Abstract
BACKGROUND.
The role of radiotherapy (RT) in the management of Merkel cell carcinoma (MCC) is controversial. The authors of this report evaluated the rates and patterns of failure in a selected group of patients who underwent RT for MCC of the head and neck (HN).
METHODS.
The records of 145 consecutive patients with MCC of the HN who presented to the authors’ institution between 1988 and 2009 were reviewed. Only patients who received RT at the institution were included. The cumulative incidence of locoregional failure (LRF), distant metastatic failure (DMF), disease progression (DP) and disease-specific death (DSD) were estimated with death as a competing risk.
RESULTS.
Forty-eight patients were identified. The median follow-up was 51 months (range, 6–220 months) for living patients. LRF developed in 5 patients (10%), and those patients had a median time to recurrence of 3 months. Two of the 5 LRFs were local and developed at the edge of the treatment field; the remaining 3 LRFs were in lymph nodes and occurred outside the treatment field. DMF developed in 12 patients (25%). The estimated 5-year cumulative incidences of LRF, DP, and DSD were 10%, 30%, and 21%, respectively. Acute toxicities included 5 episodes (10%) of grade 3 dermatitis and 1 episode (2%) of grade 3 mucositis.
CONCLUSIONS.
The authors report a site-specific series of patients with HN MCC who received RT. In this group of patients with adverse features, RT was well tolerated, and LRF was low. The propensity for MCC to recur at the edge of the treatment field suggests that generous margins are appropriate when RT is administered.
Keywords: Merkel cell, carcinoma, radiotherapy, recurrence, survival, head and neckradiation, neuroendocrine
INTRODUCTION
Merkel cell carcinoma (MCC) is the eponym for primary cutaneous neuroendocrine carcinoma, a rare and aggressive neoplasm that usually occurs in sun-exposed areas, in which approximately 30% to 50% of patients present in the head and neck. Elderly patients and those of white ethnicity are at greatest risk. MCC often has an aggressive course with early locoregional recurrence (LRR) and high distant metastatic (DM) rates of up to 25% in most series.1–4 Ultimately, approximately 25% to 36% of patients with MCC die of their disease.
The current American Joint Committee on Cancer staging for MCC is reliant on tumor size and lymph node status.5 Recently reported histopathologic features, such as the presence of lymphovascular invasion (LVI), may further enhance the ability to stratify patients according to risk6,7 and to guide treatment for this rare disease.
Typically, treatment for MCC consists of wide local excision (WLE) with generous margins (range, 1–2 cm)8–11 and lymph node staging by sentinel lymph node biopsy (SLNBx).3,7,11 However, WLE with clear margins may not always be functionally possible or cosmetically desirable in the head and neck region because of the proximity of critical structures. Therefore, postoperative radiotherapy (RT) often has been used in an attempt to reduce the risk of local recurrence at the primary tumor site and, in some instances, to address at-risk regional lymph nodes. Because of the rarity of this disease, to date, there have been no randomized trials addressing the definitive role of RT for MCC of the head and neck.
Currently, at our institution, adjuvant RT is administered selectively among patients who are deemed at high-risk for LRR because of positive/close surgical margins, LVI, or lymph node involvement. In this study, we analyzed the rates and patterns of failures in patients who selectively received RT for MCC of the head and neck.
MATERIALS AND METHODS
Patients and Tumor Characteristics
After we obtained approval from our Institutional Review Board, we reviewed the records of 145 consecutive patients with a histologically proven diagnosis of head and neck MCC who presented to our center between 1998 and 2009. Patients with primary head and neck MCC as well as those who presented with locoregionally recurrent disease were included. A Memorial Sloan-Kettering Cancer Center pathologist reviewed each case to confirm the histologic diagnosis. Seventy-two patients (50%) received RT, and 48 patients (33%) received RT at our institution as part of their management. These 48 patients were the focus of the current study. Tumor size was defined as the maximal dimension of the tumor at pathologic analysis. At the time of microscopic examination of the excision specimen, the surgical margins were defined as positive if the tumor cells extended to the margin and close when a tumor was located ≤1 cm from the surgical margin.
End Points and Statistical Analysis
The cumulative incidences of locoregional failure (LRF), disease progression (DP), and disease-specific death (DSD) were estimated with death as a competing risk. The univariate, nonparametric, competing risks method described by Gray was used to correlate prognostic factors with outcomes.12 Overall survival (OS) was estimated using the Kaplan-Meier method and was compared using the log-rank test. All events were measured from the initiation of RT.
Local recurrence was defined as recurrence at the primary site or within the primary surgical bed. Regional recurrence was defined as recurrence within draining lymph nodes. Distant recurrence was defined as any recurrence other than local or regional recurrences.
