Abstract
Depression is common but under-treated in patients with cancer, despite being a major modifiable contributor to morbidity and early mortality. Integrating psychosocial care into cancer services through the team-based Collaborative Care Management (CoCM) model has been proven to be effective in improving patient outcomes in cancer centers. However, there is currently a gap in understanding the challenges that patients and their care team encounter in managing co-morbid cancer and depression in integrated psycho-oncology care settings. Our formative study examines the challenges and needs of CoCM in cancer settings with perspectives from patients, care managers, oncologists, psychiatrists, and administrators, with a focus on technology opportunities to support CoCM. We find that: (1) patients with co-morbid cancer and depression struggle to navigate between their cancer and psychosocial care journeys, and (2) conceptualizing co-morbidities as separate and independent care journeys is insufficient for characterizing this complex care context. We then propose the parallel journeys framework as a conceptual design framework for characterizing challenges that patients and their care team encounter when cancer and psychosocial care journeys interact. We use the challenges discovered through the lens of this framework to highlight and prioritize technology design opportunities for supporting whole-person care for patients with co-morbid cancer and depression.
Keywords: cancer, depression, whole patient, collaborative care, integrated care, health technology, qualitative methods
1. INTRODUCTION
Cancer is one of the leading causes of death worldwide. An estimated 1.7 million people in the United States were diagnosed with cancer in 2018 [129]. Lifetime prevalence of cancer is expected to be approximately 38% [31]. Advances in cancer treatments have improved the survival rate such that overall cancer death rates for all ages have decreased by approximately 1 to 2% per year during 2012 to 2016 [129]. With decreased death rates and over two-thirds of people who are diagnosed surviving five years or longer, improving quality of life has become an essential component of cancer care. Depression is one of the most common challenges for patients during and after cancer treatments, and up to 24% of patients with cancer have been diagnosed with clinically significant depression [68]. Unfortunately, depression is severely under-treated in patients with cancer, including about 25% of patients with cancer and depression that receive no depression treatment at all [126].
Cancer care has increasingly moved toward integrating psychosocial care directly into cancer services [42], and care models such as Collaborative Care Management (CoCM) have been proven to be effective in treating depression and to be cost-effective in randomized control trials [3, 22, 36, 55, 121]. CoCM is a team-based care approach with an oncologist, a care manager, and a psychiatric consultant working together to improve the patient’s psychosocial health outcomes through the use of evidence-based care practices and a patient registry to deliver measurement-based and population-based care. Despite the effectiveness of such models, a recent national survey found that approximately 80% of cancer centers lack processes to follow up and adjust treatments for patients with clinical depression [135]. Implementing CoCM is also not without its own challenges. For example, recent reviews and analyses have highlighted: (1) low fidelity in adhering to principles of effective CoCM [125], (2) lack of clarity in role definitions and boundaries, (3) challenges in long-term sustainability, and (4) lack of standard care and communication pathways and tools [83, 108, 131]. Twenty to 50% of patients are known to prematurely drop out of therapy [109, 110, 120], and patients with cancer and depression face additional challenges to engaging in care due to factors that range from weakened physical conditions, competing and overwhelming numbers of appointments, unmet logistical needs (e.g., transportation), and stigma or lack of knowledge around depression treatments. Behavioral health providers (BHPs) in cancer centers, typically trained in clinical social work, are particularly overburdened with attempting to provide depression treatments in addition to helping patients meet their navigational and financial needs.
Our research goals are: (1) to understand contextual factors and to identify challenges and needs in the depression care of patients with co-morbid cancer and depression, and (2) to identify technology design opportunities to support such care and to enhance or facilitate CoCM. We took a human-centered design approach to examining existing challenges to CoCM by conducting interviews and contextual inquiries across three urban or rural cancer centers that have implemented or aspire to implement CoCM (i.e., have implemented some components of CoCM). Prior research has examined the use of technology to support CoCM (e.g., telehealth, electronic health records, web and mobile platforms), finding it to be effective in engaging patients and treating depression [44, 70, 100, 118]. Although there have been a variety of opportunities proposed for technology enhancements of CoCM [10, 96], such technologies must be carefully grounded in concrete needs identified using human-centered design approaches [7, 8]. Furthermore, a better understanding of the needs for and feasibility of such technologies in CoCM, especially for treating depression in cancer settings, is necessary to improve effectiveness and usability of such technologies [95]. Our research thus augments this growing body of work by uncovering challenges and opportunities for design in the specific context of co-morbid cancer and depression.
Although we observed that patients with co-morbid cancer and depression experience both a cancer care journey and a psychosocial care journey consistent with prior research, our analysis revealed that considering these care journeys independently was insufficient for characterizing these patients and the challenges encountered by them and their care teams. Our research therefore also proposes a framework of parallel journeys, examining challenges and opportunities for interventions and technologies through a lens of placing these journeys side-by-side and examining where they fall out of sync.
Our specific contributions therefore include:
We propose the concept of a parallel journeys framework for examining and characterizing complex co-morbidities and care contexts.
We conduct interviews with 29 stakeholders regarding the care of patients with co-morbid cancer and depression: patients, center administrators, medical providers (i.e., oncologists, psychiatrists), and BHPs (i.e., social workers, psychologists) and contextual inquiries with 8 BHPs. Interviews and contextual inquiries were conducted at three different cancer centers.
We confirm that patients with co-morbid cancer and depression experience various phases of a cancer care journey as described by Jacobs et al. [57].
We also observe that patients with cancer and depression experience various phases of a psychosocial care journey, a concept described in psychosocial care practice guidelines that we strengthen with qualitative evidence.
We apply the parallel journeys framework to analyzing our data and examining breakdowns and challenges to care that occur at the intersection of the parallel care journeys.
We present technology design opportunities for addressing these challenges and supporting whole-person care for patients with co-morbid cancer and depression.
2. BACKGROUND
2.1. Depression and Cancer
Depression is one of the most common challenges in patients with cancer. Common contributors to depression in cancer include a patient’s psychological reaction to phases of the cancer journey (e.g., diagnosis, treatment, recovery or relapse, survivorship or end of life), social stressors (e.g., loss of a job, financial burden), and physical side effects from cancer treatment (e.g., nausea, fatigue, hair loss) [94, 112]. Recent meta-analyses have found the prevalence of depression in patients with cancer to be as high as 24% [68, 94], much higher than the 8% annual prevalence in the general population [119, 128]. Clinical depression in some patients may go undetected by oncology providers and staff who lack specialized training to diagnose mental health conditions [79, 91, 113]. In patients with cancer, depression is associated with increased mortality risk [64], longer hospital stays [86], increased risk of completed suicide [50, 81, 102], increased healthcare costs [78], decreased adherence to treatment recommendations and medications [65], and decreased quality of life [94].
Despite its high prevalence and negative impacts, depression in patients with cancer is under-treated. Seventy-three percent of patients with cancer and depression do not receive potentially effective depression treatment [126]. Patients in rural areas are more likely to receive inadequate access to mental health services compared to urban residents, partly due to a limited supply of behavioral health providers (BHPs), lack of psychiatric clinicians, and lack of on-site mental health services [35, 62, 97]. In addition, patients with co-morbid conditions (i.e., cancer and depression) encounter many barriers that limit their ability to care for their health (e.g., physical limitations, high complexity in medication management, financial constraints, compounding of symptoms) [12].
2.2. Collaborative Care Management
Because of potential direct neuropsychiatric effects of cancers and their treatments on depression [94], access to BHPs trained specifically in oncology contexts is crucial in treatment of depression for patients with cancer. However, most cancer centers are not equipped to provide comprehensive mental health care. The Institute of Medicine recommends direct integration of psychosocial services in cancer settings [89] as a cost-effective way to address inadequate treatment [80, 94], and the psychosocial oncology care framework [76] provides guidelines for achieving such recommendations. The Collaborative Care Management (CoCM) model is an evidence-based strategy for implementing these recommendations [73], a proven and cost-effective standard of care for treating depression, improving quality of life, and increasing adherence to cancer treatments [3, 22, 36, 55, 121] consistent with accepted clinical practice guidelines [2].
CoCM is based on several core principles [125]: (1) it is a team-based collaborative approach to psychosocial care with a triad of providers (i.e., as described next), (2) it is a population-based care approach responsible for the outcomes of a defined population of patients (i.e., patients identified as depressed), (3) it is a measurement-based care approach that uses validated outcome measures to monitor patients and to guide treatment decisions, and (4) it is an evidence-based approach that uses scientifically proven interventions that are effective in treating depression [5, 40].
CoCM centers around the patient. A triad of providers, each with a clearly defined role, collaborate to improve the patient’s psychosocial health outcomes. Figure 1, adapted from University of Washington AIMS Center, illustrates roles and relationships of CoCM team members in the cancer context. An oncologist is responsible for overseeing the overall care of the patient. A psychiatric consultant works with the care manager and oncologist to aid with assessment and diagnosis, to develop a biopsychosocial treatment plan, and to communicate recommendations for treatment (i.e., psychotropic medications and/or evidence-based psychosocial treatments) [11]. A care manager, who is typically a clinical social worker but can also be a nurse or psychologist, is embedded in the cancer center, consults with an on-site or remote psychiatrist with expertise in treating patients with cancer. They work closely with the care team (i.e., oncology, primary care, and other supportive care providers) to coordinate and deliver comprehensive care, to provide patient education, to deliver evidence-based psychosocial treatments, to monitor patient progress using standardized scales, and to provide systematic outreach to engage patients. [28, 33, 46]. Our work specifically examines depression care situated in the context of CoCM.
Fig. 1.
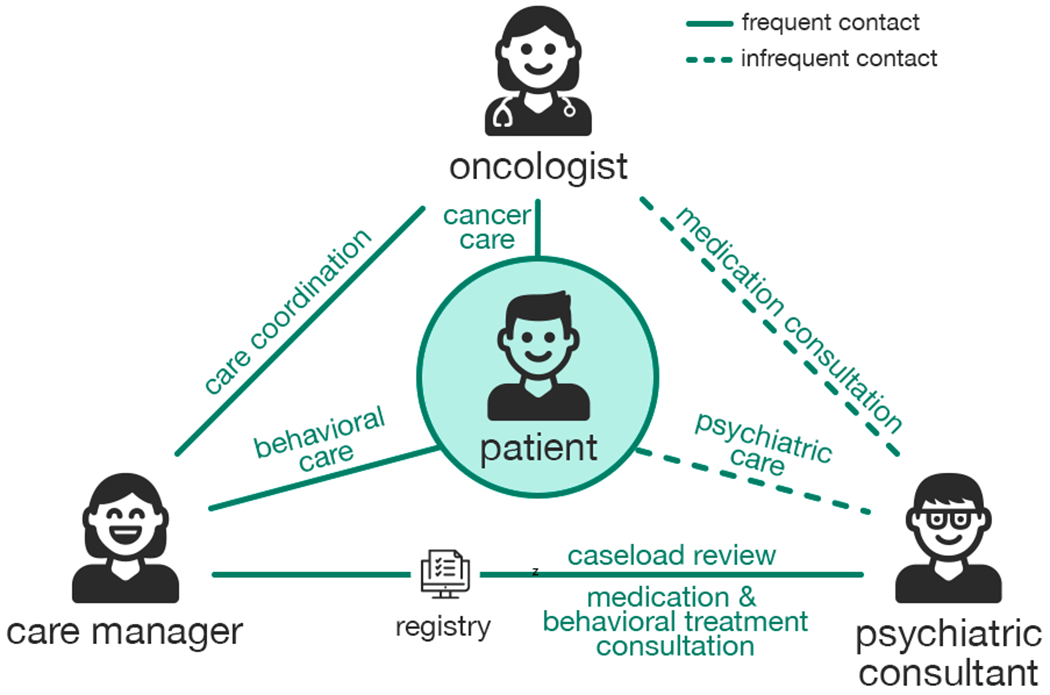
Illustration of CoCM, which centers around the patient with a triad of providers (oncologist, care manager, consulting psychiatrist) collaborating to improve the patient’s psychosocial health outcomes. Illustration is adapted from University of Washington AIMS Center (http://aims.uw.edu/).
Implementing CoCM is not without challenges. Recent reviews and analyses of CoCM have highlighted several challenges, including low fidelity to established principles of effective CoCM [125], a lack of clarity in role definitions and boundaries, challenges in the long-term sustainability of such models, and a lack of standard care and communication pathways and tools [83, 108, 131]. Other challenges are a lack of funding for non-billable services and a lack of a workforce that is sufficiently trained or available in rural areas [96]. Because these recent reviews of CoCM focus primarily on implementation of the care model in non-cancer contexts, they lack in-depth understanding of patient experiences in a co-morbid cancer and depression context and lack actionable insights for technology design to meet stakeholder needs in this context. Our research therefore aims to develop concrete technology design opportunities, focusing on identifying challenges in patient and care team experiences and developing a conceptual design framework (i.e., parallel journeys framework) to explore how and why these challenges emerge.
2.3. Technology-Supported Collaborative Care Management
Prior studies have examined addressing challenges and augmenting capabilities of CoCM with technology. For example, Kroenke et al. [70] performed automated monitoring using interactive voice response (IVR) or the Internet. Fortney et al. [44] used telephone, video conferencing, and shared electronic medical records. Rollman et al. [100] studied telephone-based communication between care managers and patients. Steel et al. [118] provided patients with web-based CoCM interventions that include written and audiovisual self-management strategies and a bulletin board. Wu et al. [133] compared usual CoCM with technology-enhanced CoCM using automated telephone assessment. A recent review of randomized controlled trials on the effect of telehealth on pain, depression, and quality of life in patients with cancer found that telehealth-enabled CoCM approaches demonstrated improvement in depression outcomes [1].
Using the core principles of CoCM as a basis for determining key tasks and processes, a comprehensive list of potential technology opportunities for enhancing CoCM has been proposed [10, 96], including a messaging platform for patient engagement, digital delivery of evidence-based psychosocial interventions and education resources, decision support tools with clinical pathways, remote self-assessment, and systematic monitoring and review of patients using a web-based registry and remote consultations. However, despite high interest in integrating technology into CoCM and promising benefits, further research is necessary in understanding the needs for and feasibility of such technologies in CoCM for various stakeholders, especially for treating depression in cancer settings [95].
2.4. Technology for Mental Health and Cancer
Computer-mediated interventions for mental health and cancer have long been of interest to the human-computer interaction (HCI) community. Early calls for research at the intersection of mental health care and HCI [29] have been met with over a decade of research investigating technological support for diverse clinical needs [103]. Computer-assisted therapy methods have been examined in a variety of contexts, including for anxiety disorders [30, 130], bipolar disorder [6], ADHD [72], phobias [132], schizophrenia [127], and depression [82, 105, 117]. The use of technology to help support mental health treatment has significant potential and has proven highly effective in a number of contexts [103].
Beyond technological interventions to address mental health, prior work has examined challenges for patient information-seeking at the individual, institutional, and infrastructural levels, uncovering challenges for providers connecting patients to local resources, a lack of mental health resources for low-income patients, and a legal system that does not address mental health needs [17]. Such research emphasizes that: (1) although technology may have significant potential to address a variety of mental health issues, such interventions need to consider the context within which they are deployed, and (2) even access to technology itself may be a barrier to mental health care.
Significant HCI research has also examined technological opportunities in cancer. For example, both patients with cancer and providers could benefit from tools that support educating the public about self-screening practices, improving accessibility of online support communities, and automatically tracking patient data [49]. Interventions for patients with cancer have included social networking features [111] and mobile applications [58] designed to surface useful information, mobile tools to support health management [59], and symptom monitoring systems [47]. Past work has examined the needs of various stakeholders across different age groups [39, 52, 77], disease types [56, 63], and geographic contexts [47]. Such interventions have generally focused on topics such as information-seeking, self-tracking, and health management, with an acknowledgement that patients in various contexts may encounter idiosyncratic challenges that require nuanced understanding to effectively address.
Given the high co-occurrence of cancer and depression [94] and given CoCM serving as the standard of care for patients with cancer and depression, our work builds on HCI research in physical and mental health interventions by highlighting and prioritizing technology opportunities specific to the context of CoCM and depression in cancer settings.
2.5. Design Framework
Prior HCI research has examined the experiences of chronic disease treatments using patient journeys as a design framework [21, 122], an approach that has been useful in guiding the design of technology interventions [38, 136]. In particular, Hayes et al. [49] describe the cancer journey in five phases: screening and diagnosis, initial information-seeking, acute care and treatment, no evidence of disease, and chronic disease and disease management. This temporal account of the cancer journey is distinct from clinical staging and trajectories of the disease and emphasizes characterizing the commonalities in patient experiences and their emotional and physical changes.
Jacobs et al. [57, 60] build on this journey metaphor to examine significant factors that contribute to an individual’s cancer journey, such as responsibilities, challenges, and personal impacts that patients face in each phase. The cancer journey framework surfaces patient challenges and needs beyond cancer treatment, including logistical issues (e.g., transportation, financial management), emotional issues (e.g., coping with diagnosis, anxiety about recurrence), clinical issues (e.g., treatment decisions and adherence), and cognitive issues (e.g., information overload, “chemo brain”). Such frameworks can be used to inform the design of technology tools specific to each phase of the cancer journey. Although this framework accounts for how cancer influences a patient’s psychosocial aspects and is useful in designing support for patients with cancer, little research has addressed the challenges of simultaneously treating cancer and its co-morbidities.
