Abstract
Background
Free mobile applications (apps) that use photoplethysmography (PPG) waveforms may extend atrial fibrillation (AF) detection to underserved populations, but they have not been rigorously evaluated.
Objective
The purpose of this study was to systematically review and evaluate the quality, functionality, and adherence to self-management behaviors of existing mobile apps for AF.
Methods
We systematically searched 3 app stores for apps that were free, available in English, and intended for use by patients to detect and manage AF. A minimum of 2 reviewers evaluated (1) app quality, using the Mobile Application Rating Scale (MARS); (2) functionality using published criteria; and (3) features that support 4 self-management behaviors (including PPG waveform monitoring) identified using evidence-based guidelines. Interrater reliability between the reviewers was calculated.
Results
Of 12 included apps, 5 (42%) scored above average for quality (MARS score ≥3.0). App quality was highest for their ease of use, navigation, layout, and visual appeal (eg, functionality and aesthetics) and lowest for their behavioral change support and subjective impressions of quality. The most common app functionalities were capturing and graphically displaying user-entered data (n = 9 [75%]). Nearly all apps (n = 11 [92%]) supported PPG waveform monitoring, but only 2 (17%) supported all 4 self-management behaviors. Interrater reliability was high (0.75–0.83).
Conclusion
The reviewed apps had wide variability in quality, functionality, and adherence to self-management behaviors. Given the accessibility of these apps to underserved populations and the tremendous potential they hold for improving AF detection and management, high priority should be given to improving app quality and functionality.
Keywords: Ambulatory electrocardiographic monitoring, Arrhythmia, Atrial fibrillation, Mobile application, Self-management
Key Findings.
-
▪
Free mobile applications that use photoplethysmography waveforms hold potential for improving atrial fibrillation (AF) detection and management, especially for underserved populations. This, in turn, may reduce thromboembolic complications from undetected atrial fibrillation.
-
▪
Our review found that there is wide variability in the objective quality and functionality of existing commercially available AF applications and the degree to which they align with self-management guidelines.
-
▪
Greater attention to the quality, functionality, and guidelines during application design and development is needed. With such attention, these technologies may become a common, safe, and effective strategy for detecting and managing AF.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia,1 but its paroxysmal nature and poor correlation with symptoms make it especially difficult to detect and manage. In fact, stroke is the first clinical presentation of AF for one-quarter of patients with asymptomatic AF.2 In response, a number of remote monitoring technologies have become more widely used for AF detection and management. These include implantable cardiac monitors, medical-grade wearables, direct-to-consumer devices, and free mobile applications (apps).3 This study focused on evaluating free mobile apps because health care access and cost are not barriers to their adoption and use. Therefore, they are accessible to a far greater proportion of the world population, including underserved adults,4 creating the potential to transform AF screening in at-risk populations.5 However, free mobile apps for AF have not been rigorously evaluated to date.
There is growing recognition that photoplethysmography (PPG), an inexpensive and widely available technology included in all smartphones, can be harnessed for PPG waveform monitoring. PPG sensors detect changes in tissue blood volume that result from peripheral pulses.6 PPG waveforms are created within smartphones when a light source (ie, light-emitting diode [LED] flash from a smartphone camera) illuminates subcutaneous tissue in the finger, and a photodetector (ie, a smartphone camera) detects changes in light intensity through the tissue.6 Small studies have demonstrated that PPG waveform measurements are sensitive (87%–100%) and specific (97%–100%) compared to the gold standard—a 12-lead electrocardiogram (ECG).7,8 When made available in smartphones, which 81% of Americans currently use,4 PPG can be used to screen large segments of the population for irregular heart rate at virtually no cost. Although confirmation of AF using a 12-lead ECG is clinically necessary, free apps that use PPG can direct individuals to seek care who might otherwise go undiagnosed.