RESULTS
Patient and tumor characteristics are summarized in Table 1. The median follow-up was 51 months (range, 6–220 months) for living patients and 35 months (range, 1–220 months) for all patients. Eighteen of 48 patients (38%) had stage III (lymph node) disease, and 17 patients (35%) presented with recurrent disease. The presence or absence of LVI was specified on pathology reports from 21 of 48 patients (44%). LVI was identified in 17 specimens (35%), and there was no LVI in 4 specimens (8%).
Table 1.
Patient Clinical and Disease Characteristics
| Characteristic | No. of Patients (%)a |
|---|---|
| Median age [range], y | 74 [25–90] |
| Sex | |
| Men | 32 (67) |
| Women | 16 (33) |
| Disease subsite | |
| Periorbital | 3 (6) |
| Forehead | 5 (10) |
| Temple | 4 (8) |
| Cheek | 12 (25) |
| Nose | 4 (8) |
| Lip/chin | 3 (6) |
| Periauricular/ear | 8 (17) |
| Scalp | 6 (13) |
| Neck | 3 (6) |
| Pre-RT evaluation | |
| PET/CT | 24 (50) |
| CT only | 13 (27) |
| MRI only | 2 (4) |
| SLNBx alone | 2 (4) |
| Clinical examination alone | 4 (8) |
| Neck dissection | 3 (6) |
| AJCC stage | |
| I | 12 (25) |
| II | 1 (2) |
| III | 18 (38) |
| Recurrent | 17 (35) |
| Status at last follow-up | |
| NED | 26 (54) |
| AWD | 3 (8) |
| DOD | 9 (19) |
| DOC | 10 (21) |
Abbreviations: AJCC, American Joint Committee on Cancer; AWD, alive with disease; CT, computed tomography; DOC, died other causes; DOD, died of disease; MRI, magnetic resonance imaging; NED, no evidence of disease; PET, positron emission tomography; RT, radiotherapy; SLNBx, sentinel lymph node biopsy;
Some percentages may not add up to 100% because of rounding.
Thirteen patients (27%) presented with stage I or II (local only) disease. The excision specimen from 4 of these 13 patients (31%) had close margins, and 3 patients (23%) had positive surgical margins. Six of the 13 patients (46%) had LVI noted on pathologic examination.
Preradiotherapy Evaluation/Imaging
Twenty-four patients (50%) underwent positron emission tomography/computed tomography (PET/CT) studies to evaluate the extent of disease before RT. Thirteen patients (27%) underwent CT imaging of the head and neck and/or chest, abdomen, and pelvis. Two patients (4%) were evaluated by magnetic resonance imaging (MRI) without PET/CT or CT. Two patients (4%) were assessed by SLNBx only. Four patients (8%) were evaluated by clinical examination alone. Three patients (6%), all treated before 1997, underwent neck dissection with no records of preoperative or pre-RT imaging evaluation identified in the patients’ charts (Table 1).
Surgery
The surgical management of all patients is summarized in Table 2. For the 31 patients who presented with primary disease, the initial surgical management of the primary tumor was WLE in 28 patients (90%) and biopsy only in 3 patients (10%). One of the 3 patients who underwent biopsy only was deemed to have unresectable disease, because operative treatment would have resulted in excessive postsurgical morbidity. Two of the 3 patients were poor surgical candidates because of significant medical comorbidities. All 3 patients received definitive RT.
Table 2.
Surgical Treatment of Patients
| Treatment | No. of Patients (%) |
|---|---|
| Patients treated for primary MCC of the head and neck | 31 |
| Primary treatment | |
| Wide local excision | 28 (90) |
| Biopsy plus definitive RT | 3 (10) |
| Lymph node treatment | |
| Pathologic staging of clinically negative lymph nodes | 19 |
| SLNBx | 10 (53) |
| None | 9 (47) |
| Patients with clinically positive lymph nodes (primary cases) | 12 |
| Neck dissection | 9 (75) |
| Excisional biopsy | 3 (25) |
| RT alone | 0 (0) |
| Patients treated for recurrent MCC of the head and neck | 17 |
| Primary treatment | |
| Wide local excision | 14 (82) |
| RT alone (primary recurrence) | 2 (12) |
| RT alone (lymph node recurrence) | 1 (6) |
| Lymph node treatment | |
| Pathologic staging of clinically negative lymph nodes | 5 |
| SLNBx | 1 (20) |
| None | 4 (80) |
| Patients with clinically positive lymph nodes (recurrent cases) | 12 |
| Neck dissection | 8 (66) |
| RT alone | 4 (33) |
Abbreviations: MCC, Merkel cell carcinoma; RT, radiotherapy; SLNBx, sentinel lymph node biopsy.
