Abstract
Objective(s):
Over the past 20 years, increasing interest in the use of medicinal plants as alternative or adjuvant treatments of several chronic diseases was observed. Accordingly, Nigella sativa or black cumin, a medicinal plant rich in bioactive compounds, has been used worldwide for food purposes or in traditional medicines. This paper aims to reveal N. sativa potential as adjunct treatment in cardiovascular diseases, diabetes, and hematological malignancies, due to their increasing prevalence and difficult management in everyday life.
Materials and Methods:
Databases like PubMed, Web of Science, Science Direct, Scopus, and Google Scholar were used to search the literature data. Keywords like anti-inflammatory effect, anti-oxidant effect, antihypertensive effects, hypolipidemic effects and hematological malignancies were used in combination with N. sativa.
Results:
Because of its numerous pharmacological actions, but especially for its anti-oxidant and anti-inflammatory properties, in vivo and in vitro studies demonstrated N. sativa positive effect against diabetes, hypertension, and hypercholesterolemia, all of them associated to cardiovascular diseases progression. Also, it was proved to have marked anti-proliferative, cytotoxic, pro-apoptotic, and anti-metastatic effects, in both solid cancers and hematological malignancies.
Conclusion:
N. sativa used as complementary treatment to classical medications can improve the management of several chronic diseases.
Key Words: Anti-inflammatory agents, Anti-oxidants, Cardiovascular diseases, Diabetes mellitus, Leukemia, Lymphoma, Multiple myeloma, Nigella sativa
Introduction
Plant bioactive compounds are receiving continuous attention because of their diverse pharmacological properties. They are considered very useful in the treatment and prevention of several common diseases and conditions like cancer, cardiovascular diseases, diabetes mellitus, obesity, and neurodegenerative diseases (1–3). Because of their strong anti-oxidant, free radical scavenging, anti-inflammatory, and anti-cancer properties, plants represent an important source of novel therapeutic drug discovery and production.
Nigella sativa L. or black cumin is one of these representative medicinal plants, used for centuries for its health benefits. Systematically N. sativa belongs to the Ranunculaceae family, and is an annual herb of 8–12 inch height, with pinnate and segmented leaves (4). Its flowers are solitary, self-pollinating, and colored in blue and white. The capsule fruits contain numerous trigonal seeds (4). Because of its seeds that have a specific aroma, a bitter and peppery taste (5, 6), this plant was used worldwide since ancient times not only as condiment and spice (4, 7–11) but also as a therapeutic plant to treat different diseases. Thus, in its originating countries in the south and east of the Mediterranean sea to Iran, Pakistan, and India (5), N. sativa is one of the most cherished medicinal species (4), being used to prepare dietary supplemental products, functional cosmetics (10, 12), and also for medicinal purposes like treating gastrointestinal disorders, rheumatism, hypertension, diabetes, obesity (13–15), and respiratory ailments (5, 16).
The health benefits of NSS different extracts and oils are given by the bioactive metabolites’ presence, either as pure molecules or in a mixture (5, 17–20). The studies show that in the seeds of N. sativa there are more than 100 chemical compounds, out of which many still have to be characterized (12, 21, 22) (Table 1).
Table 1.
General composition of Nigella sativa
| Compound class | Compound name | Content range | Source | Reference |
|---|---|---|---|---|
| Sterols | % total sterols | |||
| Cholesterol | 0.70-1.28 | s | (5, 8, 126–128) | |
| 0.48-1.01 | o | |||
| Campesterol | 9.88-13.76 | s | (5, 8, 126–128) | |
| 2.28-13.1 | o | |||
| Campestanol | 0.54-0.56 | s | (5, 8, 126–128) | |
| Stigmasterol | 10.52-20.92 | s | (5, 8, 126–128) | |
| 4.31-18.22 | o | |||
| β-sitosterol | 44.53-53.95 | s | (5, 8, 126–128) | |
| 13.24-53 | o | |||
| -sitosterol | 0.59 | o | (5, 8, 126–128) | |
| Sitostanol | 2.29-2.38 | s | (5, 8, 126–128) | |
| Δ7-Stigmasterol | 1.60-2.22 | s | (5, 8, 126–128) | |
| 0.6 | o | |||
| Δ 7-Avenasterol | 1.11-2.24 | s | (5, 8, 126–128) | |
| 2.1 | o | |||
| Δ 5- Avenasterol | 2.1 | o | (5, 8, 126–128) | |
| Tocopherols | mg/kg | |||
| α-Tocopherol | 0.8-1.3 | s | (129) | |
| β-Tocopherol | 0.5-0.9 | s | (129) | |
| -Tocopherol | 1.2-12.1 | s | (129) | |
| β-Tocotrienol | 8.2-12.1 | s | (129) | |
| Fatty acids | % | |||
| Lauric acid (C12:0) | 0.6 | o | (130–132) | |
| Myristic (C14:0) | 0.14-1 | s | (5, 128, 133, 134) | |
| 0.15-1 | o | |||
| Palmitic (C16:0) | 8.92-10.5 | s | (5, 128, 133, 134) | |
| 11.17-13.1 | o | |||
| Palmitoleic (C16:1) | 0.18 | s | (5, 128, 133, 134) | |
| 0.19-0.2 | o | |||
| Margaric acid (C17:0) | 0.061 | o | (5, 128, 133, 134) | |
| Heptadesenoic (C17:1) | 0.054 | o | (5, 128, 133, 134) | |
| Stearic (C18:0) | 2.04-2.44 | s | (5, 128, 133, 134) | |
| 2.22-3.4 | o | |||
| Oleic (C18:1) | 9.42-16.23 | s | (5, 128, 133, 134) | |
| 22.94-24.64 | o | |||
| Linoleic (C18:2) | 63.71-68.07 | s | (5, 128, 133, 134) | |
| 55.6-58.5 | o | |||
| Linolenic (C18:3) | 0.44-2.16 | s | (5, 128, 133, 134) | |
| 2.23-0.4 | o | |||
| Arachidic (C20:0) | 0.13 | s | (5, 128, 133, 134) | |
| 0.2-0.5 | o | |||
| Eicosenoic (C20:1) | 0.27 | s | (5, 128, 133, 134) | |
| 0.31 | o | |||
| Eicosedienoic acid (C20:2) | 0.33 | s | (5, 128, 133, 134) | |
| 2.55 | o | |||
| Behenic (C22:0) | 2.89 | s | (5, 128, 133, 134) | |
| 0.039 | o | |||
| Docosenoic (C22:1) | 0.047 | o | (5, 128, 133, 134) | |
| Lignoceric acid (C24:0) | 1.04 | s | (5, 128, 133, 134) | |
| Monoterpenic hydrocarbons |
mg/100gDW | |||
| α-Thujene | 4.38-109.4 | e.o. | (133, 135–137) | |
| α -Pinene | 2.19-27.65 | e.o. | (133, 135–137) | |
| Sabinene | 0.39-3.46 | e.o. | (133, 135–137) | |
| β-Pinene | 1.1-43.78 | e.o. | (133, 135–137) | |
| Myrcene | 0.21 | e.o. | (133, 135–137) | |
| α -Terpinene | 0.55-1.15 | e.o. | (133, 135–137) | |
| p-Cymene | 11.3-374.4 | e.o. | (133, 135–137) | |
| Limonene | 0.31-13.82 | e.o. | (133, 135–137) | |
| -Terpinene | 5.21-12.67 | e.o. | (133, 135–137) | |
| Monoterpene ester | Bornyl acetate | 0.04-1.15 | e.o. | (133, 135–137) |
| Monoterpenoid ketones |
Carvone | 0.02 | e.o. | (133, 135–137) |
| p-Cymen-9-ol | 12.67 | e.o. | (133, 135–137) | |
| Eugenol | 4.61 | e.o. | (133, 135–137) | |
| Thymoquinone | 3.52-413.57 | e.o. | (133, 135–137) | |
| Thymohydroquinone | 6.35-7.3 | e.o. | (133, 135–137) | |
| Monoterpenoid alcohols | Carvacrol | 2.61-12.67 | e.o. | (133, 135–137) |
| 4-Terpineol | 0.2-9.22 | e.o. | (133, 135–137) | |
| Terpenic phenols | Thymol | 1.67 | e.o. | (133, 135–137) |
| Sesquiterpene | α-Longipinene | 0.34-4.61 | e.o. | (133, 135–137) |
| Longifolene | 1.71 | e.o. | (133, 135–137) | |
| Z-γ-Bisabolene | 2.30 | e.o. | (133, 135–137) | |
| (E)-Caryophyllene | 0.02-4.61 | e.o. | (133, 135–137) | |
| Polyphenols | mg/kg | |||
| Catechin | 56.56-124.6 | o | (128) | |
| Epicatechin | 39.67-98.1 | o | (128, 138) | |
| 1.28 | sh | |||
| 0.64 | r | |||
| Catechin hydrated | 7.26 | sh | (128, 138) | |
| 3.4 | r | |||
| Rutin | 14 -117.7 | o | (128, 138) | |
| Dihydro quercitin | 4.05-31.45 | o | (128, 138) | |
| Naringine | 2.16-58.68 | o | (128, 138) | |
| Quercimeritrin | 5.89-39.89 | o | (128, 138) | |
| Quercetin | 5.03-35.67 | o | (128, 138) | |
| 2.56 | sh | |||
| 2.61 | r | |||
| Apigenin | 6.83 | sh | (128, 138) | |
| 1.77 | r | |||
| Amentoflavone | 2.91 | sh | (128, 138) | |
| Flavone | 3.4 | sh | (128, 138) | |
| 0.54 | r | |||
| Callistephin | 15.99-33.6 | o | (128, 138) | |
| Gallic acid | 27.86 | sh | (128, 138) | |
| 30.59 | r | |||
| p-Dihydroxybenzoic acid | 1.73 | r | (138) | |
| Chlorogenic acid | 1.51 | sh | (138) | |
| 0.36 | r | |||
| Vanillic acid | 143.2 | sh | (138) | |
| 89.94 | r | |||
| trans-2-Hydroxycinnamic acid | 1.25 | sh | (138) | |
| 2.58 | r | |||
| trans-Cinnamic acid | 15.47 | sh | (138) | |
| 0.98 | r | |||
| Macro elements | mg/kg DW | |||
| Potassium (K) | 708-7561 | s | (129, 130) | |
| Magnesium (Mg) | 80-1878 | s | (129, 130) | |
| Calcium (Ca) | 160-5089 | s | (129, 130) | |
| Zinc (Zn) | 2.5-48.89 | s | (129, 130) | |
| Manganese (Mn) | 1.5-22.73 | s | (129, 130) | |
| Copper (Cu) | 0.9-16.03 | s | (129, 130) | |
| Iron (Fe) | 8.65-108.1 | s | (129, 130) | |
| Phosphorus (P) | 48.9-6197 | s | (129, 130) | |
| Micro elements | mg/kg DW | |||
| Boron (B) | 19.42-23.6 | s | (129, 130) | |
| Chromium (Cr) | 0.291-0.65 | s | (129, 130) | |
| Copper (Cu) | 15.07-16.3 | s | (129) | |
| Molybdenum (Mo) | 0.221-0.40 | s | (129) | |
| Nickel (Ni) | 3.49-5.18 | s | (129) | |
S: seeds, o: oil, EO: essential oil, sh: shoots, r: roots
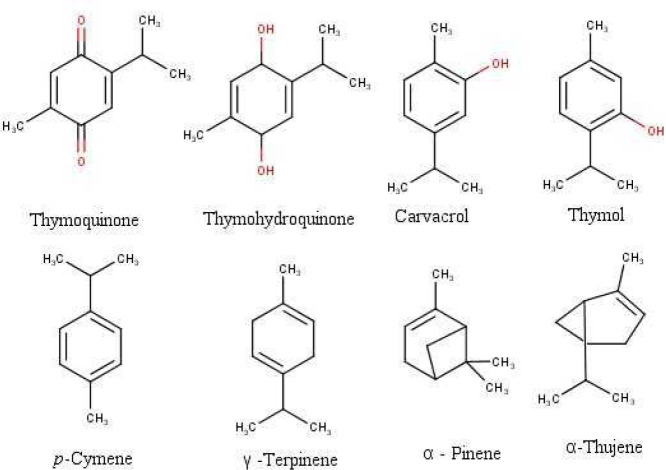
The most important due to their bioactivity are thymoquinone, nigellone, phytosterols, fatty acids, vitamins, and minerals (23). Thymoquinone (TQ) is the major seed essential oil component and most of the bioactivities have already been attributed to it (22, 24–26). Concerning the N. sativa seed oil, its primary components are monoterpenes (87.7%) like p-cymene, carvacrol, α-thujene, terpinene, α-pinene, β-pinene, and its oxygenated derivatives (9.9%) (Figure 1), the rest being sesquiterpenes and derivatives (12, 27). Isoquinoline and indazole alkaloids (7, 28), saponins (29), cycloartenols (30), and flavonoids (11, 30) were also isolated and identified in the seeds.
