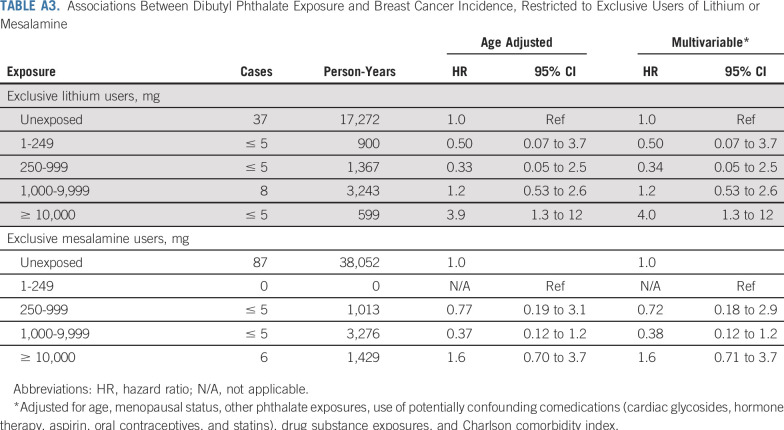
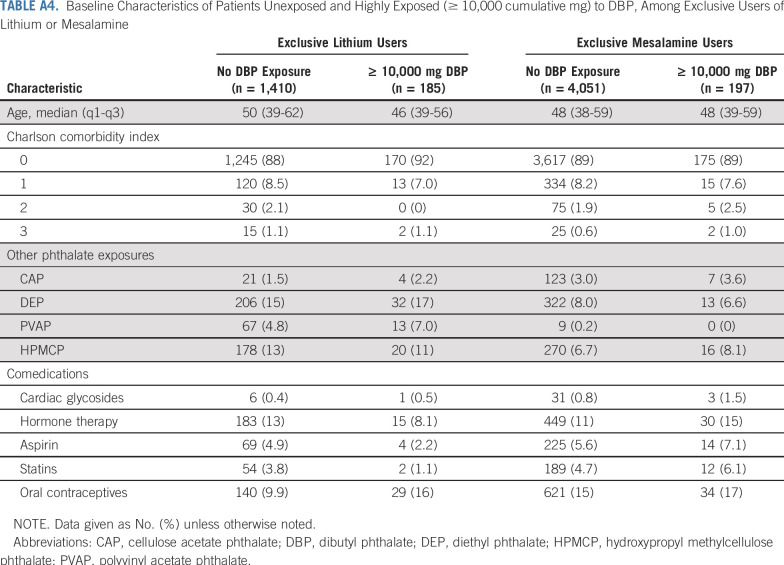
DBP associations persisted in analyses restricted to exclusive users of lithium and mesalamine (Appendix Tables A3 and A4, online only). Among the exclusive lithium users, adjustment for lithium ion exposure did not attenuate DBP associations. Results were similar under the longer 2-year exposure lag and were somewhat attenuated (as expected) under no exposure lag (Appendix Fig A3, online only).
DISCUSSION
In accordance with our a priori hypothesis, we observed an approximately two-fold increase in breast cancer incidence associated with high cumulative exposure to DBP (≥ 10,000 mg). This association was observed for ER-positive but not for ER-negative disease and was stronger among premenopausal women. No other phthalate was associated with breast cancer incidence.
We did not observe a dose response in the hazard ratio with increasing levels of DBP exposure. It is possible that environmental DBP exposure in our reference group obscured any underlying dose-response relationship. It is also possible that DBP increases breast cancer risk only after exceeding a high DBP exposure threshold. Although the association with high DBP exposure was measured with good precision, we cannot rule out that it is an artifact of random error.
The association between DBP and ER-positive breast cancer is consistent with preclinical evidence concerning the estrogenic properties of phthalates and their metabolites. Studies show that DBP increases proliferation and viability in the ER-dependent MCF-7 breast cancer cell line,20-22,25 although contradictory evidence also exists.23 Preclinical studies implicating DBP in ER potentiation also showed similar effects for BBP and DEHP—two phthalates that we could not study because they were not used as excipients in any drugs marketed in Denmark.