At the time of presentation, 19 of 31 patients (61%) with primary disease had a clinically lymph node-negative lymph node basin. Ten of these 19 patients (53%) underwent pathologic staging by SLNBx, and histologically positive lymph nodes were identified subsequently in 5 patients. Twelve of 31 patients (39%) with primary disease presented with clinically or radiographically suspicious regional lymphadenopathy. Eleven of those 12 patients (92%) with clinically or radiographically suspicious regional lymphadenopathy had pathologically positive lymph nodes identified. One patient with suspicious findings on a PET scan had pathologically negative lymph nodes identified after neck dissection.
Of the 17 patients who presented with recurrent disease, 14 patients (82%) underwent salvage WLE followed by adjuvant RT. The remaining 3 patients (18%) received salvage RT to the primary site and/or lymph node site(s). Negative margins were obtained in 11 of the 14 patients who underwent revision or salvage WLE.
Five of the 17 patients (29%) with recurrent disease had clinically negative lymph nodes, and 1 of those patients underwent SLNBx and had histologically positive lymph nodes identified. Twelve of the 17 patients with recurrent disease had clinically positive lymph nodes, and 8 of those patients (66%) underwent neck dissection. The remaining 4 patients with clinically positive lymph nodes (33%) received RT alone to the regionally recurrent site.
Radiotherapy
The radiation field encompassed the primary site alone in 15 patients (31%), the regional lymph nodes alone in 12 patients (25%), and both the primary site and regional lymph nodes in 21 patients (44%). The median prescription dose to the primary site was 60 gray (Gy) for postoperative patients and 70 Gy for definitively treated patients with a median fraction dose of 2 Gy (range, 1.8–2.8 Gy). Treatment fields encompassed the postoperative tumor bed or gross disease with generous margins of at least 2 cm with reduction near critical structures considered by the treating physician. The regional lymph nodes were not routinely targeted prophylactically in pathologically staged lymph node-negative necks. When RT was delivered to the regional lymph nodes, the median prescription dose was 54 Gy (range, 48.6–70.2 Gy) with a median fraction size of 1.8 Gy (range, 1.76–2.23 Gy), and gross lymph node disease typically was treated with a median prescription dose of 60 Gy.
The RT modality included orthovoltage or megavoltage photons alone for 1 patient and 12 patients, respectively. Electrons were used alone to treat 11 patients. Finally, a combination of photons and electrons was used to treat 24 patients. Radiation Therapy Oncology Group grade 3 acute skin toxicity was recorded in 5 patients (10%). Other grade 3 toxicities recorded included mucositis in 1 patient (2%). No grade 3 or 4 late toxicities were observed.
Chemotherapy
Eight patients (17%) received concurrent or adjuvant chemotherapy, most commonly cisplatin or carboplatin in combination with etoposide. Six patients (13%) eventually received palliative chemotherapy for metastases; all received a cisplatin-based or carboplatin-based regimen except for 1 patient treated in 1988 who received cyclophosphamide, doxorubicin, and vincristine.