Psychosocial care practice guidelines [2, 45] provide a map of how different stages or components of psychosocial care could be sequenced in cancer settings. These guidelines suggest how patients may navigate stages of psychosocial care, but primarily provide general recommendations from the perspective of care practitioners. Informed by these guidelines, our research provides greater understanding of the patient psychosocial care journey through interviews and contextual inquiries with multiple stakeholders. We also highlight additional complexities and challenges introduced by concurrent depression and cancer care.
3. METHOD
Two research questions that guided our study are:
-
RQ1
What are the challenges and needs that patients with co-morbid cancer and depression and their care team encounter in providing depression care?
-
RQ2
What are technology design opportunities for supporting patients with co-morbid cancer and depression and enhancing integrated depression care?
To answer these research questions, our study included a combination of semi-structured interviews and contextual inquiries. We conducted interviews of multiple stakeholders to obtain multiple perspectives on current practices and challenges surrounding the care of patients with co-morbid cancer and depression. We also conducted contextual inquiries to complement and contextualize our interview data by observing care practices as they unfold in real-world situations. Although our ultimate goal is to identify technology design opportunities, we ensured that our data collection was not exclusive to technology use in order to explore contextual factors and challenges that may influence the design and adoption of technology.
To ensure the safety of patients and maintain high ethical standards for the recruitment and study procedure, we worked closely with the coordinating institution’s Institutional Review Board (IRB) as the single IRB of record, with one behavioral health provider (BHP) from each site, and with psychiatry and behavioral science experts within our research team.
3.1. Study Procedure
3.1.1. Sites.
We conducted our study in three cancer centers that varied across dimensions of: (1) urban to rural according to Rural-Urban Continuum Codes (RUCC) [107], and (2) extent of existing CoCM implementation. Specifically, sites included two urban (RUCC = 1) cancer centers and one rural (RUCC = 5) cancer center. One urban site (i.e., Site 1) had implemented CoCM, including BHPs playing the role of care managers (e.g., clinical oncology social workers) assigned to different cancer specialties, embedded psychiatrists, weekly systematic caseload reviews and consultations between BHPs and psychiatrists, and an Excel-based registry to manage and track patients receiving behavioral health care at the cancer center. The other two sites (i.e., urban Site 2, rural Site 3) were aspiring to implement CoCM with limited integrated psychosocial care. Both sites offered evidence-based psychosocial treatments through BHPs who were embedded within the cancer center, but they lacked access to dedicated psychiatrists, process and workflow to support systematic follow-up and measurement-based care, and a registry for caseload management. We chose to study these sites with differing characteristics to ensure that our data derived from sites with variation in urban to rural locations and their experience with CoCM. Table 1 (top) summarizes site characteristics.
Table 1.
Site characteristics (top) and interview participants from each site (bottom). Participant categories include patients (Pt), administrators (A), behavioral health providers (BHP), medical providers (MP).
| Site 1 | Site 2 | Site 3 | ||
|---|---|---|---|---|
| Site Characteristics | Location CoCM Implemented | Urban Yes |
Urban No |
Rural No |
| Interview Participants | Patients (Pt) | 3 | 3 | 5 |
| Administrators (A) | 1 | 1 | 1 | |
| Social workers (BHP) | 4 | 2 | 2 | |
| Psychologists (BHP) | 0 | 1 | 0 | |
| Psychiatrists (MP) | 1 | 0 | 1 | |
| Primary care physician (MP) | 0 | 1 | 0 | |
| Oncologist (MP) | 1 | 1 | 1 | |
| Total | 10 | 9 | 10 | |
Due to the sensitive nature of depression and a vulnerable patient population, our study required significant coordination and collaboration from the local sites. We held an orientation meeting with various site representatives from each cancer center (i.e., site administrators, providers, medical directors) to present our study objectives and general procedures. We then identified primary points of contact (i.e., one BHP from each site) who helped finalize participant recruitment procedures, facilities use, scheduling, and compensation.
3.1.2. Participants.
At each site, we recruited four types of participants: behavioral health providers (BHP), medical providers (MP), administrators (A), and patients (Pt). BHPs are clinicians responsible for delivering psychosocial treatments and coordinating psychosocial care. These participants primarily consisted of clinical oncology social workers, with the exception of one psychologist. Medical providers consisted of oncologists as well as psychiatrists and primary care physicians. Administrators consisted of a cancer center manager, a clinical services manager, and a clinical social work manager who assumed the role of overseeing the operations and workflow of behavioral care at the cancer center. We relied on points of contact from each cancer center to identify and recruit BHPs, medical providers, and administrators.
Once identified, BHPs helped with patient recruitment in two ways. After providing BHPs with a study information handout and recruitment guidelines, we asked each BHP to identify several of their active patients whose recent PHQ-9 [69] (or equivalent) scores were 10 or above (i.e., moderate to severe depression) and to ask patient permission for the study team to contact them to participate in the interview study. In addition, each BHP contacted and obtained permission from patients whose sessions with the BHP would be observed as part of a contextual inquiry.
Across three sites, we conducted semi-structured interviews with 29 participants (11 patients, 9 BHPs, 6 medical providers, 3 administrators). Of 11 patients interviewed, 7 reported as female, 3 reported as male, and 1 did not report any gender. Six out of 11 patients interviewed reported as ethnic minority. Patient age ranged from 24 to 89 years old with mean of 47.0 and standard deviation of 18.0. Table 1 (bottom) also summarizes the number of interview participants from each site. Of the 9 BHPs interviewed, 8 were also shadowed and observed through contextual inquiry. As a result of contextual inquiry, we observed 10 unique patient sessions in Site 1, 9 in Site 2, and 7 in Site 3. We did not collect any demographic information for patients observed as part of a contextual inquiry because these patients were not the primary focus of the contextual inquiry. Of the 26 unique patients observed during contextual inquiry, one was also interviewed.
3.1.3. Interview.
We conducted semi-structured interviews of multiple stakeholders to collect their perspectives on care experiences in their own words and to triangulate common challenges and needs. The focus of each interview varied slightly based on the type of participant. With patients, our interview questions focused on understanding their experiences surrounding the depression treatment they received, coordination of psychosocial appointments, communication with their providers, and their practices and tools used in addressing their depression and symptoms. With BHPs, we focused on their workflows, tasks, and tools used in providing and coordinating psychosocial care within and outside of their sessions with patients and in communicating and collaborating with different providers. We also probed their experiences in providing evidence-based interventions, specifically behavioral activation (BA), which is an effective treatment for depression [34, 37]. With psychiatrists (or with a primary care physician if the site lacked access to a psychiatrist), interview questions were similar to those for BHPs with added attention to aspects that differentiated their roles (e.g., recommending psychopharmacological interventions or psychiatric consultations). With oncologists, we asked about their involvement in the psychosocial care of their patients, including detection of depression symptoms, communicating and coordinating care with psychosocial or psychiatric care providers, and managing treatments and medications. With administrators, our interview questions focused on operational and financial aspects of psychosocial care, monitoring the quality of psychosocial care, and adoption of new programs or technologies. These interview topics helped structure our interviews, but our interview protocol was open-ended such that we could further explore challenges in any of these topic areas with all participants.
During the interview, providers were asked to refer to a specific patient encounter to ground their responses (e.g., “please recall a last patient or session that…”). These were not necessarily the same patients we interviewed or observed while shadowing BHPs. To preserve patient confidentiality, we explicitly did not gather patient identifiers from the providers or health records. We instead captured general patient characteristics (e.g., female stem cell transplant patient), behaviors (e.g., checking texts messages on the phone), and affect (e.g., anxiety from infusion) from the conversation. Each interview lasted approximately an hour; 26 were conducted in-person and 3 by phone. Interviews were audio-recorded and later transcribed. Each interview participant was compensated with a $50 gift card.
3.1.4. Contextual Inquiry.
Contextual inquiry [15] allows for observing actual behaviors and situations as they unfold in real world contexts. The primary focus of our contextual inquiries was BHPs. We were interested in: (1) gaining a richer understanding of existing workflows and practices, of competing tasks and demands, and of technology use in the delivery of psychosocial care, and (2) observing a wide variety of patients and treatment techniques employed by the BHPs.
A typical contextual inquiry session started in the morning with a quick orientation to the day’s schedule and the patients we would be observing. During observation, we often sat next to the BHPs, listened to their conversations, watched their use of tools (e.g., desktops, mobile devices, printers, notebooks), and traveled with them to their patient sessions and meetings. When observing patient sessions, we did not interfere with the sessions and sat in the room in a way that avoided a patient’s direct line of sight. Outside of patient sessions, we asked the BHPs to think aloud if the circumstances allowed it (e.g., charting at their desks), and we asked clarifying questions in-the-moment or at a later opportune time (e.g., en route to sessions, during lunch breaks).
Each contextual inquiry session with a BHP lasted between 2 to 8 hours and consisted of one or more patient sessions ranging from approximately 15 minutes to an hour. Most patient sessions were scheduled, with the exception of 3 ad-hoc sessions. To address concerns of potentially coercing patients into being observed, each corresponding BHP obtained permission in advance from their patients for our research team to observe their sessions before our visit. We took notes throughout the observation, captured photographs of the facilities and work spaces with the permission of administrators, and received copies of psycho-education materials used in the session. No compensation was offered for participation in contextual inquiries.
3.2. Analysis
We combined interview transcripts and observation notes to create a single dataset. All potential identifiers (e.g., names, places) were manually removed by the researchers that collected the data. Our general analysis approach was to draw out challenges and needs for answering RQ1 and then to synthesize technology design opportunities for RQ2. Here we describe our analysis of challenges in greater detail, which involved two stages: (1) enumeration of challenges, and (2) characterization of challenges through patient care journeys.
3.2.1. Identification of Barriers and Facilitators.
To understand what challenges exist in our data, we identified barriers that hinder the progress of depression care and facilitators that enable depression care in cancer settings. Three researchers qualitatively coded the interview and observation data using inductive thematic analysis [19]. Two of the three researchers were already familiar with the data, having collected and anonymized most of the data. All interview transcripts and observation notes were entered into qualitative data analysis software (i.e, ATLAS.ti). Researchers independently examined a subset of assigned transcripts and notes, tagging parts of the texts with “memos” [16] that summarize the dialog or observation (e.g., “BHPs are responsible for organizing patient support groups”). We held collaborative memo extraction sessions for a subset of data to align our process and reconcile disagreements. The researchers were encouraged to reuse existing memos if the extracted quote could be described with an existing memo. Although technology-related themes emerged from our analysis, the researchers reframed the technology themes as the underlying stakeholder needs that the technology addresses. We then collaboratively merged, split, and refined extracted memos, using consensus meetings to organize them into emerging barriers and facilitators to psychosocial care. We iteratively repeated these processes of extracting memos independently and collaboratively organizing memos into emerging themes of barriers and facilitators (e.g., “BHPs have many competing demands”). At the end of the coding process, three researchers collaboratively reviewed all barriers and facilitators and the underlying memos and quotes to reach an agreed level of cohesiveness and consistency within each theme. We extracted the underlying challenges that our identified facilitators supported and merged those underlying challenges with barriers into a combined set of challenges. We incorporated technology-specific facilitators into our consideration for technology design opportunities.
3.2.2. Development of Psychosocial Care Journey.
After the enumeration of challenges, we organized our data into a temporal care journey to map each challenge to a certain point in time of patient care. Our construction of a psychosocial care journey involved mapping out the timeline of all stakeholder experiences through the care process based on three sources of data: (1) patient recollection of their own care experiences, (2) provider accounts of how their patients experience care, and (3) our observation of patient experiences. On the horizontal, temporal axis, we had various transition points and phases of care informed by psychosocial care practice guidelines [2]. On the vertical, stakeholder axis, we listed the patient and members of the care team and illustrated any actions performed by or interactions between stakeholders. We then mapped several challenges at different points of the care journey. Figure 2 provides a snapshot of this process. We found this exercise lacked the expressivity required to characterize the complex care paths of patients. This realization led to the development of the parallel journeys framework which we describe in detail in Section 4.1.
Fig. 2.
A snapshot of the psychosocial care journey mapping exercise for organizing challenges we discovered in our data. The horizontal axis illustrates different phases of psychosocial care, and the vertical axis describes different stakeholders and their interactions.
4. FINDINGS
We organize our findings according to our research questions. We first address RQ1, presenting contextual findings that helped us understand the challenges and needs that patients and their care teams face in receiving and providing depression care in cancer settings. Section 4.1 describes patient care journeys and our development of the parallel journeys framework. Section 4.2 applies the framework to characterize challenges identified in our data. We generally found contextual factors to be more prominent in our data because most participants had minimal use of technology in their current care practices. We then address RQ2, presenting technology-specific findings synthesized to address identified challenges. Section 4.3 presents technology design opportunities for supporting depression care of patients with cancer and describes why these opportunities are specifically important for our patient and care team context.
4.1. Parallel Journeys Framework
4.1.1. Cancer Care Journey.
The emotional and physical experiences of patients with cancer can commonly be characterized by phases of a cancer journey [49]: screening and diagnosis, information seeking, acute care and treatment, no evidence of disease, and chronic disease and disease management. Jacobs et al.’s cancer journey framework [57, 60] further characterizes these phases by incorporating patient responsibilities, challenges, and personal impacts in each phase.
The patients that we interviewed, that we observed during contextual inquiries, and that were described by providers, experienced these phases of a cancer care journey while managing their patient responsibilities. They described encountering challenges in care and experiencing changes in emotions and life. Pt13, whom we observed while shadowing BHP2, had recently been diagnosed with cancer and was finalizing treatment decisions and financial adjustments in preparation for active treatments; he began feeling a general sense of “gratitude and compassion” since the diagnosis. Pt1 described the process of getting her body prepared for stem cell transplant as being “really rushed,” and Pt2 explained that, in hindsight, she did not fully process all the diagnostic information given to her because “people were making [her] feel like everything was not a big deal when it was a huge deal.” Four of the patients we observed while shadowing BHPs were actively being treated in the infusion suite, and BHP5 described the care environment as “it’s best to always expect things will not go smoothly (laughs).” When we interviewed Pt1 just after she had finished her transplant procedure, she mentioned that her struggle was in “trying to figure out what [her] new normal is going to be.”
Our analysis revealed that the challenges that patients with cancer and depression expressed around depression care could not fit squarely within one or more of these phases of cancer care journey. For example, psychotropic medications used to treat depression could interfere with cancer treatment, thus requiring significant coordination between the psychiatrist and the oncologist. Pt8 developed severe anxiety towards chemotherapy infusions that required a BHP’s physical presence to continue with treatment. Patients often feel too sick from cancer treatments and may cancel their appointments with their BHPs, which impacts the continuity of their depression care. Although the cancer care journey can be used to characterize a range of emotional and psychosocial aspects of cancer (e.g., attitude change, return to normal), its phases are delineated by the physical aspect of cancer (i.e., around the diagnosis, treatment, and recovery from cancer) and does not account for the context introduced by co-morbid psychiatric disorders that include dedicated treatments and unique patient experiences. Therefore, the cancer care journey alone is insufficient in characterizing such co-morbid contexts.
4.1.2. Psychosocial Care Journey.
As described in Section 3.2.2, we generated a timeline of how patients experienced their depression care, organized into four key phases of a psychosocial care journey. We found that patients with depression experience four common phases of psychosocial care (see Figure 3): (1) identification of patients with depression, (2) initial psychosocial assessment, diagnosis, and rapport building, (3) active depression treatment, and (4) maintenance and relapse prevention planning. These phases of a psychosocial care journey align with practice guidelines recommended for treating major depressive disorder [45], psycho-oncology care practice guidelines [2], and CoCM guidelines and care responsibilities that include screening of patients for depressive symptoms, delivering evidence-based behavioral and/or pharmacological treatments to improve patient outcomes, systematic review of patients with psychiatric consultants and adjustment of treatment for patients who are not improving, and relapse prevention planning [40, 124]. Our data from patient recollection of care, BHP description of how they engaged with patients, and our observations of BHP sessions with patients at different points of care (e.g., initial engagement, active treatment, follow-up) provide qualitative evidence and a nuanced understanding of how patients with cancer and depression experience these phases. Here we formally introduce the four phases by defining their care goals and providing evidence of how our patients with cancer and depression experience the four phases of the psychosocial care journey.
Fig. 3.
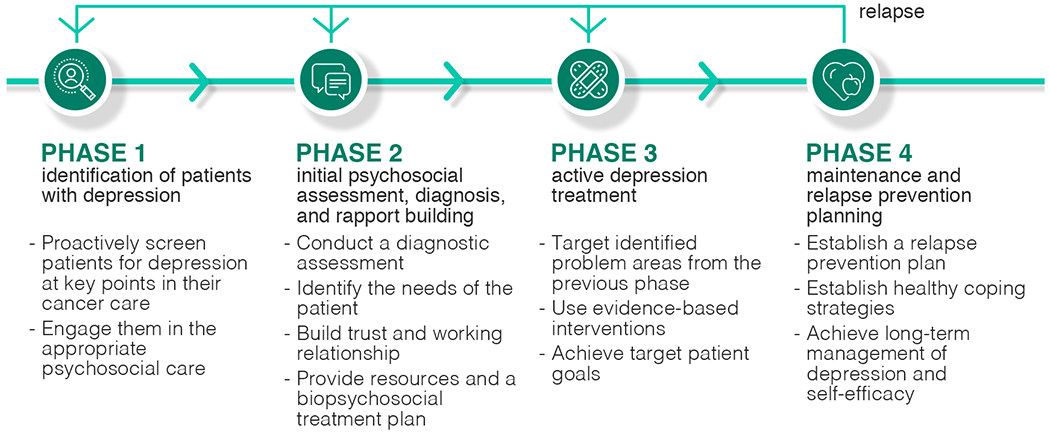
Four phases of a psychosocial care journey: (1) identification of patients with depression, (2) initial psychosocial assessment, diagnosis, and rapport building, (3) active depression treatment, and (4) maintenance and relapse prevention planning. Patients may experience a relapse and fall back to the first three phases.