Previously, literature reviews have examined mobile apps to evaluate ventricular rates in AF and to screen populations for AF,9,10 but they predominantly included proprietary and expensive technologies that may be unavailable to underserved patients. One review of free consumer apps only examined the consumer ratings and readability of text within the apps.11 Consumer ratings in the marketplace are an inadequate substitute for app quality, functionality, and adherence to evidence-based guidelines. A rigorous, standardized evaluation is critical in guiding patients and clinicians to choose high-quality, safe, and effective apps for AF. Therefore, the purpose of this study was to systematically review and evaluate existing mobile apps according to the following criteria: (1) quality based on the Mobile Application Rating Scale (MARS)12; (2) functionality based on published criteria from the IQVIA (formerly IMS Health) Institute for Healthcare Informatics13; (3) adherence to evidence-based guidelines for AF management; and (4) rigorous evaluations of apps in published research.
Methods
Systematic search and screening of available apps
In February 2019, we systematically searched the 3 major app stores: Apple AppStore for iOS Devices, Google Play Store, and Amazon Appstore for Android Devices. We identified relevant apps using the search terms “atrial fibrillation,” “ECG,” “heart rhythm,” and “cardiac arrhythmia” in each of the 3 app stores.
Apps underwent a total of 3 rounds of screening. Two reviewers evaluated apps during each round based on the prespecified criteria. During the first round, we used the titles, descriptions, and screenshots provided in the app stores to exclude apps that were not available in English or were games or books. All remaining apps were downloaded to a tablet or smartphone, and 2 additional rounds of exclusion were conducted. During the second round, we excluded apps based on the following exclusion criteria: (1) duplicates (found in multiple app stores); (2) similar versions of the same app (eg, “Pro” and “Lite” versions); (3) nonpatient facing (ie, intended for use by doctors or researchers); and (4) inaccessible to reviewers due to institutional login requirements. We added a third round of exclusion criteria in acknowledgment of the large number of apps returned in our search that were related but not directly specific to AF, including (1) apps for general heart rate (but not PPG waveform) monitoring; and (2) general medication trackers.
Evaluation measures
We evaluated app quality using MARS.12 MARS was initially developed in 2016 in response to widespread concerns about the quality, efficacy, reliability, and security of available mobile health apps. The scale was intended to provide researchers, professionals, and clinicians with a brief tool for classifying and assessing app quality.12 MARS includes 23 questions across 4 sections: classification, behavioral change, objective quality, and subjective quality. Classification involves describing app characteristics (eg, number of downloads). Behavioral change assesses the anticipated effect of the app on the user’s knowledge, attitudes, and intention/likelihood of changing health behaviors. Objective quality contains 19 items across 4 domains: engagement (entertainment and interest); functionality (ease of use and navigation); aesthetics (layout and visual appeal); and information (quality, quantity, credibility, and visual enhancement of included information). Finally, subjective quality contains 4 items evaluating the user’s overall satisfaction with the app. All items are scored using a 5-point Likert scale, with 1 being inadequate and 5 being excellent quality.
We evaluated app functionality using the 7 functionality criteria specified in the IQVIA guidelines.13 This scale differs from the functionality domain of MARS in that it evaluates specific app functions, whereas MARS evaluates general functionality. The IQVIA criteria specify the following app functionalities: communication to clinicians or care teams (communicate); visualizes user-entered data (display); provides guidance, diagnosis, or recommended actions (guide); provides information in a variety of formats (inform); provides instructions (instruct); captures user-entered data (record); and provides reminders (remind). The “record” criterion includes 4 subcategories based on whether the app allows users to enter and store personal health data (collect data); transmit personal health data (share data); evaluate personal health data (evaluate data); and send alerts and/or intervene based on personal health data (intervene). Including these 4 subcategories, the criteria include 11 possible functionalities.
We also aimed to evaluate whether apps supported lifestyle and behavioral modifications (eg, self-management) that are known to impact AF burden. Although no single set of evidence-based guidelines for AF patient self-management was identified, we reviewed both evidence-based guidelines for clinicians managing AF and evidence-based patient education resources to generate a list of recommended self-management behaviors for AF patients.14, 15, 16, 17, 18 These behaviors include self-monitoring PPG waveform, managing medications, managing symptoms, and making necessary lifestyle changes (eg, weight reduction). A description of these behaviors and the evidence-based guidelines from which they originate is provided in Table 1.
Table 1.
