Abstract
Interleukin-7 (IL-7) is required for T cell development and for maintaining and restoring homeostasis of mature T cells. IL-7 is a limiting resource under normal conditions, but it accumulates during lymphopaenia, leading to increased T cell proliferation. The administration of recombinant human IL-7 to normal or lymphopenic mice, non-human primates and humans results in widespread T cell proliferation, increased T cell numbers, modulation of peripheral T cell subsets and increased T cell receptor repertoire diversity. These effects raise the prospect that IL-7 could mediate therapeutic benefits in several clinical settings. This Review summarizes the biology of IL-7 and the results of its clinical use that are available so far to provide a perspective on the opportunities for clinical application of this cytokine.
The designation of interleukin-7 (IL-7) as an interleukin is a misnomer, as non-haematopoietic stromal cells, rather than leukocytes, are the main producers of this cytokine. T cells, B cells and natural killer (NK) cells do not produce IL-7, although small amounts of the cytokine are produced by dendritic cells (DCs)1. IL-7 signals through the IL-7 receptor (IL-7R) — a heterodimer comprised of IL-7Rα (also known as CD127) and the common cytokine receptor γ-chain (γc; also known as CD132) — and mediates anti-apoptotic and co-stimulatory proliferative signals through the activation of phosphoinositide 3-kinase (PI3K) and the Janus kinase–signal transducer and activator of transcription pathway (JAK–STAT pathway), downregulation of the cyclin-dependent kinase inhibitor p27 (also known as p27Kip1) and modulation of members of the B cell lymphoma 2 (BCL-2) family (FIG. 1). IL-7 also represses expression of Casitas B-lineage lymphoma B (CBL-B), which probably contributes to its co-stimulatory effects, and antagonizes transforming growth factor-β (TGFβ)-mediated signalling through the induction of expression of SMAD ubiquitylation regulatory factor 2 (SMURF2)2.
Figure 1 |. IL-7-mediated signalling pathways.
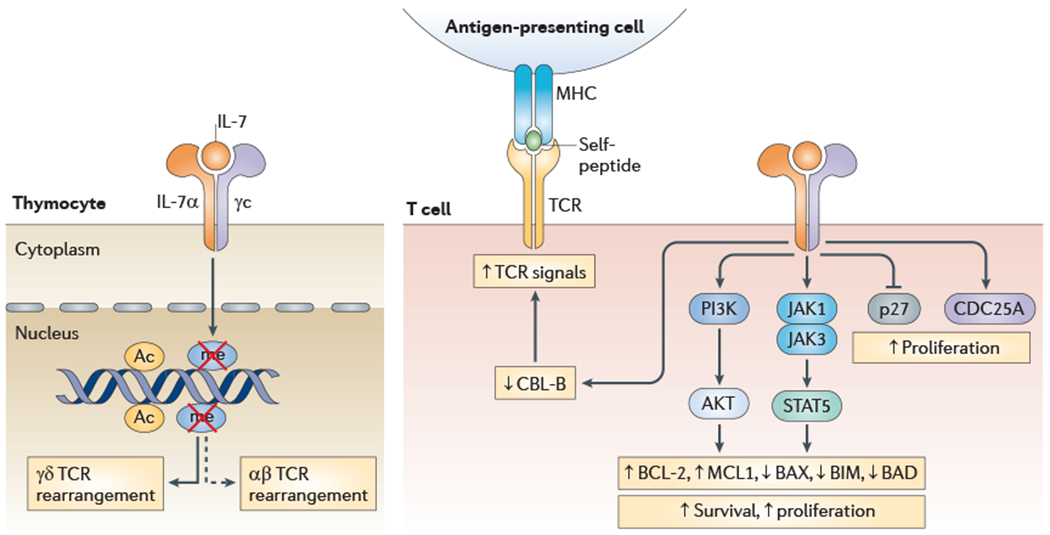
Interleukin-7 (IL-7) signals through the IL-7 receptor (IL-7R), a heterodimer comprised of IL-7Rα (also known as CD127) and the common cytokine receptor γ-chain (γc; also known as CD132). During T cell development in the thymus, IL-7-mediated signalling participates in T cell receptor (TCR) gene rearrangement through DNA demethylation and histone acetylation. In all T cells, IL-7-mediated signalling initiates downstream signalling pathways through Janus kinase 1 (JAK1), JAK3 and phosphoinositide 3-kinase (PI3K), resulting in the phosphorylation and activation of signal transducer and activator of transcription 5 (STAT5). This results in changes in the expression of B cell lymphoma 2 (BCL-2) family members, such as increased expression of the anti-apoptotic molecules BCL-2 and MCL1 (myeloid cell leukaemia seguence 1) and decreased expression of the pro-apoptotic molecules BAX (BCL-2-associated X protein), BIM (BCL-2-interacting mediator of cell death) and BAD (BCL-2 antagonist of cell death). IL-7-mediated signalling also leads to decreased levels of the cyclin-dependent kinase inhibitor p27 (also known as p27Kip1), increased levels of CDC25A (cell division cycle 25 homologue A) and changes in the expression of TCR modulators such as Casitas B-lineage lymphoma B (CBL-B).The result of IL-7-mediated signalling is increased T cell survival, increased proliferation, augmented TCR signals and, for recent thymic emigrants,TCR-independent proliferation.
In contrast to other members of the γc cytokine family, for which T cell activation and/or cytokine signalling increase expression of the respective cytokine receptor by T cells, IL-7Rα is expressed by most resting T cells and is downregulated following IL-7-mediated signalling and/or T cell activation3,4. Rather than provide a comprehensive review of IL-7 biology, which can be found elsewhere5,6, this Review briefly summarizes the biology of IL-7, with an emphasis on recent findings, and focuses on those properties that are most relevant to the use of IL-7 as a therapeutic agent.
IL-7 and lymphocytes
Effects on development.
IL-7 is absolutely required for human T cell development as illustrated by the absence of T cells in humans with severe combined immunodeficiency (SCID) owing to mutations in the IL-7Rα or γc chains7,8. IL-7R expression is tightly controlled during thymopoiesis (FIG. 2), being present on double-negative thymocytes, absent on double-positive thymocytes then re-expressed by single-positive thymocytes, and remaining present on most mature T cells (reviewed in REF. 9). IL-7 provides trophic and proliferative signals to double-negative thymocytes and also directly instructs recombination of the T cell receptor γ-chain (TCRγ) locus. As a result, αβ T cell development can be partly restored in IL-7-deficient hosts by overexpression of the anti-apoptotic molecules BCL-2 or myeloid cell leukaemia sequence 1 (MCL1), or loss of pro-apoptotic molecules, whereas γδ T cell development cannot be restored by this approach5,10–13. Some of the effects of IL-7 on T cell development can be substituted for by thymic stromal lymphopoietin (TSLP), which signals through IL-7Rα complexed with TSLP receptor (TSLPR); this finding explains the more severe immunodeficiency that is present in IL-7Rα-deficient mice compared with IL-7-deficient mice14. Recent studies have shown that IL-2 is the main factor driving the development of natural regulatory T (TReg) cells; however, either IL-7 or TSLP can mediate intrathymic TReg cell development in the absence of IL-2 (REFS 15–17).
Figure 2 |. IL-7R expression by lymphocytes.
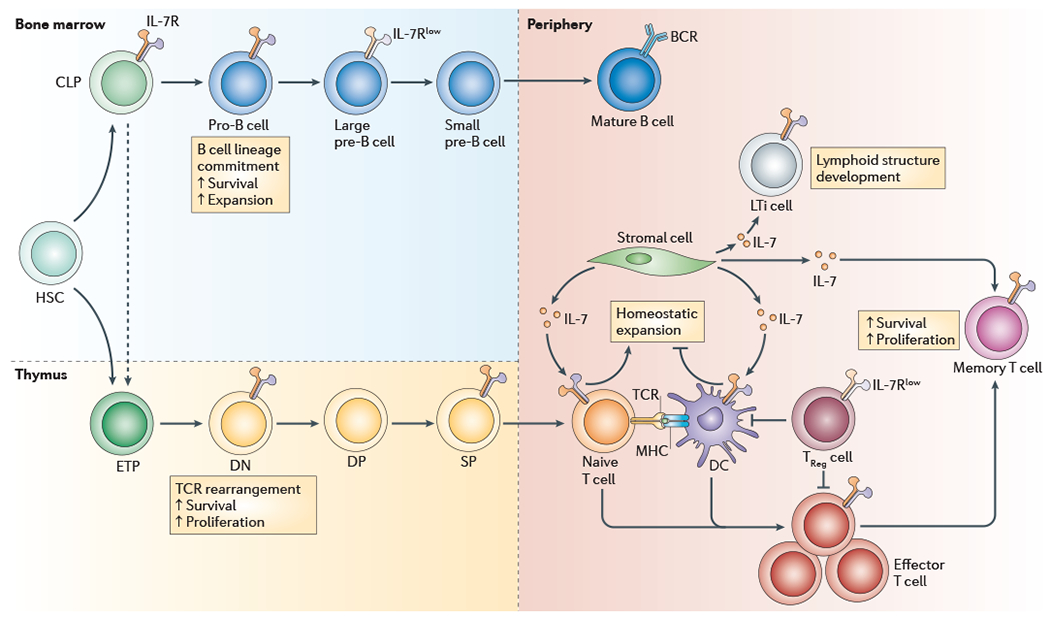
Because expression of the common cytokine receptor γ-chain (γc) is ubiquitous on developing and mature T cells and B cells, interleukin-7 receptor (IL-7R) expression is mainly determined by the presence or absence of IL-7Rα. IL-7Rα expression is tightly regulated throughout T cell and B cell lymphopoiesis. IL-7Rα is not expressed by haematopoietic stem cells (HSCs). During B cell development, IL-7Rα is expressed by common lymphoid progenitor (CLP) cells in the bone marrow and by pro-B cell and large pre-B cell progenitors; IL-7Rα expression is then downregulated on more mature B cell populations. During T cell development, IL-7Rα expression is absent from early T cell lineage progenitor (ETP) cells found in the thymus, a population of cells that is probably derived directly from HSCs but with some evidence indicating that these cells are derived from CLPs. In the thymus, IL-7Rα is expressed by double-negative (DN) thymocytes, then downregulated on double-positive (DP) thymocytes and re-expressed by single-positive (SP) thymocytes and recent thymic emigrants (not shown). During post-thymic T cell differentiation, the expression of IL-7Rα is also tightly regulated. It is expressed by naive T cells, but receptor expression is lost on most effector T cells after activation. A small population of effector T cells probably retain expression of IL-7Rα, which marks them to become long-lived memory T cells. Non-T cells, including lymphoid tissue inducer (LTi) cells and some dendritic cell (DC) subsets, also express IL-7Rα, although the expression of γc is more variable on these subsets. Importantly, IL-7 is not produced by lymphocytes but rather by stromal cells in lymphoid organs. BCR, B cell receptor; TCR, T cell receptor; TReg cell, regulatory T cell.