Phase 1:
In the identification of patients with depression phase, patients are screened for depressive symptoms at various intervals throughout their cancer care using standard and validated screening tools such as the Distress Thermometer [101] or the 2-item Patient Health Questionnaire (PHQ-2) [75]. The goal of this phase is to proactively screen patients for depression at key points in their cancer care and to engage them in appropriate psychosocial care to address potential barriers to engagement in cancer care and to improve health outcomes. When a patient reports depressive symptoms above a critical threshold, the care team refers the patient to the appropriate BHP for a thorough assessment: “The information from the distress screening device is entered [into the EHR]. If they’ve hit a certain threshold, it triggers an alert to social work. And then social work calls and follows up with them.” (BHP3). Patients with pre-existing depression that require coordination with an outside psychosocial care provider or continued management of their condition are also referred to BHPs: “If a medication seems to be indicated or if they have a more complex mental health background, then psychiatry or psychology would be an appropriate referral. But it really should happen after they’re referred to social work.” (BPH9).
Phase 2:
After patients are identified as potentially depressed and referred to a BHP, they move to the initial psychosocial assessment, diagnosis, and rapport building phase. The goal of this phase is to gain a deeper understanding of the whole patient, to assess the needs of the patient, to build trust and a working relationship, to conduct a diagnostic assessment, and to provide resources and a depression treatment plan. The patient meets with a BHP who performs a full psychosocial assessment of the patient by using validated tools to establish baseline symptom severity (e.g., 9-item Patient Health Questionnaire (PHQ-9) [69], 7-item Generalized Anxiety Disorder (GAD-7) [115]), by eliciting background information (e.g., family and medical history, psychiatric and substance use history, financial background, social support, practical needs), and by conducting a comprehensive clinical evaluation to establish a diagnosis prior to initiating treatment [4]. Through one or more interactions with the patient, the BHP builds rapport with the patient “to best know them and support them” (BHP4), “to open up or unpack what [BHPs] need to track them” (BHP6), “for patients to begin to trust more and join with [BHPs]” (BHP5), and “to get a patient buy-in [and know] whether or not they’re gonna continue to ask for your support” (MP2). This phase also provides an opportunity for patients to receive psycho-education and supportive resources: “One of the things I try and do with patients is also let them know the different supportive care resources here, and that any of them, whether it’s psychiatry, psychology… it’s okay for them to see any of… It’s really giving ‘em permission.” (BHP6).
Phase 3:
After patients have worked with BHPs and oriented themselves to available psychosocial support, the next phase of the psychosocial care journey involves active depression treatment. The goal of this phase is to target identified problem areas from the previous phase and to achieve target patient goals (e.g., remission of depression as indicated by low PHQ-9 scores, cancer treatment adherence). BHPs provide brief evidence-based treatments (e.g., problem solving therapy, motivational interviewing, cognitive behavioral therapy (CBT), behavioral activation (BA), acceptance and commitment therapy) during scheduled sessions (e.g., weekly, bi-weekly) based on the needs of the patients: “I will be doing BA with him mostly because of the way he and his wife talked about his activity level… he didn’t identify with the word depressed, but he said, ‘I just don’t feel like doing anything.’ Like the lack of motivation, and he’s less talking about like, ‘I’m thinking about X, Y and Z,’ where… if he’s having thoughts that are getting in the way, that would make a difference. I would do… be doing CBT with him.” (BHP4). Patients regularly complete patient-reported outcomes (e.g., PHQ-9, GAD-7) and perform tasks or assignments between sessions for continuous care and positive reinforcement: “I really do enjoy opening the sticker… the actual peeling of the sticker, and putting it next to my whatever it, my goal… whatever it was. It makes you feel good. So she actually prescribed that you should get this activity journal, log it, put the sticker next to it.” (Pt8). Patients that need psychotropic medication management or that have additional co-morbidities (e.g., co-morbid anxiety, substance use disorders) are typically also seen by the psychiatrists.
Throughout active depression treatment, BHPs monitor the progress of patients using assessment tools and their clinical judgement: “I kind of rely on the [assessment] tool to sort of be the indicator of how things are going.” (BHP6). BHPs systematically monitor all patients under their care, proactively reach out to patients when necessary, and conduct regular caseload reviews with psychiatric consultants to make treatment adjustments. For patients who are discharged after completing their cancer treatments or who move away from the cancer center, BHPs facilitate referrals to and coordination with community providers for continued engagement in care: “There are some patients who have serious mental illness1 and I actually think need to be in a community mental health center where they have a case manager. And so earlier on I will try to get them involved as soon as possible once they’re like physically well enough to be able to travel to that place. And then we’ll work together for a while and then I’ll try to sort of let the community mental health agency take over.” (MP2).
Phase 4:
When a patient’s depressive symptoms substantially improve and the treatment target has been reached (e.g., depression remission), patients move to the maintenance and relapse prevention planning phase. The goal of this phase is for patients to achieve long-term remission of depression and maximize self-efficacy. Patients work with their BHPs to establish a relapse prevention plan that includes identifying early warning signs (e.g., changes in sleep, mood), establishing effective coping strategies (e.g., taking medications, reducing stress, engaging in pleasurable activities), and contacting BHPs if symptoms re-emerge [53].
As with a cancer care journey, the psychosocial care journey alone is insufficient for characterizing care experiences of patients with cancer and depression. For example, depression care could continue beyond cancer treatment, but because behavioral health services are often embedded in and limited by cancer center resources, patients who move away after active cancer treatment or are discharged from the cancer center may need to search for new providers. Recurrence of depression could place patients back into the psychosocial care journey, and such patients may be engaged in different phases of cancer care, introducing complications of coordinating multiple care resources.
4.1.3. Parallel Journeys Framework.
Both the cancer care journey and the psychosocial care journey were alone limiting in characterizing the challenges of patients with cancer and depression, in part because the combination of the two journeys further complicate the patient experience by exacerbating the characteristic challenges of each of them. To address this limitation, we developed the concept of a parallel journeys framework as a conceptual design framework to examine these journeys together and to help organize our data. To illustrate this concept of parallel journeys, we describe the parallel journeys of three patients extracted from our qualitative data. Pt12’s experiences were described by MP2 during her interview. Pt1 discussed her own experiences during her interview. Pt13’s experiences were constructed based on our observation while shadowing BHP2. We also listened to BHP2 discuss Pt13 with a nutritionist, and we asked clarifying questions about Pt13 to BHP2 outside of the session.
Prolonged engagement in psychiatric care:
Pt12 (Figure 4 top) has experienced life-long depression prior to her breast cancer diagnosis. Because of the complexity of her depression and a need for psychiatric support, she is immediately referred to see a psychiatrist. Upon completion of her chemotherapy, she is placed on hormone modulating therapy for the next five years with regular monitoring for recurrence of cancer. Because she is still being treated by the cancer center, she remains actively engaged in the psychiatric care that the cancer center provides once every two months.
Fig. 4.
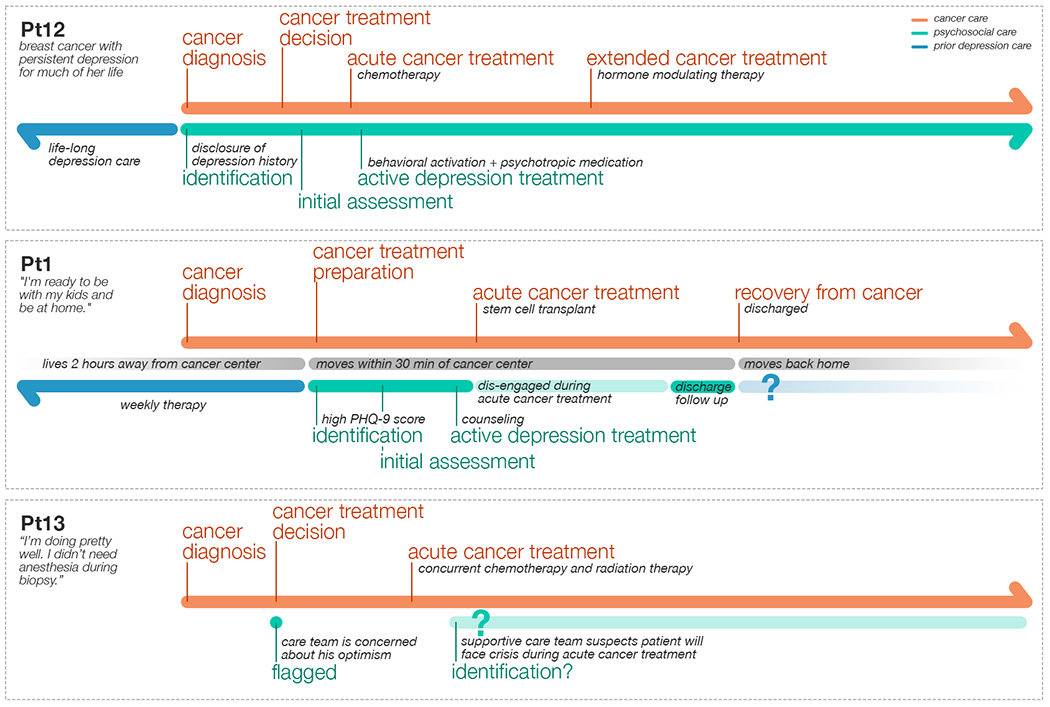
Illustration of parallel journeys for three patients with cancer and depression. Different patients engaged in the psychosocial care at different phases of their cancer care. These patient journeys were constructed from a medical provider interview (Pt12 described by MP2), a patient interview (Pt1), and observation of a BHP (Pt13 session with BHP2).
Pt12’s hormone modulating therapy will last five years, during which time she will be eligible to see the psychiatrist at the cancer center. Although the cancer diagnosis had initially caused her depression to be severely decompensated, the psychiatrist recommends she see an outside BHP regularly because the psychiatrist does not have more frequent appointment availability: “From a therapeutic standpoint… she would probably do better with more frequent therapy. We just simply don’t have the availability.” The length of her cancer treatment puts a strain on her depression as well as the psychosocial care team at the cancer center who is obligated to treat existing patients while turning away new patients due to an overbooked schedule.
Abrupt termination of depression care:
Pt1 (Figure 4 middle) moved across the state to receive a stem cell transplant2. Prior to her cancer diagnosis, she normally saw a therapist every week to manage her depression. The standard preparation phase of her transplant process includes a full psychosocial assessment by a BHP, so her initial PHQ-9 assessment revealed a high level of depression and a need for continued psychosocial follow up. Her transplant was urgent, so she was disengaged from her depression care with no support during three weeks of acute cancer treatment. Her follow-up session with the BHP happened when she was ready to be discharged from the hospital. She lived away from her home, family, and established depression care for a total of two and a half months to receive the cancer treatment. She moved back home as soon as the cancer treatment was completed, and she was not able to develop an ongoing relationship with the BHP at the cancer center or to receive a proper transition plan.
Pt1 faced two challenges during her depression care. First, her depression care was paused during transplant: “if my circumstances were a little different, because my transplant was a little rushed… I probably would’ve scheduled something with [BHP] every week.” Second, her follow-up session with the BHP was focused on her wanting to move back home earlier than recommended, and she left the cancer center without a proper transition plan to continue her depression care. She lives two hours away from the cancer center and wished for a phone follow up: “she could’ve just called me for a few minutes just to like say, check in, and see how I was doing.” Her abrupt transition out of depression care illustrates some of the missed opportunities that contribute to under-treatment of depression in patients with cancer.
Anticipated crises during active cancer treatment:
Pt13 (Figure 4 bottom) was diagnosed with head and neck cancer with a part-time income that barely covered his living expenses. He was referred to the BHP by the oncology staff because his family seemed to be more distressed than the patient himself about the diagnosis. He was scheduled to receive concurrent radiation and chemotherapy treatment that is notoriously difficult on the body. However, he had an optimistic view about his treatment and was willing to remain employed throughout treatment to support himself. Although his depression and anxiety assessment scores did not currently indicate clinically significant symptoms, the BHP flagged the patient for monitoring because she suspected he will need help when the intensity of the treatment starts to affect his physical capacity to work and may negatively impact his mental health.3
Pt13 had not yet been diagnosed with depression, but his supportive care team suspected that the severity of his cancer and treatment and possible subsequent unemployment would put him at high risk for depression4. If he develops depressive symptoms during his acute cancer treatment, as anticipated by his care team, he would need to be identified through a screening process to receive depression care. Without a proper screening process, his depressive symptoms could be easily overlooked because oncology providers are not focused on diagnosing mental health disorders and male patients often do not self-disclose their depressive symptoms [79, 91, 113].
As Figure 4 illustrates, the care experiences of these three patients are vastly different from each other. These experiences represent not only three distinct ways that patients with cancer engaged or would engage in their depression care, but also highlight unique challenges brought on by the interaction between cancer care and depression care. Patients could be engaged in depression care for a prolonged period due to the length of their cancer treatment (Pt12), or depression care could abruptly end because cancer treatment completed (Pt1). Acute cancer treatment could lead to disengagement from depression care (Pt1), or it could cause depressive symptoms (Pt13).
4.2. Two Journeys, Additional Challenges
The three patient experiences described above demonstrate that different patients engage in depression care at different phases of their cancer care according to their cancer diagnosis, treatments, physical and emotional conditions, and personal and environmental circumstances before, during, and after their cancer care journey. In order to consider the challenges that patients with cancer and depression navigate when these parallel journeys interact, we examine the cancer care journey described by Jacobs et al. [57, 60] alongside the psychosocial care journey as a design framework, which we refer to as a parallel journeys framework. This framework is used to organize our findings, specifically the impact on patients and their care team (e.g., BHPs, psychiatrists, oncologists) due to: (1) the interactions and sometimes competition between the two care journeys, and (2) challenges introduced by the current cancer-centric processes of care. Table 2 demonstrates the use of this framework by providing an example challenge that we observed at each intersection point between the two care journeys. Although we did not observe challenges for every possible intersection, these examples show how each stage of the psychosocial care journey produces challenges in at least one stage of the cancer care journey, and vice versa.
Table 2.
Challenges at the intersections between psychosocial care journeys and cancer care journeys. Columns represent phases of a psychosocial care journey, and rows represent phases of a cancer care journey. At the intersection of the two journeys, we provide an example challenge identified in our data, noting that additional unobserved challenges may exist.
| Identification of patients with depression | Initial assessment, diagnosis, and rapport building | Active depression treatment | Maintenance and relapse prevention planning | |
|---|---|---|---|---|
| Cancer screening and diagnosis | Information overload from both cancer diagnosis and supportive care resources (Pt10) | Patients under-report depressive symptoms during initial assessment (BHP1) | Depression is decompensated with cancer diagnosis (MP2) | Cancer diagnosis can exacerbate existing stressors and risk factors (MP1) |
| Initial information-seeking | Patients hide depression following cancer diagnosis (MP1) | Patients may get tired of paperwork or refuse initial assessment (MP2) | <unobserved> | <unobserved> |
| Acute cancer care and treatment | Oncology staff may not screen for depression (A1) | Initial assessment can be difficult when cancer-related stressors take precedence (BHP3) | Lack of energy impedes depression treatment (MP5) | <unobserved> |
| No evidence of disease | Depression may not be identified when not seeing oncologists or BHPs (BHP5) | <unobserved> | Patients are not transitioned to community providers or lose access to BHPs following cancer treatment (Pt1) | <unobserved> |
Such a framework can also be useful in identifying challenges that may not be directly observed in data. For example, we did not explicitly interview patients who developed depression after their cancer treatment. However, one BHP explained the importance of monitoring patients after cancer treatment [32, 84, 92]. When her previous transplant center enforced conservative post-treatment guidelines (i.e., patients are sequestered at home for 6 months to a year), she would strategize with patients on productive use of time at home to counter feelings of depression that may accompany social isolation (BHP5).
We also found that interactions between the two journeys impact the stakeholders differently, suggesting a third dimension of stakeholder role in the organization in Table 2. Because our goal is to design technology for providing behavioral health care and for supporting patients and their care team, we present these multi-dimensional challenges primarily by phases of the psychosocial care journey and according to different stakeholder perspectives. Then, in each subsection, we highlight specific interactions with phases of the cancer care journey.