AF-specific self-management behaviors
| Behavior | Description | Source supporting this criterion |
||
|---|---|---|---|---|
| AHA/ACC/HRS clinical guidelines∗ | AHA “Life’s Simple 7”† | Mayo Clinic and AHA patient resources‡ | ||
| Self-monitoring | Patient self-monitoring and/or remote monitoring of heart rate and rhythm | ✓ | ||
| Medication management | Adherence to medications; tracking medication changes (eg, warfarin based on INR) | ✓ | ||
| Symptom management | Monitoring and reporting symptoms to health care providers | ✓ | ||
| Lifestyle changes | Making healthy lifestyle changes to reduce the risk of AF recurrence and associated complications | ✓ | ✓ | |
Finally, we conducted a brief systematic review of the literature to evaluate the degree to which the included apps had been used in scientific research. App names were used as search terms in each of 3 scholarly databases: PubMed, Scopus, and ACM Digital Library (a computing and information technology database). We reviewed both peer-reviewed and grey literature (ie, literature that is not formally published in peer-reviewed journals, such as conference proceedings).19
Data extraction procedures and analysis
We created a data extraction form consisting of questions from (1) the MARS questionnaire; (2) IMS functionality guidelines; and (3) evidence-based guidelines for AF self-management. Three reviewers (VJ, SI, MA) independently evaluated the 4 randomly selected apps using the data extraction form to assess interrater reliability, which was acceptable (0.75–0.83). Domains with low agreement between reviewers (<0.7) were discussed until consensus regarding evaluation methodology was reached, apps were re-reviewed, and interrater reliability was recalculated. The remaining apps were independently evaluated by 2 reviewers.
Results
Search results
We identified 1473 potentially relevant apps across the 3 stores (Figure 1). Apps that were games or books (n = 490) or were not available in English (n = 251) based on their titles and descriptions in the app stores were excluded during the first round of screening. During the second round, apps that were nonpatient facing (n = 302); were inaccessible because they required an assigned or institutional login (n = 171); were duplicates (n = 130); or were similar versions of the same app (n = 39) were excluded. A large number of the remaining 90 apps offered only heart rate (but not PPG waveform) monitoring (n = 66) or general, non-AF–specific medication management tools (n = 12). Therefore, these apps were excluded in the third round. A total of 12 apps were ultimately included for review and analysis.
Figure 1.
Flow diagram of app screening. AF = atrial fibrillation.
Descriptive characteristics of included apps
Table 2 provides a brief description of the 12 included apps. The majority of apps (n = 10 [83%]) were available in the Apple App Store, fewer were available in the Google Play Store (n = 7 [58%]), and only 1 was available in the Amazon App Store. The number of installations ranged from 50 (EverBeat) to 500,000 (Cardiac Diagnosis). All apps were free, and 5 (42%) also had upgrades available for costs ranging from $3 to $79. Nearly all of the apps (n = 9 [75%]) included privacy policies.
Table 2.
Description of included apps
| Name | Developer | Platform | Version | Last update | Cost of upgrade | Privacy policy |
|---|---|---|---|---|---|---|
| Afib Companion | Pawel Kuklik | 0.0.41 | 2017 | 0 | No | |
| AFib Manager | At Point of Care | Apple | 10.4 | 2019 | 0 | Yes |
| BeatScanner | SuperECG | Apple | 1.2.1 | 2018 | 9.99 | No |
| Cardiac Diagnosis (heart rate, arrhythmia) | SUNG DO KIM | 122 | 2019 | 12.99 | Yes | |
| ECG Check | Cardiac Designs | Apple, Google | 2.1.4 | 2017 | 0 | Yes |
| EverBeat | GrekTek | Apple | 1.5.5 | 2018 | 0 | Yes |
| GoHeart | Tengfei Wang | Apple, Google | 1.0.4 | 2018 | 0 | No |
| Heart for Heart | Happitech B.V. | Apple | 1.7 | 2018 | 9.99 | Yes |
| Heart Rate Monitor: EKG Pulse Tracker for Cardio | Master App Solutions | Apple, Google | 1 | 2018 | 79.00 | Yes |
| Heart_Rhythm | SoftRobo | Apple, Google | 2.8 | 2018 | 3.00 | Yes |
| Photo AFib Detector | CCApp | Apple, Google | 2016.12.11 | 2016 | 0 | Yes |
| Qardio heart health | Qardio, Inc. | Apple, Google, Amazon | 1.83.1 | 2019 | 0 | Yes |
Quality of included apps
The MARS quality scores are shown in Figure 2. Less than half of the apps (n = 5 [42%]) had above-average quality, with an overall MARS score ≥3.0. The top-rated apps by total MARS score were Afib Companion (4.1), Qardio heart health (3.6), and Photo Afib Detector (3.3). In addition, Afib Companion was the highest rated app in 3 domains (functionality, subjective quality, and information).