Figure1.
Chemical structure of some principal bioactive compounds in Nigella sativa
The in vivo and in vitro studies showed that among pharmacological properties of NSS, the anti-oxidant and anti-inflammatory activities, anti-asthmatic (31), anti-cancer and anti-diabetic (18, 22, 32–37), anti-hyperlipidemic (38, 39), anti-microbial (40, 41), anti-convulsant (42, 43), and analgesic activities (23, 44, 45) were intensively studied for their therapeutic potential. Thus, these pharmacological properties can be further translated into potent and therapeutically interesting activities on the immune, endocrine, cardiovascular, and respiratory systems as well as into protective effects against different types of induced toxicity (11, 22, 46–49), dermatological (50, 51), and metabolic effects (52–54). Though, a convincing number of studies regarding N. sativa health benefits are already available its application in real life disease management is still missing. Thus, the purpose of this review was to provide the most important comprehensive scientific reports that could encourage the clinical use of N. sativa especially in cardiology and hematological malignancies. Also, the aim of this review was to analyze the most recent literature data regarding the effects of N. sativa on oxidative stress and inflammation in the pathophysiology of chronic disease management. The control of these pathophysiological processes and diseases remains problematic, despite the dedicated effort in this respect. Overall, we aim to show that the use of N. sativa finds a great scientific confirmation in preventive medical practice.
Methods
The artilcles used in the present study were searched using the following databeses: PubMed, Web of Science, Science Direct, Scopus, and Google Scholar. The search was done using the keywords mentioned above. Special foscus was applied to the most recent articles, published between 2010 to 2018, which account for aproximately 65 % out of the total articles used in this review. Articles published between 2000 to 2009 were aproximately 29%.
Nigella sativa in inflammation and oxidative stress
The anti-inflammatory and anti-oxidant effects of N. sativa were studied in various acute and chronic models of experimental inflammation and oxidative stress knowing that these two processes are interconnected (55-57). As observed in Table 2, N. sativa was used as oil, essential oil, or different types of extracts (water, hydro-alcoholic).
Table 2.
The anti-inflammatory effects of Nigella sativa and TQ, its main constituent
| Type | Substance | Administrated dose | Experimental model | Anti-inflammatory effects | Observation | Reference |
|---|---|---|---|---|---|---|
| In vitro | Thymoquinone (TQ) | Calcium ionophore stimulated peritoneal leukocytes (rats) |
COX and 5-LO pathways of arachidonic acid metabolism were inhibited. Thromboxane, leukotrienes B4, and C4 production was down-regulated. |
The effect was dose-dependent. TQ had a higher effect than NSO. |
(139) | |
| Nigella sativa oil (NSO) | ||||||
| NSO |
12.5–50 mg/m | Calcium- or ionophore-stimulated neutrophils | The effect was dose-dependent possibly through the antioxidative action. | (140) | ||
| TQ | 0.01 and 6.25 μg | |||||
| Nige llone |
6.25 and 50 μg/ml | |||||
| TQ | 1, 3, 10, 100 µM/ml | Human granulocyte suspensions | LTC4 and LTB4 formation was inhibited. LTC4 synthase activity was significantly inhibited. LTA4 into LTC4 transformation in human blood cells was suppressed. |
TQ effective concentration was close to TQ concentration used in animals. | (141) | |
| In vivo | TQ | 1 mg/kg | Allergic encephalomye litis rat model |
Perivascular inflammation was reduced. Symptoms were reduced. |
The anti-inflammatory effect was influenced by the increased glutathione level. | (142) |
| TQ | 15 mg/kg intraperitoneally (i.p.) | Multiple sclerosis mice model | Symptom developments were prevented in 90% of the subjects. Inflammation and symptoms were improved in 50% of the subjects. |
(143) | ||
| TQ | 5 and 10 mg/kg | Acetic acid-induced colitis in rats | Myeloperoxidase activity, platelet activating factor, and histamine levels were restored. Glutathione levels were reduced. |
Anti-inflammatory effect of TQ can be mediated by its antioxidant action. | (144) | |
| TQ | 75 mg/kg of | Trinitrobenzene sulfonic acid (TNBS) induced colitis in rats | Proinflammatory cytokines were not changed. mRNA expression for IL-1β, IL-6, TNF-α, and IFN-γ in the colonic tissue was not affected. Histopathological changes were not reversed. |
TQ anti-inflammatory effect in TNBS colitis was not proven. | (145) | |
| NSO | 2.5 ml/kg, orally (p.o.) | TNBS induced colitis | LDH activity, TNF-α, IL-1β, IL-6 levels in blood were significantly decreased. | Anti-inflammatory and anti-oxidant activities were proven. | (146) | |
| NSO | TNBS induced colitis | TNF-α, IL-1β, and IL-6, lactate dehydrogenase, triglycerides, and cholesterol in serum were decreased. Neutrophil infiltration was inhibited. |
Superoxide dismutase (SOD) activity was increased. Myeloperoxidase (MPO) levels were decreased. |
(147) | ||
| TQ | 100 mg/kg | Chronic pancreatitis induced in rats | Amylase and lipase levels were changed. Il-1β and IL-18 levels were decreased. |
MPO activity and the oxidative stress index (OSI) were decreased. | (148) | |
| NSO | 0.66 ml and 1.55 ml/kg i.p. |
Carrageenan-induced paw edema and cotton pellet granuloma formation in rats | Eicosanoids generation was inhibited. | Lipid peroxidation was inhibited. | (149) | |
| Nigella sativa seeds (NSS) essential oil | 100, 200 and 400 µL/kg, p.o. or i.p. | Carrageenan-induced paw edema in rats | N. sativa essential oil had anti-inflammatory effect only in the i.p. administration. | (150) | ||
| N. sativa aqueous extract | 500 mg/kg | Carrageenan-induced paw edema | Inflammation was significantly reduced. | Results were comparable to aspirin (100 mg/kg). | (151) | |
| NSO | 1g/ day | Rheumatoid arthritis patients | Anti-inflammatory IL-10 increased. Pro-inflammatory cytokines and TNF-α were non-significantly decreased. |
Malondialdehyde (MDA) and NO serum levels were reduced. | (53) |
Also, among bioactive compounds identified in N. sativa oil (NSO), TQ was intensively studied (58). Of the vast literature data, the most recent articles, analyzing both inflammation and oxidative stress, were selected to identify how these processes are influencing each other in various illnesses.
The anti-inflammatory and anti-oxidant activity of N. sativa was observed in an induced model of acute inflammation in rats (59). Within this experimental lipopolysaccharides (LPS) model, the productions of ROS (e.g., nitric oxide (NO)) and of inflammatory mediators (e.g., tumor necrosis factor alpha (TNF-α), interleukins) were stimulated, generating liver and lung inflammation. The groups treated with N. sativa [500 mg/kg, intragastric (ig)] and NSO (5 ml/kg, ig) had the FDG (8F-fluoro-deoxy-D- glucose – used to assess the liver and lung inflammation status, with an increased rate in the inflammatory processes) uptakes nearly normal. Regarding the lipid peroxidation, malondialdehyde (MDA) levels decreased in the groups treated with N. sativa and NSO. Also, effective anti-oxidant properties were observed in the groups treated with N. sativa and NSO, superoxide dismutase (SOD) and catalase (CAT) levels in erythrocyte, lung, and liver being decreased (59).
The effect of NSO on oxidative stress and inflammation was also studied in diabetes-induced rat animal model (60). The oxidative stress parameters (thiobarbituric acid reactive substances-TBARS, NO, Glutathione-GSH levels and Xanthine oxidase -XO, SOD, Glutathione peroxidise-GPx and Glutathione-S-transferase-GST activities) were determined in rat brain tissues. The combination of classical drugs used to treat type2-diabetes mellitus (metformin, glimepiride) with NSO (2.0 ml, orally) had a positive effect on the oxidative stress markers. Therefore, TBARS, NO levels and XO activities, were restored to normal conditions values when compared with the control group. Also, the combination of classical treatment with NSO determined significant elevations of anti-oxidant markers (GSH, GPx, GST, and SOD activities) when compared with diabetic non-treated groups (60). The effect of NSO on central inflammation process (TNF-α, IL-6, IL-1β, and inducible nitric oxide synthase-iNOS) as well as the expression level of nuclear factor kappa-B (NFκ-Bp65) were investigated in both serum and brain tissue. Serum anti-inflammatory levels were significantly lowered in the groups treated with NSO, metformin, glimepiride and their combination as compared with control and diabetic groups. The same trend was observed in the levels of brain inflammatory cytokines. The values were significantly decreased in the rat groups treated with NSO, classical therapy and combination of NSO with classical treatment (60). Thus, NSO, single or in combination with medical treatment suppressed both induced oxidative stress and inflammatory process in type2-diabetes mellitus.