Previous epidemiologic studies relied on urinary phthalate metabolites as a proxy for systemic exposure to parent compounds. Because of their rapid elimination, measurement of urinary metabolites captures only recent exposure. López-Carillo et al30 found a potentially causal association of similar magnitude for a metabolite of DEP and potentially preventive associations for metabolites of BBP and DOP. Holmes et al31 reported a potentially causal association for a metabolite of DEHP in a case-control study of Alaskan natives, and Morgan et al32 found near-null associations between exposure to any phthalate metabolite and breast cancer in a cross-sectional US study. None of these studies found an association between DBP metabolites and incident breast cancer. Reeves et al33 recently conducted the first prospective study of phthalate exposure and postmenopausal breast cancer incidence, using multiple measurements of urinary metabolites in 419 cases and 838 matched controls. Although they concluded that there were no positive associations between phthalate exposures and breast cancer overall, they also reported a secondary analysis in which the highest quarter of DBP exposure was associated with 10-fold higher breast cancer odds compared with the lowest quarter (odds ratio, 9.96; 95% CI, 1.93 to 51), consistent with the DBP association pattern we observed.33
Primary strengths of our study are its prospective exposure information, large size, use of an essentially unselected nationwide source population, and high-validity data from the Danish population-based registries.35,36,38,41,52 Using prescription fills to quantify phthalate exposure avoided costly biomarker assays. This not only permitted study of a large population but also facilitated the first epidemiologic study of breast cancer risk on the basis of time-varying and longitudinal measurement of phthalate exposure. In contrast, most earlier studies relied on relatively small numbers of patients and single, nonprospective exposure measurements.30-32
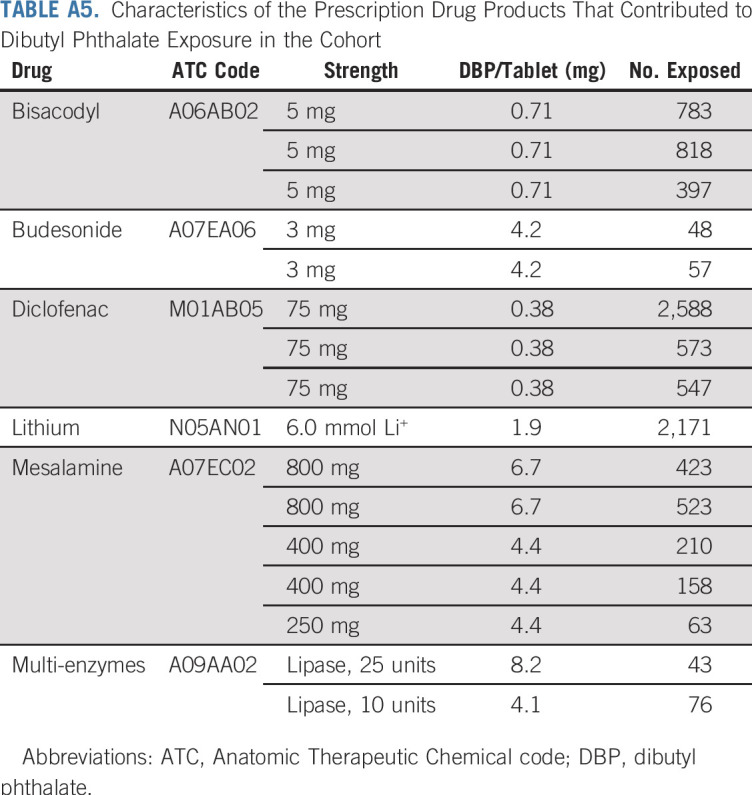
Our exposure measurement scheme has some notable tradeoffs, including misclassification of true phthalate exposure and potential confounding by active drug ingredients and the underlying medical indications for treatment. Some misclassification is expected for two reasons: first, we could not measure nonmedication sources of phthalate exposure (eg, occupational and environmental exposures); second, we cannot be certain that patients ingested all their filled prescriptions, as assumed in our exposure calculations. These concerns are allayed by the following considerations. First, on the basis of a prior exposure study, we expect environmental sources to make up a relatively small proportion of total exposure among users of phthalate-containing medications.8,11 We would expect such misclassification to bias our association estimates toward the null, which would not explain the positive association between DBP and breast cancer risk. It is possible that such misclassification masked low-magnitude breast cancer associations with other phthalates. Second, misclassification as a result of incomplete adherence to prescription durations may have led to overestimation of cumulative exposure levels. However, a record in the DNPR means that a patient paid for and collected the medication, which implies intention to adhere. Furthermore, although absolute measures of cumulative ingestion