4.2.1. Psychosocial Care Journey: Identification of Patients with Depression.
We observed a number of challenges in detecting depression in patients with cancer (Table 2, column 1). The best practice guideline for patient identification is to longitudinally screen for patient distress throughout their cancer journey using validated assessment tools [42, 85, 93], necessary because depression can be undetected in patients with cancer who may develop it at any point during their cancer journey and because oncology providers are not focused on identifying mental health conditions [79, 91, 113]. With inadequate screening, BHPs are confronted with emotional crises that arise from underlying depression. However, we observed that all three sites did not administer distress screening longitudinally (e.g., PHQ-2). Screening was instead administered at the initial oncology consultation and sometimes at other pivotal moments (e.g., after treatment, at relapse), a timeline defined by the cancer journey rather than the psychosocial care journey.
Patient Perspective:
Some patients hesitated to report depressive symptoms due to unwanted consequences: “And if you’re honest and you say that you consider depression, all the brakes come on and that’s a bad thing. Because that makes people- or it makes me not wanna rec- put down that I’m depressed. It’s gonna get negative attention. It doesn’t get positive.” (Pt5). It was common for patients with cancer to describe feeling pressured to be strong. Patients we observed felt they had to hide their vulnerability or even their disease from their children or family and did not have a safe place to be vulnerable. One patient said, “Oh my gosh. It took me forever to really admit it, ‘cause I was … I didn’t have to put on bracelets that said,… you’re strong. And I was just, like, I can’t be depressed, I can’t be depressed. And it just finally, it was, like, I give up.” (Pt8). Some patients normalized depression as an obvious side effect of cancer: “[They] laugh it off and say, ‘well I’ve got cancer of course I’m depressed.’” (MP4). Some patients expect depression to go away when cancer goes away: “They say ‘Well, this is just related to my cancer. I treat my cancer, and you know… I won’t have to worry about my mood.’” (MP5).
Because cancer demands so many resources from everyone surrounding a patient, patients often felt guilty and were hesitant to seek out help when they needed it: “I don’t have employment anymore, I get charity from the hospital, so they pay for me to see all these people and so there’s that hesitance too, where it’s like, I’m already aware of how much they’re paying. I mean, do I wanna ask for more? There are other patients here that also need resources. How much should go to me and… Balancing that and what that feels like psychologically as well.” (Pt11).
Behavioral Health Provider Perspective:
When patients are not adequately screened and identified, BHPs commented that patient distress could “explod[e] into crisis” (BHP1) at any moment and that needing to manage to such crises as they unfold is not an ideal situation for bringing patients into depression care. Social workers also described being “dumping grounds” (A1) for all problems that the oncologists cannot address: “It’s common if somebody cries, that some of them come running to us. They get uncomfortable with a lot of emotion in the room.” (BHP8). One administrator said, “In many places I’ve been, that was kind of like, if you didn’t know what to do with it, send it to the social worker. (laughs). Yeah. That’s not okay.” (A1). Even though the BHPs are trained to assess and treat anxiety and depression within the oncology context, some providers bypass the BHP and refer patients directly to psychiatry: “We’re still struggling I think in some ways in social work overall, to emphasize our role to the team as being the primary resource for mental health care versus psychiatry. We have the skills to assess and treat, you know, anxiety and depression within the oncology context. So, there’s no reason to bump up to a higher level.” (BHP9).
Medical Provider Perspective:
Primary care visits and oncology visits are common points in cancer care where depression is identified. All three oncologists we interviewed reported that they identify patients with depression through the use of their “gut feelings most of the time” (MP1) and from patients describing depressive symptoms (i.e., apathy, fatigue, forgetfulness that cannot be attributed to current treatment or medication) (A1) or due to patients “break[ing] into tears” (MP3) during their follow-up visits. However, “sensing” distress is difficult for providers because they lack sufficient psychiatric training, some patients are not forthcoming about depression, and distressed patients often present to providers with physical symptoms rather than mental health issues [42]: “I will be with the patient for months on end and then it’s not until I make a referral to palliative [care] that palliative says ‘Hey, did you know your patient’s depressed?’ I’m like ‘Oh, how did I miss that?’” (MP1). One provider said, “My suspicion would be that patients may minimize their symptoms a little bit to me. I think there’s a little bit of that they don’t want me to feel disappointed that they’re not responding [to medication].” (MP5).
We also observed that oncology nursing staff may be more attentive to patient depressive symptoms than medical oncologists: “When I first started working here, a nurse brought to my attention a patient who they thought could benefit and when the oncologist was approached about putting in a referral for this patient, he remarked, ‘Well, why? She’s one of my happiest patients.’ And, in actuality, this patient was incredibly depressed.” (BHP3). However, the staff was under pressure to improve the efficiency of oncology care processes, which limited the opportunity to take time to administer depression screening or adequately triage patient psychosocial needs for proper referral: “There’s a lot of pressure from providers to get in and out of the room so that they can get into the room and see patients. And so they are there always pressuring the MAs, LPNs, you know, to do things quickly and get out.” (A1).
4.2.2. Psychosocial Care Journey: Initial Psychosocial Assessment, Diagnosis, and Rapport Building.
We also identified challenges in the assessment phase of the psychosocial care journey, either associated with active cancer treatment (Table 2, column 2) or based on a variety of needs that patients had at different phases of their cancer care. Because cancer places such high demand on financial, physical, and practical resources [71], patients were more likely to drop out or not show up to the first appointment with their BHP or to occupy most of the initial assessment appointment with discussion around their practical needs (e.g., financial aid, transportation, housing) instead of treating their depression.
Patient Perspective:
The first visit is an opportunity for a patient to express their emotional support needs and to negotiate what kind of care they can expect from the follow-up sessions: “[we discussed] things that I want to address or that I’m struggling with … or what I’m wanting out of the sessions.” (Pt11). To some patients, it was a first time they felt validated: “She came to me when I was first admitted to the hospital. And, she’s been a godsend. Just because she would… there’s some validation that you’re going through something.” (Pt5). We also observed that the resources and support that patients received during this phase depended on where the patient is in their cancer care journey. For patients that are just entering cancer care or are in an unfamiliar environment far away from home, the initial assessment helped patients be connected with someone: “When I first went to [treatment city], I was by myself for a really long time, so I didn’t really have a whole lot of people to talk to. So, getting to sit down and talk to [BHP] about how things were going and how I was feeling about my whole transplant was really helpful for me.” (Pt1). For patients who have been fighting cancer for an extended period of time, the initial psychosocial session also helped patients establish a safe space to be vulnerable when the usual cancer culture encourages them to “be strong and keep fighting” (BHP1). For patients in later stages of cancer, it provided an opportunity to have a conversation about seemingly awkward but important topics such as the power of attorney, end of life directives, or options for physician-assisted hastened death.
Patients were sometimes not aware they were referred to see a BHP: “Sometimes they (patients) didn’t know that they were scheduled for us (BHPs) so they’re like, ‘What the heck is this about?’” (BHP1). Patients may cancel or skip initial appointments because there has not been an established relationship: “Our no shows are more common with patients that have never met us in the first place, I think partially because we don’t have a relationship.” (MP2).
Behavioral Health Provider Perspective:
When BHPs are introduced to a patient during a crisis moment, BHPs often perceive the need to drop everything to attend to those crises. In addition, patients are often overwhelmed with the cancer-related issue at hand such that BHPs cannot perform a full psychosocial assessment, share psycho-education or resources in a digestible manner, or identify goals or a treatment plan for future sessions. One BHP commented “because you’re dealing with the crisis and the emotional fallout,… I think that sometimes our assessments are jumbled and not thorough. And you can kind of try and backtrack at the next meeting to do a better assessment, but that doesn’t always happen either.” (BHP1). Another said “when you’re going into a crisis moment, it’s hard to just say, ‘Oh, wait. Let me give you this survey.’ (laughs)” (BHP2).
Cancer places such a high financial burden on patients that BHPs spend much of their time addressing patient practical needs instead of their underlying depression: “They’re always having to deal with financial issues, or this person needs housing, or lodging, or transportation.” (MP5). Social workers often are not able to establish themselves as BHPs during the initial assessment: “I think especially medical social workers, just get dragged into the practical stuff and never use our other [behavioral therapy] skills. And so I just feel like my skills are really rusty.” (BHP1).
4.2.3. Psychosocial Care Journey: Active Depression Treatment.
The intersection between active depression treatment and various stages of cancer care presents additional challenges (Table 2, column 3). During active depression treatment, BHPs must be flexible to frequently-shifting patient circumstances associated with cancer and oncology treatment. Depressive episodes vary in both length [114] and rate of recurrence [20], and cancer diagnoses and treatments present unpredictable logistical [23] and health [48] challenges. This leads to significant challenges for patients, BHPs, and medical providers who need to navigate the dual journeys of depression and cancer care. We found that these challenges can arise during the acute treatment and recovery phases of cancer care.
Patient Perspective:
Many patients with cancer and depression may disengage from depression treatment due to fatigue, which is both a symptom of depression and a common side effect of cancer treatment. The heavy burden of oncology and psychosocial appointments may be especially overwhelming for depressed patients who lack motivation or hope, reducing patient willingness to engage in one treatment or the other: “I guess I’m just tired of all these appointments happening, I’m like can I get three weeks free without an appointment, you know?” (Pt3). Patients may also feel physically or cognitively drained when going through chemotherapy, losing motivation to seek out depression treatment: “It’s hard to prioritize seeking that out if you’re already dealing with something else, because it’s like, ‘Man, I got treatment…I’m dealing with the side effects of the last treatment.’” (Pt4). In some cases, external resources such as books, apps, and support groups can be helpful to patients in this phase: “I joined this—on Instagram—a group of metastatic breast cancer people, and I look at all this thing. All this, thousands of women around the world, just sharing their thing. And, it gives me hope…” (Pt10).
Another challenge for patients arose out of a general lack of documentation of psychosocial session outcomes: “Other providers have discharge notes through [registry system]. I don’t have that. Sending home discharge notes is impractical because I can’t type during the session.” (BHP3). Often, patients may have memory challenges while going through chemotherapy: “I did have chemo and sometimes that brain, you know, remembering dates, exact times, a little hard. And side effects from a lot of the stuff I take and I hate that my memory, that’s one thing that I noticed had changed. My memory.” (Pt3). Patients therefore may have trouble keeping track of session outcomes and assignments, and some suggested that tools to help manage that information could be helpful: “It would be, it could be a useful tool. I’ve never really thought about it. He [psychologist] kind of references back like, how I was feeling at the past visit. You know, a little bit but and maybe he keeps track of it but I don’t really even remember what happened yesterday. I’d have to look back up in the calendar to see when it was…Yeah, that’s pretty cool. Some additional tool that’s specific to mental health? That tracks your fears, how you’ve been feeling? I don’t know, that’d be cool.” (Pt2). Such tracking may empower patients to develop deeper insights about their own behavior: “Usually it’s the patients where their mood is all over the place. Or they really haven’t yet linked the fact that activities or physical sensations link to their mood, and I’m trying to encourage that link—the association in their head.” (BHP1).
Behavioral Health Provider Perspective:
During the acute cancer treatment phase, patients may encounter unpredictable crises that derail depression treatment: “There will be times where we have one session, and we’ve done a lot of Behavioral Activation stuff, but then the next time they come in, maybe they’ve gotten some really bad news, or something and they’re just really distraught, and that session, rather than following-up with the Behavioral Activation, we’re kind of doing more of a kind of crisis management.” (BHP3). Patients may choose to end their depression treatment prematurely when their oncology treatment is finished, despite patients still being symptomatic from depression or despite BHP belief they could benefit from further sessions or are at high risk of recurrence: “We are, in my mind, not specifically mental health like you go and you’re coming because you want to get mental health treatment. We are coming to support you to get you through the treatment, and so if you’re starting to feel better because physically you’re feeling better…I can’t really justify telling them like, ‘Oh, you must meet me again.’” (BHP4). Clinic social workers may have limited capacity to see patients discharged after completing active cancer treatment, leaving patients to manage their own depression care: “So I don’t really know how anyone can help me at this point. I’m still just trying to figure out how I can help myself.” (Pt1).
Another challenge emerges due the need to coordinate between oncology and psychosocial appointments. Appointments with BHPs are frequently scheduled on the same day as cancer treatments, because of the convenience for a patient. However, patient psychosocial needs may not easily fit into this schedule, and it may be difficult for a patient to come in for appointments outside scheduled oncology treatments: “So, to ask them to come for an exceptional visit is very hard for them sometimes… I try to schedule it when it’s convenient for the patient because… they’ve just finished some treatment and they don’t have another stretch of treatment for two weeks, chances of them come back in here when they’re not feeling well is slim to none.” (BHP5).
Medical Provider Perspective:
Just as frequent oncology appointments may make adherence to depression treatment more difficult, depressive symptoms can likewise impair oncology treatment: “Usually I just let the patients know I am worried about this and make it clear that this could interfere with the main treatment, which is the oncology focused treatment that I’m trying to achieve for them.” (MP1). In particular, depression could potentially lead to poor decision-making around oncology care: “I get concerned at times that there are patients who are making bad decisions about that care. Possibly because of depression…I mean, there are times frankly when I’ve had patients who had a decision-making process so flawed, and they’re making such a bad decision that could have really negative impact on their outcome.” (MP3).
Nearly all participants, including both oncologists and patients, reported the lack of available appointment slots among the behavioral and psychiatric care providers as one of the main barriers for accessing mental health care: “The counseling is something that I would love to have for every patient. But the problem becomes, how do I arrange that? [psychologist] is one person. He can’t see absolutely everybody, and then when he does, and he wants to do ongoing CBT. It’s very hard to schedule appointments with people.” (MP5). When access to psychiatry is limited, the BHPs, primary care physicians, and oncologists often needed to collaborate to determine the appropriate set of psychotropic medications. This was not an ideal solution, given that none of these providers were properly trained to manage such situations: “I’m a medical oncologist so I feel a little bit over my head (laughs) in that situation. ‘Cause I wasn’t trained to manage bipolar disorder.” (MP2).
4.2.4. Psychosocial Care Journey: Maintenance and Relapse Prevention Plan.
We were not able to observe any patients moving into the maintenance phase, possibly because patients were discharged from the cancer center before their target depression treatment goals were reached. Instead, patients simply disengaged from depression care because they were discharged from cancer centers (Table 2, column 4). There were also some efforts to connect patients with community mental health specialists to continue their depression treatment outside of the cancer centers.
Patient Perspective:
Some patients needed to manage their own depression, due to a lack of proper hand-off to appropriate community BHPs that patients can access outside of the cancer centers: “I’m trying to figure out what I’m supposed to do with myself and what kind of person I’m supposed to be in this next year. This isn’t really anything that the doctors really told me would happen, that I would feel so lost and unsure what to do next.” (Pt1). In some cases, patients felt reluctant to seek treatment elsewhere: “She [patient] also feels a sense of safety at [urban site], and so going to a new place, it’s scary for her.” (MP2). This presents a challenge, as cancer center behavioral health resources may not have the capacity to continue treatment after a patient’s cancer treatment is finished: “I had a patient once who was angry, but she hadn’t had cancer in 12 years when she came here and she wanted to see a social worker. And I said, that’s just not, you know, it’s not possible.” (BHP5). One patient explicitly rejected receiving continued depression treatment at the cancer center: “I just associate this place with (laughs) not good feelings, I don’t know. It sounds mean, but I mean it’s just… the least amount of time you have to be up here, the better.” (Pt8).
Many patients, especially those needing special treatment that their local or rural cancer center did not offer (e.g., stem cell transplant), had to relocate or travel for hours for access to the urban cancer center. Unlike psychosocial care in primary care settings where patients are likely to remain in the same primary care facility in their local communities, some patients would need to travel for hours to continue their depression treatment: “It’s challenging when they don’t live here… It’s challenging that they can’t get here.” (BHP5).
Behavioral Health Provider Perspective
As much as BHPs and psychiatrists wanted to continue treating patients, they also reported their skills and expertise should be used for those who require psycho-oncology support: “This is one of our struggles. Because she wants to see me. I think she gets something out of our visits. I could probably see her for years (laughs) and she has breast cancer, and the breast oncologists like us to take care of their patients long term. But she doesn’t necessarily have to have a provider who specializes in psycho-oncology at this point unlike some of our patients. So I think each provider has a different opinion, but for me, I hope to be able to graduate her because I want to open up my clinic to new patients.” (MP2).
Although patients were welcomed to reach out to their BHPs during active cancer treatment, BHPs were also limited to only seeing patients in active cancer care: “We have a limited number of appointments that we allow our behavioral health providers to see after treatment is done, because we don’t have the staff here to keep those patients coming back day after day.” (A2). One site partnered with a startup that offers matching of patients to BHPs, but there were exclusions: “We sometimes have to find a resource for patients if they’re outside of that area or [on] Medicaid.” (A3).
4.3. Technology Design Opportunities for CoCM for Depression and Cancer
We have presented specific challenges that patients with cancer and depression encounter. Informed by these challenges, we have also identified design opportunities aimed at improving CoCM for depression in cancer settings, specifically opportunities that can be addressed through the use of technology Although some of the identified challenges may not be unique to this setting, collecting insights with this population allowed us to more saliently observe the particular issues facing those undergoing both cancer care journeys and psychosocial care journeys, in turn allowing us to better prioritize functionalities and design opportunities. Whereas prior sections are organized by phases of the psychosocial care journey, this section presents a set of technology design opportunities and how each opportunity can improve the care experiences across multiple phases of the cancer care journey and the psychosocial care journey.