Figure 2.
Mobile Application Rating Scale (MARS) app quality scores.
By domain, the functionality (ease of use and navigation) and aesthetics (layout and visual appeal) quality domains scored the highest above average across the 12 apps. The behavioral change (likelihood of changing behaviors) and subjective quality (overall impressions) domains scored the lowest, both well below average across the 12 apps.
Functionality of included apps
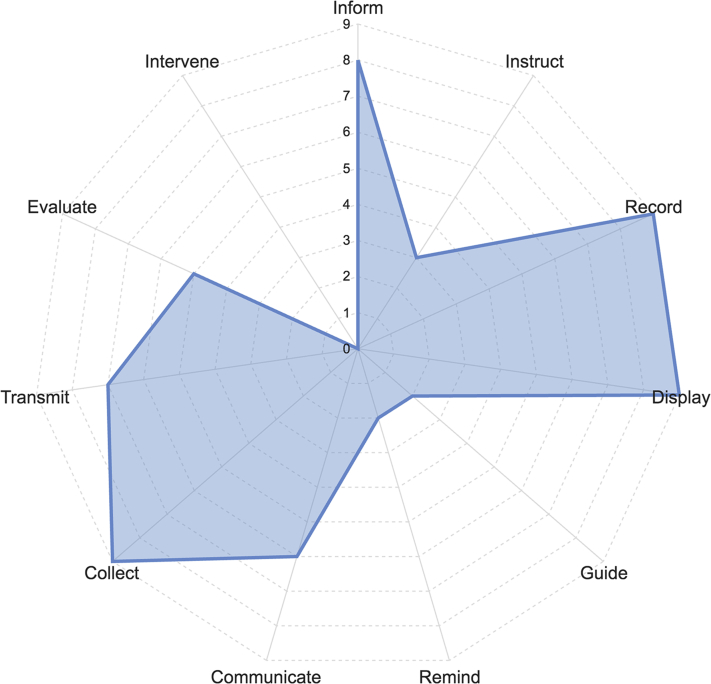
The prevalence of the distinct functionalities within the included apps is shown in Figure 3. The most common functionalities were capturing user-entered data (record; n = 9 [75%]) and graphically displaying user-entered data (display; n = 9 [75%]). Other common functionalities were providing information in a variety of formats, such as text, photo, and video (inform; n = 8 [67%]), and sharing health data via export, upload, or e-mail (transmit; n = 7 [58%]).
Figure 3.
IQVIA functionality scores.
Few apps included functionalities for providing reminders or alerts to the user (remind; n = 2 [17%]) or providing guidance, possibly including diagnosis or recommendations, based on user-entered information (guide; n = 2 [17%]). No apps offered the “intervene” functionality, which entails sending alerts based on the collected data or propose behavioral interventions.
The BeatScanner, GoHeart, Heart Rate Monitor, and Qardio heart health apps offered 7 functionalities, which was the highest number offered among the 12 apps. Cardiac Diagnosis (2 functionalities) and Heart for Heart (1 functionality) offered the fewest functionalities. AFib Companion, the highest rated app for overall quality, offered 5 functionalities, which was the average number of functionalities offered among the 12 apps.
Adherence of included apps with evidence-based self-management guidelines
The degree to which the included apps supported the 4 evidence-based self-management behaviors identified in the literature is given in Table 3. Nearly all of the apps (n = 11 [92%]) supported self-monitoring of PPG waveform (Figure 4). Many apps (n = 8 [67%]) also supported monitoring symptoms, including documenting symptoms and viewing trends over time. Few apps (n = 4 [33%]) supported lifestyle change, which involves reducing AF risk through lifestyle factors such as smoking, weight management, and blood pressure control. Additionally, few apps (n = 3 [25%]) supported medication management, which includes medication adherence and accurate dosing.