may be overestimated, we expect that the rank-order of exposure levels would remain intact and serve well for comparison of disease risks between higher and lower exposure levels. Substantial confounding by active drug ingredients and medical indications is unlikely to have influenced our estimates. First, we expect that phthalate exposure in our cohort was randomly allocated among patients exposed to specific medications, as we are unaware of any influences on whether a given prescription was filled with a phthalate-containing product versus a phthalate-free product. Unfortunately, polypharmacy in our study population prevented comparisons of phthalate exposure levels within strata of patients exposed to single medications, which would have preserved the natural randomization. However, the intermix of phthalate-containing and phthalate-free products across all medication types allowed us to adjust for drug substance exposures—and, simultaneously, for their underlying indications. Furthermore, none of the medications or treatment indications relevant to DBP exposure—bisacodyl (laxative for constipation), budesonide (corticosteroid for inflammatory bowel disease), diclofenac (nonsteroidal anti-inflammatory drug for arthritis and migraines), lithium (antipsychotic for bipolar disorder), mesalamine (aminosalicylate for inflammatory bowel disease), and multi-enzymes (for digestive aid)—has been associated with breast cancer incidence in clinical studies (Appendix Table A5).
We could not adjust for adiposity, which we expect to be higher among phthalate-exposed patients. However, adiposity is negatively associated with breast cancer risk in premenopausal women,53 and the DBP association was particularly strong in that subgroup. Likewise, we could not adjust for complete reproductive history. However, adjustment for parity did not substantially affect association estimates—a result that is concordant with the expectation that parity would not be associated with phthalate exposures from medicines. Finally, our models of ER-positive and ER-negative disease could support adjustment only for age, menopausal status, and active drug ingredient exposures. Although this leaves the possibility of residual confounding due to other phthalate exposures, comedications, and comorbidity, these factors did not substantially affect association estimates from our main outcome models.
In summary, we observed an approximately two-fold increase in ER-positive breast cancer incidence among women who were highly exposed to DBP through medications, consistent with preclinical evidence and with our a priori hypothesis. No other type of phthalate exposure, including lower-level DBP exposure, was associated with breast cancer. BBP and DEHP have similar actions to DBP in vitro but could not be measured via medication use in Denmark. Future efforts should focus on these potentially important exposures in addition to replicating the DBP association. In the meantime, it may be prudent for women to consult with prescribers and pharmacists to determine the phthalate content of their medications and whether long-term treatment with DBP-formulated pharmaceuticals can be avoided.
ACKNOWLEDGMENT
We thank Morten Olsen, MD, PhD, for his assistance with preparing data applications for this study. We also thank Elizabeth Cahn, Carol Matyka, and Judy Downey for contributing to this project as advocates for patients with breast cancer.
Appendix
FIG A1.