4.3.1. Provide tools for and around self-assessment.
From a patient perspective, we observed that patients in active cancer treatment may experience physical side-effects of the treatment (e.g., chemotherapy) and difficulties with transportation, both of which may make it more difficult to attend appointments. Furthermore, some patients in active treatment noted that they had significant downtime in the infusion suite with little to do, suggesting another opportunity for self-assessment activities. Finally, patients nearing the end of treatment described being tired of making trips to their treatment sites, suggesting they may also benefit from home self-assessment. Providing patients with self-assessment tools could help patients stay engaged in care through remote-monitoring when in-person appointments are less desirable or feasible.
For example, systems could allow patients to perform technology-supported self-assessments (e.g., universal distress screening or depression and anxiety assessment performed through electronic surveys, apps, or IVR technologies). Resulting data could be directly integrated with an EHR system, further reducing the time spent on manually entering such data. Self-assessments could also be configured to be administered at an appropriate frequency, thus avoiding a need for BHPs to determine whether the assessment needs to be administered before every patient visit. Such a system would allow the frequency of assessments to be decoupled from the appointment schedule to accommodate patients at various phases of their cancer care journey.
From a related provider perspective, we also observed that a patient’s cancer journey creates additional challenges for BHPs responsible for psychosocial care. BHPs play a critical role in coordinating patient care across many providers and supporting patient needs from multiple perspectives. Treatment of patient depression during the limited time available within sessions therefore competes with other tasks that are directly (e.g., responding to patient voicemails, handling crises) or indirectly (e.g., contacting Medicaid case managers, reviewing financial aid applications) related to patient depression. Many of these tasks are typically organized through memory, handwritten notes, or email/calendar reminders. We also saw that patient depression care is often neglected when BHP resources are constrained [83]. Technology improvements that help BHPs manage competing demands, or even improvements that allow more effective use of limited time, could allow BHPs to focus on activities critical for patient management and for providing psychosocial care.
One such opportunity is to give BHPs access to patient self-assessment data, thereby reducing logistical burdens and allowing BHPs to focus on using session time to better deliver depression treatment itself. Systems can also reduce inefficiencies in managing and distributing patient care resources (e.g., sifting through binders of care materials, photocopying resources, walking back to their desks to find materials) through a repository of resource materials that all BHPs have access to and which can be emailed or printed for patients as needed during a session. Similarly, systems can also allow BHPs to configure, schedule, and organize action items with priorities, reminders, and deadlines. Such electronic assessment systems have been shown to be feasible and effective in cancer settings [13, 14, 41, 43]. In addition to being consistent with recommendations of prior research, our results characterize these challenges as resulting from the parallel journeys of patients with co-morbid cancer and depression. Our examination of the perspectives of multiple stakeholders show this is a key priority for both patients and providers in this setting.
4.3.2. Provide tools for population-based patient monitoring.
We found challenges in a patient’s cancer journey can often influence patients to disengage from their depression care. Monitoring patient progress is a critical component to ensure an appropriate level of patient engagement in their depression care. With appropriate monitoring in place, providers can both: (1) detect when patients are not improving as expected and therefore make appropriate adjustments to treatment, and (2) identify patients who are disengaged from care and therefore initiate proactive outreach. However, all three sites lacked appropriate and efficient tooling support for population-based analysis and monitoring of patients. One site leveraged an Excel-based patient registry to systematically follow all patients, but the corresponding workflow created duplication of efforts (e.g., the registry was not directly integrated into the EHR), which discouraged proactive monitoring of patients. To streamline population-based patient monitoring, systems could allow patient self-assessment data to be directly entered into the EHR (i.e., reducing the burden of data entry) and could also provide a summary dashboard for easy access to pertinent information about a provider’s population of patients. Although prior research has proposed EHR systems that collect data from clinical registries [18] or aggregate information from various databases [106], this solution would build upon our suggestion for patient self-assessment to further address the needs of this patient population. For example, such a system could help track patients undergoing active cancer treatment, who may “fall through the cracks” when cancer care is prioritized over depression treatment. Furthermore, given that patients who have completed their cancer treatment may lose access to the cancer care center, an electronic registry could trigger action items or send reminders to BHPs to follow up with patients who have been discharged or moved away but still require depression care (e.g., to help ensure appropriate transition plans).
Patients we interviewed reported frequently checking their progress toward cancer recovery by logging into their EHR portal and viewing historical lab results and provider notes. The same system could also help patients reflect on their progress with depression treatment, which could help raise awareness of importance of treating their depression. A system might provide rewards and achievements intended to increase self-efficacy or might guide patients with appropriate interventions and psycho-education materials.
4.3.3. Provide access to digitally translated evidence-based psychosocial interventions.
We observed that patients often have significant periods of downtime across multiple stages of the cancer care journey. For example, during active treatment, patients may spend several hours at a time in the infusion suite. Furthermore, stem cell transplant patients going through recovery may not be allowed travel, left with long periods of time with potentially little to do. For those patients who are also undergoing treatment for depression, these may represent opportunities to provide electronic resources (including tools such as worksheets) for evidence-based psychosocial interventions. By translating behavioral health resources into an engaging digital format that patients can access via their phones, tablets, or computers (e.g., while at home or in an infusion suite), patients could be better supported in their psychosocial care journeys during these long stretches mandated by their cancer care journeys. Easy access to such resources could be especially important for those undergoing active cancer treatment, where we observed that unpredictable crises can be common.
Digital resources for evidence-based psychotherapy have been of interest to researchers for some time [27, 54, 67], including resources developed for anxiety and depression [98]. A tool could aggregate such existing resources and incorporate them into patient treatment (e.g., patient-facing mobile apps could include search features to identify relevant resources), which could further support patients who have difficulty accessing frequent in-person sessions (e.g., for health reasons, for financial reasons, due to a lack of cancer center depression resources). Our parallel journeys framework also suggests that existing resources or new interventions might be organized according to where patients are in their multiple journeys (e.g., presenting additional resources at times in a patient’s cancer journey when they are most at risk for depression).
4.3.4. Document shared understanding between patients and providers.
Our findings revealed that forgetfulness and fatigue negatively impact patient engagement in care, a challenge that is magnified by the additional difficulties of navigating parallel journeys. Although forgetfulness as a barrier to treatment adherence may seem obvious, we cannot dismiss the fact that the cancer treatment impacts the brain chemistry in a manner that severely reduces patient ability to remember (i.e., so-called “chemo brain”) [51, 116]. In addition, patients with cancer receive a cocktail of medications with significant toxicities and endure an overwhelming number of hospital appointments that intensify their fatigue. Although depression care sessions often generated action items or assignments for patients, these decisions were not documented or were documented in a manner such that they were not available when the patients needed them (e.g., sticky notes, paper). Furthermore, BHPs sometimes forgot they assigned anything to a patient, and the resulting lack of follow-up accountability and interest from the BHP can lead to a loss of trust and consequent nonadherence by patients. Given that prior research on patient-provider collaborations using personal informatics data has indicated that clear goal-setting is necessary for managing expectations around data collection and reducing information overload for both parties [26], there is an opportunity for a system to help patients and providers manage a shared understanding of depression treatment, especially given the high demands of this population.
Documentation of action items could be facilitated through provider-facing systems that allow quick charting during patient sessions. Such a tool could be designed for transparent collaboration in documentation of action items between patients and providers during a session, a strategy that can ensure shared understanding and has been found to build trust and reduce miscommunication in patient-provider collaboration around personal informatics data [26, 104]. Patient-facing systems can make action items accessible in a variety of modalities, especially on mobile devices, a possibility supported by one BHP participant: “people always know where their phone’s at. (laughs)” (BHP3). Such a system would need to be designed in a manner that minimizes cognitive burden, both during a session (i.e., ensuring it enhances collaborative goal-setting rather than distracting from an in-person session) and afterwards (e.g., action items could be lost in typical text-heavy after-visit summary).
Such support for patient-provider collaboration around shared understanding of patient action items could also facilitate fidelity to active components of evidence-based treatments. For example, systems could include activity and mood tracking components of behavioral activation, reminders for planned pleasant activities, or positive feedback provided either automatically, as scheduled by BHPs, or synchronously by BHPs in response to patient-entered data. Systems can also include brief behavioral strategies (e.g., goal setting, activity scheduling, problem solving to address barriers) as well as helpful tools (e.g., basic cognitive or behavioral exercises when patients report depressive symptoms). Patient documentation of their completed action items could support a sense of accomplishment and progress, and providers could use data to follow up with patients to reward and encourage positive behavioral changes or to troubleshoot barriers when a patient is not engaging with action items.
4.3.5. Support timely and appropriate communication.
Patients reported they experience intense depressive symptoms between sessions or outside of normal clinic hours and feel a need for guidance from their psychosocial providers. These needs may be exacerbated by the unpredictable course of cancer treatment, which may lead to crisis moments throughout the care journey. Patients overwhelmingly wanted a way to reach their providers outside of their sessions. However, patients hesitated to reach out during these crisis moments due to their own guilt of burdening the busy providers, fear of being on hold, or anticipation of increased anxiety from waiting for call backs. On the other hand, BHPs were hesitant to receive a flood of incoming communication requests and data that they cannot handle: “[handling suicide ideation] would be really hard. I mean, there would be, there’s, there’s just no way to ensure that there could be a timely response to that. And I think, I think that you’ve probably pointed out the biggest downside of having any kind of, um, electronic communication that is kinda counseling focused.” (BPH9). Importantly, patients with depression will experience suicidal thoughts whether or not these thoughts have been communicated to care providers and arguably patient risk is greatest if providers are unaware of patient suicidality. With careful attention to the design of both clinical processes and the technologies, a system that facilitates such communication and appropriate clinical intervention may help reduce patient risk for self-harm.
We observed that planning around communication typically happened during the initial assessment, verbally and through exchange of business cards. Systems can enhance this workflow by documenting the agreed communication plan and making care team contact information readily available in the desired modalities. Prior research has found that online patient portals can significantly improve patient satisfaction with their communication with their providers, based on increased convenience and direct physician response [74]. Building on prior research regarding the advantages and disadvantages of different communication methods [134], our results emphasize a need for shared understanding between patients and providers regarding appropriate use of and expectations for communication channels. Our parallel journeys framework also provides a lens for understanding how shared understanding regarding communication can break down (e.g., the reluctance of patients near the end of their cancer treatment to reach out for depression support, due to guilt regarding the already significant support received during their cancer journey, thus contributing to risk of relapse or unsuccessful transition in their psychosocial care journey).
Asynchronous and virtual communication methods could lower the barriers to patients reaching out and could reduce time commitment required from BHPs (e.g., messaging can be less time-consuming than scheduling and conducting a phone session). A system could allow appropriate expectation-setting by supporting a BHP in configuring the system to respond immediately with a short message (e.g., regarding when a patient might expect a response). While waiting for a BHP’s response, patients could interact with other aspects of a system to find information and interventions appropriate for their circumstances. Because patients require clear understanding of the level of support for crisis management [9], design and implementation of such a system will require transparency around the boundaries and limits of using the digital system. BHPs can develop safety plans for their patients and educate patients on real-time intervention tools for suicidal ideation which can be accessed through patient-facing systems (e.g., calling 911, a suicide hotline, a crisis text line). By providing patients with in-the-moment access to crisis resources, patients may also be less likely to show up to BHP appointments in crisis and may therefore be better able to focus on their depression treatment.
4.3.6. Improve access to resources.
Patients with cancer can be overwhelmed with information, especially during diagnosis and treatment decision phases [66]. Patients in the initial information-seeking phase may have questions around navigational or practical topics (e.g., housing, childcare, financial assistance, volunteer services, scheduling logistics, the course of treatment); as they transition into active treatment, they may have additional questions about other topics (e.g., transportation options). Patients can also be overwhelmed by emotions they experience throughout their care. We found that patients were given resources and information, but they were not able to internalize them and wanted frequent reminders: “Even if you didn’t get anything in-between and something came up, having that periodic reminder would… You would know that that resource is available if you need it. Versus just a one-time when you first come in.” (Pt11). To make patient resources more accessible, systems could intelligently recommend appropriate information based on a patient’s specific phase of a cancer care journey and of a psychosocial care journey, similar to personalized systems proposed for patients at risk for cardiovascular disease [24]. BHPs can also manage, curate, and configure appropriate sets of resources based on patient needs and make them available in patient-facing systems. Systems can also allow patients to discover relevant content (e.g., psycho-education resources, self-guided interventions) and could include peer recommendations of useful resources from patients in similar circumstances.
5. DISCUSSION AND FUTURE WORK
5.1. Characterizing the Whole Patient through a Parallel Journeys Framework
The field of psychosocial oncology has made significant progress in acknowledging the association between psychological and physiological challenges encountered by patients with cancer, highlighting the growing awareness and importance of caring for the whole patient and the need to target quality of life as the desired goal for patients with cancer [61]. Despite the increased attention to care for the whole patient and the Institute of Medicine recommendation to integrate psychosocial services into cancer care [89], there still remain 73% of patients with cancer and depression who receive inadequate or no depression treatment at all [126]. Our findings confirmed known and unmet challenges in providing psychosocial care in cancer settings [40, 83]: (1) Patients normalized depression as a side effect of cancer. (2) Oncologists did not feel they have sufficient training to manage the psychiatric and psychological aspect of cancer. (3) BHPs were overburdened with navigational and practical responsibilities that interfered with their provision of depression care. (4) Organizations lacked the psychiatric and psychosocial resources and processes to adequately support patient psychosocial needs. In examining our findings, we discovered that the commonality that underlies these unmet challenges is still rooted in treatment settings that prioritize the treatment of the disease process (i.e., cancer) over the holistic needs of the patient as a person.
We have demonstrated that patients with cancer and depression go through their cancer care journey in parallel with their psychosocial care journey. The concept of the parallel journeys framework came from our finding that cancer-centric views, both in terms of clinical staging of the cancer or the phases of a cancer care journey, inadequately describe the experiences of patients with cancer and depression whose psychosocial needs do not necessarily align with cancer-centric views. Despite all of the sites we examined having integrated psychosocial care processes, we still observed across all sites an attitude towards depression being a secondary disease, manifested in how the center structured their budget to hire psychiatric staff, how patients prioritized their limited capacity to cancer-related appointments, how oncology staff supported the distress screening process, and how BHPs over-accommodated to fit the cancer care. As our findings revealed, cancer settings still require raising the priority of mental health care. Presenting the psychosocial care journey taking place in parallel alongside the cancer care journey is one step toward better characterizing the whole patient.
In characterizing the whole patient, our work brought cancer care and psychosocial care journeys together to describe the complexity of co-morbidities, representing the needs of a particular “extreme user group” [25] who have the potential to provide deep insights about these issues [123]. We leveraged existing models of care journeys, and we did not evaluate the feasibility of our framework in other co-morbid contexts or using other models of care journeys. However, our parallel journeys framework may generalize to other conditions because it does not dictate either the types of co-morbid conditions nor the specific models of care journey. Understanding exactly how this framework can be used to describe care challenges in other co-morbidities or using other models of care journey remains an opportunity for future work.
5.2. Technology to Prevent Patients from Falling through the Cracks
Our study revealed several missed opportunities that left patients with cancer and depression to fall through the cracks and receive less than ideal or no depression care. Through the parallel journeys lens, our work exposed many of these “cracks” at the intersection of the cancer care journey and the psychosocial care journey. At a high level, the parallel journeys framework brings attention to technology design opportunities to ensure patients with cancer and depression receive adequate depression care.
Each of the four phases of psychosocial care journey is delineated by a set of concrete and measurable goals, similar to how the cancer care journey is conceptualized (i.e., with completion of tasks or changes to the physical body demarcating the phases and paths that patients with cancer experience). Our parallel journeys framework highlights the need for tools to support achieving psychosocial goals in each phase of the psychosocial care journey to help patients progress toward improved mental health. Given the goals of each phase of psychosocial care, tools also need to be flexible enough to accommodate the needs and challenges of each phase of cancer care. Having concrete goals promotes measurement-based practices and allows evaluation of potential tools based on the forward progress of each patient.
The parallel journeys framework also directs attention to the need for tools to support continuity of care within and across psychosocial care phases and beyond cancer treatment. Burdens of cancer play a significant role in patients disengaging from depression care, forcing BHPs to be flexible and balance the need to address the crises presented at sessions with interventions to address underlying depression. In addition, because psychosocial care service is integrated into the cancer center, patients discharged from cancer care often discontinue psychosocial care. Improved tools that make psychosocial care accessible outside of cancer centers could support patients that would normally disengage from depression care and could support patients that need in-the-moment help in receiving timely communication and interventions.
Ultimately, it is important to consider the broader context when designing specific policy interventions [15]. Although challenges such as high dropout rates or low adherence to depression treatment are not unique to cancer care settings [90], we observed that the difficulties of depression care are compounded by the difficulties of cancer care, including side effects of cancer and its treatment, financial burdens, and logistical burdens that accumulate from cancer treatment. These burdens are felt both by the patients and by the BHPs who are also overwhelmed by the demands placed on them in the cancer setting. There are many existing technology solutions for monitoring mood and tracking activities [25, 26], but the development of effective interventions and technologies in this context must consider the interactions between the various stakeholders and the specific challenges faced by patients on these parallel journeys and their providers.