Patients with SCID due to IL-7Rα or γc mutations generate B cells8, which shows that IL-7 is not absolutely required for primary B cell development (reviewed in REF. 18) in humans. However, recent data from an in vitro culture system comparing adult bone marrow cells with umbilical cord blood stem cells indicate that IL-7-independent B cell development in humans might be restricted to fetal life and that IL-7 is required for B cell development postnatally19. Interestingly, IL-7-dependent B cells seem to be important for the generation of antibody responses to T cell-independent polysaccharide antigens, a finding that could explain the poor responsiveness to T cell-independent antigens that is observed in infants20. Despite these caveats, IL-7 probably has an important role in normal human B cell development. IL-7R expression is tightly regulated during B cell development, being expressed by common lymphoid progenitors and by B cell progenitors through the pro-B cell stage, then downregulated on pre-B cells (FIG. 2). Unlike mature T cells, IL-7R is not re-expressed by mature B cells. IL-7-mediated signalling induces the expression of transcription factors involved in B cell lineage commitment21 and contributes to the ordering of immunoglobulin gene rearrangements by repressing Igκ transcription during pro-B cell development22–24. In vitro studies show that IL-7 drives the clonal expansion and survival of immature B cells25; the dependence on IL-7 to drive the clonal expansion of immature B cells increases when adult bone marrow is used as the source of B cell progenitors compared with cord blood19. Furthermore, the expansion of early B cell progenitors is observed in patients who are treated with recombinant human IL-7 (rhIL-7; described below). IL-7 has also been implicated as a mediator of survival and proliferation of malignant pre-B cells26. Although genetic alterations of IL-7Rα have not been reported in B cell malignancy, mutations in TSLPR (also known as CLRF2) occur in 15% of cases of human pre-B cell acute lymphoblastic leukaemia (ALL), which indicates that signalling mediated by the IL-7Rα axis can contribute to leukaemogenesis27.
IL-7Rα is also expressed by DCs, in which signalling mediated by TSLP has an important role in activating DCs that direct the differentiation of T helper 2 (TH2) cells and contribute to allergic inflammation28. However, IL-7-mediated signalling has also recently been implicated in DC development29 and in regulating CD4+ T cell proliferation during lymphopaenia1. Disruption of IL-7-mediated signalling in DCs in mice leads to increased homeostatic T cell proliferation, in particular of CD4+ T cells, thus implicating IL-7-mediated signalling in DCs in a regulatory axis that controls T cell proliferation in response to self antigens, and potentially providing a mechanism by which IL-7 can potentiate self antigen-driven T cell proliferation during lymphopaenia while avoiding autoimmunity.
With regard to NK cells, IL-15, but not IL-7, is essential for NK cell development in the bone marrow. However, IL-7 has recently been implicated in the development of IL-7Rα+CD11b−Ly49low thymic NK cells in mice, which function mainly in the secretion of cytokines rather than in cell killing30,31. Thymic NK cells have not been formally described in humans. Recent studies have also identified an essential role for IL-7 in the development of lymphoid tissue inducer (LTi) cells (BOX 1). Briefly, mouse LTi cells are CD4+CD3−IL-7Rα+ cells that are present in large numbers in the lymph node and Peyer’s patch anlagen of fetal mice. LTi cells express the nuclear hormone receptor retinoic acid receptor-related orphan receptor-γt (RORγt). They interact with lymphotoxin receptor-expressing mesenchymal cells in the developing lymph nodes through IL-7-mediated production of lymphotoxin α1β2, which presumably initiates lymph node development32,33. The number of LTi-like cells is markedly decreased in mice lacking IL-7Rα34 but these cells are present in IL-7-deficient mice and TSLPR-deficient mice, which indicates that IL-7 and TSLP have redundant roles in the maintenance and/or development of LTi cells. Although LTi cells are rare after birth, Il7-transgenic mice have an increased number of LTi cells, increased numbers of Peyer’s patches and ectopic (tertiary) lymphoid tissue development, which demonstrates the postnatal activity of IL-7-responsive LTi cells35,36. IL-7-regulated LTi-like cells in adult mice seem to be important for regulating tissue-associated immune responses37–39 and they can also mediate the development of tertiary lymphoid structures in the setting of chronic inflammation40. Human LTi cells are CD4−CD3−IL-7Rα+RORγt+ NK cell precursors that produce IL-17 and IL-22 (REFS 41,42). These RORγt+ IL-22-producing cells are absent in humans who lack IL-7Rα43. Human IL-22-producing NK cells proliferate in response to IL-7 (REF. 44), which implicates IL-7 in the biology of LTi cells in humans. Interestingly, NK cell receptor-expressing LTi-like cells can differentiate into innate effector cells that can contribute to autoimmunity, a process that might be mitigated by IL-7-mediated signalling43.
Box 1 |. Lymphoid tissue inducer cells.
Lymph node development in fetal mice depends on mesenchymal cells that express vascular cell adhesion molecule 1 (VCAM1) and intercellular adhesion molecule 1 (ICAM1), which are known as stromal organizer cells, and on CD4+ interleukin-7 receptor-α (IL-7Rα)+ lymphoid tissue inducer (LTi) cells, the development of which requires the transcription factor retinoic acid receptor-related orphan receptor-γt (RORγt)32,119. LTi cells are derived from a lymphoid precursor cell that can generate B cells, T cells, natural killer (NK) cells and dendritic cells120; commitment to the LTi cell lineage is controlled by the helix–loop–helix protein inhibitor of DNA binding 2 (ID2)121. The number of LTi cells is increased in Il7-transgenic mice, resulting in increases in the number and size of Peyer’s patches, as well as the development of ectopic (tertiary) lymphoid tissue35. Although lymphoid tissue development occurs mainly in the fetus, IL-7-regulated LTi-like cells can also be identified in adult mice36. These cells seem to be important for the generation of gut lymphoid tissue and for immune responses in the gut37,38. Linder the control of these cells, tertiary lymphoid structures can also develop in inflammatory conditions40 and contribute to the generation of productive immune responses39,122,123 and autoimmunity124 in non-lymphoid tissues. Interestingly, the attraction of LTi cells to tumours by CC-chemokine ligand 21 (CCL21) results in the development of a tertiary lymphoid structure-like environment and contributes to tumour development by inducing tolerance125. In mice and humans, a subset of IL-22-producing NK cell receptor-expressing cells have also been identified as having LTi cell-like properties41,44,126–128. Recently, using fate-mapping experiments in reporter mice in which enhanced green fluorescent protein is expressed under the control of the Rorc locus (which encodes RORγt), these cells have been shown to be derived from RORγt-expressing LTi cell precursors ratherthan from NK cells43. Interestingly, NK cell receptor-expressing LTi-like cells in mice can differentiate into RORγt− innate effector cells that can contribute to colitis, a process that is blocked by IL-7. It is not known whether the human NK cell receptor-expressing IL-22-producing cells have a similar origin and properties, but these cells are absent in humans with severe combined immunodeficiency due to IL-7Rα mutation43.
In summary, IL-7 is a non-redundant cytokine that is essential for primary T cell development and probably has an important role in normal B cell development. IL-7 can also contribute to the development of some DC subsets, and IL-7-mediated signalling in DCs has been implicated as a regulator of peripheral CD4+ T cell homeostasis. Although IL-7 has no significant role in the development of bone marrow-derived NK cells, it is required for the development of thymic NK cells and recent work has identified IL-7Rα expression on NK cell receptor-expressing cells with properties of LTi cells, some of which mediate innate immune responses.
Effects on peripheral T cell homeostasis.
The homeostatic effects of IL-7 on peripheral T cells45 can be conceptualized by emphasizing that IL-7 is distinct from typical ‘activation’ cytokines (FIG. 3). IL-2 can be considered a prototypical ‘activation’ cytokine in that it does not signal in resting naive T cells but does signal in activated effector T cells following cytokine production and receptor upregulation, and has limited effects on resting memory T cells. By contrast, IL-7 is continuously available in secondary lymphoid organs (SLOs) as a result of stromal cell production46, and IL-7R expression is maintained on resting T cells by the transcription factors forkhead box protein O1 (FOXO1)47 and ETS1 (REF. 48). T cells also probably encounter IL-7 outside of SLOs, as IL-7 can be detected in human plasma and is produced by keratinocytes, gut epithelial cells, other parenchymal cells and DCs1,49–52. Continuous IL-7-mediated signalling induces anti-apoptotic and co-stimulatory responses that are essential for the survival of naive T cells. However, when T cells become activated, the expression of IL-7Rα is downregulated, which prevents these cells from responding to IL-7. An exception to this rule occurs during primary immune responses, when IL-7Rα is selectively expressed on a small minority of effector T cells that are destined to enter the central memory T cell pool, thus implicating IL-7 as a modulator of the effector to memory cell transition53. IL-7Rα is re-expressed by resting memory T cells, but its expression is downregulated on terminally differentiated, senescent T cells. IL-15 provides homeostatic signals that are required for the maintenance of memory T cells, but IL-7 can substitute for IL-15 in this regard during lymphopaenia54,55.
Figure 3 |. Distinctions between a prototypical activation cytokine (IL-2) and a prototypical homeostatic cytokine (IL-7).
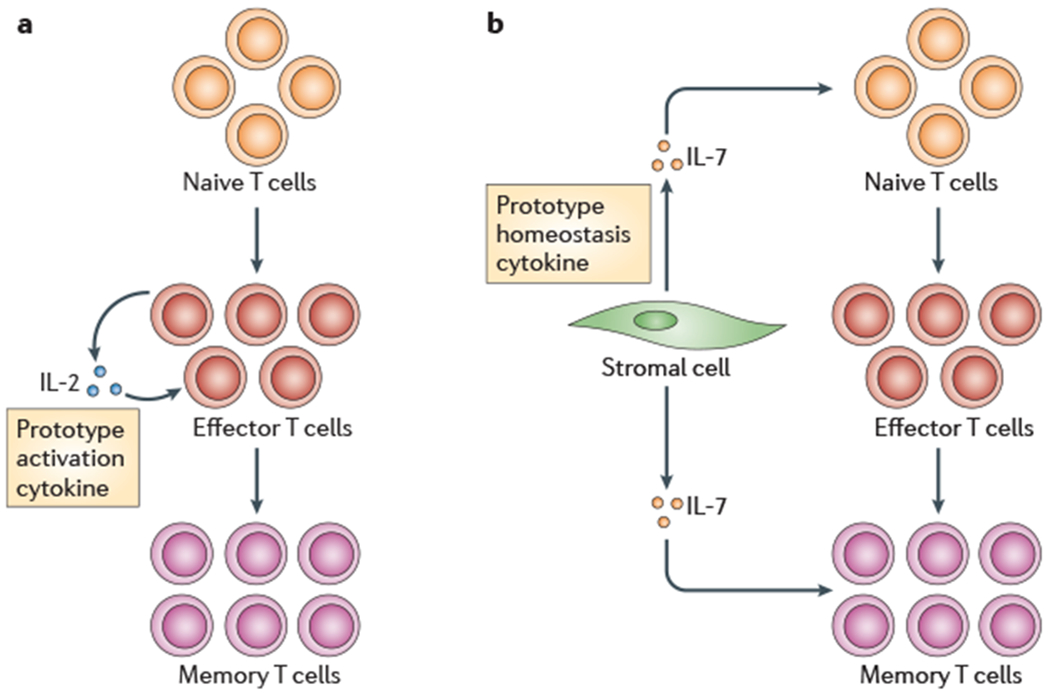
a | Interleukin-2 (IL-2) does not mediate signals for resting naive or memory T cells, but it is a crucial growth factor for activated effector T cells and is produced by activated lymphocytes, b | By contrast, IL-7 is a stromal cell-derived cytokine that provides continuous signals to resting naive and memory T cells, but does not signal to most activated effector T cells. Effector T cells that are destined to enter the memory cell pool are an exception to this rule, as they upregulate expression of interleukin-7 receptor-α (IL-7Rα) before the transition53 (not shown).