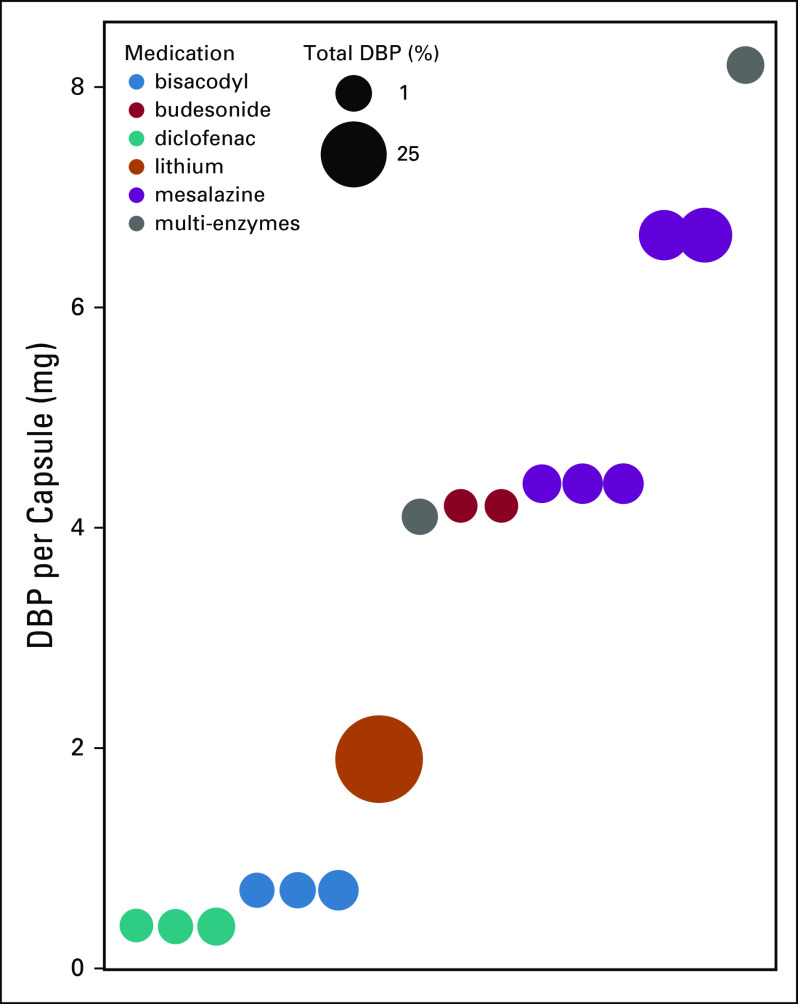
Contribution of specific drugs to the total mass of dibutyl phthalate (DBP) contained in prescriptions filled by the cohort. Lithium products make up 64% of the total DBP and mesalazine products make up 27% of the total.
FIG A2.
Distribution of cumulative moles of lithium ion in filled prescriptions for lithium drug products among exclusive users of lithium, according to dibutyl phthalate (DBP) exposure levels.
FIG A3.
Adjusted associations between high dibutyl phthalate exposure (≥ 10,000 cumulative mg v unexposed) under lag periods of 2 years, 1 year (the main analysis), and 0 years (no lag).
TABLE A1.
Characteristics of All Lithium Drug Products (ATC code: N05AN01) Prescribed to the Cohort
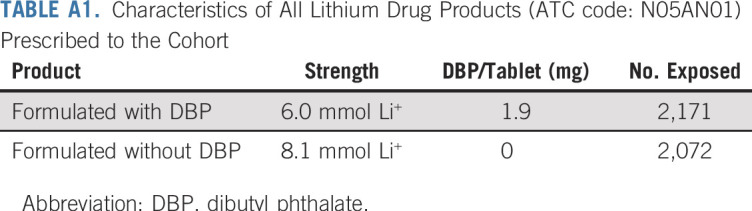
TABLE A2.
