Abstract
Chronic myeloid leukemia (CML) is a clonal hematopoietic stem cell disorder characterized by a reciprocal translocation, t(9;22) (q34.1;q11.2). This leads to fusion of the BCR and ABL1 genes, encoding an active tyrosine kinase that causes unregulated proliferation of the myeloid lineage. The BCR/ABL1 fusion protein is found not only in CML, but also in a subset of de novo B-lymphoblastic leukemia (B-LL). However, the fusion protein in CML is characteristically the slightly longer p210 variant, whereas the p190 variant is more frequently found in B-LL. Without treatment, CML will progress to accelerated and/or blast phase (BP). Disease progression is often characterized by accumulation of additional chromosomal abnormalities. The development of tyrosine kinase inhibitor (TKI) therapy that targets BCR/ABL1 has revolutionized treatment of CML and vastly improved outcomes, although the disease can still progress despite TKI therapy. Blast phase most commonly manifests as myeloid BP; however, up to 30% of BP presents as lymphoid BP (LBP), typically of the B-cell lineage. The B-lymphoblasts of LBP have a phenotype indistinguishable from that of de novo B-LL. However, LBP typically carries the p210 BCR/ABL transcript and may show distinct chromosomal anomalies, including loss of chromosome 9p. The prognosis for CML-BP is poor, although survival has improved with TKI therapy and stem cell transplant, and LBP has been associated with superior survival compared with myeloid BP. Here we present a case of CML in B-lymphoid BP and review the current literature.
Keywords: B-lymphoid blast phase, chronic myeloid leukemia, lymphoid blast phase, tyrosine kinase inhibitor
CASE REPORT
A 57-year-old man with a history of a seizure disorder presented to the emergency department following a seizure and was found to have an elevated white blood cell count of 29,210/μL (range, 4500–11,000/μL), with a normal hematocrit (41.4%; range, 41%–53%) and platelet count (288,000/μL; range 150,000–350,000/μL). The initial differential was reported as: 46% neutrophils, 3% myelocytes, 6% metamyelocytes, 1% bands, 6% monocytes, 34% lymphocytes, 4% “young unidentified” cells, 0% eosinophils, and 0% basophils (Fig. 1). Flow cytometry studies were then performed on the peripheral blood which showed 12% phenotypically abnormal B-lymphoblasts. The abnormal lymphoblasts expressed moderate-intensity CD45, along with CD19, CD34, HLA-DR, CD10 (variable), and partial CD13 and CD33. The blasts had minimal dim expression of CD20 and did not show expression of surface light chain, CD117, or other myeloid and T-cell markers (Fig. 2). Basophils comprised approximately 1% of cells on flow cytometric analysis.
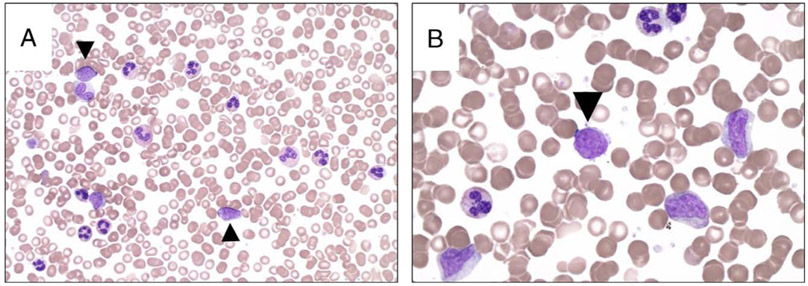
FIGURE 1.
Peripheral smears at the time of diagnosis; circulating blasts are indicated with an arrowhead. A, The peripheral blood shows a leukocytosis with a myeloid predominance and occasional circulating blasts (modified Wright-Giemsa stain, original magnification ×500, oil). B, The circulating myeloid cells are present in various stages of maturation (modified Wright-Giemsa stain, original magnification ×1000, oil).