5.3. Limitations
We only recruited patients with depression whose recent PHQ-9 score was greater than 10. These patients were also reachable by their BHPs and the researchers, potentially introducing selection bias towards patients who are more engaged in depression care. Recruiting actively distressed patients also meant we did not interview any patient in the maintenance phase of a psychosocial care journey. Our sample of patients was relatively small, such that we did not have a representative sample for age, race/ethnicity, gender, cancer type, or cancer stage. Our work also excluded caregivers who are often the driving force behind enabling care for patients. Caregivers, as well as other family members, often need psychosocial care themselves due to the high demand and emotional stress that cancer places on relationships surrounding the patient [87]. More research is therefore necessary to understand caregiver challenges in supporting patients, their own psychosocial experiences, and their participation in designing patient-facing tools.
Our contextual inquiry method involved observing sessions that were highly sensitive, private, and emotionally charged. Despite the fact that patients were reminded to ignore the observer in the room, it is possible that they behaved differently during the observed sessions. In our journey mapping exercise, we pieced together a single patient’s journey from a single stakeholder’s description or from our observation notes, either of which could potentially include biased perspectives. We leveraged existing journey paradigms and guidelines for cancer care and psychosocial care to examine patients with cancer and depression and to identify the importance of considering a parallel journeys framework for characterizing their challenges. The phases of the psychosocial care journey are consistent with clinical practice guidelines [2] and therefore have clinical validity for depression care. However, we have not applied the framework to other co-morbidities and there may be limitations on the generalizability of the phases of the psychosocial care journey in other co-morbid contexts.
6. CONCLUSION
The integration of psychosocial services in cancer care is crucial in supporting the whole patient, and yet challenges rooted in cancer-centric views continue to prevent patients with cancer and depression from receiving adequate care. We introduce the parallel journeys framework to characterize patient experiences in simultaneously navigating the cancer care journey and the psychosocial care journey. The framework helps reveal challenges at the seams of the two journeys and helps surface related technology opportunities. We encourage health technology researchers, designers, and healthcare providers to consider both cancer care journeys and psychosocial care journeys in their future design of technologies for patients with cancer and depression.
ACKNOWLEDGMENTS
The authors would like to thank all of our participants for their time and feedback. We would like to acknowledge site representatives and BHPs that made this study possible and patients who shared their stories and allowed us to observe their care in their most vulnerable contexts. We thank our reviewers for their thoughtful feedback. This research was supported by the National Institutes of Health under awards P50MH115837 and R01LM012810. Content is solely the responsibility of the authors and does not necessarily represent official views of the National Institutes of Health.
Footnotes
This discussion was in reference to a patient with major depression that persisted beyond active cancer care.
Patients undergoing a bone marrow or stem cell transplant are asked to live within a 30-minute drive of the cancer center.
Our study did not follow this patient beyond this observation.
Contributor Information
JINA SUH, University of Washington, USA and Microsoft Research, USA.
SPENCER WILLIAMS, University of Washington, USA.
JESSE R. FANN, University of Washington, USA and Seattle Cancer Care Alliance, USA
JAMES FOGARTY, University of Washington, USA.
AMY M. BAUER, University of Washington, USA
GARY HSIEH, University of Washington, USA.
REFERENCES
- [1].Agboola Stephen O, Ju Woong, Elfiky Aymen, Kvedar Joseph C, and Jethwani Kamal. 2015. The Effect of Technology-Based Interventions on Pain, Depression, and Quality of Life in Patients with Cancer: A Systematic Review of Randomized Controlled Trials. Journal of Medical Internet research 17, 3 (2015), e65 10.2196/jmir.4009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Andersen Barbara L, DeRubeis Robert J, Berman Barry S, Gruman Jessie, Champion Victoria L, Massie Mary Jane, Holland Jimmie C, Partridge Ann H, Bak Kate, Somerfield Mark R, et al. 2014. Screening, Assessment, and Care of Anxiety and Depressive Symptoms in Adults with Cancer: An American Society of Clinical Oncology Guideline Adaptation. Journal of Clinical Oncology 32, 15 (2014), 1605 10.1200/jco.2013.52.4611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Archer Janine, Bower Peter, Gilbody Simon, Lovell Karina, Richards David, Gask Linda, Dickens Chris, and Coventry Peter. 2012. Collaborative Care for Depression and Anxiety Problems. Cochrane Database of Systematic Reviews 10 (2012). 10.1002/14651858.cd006525.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].American Psychiatric Association et al. 2013. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. BMC Med 17 (2013), 133–137. 10.1176/appi.books.9780890425596.x00diagnosticclassification [DOI] [Google Scholar]
- [5].American Psychiatric Association et al. 2016. Dissemination of Integrated Care Within Adult Primary Care Settings: The Collaborative Care Model. https://www.psychiatry.org/psychiatrists/practice/professional-interests/integrated-care/learn
- [6].Bardram Jakob E, Frost Mads, Szántó Károly, Faurholt-Jepsen Maria, Vinberg Maj, and Kessing Lars Vedel. 2013. Designing Mobile Health Technology for Bipolar Disorder: A Field Trial of the Monarca System. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems ACM, 2627–2636. 10.1145/2470654.2481364 [DOI] [Google Scholar]
- [7].Bauer Amy M, Hodsdon Sarah, Bechtel Jared M, and Fortney John C. 2018. Applying the Principles for Digital Development: Case Study of a Smartphone App to Support Collaborative Care for Rural Patients with Posttraumatic Stress Disorder or Bipolar Disorder. Journal of Medical Internet Research 20, 6 (2018), e10048 10.2196/10048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bauer Amy M, Hodsdon Sarah, Hunter Suzanne, Choi Youlim, Bechtel Jared, and Fortney John C. 2017. Lessons from the Deployment of the SPIRIT App to Support Collaborative Care for Rural Patients with Complex Psychiatric Conditions. In Proceedings of the 2017 ACM International Joint Conference on Pervasive and Ubiquitous Computing and Proceedings of the 2017 ACM International Symposium on Wearable Computers ACM, 772–780. 10.1145/3123024.3125610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bauer Amy M, Iles-Shih Matthew, Ghomi Reza Hosseini, Rue Tessa, Grover Tess, Kincler Naomi, Miller Monica, and Katon Wayne J. 2018. Acceptability of mHealth Augmentation of Collaborative Care: A Mixed Methods Pilot Study. General Hospital Psychiatry 51 (2018), 22–29. 10.1016/j.genhosppsych.2017.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bauer Amy M, Thielke Stephen M, Katon Wayne, Unutzer Jurgen, and Arean Patricia. 2014. Aligning Health Information Technologies with Effective Service Delivery Models to Improve Chronic Disease Care. Preventive Medicine 66 (2014), 167–172. 10.1016/j.ypmed.2014.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bauer Amy M, Williams Mark D, Ratzliff Anna, and Unutzer Jurgen. 2019. Best Practices for Systematic Case Review in Collaborative Care. Psychiatric Services (2019), appi–ps. 10.1176/appi.ps.20190008 [DOI] [PubMed] [Google Scholar]
- [12].Bayliss Elizabeth A, Steiner John F, Fernald Douglas H, Crane Lori A, and Main Deborah S. 2003. Descriptions of Barriers to Self-Care by Persons with Comorbid Chronic Diseases. The Annals of Family Medicine 1, 1 (2003), 15–21. 10.1370/afm.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Berry Donna L, Blumenstein Brent A, Halpenny Barbara, Wolpin Seth, Fann Jesse R, Austin-Seymour Mary, Bush Nigel, Karras Bryant T, Lober William B, and McCorkle Ruth. 2011. Enhancing Patient-Provider Communication with the Electronic Self-Report Assessment for Cancer: A Randomized Trial. Journal of Clinical Oncology 29, 8 (2011), 1029 10.1200/jco.2010.30.3909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Donna L Berry Fangxin Hong, Halpenny Barbara, Partridge Ann H, Fann Jesse R, Wolpin Seth, Lober William B, Bush Nigel E, Parvathaneni Upendra, Back Anthony L, et al. 2014. Electronic Self-Report Assessment for Cancer and Self-Care Support: Results of a Multicenter Randomized Trial. Journal of Clinical Oncology 32, 3 (2014), 199 10.1200/jco.2013.48.6662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Beyer Hugh and Holtzblatt Karen. 1998. Contextual Design: Defining Customer-Centered Systems. Vol. 1 Morgan kaufmann. [Google Scholar]
- [16].Birks Melanie, Chapman Ysanne, and Francis Karen. 2008. Memoing in Qualitative Research: Probing Data and Processes. Journal of Research in Nursing 13, 1 (2008), 68–75. 10.1177/1744987107081254 [DOI] [Google Scholar]
- [17].Bogia Megan, Nurik Chloé, Ngan Andrea, Kuhn Bennett, Kumar Ila, and Lingel Jessa. 2018. Institutional Shadow Bodies in Mental Health Care Information Seeking. In Companion of the 2018 ACM Conference on Computer Supported Cooperative Work and Social Computing ACM, 269–272. 10.1145/3272973.3274072 [DOI] [Google Scholar]
- [18].Botsis Taxiarchis, Hartvigsen Gunnar, Chen Fei, and Weng Chunhua. 2010. Secondary use of EHR: Data Quality Issues and Informatics Opportunities. Summit on Translational Bioinformatics 2010 (2010), 1 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3041534/ [PMC free article] [PubMed] [Google Scholar]
- [19].Braun Virginia and Clarke Victoria. 2006. Using Thematic Analysis in Psychology. Qualitative Research in Psychology 3, 2(2006), 77–101. 10.1191/1478088706qp063oa [DOI] [Google Scholar]
- [20].Burcusa Stephanie L and Iacono William G. 2007. Risk for Recurrence in Depression. Clinical Psychology Review 27, 8 (2007), 959–985. 10.1016/j.cpr.2007.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Burgess Eleanor R, Reddy Madhu C, Davenport Andrew, Laboi Paul, and Blandford Ann. 2019. “Tricky to get your head around”: Information Work of People Managing Chronic Kidney Disease in the UK. In Proceedings of the 2019 CHI Conference on Human Factors in Computing Systems ACM, 665 10.1145/3290605.3300895 [DOI] [Google Scholar]
- [22].Castelijns Hilde, Eijsbroek Vera, Cees A Th, van Marwijk Harm WJ, van der Feltz-Cornelis Christina M, et al. 2018. Illness Burden and Physical Outcomes Associated with Collaborative Care in Patients with Comorbid Depressive Disorder in Chronic Medical Conditions: A Systematic Review and Meta-Analysis. General Hospital Psychiatry 50 (2018), 1–14. 10.1016/j.genhosppsych.2017.08.003 [DOI] [PubMed] [Google Scholar]
- [23].Charlton Mary, Schlichting Jennifer, Chioreso Catherine, Ward Marcia, and Vikas Praveen. 2015. Challenges of Rural Cancer Care in the United States. Oncology 29, 9 (2015). https://www.cancernetwork.com/oncology-journal/challenges-rural-cancer-care-united-states/page/0/1 [PubMed] [Google Scholar]
- [24].Chi Chih-Lin, Nick Street W, Robinson Jennifer G, and Crawford Matthew A. 2012. Individualized Patient-Centered Lifestyle Recommendations: An Expert System for Communicating Patient Specific Cardiovascular Risk Information and Prioritizing Lifestyle Options. Journal of Biomedical Informatics 45, 6 (2012), 1164–1174. 10.1016/j.jbi.2012.07.011 [DOI] [PubMed] [Google Scholar]
- [25].Choe Eun Kyoung, Lee Nicole B, Lee Bongshin, Pratt Wanda, and Kientz Julie A. 2014. Understanding Quantified-Selfers’ Practices in Collecting and Exploring Personal Data. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems ACM, 1143–1152. 10.1145/2556288.2557372 [DOI] [Google Scholar]
- [26].Chung Chia-Fang, Dew Kristin, Cole Allison, Zia Jasmine, Fogarty James, Julie A Kientz, and Sean A Munson. 2016. Boundary Negotiating Artifacts in Personal Informatics: Patient-Provider Collaboration with Patient-Generated Data. In Proceedings of the 19th ACM Conference on Computer-Supported Cooperative Work & Social Computing ACM, 770–786. 10.1145/2818048.2819926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cicila Larisa N, Georgia Emily J, and Doss Brian D. 2014. Incorporating Internet-Based Interventions into Couple Therapy: Available Resources and Recommended Uses. Australian and New Zealand Journal of Family Therapy 35, 4 (2014), 414–430. 10.1002/anzf.1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Coluzzi Paul H, Grant Marcia, Doroshow James H, Rhiner Michelle, Ferrell Betty, and Rivera Lynne. 1995. Survey of the Provision of Supportive Care Services at National Cancer Institute-Designated Cancer Centers. Journal of Clinical Oncology 13, 3 (1995), 756–764. 10.1200/jco.1995.13.3.756 [DOI] [PubMed] [Google Scholar]
- [29].Coyle David, Doherty Gavin, Matthews Mark, and Sharry John. 2007. Computers in Talk-Based Mental Health Interventions. Interacting with Computers 19, 4 (2007), 545–562. 10.1016/j.intcom.2007.02.001 [DOI] [Google Scholar]
- [30].Coyle David, McGlade Nicola, Doherty Gavin, and O’Reilly Gary. 2011. Exploratory Evaluations of a Computer Game Supporting Cognitive Behavioural Therapy for Adolescents. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems ACM, 2937–2946. 10.1145/1978942.1979378 [DOI] [Google Scholar]
- [31].Cronin Kathleen A, Lake Andrew J, Scott Susan, Sherman Recinda L, Noone Anne-Michelle, Howlader Nadia, Henley S Jane, Anderson Robert N, Firth Albert U, Ma Jiemin, et al. 2018. Annual Report to the Nation on the Status of Cancer, part I: National Cancer Statistics. Cancer 124, 13 (2018), 2785–2800. 10.1002/cncr.31551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Deshields Teresa, Tibbs Tiffany, Fan Ming-Yu, and Taylor Marie. 2006. Differences in Patterns of Depression After Treatment for Breast Cancer. Psycho-Oncology: Journal of the Psychological, Social and Behavioral Dimensions of Cancer 15, 5 (2006), 398–406. 10.1002/pon.962 [DOI] [PubMed] [Google Scholar]
- [33].Deshields Teresa, Zebrack Brad, and Kennedy Vicki. 2013. The State of Psychosocial Services in Cancer Care in the United States. Psycho-Oncology 22, 3 (2013), 699–703. 10.1002/pon.3057 [DOI] [PubMed] [Google Scholar]
- [34].Dimidjian Sona, Hollon Steven D, Dobson Keith S, Schmaling Karen B, Kohlenberg Robert J, Addis Michael E, Gallop Robert, McGlinchey Joseph B, Markley David K, Gollan Jackie K, et al. 2006. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. Journal of consulting and clinical psychology 74, 4 (2006), 658 10.1037/0022-006x.74.4.658 [DOI] [PubMed] [Google Scholar]
- [35].Druss Benjamin G, Bornemann Thomas, Fry-Johnson Yvonne W, McCombs Harriet G, Politzer Robert M, and Rust George. 2008. Trends in Mental Health and Substance Abuse Services at the Nation’s Community Health Centers: 1998–2003. American Journal ofPublicHealth 98, Supplement_1 (2008), S126–S131. 10.2105/ajph.2005.076943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Duarte A, Walker J, Walker S, Richardson G, Holm Hansen C, Martin P, Murray G, Sculpher M, and Sharpe M. 2015. Cost-Effectiveness of Integrated Collaborative Care for Comorbid Major Depression in Patients with Cancer. Journal of Psychosomatic Research 79, 6 (2015), 465–470. 10.1016/j.jpsychores.2015.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ekers David, Webster Lisa, Van Straten Annemieke, Cuijpers Pim, Richards David, and Gilbody Simon. 2014. Behavioural Activation for Depression; An Update of Meta-Analysis of Effectiveness and Sub Group Analysis. PloS One 9, 6 (2014), e100100 10.1371/journal.pone.0100100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].El-Gayar Omar, Timsina Prem, Nawar Nevine, and Eid Wael. 2013. Mobile Applications for Diabetes Self-Management: Status and Potential. Journal of Diabetes Science and Technology 7, 1 (2013), 247–262. 10.1177/193229681300700130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Eschler Jordan and Pratt Wanda. 2017. I’m So Glad I Met You: Designing Dynamic Collaborative Support for Young Adult Cancer Survivors. In Proceedings of the 2017 ACM Conference on Computer Supported Cooperative Work and Social Computing ACM, 1763–1774. 10.1145/2998181.2998326 [DOI] [Google Scholar]