Recurrence and Survival
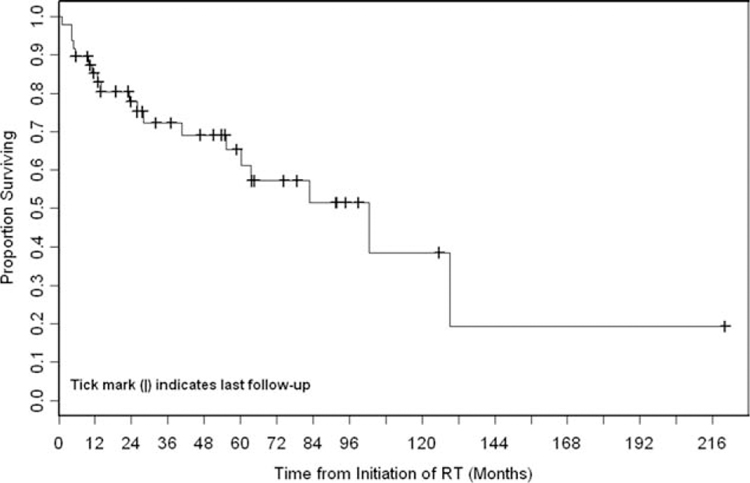
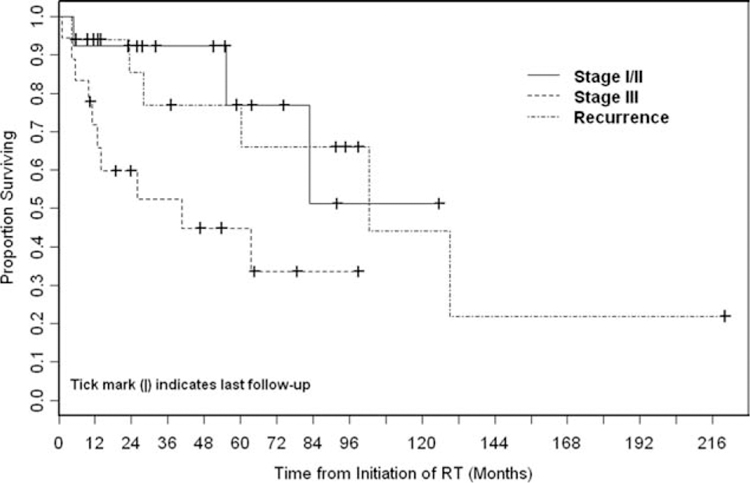
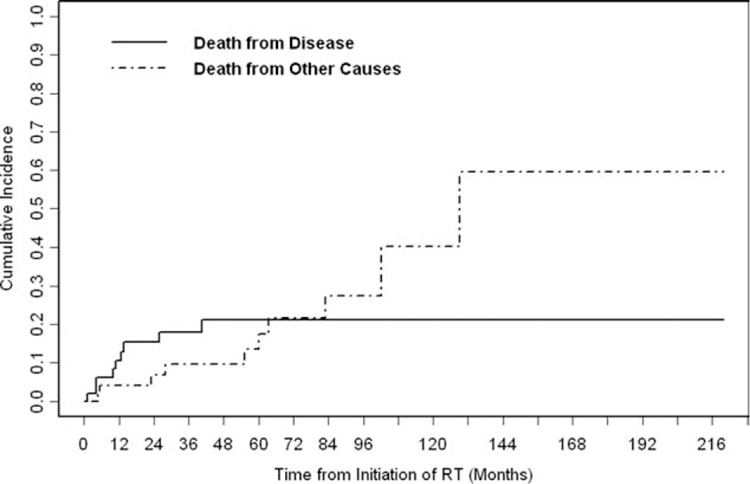
The 5-year OS rate was 65% (Fig. 1). Stage III disease was associated significantly with worse OS compared with stage I/II disease (P = .04). The association of recurrent disease with OS did not differ significantly compared with stage I/II disease (P = .84) or stage III disease (P = .06) (Fig. 2). The 5-year cumulative incidence of DSD was 21% (Fig. 3).
Figure 1.
Overall survival (OS) is illustrated for all patients. During the follow-up period, 19 patients died. The median OS was 102.4 months (95% confidence interval, 55.2 months to not reached), and the 5-year OS rate was 65% (95% confidence interval, 48%–78%). RT indicates radiotherapy.
Figure 2.
Overall survival (OS) was stratified according to disease stage. Stage III disease was associated significantly with worse OS compared with stage I/II disease (P =.04). The OS for patients with recurrent disease did not differ significantly compared with the OS of patients with stage I/II disease (P =.84) or patients with stage III disease (P =.06). RT indicates radiotherapy.
Figure 3.
The cumulative incidence of disease-specific death is illustrated. During the follow-up period, 9 patients died from disease. The 5-year cumulative incidence of disease-specific death was 21% (95% confidence interval, 8%–34%). RT indicates radiotherapy.
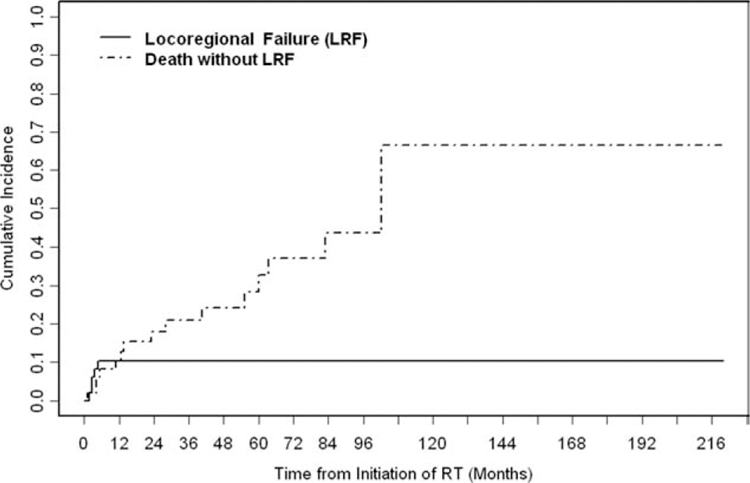
The 5-year cumulative incidence of LRF was 10% (Fig. 4). Of the 48 patients with head and neck MCC who received RT in this study, LRF developed in 5 patients (10%). The recurrence site was local in 2 patients (4%) and regional in 3 patients (6%). The median time to recurrence for the 5 patients was 3 months (range, 2–5 months). Both local failures occurred at the edge of the radiation field. One patient was salvaged successfully by WLE plus adjuvant RT and was rendered disease free as of the last follow-up 9 years later. The other patient with local recurrence underwent successful salvage of the local failure but eventually recurred distantly. The 3 regional recurrences occurred in patients who did not receive RT to the regional lymph nodes and, thus, were out of field. Two of those 3 patients (66%) presented with pathologically negative lymph nodes determined by SLNBx. All 3 patients were underwent successful regional salvage with surgery and adjuvant RT. One of these 3 patients later developed a distant recurrence. In a univariate competing-risk analysis, tumor size, positive/close margins, lymph node status, disease stage, concurrent/adjuvant chemotherapy, and RT dose (<60 Gy vs ≥60 Gy) were not associated significantly with locoregional recurrence (LRR) (Table 3). LVI was not included in the univariate competing-risk analysis, because, in the majority of patients (56%), LVI status was not specified on the pathology report.