Beyond these general principles, several details regarding the role of IL-7 in peripheral T cell homeostasis deserve consideration. Although a wide array of mature T cells express IL-7R, IL-7 has preferential effects on specific T cell subsets, the biology of which is not fully understood. For example, the effects of IL-7 are most potent on recent thymic emigrants (RTEs), in which IL-7-mediated signalling can induce proliferation in the absence of TCR signalling56,57. IL-7 also has potent co-stimulatory effects on naive non-RTE T cell populations, and IL-7-mediated induction of T cell proliferation in response to low-affinity, self antigens is essential for the homeostatic proliferative response observed during lymphopaenia58,59. Both RTEs and naive T cells express high levels of IL-7Rα, and it remains unclear why IL-7 induces TCR-independent proliferation of RTEs but not naive T cell subsets. Similarly, IL-7R is expressed at similar levels by CD4+ and CD8+ T cells — with the exception of senescent terminally differentiated IL-7Rα− T cells, which make up a sizable fraction of the CD8+ T cell pool in humans but are rare in the peripheral CD4+ T cell pool60. Despite this, IL-7 generally has more potent effects on CD8+ T cells than it does on CD4+ T cells, a feature of its biology that remains poorly understood. IL-7Rα expression is notably low on FOXP3+ TReg cells compared with non-regulatory T cell subsets, such that cells with a CD4+CD25hiIL-7Rαlow phenotype are highly enriched for TReg cells61,62. Interestingly, however, activated FOXP3+ TReg cells have recently been shown to upregulate expression of IL-7R, which is the inverse of the pattern of IL-7R expression that is found on non-TReg cell populations of T cells63.
Although IL-7 is continuously available in SLOs, the level of IL-7 has an essential role in modulating T cell homeostasis. A model of T cell homeostasis that emphasizes IL-7 as a limiting resource3 has been assembled from data showing that many T cells compete for access to IL-7 at any given time, that IL-7 signalling leads to downregulation of IL-7Rα expression and that increasing the availability of IL-7 through exogenous administration increases the size of the T cell pool. However, when T cell populations are decreased in size, circulating and tissue levels of IL-7 increase, resulting in a strong inverse correlation between circulating levels of IL-7 and the numbers of CD4+ T cells64–66. Increases in the circulating level of IL-7 can be readily measured in lymphopenic humans, who typically have peak elevations that generally do not exceed 60 pg of IL-7 per ml of plasma but that are substantially above normal levels of 2–8 pg per ml. Increased IL-7 levels during lymphopaenia result from decreased use, and the production of IL-7 actually decreases in this setting as a result of a regulatory feedback loop mediated by IL-7Rα1. Depending on the cause of lymphopaenia, IL-7 levels can remain increased for months to years, but they readily decrease after the recovery of CD4+ T cell populations, which leads to receptor-mediated clearance of IL-7 and a return to homeostatic levels of IL-7. This pattern of regulation is similar to that of hormone–receptor interactions, and it mirrors the relationships seen between circulating levels of erythropoietin and red blood cell counts67, circulating thrombopoietin levels and platelet counts68 and circulating granulocyte colony-stimulating factor (G-CSF) levels and neutrophil counts69. The physiological changes in T cell biology that occur during lymphopaenia include increased proliferative rates of both naive and memory T cell populations, and increased proliferative responses to low-affinity antigens. As described below, the administration of IL-7 in mice, non-human primates and humans largely replicates the lymphopenic physiology, thus implicating IL-7 as an important mediator of altered T cell reactivity during lymphopaenia.
IL-7 therapy
Preclinical data supporting therapeutic development.
Following a lymphopaenia-inducing insult, humans typically experience prolonged T cell depletion, with the most profound effects being on CD4+ T cells70. This is a result of impaired thymic function in most clinical scenarios that are associated with lymphopaenia, as thymic-independent homeostatic proliferation of peripheral CD4+ T cells is not efficiently supported by physiological increases in IL-7 levels1. Although the total number of CD8+ T cells often recovers quickly, the naive CD8+ T cell subset and TCR repertoire diversity remain abnormal for months to years71. Numerous animal studies have shown that IL-7 administration augments immune reconstitution, both in the context of stem cell transplantation and after intensive chemotherapy72–76. Although this might seem counter-intuitive as physiological increases in the level of IL-7 are already present during lymphopaenia, the circulating levels of IL-7 obtained after pharmacological administration are far greater than those that are present in lymphopenic hosts. Levels of IL-7 have not been measured in the tissues, but it is reasonable to presume that the tissue levels of IL-7 achieved with IL-7 therapy are also substantially greater than those which accumulate as a result of lymphopaenia. The main pathway by which IL-7 enhances immune reconstitution is the thymus-independent homeostatic expansion of peripheral T cell populations, although some reports have shown that IL-7 can also increase thymopoiesis77–79. Several other cytokines, including keratinocyte growth factor80,81, IL-15 (REF. 82) and FMS-related tyrosine kinase 3 ligand (FLT3L)83, have immunorestorative properties, but none has been shown to have such potent effects as IL-7 in as many model systems. Together, the limited thymic reserve in most lymphodepleted humans and the robust capacity of IL-7 to enhance immune reconstitution of both CD4+ and CD8+ T cells through thymic-independent pathways make IL-7 an attractive candidate for clinical development as an immunorestorative factor.
IL-7 therapy also augments antigen-specific T cell responses after vaccination, viral infection and adoptive cell therapy2,84–88. Vaccination and viral infection studies in mice show that the transient administration of IL-7 around the time of vaccination or initial viral infection increases the size of antigen-specific effector and memory T cell populations and has long-lasting effects on the memory T cell pool2,84,88,89. IL-7 enhances CD4+ and CD8+ effector T cell responses and CD8+ memory T cell responses after vaccination, with the greatest effect being on T cell responses specific for subdominant antigens; this suggests that the administration of IL-7 with tumour vaccines could increase immunity towards weak tumour antigens. The effectiveness of IL-7 as a vaccine adjuvant depends on the timing of administration, as durable effects of IL-7 on the memory T cell pool were only observed when IL-7 was administered during the contraction phase of the CD8+ T cell response89. Importantly, in a model in which a viral infection induced a primary immune response that mediated antitumour effects, IL-7 did not enhance the antitumour immune response in the absence of concomitant viral infection, which shows that the adjuvant effects of IL-7 require concomitant innate immune activation and are not simply the result of homeostatic T cell proliferation2. In this model, IL-7 increased the number of antigen-specific CD4+ and CD8+ T cells in the draining lymph nodes of the inflamed pancreas, which is consistent with the known capacity of IL-7 to augment the number of antigen-specific effector T cells during an acute immune response. However, IL-7 also induced marked qualitative effects in the immune milieu, including the increased production of pro-inflammatory cytokines and the induction of antigen-specific TH17 cells. Antigen-specific T cells in virus-infected mice treated with IL-7 also became more potent on a per cell basis. They were resistant to the suppressive effects of TGFβ and TReg cells; this resistance was associated with IL-7-mediated upregulation of expression of SMURF2 (an E3 ubiquitin protein ligase that targets TGFβ receptor II and SMAD2 for degradation) and of NEDD4 (an E3 ubiquitin protein ligase that targets CBL-B for degradation). A more recent report showed that therapy with rhIL-7 also enhances viral clearance in mice during chronic infection with lymphocytic choriomeningitis virus, which mimics chronic viral infections in humans such as with HIV or hepatitis C virus. Here, the effects of IL-7 involved the downregulation of expression of programmed cell death 1 (PD1) and of suppressor of cytokine signalling 3 (SOCS3) in T cells, together with increased IL-22 production, mediated in part through the suppression of FOXO transcription factors90. However, in a similar study using the same chronic viral infection model, although IL-7 therapy enhanced T cell-mediated viral clearance, the effect was mediated by increased numbers of virus-specific T cells through increased proliferation and attenuated contraction of the T cell response, associated with increased BCL-2 expression but without any change in the expression of CBL-B or PD1 (REF 91). So, in addition to increasing the number of antigen-specific T cells by modulating T cell reactivity, IL-7 seems to antagonize several inhibitory networks in the setting of acute and chronic viral infection2,90.
IL-7 has also recently been implicated in mediating protective effects in the setting of sepsis. Recent literature has shown that sepsis is more severe in T cell-deficient mice and that the pathophysiology of sepsis involves a decrease in the number of T cells and hypofunctionality of the remaining T cells. Following caecal ligation and puncture, IL-7 therapy: blocked T cell apoptosis through the upregulation of BCL-2 expression and decreased expression of PUMA (p53-upregulated modulator of apoptosis); prevented the loss of delayed-type hypersensitivity (DTH) responses; and increased leukocyte trafficking to sites of infection through increased expression of the integrin molecules lymphocyte function-associated antigen 1 (LFA1) and very late antigen 4 (VLA4), which resulted in a concomitant decrease in bacterial load92. The beneficial effects of IL-7 in sepsis seem to involve increased production of interferon-γ (IFNγ) and CXC-chemokine ligand 10 (CXCL10), and the generation of IL-17-producing γδ T cells, all of which increase the recruitment of neutrophils.
Clinical studies.
IL-7 has been produced for human administration using recombinant technology. The first studies in humans were carried out with rhIL-7 produced in Escherichia coli (designated CYT99007 (Cythersis, Inc.)); current trials use rhIL-7 produced in eukaryotic cells (designated CYT107 (Cythersis, Inc.)), which replicates the glycosylation profiles found in the native protein and might decrease the risk of immunogenicity. So far, 15 clinical trials have been registered worldwide that incorporate the administration of rhIL-7 (TABLE 1) for treating patients with cancer, idiopathic CD4+ T cell lymphocytopaenia and chronic viral infections, including HIV, hepatitis B virus and hepatitis C virus infections, and for use after haematopoietic stem cell (HSC) transplantation. All of the trials have administered rhIL-7 subcutaneously, with a dose range of 3–60 μg per kg of body weight and administration schedules ranging from a single dose of rhIL-7 to administration every other day for 8 doses, to weekly or every other week for 3–4 doses, in some cases repeated after a treatment-free interval of several months. So far, rhIL-7 has been well tolerated, with consistent biological effects seen at doses that are associated with limited toxicity in patients with advanced malignancy or HIV infection. The most common side effects observed have been low-grade fever, malaise, transient increases in liver enzyme levels, and erythema and induration at the site of administration. No significant capillary leak or acute toxicity has been observed so far after rhIL-7 therapy, and the drug has mainly been administered in the outpatient setting.
