Abstract
Liver injury and acute liver failure caused by acetaminophen (APAP) overdose is the clinically most important drug toxicity in western countries. Mechanistic investigations have revealed a central role of mitochondria in the pathophysiology. Excess formation of the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI) after an overdose leads to hepatic glutathione depletion, mitochondrial protein adducts formation and an initial oxidant stress, which triggers the activation of mitogen activated protein (MAP) kinase cascade ultimately leading to c-jun N-terminal kinase (JNK) phosphorylation. Phospho-JNK translocates to the mitochondria and amplifies the oxidative and nitrosative stress eventually causing the mitochondrial membrane permeability transition pore opening and cessation of ATP synthesis. In addition, mitochondrial matrix swelling ruptures the outer membrane and releases endonucleases, which cause nuclear DNA fragmentation. Together, the nuclear DNA damage and the extensive mitochondrial dysfunction result in necrotic cell death. However, the pro-cell death signaling events are counteracted by adaptive responses such as autophagy and mitochondrial biogenesis. The improved mechanistic insight into the pathophysiology leads to better understanding of the mechanisms of action of the existing antidote N-acetylcysteine and justifies the clinical testing of novel therapeutics such as 4-methylpyrazole and calmangafodipir.
Keywords: Acetaminophen hepatotoxicity, mitochondrial dysfunction, biogenesis, oxidant stress, peroxynitrite, c-jun N-terminal kinase
1. Introduction
Acetaminophen (APAP) is one of the most widely used analgesic and anti-pyretic drugs. At therapeutically recommended doses, APAP is generally considered a safe drug,1,2 even in high risk populations such as alcoholics3 or patients with liver disease.4 More than 90% of a therapeutic dose is directly glucuronidated or sulfated and rapidly excreted.5 Only a small fraction is metabolized by cytochrome P450 enzymes, especially Cyp2E, to form the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI), which is effectively detoxified by hepatocellular glutathione (GSH); the GSH conjugate is then excreted into bile or blood.6 The effectiveness of the protective system is documented by the very low levels of protein adducts formed after therapeutic doses in both humans and animals.7–9 However, due to the availability of APAP in numerous prescription and over-the-counter medications, intentional and unintentional overdosing is a significant clinical problem.10 APAP overdose can induce severe liver injury and even acute liver failure. In fact, APAP overdose is the most common cause of acute liver failure in most western countries.11 After an overdose, the phase II conjugation reactions are either saturated (sulfation) or, despite a dramatic increase, the glucuronidation cannot keep up with the high levels of APAP.12 This leads to enhanced NAPQI formation, which causes depletion of the cellular GSH stores and a dramatic increase in hepatic protein adducts.5 In the 1970s, when GSH depletion and protein adduct formation in response to APAP overdose in mice was first discovered, it was assumed that this is the main mechanism of cell death.13,14 However, over the years it became obvious that the early protein adduct formation is just an initiating event, which triggers complex cell death signaling pathways and adaptive reactions centered around mitochondria. The critical role of mitochondria in the pathophysiology will be discussed in this review.
2. Mitochondrial Dysfunction and Damage after APAP Overdose
2.1. Acetaminophen-induced generation of mitochondrial protein adducts
Although mitochondrial protein adducts after APAP overdose in mice were described during the early studies by Mitchell and coworkers,13 a functional relevance was not recognized. However, later studies by Sid Nelson’s group comparing protein adducts and injury in mice after treatment with APAP (N-acetyl-para-aminophenol) versus its regional isomer AMAP (N-acetyl-meta-aminophenol) suggested significant differences between the two compounds. Although AMAP formed reactive metabolites and overall protein adducts in the liver similar to APAP,15 AMAP in contrast to APAP did not form adducts in mitochondria and did not cause liver injury.16,17 These observations suggested that mitochondrial protein adducts are relevant for the injury process. In addition, increased oxidant stress inside liver mitochondria in APAP- but not AMAP-treated animals implicated the mitochondrial protein adducts in the generation of a mitochondrial oxidant stress during APAP hepatotoxicity.18 More recent studies demonstrated that AMAP can cause cell injury in human liver slices19 and in primary human hepatocytes.20 This susceptibility of human cells to AMAP compared to mouse hepatocytes correlated with the formation of mitochondrial protein adducts.20 Furthermore, comparison between APAP treatment of rats and mice showed delayed formation and significantly less protein adducts in rat liver mitochondria, which may explain the lower susceptibility of rats to an APAP overdose.21 Together, these observations support the hypothesis that not the overall protein adduct levels in the cell but rather the protein adducts on the mitochondria are the most critical initiating events in the pathophysiology of APAP-induced cell death (Figure 1).
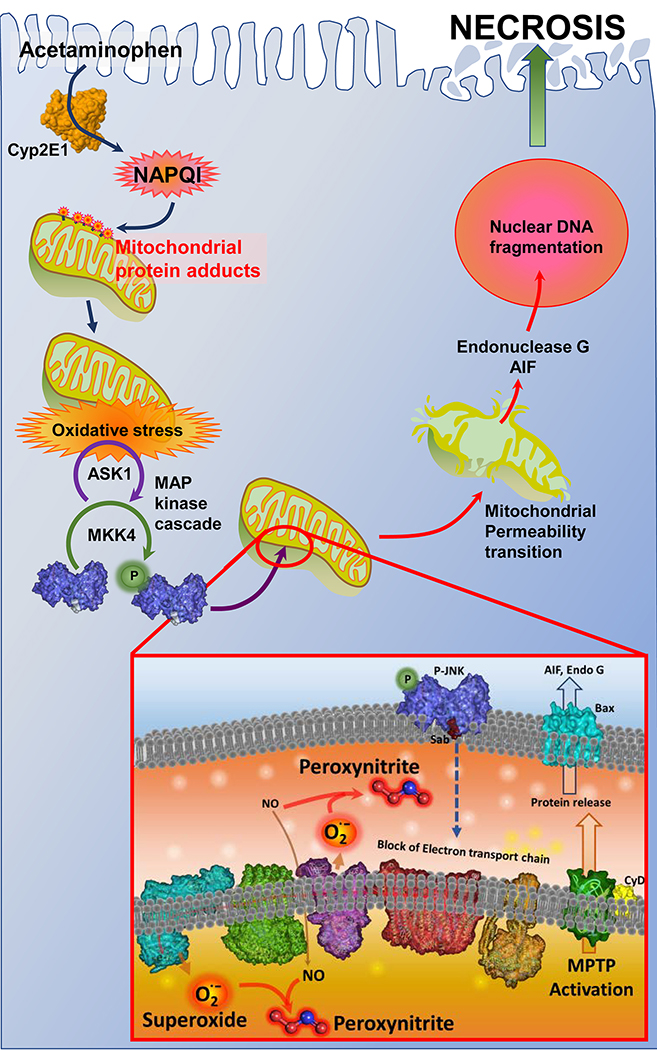
Figure 1: Mitochondria are an important target of the reactive metabolite of APAP.