Figure 4.
The cumulative incidence of locoregional failure (LRF) is illustrated. During the follow-up period, 5 patients experienced LRF. The 5-year cumulative incidence of LRF was 10% (95% confidence interval, 2%–19%). RT indicates radiotherapy.
Table 3.
Clinicopathologic Characteristics at Presentation for Radiotherapy: Log-Rank Tests of Factors Predictive of Locoregional Recurrence-Free, Progression-Free, and Disease-Specific Survival
| Prognostic Factor | No. of Patients (%) | Survival Analysis: Pa | |||
|---|---|---|---|---|---|
| OS | DSD | LRR | DP | ||
| Stage | .03b | .003b | .68 | .29 | |
| I and II | 13 (27) | ||||
| III | 18 (38) | ||||
| Recurrent | 17 (35) | ||||
| Lymph node status before RT | .11 | .01b | .48 | .009b | |
| Negative | 17 (35) | ||||
| Positive | 31 (65) | ||||
| Positive/close margins | .70 | .42 | .87 | .31 | |
| No | 31 (65) | ||||
| Yes | 13 (27) | ||||
| Unknown | 4 (8) | ||||
| Concurrent/adjuvant chemotherapy | .83 | .26 | .38 | .99 | |
| No | 42 (88) | ||||
| Yes | 6 (13) | ||||
| RT dose to primary, Gy | .24 | .89 | .26 | .54 | |
| <60 | 16 (33) | ||||
| ≥60 | 21 (44) | ||||
| None | 11 (23) | ||||
Abbreviations: DP, disease progression; DSD, disease-specific death; Gy, gray; LRR, locoregional recurrence; OS, overall survival; RT, radiotherapy.
P values were determined with the log-rank test (OS) or the Gray test (DSD, LRR, and DP).
Statistically significant.
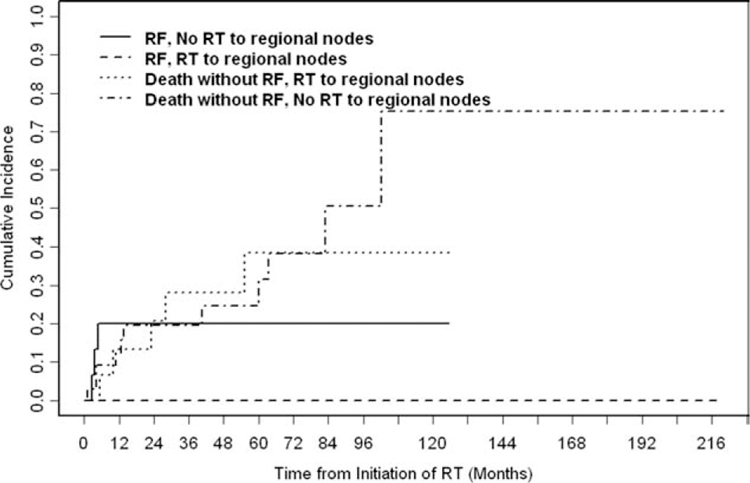
Adjuvant RT to the regional cervical lymph nodes was associated significantly with improved regional control (80% without regional RT vs 100% with regional RT; P = .01) (Fig. 5).
Figure 5.
The cumulative incidence of regional failure (RF) was stratified according to the receipt of radiotherapy (RT) to regional lymph nodes. Adjuvant RT to regional cervical lymph nodes improved regional control (100% achieved regional control with regional RT vs 80% without regional RT; P =.01).