Characteristics of All Mesalamine Drug Products (ATC: A07EC02) Prescribed to the Cohort
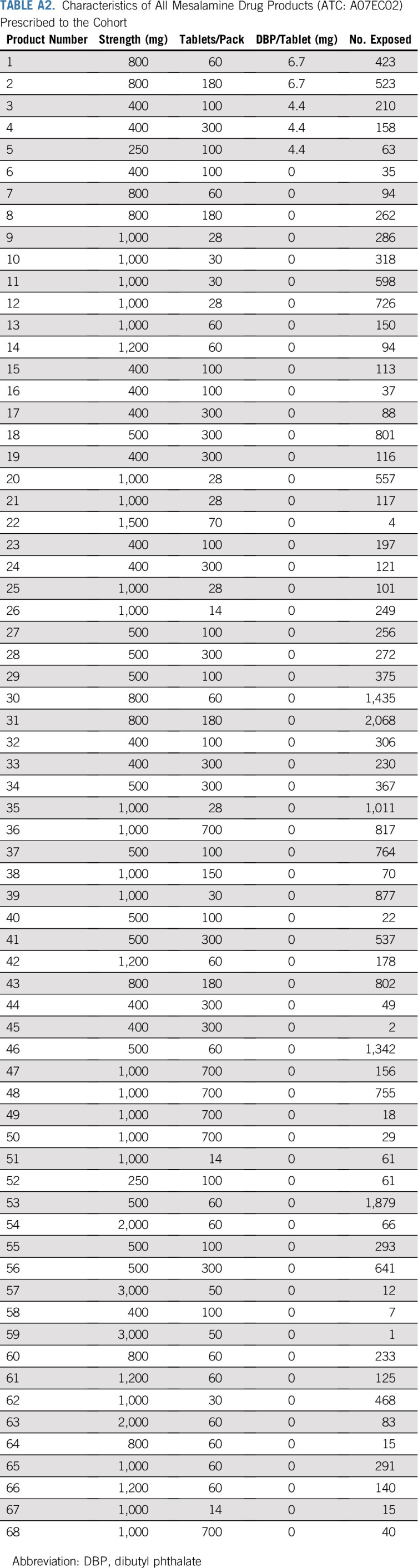
TABLE A3.
Associations Between Dibutyl Phthalate Exposure and Breast Cancer Incidence, Restricted to Exclusive Users of Lithium or Mesalamine
TABLE A4.
Baseline Characteristics of Patients Unexposed and Highly Exposed (≥ 10,000 cumulative mg) to DBP, Among Exclusive Users of Lithium or Mesalamine
TABLE A5.
Characteristics of the Prescription Drug Products That Contributed to Dibutyl Phthalate Exposure in the Cohort
Footnotes
Supported by Susan G. Komen for the Cure Grant No. CCR13264024 (T.P.A.), by funding from the National Institute of General Medical Sciences Grant No. P20 GM103644 (T.P.A.) and R01 CA166825 (T.L.L.).
Funding agencies had no role in the design, execution, interpretation, or reporting of this research study.
See accompanying Editorial on page 1775
AUTHOR CONTRIBUTIONS
Conception and design: Thomas P. Ahern, Timothy L. Lash, Per Damkier
Financial support: Thomas P. Ahern
Provision of study material or patients: Anne Broe, Deirdre P. Cronin-Fenton, Peer M. Christiansen, Henrik Toft Sørensen
Collection and assembly of data: Thomas P. Ahern, Anne Broe, Deirdre P. Cronin-Fenton, Sinna Pilgaard Ulrichsen, Peer M. Christiansen, Per Damkier
Data analysis and interpretation: Thomas P. Ahern, Timothy L. Lash, Deirdre P. Cronin-Fenton, Bernard F. Cole, Rulla M. Tamimi, Henrik Toft Sørensen, Per Damkier
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phthalate Exposure and Breast Cancer Incidence: A Danish Nationwide Cohort Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Timothy L. Lash
Consulting or Advisory Role: Amgen
Travel, Accommodations, Expenses: Amgen
Peer M. Christiansen
Honoraria: Roche Denmark
Consulting or Advisory Role: Roche Denmark
Travel, Accommodations, Expenses: Roche Denmark
Bernard F. Cole
Consulting or Advisory Role: Karyopharm Therapeutics, Daiichi Sankyo, Aperture Bio
No other potential conflicts of interest were reported.