Table 1 |.
Clinical trials of recombinant human interleukin-7
| Protocol | ClinicalTrials.gov identifier | Eligible patient population | rhIL-7 dose and administration schedule | Date initiated | Status | Refs |
|---|---|---|---|---|---|---|
| A Phase I study of subcutaneous rhIL-7 (CYT99007) in patients with refractory non-haematological malignancy | NCT00062049 | Refractory cancer | 3–60 μg per kg every other day for 8 doses | April 2003 | Not recruiting | 93,94 |
| A study of subcutaneous rhIL-7 (CYT99007) in conjunction with peptide immunization in patients with metastatic melanoma | NCT00091338 | Melanoma | 3–60 μg per kg every 3 days for 8 doses | September 2004 | Not recruiting | 97 |
| A Phase I, randomized, placebo-controlled, double-blind study evaluating the safety of subcutaneous, single-dose rhIL-7 in HIV-1-infected patients who are receiving antiretroviral therapy | NCT00099671 | HIV infection | 3–60 μg per kg for 1 dose | December 2004 | Not recruiting | 98 |
| A Phase I, randomized, placebo-controlled, double-blind study evaluating the safety of subcutaneous, single-dose rhIL-7 in HIV-1-infected patients who are receiving antiretroviral therapy | NCT00105417 (A5214) | HIV infection | 3–100 μg per kg for 1 dose | March 2005 | Not recruiting | – |
| A Phase I study of subcutaneous rhIL-7 (CYT107) in patients with refractory metastatic melanoma or renal cell carcinoma | NCT00492440 | Kidney cancer or melanoma | 30–120 μg per kg per week for 3 doses | May 2007 | Recruiting | – |
| A Phase I/IIa randomized, placebo-controlled, single-blind, multicentre, dose-escalation study of subcutaneous, intermittent rhIL-7 (CYT107) in chronically HIV-infected patients with CD4+ T cell counts of 101–400 cells per mm3 and plasma HIV RNA levels of less than 50 copies per ml after at least 12 months of HAART | NCT00477321 (INSPIRE) | HIV infection | 3–10 μg per kg every other day for 8 doses | May 2007 | Not recruiting | 101 |
| A Phase I/IIa dose-escalation study of repeated administration of rhIL-7 (CYT107) add-on treatment in genotype 1 or 4 HCV-infected patients who are resistant to pegylated IFNα and ribavirin | NCT01025297 | HCV infection | 3–20 μg per kg every week for 4 doses | July 2008 | Recruiting | – |
| A Phase I study of rhIL-7 (CYT107) in recipients of HLA-matched ex vivo T cell-depleted bone marrow or peripheral blood stem cell transplants | NCT00684008 | Haematopoietic stem cell transplantation | 10–30 μg per kg every week for 3 doses | March 2008 | Recruiting | – |
| A Phase I/IIa dose-escalation study in Asia of repeated administration of rhIL-7 (CYT107) add-on treatment in genotype 1 HCV-infected patients who are resistant to pegylated IFNα and ribavirin | NCT01024894 | HCV infection | 3–20 μg per kg every week for 4 doses | January 2009 | Recruiting | – |
| A Phase I/IIa dose-escalation study in Asia of repeated administration of rhIL-7 (CYT107) add-on treatment in genotype 1 HCV-infected patients who are resistant to pegylated IFNα and ribavirin | NCT01025596 (ECLIPSE-1) | HCV infection | 3–30 μg per kg every week for 4 doses | January 2009 | Recruiting | – |
| rhIL-7 (CYT107) treatment of patients with idiopathic CD4+ T cell lymphocytopaenia: expansion of CD4+ T cell populations | NCT00839436 (ICICLE) | Idiopathic CD4+ T cell lymphocytopaenia | 3–20 μg per kg every week for 3 doses, from week 1 a nd t hen repeated from week 24 | February 2009 | Recruiting | – |
| A Phase I/II randomized, open labelled, controlled, dose-escalation study of repeated administration of rhIL-7 (CYT107) add-on antiviral treatment and vaccination in HBeAg-negative chronic HBV-infected patients | NCT01027065 | HBV infection | Not known | December 2009 | Recruiting | – |
| A pilot study of tumour vaccination and rhIL-7 following standard multimodality therapy in patients with high-risk paediatric solid tumours | NCT00923351 | Paediatric solid tumours | 20 μg per kg every other week for 4 doses | December 2009 | Recruiting | – |
| An open-labelled, multicentre study of subcutaneous intermittent rhIL-7 (CYT107) in chronically HIV-infected patients with CD4+T cell counts of 101–400 cells per mm3 and plasma HIV RNA levels of less than 50 copies per ml after at least 12 months of HAART | NCT01190111 (Inspire-2) | HIV infection | 20 μg per kg every week for 3 doses | January 2010 | Not recruiting | – |
| A multicentre, open-labelled, controlled, randomized study of rhIL-7 (CYT107) treatment to restore and maintain CD4+ T cell counts above 500 cells per μl in HIV-infected patients with CD4+ T cell counts of 101–350 cells per μl after at least 2 years of HAART and with plasma HIV RNA levels of less than 50 copies per ml for 18 months | NCT01241643 (Inspire-3) | HIV infection | 20 μg per kg every week for 2 weeks; maximum of 4 cycles within 21 months and maximum of 3 cycles within 12 months | September 2010 | Recruiting | – |
| A Phase II study of enhancement of immune reconstitution and vaccine responses with administration of rhIL-7 (CYT107) in older subjects after chemotherapy | Not assigned | Healthy adults of less than 60 years of age | 20 μg per kg every week for 3 doses | Set to open in 2011 | Set to open in 2011 | – |
HAART, highly active antiretroviral therapy; HBeAg, hepatitis B virus e antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; IFNα, interferon-α; rhIL-7, recombinant human interleukin-7.
In the first study in humans, rhIL-7 (CYT99007) was administered every other day for a total of 14 days to 16 patients with refractory cancer93,94. IL-7 induced a transient decrease in the number of circulating lymphocytes 1–2 days after administration, presumably related to the trafficking of T cells to the tissues; this effect is also observed after administration of recombinant simian IL-7 to macaques95 and might be related to the known effects of IL-7 on integrin activation96. This was followed by substantial dose-dependent increases in the numbers of circulating CD4+ and CD8+ T cells, which peaked on day 21 after the start of IL-7 administration and were sustained for at least 8 weeks. Doses of 30–60 μg of IL-7 per kg induced 3–4-fold increases in the numbers of circulating CD4+ and CD8+ T cells, often resulting in supranormal values for these cell counts. It is important to note that the effects of therapeutic administration of rhIL-7 were not limited to circulating T cells, which could be confounded by changes in T cell trafficking, but also correlated with increases in the size of SLOs, including the spleen and lymph nodes, as well as evidence of increased metabolic activity in these sites.
The measured half-life of rhIL-7 in this study was 6–10 hours, but the biological effects persisted well beyond the time when circulating levels of IL-7 returned to baseline94. As IL-7 is known to bind to components of the extracellular matrix, the long-lasting effects of therapy with rhIL-7 could result from saturation of this tissue compartment followed by the slow release of IL-7 such that it is not readily detectable in the serum, and/or from pharmacodynamic changes in lymphocyte proliferation and/or apoptosis that persist beyond the period of exposure to rhIL-7. Significant increases in the cell cycle rates of both CD4+ and CD8+ T cells and increased expression of BCL-2 by T cells were observed after therapy with rhIL-7. T cell proliferation rates were tightly correlated with IL-7Rα expression, both of which decreased markedly by day 7 after the start of therapy despite continued rhIL-7 administration until day 14. These results indicate that modulation of IL-7Rα expression potently regulates the T cell proliferative effects induced by IL-7 therapy, providing a ‘built-in’ safety valve to prevent uncontrolled IL-7-induced lymphoproliferation. Interestingly, BCL-2 expression levels remained high until day 14, despite the downregulation of IL-7Rα expression.
The effects of rhIL-7 were selective with regard to T cell subsets. CD8+ T cell populations were expanded to a greater extent than were CD4+ T cells, and FOXP3+CD4+ TReg cells had lower rates of proliferation after rhIL-7 therapy compared with CD4+FOXP3− T cell subsets, resulting in a decreased frequency of TReg cells after rhIL-7 therapy. Similar findings were seen when rhIL-7 was administered to patients with melanoma97 and to HIV-infected patients98. Notable selectivity was seen with regard to the effects of IL-7 on naive versus non-naive T cells (FIG. 4). Although naive T cells rarely proliferate, rhIL-7 therapy preferentially induced the proliferation of this subset, which induced a marked increase in the relative proportion of naive T cell populations present. This was not the result of clonal expansion or altered T cell phenotypes and, as a result, patients treated with a short course of rhIL-7 had marked broadening of TCR repertoire diversity. Such findings had previously only been shown coincident with increased thymopoiesis99, but the effects of IL-7 on thymopoiesis seemed to be marginal or nonexistent in this study. Rather, rhIL-7 therapy increased TCR repertoire diversity by modulating the subset composition of the peripheral T cell pool such that the more diverse components made up a greater proportion of the total population. Although patient numbers were small in this study, the preferential expansion of naive CD4+ and CD8+ T cell populations by rhIL-7 was age independent93. Similar effects have been observed after IL-7 therapy in mice100.
Figure 4 |. Recombinant human IL-7 diversifies the TCR repertoire by preferential expansion of naive T cell populations and recent thymic emigrants.
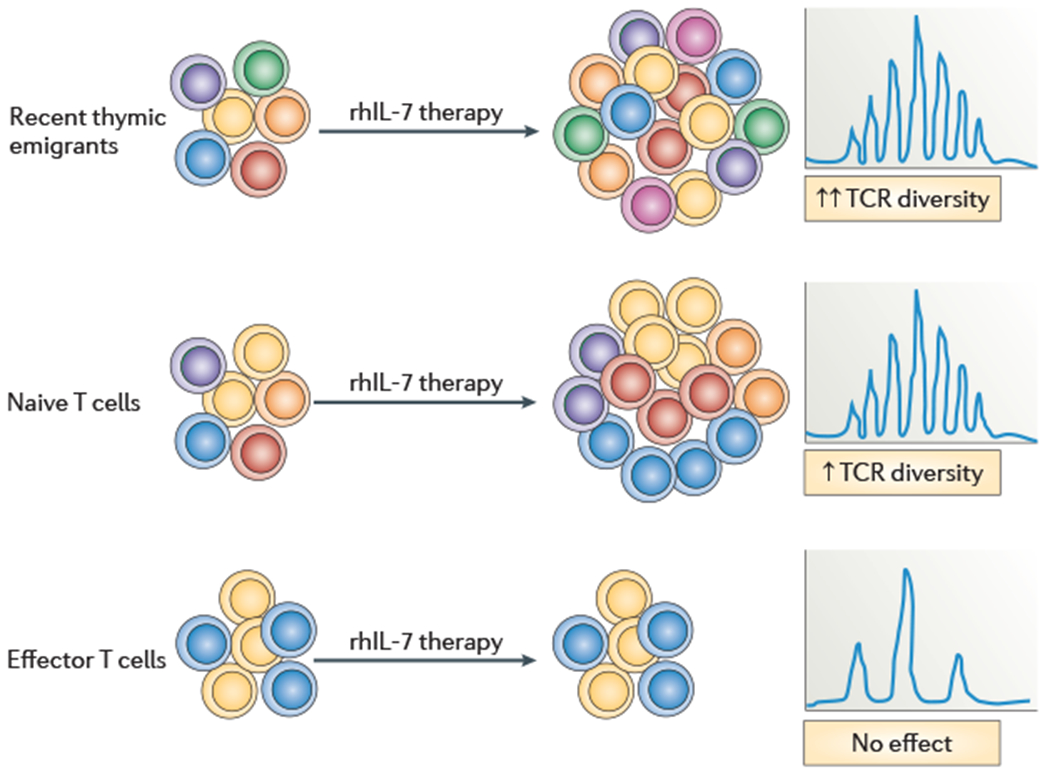
Recombinant human interleukin-7 (rhIL-7) therapy inducesthe proliferation of mature T cell populations, with differential effects on various subsets. For both CD4+ and CD8+T cells, rhIL-7 induces greater proliferation of the more diverse recent thymic emigrant and naive T cell subsets as compared with the less diverse, effector T cell subsets. This results in a preferential increase in the size of T cell populations that have a diverse T cell receptor (TCR) repertoire in the peripheral T cell pool and a net increase in TCR repertoire diversity overall. This effect seems to be age independent and does not reguire increased thymopoiesis, although a thymopoietic effect of rhIL-7, if it were to occur, would be predicted to enhance the potency of the overall TCR repertoire diversification.