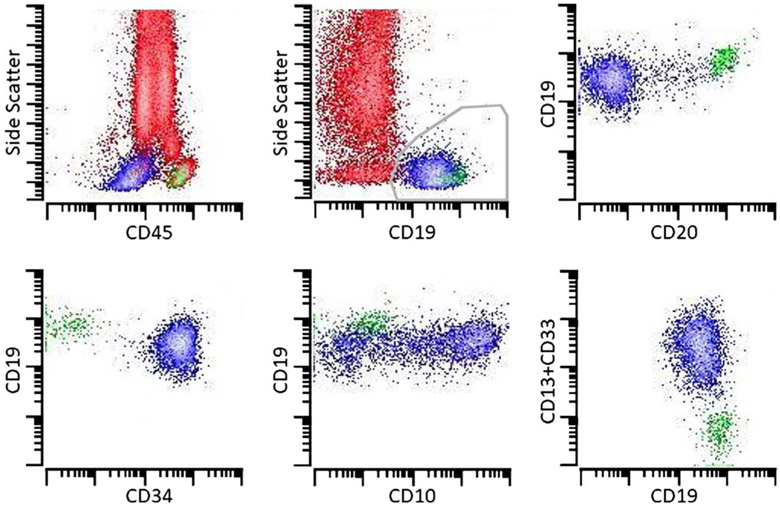
FIGURE 2.
Flow cytometric analysis of the peripheral blood shows numerous granulocytes (73%), as well as some monocytes (4%) and lymphocytes (10%). CD19+ blasts (approximately 12%) largely lack expression of CD20 but are positive for CD34 and variable CD10. The blasts also show partial expression of CD13 and CD33. Background granulocytes, monocytes, T cells, and natural killer cells are red; B-lymphoblasts are blue, and mature B cells are green.
A bone marrow biopsy was then performed. The marrow was hypercellular with a patchy increase in immature cells. The background marrow showed an elevated myeloid-to-erythroid ratio (approximately 8:1), and both lineages showed a full range of maturation. Megakaryocytes were normal in number but had variable morphology, including some small forms with hypolobated nuclei. The bone marrow aspirate smear was notable for 20% to 25%blasts with a moderate amount of lightly basophilic agranular cytoplasm, fine chromatin, and conspicuous nucleoli (Fig. 3). The background marrow elements showed a myeloid predominance with no significant dysplasia. Flow cytometry studies on the bone marrow showed approximately 24% abnormal B-lymphoblasts. Fluorescence in situ hybridization (FISH) analysis performed on the bone marrow specimen demonstrated BCR-ABL1 fusion in 93% of cells, and multiplex polymerase chain reaction analysis detected the BCR e1/a2 (p190) transcript. A diagnosis of chronic myeloid leukemia (CML) in B-lymphoid blast phase (BP) was rendered.
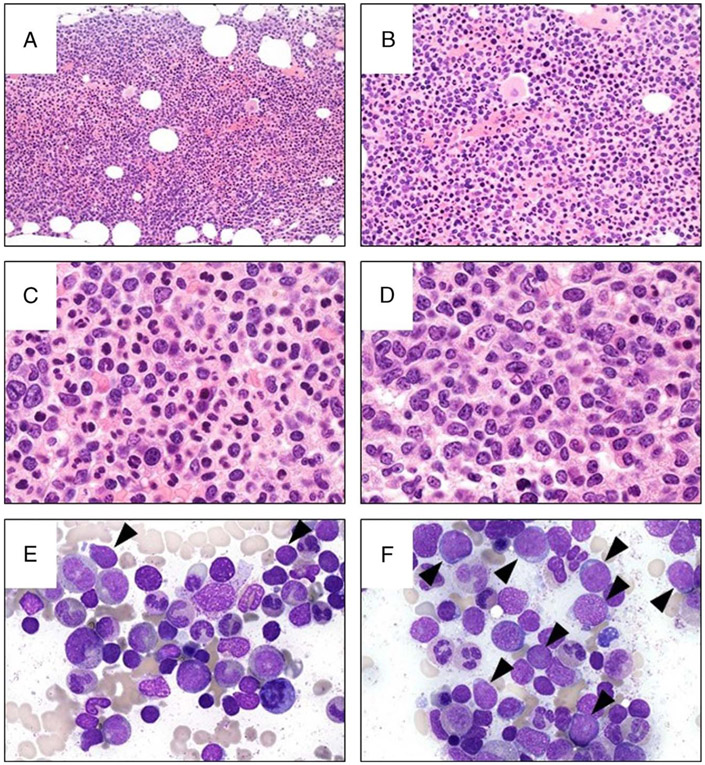
FIGURE 3.