Metabolism of APAP by Cytochrome P450 2E1 (Cyp2E1) results in formation of NAPQI, which forms protein adducts on mitochondria and induces mitochondrial oxidant stress. This initiates a MAP kinase cascade, which ultimately results in phosphorylation and activation of JNK. JNK translocation to the outer mitochondrial membrane and binding to Sab then triggers inhibition of the mitochondrial respiratory chain. The subsequent reaction of mitochondrial derived superoxide with nitric oxide within mitochondria forms the reactive peroxynitrite, which ultimately induces the mitochondrial permeability transition with release of mitochondrial proteins such as endonuclease G and apoptosis inducing factor (AIF). These then translocate to the nucleus and induce DNA fragmentation and ultimately hepatocyte necrosis (Adapted from reference93).
2.2. Mitochondrial oxidant stress-induced MAP kinase activation
The initial discovery of a mitochondrial oxidant stress was based on elevated glutathione disulfide (GSSG) levels inside mitochondria and the absence of relevant GSSG levels in the cytosol or GSSG excreted into bile.18,22 It is known that GSSG formed inside mitochondria can only be reduced to GSH but cannot be exported into the cytosol.23 This suggested a selective formation of reactive oxygen in the matrix of mitochondria, which was later confirmed by the use of MitoSOX Red, a mitochondrial superoxide indicator.24 In addition, the formation of peroxynitrite, a reaction product of superoxide and nitric oxide, was also located predominantly inside mitochondria.25 Interestingly, other cellular sources of reactive oxygen such as cytochrome P450, as hypothesized earlier,26 were excluded based on the fact that neither GSSG formation nor 2’,7’-dichlorodihydrofluorescein diacetate fluorescence increased during the drug metabolism phase.27,28 Despite the convincing evidence of a mitochondrial oxidant stress and peroxynitrite formation during APAP hepatotoxicity,18,22,24,25 the oxidant and nitrosative stress appeared to be a late event that correlated closely with cell injury and not with the early mitochondrial protein adduct formation.8
The evidence for an early oxidant stress emerged after it was recognized that the mitogen activated protein (MAP) kinase c-jun N-terminal kinase (JNK) is involved in APAP-induced liver injury29 and the JNK inhibitor SP600125 prevented the APAP-induced oxidant stress (Figure 1).30 An upstream mediator of this JNK activation seems to be the redox-sensitive apoptosis signal-regulating kinase 1 (ASK1), a member of MAP kinase kinase kinase (MAP3K) family, which exists in the cytosol in a complex with thioredoxin and can be liberated and activated by oxidation of thioredoxin.31 Deficiency of ASK-1 and inhibitors of ASK-1 were shown to attenuate JNK activation and reduce APAP-induced liver injury.32,33 Other upstream redox-sensitive MAP3K involved in the process include mixed-lineage kinase 3 (MLK3)34 and glycogen synthase kinase-3beta (GSK-3β)35 all of which inhibit JNK activation and reduce injury. These upstream kinases do not directly interact with JNK but act through the MAP2K MKK4, which then phosphorylates JNK (Figure 1).36 JNK activation is counteracted by mitogen-activated protein kinase phosphatase (Mkp)-1, which inhibits APAP-induced liver injury.37 Together these data suggest that a mitochondrial protein adduct-induced mild initial oxidant stress, which is not readily detected by even sensitive oxidant stress markers, activates redox-sensitive MAP3 kinases and triggers a kinase cascade resulting in the extensive activation of JNK. Importantly, this initial oxidant stress is insufficient to cause direct damage to mitochondria or trigger the mitochondrial membrane permeability transition (MPT).