rhIL-7 (CYT99007) was also administered as a single dose, in the context of a placebo-controlled trial, to 18 patients with HIV infection98. This study confirmed that rhIL-7 induced early lymphopaenia, followed by a significant increase in the rate of T cell cycling on day 4 after IL-7 administration and increases in circulating CD4+ and CD8+ T cell counts that persisted for at least 14 days after rhIL-7 administration. Given that serum rhIL-7 levels peaked at 4 hours after the dose was administered, that the half-life of IL-7 was 7–23 hours and that circulating IL-7 levels had returned to baseline by 72 hours after therapy, the results also clearly show that the biological effects of rhIL-7 continue well after serum cytokine levels return to baseline. In this study of patients with HIV infection, increases in the numbers of central memory CD4+ and CD8+ T cells were most prominent, but naive CD4+ and CD8+ T cells also increased in number, whereas no increase in the number of effector memory CD8+ T cells was observed. No detectable effects on the frequency or cell cycle rates of T cells specific for HIV, Epstein–Barr virus, cytomegalovirus (CMV) or influenza virus were observed in this single-dose study. In a second study that administered 8 doses of rhIL-7 every other day in 14 patients infected with HIV, similar increases in the total number of T cells and similar expansion of naive and central memory T cell populations were observed101. Despite transient increases in HIV replication, the production of IL-2 and IFNγ in response to CMV and HIV antigens in vitro was increased in four subjects after rhIL-7 therapy, which might indicate improved antiviral immunity. Similarly, a recent case report described early immune reconstitution in one patient treated with rhIL-7 as part of an antiviral regimen for progressive multifocal leukoencephalopathy associated with idiopathic CD4+ T cell leukopaenia102. So, rhIL-7 administration consistently results in patterns of immune reconstitution in various settings associated with lymphopaenia, and preliminary data indicate that IL-7 might augment virus-specific immune responses, which could provide clinical benefit for immunosuppressed individuals.
These clinical studies have also provided insight into the effects of IL-7 on human B cell lymphopoiesis. Administration of rhIL-7 had minimal effects on circulating mature B cells; however, transient increases in the numbers of circulating immature/transitional B cells after rhIL-7 administration were noted in two studies94,98. Examination of the bone marrow in some patients showed marked expansion of the lymphoid compartment, which was mainly comprised of early pre-B cells at various stages of differentiation. Molecular studies showed no evidence for clonal dominance of B cell populations, and in all patients the bone marrow morphology normalized following cessation of rhIL-7 therapy94. So, rhIL-7 can induce marked early B cell proliferation in the bone marrow, which is consistent with the appearance of haematogones; these cells are often observed following recovery from dose-intensive chemotherapy but can be confused with malignant cells by light microscopy. These results show the direct effects of IL-7 on human B cell development and illustrate why long-term, uninterrupted IL-7 exposure at high levels might predispose to lymphoproliferation; extreme caution is therefore warranted with regard to the use of rhIL-7 in patients with B cell malignancies, which often express IL-7R.
Promise, challenges and notes of caution
The published clinical experience with the use of rhIL-7 therapy so far is modest, but there is consistent evidence that rhIL-7 is safe, is bioactive and has a unique capacity to expand naive T cell populations and augment immune reconstitution with TCR repertoire diversification. So, the clinical development of rhIL-7 is compelling for several conditions associated with T cell immunodeficiency, in which impaired immune reconstitution contributes to morbidity or mortality (TABLE 2). These include some rare diseases such as idiopathic CD4+ T cell lymphocytopaenia and congenital immunodeficiencies, as well as more common conditions such as the immunodeficiency that results after chemotherapy for cancer, HIV infection or HSC transplantation. The ability of IL-7 to increase TCR repertoire diversity might also provide a clinical benefit for the largest subset of the human population with progressive immune deficiencies, namely aged individuals. As thymus activity decreases with age, the frequency of naive T cells originating from the thymus decreases. The decreasing proportion of naive cells in the total T cell population contributes to a progressively restricted, oligoclonal TCR repertoire as effector T cell populations, which are skewed in their TCR specificity, increase in frequency. Such TCR skewing is particularly marked for CD8+ T cells. The administration of rhIL-7 would be predicted to reverse the decreased representation of naive T cells over time in elderly individuals and restore TCR repertoire diversity. It is not known whether this TCR diversity would result in a functional improvement in T cell responses in elderly individuals.
Table 2 |.
Human immunodeficiencies that are potentially treatable with IL-7 therapy
| Clinical condition | Specific populations predicted to benefit from rhIL-7 | Promise | Pitfalls |
|---|---|---|---|
| HIV infection | Immune non-responders: these individuals have persistent severe CD4+ T cell depletion after viral suppression with HAART and have increased mortality rates compared with patients with CD4+ T cell reconstitution after HAART129–131 | Early results show persistent increases in CD4+ T cell counts even after short-course IL-7 administration. Concomitant HAART seems to prevent IL-7-induced increases in viral load | Large studies will be needed to determine whether IL-7-induced increases in CD4+ T cell counts translate into increased survival and/or decreased mortality |
| Idiopathic CD4+ T cell lymphocytopaenia | Individuals with opportunistic infections and/or significant morbidity and mortality associated with CD4+ T cell deficiency | IL-7 therapy is predicted to increase CD4+ T cell counts | Rare, heterogeneous population of patients, who are difficult to study systematically |
| Congenital immunodeficiency | Individuals with incomplete DiGeorge syndrome or other subsets of patients with a ‘leaky phenotype’such that some T cells are present; this T cell population could be expanded with rhIL-7 | IL-7 therapy is predicted to increase the size of T cell subsets, particularly of naive recent thymic emigrants | • Not likely to benefit patients with a complete block in T cell development • Potential increased risk of Omenn syndrome, a GVH D-like autoimmune condition in patients with congenital immunodeficiency |
| After haematopoietic stem cell transplantation | Individuals who receive profoundly T cell-depleted stem cell transplants following haploidenticaltransplantation and patients who receive an umbilical cord blood transplant who have slow and incomplete immune reconstitution. Impaired immune reconstitution correlates with increased post-transplant morbidity and mortality132 | IL-7 therapy is predicted to increase the rate and degree of T cell reconstitution | Potential increased risk of GVHD, EBV-associated lymphoproliferation or graft rejection |
| After chemotherapy for cancer | Individuals who receive dose-intensive lymphotoxic agents, particularly with increased age and mediastinal irradiation | IL-7 therapy is predicted to increase the rate and degree of T cell reconstitution | • Careful clinical studies of targeted populations will be needed to determine whether enhanced immune reconstitution translates into improved overall or event-free survival • IL-7-mediated signalling on leukaemias and lymphomas precludes its use in this high-risk population |
| Ageing | Elderly individuals who are poor responders to vaccines and are susceptible to viral reactivation | Even short-term IL-7 therapy is predicted to enhance TCR repertoire diversity through the preferential proliferation of recent thymic emigrants and naive T cells. Combining IL-7 with vaccines might increase the probability of a vaccine response | Careful clinical studies will be needed to determine whether improved immune parameters translate into improved survival or decreased infection-related morbidity or mortality |
EBV, Epstein–Barr virus; GVHD, graft-versus-host disease; HAART, highly active antiretroviral therapy; rhIL-7, recombinant human interleukin-7; TCR, T cell receptor.
In a clinical scenario in which T cell immunodeficiency is the main barrier to cure or to improved clinical outcome, it seems probable that rhIL-7 could have therapeutic benefit. In reality, however, such clinical scenarios are by their nature complex and showing that the effects of rhIL-7 on immune homeostasis will translate to an improved clinical outcome remains a challenge (TABLE 2). For example, in settings of acquired immune deficiency such as HIV infection, after HSC transplantation or idiopathic CD4+ T cell lymphocytopaenia, it remains to be seen whether rhIL-7-mediated increases in the numbers of CD4+ T cells will directly improve survival rates or the length of disease-free intervals. For example, previous work had shown that rhIL-2 increased CD4+ T cell counts in HIV infected individuals, but two large randomized studies failed to show a survival benefit after rhIL-2 administration despite increases in the CD4+ T cell counts103. As rhIL-2 preferentially expands TReg cell populations, whereas rhIL-7 expands non-regulatory T cell populations and increases TCR repertoire diversity, the overall results with rhIL-7 might be different. However, carefully controlled clinical trials targeting those patients who are most at risk for opportunistic complications are essential to determine whether the biological effects of rhIL-7 will translate into improved survival or decreased morbidity of patients. Similarly, it seems probable that rhIL-7 would enhance immune reconstitution after HSC transplantation, but care will be needed to identify patient populations for whom this effect is most likely to result in clinical benefit without potential adverse effects, such as graft-versus-host disease (GVHD). Indeed, polymorphisms in the gene encoding IL-7Rα are associated with the risk of mortality after allogeneic HSC transplantation104, serum levels of IL-7 have been shown to correlate with the development of GVHD in humans105,106 and IL-7 administration exacerbates GVHD in mice107.
IL-7 is also a potent vaccine adjuvant and it increases the effectiveness of adoptive transfer therapies, properties that it shares with IL-15, IL-21 and, in some cases, IL-2 (REF. 108). It remains possible that IL-7 will have a therapeutic role on the basis of its capacity to augment antigen-specific immune responses, either in the context of preventative or therapeutic vaccines or in the setting of chronic viral infection. Animal models indicate that IL-7 is a potent agent in this regard, and several clinical trials incorporating rhIL-7 with antiviral agents, tumour vaccines and/or adoptive cell therapy are underway. Finally, the recent data implicating IL-7 as an agent that can decrease mortality associated with sepsis add a new dimension to the therapeutic potential of rhIL-7 (REFS 92,109).