Table 3.
AF self-management guidelines
| App name | Self-monitoring using PPG waveform | Medication management | Symptom management | Lifestyle changes |
|---|---|---|---|---|
| Afib Companion | ✓ | ✓ | ✓ | |
| AFib Manager | ✓ | ✓ | ✓ | |
| BeatScanner | ✓ | |||
| Cardiac diagnosis | ✓ | ✓ | ||
| ECG Check | ✓ | |||
| EverBeat | ✓ | ✓ | ✓ | ✓ |
| GoHeart | ✓ | ✓ | ||
| Heart for Heart | ✓ | |||
| Heart Rate Monitor: EKG Pulse Tracker for Cardio | ✓ | ✓ | ||
| Heart_Rhythm | ✓ | |||
| Photo AFib Detector | ✓ | ✓ | ||
| Qardio heart health | ✓ | ✓ | ✓ | ✓ |
AF = atrial fibrillation; PPG = photoplethysmography.
Figure 4.
Example of photoplethysmography (PPG) recording using the Heart for Heart app.
Nearly all of the apps (n = 11 [92%]) supported PPG waveform monitoring. Two apps (Qardio heart health, EverBeat) supported all 4 self-management behaviors, whereas 4 apps (BeatScanner, ECG Check, Heart for Heart, Heart_Rhythm) supported only 1 behavior—PPG waveform monitoring.
Evaluation of included apps in research
No articles including any of the 12 apps were identified in the 3 scholarly databases searched.
Discussion
In this review, we systematically searched and evaluated 12 mobile apps available in consumer app stores against 2 established metrics for app quality and functionality, and for adherence to recognized AF self-management support.14, 15, 16, 17, 18 We found that more than half of the apps had below average quality, and most offered only a few distinct functionalities. Although PPG waveform monitoring was widely offered, other areas of AF self-management were less supported. These findings suggest there is much room for improvement in the development of mobile apps for AF.
Free mobile apps hold tremendous potential to improve the screening and management of AF, particularly in underserved populations. In a recent study, a mobile app used to screen >12,000 individuals for AF in 7 days detected possible AF in 136 individuals (1%), who then sought a confirmatory diagnosis.20 The STROKESTOP study found that intermittent, long-term, home-based ECG screening resulted in a 4-fold increase in the number of detected AF cases compared to current practice of single time-point ECGs.21 As such, mobile app-based PPG waveform monitoring has been recognized as a potential pathway to reduce avoidable emergency department visits and strokes attributable to AF.22 In addition, the widespread adoption of these apps can be leveraged so that data can be aggregated to inform population-level knowledge about AF. The Heart for Heart app reviewed in our study is part of a larger crowdsourcing effort in which PPG waveform data are compiled and visualized so that population-level patterns can be identified. To date, they have aggregated data from more than 1 million users worldwide, and visualized trends by demographics, country, weight, medical history, smoking status, and lifestyle in a publicly available dashboard.
At the same time, the performance of smartphone-based PPG waveform detection of AF has been variable among populations to date. For example, among hospitalized patients with ECG-confirmed cardiac rhythms of AF or normal sinus rhythm, the positive predictive value (PPV) is high (97.5%–99.6%), as is the negative predictive value (NPV) (96.0%).23 However, when used for screening in the general population of adults age ≥18 years, PPV is slightly lower (91.6%).24 Moreover, when used among adults at higher risk for developing AF (including age ≥65 years, hypertension, or diabetes), PPV is markedly more variable (51.3%–87.5%) yet NPV is high (99.8%–100%).25,26
Given this variability, the potential for these apps to cause harm should be more carefully considered. Patients may assume PPG waveforms are an appropriate diagnostic substitute for a 12-lead ECG, when instead they should only be a first step to confirmation of an AF diagnosis by 12-lead ECG. Importantly, therefore, false-negative findings from an app may offer false confidence and prevent patients from seeking care. Few of the apps we reviewed paired PPG with interpretation or explicit recommended actions (ie, seek care). This may result in missed opportunities for confirmatory diagnosis, thus undermining the primary benefit of population screening.