2.3. Mitochondrial JNK translocation and amplification of the mitochondrial oxidant stress
After the importance of JNK activation in APAP toxicity was recognized,29,38,39 it became clear that JNK seems to regulate the main mitochondrial oxidant/nitrosative stress.30 However, the mechanism by which JNK might amplify the initial oxidant stress remained unclear.40 Follow-up studies from Neil Kaplowitz’s laboratory shed light on this mystery. First, it was established that activated JNK (phospho-JNK) translocates from the cytosol to the mitochondria41 and binds to the anchor protein Sab on the outer mitochondrial membrane.42 The mitochondrial JNK translocation is responsible for the oxidative/nitrosative stress and prolonged JNK activation.42 The mechanism by which P-JNK binding and phosphorylation of Sab enhances the superoxide formation involves the release of SHP1 (protein tyrosine phosphatase, nonreceptor type 6, PTPN6) from Sab on the inside of the mitochondrial outer membrane (Figure 1). This triggers its activation and transfer of P-SHP1 to the inner membrane, where it dephosphorylates P-Src (active).43 This process requires docking protein 4 (DOK4) on the inner membrane. Src (inactive) further inhibits the electron transport chain and promotes release of superoxide, which gives rise to an amplified oxidative/nitrosative stress that is responsible for further JNK activation and cell death.43
Since the first reports of a mitochondrial oxidant stress during APAP hepatotoxicity18,22 it was demonstrated that the reactive oxygen did not trigger extensive lipid peroxidation and loading the liver with vitamin E did not protect.44 In contrast, superoxide derived from the electron transport chain reacts with nitric oxide to form the potent oxidant peroxynitrite,45 which was recognized as the actual oxidant responsible for the injury (Figure 1).46 The mitochondrial superoxide dismutase (SOD2, MnSOD), which accelerates the dismutation of superoxide to hydrogen peroxide and oxygen, is actually a protective factor as it limits peroxynitrite formation and promotes the detoxification of hydrogen peroxide by glutathione peroxidase.47 Thus, partial deficiency of MnSOD enhanced peroxynitrite formation and aggravated APAP-induced liver injury.48,49 Unfortunately, during APAP toxicity, MnSOD is in part inactivated by peroxynitrite50 and hepatic GSH, a direct scavenger of peroxynitrite, is severely depleted.46 Thus, the accelerated recovery of hepatic GSH levels after treatment with antidotes like GSH or N-acetylcysteine restores mitochondrial GSH levels, which scavenge peroxynitrite and protect against APAP-induced liver injury.46,51–53 In addition, Mito-TEMPO, a mitochondrial-targeted SOD mimetic, eliminated nitro-tyrosine staining and effectively protected against APAP toxicity.54,55 Furthermore, it was shown that APAP causes a block of complex II of the electron transport chain (ETC), which triggers the oxidant stress and causes ATP depletion.56 Methylene blue, a redox-active compound that can accumulate in mitochondria, was able to accept electrons from NAPQI-altered, succinate-energized complex II and transfer them to cytochrome c, thereby overcoming the block of the ETC.56 Thus, methylene blue effectively prevented APAP hepatotoxicity without affecting metabolic activation.56 In addition to the oxidant stress, lysosome-derived iron is taken up into mitochondria through the mitochondrial electrogenic Ca2+, Fe2+ uniporter during APAP-induced cell death.57,58. Chelation of lysosomal iron and inhibition of mitochondrial iron uptake prevented mitochondrial dysfunction and cell death.58 Taken together, there is extensive evidence for a critical role of a mitochondrial oxidant stress being responsible for mitochondrial dysfunction leading to the MPT pore opening, breakdown of the membrane potential and cessation of ATP synthesis.59,60 These events also lead to mitochondrial matrix swelling with rupture of the outer membrane, release of intermembrane proteins and nuclear translocation of endonuclease G and apoptosis-inducing factor (AIF), which cause nuclear DNA fragmentation as indicated by the TUNEL assay61–63 and nuclear DNA fragments of various sizes (Figure 1).64 Other intermembrane proteins released from mitochondria into the cytosol include cytochrome c and second mitochondria-derived activator of caspase (Smac)62,65,66 but this does not trigger apoptotic cell death.67
2.4. New therapeutic options for APAP toxicity
The only clinically approved antidote against APAP overdose is N-acetylcysteine (NAC), which was discovered based on the initial mechanistic insight of reactive metabolite formation, GSH depletion and protein adduct formation.13,14 When treated very early after the overdose, NAC protects mainly through scavenging of NAPQI and preventing protein adduct formation.68 However, NAC does not react directly with NAPQI but acts through promoting hepatic GSH synthesis.69 At later time points, the newly synthesized mitochondrial GSH scavenges peroxynitrite and assists in detoxification of hydrogen peroxide by glutathione peroxidase.46,51–53 In addition, any excess NAC not used for GSH synthesis will be converted to Krebs cycle intermediates, which support the mitochondrial bioenergetics and enhance ATP levels.53 Thus, NAC has multiple modes of action over a significant therapeutic window. As a result, it was shown that NAC is most effective in human overdose patients during the first 8 h after APAP but is still partially effective up to 24 h.70,71
Despite the effectiveness of NAC, there are some side-effects including anaphylactic reactions, that limit the dose that can be given to a patient. Therefore, additional antidotes with complementary modes of action are desirable. The clinically approved antidote for methanol and polyethylene glycol poisoning 4-methylpyrazole (4MP) is a potential candidate.72 Recent preclinical studies showed that 4MP co-treatment with APAP eliminated the oxidative metabolism through inhibition of P450 enzymes and completely prevented the injury in a murine model of APAP hepatotoxicity.73 Interestingly, a delayed treatment, which did not affect oxidative metabolism and protein adduct formation, was also highly effective.74 These studies demonstrated that 4MP can also inhibit the activation and mitochondrial translocation of JNK.74 Furthermore, studies in human volunteers indicated that 4MP co-treatment with supratherapeutic doses of APAP effectively prevented any oxidative metabolism of APAP but had no adverse effects in humans.75 Thus, 4MP can have complementary mechanisms of action compared to NAC. However, in contrast to NAC, 4MP acts directly, i.e. does not need to be metabolized to be effective and does not appear to have any relevant side-effects. A co-treatment with NAC and 4MP may be implicated in patients with extremely high overdoses of APAP.72
Another antidote which is currently in clinical development is the mitochondrial SOD mimetic calmangafodipir.76 As discussed above, SOD mimetics targeted to mitochondria are highly effective in preventing peroxynitrite formation and therefore are protective in the mouse APAP toxicity model.54,55 Calmangafodipir has recently been tested in APAP overdose patients.77 Although these were early presenting patients receiving the standard of care NAC and therefore were at low risk of developing liver injury, treatment with calmangafodipir proved to be safe and without adverse effect.77 In addition, using highly sensitive injury parameters, e.g. cytokeratin-18, showed a trend to less liver injury.77 Thus, if efficacy in high overdose patients can be established, calmangafodipir may be a promising complementary antidote to NAC.