The potent effects of IL-7 on T cell development and homeostasis provide ample opportunities for clinical translation. However, such diverse and potent effects of IL-7 on the immune system could also have adverse consequences, particularly in high-risk individuals or during chronic administration. Indeed, the evidence that IL-7 therapy potently expands early B cell and T cell progenitor populations means that IL-7 is contraindicated for patients with lymphoid malignancies and/or lymphoproliferation. As profoundly lymphopenic individuals are predisposed to lymphoproliferation, mainly as a result of virus-induced effects, the very patient population who could potentially benefit most from IL-7 therapy might also have an increased risk of adverse effects from this therapy.
Studies in mouse models indicate that the effects of IL-7 on T cell cycling probably involve enhanced proliferative responses to self antigens58,59, raising the possibility that rhIL-7 therapy might predispose to autoimmune disease. Indeed, Il7-transgenic mice, which constitutively express high levels of IL-7, develop severe immune-mediated dermatitis110, and polymorphisms in the sixth exon of the gene encoding IL-7Rα modulate susceptibility to multiple sclerosis, which implicates the IL-7-mediated signalling axis in predisposition to autoimmunity111,112. IL-7 also has a role in the immunobiology of rheumatoid arthritis113 and blocking signalling through IL-7Rα can decrease the severity of joint inflammation in mouse models114. In mouse models of colitis, IL-7 produced by human intestinal epithelial cells51 contributes to the development of intestinal inflammation115. Adoptive transfer of mucosal T cells expressing high levels of IL-7Rα induces colitis in recipient mice and toxin-conjugated depletion of IL-7Rα-expressing cells can abrogate established colitis116. So, IL-7 has been implicated in the pathophysiology of multiple autoimmune diseases, which is consistent with its known capacity to induce T cell proliferation in response to low-affinity antigens and self antigens during lymphopaenia. In the context of cancer therapy, the capacity of IL-7 to break tolerance to self antigens might be a desirable effect if the toxicity is tolerable and treatable, as observed with cytotoxic T lymphocyte antigen 4 (CTLA4)-targeted therapy117. But such an analysis will require substantial clinical experience with rhIL-7 and will vary depending on the nature and severity of the underlying disease. Finally, although neutralizing antibodies specific for rhIL-7 have not been reported so far, it remains possible that rhIL-7 therapy could induce the production of crossreactive neutralizing antibodies that could adversely affect endogenous levels of IL-7 and paradoxically worsen lymphopaenia, a scenario that has been reported in the context of erythropoietin administration118.
Concluding remarks
Preclinical data generated from numerous model systems over the past two decades have shown that IL-7 has potent immunorestorative effects, as well as vaccine adjuvant effects and beneficial effects in the setting of adoptive cell therapy. Several other cytokines have immunorestorative properties, but no other single agent has the same potency and breadth of effects on immune reconstitution as IL-7. Because several clinical scenarios of immunodeficiency are associated with substantial morbidity and mortality, the immunorestorative properties of IL-7 have led to interest regarding its therapeutic translation. Several clinical trials are ongoing (TABLE 1) in settings of acquired immunodeficiency, chronic viral infection and cancer. The clinical experience obtained so far, in a relatively small number of patients, shows that rhIL-7 therapy is safe, is well tolerated and results in potent immunorestorative effects. Therapy with rhIL-7 increases T cell mass, both circulating and in SLOs, and its effects persist following clearance of the agent. Despite the proven importance of IL-7 in thymopoiesis, the beneficial effects of IL-7 on TCR repertoire diversification occur mainly as a result of the preferential post-thymic proliferation of naive T cells. rhIL-7 therapy also leads to a relative decrease in the frequency of TReg cells in the peripheral T cell pool and induces the expansion of immature B cell populations in the bone marrow in some patients. Despite the impressive biological effects of IL-7 on T cell populations, the essential issue regarding clinical development is the need to show that the biological effects of rhIL-7 translate to improved clinical outcomes such as prolonged survival or cure. Such proof of concept can only be obtained by carrying out careful clinical trials in targeted populations who are at greatest risk owing to T cell immunodeficiency and/or in clinical scenarios where the adjuvant effects of IL-7 markedly increase the potency of a multi-agent immunotherapeutic regimen. As with all clinical agents, the ultimate value of rhIL-7 therapy will be determined based on its therapeutic index, which is a measure of the relative benefits versus relative risks for each individual patient.
Acknowledgements
We would like to thank S. Durum for his critical review of the manuscript and his helpful discussions. This work was supported by the Intramural Research Program of the National Institutes of Health, USA.
Glossary
- Janus kinase–signal transducer and activator of transcription pathway (JAK–STAT pathway)
An evolutionarily conserved signalling pathway that is associated with type I and type II cytokines. Receptor ligation by these cytokines leads to a series of events that includes the recruitment and activation of JAKs and the phosphorylation of various STATs, which in turn translocate to the nucleus where they transactivate various genes involved in cell differentiation, survival, apoptosis and proliferation
- Severe combined immunodeficiency (SCID)
A primary (inherited) immunodeficiency characterized by defects in cell-mediated and humoral immune responses. Affected infants commonly die within the first year of life owing to recurrent infections. Mutations in approximately ten different genes have been described to cause this condition, but defects in the common cytokine receptor γ-chain (γc) are the most common and result in X-linked SCID. Other genes that are mutated in patients with SCID include those encoding Janus kinase 3 (JAK3), recombination activating gene 1 (RAG1) and RAG2, IL-7 receptor α-chain (IL-7Rα) and adenosine deaminase
- pro-B cell
A cell at the earliest stage of B cell development in the bone marrow. These cells are characterized by incomplete immunoglobulin heavy-chain gene rearrangement and are defined as being CD19+ cytoplasmic IgM− or, sometimes, as B220+CD43+ (by the Hardy classification scheme)
- pre-B cell
A cell at a stage of B cell development in the bone marrow that is characterized by complete immunoglobulin heavy-chain gene rearrangement in the absence of immunoglobulin light-chain gene rearrangement. These cells, express the pre-B cell receptor, which comprises a pseudo light chain and a heavy chain. They are phenotypically CD19+ cytoplasmic IgM+ or are sometimes defined as being B220+CD43−cell surface IgM− (by the Hardy classification scheme)
- Sepsis
A systemic response to severe infection or tissue damage, leading to a hyperactive and unbalanced network of pro-inflammatory mediators. Vascular permeability, cardiac function and metabolic balance are affected, resulting in tissue necrosis, multi-organ failure and death
- Delayed-type hypersensitivity (DTH)
A cellular immune response to antigen that develops over ~24–72 hours with the infiltration of T cells and monocytes, and is dependent on the production of T helper 1 cell-specific cytokines
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- 1.Guimond M et al. Interleukin 7 signaling in dendritic cells regulates the homeostatic proliferation and niche size of CD4+ T cells. Nature Immunol. 10, 149–157 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]; The first definitive evidence that the increased levels of IL-7 during lymphopaenia are the result of decreased consumption rather than increased production. This study also identifies IL-7-mediated signalling on DCs as a modulator of T cell homeostasis.
- 2.Pellegrini M et al. Adjuvant IL-7 antagonizes multiple cellular and molecular inhibitory networks to enhance immunotherapies. Nature Med. 15, 528–536 (2009). [DOI] [PubMed] [Google Scholar]; This study provides mechanistic insight into the vaccine adjuvant effect of IL-7 and increases the known targets of IL-7-mediated signalling to include negative regulators of the T cell response such as CBL-B and SMURF2.
- 3.Park JH et al. Suppression of IL7Ra transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity 21, 289–302 (2004). [DOI] [PubMed] [Google Scholar]; This study established IL-7 as a limiting resource for T cells.
- 4.Fry TJ et al. IL-7 therapy dramatically alters peripheral T-cell homeostasis in normal and SIV-infected nonhuman primates. Blood 101,2294–2299 (2003). [DOI] [PubMed] [Google Scholar]; The first demonstration that the effects of in vivo IL-7 administration extend to non-human primates. This study also showed that IL-7-mediated signalling downregulates expression of IL-7Rα.
- 5.Khaled AR & Durum SK Death and Baxes: mechanisms of lymphotrophic cytokines. Immunol. Rev 193, 48–57 (2003). [DOI] [PubMed] [Google Scholar]
- 6.Jiang Q et al. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 16, 513–533 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Puel A, Ziegler SF, Buckley RH & Leonard WJ Defective IL7R expression in T−B+NK+ severe combined immunodeficiency. Nature Genet. 20, 394–397 (1998). [DOI] [PubMed] [Google Scholar]; The first description in humans of SCID due to deficiency of IL-7Rα signalling. This study identified important differences in lymphocyte development between mice and humans.
- 8.Cunningham-Rundles C & Ponda P P Molecular defects in T- and B-cell primary immunodeficiency diseases. Nature Rev. Immunol. 5, 880–892 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Mazzucchelli R & Durum SK Interleukin-7 receptor expression: intelligent design. Nature Rev. Immunol. 7, 144–154 (2007). [DOI] [PubMed] [Google Scholar]; A definitive review of the role of IL-7Rα in T cell development.