- [40].Fann JR and Sexton J. 2015. Collaborative Psychosocial Oncology Care Models In Psycho-Oncology. Oxford University Press, London, 1–16. 10.1093/med/9780199363315.003.0099 [DOI] [Google Scholar]
- [41].Fann Jesse R, Berry Donna L, Wolpin Seth, Austin-Seymour Mary, Bush Nigel, Halpenny Barbara, Lober William B, and McCorkle Ruth. 2009. Depression Screening Using the Patient Health Questionnaire-9 Administered on a Touch Screen Computer. Psycho-Oncology: Journal of the Psychological, Social and Behavioral Dimensions of Cancer 18, 1 (2009), 14–22. 10.1002/pon.1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Fann Jesse R, Ell Kathleen, and Sharpe Michael. 2012. Integrating Psychosocial Care into Cancer Services. Journal of Clinical Oncology 30, 11 (2012), 1178–1186. 10.1200/jco.2011.39.7398 [DOI] [PubMed] [Google Scholar]
- [43].Fann Jesse R, Hong Fangxin, Halpenny Barbara, Blonquist Traci M, and Berry Donna L. 2017. Psychosocial Outcomes of an Electronic Self-Report Assessment and Self-Care Intervention for Patients with Cancer: A Randomized Controlled Trial. Psycho-Oncology 26, 11 (2017), 1866–1871. 10.1002/pon.4250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fortney John C, Pyne Jeffrey M, Kimbrell Timothy A, Hudson TeresaJ, Robinson Dean E, Schneider Ronald, Moore William M, Custer Paul J, Grubbs Kathleen M, and Schnurr Paula P. 2015. Telemedicine-Based Collaborative Care for Posttraumatic Stress Disorder: A Randomized Clinical Trial. JAMA Psychiatry 72, 1 (2015), 58–67. 10.1001/jamapsychiatry.2014.1575 [DOI] [PubMed] [Google Scholar]
- [45].Gelenberg AJ, Freeman MP, Markowitz JC, Rosenbaum JF, Thase ME, Trivedi MH, and Van Rhoads RS. 2010. American Psychiatric Association Practice Guidelines for the Treatment of Patients with Major Depressive Disorder. Am J Psychiatry 167, Suppl. 10 (2010), 9–118. 10.1176/appi.books.9780890423387.654001 [DOI] [Google Scholar]
- [46].Hammer Sheila L, Clark Karen, Grant Marcia, and Loscalzo Matthew J. 2015. Seventeen Years of Progress for Supportive Care Services: A Resurvey of National Cancer Institute-Designated Comprehensive Cancer Centers. Palliative & Supportive Care 13, 4 (2015), 917–925. 10.1017/s1478951514000601 [DOI] [PubMed] [Google Scholar]
- [47].Munirul Haque Md, Kawsar Ferdaus, Adibuzzaman Md, Miftah Uddin Md, Ahamed Sheikh I, Love Richard, Hasan Ragib, Dowla Rumana, Ferdousy Tahmina, and Salim Reza. 2015. e-ESAS: Evolution of a Participatory Fesign-Based Solution forBreast Cancer (BC) Patients inRuralBangladesh. Personal and Ubiquitous Computing 19, 2 (2015), 395–413. 10.1007/s00779-014-0828-6 [DOI] [Google Scholar]
- [48].Harding R, Epiphaniou E, Hamilton D, Bridger S, Robinson V, George R, Beynon T, and Higginson IJ. 2012. What are the Perceived Needs and Challenges of Informal Caregivers in Home Cancer Palliative Care? Qualitative Data to Construct a Feasible Psycho-Educational Intervention. Supportive Care in Cancer 20, 9 (2012), 1975–1982. 10.1007/s00520-011-1300-z [DOI] [PubMed] [Google Scholar]
- [49].Hayes Gillian R, Abowd Gregory D, Davis John S, Blount Marion L, Ebling Maria, and Mynatt Elizabeth D. 2008. Opportunities for Pervasive Computing in Chronic Cancer Care. In International Conference on Pervasive Computing Springer, 262–279. 10.1007/978-3-540-79576-6_16 [DOI] [Google Scholar]
- [50].Henson Katherine E, Brock Rachael, Charnock James, Wickramasinghe Bethany, Will Olivia, and Pitman Alexandra. 2019. Risk of Suicide after Cancer Diagnosis in England. JAMA Psychiatry 76, 1 (2019), 51–60. 10.1001/jamapsychiatry.2018.3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hess Lisa M and Insel Kathleen C. 2007. Chemotherapy-Related Change in Cognitive Function: A Conceptual Model In Oncology Nursing Forum, Vol. 34 10.1188/07.onf.981-994 [DOI] [PubMed] [Google Scholar]
- [52].Hong Matthew K, Wilcox Lauren, Machado Daniel, Olson Thomas A, and Simoneaux Stephen F. 2016. Care Partnerships: Toward Technology to Support Teens’ Participation in Their Health Care. In Proceedings of the 2016 CHI Conference on Human Factors in Computing Systems ACM, 5337–5349. 10.1145/2858036.2858508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hunkeler Enid M, Katon Wayne, Tang Lingqi, Williams John W, Kroenke Kurt, Lin Elizabeth HB, Harpole Linda H, Arean Patricia, Levine Stuart, Grypma Lydia M, et al. 2006. Long Term Outcomes from the IMPACT Randomised Trial for Depressed Elderly Patients in Primary Care. British Medical Journal 332, 7536 (2006), 259–263. 10.1136/bmj.38683.710255.be [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Iana Roumenova Ianakieva. 2015. A Task Analysis of Therapeutic Engagement in a Professionally Facilitated Online Intervention for Young Couples Affected by Breast Cancer. Ph.D. Dissertation York University; Toronto, ON: http://hdl.handle.net/10315/30677 [Google Scholar]
- [55].Irwin Kelly E, Park Elyse R, Fields Lauren E, Corveleyn Amy E, Greer Joseph A, Perez Giselle K, Callaway Catherine A, Jacobs Jamie M, Nierenberg Andrew A, Temel Jennifer S, et al. 2019. Bridge: Person-Centered Collaborative Care for Patients with Serious Mental Illness and Cancer. The Oncologist 24, 7 (2019), 901–910. 10.1634/theoncologist.2018-0488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Jacobs Maia, Clawson James, and Mynatt Elizabeth D. 2015. Lessons Learned from a Yearlong Deployment of Customizable Breast Cancer Tablet Computers. In Proceedings of the conference on Wireless Health ACM, 7 10.1145/2811780.2811951 [DOI] [Google Scholar]
- [57].Jacobs Maia, Clawson James, and Mynatt Elizabeth D. 2016. A Cancer Journey Framework: Guiding the Design of Holistic Health Technology. In Proceedings of the 10th EAI International Conference on Pervasive Computing Technologies for Healthcare 114–121. 10.4108/eai.16-5-2016.2263333 [DOI] [Google Scholar]
- [58].Jacobs Maia, Johnson Jeremy, and Mynatt Elizabeth D. 2018. MyPath: Investigating Breast Cancer Patients’ Use of Personalized Health Information. Proceedings of the ACM on Human-Computer Interaction 2, CSCW (2018), 78 10.1145/3274347 [DOI] [Google Scholar]
- [59].Jacobs Maia L, Clawson James, and Mynatt Elizabeth D. 2014. My Journey Compass: A Preliminary Investigation of a Mobile Tool for Cancer Patients. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems ACM, 663–672. 10.1145/2556288.2557194 [DOI] [Google Scholar]
- [60].Jacobs Maia L, Clawson James, and Mynatt Elizabeth D. 2017. Articulatinga Patient-Centered Design Space for Cancer Journeys. EAI Endorsed Transactions on Pervasive Health and Technology 3, 9 (2017). 10.4108/eai.21-3-2017.152394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Jacobsen Paul B, Holland Jimmie C, and Steensma David P. 2012. Caring for the Whole Patient: The Science of Psychosocial Care. American Society of Clinical Oncology. 10.1200/jco.2011.41.4078 [DOI] [PubMed] [Google Scholar]
- [62].Jameson John Paul and Blank Michael B. 2010. Diagnosis and Treatment of Depression and Anxiety in Rural and Nonrural Primary Care: National Survey Results. Psychiatric Services 61, 6 (2010), 624–627. 10.1176/ps.2010.61.6.624 [DOI] [PubMed] [Google Scholar]
- [63].Karampela Maria, Porat Talya, and Mylonopoulou Vasiliki. 2019. Needs of Head and Neck Cancer Patients and Stakeholders During Rehabilitation. In Proceedings of the 13th EAI International Conference on Pervasive Computing Technologies for Healthcare ACM, 415–421. 10.1145/3329189.3329236 [DOI] [Google Scholar]
- [64].Kisely Stephen, Crowe Elizabeth, and Lawrence David. 2013. Cancer-Related Mortality in People with Mental Illness. JAMA Psychiatry 70, 2 (2013), 209–217. 10.1001/jamapsychiatry.2013.278 [DOI] [PubMed] [Google Scholar]
- [65].Kissane David. 2009. Beyond the Psychotherapy and Survival Debate: The Challenge of Social Disparity, Depression and Treatment Adherence in Psychosocial Cancer Care. Psycho-Oncology: Journal of the Psychological, Social and Behavioral Dimensions of Cancer 18, 1 (2009), 1–5. 10.1002/pon.1493 [DOI] [PubMed] [Google Scholar]
- [66].Klasnja Predrag, Hartzler Andrea Civan, Unruh Kent T, and Pratt Wanda. 2010. Blowing in the Wind: Unanchored Patient Information Work During Cancer Care. In Proceedings of the SIGCHI Conference on HumanFactors in Computing Systems 193–202. 10.1145/1753326.1753355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Kraft Pal, Drozd Filip, and Olsen Elin. 2008. Digital Therapy: Addressing Willpower as Part of the Cognitive-Affective Processing System in the Service of Habit Change. In International Conference on Persuasive Technology Springer, 177–188. 10.1007/978-3-540-68504-3_16 [DOI] [Google Scholar]
- [68].Krebber AMH, Buffart LM, Kleijn G, Riepma IC, De Bree R, Leemans CR, Becker A, Brug J, Van Straten A, Cuijpers P, et al. 2014. Prevalence of Depression in Cancer Patients: a Meta-Analysis of Diagnostic Interviews and Self-Report Instruments. Psycho-Oncology 23, 2 (2014), 121–130. 10.1002/pon.3409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kroenke Kurt, Spitzer Robert L, and Williams Janet BW. 2001. The PHQ-9: Validity of a BriefDepressionSeverity Measure. Journal of General Internal medicine 16, 9 (2001), 606–613. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kroenke Kurt, Theobald Dale, Wu Jingwei, Norton Kelli, Morrison Gwendolyn, Carpenter Janet, and Tu Wanzhu. 2010. Effect of Telecare Management on Pain and Depression in Patients with Cancer: A Randomized Trial. Journal of the American Medical Association 304, 2(2010), 163–171. 10.1001/jama.2010.944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Landwehr Michelle S, Watson Samantha E, Macpherson Catherine F, Novak Katherine A, and Johnson Rebecca H. 2016. The Cost of Cancer: A Retrospective Analysis of the Financial Impact of Cancer on Young Adults. Cancer Medicine 5, 5 (2016), 863–870. 10.1002/cam4.657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Lederman Reeva, Wadley Greg, Gleeson John, Bendall Sarah, and Alvarez-Jimenez Mario. 2014. Moderated Online Social Therapy: Designing and Evaluating Technology for Mental Health. ACM Transactions on Computer-Human Interaction (TOCHI) 21, 1 (2014), 5 10.1145/2513179 [DOI] [Google Scholar]
- [73].Li Madeline, Kennedy Erin B, Byrne Nelson, Gerin-Lajoie Caroline, Katz Mark R, Keshavarz Homa, Sellick Scott, and Green Esther. 2017. Systematic Review and Meta-Analysis of Collaborative Care Interventions for Depression in Patients with Cancer. Psycho-Oncology 26, 5 (2017), 573–587. 10.1002/pon.4286 [DOI] [PubMed] [Google Scholar]
- [74].Lin Chen-Tan, Wittevrongel Loretta, Moore Laurie, Beaty Brenda L, and Ross Stephen E. 2005. An Internet-Based Patient-Provider Communication System: Randomized Controlled Trial. Journal of Medical Internet Research 7, 4 (2005), e47 10.2196/jmir.7.4.e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Löwe Bernd, Kroenke Kurt, and Grafe Kerstin. 2005. Detecting and Monitoring Depression with a Two-Item Questionnaire (PHQ-2). Journal of Psychosomatic Research 58, 2 (2005), 163–171. 10.1016/j.jpsychores.2004.09.006 [DOI] [PubMed] [Google Scholar]
- [76].Macdonald GC Turnbull, Baldassarre F, Brown P, Hatton-Bauer J, Li M, Green E, and Lebel S. 2012. Psychosocial Care for Cancer: A Framework to Guide Practice, and Actionable Recommendations for Ontario. Current Oncology 19, 4 (2012), 209 10.3747/co.19.981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Massimi Michael, Odom William, Banks Richard, and Kirk David. 2011. Matters of Life and Death: Locating the End of Life in Lifespan-Oriented HCI Research. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems ACM, 987–996. 10.1145/1978942.1979090 [DOI] [Google Scholar]
- [78].Mausbach Brent T, Yeung Philip, Bos Taylor, and Irwin Scott A. 2018. Health Care Costs of Depression in Patients Diagnosed with Cancer. Psycho-Oncology 27, 7 (2018), 1735–1741. 10.1002/pon.4716 [DOI] [PubMed] [Google Scholar]
- [79].McDonald Margaret V, Passik Steven D, Dugan William, Rosenfeld Barry, Theobald Dale E, and Edgerton Sara. 1999. Nurses’ Recognition of Depression in their Patients with Cancer. In Oncology Nursing Forum, Vol. 26 593–599. 10.1002/pon.3191 [DOI] [PubMed] [Google Scholar]
- [80].Meijer Anna, Roseman Michelle, Milette Katherine, Coyne James C, Stefanek Michael E, Ziegelstein Roy C, Arthurs Erin, Leavens Allison, Palmer Steven C, Stewart Donna E, et al. 2011. Depression Screening and Patient Outcomes in Cancer: A Systematic Review. PLoS One 6, 11 (2011), e27181 10.1371/journal.pone.0027181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Misono Stephanie, Weiss Noel S, Fann Jesse R, Redman Mary, and Yueh Bevan. 2008. Incidence of Suicide in Persons with Cancer. Journal of Clinical Oncology 26, 29 (2008), 4731 10.1200/jco.2007.13.8941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Mohr David C, Montague Enid, Stiles-Shields Colleen, Kaiser Susan M, Brenner Christopher, Carty-Fickes Eric, Palac Hannah, and Duffecy Jenna. 2015. MedLink: A Mobile Intervention to Address Failure Points in the Treatment of Depression in General Medicine. In Proceedings of the 9th International Conference on Pervasive Computing Technologies for Healthcare ICST (Institute for Computer Sciences, Social-Informatics and Telecommunications Engineering, 100–107. 10.4108/icst.pervasivehealth.2015.259042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Moise Nathalie, Shah Ravi N, Essock Susan, Jones Amy, Carruthers Jay, Handley Margaret A, Peccoralo Lauren, and Sederer Lloyd. 2018. Sustainability of Collaborative Care Management for Depression in Primary Care Settings with Academic Affiliations across New York State. Implementation Science 13, 1 (2018), 128 10.1186/s13012-018-0818-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Mosher Catherine E, DuHamel Katherine N, Rini Christine, Corner Geoffrey, Lam Joanne, and Redd William H. 2011. Quality of Life Concerns and Depression among Hematopoietic Stem Cell Transplant Survivors. Supportive Care in Cancer 19, 9 (2011), 1357–1365. 10.1007/s00520-010-0958-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].National Comprehensive Cancer Network et al. 2003. Distress Management. Clinical Practice Guidelines. Journal of the National Comprehensive Cancer Network: JNCCN 1, 3 (2003), 344 10.6004/jnccn.2003.0031 [DOI] [PubMed] [Google Scholar]
- [86].Nipp Ryan D, El-Jawahri Areej, Moran Samantha M, D’arpino Sara M, Johnson P Connor, Lage Daniel E, Wong Risa L, Pirl William F, Traeger Lara, Lennes Inga T, et al. 2017. The Relationship Between Physical and Psychological Symptoms and Health Care Utilization in Hospitalized Patients with Advanced Cancer. Cancer 123, 23 (2017), 4720–4727. 10.1002/cncr.30912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Northouse Laurel, Williams Anna-leila, Given Barbara, and McCorkle Ruth. 2012. Psychosocial Care for Family Caregivers of Patients with Cancer. Journal of Clinical Oncology 30, 11 (2012), 1227–1234. 10.1200/jco.2011.39.5798 [DOI] [PubMed] [Google Scholar]