In general, false positives are also common with PPG waveforms, which cannot distinguish from among AF, atrial or ventricular premature contractions, and variable atrioventricular conduction (atrioventricular block).20 False positives may increase anxiety and lead patients to seek care unnecessarily, which in high numbers would negate the cost-effectiveness of app-based screening. They also may be misinterpreted by clinicians, who may not recognize the limitations of PPG waveforms and may inappropriately start anticoagulation therapy before confirming the diagnosis of AF. As such, education needs to focus on the strengths and limitations of PPG waveforms for AF screening.
Given the risk of thromboembolic complications from undiagnosed and therefore untreated AF and the known benefits of anticoagulation therapy to minimize this risk, screening of at-risk populations remains a strategy of interest. Lack of rigorous clinical trial data has precluded evidence-based guidelines from being able to provide guidance on screening techniques. As a result, recommendations in the US and European guidelines currently are limited and somewhat conflicting. For example, the 2016 European Society of Cardiology Guidelines for Management of Atrial Fibrillation support opportunistic, community-based ECG screening for silent AF as a cost-effective measure in adults age ≥65 years and other at-risk populations (S5.2).27 However, the 2019 American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation acknowledges a role for “‘smart’ worn or handheld WiFi-enabled device(s)” in the context of screening for silent AF only among patients with cryptogenic stroke (S7.12-7, S7.12-8).15 The US Preventive Services Task Force 2018 recommendation statement concluded that current evidence is insufficient to evaluate the benefits and harms of ECG screening among asymptomatic individuals age ≥65 years without previously diagnosed AF.28 As a result, the US Preventive Services Task Force has called for rigorous randomized controlled trials of asymptomatic patients comparing outcomes with and without screening ECGs to produce higher-quality evidence. Several ongoing trials are poised to address this evidence gap, including the STROKESTOP,21 SCREEN-AF (NCT02392754), IDEAL-MD (NCT02270151), and D2AF29 studies. As individuals continue to adopt and use mobile apps and other “smart” devices at rapid rates, this evidence will be critical in ensuring that screening and practice guidelines address the changing landscape of clinical AF management and may guide the development of safer, more effective strategies for detecting AF.
Finally, our results suggest that existing apps widely support PPG waveform monitoring but may be lacking in other areas equally important for AF prevention and management. For example, individuals at risk for AF would benefit from reduction of lifestyle-related AF risk factors, including obesity and hypertension. Extensive educational and motivational material has already been developed as part of the American Heart Association’s Life’s Simple 7 (LS7) campaign, which can be incorporated into an AF-focused app. The core concepts of LS7 were embedded in the Afib Companion app, which provides a 90-day educational and exercise program for individuals with AF. The program consists of short, daily lessons about the disease, promoting a healthy lifestyle and anxiety-reduction techniques (eg, mindfulness, breathing lessons). In the iHEART (iPhone Helping Evaluate Atrial Fibrillation Rhythm Through Technology) trial, LS7 content was tailored into automated text messages to promote behavioral change among AF patients.30 Regarding other important self-management behaviors, digital devices to promote medication adherence, such as smart pill boxes, have demonstrated the potential to significantly impact outcomes such as stroke in individuals with AF.31 Finally, tracking and visualizing trends among AF, symptoms, and behaviors (eg, caffeine intake) may help personalize selection of medical therapies and motivate positive behavioral change to reduce symptom burden.32
Study limitations
Although clear guidelines for self-management have been published for other chronic cardiovascular conditions, such as the Heart Failure Society of America 2010 Comprehensive Heart Failure Practice Guideline,33 none have been published for AF. Rather, the authors searched multiple evidence-based sources to compile a list of self-management behaviors. A lack of self-management guidelines may be a reason for the lack of comprehensive support for self-management behaviors observed among the 12 apps. As the prevalence of AF is projected to double in prevalence to an estimated 12 million individuals in the United States by 2030,1 clear guidelines for patient self-management are critically needed.
Additionally, the ever-evolving nature of available mobile apps makes the findings of this review somewhat transient. New apps for AF may soon become available, while some of the apps we reviewed may no longer be offered in the future. Nonetheless, the benefit of this review is not limited to the specific apps evaluated but rather is the methodology as well as identification of critical gaps. The methodology we describe can be used by clinicians and researchers seeking to establish the quality of current mobile apps. The Food and Drug Administration currently has limited oversight over most patient-facing apps, leaving few safeguards ensuring the clinical quality and safety of available apps. Clinicians are likely to feel more comfortable recommending to their AF patients those apps that meet minimum quality thresholds and support a range of functionalities and self-management behaviors.