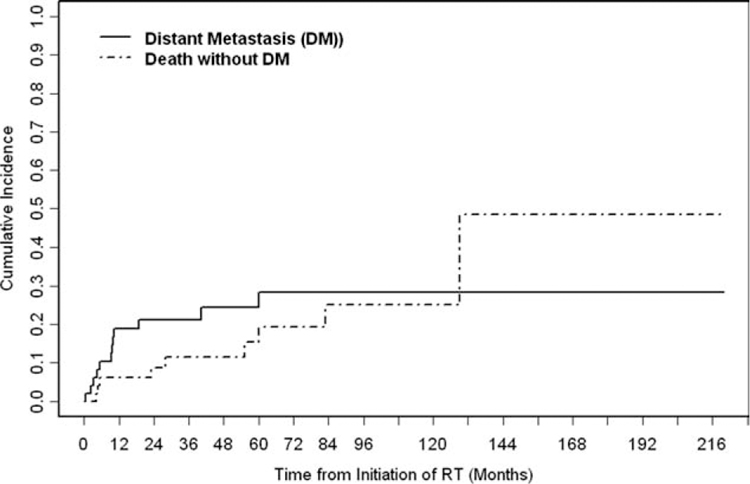
The 5-year cumulative incidence of distant failure was 24% (Fig. 6). Distant metastases developed in 12 patients (25%). The median time to distant recurrence for those 12 patients was 9 months (range, 1–60 months). The sites of initial distant metastases were the liver (n = 3), abdomen (n = 3), bone (n = 2), and chest wall (n = 1). The remaining 4 patients had diffuse metastatic disease at the time of recurrence.
Figure 6.
The cumulative incidence of distant metastasis (DM) is illustrated. During the follow-up period, 12 patients developed DM. The 5-year cumulative incidence of distant failure was 24% (95% confidence interval, 11%–37%). RT indicates radiotherapy.
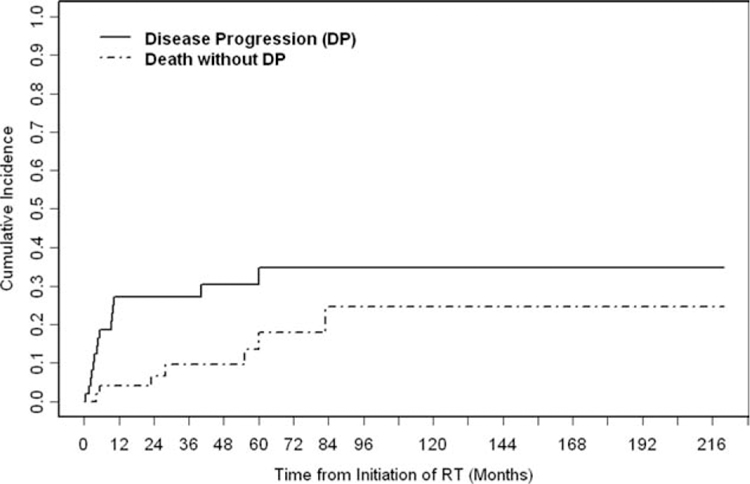
The 5-year cumulative incidence of DP was 30% (Fig. 7). Lymph node disease was significantly predictive of DP (P = .009). Disease stage (P = .003) and the presence of regional lymphadenopathy (P = .01) were associated significantly with DSD. Positive/close margins, concurrent/adjuvant chemotherapy, and RT dose (<60 Gy vs ≥60 Gy) were not associated significantly with DSD (Table 3).
Figure 7.
The cumulative incidence of disease progression (DP) is illustrated. Fifteen patients experienced DP (locoregional and/or distant failure). The 5-year cumulative incidence of disease progression was 30% (95% confidence interval, 17%–42%). RT indicates radiotherapy.
DISCUSSION
In this study, we observed that patients with MCC of the head and neck who underwent surgical resection followed by adjuvant RT achieved excellent locoregional control. The low LRR cumulative incidence of 10% at 5 years was obtained despite the presence of adverse clinical and pathologic features in many patients. For example, 73% of patients presented with stage III or recurrent disease. Moreover, the majority of patients who presented with stage I or II disease had evidence of LVI or positive/close margins.
In patients with MCC of the head and neck, despite the general presentation with smaller primary tumors compared with other disease sites (1.3 cm vs 2.6 cm, respectively),3 it can be difficult to obtain margin-negative excisions while maintaining functionally acceptable outcomes. Allen et al. and Hui et al. observed 94% and 93% margin-negative excision rates, respectively, in studies of primary MCC of all sites. In the currently study, only 71% of head and neck MCC excisions had negative margins. Radiation offered a high level of locoregional control in a multimodal setting in this selected group of high-risk patients with MCC of the head and neck. The treatment was well tolerated, with minimal acute or late grade 3 or greater toxicity.
MCC is an exceedingly radiosensitive tumor in vitro.13 Our study further suggests it is radiosensitive in vivo, and not a single LRR occurred in-field in our patients. These results are similar to those from another head and neck MCC series of 36 patients by Lawenda et al., who reported a 95% local control rate at 2 years when RT was delivered to the primary site.14 The current study’s 2 local recurrences occurred near the radiation field margin, suggesting that, when radiation is administered in the treatment of this disease, generous treatment margins may be indicated. It is noteworthy that, in the trunk or extremities, the National Comprehensive Cancer Network recommends wide RT margins (5 cm).11 However, this goal is difficult to achieve in the head and neck because of the proximity of critical anatomic structures; therefore, we recommend treatment margins of ≥2 cm, although, in areas near critical structures, reduction of the field margin may have to be considered. Finally, in patients who are unfit operative candidates or who refuse surgery, definitive RT with or without chemotherapy15 may be a reasonable alternative.