- [88].Osazuwa-Peters Nosayaba, Simpson Matthew C., Zhao Longwen, Boakye Eric Adjei, Olomukoro Stephanie I., Deshields Teresa, Loux Travis M., Varvares Mark A., and Schootman Mario. 2018. Suicide Risk among Cancer Survivors: Head and Neck versus Other Cancers. Cancer 124, 20(2018), 4072–4079. https://doi.org/10.1002/cncr.31675 arXiv:https://doi.org/10.1002/cncr.31675https://acsjournals.onlinelibrary.wiley.com/doi/pdf/10.1002/cncr.31675 arXiv: https://acsjournals.onlinelibrary.wiley.com/doi/pdf/10.1002/cncr.31675 [DOI] [PubMed] [Google Scholar]
- [89].Page Ann EK, Adler Nancy E, et al. 2008. Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. National Academies Press; 10.17226/11993 [DOI] [PubMed] [Google Scholar]
- [90].Pampallona Sandro, Bollini P, Tibaldi G, Kupelnick B, and Munizza Carmine. 2002. Patient Adherence in the Treatment of Depression. The British Journal of Psychiatry 180, 2 (2002), 104–109. https://doi.org/10T192/bjp.180.2T04 [DOI] [PubMed] [Google Scholar]
- [91].Passik Steven D, Dugan William, McDonald Margaret V, Rosenfeld Barry, Theobald Dale E, and Edgerton Sara. 1998. Oncologists’ Recognition ofDepression in their Patients with Cancer. Journal of Clinical Oncology 16, 4 (1998), 1594–1600. 10.1200/JCO.1998.16.4T594 [DOI] [PubMed] [Google Scholar]
- [92].Philip Errol J, Merluzzi Thomas V, Zhang Zhiyong, and Heitzmann Carolyn A. 2013. Depression and Cancer Survivorship: Importance of Coping Self-Efficacy in Post-Treatment Survivors. Psycho-Oncology 22, 5 (2013), 987–994. 10.1002/pon.3088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Pirl William F, Fann Jesse R, Greer Joseph A, Braun Ilana, Deshields Teresa, Fulcher Caryl, Harvey Elizabeth, Holland Jimmie, Kennedy Vicki, Lazenby Mark, et al. 2014. Recommendations for the Implementation of Distress Screening Programs in Cancer Centers: Report from the American Psychosocial Oncology Society (APOS), Association of Oncology Social Work (AOSW), and Oncology Nursing Society (ONS) Joint Task Force. Cancer 120, 19 (2014), 2946–2954. 10.1002/cncr.28750 [DOI] [PubMed] [Google Scholar]
- [94].Pitman Alexandra, Suleman Sahil, Hyde Nicholas, and Hodgkiss Andrew. 2018. Depression and Anxiety in Patients with Cancer. Bmj 361 (2018), k1415 10.1136/bmj.k1415 [DOI] [PubMed] [Google Scholar]
- [95].Randhawa Gurvaneet S, Ahern David K, and Hesse Bradford W. 2017. Information Technology-Enabled Team-Based, Patient-Centered Care: The Example ofDepression Screening and Management in Cancer Care. Health Policy and Technology 6, 1 (2017), 67–71. 10.1016/j.hlpt.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Raney Lori, Bergman David, Torous John, and Hasselberg Michael. 2017. Digitally Driven Integrated Primary Care and Behavioral Health: How Technology Can Expand Access to Effective Treatment. Current Psychiatry Reports 19, 11 (2017), 86 10.1007/s11920-017-0838-y [DOI] [PubMed] [Google Scholar]
- [97].Reschovsky James D and Staiti Andrea B. 2005. Access and Quality: Does Rural America Lag Behind? Health Affairs 24,4(2005), 1128–1139. 10.1377/hlthaff.24.4T128 [DOI] [PubMed] [Google Scholar]
- [98].Reynolds Julia, Griffiths Kathleen, Christensen Helen, et al. 2011. Anxiety and Depression: Online Resources and Management Tools. Australian Family Physician 40, 6 (2011), 382 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=21655483 [PubMed] [Google Scholar]
- [99].Rieke Katherine, Schmid Kendra K., Lydiatt William, Houfek Julia, Boilesen Eugene, and Watanabe-Galloway Shinobu. 2017. Depression and Survival in Head and Neck Cancer Patients. Oral Oncology 65 (2017), 76–82. 10.1016/j.oraloncology.2016.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Rollman Bruce L, Belnap Bea Herbeck, Mazumdar Sati, Abebe Kaleab Z, Karp Jordan F, Lenze Eric J, and Schulberg Herbert C. 2017. Telephone-Delivered Stepped Collaborative Care for Treating Anxiety in Primary Care: A Randomized Controlled Trial. Journal of General Internal Medicine 32, 3 (2017), 245–255. 10.1007/s11606-016-3873-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Roth Andrew J, Kornblith Alice B, Batel-Copel Laure, Peabody Elizabeth, Scher Howard I, and Holland Jimmie C. 1998. Rapid Screening for Psychologic Distress in Men with Prostate Carcinoma: a Pilot Study. Cancer: Interdisciplinary International Journal of the American Cancer Society 82, 10 (1998), 1904–1908. [DOI] [PubMed] [Google Scholar]
- [102].Saad Anas M, Gad Mohamed M, Al-Husseini Muneer J, AlKhayat Mohamad A, Rachid Ahmad, Samir Alfaar Ahmad, and Hamoda Hesham M. 2019. Suicidal Death Within a Year of a Cancer Diagnosis: A Population-Based Study. Cancer 125, 6 (2019), 972–979. 10.1002/cncr.31876 [DOI] [PubMed] [Google Scholar]
- [103].Sanches Pedro, Janson Axel, Karpashevich Pavel, Nadal Camille, Qu Chengcheng, Dauden Roquet Claudia, Umair Muhammad, Windlin Charles, Doherty Gavin, Hook Kristina, et al. 2019. HCI and Affective Health: Taking stock of a decade of studies and charting future research directions. In Proceedings of the 2019 CHI Conference on Human Factors in Computing Systems ACM, 245 10.1145/3290605.3300475 [DOI] [Google Scholar]
- [104].Schroeder Jessica, Hoffswell Jane, Chung Chia-Fang, Fogarty James, Munson Sean, and Zia Jasmine. 2017. Supporting Patient-Provider Collaboration to Identify Individual Triggers Using Food and Symptom Journals. In Proceedings of the 2017 ACM Conference on Computer Supported Cooperative Work and Social Computing ACM, 1726–1739. 10.1145/2998181.2998276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Schroeder Jessica, Wilkes Chelsey, Rowan Kael, Toledo Arturo, Paradiso Ann, Czerwinski Mary, Mark Gloria, and Linehan Marsha M. 2018. Pocket Skills: A Conversational Mobile Web App to Support Dialectical Behavioral Therapy. In Proceedings of the 2018 CHI Conference on Human Factors in Computing Systems ACM, 398 10.1145/3173574.3173972 [DOI] [Google Scholar]
- [106].Schuler Thilo, Garde Sebastian, Heard Sam, and Beale Thomas. 2006. Towards Automatically Generating Graphical User Interfaces from openEHR Archetypes. Studies in Health Technology and Informatics 124 (2006), 221 http://www.academia.edu/download/41884688/Towards_automatically_generating_graphic20160202-27158-1hgx8j1.pdf [PubMed] [Google Scholar]
- [107].USDA Economic Research Service. 2013. 2013 Rural-Urban Continuum Codes. https://data.nal.usda.gov/dataset/rural-urban-continuum-codes
- [108].Shaw Joanne, Sethi Suvena, Vaccaro Lisa, Beatty Lisa, Kirsten Laura, Kissane David, Kelly Brian, Mitchell Geoff, Sherman Kerry, and Turner Jane. 2019. Is Care Really Shared? A Systematic Review of Collaborative Care (Shared Care) Interventions for Adult Cancer Patients with Cepression. BMC Health Services Research 19, 1 (2019), 120 10.1186/s12913-019-3946-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Simon Gregory E, Ding Victoria, Hubbard Rebecca, Fishman Paul, Ludman Evette, Morales Leo, Operskalski Belinda, and Savarino James. 2012. Early Dropout from Psychotherapy for Depression with Group-and Network-Model Therapists. Administration and Policy in Mental Health and Mental Health Services Research 39, 6 (2012), 440–447. 10.1007/s10488-011-0364-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Simon Gregory E and Ludman Evette J. 2010. Predictors of Early Dropout from Psychotherapy for Depression in Community Practice. Psychiatric Services 61, 7 (2010), 684–689. 10.1176/ps.2010.61.7.684 [DOI] [PubMed] [Google Scholar]
- [111].Skeels Meredith M, Unruh Kenton T, Powell Christopher, and Pratt Wanda. 2010. Catalyzing Social Support for Breast Cancer Patients. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems ACM, 173–182. 10.1145/1753326.1753353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Smith Hamish R. 2015. Depression in Cancer Patients: Pathogenesis, Implications and Treatment. Oncology Letters 9, 4 (2015), 1509–1514. 10.3892/ol.2015.2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Sollner W, DeVries A, Steixner E, Lukas P, Sprinzl G, Rumpold G, and Maislinger S. 2001. How Successful are Oncologists in Identifying Patient Distress, Perceived Social Support, and Need for Psychosocial Counselling? British Journal of Cancer 84, 2 (2001), 179 10.1054/bjoc.2000.1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Spijker JAN, De Graaf Ron, Bijl Rob V, Beekman Aartjan TF, Ormel Johan, and Nolen Willem A. 2002. Duration of Major Depressive Episodes in the General Population: Results from The Netherlands Mental Health Survey and Incidence Study (NEMESIS). The British Journal of Psychiatry 181, 3 (2002), 208–213. 10.1192/bjp.181.3.208 [DOI] [PubMed] [Google Scholar]
- [115].Spitzer Robert L, Kroenke Kurt, Williams Janet BW, and Lowe Bernd. 2006. A Brief Measure for Assessing Generalized Anxiety Disorder: The GAD-7. Archives of Internal Medicine 166, 10 (2006), 1092–1097. 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- [116].Staat Kari and Segatore Milena. 2005. The Phenomenon of Chemo Brain. Clinical Journal of Oncology Nursing 9, 6 (2005), 713 10.1188/05.cjon.713-721 [DOI] [PubMed] [Google Scholar]
- [117].Stallard Paul, Richardson Thomas, Velleman Sophie, and Attwood Megan. 2011. Computerized CBT (Think, Feel, Do) for Depression and Anxiety in Children and Adolescents: Outcomes and Feedback from a Pilot Randomized Controlled Trial. Behavioural and Cognitive Psychotherapy 39, 3 (2011), 273–284. 10.1017/s135246581000086x [DOI] [PubMed] [Google Scholar]
- [118].Steel Jennifer L, Geller David A, Kim Kevin H, Butterfield Lisa H, Spring Michael, Grady Jonathan, Sun Weiing, Marsh Wallis, Antoni Michael, Dew Mary Amanda, et al. 2016. Web-Based Collaborative Care Intervention to Manage Cancer-Related Symptoms in the Palliative Care Setting. Cancer 122, 8 (2016), 1270–1282. 10.1002/cncr.29906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Steel Zachary, Marnane Claire, Iranpour Changiz, Chey Tien, Jackson John W, Patel Vikram, and Silove Derrick. 2014. The Global Prevalence of Common Mental Disorders: A Systematic Review and Meta-Analysis 1980–2013. International Journal of Epidemiology 43, 2 (2014), 476–493. 10.1093/ije/dyu038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Swift Joshua K and Greenberg Roger P. 2012. Premature Discontinuation in Adult Psychotherapy: A Meta-Analysis. Journal of Consulting and Clinical Psychology 80, 4 (2012), 547 10.1037/a0028226 [DOI] [PubMed] [Google Scholar]
- [121].Thota Anilkrishna B, Ann Sipe Theresa, Byard Guthrie J, Zometa Carlos S, Hahn Robert A, McKnight-Eily Lela R, Chapman Daniel P, Abraido-Lanza Ana F, Pearson Jane L, Anderson Clinton W, et al. 2012. Collaborative Care to Improve the Management of Depressive Disorders: A Community Guide Systematic Review and Meta-Analysis. American Journal of Preventive Medicine 42, 5 (2012), 525–538. https://doi.org/0.1016/j.amepre.2012.01.019 [DOI] [PubMed] [Google Scholar]
- [122].Torsi Silvia, Nasr Nasrin, Wright Peter C, Mawson Sue J, and Mountain Gail A. 2009. User-Centered Design for Supporting the Self-Management of Chronic Illnesses: an Interdisciplinary Approach. In Proceedings of the 2nd International Conference on PErvasive Technologies Related to Assistive Environments ACM, 43 10.1145/1579114.1579157 [DOI] [Google Scholar]
- [123].Troshynski Emily, Lee Charlotte, and Dourish Paul. 2011. Accountabilities of Presence: Reframing Location-Based Systems. Droit et Cultures. Revue Internationale Interdisciplinaire 61 (2011), 171–193. 10.1145/1357054.1357133 [DOI] [Google Scholar]
- [124].Unützer Jürgen, Harbin Henry, Schoenbaum Michael, and Druss Benjamin. 2013. The Collaborative Care Model: An Approach for Integrating Physical and Mental Health Care in Medicaid Health Homes. HEALTH HOME, Information Resource Center (2013), 1–13. 10.1176/appi.ps.57.1.37 [DOI] [Google Scholar]
- [125].Vanderlip ER, Rundell J, Avery M, Alter C, Engel C, Fortney J, and Williams M. 2016. Dissemination of Integrated Care within Adult Primary Care Settings: The Collaborative Care Model. Washington DC: American Psychiatric Association and Academy of Psychosomatic Medicine; (2016). [Google Scholar]
- [126].Walker Jane, Hansen Christian Holm, Martin Paul, Symeonides Stefan, Ramessur Ravi, Murray Gordon, and Sharpe Michael. 2014. Prevalence, Associations, and Adequacy of Treatment of Major Depression in Patients with Cancer: A Cross-Sectional Analysis of Routinely Collected Clinical Data. The Lancet Psychiatry 1, 5 (2014), 343–350. 10.1016/s2215-0366(14)70313-x [DOI] [PubMed] [Google Scholar]
- [127].Wang Rui, Aung Min SH, Abdullah Saeed, Brian Rachel, Campbell Andrew T, Choudhury Tanzeem, Hauser Marta, Kane John, Merrill Michael, Scherer Emily A, et al. 2016. CrossCheck: Toward Passive Sensing and Detection of Mental Health Changes in People with Schizophrenia. In Proceedings of the 2016 ACM International Joint Conference on Pervasive and Ubiquitous Computing ACM, 886–897. 10.1145/2971648.2971740 [DOI] [Google Scholar]
- [128].Waraich Paul, Goldner Elliot M, Somers Julian M, and Hsu Lorena. 2004. Prevalence and Incidence Studies of Mood Disorders: A Systematic Review of the Literature. The Canadian Journal of Psychiatry 49, 2 (2004), 124–138. 10.1177/070674370404900208 [DOI] [PubMed] [Google Scholar]
- [129].Ward Elizabeth, Sherman Recinda L, Henley S Jane, Jemal Ahmedin, Siegel David A, Feuer Eric J, Firth Albert U, Kohler Betsy A, Scott Susan, Ma Jiemin, et al. 2019. Annual Report to the Nation on the Status of Cancer, 1999–2015, Featuring Cancer in Men and Women ages 20–49. JNCI: Journal of the National Cancer Institute (2019). 10.1093/jnci/djz106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Wehbe Rina R, Watson Diane K, Tondello Gustavo F, Ganaba Marim, Stocco Melissa, Lee Alvin, and Nacke Lennart E. 2016. ABOVE WATER: An Educational Game for Anxiety. In Proceedings of the 2016 Annual Symposium on Computer-Human Interaction in Play Companion Extended Abstracts ACM, 79–84. 10.1145/2968120.2971804 [DOI] [Google Scholar]
- [131].Wood Emily, Ohlsen Sally, and Ricketts Thomas. 2017. What are the Barriers and Facilitators to Implementing Collaborative Care for Depression? A Systematic Review. Journal of Affective Disorders 214 (2017), 26–43. 10.1016/j.jad.2017.02.028 [DOI] [PubMed] [Google Scholar]
- [132].Wrzesien Maja, Alcañiz Mariano, Botella Cristina, Burkhardt Jean-Marie, Lopez Juana Breton, and Rodriguez Ortega Alejandro. 2014. A Pilot Evaluation of a Therapeutic Game Applied to Small Animal Phobia Treatment. In International Conference on Serious Games Development and Applications Springer, 10–20. 10.1007/978-3-319-11623-5_2 [DOI] [Google Scholar]
- [133].Wu Shinyi, Ell Kathleen, Jin Haomiao, Vidyanti Irene, Chou Chih-Ping, Lee Pey-Jiuan, Gross-Schulman Sandra, Myerchin Sklaroff Laura, Belson David, Nezu Arthur M, et al. 2018. Comparative Effectiveness of a Technology-Facilitated Depression Care Management Model in Safety-Net Primary Care Patients with Type 2 Diabetes: 6-Month Outcomes of a Large Clinical Trial. Journal of Medical Internet Research 20, 4 (2018), e147 10.2196/jmir.7692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Ye Jiali, Rust George, Fry-Johnson Yvonne, and Strothers Harry. 2010. E-mail in Patient-Provider Communication: A Systematic Review. Patient Education and Counseling 80, 2 (2010), 266–273. 10.1016/j.pec.2009.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Zebrack Brad, Kayser Karen, Padgett Lynne, Sundstrom Laura, Jobin Chad, Nelson Krista, and Fineberg Iris C. 2016. Institutional Capacity to Provide Psychosocial Oncology Support Services: A Report from the Association of Oncology Social Work. Cancer 122, 12 (2016), 1937–1945. 10.1002/cncr.30016 [DOI] [PubMed] [Google Scholar]
- [136].Zheng Huiru, Nugent Chris, McCullagh Paul, Huang Yan, Zhang Shumei, Burns William, Davies Richard, Black Norman, Wright Peter, Mawson Sue, et al. 2010. Smart Self Management: Assistive Technology to Support People with Chronic Disease. Journal of Telemedicine and Telecare 16, 4 (2010), 224–227. 10.1258/jtt.2010.004017 [DOI] [PubMed] [Google Scholar]