Bone marrow biopsy and aspirate. A, The marrow is hypercellular (hematoxylin-eosin [H&E] stain, original magnification × 100). B, The layer of immature granulocytes adjacent to the bone (bottom of the picture) is thickened. There are occasional small megakaryocytes with hypolobated nuclei (H&E stain, original magnification ×500, oil). Some areas of the marrow show a marked myeloid predominance with progressive maturation and relatively few immature cells (C, H&E stain, and E, modified Wright-Giemsa stain, original magnification both × 1000, oil), whereas other areas show increased immature cells (D, H&E stain, and F, modified Wright-Giemsa stain, original magnification both × 1000, oil). E and F, Blasts in the aspirate are indicated with an arrow, and the background marrow shows a myeloid predominance with no overt dysplasia.
Therapy was initiated with weekly vincristine and dexamethasone plus daily dasatinib. As the lymphoblasts expressed CD20, rituximab was added on days 1 and 15. The patient also received prophylactic intrathecal methotrexate. His circulating blasts cleared and he was then treated with high-dose methotrexate and cytarabine. Dasatinib was continued throughout. A follow-up bone marrow evaluation, performed approximately 54 days following presentation, showed a minute population of abnormal B-lymphoblasts by flow cytometry (0.065% of cells), consistent with residual disease, and polymerase chain reaction analysis again detected the p190 transcript. The T315I mutation was not detected. The patient then received 2 cycles of blinatumomab therapy, while continuing dasatinib. A subsequent bone marrow evaluation, performed approximately 94 days following presentation, was negative for residual disease by flow cytometry and FISH analysis. The patient declined bone marrow transplant.
DISCUSSION
Chronic myeloid leukemia is a clonal hematopoietic stem cell disorder that is characterized by a reciprocal translocation, t(9;22)(q34.1;q11.2), which causes fusion of the BCR gene on chromosome 9q34.1 and the ABL1 gene on chromosome 22q11.2.1-6 The BCR-ABL1 fusion gene encodes a tyrosine kinase that is constitutively active, leading to marked myeloid hyperplasia in the bone marrow. Disease onset can be subtle, and patients may be diagnosed when they are found to have an increase in myeloid cells, or sometimes platelets, in the peripheral blood.1-7 Chronic myeloid leukemia is separated into 3 phases: chronic phase (CP), accelerated phase (AP), and BP. The division is determined by a set of pathologic, cytogenetic, and clinical criteria. The most frequently invoked pathologic criterion is the percentage of blasts in the blood or marrow.7 In CP, blasts must account for less than 10% of the blood and bone marrow cellularity, but they are typically less numerous (<5%). Patients who progress to AP because of increased blasts have 10% to 19% blasts in the blood or bone marrow, and BP is characterized by 20% blasts or greater in the blood or bone marrow.7-11 If therapy with a tyrosine kinase inhibitor (TKI) directed against the BCR/ABL1 fusion protein is not initiated, then CML-CP typically progresses to BP within 3 to 5 years,12 although approximately 5% of cases of CML are diagnosed when the patient is already in AP or BP with no documented preceding CP7 While the advent of TKIs has greatly improved outcomes in CML,7,13-15 some patients may still progress to AP or BP despite TKI therapy.6,7,12,16
The mechanism of transformation from CML-CP to BP is not well understood, but acquisition of additional chromosomal abnormalities appears to play a role in disease progression.1-4,6,8,12,17-21 At diagnosis, approximately only 5% to 10% of cases of CML will show chromosomal abnormalities in addition to t(9;22); however, additional chromosomal anomalies are identified in 50% to 80% of CML-AP3,17 Some of the most commonly identified abnormalities in CML-AP include additional copies of the Philadelphia chromosome, isochromosome 17q, trisomy 8,3-5,16,19,20 and gains in c-Myc copy number.4,19 In fact, the acquisition of specific additional chromosomal abnormalities can be used, by itself, as a criterion for advancement from CML-CP to CML-AP. The chromosomal changes that define transformation to CML-AP include a second Philadelphia chromosome, isochromosome 17q, trisomy 8, trisomy 19, abnormalities of 3q26.2, or a complex karyotype. Molecular studies do not play a major role in the diagnosis of CML-AP, but an increase in BCR-ABL1 mRNA expression may be detected in patients prior to any clinical or laboratory evidence of transformation.22
Chronic myeloid leukemia in BP may manifest as myeloid BP (MBP), lymphoid BP (LBP),1,7,12 or, rarely, mixed lineage BP1 Chronic myeloid leukemia most frequently progresses to MBP, but LBP accounts for up to 30% of CML-BP The lymphoblasts are most commonly of the B-cell lineage, although T-lymphoblastic transformation is rarely seen.1,7,23,24 Chronic myeloid leukemia–LBP patients may present with sheets of blasts that largely replace the marrow, but a diagnosis of CML-LBP is appropriate if lymphoblasts account for 20% or more of the blood or bone marrow cellularity. Occasionally, patients with CML have fewer than 20%lymphoblasts in the marrow or peripheral blood. Although this finding theoretically meets the criteria for CML-AP, it is worrisome for an impending transformation to frank CML-BP, and the phenotype of the blasts should be noted prominently in the report.