The inflammatory processes, as well as oxidative stress parameters, were also investigated in an experimental autoimmune encephalomyelitis (EAE) animal model in rats (61). The aim was to control the inflammation and demyelination processes in EAE by using N. sativa seeds (NSS). As expected, NSS (2.8 g/kg) reduced inflammation and enhanced cerebellum remyelination as confirmed by the histopathological, immunohistochemical, and ultrastructural analysis (61). The anti-inflammatory effect was explained by NSS inhibitory effect on astrocytes expression affecting transforming growth factor beta 1 (TGF-β1) production. The effect of NSS on oxidative stress process was investigated by analyzing the levels of MDA, GSH, GST, NO, and catalase activity in the cerebellum and medulla of rats. A significant decrease of MDA levels was detected in the group fed with NSS 2 weeks prior to EAE induction (cerebellar samples) and in the group fed with NSS after the appearance of first clinical signs (medulla samples). Decreased catalase activity was observed for both cerebellar (groups fed with N.sativa after EAE induction) and medulla (groups fed with N. sativa both prior and after EAE induction) samples. Regarding GSH levels, the cerebellum samples had a significant increase in both N. sativa groups as compared with the EAE group. Significant increases were observed in the medulla samples too, but just for the group treated with N. sativa after EAE induction as compared with control levels (61). The promising results obtained in both groups treated with N. sativa prior to and after EAE induction suggests that N. sativa can be used in prevention and treatment of EAE.
The effect of methanolic extract of N. sativa on inflammation and oxidative stress parameters were investigated in clinically endometritic cows (62). The anti-inflammatory effect of N. sativa was determined in relation to haptoglobin (Hp) and serum amyloid A (SAA) levels while under oxidative stress through MDA, SOD, and GSH-Px levels (62). The intrauterine infusion of N. sativa methanolic extract (100 ml, once a day) exerted anti-inflammatory effects. Thus, the levels of Hp and SAA significantly decreased in the group treated with N. sativa as compared with control and ceftiofur hydrochloride groups (62). Oxidative stress induced process within the inflammation model was characterized by significant increases of GSH-Px and SOD levels and by the significant decrease of MDA levels in the groups treated with N. sativa and ceftiofur as compared with the control group C. Also, the insignificant differences between N. sativa and ceftiofur groups in relation with MDA, SOD, and GSH-Px levels were attributed to the anti-oxidant property of N. sativa (62).
The effect of N. sativa hydro-alcoholic extract on inflammatory (IL 6 and TNF-a) and oxidative stress markers (MDA, total thiol, and SOD) in a lipopolysaccharide inflammation-induced myocardial fibrosis in rats was also studied (63) . Levels of heart IL-6 and TNF α cytokines were reduced when the highest dose of N. sativa extract (400 mg/kg) was administrated, as compared with control groups (63). Oxidative stress parameters were characterized by MDA reduction and total thiol, SOD, and catalase concentrations increase in heart samples. Because of these dose-dependent N. sativa changes it was concluded that administration of N. sativa improved myocardial fibrosis evolution through alteration of oxidative/anti-oxidative balance.
TQ, the major bioactive compound in N. sativa was also studied in different models of inflammation in relation to the oxidative stress processes (16, 64). The response modulation of oxidative stress and inflammatory cytokine profile by TQ was investigated using the collagen-induced arthritis animal model in rats (65). Next, oxidative stress was monitored through myeloperoxidase-MPO, TBARS, lipid peroxidation-LPO, GSH, CAT, SOD, and NO measurement (65). The inflammatory process was followed through inflammatory mediators like IL-1β, IL-6, TNF-α, IL-10, interferon-gamma-IFN-γ, and Prostaglandin E2 - PGE2. They suggested that TQ (5 mg/kg body weight) modulated the antiarthritic effect in joint cartilage through significant suppression of LPO and MPO activity. Decreased NO levels, increased anti-oxidant enzymes activity, the cytokine imbalance, as well the inhibitory effect on the accumulation and activation of polymorphonuclear cells (PMNs) suggested TQ’s antiarthritic effect (65).
The anti-inflammatory molecular mechanism of TQ was studied using complex in vivo and in vitro experimental settings (66). Within the in vitro lipopolysaccharide (LPS) -activated macrophages, and in vivo induced hepatitis (LPS/D-galactosamine and induced gastritis (EtOH/HCl) mouse models, TQ anti-inflammatory effect and its target proteins were approached (66). They showed that TQ (25 µM) strongly inhibited NO production, repressed NO synthase, TNF-α, cyclooxygenase (COX−2), IL−6, and IL-1β expression during in vitro experiments (66). During in vivo experiments, TQ (5 and 25 mg/kg) inhibited NO and PGE2, as well as IL-6 and TNF-α, and the genes responsible for iNOS and COX-2 production (66). They also showed that the inflammatory response in the pancreatic ductal adenocarcinoma was reduced due to suppression of inflammatory gene expression mediated through NF-κB (66). Finally, they proposed that TQ anti-inflammatory effects are probably due to degradation of interleukin-1 receptor-associated kinase 1(IRAK1) and by this the downstream reduction of NF-κB and of activator protein (AP-1) (66).
It can be concluded that N. sativa and its derivatives (oil, seeds, extracts, and its active ingredients) exert anti-inflammatory and anti-oxidant effects in the chronic inflammation processes, commonly encountered in many pathological diseases. Generally, the inflammatory process is described in relation with the specific changes in the central or local inflammatory cytokine profile (e.g., IL-6, Il-1β, TNF α, IL-10, INF-γ, and others) while, the oxidative stress process is commonly characterized using parameters like MDA, SOD, CAT, TBARS, NO, GSH, GST, and XO.
Therefore, according to the literature studies, the use of N. sativa in the prevention and treatment of chronic inflammatory disorders represents a very good promise. However, more studies are needed to fully understand how N. sativa could be used to control and reduce the inflammation and oxidative stress processes. So far, no clinical trial data are available, thus active research is essential. Since both processes are strongly linked and simultaneously described to initiate and maintain many pathological conditions, it would be very useful that clinical studies would approach the role of N. sativa in the chronic disease management from this perspective.
Nigella sativa and cardiovascular diseases
Cardiovascular diseases (CVD’s) are still considered the leading cause of morbidity and mortality in both developed and developing countries. According to the World Health Organization (WHO), every year about 17.1 million people die with cardiovascular problems like heart attack, stroke, and heart failure (67, 68). It is supposed that the occurrence of deaths may increase to 23.6 million in 2030 (WHO 2009). CVD is the leading cause of death worldwide mainly associated with various factors such as elevated total cholesterol serum, raised LDL and increase in LDL oxidation, high platelet aggregation, hypertension, and smoking (69). Besides this, augmented oxidative stress also plays a crucial role in the pathophysiology of CVD such as atherosclerosis, heart failure, hypertension, cardiac hypertrophy, and ischemia-reperfusion (70). Also, oxidative stress and inflammation are responsible for vascular damage common to hypertension, hyperlipidemia, and diabetes. The control of these pathologies is of fundamental importance as they can degenerate into CVDs that are the main cause of death in Western countries.
There are several synthetic therapeutic agents available for the treatment of different cardiovascular disorders at the clinical level, but most of them are facing the problem of unwanted or side effects, inefficacy in a variety of patients, and pharmacokinetic problems. Moreover, the interaction of cardiovascular medication with other drugs also limits the use of certain medications. In this regard, alternative therapies with diverse clinical applications, their effect in multiple patients together with their reasonable safety profile are the target of several research groups around the world. Thus natural healing agents could be considered an ideal approach to these limitations.
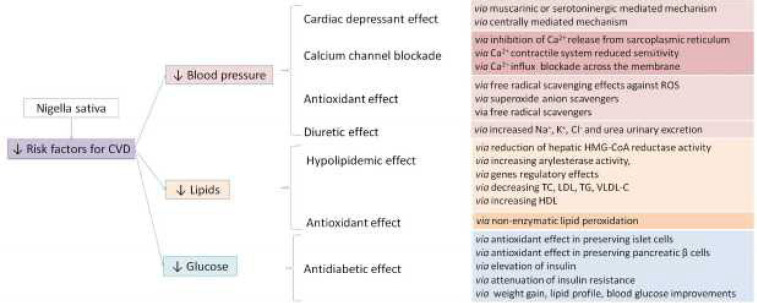
Among all natural products used in traditional medicine, N. sativa and its constituents showed interesting activity against all cardiovascular risk factors both for their direct pharmacological actions and for its anti-oxidant capacity (Figure 2).
Figure 2.
Proposed mechanism of Nigella sativa in relation to cardiovascular diseases
↑: increase; ↓: decrease; Ca2+, calcium (II) ion; Na+, sodium ion; K+, potassium ion; Cl-, chloride ion; HMG-CoA reductase hydroxyl methyl glutaryl reductase, TC: total cholesterol; LDL: low-density lipoprotein cholesterol; TG: triglyceride; VLDL-C: very low-density lipoprotein cholesterol; HDL: high-density lipoprotein cholesterol
Antihypertensive effects of Nigella sativa
It has been reported that the volatile oil of N. sativa and TQ, the most pharmacologically active ingredients found in the black seed, decrease both the arterial blood pressure (BP) and heart rate (71)including stroke, coronary artery disease, heart failure, and peripheral vascular disease. The increase in oxidative stress has been associated with the pathogenesis of hypertension. Increase of blood pressure is due to an imbalance between anti-oxidants defence mechanisms and free radical productions. Excessive production of reactive oxygen species reduces nitric oxide bioavailability leading to an endothelial dysfunction and a subsequent increase in total peripheral resistance. Hypertension can cause few symptoms until it reaches the advanced stage and poses serious health problems with lifelong consequences. Hypertensive patients are required to take drugs for life to control the hypertension and prevent complications. Some of these drugs are expensive and may have adverse reactions. Hence, it is timely to examine scientifically, complimentary therapies that are more effective and with minimal undesirable effects. Nigella sativa (NS. In a recent systematic review , the results of 11 published studies on humans exploring the BP-lowering effects of N. sativa treatment have been reported (72). Meta-analysis showed that N. sativa treatment for a period of 4–12 weeks is able to reduce both systolic BP and diastolic BP (72). Furthermore, a subgroup analysis revealed that N. sativa powder seems to have more beneficial effects compared with NSO. The exact mechanism involved in the N. sativa antihypertensive action is not well established. Many hypotheses have been extrapolated from the results of animal studies and they include a cardiac depressant effect, calcium channel blocking property, and a diuretic effect. The cardiac depressant effect of N. sativa seems to be mediated via central mechanisms involving vasomotor center in the medulla and sympathetic outflow to periphery rather than to NO or eicosanoid (71) including stroke, coronary artery disease, heart failure, and peripheral vascular disease. The increase in oxidative stress has been associated with the pathogenesis of hypertension. Increase of blood pressure is due to an imbalance between anti-oxidants defence mechanisms and free radical productions. Excessive production of reactive oxygen species reduces nitric oxide bioavailability leading to an endothelial dysfunction and a subsequent increase in total peripheral resistance. Hypertension can cause few symptoms until it reaches the advanced stage and poses serious health problems with lifelong consequences. Hypertensive patients are required to take drugs for life to control the hypertension and prevent complications. Some of these drugs are expensive and may have adverse reactions. Hence, it is timely to examine scientifically, complimentary therapies that are more effective and with minimal undesirable effects. Nigella sativa (NS. The blockage of calcium channels is mainly due to thymol, one of the active compounds of NS. Thymol reduces Ca2+ currents through L-type Ca2+ channels, thus inducing vascular smooth muscle cell relaxation. It has also been observed that thymol exerts a negative inotropic action on canine and guinea pig-isolate cardiac preparations (73). Finally, it has been demonstrated that N. sativa increases Na+, K+, and Cl- excretion (74). This diuretic effect enhances the other hypotensive effects described above.