Conclusion
In this study, we systematically reviewed and evaluated 12 mobile apps for AF management. We found wide variability in quality and functionality offered, generally poor alignment with evidence-based recommendations for self-management behaviors, and no rigorous research evaluation of the apps in the peer-reviewed or grey literature. These findings point to opportunities to improve the quality of mobile apps for patients with AF who may lack the resources to use expensive or invasive remote monitoring technologies. High-quality mobile apps for AF have the potential to increase screening and monitoring of AF with the goal of reducing negative sequelae of the disease (eg, stroke) and bolstering positive lifestyle changes.
Footnotes
This work was supported by the National Institute of Nursing Research of the National Institutes of Health (Award Number R00NR016275).
References
- 1.Colilla S., Crow A., Petkun W., Singer D.E., Simon T., Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112:1142–1147. doi: 10.1016/j.amjcard.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 2.Jaakkola J., Mustonen P., Kiviniemi T. Stroke as the first manifestation of atrial fibrillation. PloS One. 2016;11 doi: 10.1371/journal.pone.0168010. e0168010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hickey K.T., Riga T.C., Mitha S.A., Reading M.J. Detection and management of atrial fibrillation using remote monitoring. Nurse Pract. 2018;43:24–30. doi: 10.1097/01.NPR.0000530214.17031.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pew Research Center Mobile Fact Sheet. 2018. http://www.pewinternet.org/fact-sheet/mobile/# Available at: Accessed October 25, 2019.
- 5.Turakhia M.P., Kaiser D.W. Transforming the care of atrial fibrillation with mobile health. J Interv Card Electrophysiol. 2016;47:45–50. doi: 10.1007/s10840-016-0136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas. 2007;28:R1–R39. doi: 10.1088/0967-3334/28/3/R01. [DOI] [PubMed] [Google Scholar]
- 7.De Ridder B., Van Rompaey B., Kampen J.K., Haine S., Dilles T. Smartphone apps using photoplethysmography for heart rate monitoring: meta-analysis. JMIR Cardio. 2018;2:e4. doi: 10.2196/cardio.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krivoshei L., Weber S., Burkard T. Smart detection of atrial fibrillation. Europace. 2017;19:753–757. doi: 10.1093/europace/euw125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giebel G.D., Gissel C. Accuracy of mHealth devices for atrial fibrillation screening: systematic review. JMIR Mhealth Uhealth. 2019;7 doi: 10.2196/13641. e13641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li K.H.C., White F.A., Tipoe T. The current state of mobile phone apps for monitoring heart rate, heart rate variability, and atrial fibrillation: narrative review. JMIR Mhealth Uhealth. 2019;7 doi: 10.2196/11606. e11606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayyaswami V., Padmanabhan D.L., Crihalmeanu T., Thelmo F., Prabhu A.V., Magnani J.W. Mobile health applications for atrial fibrillation: a readability and quality assessment. Int J Cardiol. 2019;293:288–293. doi: 10.1016/j.ijcard.2019.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoyanov S.R., Hides L., Kavanagh D.J., Zelenko O., Tjondronegoro D., Mani M. Mobile app rating scale: a new tool for assessing the quality of health mobile apps. JMIR Mhealth Uhealth. 2015;3:e27. doi: 10.2196/mhealth.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aitken M., Gauntlett C. IMS Institute for Healthcare Informatics; Parsippany, NJ: 2013. Patient Apps for Improved Healthcare: From Novelty to Mainstream. [Google Scholar]
- 14.January C.T., Wann L.S., Alpert J.S. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 15.January C.T., Wann L.S., Calkins H. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 16.American Heart Association Atrial Fibrillation: Treatment and Prevention of Atrial Fibrillation. 2016. https://www.heart.org/en/health-topics/atrial-fibrillation/treatment-and-prevention-of-atrial-fibrillation Available at: Accessed January 20, 2019.