The B-lymphoblasts of CML-BP have a phenotype that is essentially indistinguishable from de novo B-lymphoblastic leukemia (B-LL), with expression of B-cell antigens (CD79a, CD19, and PAX5) along with markers of immaturity (TdT, CD34, and/ or CD10).1,4,7,23,25,26 As seen in the presented case, the blasts of CML in B-lymphoid BP often show expression of myeloid antigens, most frequently CD13 and/or CD33. While the vast majority of cases of CML-BP gain additional chromosomal abnormalities, overall LBP typically has fewer additional anomalies than MBP.3 Of note, B-lymphoid BP has been associated with loss of the short arm of chromosome 9. This chromosomal abnormality is postulated to play an important role in CML-LBP because 9p is home to genes important in B-cell differentiation (PAX5 and CDKN1A).1,13,17,20,27,28 Lymphoid BP has also been associated with deletion of p16/CDKN2A20,21,28,29 and numerical gains and breakpoints involving chromosomes 1q and 7p.17
When CML-LPB presents without a known history of CML-CP, it can be difficult to distinguish from de novo B-LL with t (9;22).2,9,17,27,30 There are some features that may help differentiate the 2 processes. Basophilia and increased myelocytes and metamyelocytes within the peripheral blood raise the possibility of an underlying CML-LBP.9 It should be noted that basophilia is not specific to CML-LBP and can be also seen in B-LL with t(5;14)(q31.1;q32.1); however, B-LL with t(5;14) is typically associated with a pronounced eosinophilia that is not a hallmark of CML-BP.7 If the marrow is not entirely overrun by blasts, then background myeloid hyperplasia and the presence of micromegakaryocytes may also help to suggest an underlying CML. Unfortunately, the immunophenotype of the blasts cannot be used to distinguish between CML-LBP, and de novo B-LL, as both may show expression of one or more myeloid antigens, typically CD13 or CD33, on blasts.7,17
In cases where there is no clear history of CML, molecular studies are often helpful in suggesting an underlying CML. In CML, the breakpoint within BCR is almost exclusively in the major breakpoint cluster region (Mbcr), which creates a 210-kD fusion protein (p210). In contrast, in up to 77% of cases of B-ALL with BCR-ABL1, the breakpoint is within the minor breakpoint cluster region (mbcr), forming a smaller 190-kD fusion protein (p190).2,30 Thus, the presence of a p210 fusion protein in a patient with B-lymphoblasts increases the possibility of, but is not diagnostic for, an underlying CML. The p190 transcript is only rarely seen in CML (1%–2% of patients). Chronic myeloid leukemia with the p190 isoform often has relatively increased monocytes (>3%), as compared with CML with the p210 isoform, and can mimic chronic myelomonocytic leukemia.