Alongside these described effects, it is important to underline how the anti-oxidant effect of N. sativa is important for limiting hypertension. It is well known that oxidative stress influences the pathogenesis of essential hypertension or the arterial damage produced during essential hypertension (75). In fact, oxidative stress may play a pivotal role in the development of hypertension via the following mechanisms: enhanced sequestration of NO by ROS (76), formation of lipid peroxidation products (77), and depletion of NOS cofactors such as tetrahydrobiopterin (78). Lastly, it may cause functional and structural changes in the vascular wall and blood vessels (79). Among all constituents of NS, TQ seems to be the one mainly involved in this anti-oxidant effect at the base of the anti-hypertensive effect. TQ improves endothelial function by reducing oxidative stress and improving the expression levels of eNOS (17). Furthermore, it has been proven that NSO is able to mediate an anti-hypertensive effect as it reduces the angiotensin-converting enzyme (ACE) activity and increases the heme oxygenase (HO-1) activity. ACE promotes the production of angiotensin II (Ang II), which has a direct vasoconstrictor effect on the vessels but which is also responsible for the production of ROS, which exacerbates vascular damage, while HO-1 reduces the activity of Ang II (75).
Hypolipidemic effects of Nigella sativa
Many studies report that N. sativa significantly affects the lipid profile by reducing serum cholesterol LDL and triglycerides levels (80) both in animals and in humans. In fact, it has been reported that the petroleum ether extract of N. sativa (2 g/kg/day) reduces plasma triglycerides and increases HDL-cholesterol in rats (37) and similar effects have been observed in normal rats treated with NSO. In rabbits fed a cholesterol-rich diet, TQ consumption (3.5 mg/kg, orally) reduces total cholesterol, LDL, and triglycerides and increases HDL-cholesterol concentration (80, 81). A randomized placebo-controlled clinical trial showed that N. sativa consumption (2 g/day for 4 weeks) has beneficial effects in the treatment of hypercholesterolemia (82). Another study conducted on hypercholesterolemic patients showed that a dose of 1 g/day of N. sativa powder for a period of two months induces a significant decrease in triglyceride and LDL-cholesterol levels, as well as an increase in HDL-cholesterol levels (83). The effects of powdered N. sativa (1 g/day) have also been evaluated in menopausal women, one of the high-risk groups for developing dyslipidemia. Results showed a significant decrease in TG, LDL, and total cholesterol levels (84). A large randomized clinical trial evaluated if N. sativa supplement (500 mg/day) increases the effect of simvastatin, a widely used hypolipidemic drug. Data from this study showed a significant reduction in cholesterol, TG, and LDL levels in N. sativa plus simvastatin-treated patients compared with patients treated with simvastatin alone (85). The mechanisms by which N. sativa exerts this hypolipidemic effect are numerous but are all hypothesized based on animal studies. It has been proposed that N. sativa reduces the synthesis of cholesterol by hepatocytes and lowers its adsorption from the small intestine (86). It was also proposed that N. sativa stimulates cholesterol secretion in the bile (87). Furthermore, anti-oxidant components present in N. sativa can prevent non-enzymatic lipid peroxidation, which is a key factor in the atherosclerotic process (88). In addition, the inhibition of the inflammatory mediator’s production plays an important role in the prevention of endothelial dysfunction. Inflammatory cells such as polymorphonuclear leukocytes and vascular endothelial cells are activated in hypercholesterolemia and cause overproduction of free radicals. These free radicals are, in turn, implicated in the initial and development stages of atherosclerosis by oxidation of Ox-LDL and contributed to the inflammatory state of atherosclerosis (89). N. sativa reduces the foam cell formation in the blood vessel wall that accelerates the local inflammatory response that eventually leads to atherosclerotic plaque formation (90). Furthermore, it has been reported that TQ improved high cholesterol in the blood and prevented making plaque through decreasing oxidative stress and lipid profiles (91).
Anti-diabetic effects of Nigella sativa
N. sativa shows interesting anti-diabetic properties, as reported by several studies. In patients with type 2 diabetes, supplementation of 26.7 mg/kg/day of N. sativa seed for 12 weeks resulted in a significant reduction in fasting blood glucose, 2 hr post-prandial blood glucose, glycosylated hemoglobin, and insulin resistance, without affecting renal or hepatic functions (87). A subsequent study, conducted by the same research group, confirmed these favorable effects and showed that they also persist along the one year study period (92). Similar results were obtained in another study in which patients with type 2 diabetes received 30 mg/kg/day of NSO for 3 months in addition to their anti-diabetic drug (93). Studies conducted on animals have allowed highlighting that N. sativa inhibits sodium-dependent absorption of D-glucose (54), enhances glucose-induced insulin release from Langherans islets (94), and stimulates glucose uptake in skeletal muscles and adipocytes (95). The common denominator of all these observed effects is the reduction of oxidative stress. In fact, free radicals cause over generation of ROS, which initiates several pathways related to the inflammatory signaling cascades which will lead to inflammation (96) finally causing the apoptosis of pancreatic β cell (97, 98). Thus, N. sativa seed, NSO and TQ decrease oxidative stress therefore preserving the pancreatic β cell integrity (80, 99) and increasing peripheral insulin sensitivity (92). Furthermore, N. sativa reduces the cardiovascular complications associated with diabetes as it preserves the integrity of the vessel wall as described above (17).
The multiple uses of N. sativa in folk medicine encouraged many investigators to isolate its active components in order to evaluate their effects in cardiovascular disorders. The analysis of the data reported in the literature shows that the efficacy of N. sativa in these pathologies is mainly ascribed to its anti-oxidant activity. However, some aspects limit the possibility to draw unequivocal conclusions. First aspect refers to the large number of in vitro and in vivo studies that have been conducted and by contrast to the few in humans. Second aspect concerns the fact that there is not a general formulation for N. sativa administration, sometimes being used as powder, other times as an oil, and in many cases only TQ from N. sativa was analyzed. The third aspect refers to the different doses used, and to the fact that sometimes these are not well defined. So, long-term human trials are required to better establish the pharmacological activity of N. sativa in cardiovascular disorders, diabetes, or to a cluster of metabolic conditions that eventually affects the heart disease management.
Nigella sativa and hematological malignancies
Hematological malignancies are cancers broadly divided based on their origin (myeloid and lymphoid) and evolution (acute or chronic). They represent around 10% of all cancers, representing thus an important problem (100)>2000 diagnoses annually, socio-demographically representative of the UK. Their prevalence is expected to increase, as the life expectancy increases and the population gets older. Major types of hematological malignancies include non-Hodgkin lymphoma, multiple myeloma, chronic myeloid leukemia, acute myeloid leukemia, chronic myeloid leukemia, and myelodysplastic syndrome. Although sometimes the hematological malignancies are indolent, most of the patients will need treatment. Treatment-related toxicity can be a major issue in some patients, usually the treatment consisting in prolonged exposure to various combined chemotherapy regimens.
The anti-cancer effects of N. sativa and TQ were analyzed in vitro and in vivo, in relationship with various types of cancers, mostly solid ones. Most of them showed unequivocally anti-proliferative, cytotoxic, pro-apoptotic, and anti-metastatic effects (101). A limited number of studies evaluated the relationship between TQ and hematological malignancies. As these studies were not systematically reviewed, we chose to focus on them in this review, rather than on studies performed on solid cancers.
TQ effects on acute leukemia cells
Acute myeloid and acute lymphoblastic leukemias have a poor prognosis, despite the progress made regarding diagnosis and treatment. Acute myeloid leukemia is the most frequent leukemia in adults, its incidence increasing with age. However, in children, acute lymphoblastic leukemia is several times more frequent than acute myeloid leukemia. El-Mahdy et al., (2005) (102) were among the first to provide evidence on how TQ induces apoptosis in leukemic cells. They analyzed the p53-null myeloblastic leukemia HL-60 cell line. They showed that TQ (25, 50 and 100 µM) had a dose- and time-dependent cytotoxic effect on the cells, the IC50 (half-maximal inhibitory concentration) being achieved at 23 μM, after 24 hr of TQ exposure. TQ also disrupted the mitochondrial membrane, favored cytochrome c release into the cytoplasm, activated caspases 3, 8, and 9, pro-apoptotic protein Bax up-regulation, and anti-apoptotic protein Bcl-2 down-regulation (102). The effects of TQ (0, 1, 3, 10, 20, 30, 50, 100 µM) on proliferation and apoptosis, using a p53-mutated clone of the human leukemic T-cell line Jurkat were investigated (103). Following the exposure to TQ for 24 hr, both the proliferation and viability of the Jurkat cells were diminished in a dose-dependent manner, the IC50 being 24.2 +/- 0.2 μM. Next, the authors focused on the apoptotic mechanisms triggered by TQ. First, they observed an increasing number of sub-G0/G1 cells (a sign of apoptosis), in parallel with increasing doses of TQ. They went on to demonstrate that TQ stimulates the production of ROS, which destabilizes the mitochondrial membrane. Then, they noted the expression of p73 increment, a protein that regulates cell cycle progression. This was accompanied by a decrease in the expression of UHRF1, HDAC1, and DNMT1 proteins (103)by focusing on the anti-apoptotic and epigenetic integrator UHRF1 which is essential for cell cycle progression. For this purpose, we analyzed the effects of a known anti-neoplastic drug, thymoquinone (TQ. UHRF1 has been shown to have anti-apoptotic properties (104). Thus, its down-regulation would favor apoptosis. In fact, UHRF1 gained considerable interest during recent years. Since it is expressed in most of the cancers, it has the potential to become a “universal” cancer biomarker (104). The same research team that described the relationship between TQ, and p73 and UHRF1 deregulation demonstrated later that this process was preceded by a down-regulation of PDE1A, a cyclic nucleotide phosphodiesterase (105)the active principle of Nigella sativa black seeds, has anti-proliferative properties on numerous cancer cell types. Others and we have previously reported that TQ acts as agent that triggers cell cycle arrest and apoptosis through either a p53- or p73-dependent pathway. However, the immediate targets recruited upon TQ-induced cytotoxicity have not yet been clearly identified. We therefore asked whether cyclic nucleotide phosphodiesterases (PDEs. Among the proteins down-regulated by TQ in Jurkat cells, DNMT1 was mentioned too (103)by focusing on the anti-apoptotic and epigenetic integrator UHRF1 which is essential for cell cycle progression. For this purpose, we analyzed the effects of a known anti-neoplastic drug, thymoquinone (TQ. The effects exerted by TQ on DNMT1 in acute myeloid leukemia cells were demonstrated using in vitro, ex vivo and in vivo models - Kasumi-1, MV4-11, THP-1, and ML- 1 cell lines, primary cells from leukemia patients (with 1, 10, 30, and 300 nM, 1, 3, 10, 30, and 100 μM of TQ) and a murine leukemia model (with 0, 15, and 30 mg/kg TQ, intravenous injection) (106). It was observed that TQ not only binds directly to the catalytic domain of DNMT1, but also diminishes DNMT1 expression. Thus, TQ produces DNA hypomethylation, promoting apoptosis in the leukemic cells. In the light of these findings, TQ could become a DNA hypomethylating adjuvant agent used in the therapy of acute myeloid leukemia, along with the already approved DNA hypomethylating drugs, namely azacitidine and decitabine (106).