REFERENCES
- 1.Blount BC, Silva MJ, Caudill SP, et al. Levels of seven urinary phthalate metabolites in a human reference population. Environ Health Perspect. 2000;108:979–982. doi: 10.1289/ehp.00108979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hauser R, Duty S, Godfrey-Bailey L, et al. Medications as a source of human exposure to phthalates. Environ Health Perspect. 2004;112:751–753. doi: 10.1289/ehp.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meeker JD, Sathyanarayana S, Swan SH. Phthalates and other additives in plastics: Human exposure and associated health outcomes. Philos Trans R Soc Lond B Biol Sci. 2009;364:2097–2113. doi: 10.1098/rstb.2008.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wittassek M, Koch HM, Angerer J, et al. Assessing exposure to phthalates - the human biomonitoring approach. Mol Nutr Food Res. 2011;55:7–31. doi: 10.1002/mnfr.201000121. [DOI] [PubMed] [Google Scholar]
- 5.Barr DB, Silva MJ, Kato K, et al. Assessing human exposure to phthalates using monoesters and their oxidized metabolites as biomarkers. Environ Health Perspect. 2003;111:1148–1151. doi: 10.1289/ehp.6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silva MJ, Barr DB, Reidy JA, et al. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999-2000. Environ Health Perspect. 2004;112:331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zota AR, Calafat AM, Woodruff TJ. Temporal trends in phthalate exposures: Findings from the National Health and Nutrition Examination Survey, 2001-2010. Environ Health Perspect. 2014;122:235–241. doi: 10.1289/ehp.1306681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hernández-Díaz S, Mitchell A, Kelley K, Calafat A, Hauser R: Medications as a potential source of exposure to phthalates in the U.S. population. Env Health Perspect 117:185-189, 2009. [DOI] [PMC free article] [PubMed]
- 9. Kelley K, Hernández-Díaz S, Chaplin E, Hauser R, Mitchell A: Identification of phthalates in medications and dietary supplement formulations in the United States and Canada. Env Health Perspect 120:379-384, 2012. [DOI] [PMC free article] [PubMed]
- 10.Hait EJ, Calafat AM, Hauser R. Urinary phthalate metabolite concentrations among men with inflammatory bowel disease on mesalamine therapy. Endocr Disruptors (Austin) 2014;1:e25066. doi: 10.4161/endo.25066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ennis ZN, Broe A, Pottegård A, et al. Cumulative exposure to phthalates from phthalate-containing drug products: A Danish population-wide study. Br J Clin Pharmacol. 2018;84:1798–1805. doi: 10.1111/bcp.13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broe A, Ennis ZN, Pottegård A, et al. Population exposure to phthalate-containing drugs. Basic Clin Pharmacol Toxicol. 2017;121:153–158. doi: 10.1111/bcpt.12781. [DOI] [PubMed] [Google Scholar]
- 13.Buck Louis GM, Sundaram R, Sweeney AM, et al. Urinary bisphenol A, phthalates, and couple fecundity: the Longitudinal Investigation of Fertility and the Environment (LIFE) study. Fertil Steril. 2014;101:1359–1366. doi: 10.1016/j.fertnstert.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duty SM, Silva MJ, Barr DB, et al. Phthalate exposure and human semen parameters. Epidemiology. 2003;14:269–277. [PubMed] [Google Scholar]
- 15. Broe A, Pottegård A, Hallas J, Ahern TP, Fedder J, Damkier P: Association between use of phthalate-containing medication and semen quality among men in couples referred for assisted reproduction. Hum Reprod 33:503-511, 2018. [DOI] [PMC free article] [PubMed]
- 16.Jensen MS, Anand-Ivell R, Nørgaard-Pedersen B, et al. Amniotic fluid phthalate levels and male fetal gonad function. Epidemiology. 2015;26:91–99. doi: 10.1097/EDE.0000000000000198. [DOI] [PubMed] [Google Scholar]