Fluorescence in situ hybridization studies for BCR/ABL1 can also be helpful in ascertaining whether lymphoblasts of the B-cell lineage represent CML-LBP or de novo B-LL. Specifically, if the percentage of cells that are positive for BCR/ABL1 via FISH is significantly greater than the lymphoblast percentage, then it strongly suggests an underlying CML, as this finding implies that the background myeloid cells also carry the translocation. A few groups have advocated using FISH to evaluate neutrophils for the BCR/ABL1 rearrangement in order to differentiate between CML-LBP and de novo B-LL with t(9;22), because neutrophils would be negative for the translocation in de novo B-LL, but would carry the translocation in CML-LBP. However, this is not routinely done. While CML-BP and de novo B-LL do show some characteristic cytogenetic abnormalities, the karyotype is typically not helpful for distinguishing between the entities. Some studies have shown that B-LL with BCR-ABL1 is associated with a greater number of additional chromosomal changes when compared with CML in LBP.2,17 Additionally, gains in chromosome 9 are associated with B-LL with BCR-ABL1, whereas deletions in chromosome 9p are more commonly seen in CML in LBP; however, neither of these changes can be used to definitively distinguish between CML-LBP and B-LL.27
Treatment for CML in B-lymphoid BP is effectively the same as de novo Philadelphia chromosome–positive B-LL in adults. It is recommended that patients with CML-BP receive TKI therapy combined with chemotherapy, which for LBP typically includes vincristine- and prednisone-based protocols.1,5,10,18,31,32 Second-generation TKIs, such as dasatinib, have been proven to have superior blood-brain barrier penetration compared with imatinib and may be the favored therapeutic agent in patients with central nervous system disease.10,33 If CML evolves to BP despite imatinib therapy, then a second-generation TKI is recommended.10 Additionally, dasatinib or nilotinib is preferred to imatinib in cases of resistance-conferring mutations.10,34 Although TKI combination therapy is effective for short-term treatment of CML-BP, hematopoietic stem cell transplant (HSCT) is regarded as the only potential cure for this disease.2,3,5,6,10,18,30,34 While in adult patients the distinction between B-lymphoid CML-BP and de novo B-LL with t(9;22) does not affect the treatment paradigm, in children this distinction has significant clinical ramifications. Pediatric patients with B-lymphoid CML-BP are typically offered HSCT as consolidation after successful induction of remission of BP. In contrast, pediatric patients with B-ALL with t(9;22) are not routinely offered HSCT in first remission, particularly if they are able to achieve negativity for minimal residual disease.35,36
Without proper treatment, CML-BP is fatal, with a median survival of only 7 to 11 months.10 However, with TKI therapy, survival is greatly improved.7,10 Factors that improve rates of overall survival include younger age at diagnosis, rapid treatment of BP, and fewer additional chromosomal abnormalities.25 Interestingly, when compared with MBP, LBP has a better response to therapy and longer overall survival.3,18
CONCLUSIONS
Here, we present a patient whose initial presentation of CML was in B-lymphoid BP (CML-LBP). The morphologic findings in the peripheral blood and marrow suggested an underlying CML, and the discordance between the percentage of cells in the marrow that carried BCR-ABL1 (93%) versus the percentage of blasts in the marrow (approximately 25%) also favored a diagnosis of CML in LBP. Interestingly, this case carried a p190 BCR/ ABL1 transcript, which is more frequently associated with de novo B-LL, but can rarely be seen in CML. Thus, while the presence of the p210 transcript may be helpful in making the distinction between CML-LBP and B-LL, the length of the transcript cannot be used by itself to distinguish between these entities. Despite the patient's lack of a known CML-CP as well as the presence of the p190 transcript, a diagnosis of CML was favored over de novo B-LL because of the morphologic and cytogenetic findings. The patient received therapy appropriate for CML-BP, but refused a potentially curative bone marrow transplant. This case underscores the importance of bone marrow findings in patients presenting with lymphoblastic leukemia and also emphasizes the potential difficulties in differentiating CML-LBP from de novo B-LL.
Acknowledgments
This work was supported in part by the Sidney Kimmel Comprehensive Cancer Center grant from the National Institutes of Health (P30 CA006973; J.A.W., B.D.S., A.S.D.).
Footnotes
The authors have no conflicts to declare.