3. The role of mitochondrial biogenesis in APAP Hepatotoxicity
3.1. Mitochondrial biogenesis in cellular homeostasis
Mitochondrial biogenesis is a fundamental process in cell biology which is critical for normal cellular regeneration and homeostasis. It is essential for recovery of cellular function by facilitating controlled regeneration of mitochondria so that critical cellular processes such as respiration and metabolism can be maintained. Mitochondrial biogenesis is a complex coordinated process which ensures that new protein synthesis in the mitochondria is coupled to that from the nucleus and acts in concert with mitochondrial fission and fusion to ensure proper functioning of newly synthesized mitochondria (Figure 2). Since mitochondrial biogenesis is an energy intensive process requiring a large number of coordinated changes in gene expression and protein synthesis, it is a tightly controlled process which can be initiated by a number of stimuli indicating a shift in cellular homeostasis. These could include fasting, exercise and oxidative stress.78 Despite the large variety of initiating signals, however, they all converge at peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC1α), which is considered to be the central mediator for the process (Figure 2). PGC1α controls a number of downstream targets including nuclear respiratory factor (NRF) 1 & 2 and Transcription Factor A, Mitochondrial (Tfam)79 and while the complex transcriptional regulation of mitochondrial biogenesis is outside the scope of this review, it has been comprehensively examined.80 Among the downstream targets of PGC1α, the nuclear respiratory factors NRF-1 and NRF-2 were the first nuclear transcription factors implicated in induction of multiple facets of mitochondrial function.80 While NRF-1 acts on genes encoding respiratory subunits as well as Tfam, in addition to other genes whose products regulate mitochondrial transcription and ribosome assembly, NRF-2 was initially identified as a transcriptional upregulator of the cytochrome oxidase subunit IV (COXIV) promoter.80 Both factors cooperate in the expression of mitochondrial import proteins as well, and their coordinated control of mitochondrial related gene expression has been suggested to link expression of the respiratory chain complexes with the biogenesis of the organelle itself.80 It should also be noted that the nuclear respiratory factor NRF-2 is distinct from the nuclear factor erythroid 2-related factor 2, which is also often abbreviated as Nrf2, and regulates the expression of antioxidant proteins. Tfam is a transcription factor which plays a central role in maintenance of the mitochondrial genome and biogenesis. Tfam was the first mammalian protein demonstrated to regulate mtDNA copy number in vivo and is essential for mitochondrial biogenesis and embryonic development.81 Tfam coats the mitochondrial genome and compacts it by inducing U turns on the DNA molecule82 and interactions between Tfam and mtDNA participate in regulation of mitochondrial biogenesis.83 The essential role of Tfam in mitochondrial maintenance is illustrated by the fact that whole body disruption of Tfam is embryonically lethal,81 and animals with tissue specific knockout of Tfam in the heart and muscle are viable but develop a mosaic cardiac-specific progressive respiratory chain deficiency and functional deficits.84
Figure 2: Mitochondrial biogenesis as an adaptive response to APAP-induced injury.
APAP overdose induces a mitochondrial oxidant stress, which results in the amplification of mitochondrial defects to ultimately induce hepatocyte necrosis around the central vein. In surviving cells around this area of necrosis, the mitochondrial dysfunction induces upregulation of PGC1α, the central regulator of mitochondrial biogenesis. This in turn enhances levels of important transcription factors such as NRF-1, NRF-2 and Tfam, which promote mitochondrial biogenesis to compensate for APAP induced mitochondrial defects and facilitate regeneration and recovery.
3.2. Mitochondrial biogenesis in recovery after APAP-induced liver injury
As discussed in the initial sections, mitochondria are critical organelles involved in APAP induced liver injury, though it is now becoming evident that mitochondrial biogenesis plays an important role in liver recovery and regeneration after an APAP overdose. While mitochondrial biogenesis measured by amounts of mtDNA and proteins increased significantly in HepG2 cells exposed to low, non-cytotoxic concentrations of APAP, accompanied by upregulated expression of PGC-1α, NRF-1 and TFAM,85 these studies have important issues. The caveat with this study in HepG2 cells is that since APAP-induced liver injury requires metabolic activation to a reactive metabolite, the lack of metabolic competency of HepG2 cells deviates from the mechanism of injury in mice and humans and thus HepG2 cells are not a relevant model of APAP hepatotoxicity.86 More relevant in vivo studies in the mouse clearly demonstrated that APAP overdose triggers unique zonated changes in the mouse liver, with necrosis around the central vein (zone 1), mitochondrial spheroid formation beyond that (zone 2), followed by autophagy (zone 3) and mitochondrial biogenesis in cells farthest from the central vein (zone 4).87 Our earlier study in the mouse model demonstrated that both electron transport chain activity as well as mtDNA content, which decrease during the APAP-induced injury phase, begin to recover at later time points (12 h after a 300 mg/kg dose of APAP), suggesting that mitochondrial biogenesis occurs at late time points after APAP overdose (Figure 2).88 Immunofluorescence staining for the mitochondrial outer membrane protein Tom20 in liver sections at various time points demonstrated that elevation in mitochondrial mass occurs selectively in hepatocytes surrounding necrotic areas.88 This spatially selective increase in mitochondrial abundance was accompanied by induction of mitochondrial biogenesis signaling mediators including PGC1α and NRF-1, with PGC1α upregulation selectively in hepatocytes surrounding necrotic areas.88 The importance of mitochondrial biogenesis for liver recovery after APAP induced injury was further illustrated by the enhanced recovery and regeneration after post-treatment with the known mitochondrial biogenesis inducer SRT1720.88 Other interventions such as diphenyl diselenide treatment was also shown to enhance levels of PGC-1α and help to restore NRF-1 levels associated with mitochondrial biogenesis in the context of APAP-induced liver injury.89 One of the signaling mediators implicated in upregulation of mitochondrial biogenesis is fibroblast growth factor 21 (FGF21), which is a hepatocyte-secreted hormone with pleiotropic effects on glucose and lipid metabolism upregulated in response to APAP overdose.90 FGF21 was shown to induce hepatic expression of PGC-1α, thereby increasing the nuclear abundance of NRF-2 and FGF21 knockout mice showed aggravated liver damage after APAP overdose.90 In addition to FGF21, sestrin 2, a stress inducible protein, was also shown to be a regulator of PGC1α that confers survival and facilitates recovery of liver cancer cells from APAP‐induced mitochondrial damage under glucose‐starved conditions.91 These data clearly indicate that induction of mitochondrial biogenesis in the unique population of surviving cells around the necrotic area play a critical role in liver recovery and regeneration after acute APAP-induced liver injury (Figure 2).