The effects of TQ on acute lymphocyte leukemic cell line (CEMss) were also investigated (107). TQ (0, 5, 10, and 20 μg/ml), had cytotoxic effects, with an IC50 value of 1.5±0.04 μg/ml, after 24 hr of TQ exposure. TQ also increased ROS production and favored apoptosis, in a dose- and time-dependent manner, with increased activities for caspases 3, 8, and 9, decreased expression of Bcl-2, and increased expression of Bax (107). The same research team expanded their work on WEHI-3 cell line, which has features of myelomonocytic leukemia. They analyzed the relationship between TQ and this cell line. They also injected WEHI-3 cells to BALB/c mice, in order to assess the in vivo effects of TQ. TQ had cytotoxic and pro-apoptotic effects on WEHI-3 cells, in a dose-dependent manner, the IC50 being 2.0±0.04 µg/ml, after 24 hr of exposure. TQ also reduced the size of spleen and liver of BALB/c mice injected with WEHI-3 cells (108).
The propensity of TQ to trigger apoptosis by generating ROS was demonstrated by using TQ (1, 5, 10, 40, or 100 µM) on several leukemic T-cell lines, HTLV-1 positive (MT-2 and HuT-102), and HTLV-1 negative (CEM and Jurkat) (109). TQ were toxic to HTLV-1 negative cells and also to HTLV-2 positive cells, although to a lesser extent. After 48 hr of TQ exposure, IC50 had the following values: 8 μM (CEM), 28 μM (Jurkat), 35 μM (MT-2), and 85 μM (HuT-102). More HTLV-1 negative cells than HTLV1 positive accumulated in sub-G1 phase, suggesting that the malignant HTLV-1 negative cells are more prone to TQ-induced apoptosis. This was confirmed by several other data generated by the study. While TQ induced modifications in both HLTV-1 positive and negative cells, the effects were stronger in HLTV1-positive cells, which displayed: more mitochondrial membrane potential disruption and release of cytochrome c, more activation of caspases 3 and 9, more production of ROS, and a greater depletion of intracellular glutathione. These effects were not seen in normal PMBC (peripheral mononuclear blood cells), an observation that suggests a selective action of the TQ on the malignant lymphocytes (109). Other two recent studies revealed similar findings regarding the effects of TQ on leukemic T cell lines, namely decreased cell viability and induction of apoptosis (110, 111)a natural compound isolated fromNigella sativa, induces growth inhibition and apoptosis in several cancer cell lines. The aim of the present study was to investigate the effect of TQ alone and in combination with doxorubicine on the proliferation inhibition and apoptosis induction of TQ in a lymphoblastic leukemia cell line. Jurkat cell line was cultured in standard condition and with concentrations of TQ (0-30 μm. The involvement of the TGF family, TQ producing up-regulation of TGFβ1, and down-regulation of TGFα (111)there is no effective treatment for ATL. Thymoquinone has been reported to have anti-cancer properties. Objective The aim of this study is to investigatthe effects of TQ on proliferation, apoptosis induction and the underlying mechanism of action in both HTLV-1 positive (C91-PL and HuT-102 was also shown.
TQ effects on lymphoma cells
The possible inhibitory role of TQ on lymphoma cells was analyzed through the effects of TQ on several pleural effusion lymphoma (PEL) cell lines such as BC-1, BC-3, BCBL-1, and HBL-6 (112). PEL is a rare type of lymphoma, appearing mostly in HIV-infected patients. It is associated always with human herpes virus 8. After 24 hr exposure of the PEL cell lines to increasing TQ concentrations (10, 25, and 50 μM), the growth of all PEL cell lines was inhibited by TQ in a dose-dependent manner. Moreover, TQ was non-toxic to non-malignant cells, namely peripheral mononuclear blood cells obtained from healthy individuals. TQ also induced in PEL cell lines several apoptotic mechanisms: inhibition of the PKB/AKT signaling pathway, Bax up-regulation and Bcl-2 down-regulation, mitochondrial membrane disruption and cytochrome c release, caspases 3 and 9 activation, and PARP cleavage. As pre-treatment with N-acetyl-cysteine (a ROS scavenger) abrogates most of these phenomena, it is clear that the apoptosis triggered by TQ greatly depends on ROS produced (112).
NFkB activation is the hallmark of activated B-cell lymphoma (ABC), one of the main types of diffuse large B-cell lymphoma (DLBCL), which in turn represents the most frequent type of non-Hodgkin lymphoma. The anti-apoptotic role of the NFkB signaling pathway in ABC was investigated based on TQ previously described pro-apoptotic properties (113). Thus, two ABC cell lines, namely HBL-1 and RIVA, were exposed to TQ using concentrations of 5 and 10 μM. TQ shut down the phosphorylation of p65 (a subunit of NFkB). On the other hand, the down-regulation of the p65 targets such as Bcl-2, Bcl-xL, or XIAP favored mytochondrial-induced apoptosis. All these consequences depended on the TQ dose used. TQ produced the release of ROS in ABC cells, mostly superoxide anion and hydrogen peroxide. Pre-exposure of the ABC cells to N-acetyl-cysteine inhibited the release of reactive oxygen species (113).
Epstein-Barr virus, while asymptomatic in most of the individuals infected, is able to induce several types of lymphoid and epithelial neoplasms, such as Burkitt lymphoma, diffuse large B-cell lymphoma, Hodgkin lymphoma, or nasopharyngeal carcinoma. The relationship between TQ and EBV infected B lymphocytes was investigated (114). Several types of cells: EBV transformed B-cell lines (lymphoblastoid cell lines), an EBV-positive cell line (Raji), two EBV-negative cell lines (DG-75 and BL41), normal PBMC, and periodontal ligament fibroblasts were exposed to TQ (0.4-200 µmol/l) (114). TQ inhibited the growth of both EBV-positive and EBV-negative cell lines. After 24 hr of TQ exposure, the greatest inhibitory effect was seen in the case of lymphoblastoid cell lines (minimum IC50 was 0.99 +/- 0.02 μmol/l), followed by the Raji (EBV-positive) cell line (IC50 was 11 +/- 2.1 μmol/l), followed by the two EBV-negative cell lines (DG-75 and BL41) (IC50 were 20 +/- 2.1 and 16.24 +/- 0.31 μmol/l, respectively). TQ had the lowest toxicity against the normal fibroblasts from the periodontal ligament (IC50 was 85 +/- 6.5 μmol/l). The study revealed also other interesting findings. For instance, TQ induced apoptosis in lymphoblastoid cells, as demonstrated by TUNEL and caspase-3 assays. This effect was dose- and time-dependent. Moreover, TQ also inhibited the expression of several EBV genes, namely EBNA1, EBNA2, and LMP1, the effect being the strongest in the case of EBNA2 (114).
TQ effects on multiple myeloma cells
Multiple myeloma (MM) is a mature B-cell lymphoid neoplasm, arising from plasma cells. Although progress has been made regarding the treatment options, it remains a disease with a poor prognosis. Signal transducer and activator of transcription-3 (STAT-3) activation signaling is common to several cancers, including MM. The effects of TQ on STAT-3 signalling, using U266 and RPMI 8226 MM cell lines were analysed (115). TQ had various, multiple effects on MM cells. Firstly, TQ markedly inhibited the constitutive phosphorylation of STAT-3, this effect being dose - and time-dependent. TQ displayed the greatest inhibition at around 15 μM, after around 4 hr of exposure. TQ inhibited not only the constitutive STAT-3 phosphorylation, but also that IL-6-dependent, a cytokine that is crucial in the pathogenesis of MM. The level of several proteins regulated by STAT-3, like cyclin D1, Bcl-2, Bcl-xL, survivin, Mcl-1, and VEGF significantly decreased upon TQ exposure, the effect being the greatest after 36-48 hr (115). Regarding the effects on cell cycle, TQ forced the MM cells to distribute in the pre-G1 phase, a phenomenon indicating apoptosis. In fact, it seems that STAT-3 suppression by TQ promotes apoptosis by activation of caspase-3, which in turn cleaves the PARP protein.
The effects of TQ on MDN and XG2-MM cell lines was also investigated (116). In general, they observed similar effects of TQ on MM cells, as those reported by Li et al. (2010). TQ had dose- and time-dependent cytotoxic effects on both cell lines, the IC50 being 10 μM (MDN cell line) and 8.5 μM (XG2 cell line), respectively. The effect was at peak intensity after 12 hr of TQ exposure. In both MDN and XG2 cells, TQ also inhibited the IL-6-dependent growth. In both MDN and XG2 cell lines, TQ markedly decreased the level of CXCL-12-dependent actin polimerization, a process essential for the dynamics of multiple myeloma cells. Finally, TQ decreased the level of STAT-3 phosphorylation, but not of STAT-5. The level of several proteins regulated by STAT-3 activation, such as Bcl-2 and Bcl-XL was also diminished (116)the major active component of the medicinal herb Nigella sativa Linn., has been described as a chemopreventive and chemotherapeutic compound. METHODS In this study, we investigated the effect of TQ on survival, actin cytoskeletal reorganization, proliferation and signal transduction in multiple myeloma (MM. These observations also parallel the data reported by Li et al. (2010).
TQ as a synergic therapeutic agent in the treatment of hematological malignancies and other cancers
The combined effects of TQ and doxorubicin were investigated, and it was found that the two had a synergistic effect (110). This is not surprising, taking into account that the synergism between TQ and various chemotherapeutic agents has been described previously. For instance, Effenberger-Neidnicht and Schobert (2011) analyzed the combined effects of TQ and doxorubicin on several cell lines originating from various human cancers. A synergistic effect between TQ and doxorubicin was noted in the case of HL-60 leukemia cell line and multidrug resistant MCF7/TOPO (topotecan resistant) breast carcinoma cell line (117). A synergism between TQ and gemcitabine and oxaliplatin in pancreatic cancer cells was also reported (118). Importantly, pre-exposure to TQ favored chemosensitization to gemcitabine and oxaliplatin, rather than their co-administration (118). The synergism between TQ and cisplatin in both non-small cell and small cell lung cancer cell lines was described (119). They replicated these in vitro data in an in vivo mouse xenograft model (119). Next, the synergism between TQ and topotecan, a topoisomerase I inhibitor, in acute myelogenous leukemia cell lines was reported (120). Again, it seems that pre-exposure to TQ before topotecan exposure has more cytotoxic effects than their co-administration (120). TQ also potentiates thalidomide and bortezomib in inducing apoptosis in MM cells (115). The synergism between TQ and bortezomib both in vitro, on various MM cell lines, and in vivo, on a xenograft mouse model were also investigated (121). Also, the synergistic effect between TQ and temozolomide (TMZ) was reported (122). A higher decrease was observed in brain cancer cell (human glioblastoma cell line (U87MG)) viability when these drugs were used in combination (20 µM of TMZ and 50 µM of TQ) rather than when they were used alone (122). It was suggested that autophagy blockage at the transcriptional level by TQ reduced the resistance mechanism of these cells to TMZ.