- 17.Kay VR, Chambers C, Foster WG. Reproductive and developmental effects of phthalate diesters in females. Crit Rev Toxicol. 2013;43:200–219. doi: 10.3109/10408444.2013.766149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolff MS, Teitelbaum SL, McGovern K, et al. Phthalate exposure and pubertal development in a longitudinal study of US girls. Hum Reprod. 2014;29:1558–1566. doi: 10.1093/humrep/deu081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giulivo M, Lopez de Alda M, Capri E, et al. Human exposure to endocrine disrupting compounds: Their role in reproductive systems, metabolic syndrome and breast cancer. A review. Environ Res. 2016;151:251–264. doi: 10.1016/j.envres.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Chen FP, Chien MH, Chern IY. Impact of low concentrations of phthalates on the effects of 17β-estradiol in MCF-7 breast cancer cells. Taiwan J Obstet Gynecol. 2016;55:826–834. doi: 10.1016/j.tjog.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Chen FP, Chien MH. Lower concentrations of phthalates induce proliferation in human breast cancer cells. Climacteric. 2014;17:377–384. doi: 10.3109/13697137.2013.865720. [DOI] [PubMed] [Google Scholar]
- 22.van Meeuwen JA, Ter Burg W, Piersma AH, et al. Mixture effects of estrogenic compounds on proliferation and pS2 expression of MCF-7 human breast cancer cells. Food Chem Toxicol. 2007;45:2319–2330. doi: 10.1016/j.fct.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Okubo T, Suzuki T, Yokoyama Y, et al. Estimation of estrogenic and anti-estrogenic activities of some phthalate diesters and monoesters by MCF-7 cell proliferation assay in vitro. Biol Pharm Bull. 2003;26:1219–1224. doi: 10.1248/bpb.26.1219. [DOI] [PubMed] [Google Scholar]
- 24.Harris CA, Henttu P, Parker MG, et al. The estrogenic activity of phthalate esters in vitro. Environ Health Perspect. 1997;105:802–811. doi: 10.1289/ehp.97105802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong EJ, Ji YK, Choi KC, et al. Conflict of estrogenic activity by various phthalates between in vitro and in vivo models related to the expression of Calbindin-D9k. J Reprod Dev. 2005;51:253–263. doi: 10.1262/jrd.16075. [DOI] [PubMed] [Google Scholar]
- 26.Hsieh TH, Tsai CF, Hsu CY, et al. Phthalates induce proliferation and invasiveness of estrogen receptor-negative breast cancer through the AhR/HDAC6/c-Myc signaling pathway. FASEB J. 2012;26:778–787. doi: 10.1096/fj.11-191742. [DOI] [PubMed] [Google Scholar]
- 27.Hsieh TH, Tsai CF, Hsu CY, et al. n-Butyl benzyl phthalate promotes breast cancer progression by inducing expression of lymphoid enhancer factor 1. PLoS One. 2012;7:e42750. doi: 10.1371/journal.pone.0042750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh S, Li SS. Epigenetic effects of environmental chemicals bisphenol A and phthalates. Int J Mol Sci. 2012;13:10143–10153. doi: 10.3390/ijms130810143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang YC, Tsai CF, Chuang HL, et al. Benzyl butyl phthalate promotes breast cancer stem cell expansion via SPHK1/S1P/S1PR3 signaling. Oncotarget. 2016;7:29563–29576. doi: 10.18632/oncotarget.9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. López-Carrillo L, Hernández-Ramírez R, Calafat AM, et al: Exposure to phthalates and breast cancer risk in northern Mexico. Env Health Perspect 118:539-544, 2010. [DOI] [PMC free article] [PubMed]
- 31.Holmes AK, Koller KR, Kieszak SM, et al. Case-control study of breast cancer and exposure to synthetic environmental chemicals among Alaska Native women. Int J Circumpolar Health. 2014;73:25760. doi: 10.3402/ijch.v73.25760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgan M, Deoraj A, Felty Q, et al. Environmental estrogen-like endocrine disrupting chemicals and breast cancer. Mol Cell Endocrinol. 2017;457:89–102. doi: 10.1016/j.mce.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 33. doi: 10.1093/jnci/djz002. Reeves KW, Santana MD, Manson JE, et al: Urinary phthalate biomarker concentrations and postmenopausal breast cancer risk. J Natl Cancer Inst 10.1093/jnci/djz002 [epub ahead of print on January 10, 2019] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frank L. Epidemiology. When an entire country is a cohort. Science. 2000;287:2398–2399. doi: 10.1126/science.287.5462.2398. [DOI] [PubMed] [Google Scholar]