- 10.Akashi K, Kondo M, von Freeden-Jeffry U, Murray R & Weissman IL Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell 89, 1033–1041 (1997). [DOI] [PubMed] [Google Scholar]
- 11.Maraskovsky E et al. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1−/− mice. Cell 89, 1011–1019 (1997). [DOI] [PubMed] [Google Scholar]
- 12.Pellegrini M et al. Loss of Bim increases T cell production and function in interleukin 7 receptor-deficient mice. J. Exp. Med 200, 1189–1195 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khaled AR et al. Bax deficiency partially corrects interleukin-7 receptor-α deficiency. Immunity 17, 561–573 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Al-Shami A et al. A role for thymic stromal lymphopoietin in CD4+ T cell development. J. Exp. Med 200, 159–168 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vang KB et al. IL-2, -7, and -15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. J. Immunol 181, 3285–3290 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazzucchelli R et al. Development of regulatory T cells requires IL-7Rα stimulation by IL-7 or TSLP. Blood 112, 3283–3292 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bayer AL, Lee JY, de la Barrera A, Surh CD & Malek TR A function for IL-7R for CD4+CD25+Foxp3+ T regulatory cells. J. Immunol 181,225–234 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herzog S, Reth M & Jumaa H Regulation of B-cell proliferation and differentiation by pre-B-cell receptor signalling. Nature Rev. Immunol. 9, 195–205 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Parrish YK et al. IL-7 dependence in human B lymphopoiesis increases during progression of ontogeny from cord blood to bone marrow. J. Immunol 182, 4255–4266 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shriner AK, Liu H, Sun G, Guimond M & Alugupalli KR IL-7-dependent B lymphocytes are essential for the anti-polysaccharide response and protective immunity to Streptococcus pneumoniae. J. Immunol 185, 525–531 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Kikuchi K, Lai AY, Hsu CL & Kondo M IL-7 receptor signaling is necessary for stage transition in adult B cell development through up-regulation of EBF. J. Exp. Med 201, 1197–1203 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson K et al. Regulation of immunoglobulin light-chain recombination by the transcription factor IRF-4 and the attenuation of interleukin-7 signaling. Immunity 28, 335–345 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Corcoran AE, Riddell A, Krooshoop D & Venkitaraman AR Impaired immunoglobulin gene rearrangement in mice lacking the IL-7 receptor. Nature 391,904–907 (1998). [DOI] [PubMed] [Google Scholar]
- 24.Bertolino E et al. Regulation of interleukin 7-dependent immunoglobulin heavy-chain variable gene rearrangements by transcription factor STAT5. Nature Immunol. 6, 836–843 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Malin S et al. Role of STAT5 in controlling cell survival and immunoglobulin gene recombination during pro-B cell development. Nature Immunol. 11, 171–179 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown VI et al. Rapamycin is active against B-precursor leukemia in vitro and in vivo, an effect that is modulated by IL-7-mediated signaling. Proc. Natl Acad. Sci. USA 100, 15113–15118 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoda A et al. Functional screening identifies CRLF2 in precursor B-cell acute lymphoblastic leukemia. Proc. Natl Acad. Sci. USA 107, 252–257 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu YJ et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu. Rev. Immunol 25, 193–219 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Vogt TK, Link A, Perrin J, Finke D & Luther SA Novel function for interleukin-7 in dendritic cell development. Blood 113, 3961–3968 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Vosshenrich CA et al. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nature Immunol. 7, 1217–1224 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Ribeiro VS et al. Cutting edge: Thymic NK cells develop independently from T cell precursors. J. Immunol 185, 4993–4997 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Eberl G et al. An essential function for the nuclear receptor RORγt in the generation of fetal lymphoid tissue inducer cells. Nature Immunol. 5, 64–73 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Luther SA, Ansel KM & Cyster JG Overlapping roles of CXCL13, interleukin 7 receptor-α, and CCR7 ligands in lymph node development. J. Exp. Med 197, 1191–1198 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishikawa S, Honda K, Vieira P & Yoshida H Organogenesis of peripheral lymphoid organs. Immunol Rev. 195, 72–80 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Meier D et al. Ectopic lymphoid-organ development occurs through interleukin 7-mediated enhanced survival of lymphoid-tissue-inducer cells. Immunity 26, 643–654 (2007). [DOI] [PubMed] [Google Scholar]; This study identifies the importance of IL-7 for the homeostasis of LTi cells and, therefore, the ability of IL-7 to regulate SLO development.
- 36.Schmutz S et al. Cutting edge: IL-7 regulates the peripheral pool of adult RORγ+ lymphoid tissue inducer cells. J. Immunol 183, 2217–2221 (2009). [DOI] [PubMed] [Google Scholar]
- 37.Tsuji M et al. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity 29, 261–271 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Bouskra D et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 456, 507–510 (2008). [DOI] [PubMed] [Google Scholar]
- 39.Moyron-Quiroz JE et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nature Med. 10, 927–934 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Aloisi F & Pujol-Borrell R Lymphoid neogenesis in chronic inflammatory diseases. Nature Rev. Immunol. 6, 205–217 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Cella M et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457, 722–725 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cupedo T et al. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+CD127+ natural killer-like cells. Nature Immunol. 10, 66–74 (2009). [DOI] [PubMed] [Google Scholar]
- 43.Vonarbourg C et al. Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt+ innate lymphocytes. Immunity 33, 736–751 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cella M, Otero K & Colonna M Expansion of human NK-22 cells with IL-7, IL-2, and IL-1β reveals intrinsic functional plasticity. Proc. Natl Acad. Sci. USA 107, 10961–10966 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schluns KS, Kieper WC, Jameson SC & Lefrancois L Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nature Immunol. 1, 426–432 (2000). [DOI] [PubMed] [Google Scholar]; The first demonstration that IL-7 is required for the homeostatic proliferation of CD8+ T cells during lymphopenic conditions.
- 46.Takada K & Jameson SC Naive T cell homeostasis: from awareness of space to a sense of place. Nature Rev. Immunol. 9, 823–832 (2009). [DOI] [PubMed] [Google Scholar]
- 47.Ouyang W, Beckett O, Flavell RA & Li MO An essential role of the Forkhead-box transcription factor Foxo1 in control of T cell homeostasis and tolerance. Immunity 30, 358–371 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grenningloh R et al. Ets-1 maintains IL-7 receptor expression in peripheral T cells. J. Immunol 186, 969–976 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsue H, Bergstresser PR & Takashima A Keratinocyte-derived IL-7 serves as a growth factor for dendritic epidermal T cells in mice. J. Immunol 151, 6012–6019 (1993). [PubMed] [Google Scholar]
- 50.Thang PH et al. The role of IL-1 β in reduced IL-7 production by stromal and epithelial cells: a model for impaired T-cell numbers in the gut during HIV-1 infection. J. Intern. Med 268, 181–193 (2010). [DOI] [PubMed] [Google Scholar]
- 51.Watanabe M et al. Interleukin 7 is produced by human intestinal epithelial cells and regulates the proliferation of intestinal mucosal lymphocytes. J. Clin. Invest 95, 2945–2953 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sawa Y et al. Hepatic interleukin-7 expression regulates T cell responses. Immunity 30, 447–457 (2009). [DOI] [PubMed] [Google Scholar]
- 53.Kaech SM et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nature Immunol. 4, 1191–1198 (2003). [DOI] [PubMed] [Google Scholar]
- 54.Tan JT et al. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J. Exp. Med 195, 1523–1532 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kieper WC et al. Overexpression of interleukin (IL)-7 leads to IL-15-independent generation of memory phenotype CD8+ T cells. J. Exp. Med 195, 1533–1539 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dardalhon V et al. IL-7 differentially regulates cell cycle progression and HIV-1-based vector infection in neonatal and adult CD4+ T cells. Proc. Natl Acad. Sci. USA 98, 9277–9282 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Swainson L et al. IL-7-induced proliferation of recent thymic emigrants requires activation of the PI3K pathway. Blood 109, 1034–1042 (2007). [DOI] [PubMed] [Google Scholar]
- 58.Ernst B, Lee DS, Chang JM, Sprent J & Surh CD The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity 11, 173–181 (1999). [DOI] [PubMed] [Google Scholar]
- 59.Goldrath AW & Bevan MJ Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity 11, 183–190 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paiardini M et al. Loss of CD127 expression defines an expansion of effector CD8+ T cells in HIV-infected individuals. J. Immunol 174, 2900–2909 (2005). [DOI] [PubMed] [Google Scholar]
- 61.Seddiki N et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med 203, 1693–1700 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu W et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ TReg cells. J. Exp. Med 203, 1701–1711 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simonetta F et al. Increased CD127 expression on activated FOXP3+CD4+ regulatory T cells. Eur. J. Immunol 40, 2528–2538 (2010). [DOI] [PubMed] [Google Scholar]
- 64.Bolotin E, Annett G, Parkman R & Weinberg K Serum levels of IL-7 in bone marrow transplant recipients: relationship to clinical characteristics and lymphocyte count. Bone Marrow Transplant. 23, 783–788 (1999). [DOI] [PubMed] [Google Scholar]; The first observation that serum IL-7 levels are increased during lymphopaenia after bone marrow transplantation. These findings were identified as a general feature of lymphopaenia with the discovery of increased IL-7 levels in other clinical conditions associated with T cell deficiency in references 65 and 66.
- 65.Fry TJ et al. A potential role for interleukin-7 in T-cell homeostasis. Blood 97, 2983–2990 (2001). [DOI] [PubMed] [Google Scholar]
- 66.Napolitano LA et al. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nature Med. 7, 73–79 (2001). [DOI] [PubMed] [Google Scholar]
- 67.Spivak JL Erythropoietin: from bench to bedside. Trans. Am. Clin. Climatol. Assoc 102, 232–242 (1991). [PMC free article] [PubMed] [Google Scholar]
- 68.Kuter DJ & Begley CG Recombinant human thrombopoietin: basic biology and evaluation of clinical studies. Blood 100, 3457–3469 (2002). [DOI] [PubMed] [Google Scholar]
- 69.Takatani H et al. Levels of recombinant human granulocyte colony-stimulating factor in serum are inversely correlated with circulating neutrophil counts. Antimicrob. Agents Chemother. 40, 988–991 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hakim FT et al. Age-dependent incidence, time course, and consequences of thymic renewal in adults. J. Clin. Invest 115, 930–939 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mackall CL et al. Distinctions between CD8+ and CD4+ T-cell regenerative pathways result in prolonged T-cell subset imbalance after intensive chemotherapy. Blood 89, 3700–3707 (1997). [PubMed] [Google Scholar]
- 72.Komschlies KL, Grzegorzewski KJ & Wiltrout RH Diverse immunological and hematological effects of interleukin 7: implications for clinical application. J. Leukoc. Biol 58, 623–633 (1995). [DOI] [PubMed] [Google Scholar]
- 73.Storek J et al. Interleukin-7 improves CD4 T-cell reconstitution after autologous CD34 cell transplantation in monkeys. Blood 101,4209–4218 (2003). [DOI] [PubMed] [Google Scholar]
- 74.Fry TJ, Christensen BL, Komschlies KL, Gress RE & Mackall CL Interleukin-7 restores immunity in athymic T-cell-depleted hosts. Blood 97, 1525–1533 (2001). [DOI] [PubMed] [Google Scholar]
- 75.Mackall CL et al. IL-7 increases both thymic-dependent and thymic-independent T-cell regeneration after bone marrow transplantation. Blood 97, 1491–1497 (2001). [DOI] [PubMed] [Google Scholar]
- 76.Morrissey PJ et al. Administration of IL-7 to mice with cyclophosphamide-induced lymphopenia accelerates lymphocyte repopulation. J. Immunol 146, 1547–1552 (1991). [PubMed] [Google Scholar]
- 77.Bolotin E, Smogorzewska M, Smith S, Widmer M & Weinberg K Enhancement of thymopoiesis after bone marrow transplant by in vivo interleukin-7. Blood 88, 1887–1894 (1996). [PubMed] [Google Scholar]
- 78.Andrew D & Aspinall R IL-7 and not stem cell factor reverses both the increase in apoptosis and the decline in thymopoiesis seen in aged mice. J. Immunol 166, 1524–1530 (2001). [DOI] [PubMed] [Google Scholar]
- 79.Okamoto Y, Douek DC, McFarland RD & Koup RA Effects of exogenous interleukin-7 on human thymus function. Blood 99, 2851–2858 (2002). [DOI] [PubMed] [Google Scholar]
- 80.Min D et al. Protection from thymic epithelial cell injury by keratinocyte growth factor: a new approach to improve thymic and peripheral T-cell reconstitution after bone marrow transplantation. Blood 99, 4592–4600 (2002). [DOI] [PubMed] [Google Scholar]
- 81.Seggewiss R et al. Keratinocyte growth factor augments immune reconstitution after autologous hematopoietic progenitor cell transplantation in rhesus macaques. Blood 110, 441–449 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alpdogan O et al. Interleukin-15 enhances immune reconstitution after allogeneic bone marrow transplantation. Blood 105, 865–873 (2005). [DOI] [PubMed] [Google Scholar]
- 83.Fry TJ et al. Flt3 ligand enhances thymic-dependent and thymic-independent immune reconstitution. Blood 104, 2794–2800 (2004). [DOI] [PubMed] [Google Scholar]
- 84.Melchionda F et al. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J. Clin. Invest 115, 1177–1187 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]; The first description of the vaccine adjuvant effect of IL-7 and its preferential effects on subdominant antigens.