Also, this synergism can be translated in real life by TQ potential to reduce the side effects in different therapies. In this sense, some studies have already revealed promising results. For instance, cardiotoxicity might appear following treatment with anthracyclines, such as doxorubicin. Accordingly, it was demonstrated that TQ could lower the risk of doxorubicin-induced cardiotoxicity, without compromising its anti-cancer properties (123-125).
Although the number of studies performed on the relationship between N. sativa and hematological malignancies is limited, they all demonstrated the anti-proliferative, cytotoxic, and pro-apoptotic effects of its bioactive compound, namely the TQ. However, we should take into consideration that these studies mainly used in vitro models. These results should be further explored in in vivo models, and then in clinical trials. These clinical trials should probably combine TQ and various chemotherapeutic agents used in the treatment of the hematological malignancies. We could identify several reasons for this approach: first, there is evidence of a synergism between these medicines and TQ, at least in vitro. Second, TQ could have the potential to reduce the toxicity associated with various chemotherapeutic agents. Third, the evaluation of TQ alone, as single agent in the treatment of hematological malignancies, would not be acceptable right now, as data regarding TQ action in vivo are lacking.
Conclusion
Medicinal plants are used as alternative treatment therapies in several chronic diseases because of their reduced side effects and costs. Within this study, the potential anti-inflammatory and anti-oxidant effects of N. sativa in relation to different types of chronic conditions were investigated. Since inflammation and oxidative stress are strongly interconnected pathophysiological processes that are influencing themselves, the most recent references in regards to the experimental settings focused on both processes were analyzed. Studies have indicated that the use of black cumin in its different forms (as seeds, oil, or different extracts) can be used successfully in the treatment of various inflammatory diseases. Also, it was shown that because of its pharmacological properties like anti-inflammatory, anti-oxidant, and pro-apoptotic, N. sativa can be a valuable ally against cardiovascular diseases, diabetes and hematological malignancies. Future evidence based studies are needed to encourage the use of N. sativa in the management of these diseases or in the management of the side effects caused by the aggressive treatments.
Acknowledgment
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Pop RM, Puia IC, Puia A, Chedea VS, Leopold N, Bocsan IC, et al. Characterization of Trametes versicolor: Medicinal Mushroom with Important Health Benefits. Not Bot Horti Agrobot Cluj-Napoca. 2018;46:343–349. [Google Scholar]
- 2.Zavoi S, Fetea F, Ranga F, Pop RM, Baciu A, Socaciu C. Comparative Fingerprint and Extraction Yield of Medicinal Herb Phenolics with Hepatoprotective Potential, as Determined by UV-Vis and FT-MIR Spectroscopy. Not Bot Horti Agrobot Cluj-Napoca. 2011;39:82–89. [Google Scholar]
- 3.Mocan A, Pop RM, Bocsan CI, Echeverría J, Buzoianu AD, Neag MA, et al. Berberine: Botanical Occurrence, Traditional Uses, Extraction Methods, and Relevance in Cardiovascular, Metabolic, Hepatic, and Renal Disorders. Front Pharmacol. 2018;9:1–30. doi: 10.3389/fphar.2018.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akram KM, Afzal M. Chemical composition of Nigella sativa Linn: Part 2 Recent advances. Inflammopharmacology. 2016;24:67–79. doi: 10.1007/s10787-016-0262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gharby S, Harhar H, Guillaume D, Roudani A, Boulbaroud S, Ibrahimi M, et al. Chemical investigation of Nigella sativa L seed oil produced in Morocco. J Saudi Soc Agric Sci. 2015;14:172–177. [Google Scholar]
- 6.Sturtevant EL, Hedrick UP. Sturtevant’s edible plants of the world. Dover Publications; 1972. [Google Scholar]
- 7.Atta BM. Some characteristics of nigella (Nigella sativa L) seed cultivated in Egypt and its lipid profile. Food Chem. 2003;83:63–68. [Google Scholar]
- 8.Cheikh-Rouhou S, Besbes S, Lognay G, Blecker C, Deroanne C, Attia H. Sterol composition of black cumin (Nigella sativa L) and Aleppo pine (Pinus halepensis Mill) seed oils. J Food Compos Anal. 2008;21:162–168. [Google Scholar]
- 9.D’Antuono LF, Moretti A, Lovato AFS. Seed yield, yield components, oil content and essential oil content and composition of Nigella sativa L and Nigella damascena L. Ind Crop Prod. 2002;15:59–69. [Google Scholar]
- 10.Kiralan M, Özkan G, Bayrak A, Ramadan MF. Physicochemical properties and stability of black cumin (Nigella sativa) seed oil as affected by different extraction methods. Ind Crops Prod. 2014;57:52–58. [Google Scholar]
- 11.Maulidiani M, Sheikh BY, Mediani A, Wei LS, Ismail IS, Abas F, et al. Differentiation of Nigella sativa seeds from four different origins and their bioactivity correlations based on NMR-metabolomics approach. Phytochem Lett. 2015;13:308–318. [Google Scholar]
- 12.Pop RM, Militaru C, Chedea VS. Phytochemicals of Nigella sativa: Structure, Detection and Conditioning. In: Pop RM, editor. Future Perspectives on Nigella Sativa: Characterization and Pharmacological Properties. Nova Science Publishers, Inc; 2018. pp. 1–46. [Google Scholar]
- 13.Tariq M. Nigella sativa seeds: folklore treatment in modern day medicine. Saudi J Gastroenterol. 2008;14:105–6. doi: 10.4103/1319-3767.41725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neag M, Bocşan CI, Crăciun CI, Catinean A. Nigella sativa, Microbiota and Gastro-Intestinal Cancer. In: Pop RM, editor. Future Perspectives on Nigella Sativa: Characterization and Pharmacological Properties. Nova Science Publishers, Inc; 2018. pp. 197–218. [Google Scholar]
- 15.Mousavi SM, Sheikhi A, Varkaneh HK, Zarezadeh M, Rahmani J, Milajerdi A. Effect of Nigella sativa supplementation on obesity indices: A systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. 2018;38:48–57. doi: 10.1016/j.ctim.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Bocşan CI, Neag M, Zdrenghea MT, Buzoianu AD. Anti-Inflammatory, Immunomodulatory and Antiallergic Effects of Nigella sativa. In: Pop RM, editor. Future Perspectives on Nigella Sativa: Characterization and Pharmacological Properties. Nova Science Publishers, Inc; 2018. pp. 103–148. [Google Scholar]
- 17.Ahmad A, Husain A, Mujeeb M, Khan SA, Najmi AK, Siddique NA, et al. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac J Trop Biomed. 2013;3:337–352. doi: 10.1016/S2221-1691(13)60075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ait Mbarek L, Ait Mouse H, Elabbadi N, Bensalah M, Gamouh A, Aboufatima R, et al. Anti-tumor properties of blackseed (Nigella sativa L) extracts. Brazilian J Med Biol Res = Rev Bras Pesqui medicas e Biol. 2007;40:839–47. doi: 10.1590/s0100-879x2006005000108. [DOI] [PubMed] [Google Scholar]
- 19.Gilani AH, Aziz N, Khurram IM, Chaudhary KS, Iqbal A. Bronchodilator, spasmolytic and calcium antagonist activities of Nigella sativa seeds (Kalonji): a traditional herbal product with multiple medicinal uses. J Pak Med Assoc. 2001;51:115–20. [PubMed] [Google Scholar]
- 20.Khan SU, Khan RA, Khan WU. Phytochemical screening and in vitro antioxidant activities of methanolic extract of nigella sativa seeds. World Appl Sci J. 2017;35:971–975. [Google Scholar]
- 21.Khan SA, Khan AM, Karim S, Kamal MA, Damanhouri GA, Mirza Z. Panacea seed ‘Nigella’: A review focusing on regenerative effects for gastric ailments. Saudi J Biol Sci. 2016;23:542–553. doi: 10.1016/j.sjbs.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chedea VS, Pop RM, Rotar MC. Nigella sativa Bioactive Components: Key Aspects of Pharmacological Effects. In: Pop RM, editor. Future Perspectives on Nigella Sativa: Characterization and Pharmacological Properties. Nova Science Publishers, Inc; 2018. pp. 47–78. [Google Scholar]
- 23.Amin B, Hosseinzadeh H. Black Cumin (Nigella sativa) and Its Active Constituent, Thymoquinone: An Overview on the Analgesic and Anti-inflammatory Effects. Planta Med. 2016;82:8–16. doi: 10.1055/s-0035-1557838. [DOI] [PubMed] [Google Scholar]
- 24.Oskouei Z, Akaberi M, Hosseinzadeh H. A glance at black cumin (Nigella sativa) and its active constituent, thymoquinone, in ischemia: a review. Mashhad Univ Med Sci. 2018;21:1200–1209. doi: 10.22038/ijbms.2018.31703.7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tavakkoli A, Mahdian V, Razavi BM, Hosseinzadeh H. Review on Clinical Trials of Black Seed (Nigella sativa ) and Its Active Constituent, Thymoquinone. J pharmacopuncture. 2017;20:179–193. doi: 10.3831/KPI.2017.20.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gholamnezhad Z, Havakhah S, Boskabady MH. Preclinical and clinical effects of Nigella sativa and its constituent, thymoquinone: A review. J Ethnopharmacol. 2016;190:372–386. doi: 10.1016/j.jep.2016.06.061. [DOI] [PubMed] [Google Scholar]
- 27.Wajs A, Bonikowski R, Kalemba D. Composition of essential oil from seeds of Nigella sativa L cultivated in Poland. Flavour Fragr J. 2008;23:126–132. [Google Scholar]
- 28.Atta-ur-Rahman , Malik S, Hasan SS, Choudhary MI, Ni CZ, Clardy J. Nigellidine — A new indazole alkaloid from the seeds of Nigella sativa. Tetrahedron Lett. 1995;36:1993–1996. [Google Scholar]
- 29.Mehta BK, Mehta P, Gupta M. A new naturally acetylated triterpene saponin from Nigella sativa. Carbohydr Res. 2009;344:149–151. doi: 10.1016/j.carres.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Mehta BK, Pandit V, Gupta M. New principles from seeds of Nigella sativa. Nat Prod Res. 2009;23:138–148. doi: 10.1080/14786410801892078. [DOI] [PubMed] [Google Scholar]