- 35.Pottegård A, Schmidt SAJ, Wallach-Kildemoes H, et al. Data resource profile: The Danish National Prescription Registry. Int J Epidemiol. 2017;46:798–798f. doi: 10.1093/ije/dyw213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39:42–45. doi: 10.1177/1403494810393562. [DOI] [PubMed] [Google Scholar]
- 37.Storm HH, Michelsen EV, Clemmensen IH, et al. The Danish Cancer Registry--history, content, quality and use. Dan Med Bull. 1997;44:535–539. [PubMed] [Google Scholar]
- 38.Christiansen P, Ejlertsen B, Jensen M-B, et al. Danish Breast Cancer Cooperative Group. Clin Epidemiol. 2016;8:445–449. doi: 10.2147/CLEP.S99457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jensen AR, Storm HH, Møller S, et al. Validity and representativity in the Danish Breast Cancer Cooperative Group--a study on protocol allocation and data validity from one county to a multi-centre database. Acta Oncol. 2003;42:179–185. doi: 10.1080/02841860310000737. [DOI] [PubMed] [Google Scholar]
- 40.Pedersen CB, Gøtzsche H, Møller JO, et al. The Danish Civil Registration System. A cohort of eight million persons. Dan Med Bull. 2006;53:441–449. [PubMed] [Google Scholar]
- 41.Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–549. doi: 10.1007/s10654-014-9930-3. [DOI] [PubMed] [Google Scholar]
- 42.Ahern TP, Tamimi RM, Rosner BA, et al. Digoxin use and risk of invasive breast cancer: Evidence from the Nurses’ Health Study and meta-analysis. Breast Cancer Res Treat. 2014;144:427–435. doi: 10.1007/s10549-014-2886-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beral V, Million Women Study Collaborators Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 44.Clarke CA, Canchola AJ, Moy LM, et al. Regular and low-dose aspirin, other non-steroidal anti-inflammatory medications and prospective risk of HER2-defined breast cancer: The California Teachers Study. Breast Cancer Res. 2017;19:52. doi: 10.1186/s13058-017-0840-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mørch LS, Skovlund CW, Hannaford PC, et al. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377:2228–2239. doi: 10.1056/NEJMoa1700732. [DOI] [PubMed] [Google Scholar]
- 46. Bonovas S, Filioussi K, Tsavaris N, et al: Use of statins and breast cancer: A meta-analysis of seven randomized clinical trials and nine observational studies. J Clin Oncol 23:8606-8612, 2005. [DOI] [PubMed]
- 47.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 48.Bliddal M, Broe A, Pottegård A, et al. The Danish Medical Birth Register. Eur J Epidemiol. 2018;33:27–36. doi: 10.1007/s10654-018-0356-1. [DOI] [PubMed] [Google Scholar]
- 49. Allison P. Survival Analysis Using SAS. 1st ed. Cary, NC: SAS Institute; 2010.
- 50.Sullivan KM, Foster DA. Use of the confidence interval function. Epidemiology. 1990;1:39–42. doi: 10.1097/00001648-199001000-00009. [DOI] [PubMed] [Google Scholar]
- 51.Welshons WV, Engler KS, Taylor JA, et al. Lithium-stimulated proliferation and alteration of phosphoinositide metabolites in MCF-7 human breast cancer cells. J Cell Physiol. 1995;165:134–144. doi: 10.1002/jcp.1041650116. [DOI] [PubMed] [Google Scholar]
- 52.Andersen TF, Madsen M, Jørgensen J, et al. The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull. 1999;46:263–268. [PubMed] [Google Scholar]
- 53.Premenopausal Breast Cancer Collaborative Group. Schoemaker MJ, Nichols HB, et al. Association of body mass index and age with subsequent breast cancer risk in premenopausal women. JAMA Oncol. 2018;4:e181771. doi: 10.1001/jamaoncol.2018.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]