REFERENCES
- 1.Al-Khallaf H, Alali H, Alkhatti A. Precursor B cell lymphoid blast crisis of chronic myeloid leukemia with novel chromosomal abnormalities: a case report. Oncol Lett 2018;16:6691–6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balducci E, Loosveld M, Rahal I, et al. Interphase FISH for BCR-ABL1 rearrangement on neutrophils: a decisive tool to discriminate a lymphoid blast crisis of chronic myeloid leukemia from a de novo BCR-ABL1 positive acute lymphoblastic leukemia. Hematol Oncol 2018;36:344–348. [DOI] [PubMed] [Google Scholar]
- 3.Chen Z, Cortes JE, Jorgensen JL, et al. Differential impact of additional chromosomal abnormalities in myeloid vs lymphoid blast phase of chronic myelogenous leukemia in the era of tyrosine kinase inhibitor therapy. Leukemia 2016;30:1606–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faderl S, Talpaz M, Estrov Z, et al. The biology ofchronic myeloid leukemia. N Engl JMed 1999;341:164–172. [DOI] [PubMed] [Google Scholar]
- 5.Hossain A, Gupta K, Mener A, et al. Case of CML lymphoid blast crisis presenting as bilateral breast masses. BMJ Case Rep 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2018 update on diagnosis, therapy and monitoring. Am J Hematol 2018;93:442–459. [DOI] [PubMed] [Google Scholar]
- 7.Swerdlow S, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues 8, Lyon, France: International Agency for Research on Cancer; 2017. [Google Scholar]
- 8.Jabbour EJ, Hughes TP, Cortés JE, et al. Potential mechanisms of disease progression and management of advanced-phase chronic myeloid leukemia. Leuk Lymphoma 2014;55:1451–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iyer P, Carey P, Bown N, et al. Pediatric chronic myeloid leukemia with B-cell lymphoid blast crisis at presentation. Blood Res 2013;48:151–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hehlmann R How I treat CML blast crisis. Blood 2012;120:737–747. [DOI] [PubMed] [Google Scholar]
- 11.Cortes JE, Talpaz M, O'Brien S, et al. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer 2006;106:1306–1315. [DOI] [PubMed] [Google Scholar]
- 12.Askmyr M, Ågerstam H, Lilljebjörn H, et al. Modeling chronic myeloid leukemia in immunodeficient mice reveals expansion of aberrant mast cells and accumulation of pre-B cells. Blood Cancer J 2014;4:e269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baccarani M, Saglio G, Goldman J, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood 2006;108: 1809–1820. [DOI] [PubMed] [Google Scholar]
- 14.Kalmanti L, Saussele S, Lauseker M, et al. Safety and efficacy of imatinib in CML over a period of 10 years: data from the randomized CML-Study IV. Leukemia 2015;29:1123–1132. [DOI] [PubMed] [Google Scholar]
- 15.Palandri F, Castagnetti F, Testoni N, et al. Chronic myeloid leukemia in blast crisis treated with imatinib 600 mg: outcome of the patients alive after a 6-year follow-up. Haematologica 2008;93:1792–1796. [DOI] [PubMed] [Google Scholar]
- 16.Kantarjian HM, Keating MJ, Talpaz M, et al. Chronic myelogenous leukemia in blast crisis. Analysis of 242 patients. Am J Med 1987;83:445–54. [DOI] [PubMed] [Google Scholar]
- 17.Bacher U, Haferlach T, Hiddemann W, et al. Additional clonal abnormalities in Philadelphia-positive ALL and CML demonstrate a different cytogenetic pattern at diagnosis and follow different pathways at progression. Cancer Genet Cytogenet 2005;157:53–61. [DOI] [PubMed] [Google Scholar]
- 18.Derderian PM, Kantarjian HM, Talpaz M, et al. Chronic myelogenous leukemia in the lymphoid blastic phase: characteristics, treatment response, and prognosis. Am J Med 1993;94:69–74. [PubMed] [Google Scholar]
- 19.Greulich-Bode KM, Heinze B. On the power of additional and complex chromosomal aberrations in CML. Curr Genom 2012;13:471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radich JP. The biology of CML blast crisis. Hematol Am Soc Hematol Educ Program 2007;3 84–391. [DOI] [PubMed] [Google Scholar]