4. Summary and Conclusions
Oxidative metabolism of APAP forms the reactive metabolite NAPQI, which depletes GSH and binds to cellular proteins. Mitochondrial dysfunction starts with early protein adducts on mitochondria triggering a mild oxidant stress, which activates a MAP kinase signaling cascade ultimately phosphorylating JNK. Phospho-JNK translocates to the mitochondria and amplifies the oxidative and nitrosative stress triggering the MPT pore opening. Mitochondrial dysfunction is also responsible for the release of mitochondrial intermembrane endonucleases, which cause nuclear DNA fragmentation. Hence, mitochondrial dysfunction and damage is central to the pathophysiology of APAP-induced cell death. However, these events are counteracted by removal of damaged mitochondria and protein adducts by autophagy (reviewed recently92) and replacement of these mitochondria by biogenesis. Thus, the ultimate outcome of cell death or survival is determined by the extent of pro-death signaling and the opposing effects of autophagy and biogenesis. This new mechanistic insight explains the protective effect of the existing antidote N-acetylcysteine and provides the rationale for the testing of novel therapeutics focusing on mitochondrial dysfunction.
Acknowledgements
This work was supported by USA National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01 NIDDK102142 and R01 NIDDK 070195.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
REFERENCES
- 1.Temple AR, Lynch JM, Vena J, Auiler JF, Gelotte CK. Aminotransferase activities in healthy subjects receiving three-day dosing of 4, 6, or 8 grams per day of acetaminophen. Clin Toxicol (Phila). 2007;45(1):36–44. [DOI] [PubMed] [Google Scholar]
- 2.Heard K, Green JL, Anderson V, Bucher-Bartelson B, Dart RC. A randomized, placebo-controlled trial to determine the course of aminotransferase elevation during prolonged acetaminophen administration. BMC Pharmacol Toxicol. 2014;15:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuffner EK, Green JL, Bogdan GM, Knox PC, Palmer RB, Heard K, Slattery JT, Dart RC. The effect of acetaminophen (four grams a day for three consecutive days) on hepatic tests in alcoholic patients--a multicenter randomized study. BMC Med. 2007;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayward KL, Powell EE, Irvine KM, Martin JH. Can paracetamol (acetaminophen) be administered to patients with liver impairment? Br J Clin Pharmacol. 2016;81(2):210–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGill MR, Jaeschke H. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res. 2013;30(9):2174–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gelotte CK, Auiler JF, Lynch JM, Temple AR, Slattery JT. Disposition of acetaminophen at 4, 6, and 8 g/day for 3 days in healthy young adults. Clin Pharmacol Ther. 2007;81(6):840–8. [DOI] [PubMed] [Google Scholar]
- 7.Heard KJ, Green JL, James LP, Judge BS, Zolot L, Rhyee S, Dart RC. Acetaminophen-cysteine adducts during therapeutic dosing and following overdose. BMC Gastroenterol. 2011;11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGill MR, Lebofsky M, Norris HR, Slawson MH, Bajt ML, Xie Y, Williams CD, Wilkins DG, Rollins DE, Jaeschke H. Plasma and liver acetaminophen-protein adduct levels in mice after acetaminophen treatment: dose-response, mechanisms, and clinical implications. Toxicol Appl Pharmacol. 2013;269(3):240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heard K, Green JL, Anderson V, Bucher-Bartelson B, Dart RC. Paracetamol (acetaminophen) protein adduct concentrations during therapeutic dosing. Br J Clin Pharmacol. 2016;81(3):562–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher ES, Curry SC. Evaluation and treatment of acetaminophen toxicity. Adv Pharmacol 2019;85:263–272. [DOI] [PubMed] [Google Scholar]
- 11.Lee WM. Drug-induced acute liver failure. Clin Liver Dis. 2013;17(4):575–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie Y, McGill MR, Cook SF, Sharpe MR, Winefield RD, Wilkins DG, Rollins DE, Jaeschke H. Time course of acetaminophen-protein adducts and acetaminophen metabolites in circulation of overdose patients and in HepaRG cells. Xenobiotica. 2015;45(10):921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jollow DJ, Mitchell JR, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther. 1973;187(1):195–202. [PubMed] [Google Scholar]
- 14.Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973;187(1):211–7. [PubMed] [Google Scholar]
- 15.Rashed MS, Myers TG, Nelson SD. Hepatic protein arylation, glutathione depletion, and metabolite profiles of acetaminophen and a non-hepatotoxic regioisomer, 3’-hydroxyacetanilide, in the mouse. Drug Metab Dispos. 1990;18(5):765–70. [PubMed] [Google Scholar]
- 16.Tirmenstein MA, Nelson SD. Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3’-hydroxyacetanilide, in mouse liver. J Biol Chem. 1989;264(17):9814–9. [PubMed] [Google Scholar]
- 17.Qiu Y, Benet LZ, Burlingame AL. Identification of hepatic protein targets of the reactive metabolites of the non-hepatotoxic regioisomer of acetaminophen, 3’-hydroxyacetanilide, in the mouse in vivo using two-dimensional gel electrophoresis and mass spectrometry. Adv Exp Med Biol. 2001;500:663–73. [DOI] [PubMed] [Google Scholar]
- 18.Tirmenstein MA, Nelson SD. Acetaminophen-induced oxidation of protein thiols. Contribution of impaired thiol-metabolizing enzymes and the breakdown of adenine nucleotides. J Biol Chem. 1990;265(6):3059–65. [PubMed] [Google Scholar]