In our current study, as expected, we observed that lymph node involvement as a marker of disease stage was a significant prognostic indicator of disease-specific survival in patients with MCC. It is noteworthy that, of the 3 patients who developed regional recurrences, 2 patients (66%) presented with pathologically negative lymph nodes determined at SLNBx. This may have been a result of variable lymph node drainage patterns in the head and neck that can lead to false-negative results with SLNBx.16 In our series, of 10 clinically N0 necks assessed by SLNBx, 5 harbored occult disease. Therefore, we recommend addressing at-risk lymph node basins even in the clinically N0 neck.
Distant failure accounted for a large proportion of DP in our study (25%), attesting to the aggressive nature of this disease and corresponding with other reports.3,17 Some authors have suggested that, because of the high propensity of MCC to spread hematogenously and the high response rates associated with palliative chemotherapy, chemotherapy may be considered in the definitive treatment of MCC.15,18 However, the precise indications for adjuvant chemotherapy in the metastasis-free (M0) setting and the evidence supporting improved efficacy with its incorporation are not well defined by the limited available data. Chemotherapy has its own added toxicities. Currently, National Comprehensive Cancer Network guidelines indicate that, for patients with regional lymph node involvement, the addition of adjuvant chemotherapy is associated with only a ‘‘may consider’’ recommendation.11
Like in MCC that involves other sites, much work remains to identify which patients with head and neck MCC may benefit most from adjuvant RT. Recently, various histopathologic characteristics have been identified as predictive of survival in patients with MCC.6,19–21 Andea et al. reported a series of 156 patients and identified 3 histologic features that had prognostic significance for OS: tumor thickness, the presence of LVI, and the tumor growth pattern.6 In the current study, sufficient data on these features were not available from pathology reports to assess their prognostic impact.
Although it was not examined in this study, a previously unknown polyomavirus called Merkel cell polyomavirus (MCV) recently was detected that is integrated clonally into 80% of MCC tumors,22 and the authors suggest that MCV may contribute to the pathogenesis of MCC. Ongoing investigation is needed to determine the clinical and etiologic significance of MCV and its impact on LRR and survival.
Our study has several limitations, including its retrospective nature and the lack of uniformity in which patients were selected for adjuvant RT. However, for the patients who did receive RT, treatment was well tolerated, and an excellent rate of locoregional control was achieved. The data still are not conclusive for determining whether adjuvant RT improves disease control, but our current results suggest that, in a high-risk group receiving adjuvant RT after definitive surgical management, RT may offer a benefit.
Because of the complexity of managing MCC in the head and neck site, a multidisciplinary discussion is warranted for optimal treatment. Overall, we believe the following are relative indications for RT in patients with MCC of the head and neck: 1) large tumors (eg, T2–T4 disease), 2) positive/close surgical margins, 3) invasive histologic pattern or the presence of LVI, and 4) lymph node positive/stage III disease in which completion lymph node dissection is not performed. We do not routinely recommend RT for patients with pathologically staged stage IA disease (T1pN0) who have clear surgical margins after WLE; however, pathologic assessment of regional lymph nodes is recommended for accurate staging in head and neck MCC.3,4,7,23 In the setting of imaging or clinical assessment of the regional lymph nodes alone, RT to at-risk lymph node basins should be considered.
In conclusion, in this selected group of patients with MCC of the head and neck who had adverse features, RT was well tolerated, and LRR was low. Because there were no in-field recurrences in our series, despite the high-risk nature of our patients, we conclude that RT offers benefit in patients with high-risk MCC. The propensity for MCC to recur at the edge of the treatment field suggests that generous margins are appropriate when RT is administered. Further work is needed to determine clinical, histopathologic, and molecular features that identify the patients who are most likely to benefit from adjuvant RT.