- 17.American Heart Association My Life Check: Life’s Simple 7. 2018. https://www.heart.org/en/healthy-living/healthy-lifestyle/my-life-check--lifes-simple-7 Available at: Accessed January 20, 2019.
- 18.Mayo Clinic Atrial Fibrillation: Diagnosis and Treatment. 2019. https://www.mayoclinic.org/diseases-conditions/atrial-fibrillation/diagnosis-treatment/drc-20350630 Available at: Accessed January 20, 2019.
- 19.Higgins J.P.T., Green S., editors. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. Part 2: General Methods for Cochrane Reviews. 2011. Grey literature databases.https://handbook-5-1.cochrane.org/chapter_6/6_2_1_8_grey_literature_databases.htm Available at: [Google Scholar]
- 20.Verbrugge F.H., Proesmans T., Vijgen J. Atrial fibrillation screening with photo-plethysmography through a smartphone camera. Europace. 2019;21:1167–1175. doi: 10.1093/europace/euz119. [DOI] [PubMed] [Google Scholar]
- 21.Svennberg E., Engdahl J., Al-Khalili F., Friberg L., Frykman V., Rosenqvist M. Mass screening for untreated atrial fibrillation: the STROKESTOP Study. Circulation. 2015;131:2176–2184. doi: 10.1161/CIRCULATIONAHA.114.014343. [DOI] [PubMed] [Google Scholar]
- 22.Kramer D.B., Yeh R.W., Zimetbaum P.J. Editorial commentary: hard questions for mobile technology in cardiology. Trends Cardiovasc Med. 2016;26:387–388. doi: 10.1016/j.tcm.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valiaho E.S., Kuoppa P., Lipponen J.A. Wrist band photoplethysmography in detection of individual pulses in atrial fibrillation and algorithm-based detection of atrial fibrillation. Europace. 2019;21:1031–1038. doi: 10.1093/europace/euz060. [DOI] [PubMed] [Google Scholar]
- 24.Guo Y., Wang H., Zhang H. Mobile photoplethysmographic technology to detect atrial fibrillation. J Am Coll Cardiol. 2019;74:2365–2375. doi: 10.1016/j.jacc.2019.08.019. [DOI] [PubMed] [Google Scholar]
- 25.Chan P.H., Wong C.K., Poh Y.C. Diagnostic performance of a smartphone-based photoplethysmographic application for atrial fibrillation screening in a primary care setting. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003428. e003428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poh M.Z., Poh Y.C., Chan P.H. Diagnostic assessment of a deep learning system for detecting atrial fibrillation in pulse waveforms. Heart. 2018;104:1921–1928. doi: 10.1136/heartjnl-2018-313147. [DOI] [PubMed] [Google Scholar]
- 27.Kirchhof P., Benussi S., Kotecha D. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 28.US Preventive Services Task Force. Curry S.J., Krist A.H. Screening for atrial fibrillation with electrocardiography: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:478–484. doi: 10.1001/jama.2018.10321. [DOI] [PubMed] [Google Scholar]
- 29.Uittenbogaart S.B., Verbiest-van Gurp N., Erkens P.M. Detecting and Diagnosing Atrial Fibrillation (D2AF): study protocol for a cluster randomised controlled trial. Trials. 2015;16:478. doi: 10.1186/s13063-015-1006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hickey K.T., Hauser N.R., Valente L.E. A single-center randomized, controlled trial investigating the efficacy of a mHealth ECG technology intervention to improve the detection of atrial fibrillation: the iHEART study protocol. BMC Cardiovasc Disord. 2016;16:152. doi: 10.1186/s12872-016-0327-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boehme P., Wienand P., Herrmann M., Truebel H., Mondritzki T. New digital adherence devices could prevent millions of strokes from atrial fibrillation by the end of the next century. Med Hypotheses. 2017;108:46–50. doi: 10.1016/j.mehy.2017.07.034. [DOI] [PubMed] [Google Scholar]
- 32.Heidt S.T., Kratz A., Najarian K. Symptoms in atrial fibrillation: a contemporary review and future directions. J Atr Fibrillation. 2016;9:1422. doi: 10.4022/jafib.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindenfeld J., Albert N.M., Boehmer J.P. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16:e1–e194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]