- 85.Frankenberger B et al. Influence of CD80, interleukin-2, and interleukin-7 expression in human renal cell carcinoma on the expansion, function, and survival of tumor-specific CTLs. Clin. Cancer Res. 11, 1733–1742 (2005). [DOI] [PubMed] [Google Scholar]
- 86.Wittig B et al. Therapeutic vaccination against metastatic carcinoma by expression-modulated and immunomodified autologous tumor cells: a first clinical phase I/II trial. Hum. Gene Ther. 12, 267–278 (2001). [DOI] [PubMed] [Google Scholar]
- 87.Kim TS, Chung SW & Hwang SY Augmentation of antitumor immunity by genetically engineered fibroblast cells to express both B7.1 and interleukin-7. Vaccine 18, 2886–2894 (2000). [DOI] [PubMed] [Google Scholar]
- 88.Colombetti S, Levy F & Chapatte L IL-7 adjuvant treatment enhances long-term tumor-antigen-specific CD8+ T-cell responses after immunization with recombinant lentivector. Blood 113, 6629–6637 (2009). [DOI] [PubMed] [Google Scholar]
- 89.Nanjappa SG, Walent JH, Morre M & Suresh M Effects of IL-7 on memory CD8 T cell homeostasis are influenced by the timing of therapy in mice. J. Clin. Invest 118, 1027–1039 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pellegrini M et al. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell 144, 601–613 (2011). [DOI] [PubMed] [Google Scholar]
- 91.Nanjappa SG, Kim EH & Suresh M Immunotherapeutic effects of IL 7 during a chronic viral infection in mice. Blood 23 March 2011. (doi: 10.1182/blood-2010-12-323154). [DOI] [PMC free article] [PubMed] [Google Scholar]; References 90 and 91 were the first descriptions of the postive effect of IL-7 on T cell-dependent immunity and viral clearance in chronic infection models.
- 92.Unsinger J et al. IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J. Immunol 184, 3768–3779 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes the potential for a positive effect of IL-7 therapy in bacterial infection.
- 93.Sportes C et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J. Exp. Med 205, 1701–1714 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]; The first complete description of the immunological effects of rhIL-7 therapy in humans, including the important observation that TCR repertoire diversification occurs, at least in part, through the preferential expansion of RTEs and naive T cell populations.
- 94.Sportes C et al. Phase I study of recombinant human interleukin-7 administration in subjects with refractory malignancy. Clin. Cancer Res. 16, 727–735 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beq S et al. Injection of glycosylated recombinant simian IL-7 provokes rapid and massive T-cell homing in rhesus macaques. Blood 114, 816–825 (2009). [DOI] [PubMed] [Google Scholar]; This study shows that glycosylation of IL-7 can decrease its immunogenicity.
- 96.Kitazawa H et al. IL-7 activates α4β1 integrin in murine thymocytes. J. Immunol 159, 2259–2264 (1997). [PubMed] [Google Scholar]
- 97.Rosenberg SA et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J. Immunother 29, 313–319 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sereti I et al. IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood 113, 6304–6314 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]; The first report of IL-7 therapy in HIV-infected individuals. In this study, patients were administered a single dose of IL-7, thereby allowing a definitive description of the length of biological effect of IL-7 in humans.
- 99.Hakim FT & Gress RE Reconstitution of thymic function after stem cell transplantation in humans. Curr. Opin. Hematol 9, 490–496 (2002). [DOI] [PubMed] [Google Scholar]
- 100.Chu YW et al. Exogenous IL-7 increases recent thymic emigrants in peripheral lymphoid tissue without enhanced thymic function. Blood 104, 1110–1119 (2004). [DOI] [PubMed] [Google Scholar]
- 101.Levy Y et al. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J. Clin. Invest 119, 997–1007 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]; The first report of the effect of multiple doses of IL-7 in HIV-infected individuals.
- 102.Patel A, Patel J & Ikwuagwu J A case of progressive multifocal leukoencephalopathy and idiopathic CD4+ lymphocytopenia. J. Antimicrob. Chemother 65, 2697–2698 (2010). [DOI] [PubMed] [Google Scholar]
- 103.Abrams D et al. Interleukin-2 therapy in patients with HIV infection. N. Engl. J. Med 361, 1548–1559 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shamim Z et al. Genetic polymorphisms in the genes encoding human interleukin-7 receptor-α: prognostic significance in allogeneic stem cell transplantation. Bone Marrow Transplant. 37, 485–491 (2006). [DOI] [PubMed] [Google Scholar]; The first description that polymorphisms in the gene encoding IL-7Rα can contribute to human immune-mediated disease.
- 105.Dean RM et al. Association of serum interleukin-7 levels with the development of acute graft-versus-host disease. J. Clin. Oncol 26, 5735–5741 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]; The first demonstration that serum IL-7 levels are associated with human immune-mediated disease.
- 106.Thiant S et al. Plasma levels of IL-7 and IL-15 in the first month after myeloablative BMT are predictive biomarkers of both acute GVHD and relapse. Bone Marrow Transplant. 45, 1546–1552 (2010). [DOI] [PubMed] [Google Scholar]
- 107.Sinha ML, Fry TJ, Fowler DH, Miller G & Mackall CL Interleukin 7 worsens graft-versus-host disease. Blood 100, 2642–2649 (2002). [DOI] [PubMed] [Google Scholar]
- 108.Fewkes NM & Mackall CL Novel γ-chain cytokines as candidate immune modulators in immune therapies for cancer. Cancer J. 16, 392–398 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kasten KR et al. Interleukin-7 (IL-7) treatment accelerates neutrophil recruitment through γδ T-cell IL-17 production in a murine model of sepsis. Infect. Immun 78, 4714–4722 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Uehira M et al. The development of dermatitis infiltrated by γδ T cells in IL-7 transgenic mice. Int. Immunol 5, 1619–1627 (1993). [DOI] [PubMed] [Google Scholar]
- 111.Lundmark F et al. Variation in interleukin 7 receptor α-chain (IL7R) influences risk of multiple sclerosis. Nature Genet. 39, 1108–1113 (2007). [DOI] [PubMed] [Google Scholar]
- 112.Gregory SG et al. Interleukin 7 receptor α-chain (IL7R) shows allelic and functional association with multiple sclerosis. Nature Genet. 39, 1083–1091 (2007). [DOI] [PubMed] [Google Scholar]; References 111 and 112 provide definitive evidence that IL-7Rα polymorphisms contribute to the genetic risk of autoimmune disease.
- 113.Hartgring SA, Bijlsma JW, Lafeber FP & van Roon JA Interleukin-7 induced immunopathology in arthritis. Ann. Rheum. Dis 65 (Suppl. 3), 69–74 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hartgring SA et al. Blockade of the interleukin-7 receptor inhibits collagen-induced arthritis and is associated with reduction of T cell activity and proinflammatory mediators. Arthritis Rheum. 62, 2716–2725 (2010). [DOI] [PubMed] [Google Scholar]
- 115.Totsuka T et al. IL-7 is essential for the development and the persistence of chronic colitis. J. Immunol 178, 4737–4748 (2007). [DOI] [PubMed] [Google Scholar]
- 116.Yamazaki M et al. Mucosal T cells expressing high levels of IL-7 receptor are potential targets for treatment of chronic colitis. J. Immunol 1 71, 1556–1563 (2003). [DOI] [PubMed] [Google Scholar]
- 117.Kirkwood JM et al. Next generation of immunotherapy for melanoma. J. Clin. Oncol 26, 3445–3455 (2008). [DOI] [PubMed] [Google Scholar]
- 118.Casadevall N et al. Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N. Engl. J. Med 346, 469–475 (2002). [DOI] [PubMed] [Google Scholar]
- 119.van de Pavert SA & Mebius RE New insights into the development of lymphoid tissues. Nature Rev. Immunol. 10, 664–674 (2010). [DOI] [PubMed] [Google Scholar]
- 120.Mebius RE et al. The fetal liver counterpart of adult common lymphoid progenitors gives rise to all lymphoid lineages, CD45+CD4+CD3− cells, as well as macrophages. J. Immunol 166, 6593–6601 (2001). [DOI] [PubMed] [Google Scholar]
- 121.Boos MD, Yokota Y, Eberl G & Kee BL Mature natural killer cell and lymphoid tissue-inducing cell development requires Id2-mediated suppression of E protein activity. J. Exp. Med 204, 1119–1130 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.GeurtsvanKessel CH et al. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J. Exp. Med 206, 2339–2349 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rangel-Moreno J, Moyron-Quiroz JE, Hartson L, Kusser K & Randall TD Pulmonary expression of CXC chemokine ligand 13, CC chemokine ligand 19, and CC chemokine ligand 21 is essential for local immunity to influenza. Proc. Natl Acad. Sci. USA 104, 10577–10582 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee Y et al. Recruitment and activation of naive T cells in the islets by lymphotoxin-β receptor-dependent tertiary lymphoid structure. Immunity 25, 499–509 (2006). [DOI] [PubMed] [Google Scholar]
- 125.Shields JD, Kourtis IC, Tomei AA, Roberts JM & Swartz MA Induction of lymphoid-like stroma and immune escape by tumors that express the chemokine CCL21. Science 328, 749–752 (2010). [DOI] [PubMed] [Google Scholar]
- 126.Hughes T et al. Stage 3 immature human natural killer cells found in secondary lymphoid tissue constitutively and selectively express the TH17 cytokine interleukin-22. Blood 113, 4008–4010 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Luci C et al. Influence of the transcription factor RORγt on the development of NKp46+ cell populations in gut and skin. Nature Immunol. 10, 75–82 (2009). [DOI] [PubMed] [Google Scholar]
- 128.Satoh-Takayama N et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 29, 958–970 (2008). [DOI] [PubMed] [Google Scholar]
- 129.Piketty C et al. Long-term clinical outcome of human immunodeficiency virus-infected patients with discordant immunologic and virologic responses to a protease inhibitor-containing regimen. J. Infect. Dis 183, 1328–1335 (2001). [DOI] [PubMed] [Google Scholar]
- 130.Engsig FN et al. Long-term mortality in HIV patients virally suppressed for more than three years with incomplete CD4 recovery: a cohort study. BMC Infect. Dis. 10, 318 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gutierrez F et al. Patients’ characteristics and clinical implications of suboptimal CD4 T-cell gains after 1 year of successful antiretroviral therapy. Curr. HIV Res 6, 100–107 (2008). [DOI] [PubMed] [Google Scholar]
- 132.Mackall C et al. Background to hematopoietic cell transplantation, including post transplant immune recovery. Bone Marrow Transplant. 44, 457–462 (2009). [DOI] [PubMed] [Google Scholar]