Acknowledgments
FUNDING SOURCES
No specific funding was disclosed.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.Goepfert H, Remmler D, Silva E, Wheeler B. Merkel cell carcinoma (endocrine carcinoma of the skin) of the head and neck. Arch Otolaryngol. 1984;110:707–712. [DOI] [PubMed] [Google Scholar]
- 2.Takes RP, Balm AJ, Loftus BM, Baris G, Hilgers FJ, Gregor RT. Merkel cell carcinoma of the head and neck. Clin Otolaryngol Allied Sci. 1994;19:222–229. [DOI] [PubMed] [Google Scholar]
- 3.Allen PJ, Bowne WB, Jaques DP, Brennan MF, Busam K, Coit DG. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23:2300–2239. [DOI] [PubMed] [Google Scholar]
- 4.Fields RC, Busam KJ, Chou JF, et al. Five hundred patients with Merkel cell carcinoma evaluated at a single institution. Ann Surg. 2011;254:465–473; discussion 473–475. [DOI] [PubMed] [Google Scholar]
- 5.Edge SB, Byrd DR, Compton CC, et al. , eds. AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010. [Google Scholar]
- 6.Andea AA, Coit DG, Amin B, Busam KJ. Merkel cell carcinoma: histologic features and prognosis. Cancer. 2008;113:2549–2558. [DOI] [PubMed] [Google Scholar]
- 7.Fields RC, Busam KJ, Chou JF, et al. Recurrence and survival in patients undergoing sentinel lymph node biopsy for Merkel cell carcinoma: analysis of 153 patients from a single institution. Ann Surg Oncol. 2011;18:2529–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Ghazal SK, Arora DS, Simpson RH, Saxby P. Merkel cell carcinoma of the skin. Br J Plast Surg. 1996;49:491–496. [DOI] [PubMed] [Google Scholar]
- 9.Allen PJ, Zhang ZF, Coit DG. Surgical management of Merkel cell carcinoma. Ann Surg. 1999;229:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hitchcock CL, Bland KI, Laney RG 3rd, Franzini D, Harris B, Copeland EM 3rd. Neuroendocrine (Merkel cell) carcinoma of the skin. Its natural history, diagnosis, and treatment. Ann Surg. 1988; 207:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller SJ, Alam M, Andersen J, et al. Merkel cell carcinoma. J Natl Compr Canc Netw. 2009;7:322–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Statist. 1988;16:1141–1154. [Google Scholar]
- 13.Leonard JH, Ramsay JR, Kearsley JH, Birrell GW. Radiation sensitivity of Merkel cell carcinoma cell lines. Int J Radiat Oncol Biol Phys. 1995;32:1401–1147. [DOI] [PubMed] [Google Scholar]
- 14.Lawenda B, Arnold M, Tokarz V, et al. Analysis of radiation therapy for the control of Merkel cell carcinoma of the head and neck based on 36 cases and a literature review. Ear Nose Throat J. 2008; 87:634–643. [PubMed] [Google Scholar]
- 15.Poulsen M, Rischin D, Walpole E, et al. High-risk Merkel cell carcinoma of the skin treated with synchronous carboplatin/etoposide and radiation: a Trans-Tasman Radiation Oncology Group Study— TROG 96:07. J Clin Oncol. 2003;21:4371–4436. [DOI] [PubMed] [Google Scholar]
- 16.Willis AI, Ridge JA. Discordant lymphatic drainage patterns revealed by serial lymphoscintigraphy in cutaneous head and neck malignancies. Head Neck. 2007;29:979–985. [DOI] [PubMed] [Google Scholar]
- 17.Hui AC, Stillie AL, Seel M, Ainslie J. Merkel cell carcinoma: 27-year experience at the Peter MacCallum Cancer Centre. Int J Radiat Oncol Biol Phys. 2011;80:1430–1145. [DOI] [PubMed] [Google Scholar]
- 18.Poulsen M, Walpole E, Harvey J, et al. Weekly carboplatin reduces toxicity during synchronous chemoradiotherapy for Merkel cell carcinoma of skin. Int J Radiat Oncol Biol Phys. 2008;72:1070–1104. [DOI] [PubMed] [Google Scholar]
- 19.Llombart B, Monteagudo C, Lopez-Guerrero JA, et al. Clinicopathological and immunohistochemical analysis of 20 cases of Merkel cell carcinoma in search of prognostic markers. Histopathology. 2005;46:622–634. [DOI] [PubMed] [Google Scholar]
- 20.Mott RT, Smoller BR, Morgan MB. Merkel cell carcinoma: a clinicopathologic study with prognostic implications. J Cutan Pathol. 2004;31:217–223. [DOI] [PubMed] [Google Scholar]
- 21.Skelton HG, Smith KJ, Hitchcock CL, McCarthy WF, Lupton GP, Graham JH. Merkel cell carcinoma: analysis of clinical, histologic, and immunohistologic features of 132 cases with relation to survival. J Am Acad Dermatol. 1997;37(5 Pt 1):734–739. [DOI] [PubMed] [Google Scholar]
- 22.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319: 1096–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta SG, Wang LC, Penas PF, Gellenthin M, Lee SJ, Nghiem P. Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: The Dana-Farber experience and meta-analysis of the literature. Arch Dermatol. 2006;142:685–690. [DOI] [PubMed] [Google Scholar]