- 31.Boskabady MH, Mohsenpoor N, Takaloo L. Antiasthmatic effect of Nigella sativa in airways of asthmatic patients. Phytomedicine. 2010;17:707–713. doi: 10.1016/j.phymed.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Mollazadeh H, Afshari AR, Hosseinzadeh H. Review on the Potential Therapeutic Roles of Nigella sativa in the Treatment of Patients with Cancer: Involvement of Apoptosis: - Black cumin and cancer. J pharmacopuncture. 2017;20:158–172. doi: 10.3831/KPI.2017.20.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dubey PN, Singh B, Mishra BK, Kant K, Solanki RK. Nigella (Nigella sativa): A high value seed spice with immense medicinal potential. Indian J Agric Sci. 2016;86:967–979. [Google Scholar]
- 34.Gholamnezhad Z, Keyhanmanesh R, Boskabady MH. Anti-inflammatory, antioxidant, and immunomodulatory aspects of Nigella sativa for its preventive and bronchodilatory effects on obstructive respiratory diseases: A review of basic and clinical evidence. J Funct Foods. 2015;17:910–927. [Google Scholar]
- 35.Mariod AA, Ibrahim RM, Ismail M, Ismail N. Antioxidant activity and phenolic content of phenolic rich fractions obtained from black cumin (Nigella sativa) seedcake. Food Chem. 2009;116:306–312. [Google Scholar]
- 36.Periasamy VS, Athinarayanan J, Alshatwi AA. Anticancer activity of an ultrasonic nanoemulsion formulation of Nigella sativa L essential oil on human breast cancer cells. Ultrason Sonochem. 2016;31:449–455. doi: 10.1016/j.ultsonch.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 37.Le PM, Benhaddou-Andaloussi A, Elimadi A, Settaf A, Cherrah Y, Haddad PS. The petroleum ether extract of Nigella sativa exerts lipid-lowering and insulin-sensitizing actions in the rat. J Ethnopharmacol. 2004;94:251–259. doi: 10.1016/j.jep.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 38.Asgary S, Ghannadi A, Dashti G, Helalat A, Sahebkar A, Najafi S. Nigella sativa L. improves lipid profile and prevents atherosclerosis: Evidence from an experimental study on hypercholesterolemic rabbits. J Funct Foods. 2013;5:228–234. [Google Scholar]
- 39.Kooti W, Hasanzadeh-Noohi Z, Sharafi-Ahvazi N, Asadi-Samani M, Ashtary-Larky D. Phytochemistry, pharmacology, and therapeutic uses of black seed (Nigella sativa) Chin J Nat Med. 2016;14:732–745. doi: 10.1016/S1875-5364(16)30088-7. [DOI] [PubMed] [Google Scholar]
- 40.Rafati S, Niakan M, Naseri M. Anti-microbial effect of Nigella sativa seed extract against staphylococcal skin Infection. Med J Islam Repub Iran. 2014;28 [PMC free article] [PubMed] [Google Scholar]
- 41.Forouzanfar F, Bazzaz BSF, Hosseinzadeh H. Black cumin (Nigella sativa) and its constituent (thymoquinone): a review on antimicrobial effects. Iran J Basic Med Sci. 2014;17:929–38. [PMC free article] [PubMed] [Google Scholar]
- 42.Hosseinzadeh H, Parvardeh S. Anticonvulsant effects of thymoquinone, the major constituent of Nigella sativa seeds, in mice. Phytomedicine. 2004;11:56–64. doi: 10.1078/0944-7113-00376. [DOI] [PubMed] [Google Scholar]
- 43.Bepari A, Parashivamurthy M, Niazi S. Evaluation of anticonvulsant activity of volatile oil extract of Nigella sativa seeds by chemically induced seizure model in albino rats. Int J Basic Clin Pharmacol. 2016;5:1300–1307. [Google Scholar]
- 44.Bashir MU, Qureshi HJ. Analgesic effect of Nigella sativa seeds extract on experimentally induced pain in albino mice. J Coll Physicians Surg Pak. 2010;20:464–7. [PubMed] [Google Scholar]
- 45.Parvardeh S, Sabetkasaei M, Moghimi M, Masoudi A, Ghafghazi S, Mahboobifard F. Role of L-arginine/NO/cGMP/KATP channel signaling pathway in the central and peripheral antinociceptive effect of thymoquinone in rats. Iran J Basic Med Sci. 2018;21:625–633. doi: 10.22038/IJBMS.2018.26255.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tavakkoli A, Ahmadi A, Razavi BM, Hosseinzadeh H. Black Seed (Nigella Sativa) and its Constituent Thymoquinone as an Antidote or a Protective Agent Against Natural or Chemical Toxicities. Iran J Pharm Res IJPR. 2017;16:2–23. [PMC free article] [PubMed] [Google Scholar]
- 47.Hosseini SM, Taghiabadi E, Abnous K, Hariri AT, Pourbakhsh H, Hosseinzadeh H. Protective effect of thymoquinone, the active constituent of Nigella sativa fixed oil, against ethanol toxicity in rats. Iran J Basic Med Sci. 2017;20:927–939. doi: 10.22038/IJBMS.2017.9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harzallah JH, Kouidhi B, Flamini G, Bakhrouf A, Mahjoub T, Jrah Harzallah H, et al. Chemical composition, antimicrobial potential against cariogenic bacteria and cytotoxic activity of Tunisian Nigella sativa essential oil and thymoquinone. Food Chem. 2011;129:1469–1474. [Google Scholar]
- 49.Mohamadin AM, Sheikh B, Abd El-Aal AA, Elberry AA, Al-Abbasi FA. Protective effects of Nigella sativa oil on propoxur-induced toxicity and oxidative stress in rat brain regions. Pestic Biochem Physiol. 2010;98:128–134. [Google Scholar]
- 50.Aljabre SHM, Alakloby OM, Randhawa MA. Dermatological effects of Nigella sativa. J Dermatology Dermatologic Surg. 2015;19:92–98. [Google Scholar]
- 51.Boca AN. The Benefits of Nigella sativa in Various Skin Conditions. In: Pop RM, editor. Future Perspectives on Nigella Sativa: Characterization and Pharmacological Properties. Nova Science Publishers, Inc; 2018. pp. 219–240. [Google Scholar]
- 52.Razavi BM, Hosseinzadeh H. A review of the effects of Nigella sativa L and its constituent, thymoquinone, in metabolic syndrome. J Endocrinol Invest. 2014;37:1031–1040. doi: 10.1007/s40618-014-0150-1. [DOI] [PubMed] [Google Scholar]
- 53.Hadi V, Kheirouri S, Alizadeh M, Khabbazi A, Hosseini H. Effects of Nigella sativa oil extract on inflammatory cytokine response and oxidative stress status in patients with rheumatoid arthritis: a randomized, double-blind, placebo-controlled clinical trial. Avicenna J phytomedicine. 2016;6:34–43. [PMC free article] [PubMed] [Google Scholar]
- 54.Meddah B, Ducroc R, El Abbes Faouzi M, Eto B, Mahraoui L, Benhaddou-Andaloussi A, et al. Nigella sativa inhibits intestinal glucose absorption and improves glucose tolerance in rats. J Ethnopharmacol. 2009;121:419–424. doi: 10.1016/j.jep.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 55.Halliwell B, Gutteridge JM. The importance of free radicals and catalytic metal ions in human diseases. Mol Aspects Med. 1985;8:89–193. doi: 10.1016/0098-2997(85)90001-9. [DOI] [PubMed] [Google Scholar]
- 56.Halliwell B, Houltt JR, Blaket DR. Oxidants, inflammation, and anti-inflammatory drugs. Fed Am Soc Exp Biol J. 1988;2:2867–2873. doi: 10.1096/fasebj.2.13.2844616. [DOI] [PubMed] [Google Scholar]
- 57.Stadtman ER. Role of Oxidant Species in Aging. Curr Med Chem. 2004;11:1105–1112. doi: 10.2174/0929867043365341. [DOI] [PubMed] [Google Scholar]
- 58.Mollazadeh H, Mahdian D, Hosseinzadeh H. Medicinal plants in treatment of hypertriglyceridemia: A review based on their mechanisms and effectiveness. 2018;53:43–52. doi: 10.1016/j.phymed.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 59.Entok E, Ustuner MC, Ozbayer C, Tekin N, Akyuz F, Yangi B, et al. Anti-inflammatuar and anti-oxidative effects of Nigella sativa L: 18FDG-PET imaging of inflammation. Mol Biol Rep. 2014;41:2827–2834. doi: 10.1007/s11033-014-3137-2. [DOI] [PubMed] [Google Scholar]
- 60.Balbaa M, Abdulmalek SA, Khalil S. Oxidative stress and expression of insulin signaling proteins in the brain of diabetic rats: Role of Nigella sativa oil and antidiabetic drugs. Holscher C, editor. PLoS One. 2017;12:e0172429. doi: 10.1371/journal.pone.0172429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Noor NA, Fahmy HM, Mohammed FF, Elsayed AA, Radwan NM. Nigella sativa amliorates inflammation and demyelination in the experimental autoimmune encephalomyelitis-induced Wistar rats. Int J Clin Exp Pathol. 2015;8:6269–86. [PMC free article] [PubMed] [Google Scholar]
- 62.Yildiz A, Balikci E. Antimicrobial, anti-inflammatory and antioxidant activity of Nigella sativa in clinically endometritic cows. J Appl Anim Res. 2016;44:431–435. [Google Scholar]
- 63.Norouzi F, Abareshi A, Asgharzadeh F, Beheshti F, Hosseini M, Farzadnia M, et al. The effect of Nigella sativa on inflammation-induced myocardial fibrosis in male rats. Res Pharm Sci. 2017;12:74–81. doi: 10.4103/1735-5362.199050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schiotis RE, Goşa D. Nigella sativa as an Adjunctive Therapy for Rheumatic Diseases. In: Pop RM, editor. Future Perspectives on Nigella Sativa: Characterization and Pharmacological Properties. Nova Science Publishers, Inc; 2018. pp. 149–180. [Google Scholar]
- 65.Umar S, Zargan J, Umar K, Ahmad S, Katiyar CK, Khan HA. Modulation of the oxidative stress and inflammatory cytokine response by thymoquinone in the collagen induced arthritis in Wistar rats. Chem Biol Interact. 2012;197:40–46. doi: 10.1016/j.cbi.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 66.Hossen MJ, Yang WS, Kim D, Aravinthan A, Kim JH, Cho JY. Thymoquinone: An IRAK1 inhibitor with in vivo and in vitro anti-inflammatory activities. Sci Rep. 2017;7:42995. doi: 10.1038/srep42995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reddy KS. Cardiovascular diseases in the developing countries: dimensions, determinants, dynamics and directions for public health action. Public Health Nutr. 2002;5:231–237. doi: 10.1079/phn2001298. [DOI] [PubMed] [Google Scholar]
- 68.Prabhakaran D, Jeemon P, Roy A. Cardiovascular Diseases in India: Current Epidemiology and Future Directions. Circulation. 2016;133:1605–20. doi: 10.1161/CIRCULATIONAHA.114.008729. [DOI] [PubMed] [Google Scholar]
- 69.Rahman K, Lowe GM. Garlic and cardiovascular disease: a critical review. J Nutr. 2006;136:736S–740S. doi: 10.1093/jn/136.3.736S. [DOI] [PubMed] [Google Scholar]