- 19.Hadi M, Dragovic S, van Swelm R, Herpers B, van de Water B, Russel FG, Commandeur JN, Groothuis GM. AMAP, the alleged non-toxic isomer of acetaminophen, is toxic in rat and human liver. Arch Toxicol. 2013;87(1):155–65. [DOI] [PubMed] [Google Scholar]
- 20.Xie Y, McGill MR, Du K, Dorko K, Kumer SC, Schmitt TM, Ding WX, Jaeschke H. Mitochondrial protein adducts formation and mitochondrial dysfunction during N-acetyl-m-aminophenol (AMAP)-induced hepatotoxicity in primary human hepatocytes. Toxicol Appl Pharmacol. 2015;289(2):213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGill MR, Williams CD, Xie Y, Ramachandran A, Jaeschke H. Acetaminophen-induced liver injury in rats and mice: comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicol Appl Pharmacol. 2012;264(3):387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaeschke H Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J Pharmacol Exp Ther. 1990;255(3):935–41. [PubMed] [Google Scholar]
- 23.Olafsdottir K, Reed DJ. Retention of oxidized glutathione by isolated rat liver mitochondria during hydroperoxide treatment. Biochim Biophys Acta. 1988;964(3):377–82. [DOI] [PubMed] [Google Scholar]
- 24.Yan HM, Ramachandran A, Bajt ML, Lemasters JJ, Jaeschke H. The oxygen tension modulates acetaminophen-induced mitochondrial oxidant stress and cell injury in cultured hepatocytes. Toxicol Sci. 2010;117(2):515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2005;315(2):879–87. [DOI] [PubMed] [Google Scholar]
- 26.Wendel A, Feuerstein S. Drug-induced lipid peroxidation in mice--I. Modulation by monooxygenase activity, glutathione and selenium status. Biochem Pharmacol. 1981;30(18):2513–20. [DOI] [PubMed] [Google Scholar]
- 27.Smith CV, Jaeschke H. Effect of acetaminophen on hepatic content and biliary efflux of glutathione disulfide in mice. Chem Biol Interact. 1989;70(3–4):241–8. [DOI] [PubMed] [Google Scholar]
- 28.Bajt ML, Knight TR, Lemasters JJ, Jaeschke H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol Sci. 2004;80(2):343–9. [DOI] [PubMed] [Google Scholar]
- 29.Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131(1):165–78. [DOI] [PubMed] [Google Scholar]
- 30.Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2010;246(1–2):8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, Sakamoto K, Ogura K, Noguchi T, Karin M, Ichijo H, Omata M. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology. 2008;135(4):1311–21. [DOI] [PubMed] [Google Scholar]
- 33.Xie Y, Ramachandran A, Breckenridge DG, Liles JT, Lebofsky M, Farhood A, Jaeschke H. Inhibitor of apoptosis signal-regulating kinase 1 protects against acetaminophen-induced liver injury. Toxicol Appl Pharmacol. 2015;286(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma M, Gadang V, Jaeschke A. Critical role for mixed-lineage kinase 3 in acetaminophen-induced hepatotoxicity. Mol Pharmacol. 2012;82(5):1001–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shinohara M, Ybanez MD, Win S, Than TA, Jain S, Gaarde WA, Han D, Kaplowitz N. Silencing glycogen synthase kinase-3beta inhibits acetaminophen hepatotoxicity and attenuates JNK activation and loss of glutamate cysteine ligase and myeloid cell leukemia sequence 1. J Biol Chem. 2010;285(11):8244–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J, Min RWM, Le K, Zhou S, Aghajan M, Than TA, Win S, Kaplowitz N. The role of MAP2 kinases and p38 kinase in acute murine liver injury models. Cell Death Dis. 2017;8(6):e2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wancket LM, Meng X, Rogers LK, Liu Y. Mitogen-activated protein kinase phosphatase (Mkp)-1 protects mice against acetaminophen-induced hepatic injury. Toxicol Pathol. 2012;40(8):1095–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henderson NC, Pollock KJ, Frew J, Mackinnon AC, Flavell RA, Davis RJ, Sethi T, Simpson KJ. Critical role of c-jun (NH2) terminal kinase in paracetamol- induced acute liver failure. Gut. 2007;56(7):982–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du K, Xie Y, McGill MR, Jaeschke H. Pathophysiological significance of c-jun N-terminal kinase in acetaminophen hepatotoxicity. Expert Opin Drug Metab Toxicol. 2015;11(11):1769–7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaplowitz N, Shinohara M, Liu ZX, Han D. How to protect against acetaminophen: don’t ask for JUNK. Gastroenterology. 2008;135(4):1047–51. [DOI] [PubMed] [Google Scholar]
- 41.Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283(20):13565–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Win S, Than TA, Han D, Petrovic LM, Kaplowitz N . c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J Biol Chem. 2011;286(40):35071–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Win S, Than TA, Min RW, Aghajan M, Kaplowitz N. c-Jun N-terminal kinase mediates mouse liver injury through a novel Sab (SH3BP5)-dependent pathway leading to inactivation of intramitochondrial Src. Hepatology. 2016;63(6):1987–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knight TR, Fariss MW, Farhood A, Jaeschke H. Role of lipid peroxidation as a mechanism of liver injury after acetaminophen overdose in mice. Toxicol Sci. 2003;76(1):229–36. [DOI] [PubMed] [Google Scholar]
- 45.Hinson JA, Pike SL, Pumford NR, Mayeux PR. Nitrotyrosine-protein adducts in hepatic centrilobular areas following toxic doses of acetaminophen in mice. Chem Res Toxicol. 1998;11(6):604–7. [DOI] [PubMed] [Google Scholar]
- 46.Knight TR, Ho YS, Farhood A, Jaeschke H. Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: protection by glutathione. J Pharmacol Exp Ther. 2002;303(2):468–75. [DOI] [PubMed] [Google Scholar]