- 21.Radich JP, Dai H, Mao M, et al. Gene expression changes associated with progression and response in chronic myeloid leukemia. Proc Natl Acad Sci U S A 2006;103:2794–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaiger A, Henn T, Hörth E, et al. Increase of BCR-ABL chimeric mRNA expression in tumor cells of patients with chronic myeloid leukemia precedes disease progression. Blood 1995;86:2371–2378. [PubMed] [Google Scholar]
- 23.Khalidi HS, Brynes RK, Medeiros LJ, et al. The immunophenotype of blast transformation of chronic myelogenous leukemia: a high frequency of mixed lineage phenotype in “lymphoid” blasts and a comparison of morphologic, immunophenotypic, and molecular findings. Mod Pathol 1998;11:1211–1221. [PubMed] [Google Scholar]
- 24.Padhi P, Topalovski M, El Behery R, et al. A rare case of chronic myelogenous leukemia presenting as T-cell lymphoblastic crisis. Case Rep Oncol Med 2018;2018:7276128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sureda A, Carrasco M, de Miguel M, et al. Imatinib mesylate as treatment for blastic transformation of Philadelphia chromosome positive chronic myelogenous leukemia. Haematologica 2003;88:1213–1220. [PubMed] [Google Scholar]
- 26.Soma L, Oehler VG, Ding C, et al. Small, abnormal B lymphoid blast populations in chronic myelogenous leukemia at diagnosis: does this finding indicate an accelerated course? Cytometry B Clin Cytom 2016;90: 440–448. [DOI] [PubMed] [Google Scholar]
- 27.Grace C, Nacheva EP. Significance analysis of microarrays (SAM) offers clues to differences between the genomes of adult Philadelphia positive ALL and the lymphoid blast transformation of CML. Cancer Inform 2012;11:173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sill H, Goldman JM, Cross NC. Homozygous deletions of the p16 tumor-suppressor gene are associated with lymphoid transformation of chronic myeloid leukemia. Blood 1995;85:2013–2016. [PubMed] [Google Scholar]
- 29.Serra A, Gottardi E, Della Ragione F, et al. Involvement of the cyclin-dependent kinase-4 inhibitor (CDKN2) gene in the pathogenesis of lymphoid blast crisis of chronic myelogenous leukaemia. Br J Haematol 1995;91:625–629. [DOI] [PubMed] [Google Scholar]
- 30.Kolenova A, Maloney KW, Hunger SP. Philadelphia chromosome–positive acute lymphoblastic leukemia or chronic myeloid leukemia in lymphoid blast crisis. J Pediatr Hematol Oncol 2016;38:e193–e195. [DOI] [PubMed] [Google Scholar]
- 31.Rea D, Legros L, Raffoux E, et al. High-dose imatinib mesylate combined with vincristine and dexamethasone (DIV regimen) as induction therapy in patients with resistant Philadelphia-positive acute lymphoblastic leukemia and lymphoid blast crisis of chronic myeloid leukemia. Leukemia 2006;20: 400–403. [DOI] [PubMed] [Google Scholar]
- 32.Saußele S, Silver RT. Management of chronic myeloid leukemia in blast crisis. Ann Hematol 2015;94(Suppl 2):S159–S165. [DOI] [PubMed] [Google Scholar]
- 33.Porkka K, Koskenvesa P, Lundán T, et al. Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome–positive leukemia. Blood 2008;112:1005–1012. [DOI] [PubMed] [Google Scholar]
- 34.Cortes J, Rousselot P, Kim DW, et al. Dasatinib induces complete hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in blast crisis. Blood 2007;109: 3207–3213. [DOI] [PubMed] [Google Scholar]
- 35.Biondi A, GandemerV, de Lorenzo P, et al. Imatinib treatment of paediatric Philadelphia chromosome–positive acute lymphoblastic leukaemia (EsPhALL2010): a prospective, intergroup, open-label, single-arm clinical trial. Lancet Haematol 2018;5:e641–e652. [DOI] [PubMed] [Google Scholar]
- 36.Schultz KR, Carroll A, Heerema NA, et al. Long-term follow-up of imatinib in pediatric Philadelphia chromosome–positive acute lymphoblastic leukemia: Children's Oncology Group study AALL0031. Leukemia 2014;28:1467–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]