- 47.Jaeschke H, and Ramachandran A: Antioxidant defense mechanisms In: McQueen CA, Comprehensive Toxicology, Third Edition. Vol. 2, pp. 277–295. Oxford: Elsevier Ltd, 2018. [Google Scholar]
- 48.Fujimoto K, Kumagai K, Ito K, Arakawa S, Ando Y, Oda S, Yamoto T, Manabe S. Sensitivity of liver injury in heterozygous Sod2 knockout mice treated with troglitazone or acetaminophen. Toxicol. Pathol 2009;37:193–200. [DOI] [PubMed] [Google Scholar]
- 49.Ramachandran A, Lebofsky M, Weinman SA, Jaeschke H. The impact of partial manganese superoxide dismutase (SOD2)-deficiency on mitochondrial oxidant stress, DNA fragmentation and liver injury during acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2011;251(3):226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agarwal R, MacMillan-Crow LA, Rafferty TM, Saba H, Roberts DW, Fifer EK, James LP, Hinson JA. Acetaminophen-induced hepatotoxicity in mice occurs with inhibition of activity and nitration of mitochondrial manganese superoxide dismutase. J Pharmacol Exp Ther. 2011;337(1):110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bajt ML, Knight TR, Farhood A, Jaeschke H. Scavenging peroxynitrite with glutathione promotes regeneration and enhances survival during acetaminophen-induced liver injury in mice. J Pharmacol Exp Ther. 2003;307(1):67–73. [DOI] [PubMed] [Google Scholar]
- 52.James LP, McCullough SS, Lamps LW, Hinson JA. Effect of N-acetylcysteine on acetaminophen toxicity in mice: relationship to reactive nitrogen and cytokine formation. Toxicol Sci. 2003;75(2):458–67. [DOI] [PubMed] [Google Scholar]
- 53.Saito C, Zwingmann C, Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology. 2010;51(1):246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Du K, Farhood A, Jaeschke H. Mitochondria-targeted antioxidant Mito-Tempo protects against acetaminophen hepatotoxicity. Arch Toxicol. 2017. February;91(2):761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Du K, Ramachandran A, Weemhoff JL, Woolbright BL, Jaeschke AH, Chao X, Ding WX, Jaeschke H. Mito-tempo protects against acute liver injury but induces limited secondary apoptosis during the late phase of acetaminophen hepatotoxicity. Arch Toxicol. 2019;93(1):163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee KK, Imaizumi N, Chamberland SR, Alder NN, Boelsterli UA. Targeting mitochondria with methylene blue protects mice against acetaminophen-induced liver injury. Hepatology. 2015;61(1):326–36. [DOI] [PubMed] [Google Scholar]
- 57.Hu J, Kholmukhamedov A, Lindsey CC, Beeson CC, Jaeschke H, Lemasters JJ. Translocation of iron from lysosomes to mitochondria during acetaminophen-induced hepatocellular injury: Protection by starch-desferal and minocycline. Free Radic Biol Med. 2016;97:418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kon K, Kim JS, Uchiyama A, Jaeschke H, Lemasters JJ. Lysosomal iron mobilization and induction of the mitochondrial permeability transition in acetaminophen-induced toxicity to mouse hepatocytes. Toxicol Sci. 2010;117(1):101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40(5):1170–9. [DOI] [PubMed] [Google Scholar]
- 60.Masubuchi Y, Suda C, Horie T. Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J Hepatol. 2005;42(1):110–6. [DOI] [PubMed] [Google Scholar]
- 61.Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol Sci. 2006;94(1):217–25. [DOI] [PubMed] [Google Scholar]
- 62.Bajt ML, Farhood A, Lemasters JJ, Jaeschke H. Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2008;324(1):8–14. [DOI] [PubMed] [Google Scholar]
- 63.Bajt ML, Ramachandran A, Yan HM, Lebofsky M, Farhood A, Lemasters JJ, Jaeschke H. Apoptosis-inducing factor modulates mitochondrial oxidant stress in acetaminophen hepatotoxicity. Toxicol Sci. 2011;122(2):598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jahr S, Hentze H, Englisch S, Hardt D, Fackelmayer FO, Hesch RD, Knippers R. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61(4):1659–65. [PubMed] [Google Scholar]
- 65.El-Hassan H, Anwar K, Macanas-Pirard P, Crabtree M, Chow SC, Johnson VL, Lee PC, Hinton RH, Price SC, Kass GE. Involvement of mitochondria in acetaminophen-induced apoptosis and hepatic injury: roles of cytochrome c, Bax, Bid, and caspases. Toxicol Appl Pharmacol. 2003;191(2):118–29. [DOI] [PubMed] [Google Scholar]
- 66.Adams ML, Pierce RH, Vail ME, White CC, Tonge RP, Kavanagh TJ, Fausto N, Nelson SD, Bruschi SA. Enhanced acetaminophen hepatotoxicity in transgenic mice overexpressing BCL-2. Mol Pharmacol. 2001;60(5):907–15. [DOI] [PubMed] [Google Scholar]
- 67.Jaeschke H, Duan L, Akakpo JY, Farhood A, Ramachandran A. The role of apoptosis in acetaminophen hepatotoxicity. Food Chem Toxicol. 2018;118:709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Corcoran GB, Racz WJ, Smith CV, Mitchell JR. Effects of N-acetylcysteine on acetaminophen covalent binding and hepatic necrosis in mice. J Pharmacol Exp Ther. 1985;232(3):864–72. [PubMed] [Google Scholar]
- 69.Corcoran GB, Wong BK. Role of glutathione in prevention of acetaminophen-induced hepatotoxicity by N-acetyl-L-cysteine in vivo: studies with N-acetyl-D-cysteine in mice. J Pharmacol Exp Ther. 1986;238(1):54–61. [PubMed] [Google Scholar]
- 70.Rumack BH, Bateman DN. Acetaminophen and acetylcysteine dose and duration: past, present and future. Clin Toxicol (Phila) 2012;50(2):91–8. [DOI] [PubMed] [Google Scholar]
- 71.Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N Engl J Med. 1988;319(24):1557–62. [DOI] [PubMed] [Google Scholar]
- 72.Yip L, Heard K. Potential adjunct treatment for high-risk acetaminophen overdose. Clin Toxicol (Phila). 2016;54(5):459. [DOI] [PubMed] [Google Scholar]
- 73.Akakpo JY, Ramachandran A, Kandel SE, Ni HM, Kumer SC, Rumack BH, Jaeschke H. 4-Methylpyrazole protects against acetaminophen hepatotoxicity in mice and in primary human hepatocytes. Hum Exp Toxicol. 2018;37(12):1310–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Akakpo JY, Ramachandran A, Duan L, Schaich MA, Jaeschke MW, Freudenthal BD, Ding WX, Rumack BH, Jaeschke H. Delayed Treatment With 4-Methylpyrazole Protects Against Acetaminophen Hepatotoxicity in Mice by Inhibition of c-Jun n-Terminal Kinase. Toxicol Sci. 2019;170(1):57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kang AM, Padilla-Jones A, Jaeschke H, Fisher E, Rumack BH, Ceretto V, Thompson MG, Curry S. The Effect of 4-Methylpyrazole on Oxidative Metabolism of Acetaminophen in Human Volunteers (abstract). Clin Toxicol 2019;57(10): 871–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karlsson JO, Ignarro LJ, Lundström I, Jynge P, Almén T. Calmangafodipir [Ca4Mn(DPDP)5], mangafodipir (MnDPDP) and MnPLED with special reference to their SOD mimetic and therapeutic properties. Drug Discov Today. 2015;20(4):411–21. [DOI] [PubMed] [Google Scholar]
- 77.Morrison EE, Oatey K, Gallagher B, Grahamslaw J, O’Brien R, Black P, Oosthuyzen W, Lee RJ, Weir CJ, Henriksen D, Dear JW; POP Trial Investigators. Principal results of a randomised open label exploratory, safety and tolerability study with calmangafodipir in patients treated with a 12 h regimen of N-acetylcysteine for paracetamol overdose (POP trial). EBioMedicine. 2019;46:423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cherry AD, Piantadosi CA. Regulation of mitochondrial biogenesis and its intersection with inflammatory responses. Antioxid Redox Signal. 2015;22(12): 965–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jornayvaz FR, Shulman GI. Regulation of mitochondrial biogenesis. Essays Biochem. 2010;47(69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scarpulla RC, Vega RB, Kelly DP. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab. 2012;23(9): 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Larsson NG, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, Barsh GS, Clayton DA. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet. 1998;18(3): 231–236. [DOI] [PubMed] [Google Scholar]
- 82.Ngo HB, Lovely GA, Phillips R, Chan DC. Distinct structural features of TFAM drive mitochondrial DNA packaging versus transcriptional activation. Nat Commun. 2014;5:3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Picca A, Lezza AM. Regulation of mitochondrial biogenesis through TFAM-mitochondrial DNA interactions: Useful insights from aging and calorie restriction studies. Mitochondrion. 2015;25:67–75. [DOI] [PubMed] [Google Scholar]
- 84.Wang J, Wilhelmsson H, Graff C, Li H, Oldfors A, Rustin P, Brüning JC, Kahn CR, Clayton DA, Barsh GS, Thorén P, Larsson NG. Dilated cardiomyopathy and atrioventricular conduction blocks induced by heart-specific inactivation of mitochondrial DNA gene expression. Nat Genet. 1999;21(1):133–137. [DOI] [PubMed] [Google Scholar]
- 85.Zhang T, Zhang Q, Guo J, Yuan H, Peng H, Cui L, Yin J, Zhang L, Zhao J, Li J, White A, Carmichael PL, Westmoreland C, Peng S. Non-cytotoxic concentrations of acetaminophen induced mitochondrial biogenesis and antioxidant response in HepG2 cells. Environ Toxicol Pharmacol. 2016;46:71–79. [DOI] [PubMed] [Google Scholar]
- 86.McGill MR, Yan HM, Ramachandran A, Murray GJ, Rollins DE, Jaeschke H. HepaRG cells: a human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology. 2011;53(3):974–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ni HM, Williams JA, Jaeschke H, Ding WX. Zonated induction of autophagy and mitochondrial spheroids limits acetaminophen-induced necrosis in the liver. Redox Biol. 2013;1:427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Du K, Ramachandran A, McGill MR, Mansouri A, Asselah T, Farhood A, Woolbright BL, Ding WX, Jaeschke H. Induction of mitochondrial biogenesis protects against acetaminophen hepatotoxicity. Food Chem Toxicol. 2017;108(Pt A):339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carvalho NR, Tassi CC, Dobraschinski F, Amaral GP, Zemolin AP, Golombieski RM, Dalla Corte CL, Franco JL, Mauriz JL, González-Gallego J, Soares FA. Reversal of bioenergetics dysfunction by diphenyl diselenide is critical to protection against the acetaminophen-induced acute liver failure. Life Sci. 2017;180:42–50. [DOI] [PubMed] [Google Scholar]
- 90.Ye D, Wang Y, Li H, Jia W, Man K, Lo CM, Wang Y, Lam KS, Xu A. Fibroblast growth factor 21 protects against acetaminophen-induced hepatotoxicity by potentiating peroxisome proliferator-activated receptor coactivator protein-1alpha-mediated antioxidant capacity in mice. Hepatology. 2014;60(3):977–989. [DOI] [PubMed] [Google Scholar]
- 91.Kumar A, Giri S, Shaha C. Sestrin2 facilitates glutamine-dependent transcription of PGC-1alpha and survival of liver cancer cells under glucose limitation. FEBS J. 2018;285(7):1326–1345. [DOI] [PubMed] [Google Scholar]
- 92.Chao X, Wang H, Jaeschke H, Ding WX. Role and mechanisms of autophagy in acetaminophen-induced liver injury. Liver Int. 2018;38(8):1363–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ramachandran A, Jaeschke H. Acetaminophen hepatotoxicity: A mitochondrial perspective. Adv Pharmacol. 2019;85:195–219. [DOI] [PMC free article] [PubMed] [Google Scholar]