- 70.Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, et al. NADPH Oxidases in Cardiovascular Health and Disease. Antioxid Redox Signal. 2006;8:691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- 71.Leong XF, Rais MM, Jaarin K. Nigella sativa and Its Protective Role in Oxidative Stress and Hypertension. Evid Based Complement Alternat Med. 2013;2013:120732. doi: 10.1155/2013/120732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sahebkar A, Beccuti G, Simental-Mendía LE, Nobili V, Bo S. Nigella sativa (black seed) effects on plasma lipid concentrations in humans: A systematic review and meta-analysis of randomized placebo-controlled trials. Pharmacol Res. 2016;106:37–50. doi: 10.1016/j.phrs.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 73.Szentandrássy N, Szigeti G, Szegedi C, Sárközi S, Magyar J, Bányász T, et al. Effect of thymol on calcium handling in mammalian ventricular myocardium. Life Sci. 2004;74:909–21. doi: 10.1016/j.lfs.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 74.Zaoui A, Cherrah Y, Lacaille-Dubois MA, Settaf A, Amarouch H, Hassar M. Diuretic and hypotensive effects of Nigella sativa in the spontaneously hypertensive rat. Therapie. 2000;55:379–82. [PubMed] [Google Scholar]
- 75.Jaarin K, Foong WD, Yeoh MH, Kamarul ZYN, Qodriyah HMS, Azman A, et al. Mechanisms of the antihypertensive effects of Nigella sativa oil in L-NAME-induced hypertensive rats. Clinics (Sao Paulo) 2015;70:751–7. doi: 10.6061/clinics/2015(11)07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guzik TJ, West NEJ, Pillai R, Taggart DP, Channon KM. Nitric oxide modulates superoxide release and peroxynitrite formation in human blood vessels. Hypertension. 2002;39:1088–1094. doi: 10.1161/01.hyp.0000018041.48432.b5. [DOI] [PubMed] [Google Scholar]
- 77.Cracowski JL, Degano B, Chabot F, Labarère J, Schwedhelm E, Monneret D, et al. Independent Association of Urinary F2 - Isoprostanes With Survival in Pulmonary Arterial Hypertension. Chest. 2012;142:869–876. doi: 10.1378/chest.11-1267. [DOI] [PubMed] [Google Scholar]
- 78.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–9. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zalba G, José GS, Moreno MU, Fortuño MA, Fortuño A, Beaumont FJ, et al. Oxidative Stress in Arterial Hypertension. Hypertension. 2001;38:1395–1399. doi: 10.1161/hy1201.099611. [DOI] [PubMed] [Google Scholar]
- 80.Shafiq H, Ahmad A, Masud T, Kaleem M. Cardio-protective and anti-cancer therapeutic potential of Nigella sativa. Iran J Basic Med Sci. 2014;17:967–980. [PMC free article] [PubMed] [Google Scholar]
- 81.Nader MA, El-Agamy DS, Suddek GM. Protective effects of propolis and thymoquinone on development of atherosclerosis in cholesterol-fed rabbits. Arch Pharm Res. 2010;33:637–643. doi: 10.1007/s12272-010-0420-1. [DOI] [PubMed] [Google Scholar]
- 82.Sabzghabaee AM, Dianatkhah M, Sarrafzadegan N, Asgary S, Ghannadi A. Clinical evaluation of Nigella sativa seeds for the treatment of hyperlipidemia: a randomized, placebo controlled clinical trial. Med Arch (Sarajevo, Bosnia Herzegovina) 2012;66:198–200. doi: 10.5455/medarh.2012.66.198-200. [DOI] [PubMed] [Google Scholar]
- 83.Bhatti I, Rehman F, Khan M, Marwat S. Effect of prophetic medicine kalonji (Nigella sativa L) on lipid profile of human beings An in vivo approach. World Appl Sci J. 2009;6:1053–1057. [Google Scholar]
- 84.Ibrahim RM, Hamdan NS, Ismail M, Saini SM, Abd Rashid SN, Abd Latiff L, et al. Protective Effects of Nigella sativa on Metabolic Syndrome in Menopausal Women. Adv Pharm Bull. 2014;4:29–33. doi: 10.5681/apb.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alobaidi AAH. Effect of Nigella Sativa and Allium Sativum Coadminstered with Simvastatin in Dyslipidemia Patients: A Prospective, Randomized, Double-Blind Trial. Antiinflamm Antiallergy Agents Med Chem. 2014;13:68–74. doi: 10.2174/18715230113129990013. [DOI] [PubMed] [Google Scholar]
- 86.de Jong A, Plat J, Mensink RP. Metabolic effects of plant sterols and stanols (Review) J Nutr Biochem. 2003;14:362–369. doi: 10.1016/s0955-2863(03)00002-0. [DOI] [PubMed] [Google Scholar]
- 87.Bamosa AO, Ali BA, al-Hawsawi ZA. The effect of thymoquinone on blood lipids in rats. Indian J Physiol Pharmacol. 2002;46:195–201. [PubMed] [Google Scholar]
- 88.Kanter M, Coskun O, Budancamanak M. Hepatoprotective effects of Nigella sativa L and Urtica dioica L on lipid peroxidation, antioxidant enzyme systems and liver enzymes in carbon tetrachloride-treated rats. World J Gastroenterol. 2005;11:6684. doi: 10.3748/wjg.v11.i42.6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu H, Liu HY, Jiang YN, Li N. Protective effect of thymoquinone improves cardiovascular function, and attenuates oxidative stress, inflammation and apoptosis by mediating the PI3K/Akt pathway in diabetic rats. Mol Med Rep. 2016;13:2836–2842. doi: 10.3892/mmr.2016.4823. [DOI] [PubMed] [Google Scholar]
- 90.Shabana A, El-Menyar A, Asim M, Al-Azzeh H, Al Thani H. Cardiovascular Benefits of Black Cumin (Nigella sativa) Cardiovasc Toxicol. 2013;13:9–21. doi: 10.1007/s12012-012-9181-z. [DOI] [PubMed] [Google Scholar]
- 91.Ragheb A, Elbarbry F, Prasad K, Mohamed A, Ahmed MS, Shoker A. Attenuation of the development of hypercholesterolemic atherosclerosis by thymoquinone. Int J Angiol. 2008;17:186–92. doi: 10.1055/s-0031-1278307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kaatabi H, Bamosa AO, Badar A, Al-Elq A, Abou-Hozaifa B, Lebda F, et al. Nigella sativa improves glycemic control and ameliorates oxidative stress in patients with type 2 diabetes mellitus: Placebo controlled participant blinded clinical trial. PLoS One. 2015;10:1–15. doi: 10.1371/journal.pone.0113486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hosseini SM, Taghiabadi E, Abnous K, Hariri AT, Pourbakhsh H. Protective effect of thymoquinone, the active constituent of Nigella sativa fixed oil, against ethanol toxicity in rats. Iran J Basic Med Sci. 2017;20:927–939. doi: 10.22038/IJBMS.2017.9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rchid H, Chevassus H, Nmila R, Guiral C, Petit P, Chokairi M, et al. Nigella sativa seed extracts enhance glucose-induced insulin release from rat-isolated Langerhans islets. Fundam Clin Pharmacol. 2004;18:525–529. doi: 10.1111/j.1472-8206.2004.00275.x. [DOI] [PubMed] [Google Scholar]
- 95.Benhaddou-Andaloussi A, Martineau L, Spoor D, Vuong T, Leduc C, Joly E, et al. Antidiabetic Activity of Nigella sativa Seed Extract in Cultured Pancreatic β-cells, Skeletal Muscle Cells, and Adipocytes. Pharm Biol. 2008;46:96–104. [Google Scholar]
- 96.Samarghandian S, Farkhondeh T, Samini F, Borji A. Protective Effects of Carvacrol against Oxidative Stress Induced by Chronic Stress in Rat’s Brain, Liver, and Kidney. Biochem Res Int. 2016;2016:2645237. doi: 10.1155/2016/2645237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zheng Y, Wang X, Ji S, Tian S, Wu H, Luo P, et al. Mepenzolate bromide promotes diabetic wound healing by modulating inflammation and oxidative stress. Am J Transl Res. 2016;8:2738–2747. [PMC free article] [PubMed] [Google Scholar]
- 98.Dias JA, Fredrikson GN, Ericson U, Gullberg B, Hedblad B, Engström G, et al. Low-Grade Inflammation, Oxidative Stress and Risk of Invasive Post-Menopausal Breast Cancer - A Nested Case-Control Study from the Malmö Diet and Cancer Cohort. PLoS One. 2016;11:e0158959. doi: 10.1371/journal.pone.0158959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.AI-Trad B, AI-Batayneh K, EI-Metwally S, Alhazimi A, Ginawi I, Alaraj M, et al. Albuminuria and Renal Extracellular Matrix. Eur Rev Med Pharmacol Sci. 2016;20:2680–2688. [PubMed] [Google Scholar]
- 100.Smith A, Howell D, Patmore R, Jack A, Roman E. Incidence of haematological malignancy by sub-type: a report from the Haematological Malignancy Research Network. Br J Cancer. 2011;105:1684–1692. doi: 10.1038/bjc.2011.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Majdalawieh AF, Fayyad MW, Nasrallah GK. Anti-cancer properties and mechanisms of action of thymoquinone, the major active ingredient of Nigella sativa. Crit Rev Food Sci Nutr. 2017;57:3911–3928. doi: 10.1080/10408398.2016.1277971. [DOI] [PubMed] [Google Scholar]
- 102.El-Mahdy MA, Zhu Q, Wang QE, Wani G, Wani AA. Thymoquinone induces apoptosis through activation of caspase-8 and mitochondrial events in p53-null myeloblastic leukemia HL-60 cells. Int J Cancer. 2005;117:409–417. doi: 10.1002/ijc.21205. [DOI] [PubMed] [Google Scholar]
- 103.Alhosin M, Abusnina A, Achour M, Sharif T, Muller C, Peluso J, et al. Induction of apoptosis by thymoquinone in lymphoblastic leukemia Jurkat cells is mediated by a p73-dependent pathway which targets the epigenetic integrator UHRF1. Biochem Pharmacol. 2010;79:1251–1260. doi: 10.1016/j.bcp.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 104.Ashraf W, Ibrahim A, Alhosin M, Zaayter L, Ouararhni K, Papin C, et al. The epigenetic integrator UHRF1: on the road to become a universal biomarker for cancer. Oncotarget. 2017;8:51946–51962. doi: 10.18632/oncotarget.17393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Abusnina A, Alhosin M, Keravis T, Muller CD, Fuhrmann G, Bronner C, et al. Down-regulation of cyclic nucleotide phosphodiesterase PDE1A is the key event of p73 and UHRF1 deregulation in thymoquinone-induced acute lymphoblastic leukemia cell apoptosis. Cell Signal. 2011;23:152–60. doi: 10.1016/j.cellsig.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 106.Pang J, Shen N, Yan F, Zhao N, Dou L, Wu LC, et al. Thymoquinone exerts potent growth-suppressive activity on leukemia through DNA hypermethylation reversal in leukemia cells. Oncotarget. 2017;8:34453–34467. doi: 10.18632/oncotarget.16431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Salim LZA, Mohan S, Othman R, Abdelwahab SI, Kamalidehghan B, Sheikh BY, et al. Thymoquinone induces mitochondria-mediated apoptosis in acute lymphoblastic leukaemia in vitro. Molecules. 2013;18:11219–40. doi: 10.3390/molecules180911219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ali Salim LZ, Othman R, Abdulla MA, Al-Jashamy K, Mohd AH, Hassandarvish P, et al. Thymoquinone Inhibits Murine Leukemia WEHI-3 Cells In Vivo and In Vitro. Wang Z, editor. PLoS One. 2014;9:e115340. doi: 10.1371/journal.pone.0115340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dergarabetian EM, Ghattass KI, El-Sitt SB, Al-Mismar RM, El-Baba CO, Itani WS, et al. Thymoquinone induces apoptosis in malignant T-cells via generation of ROS. Front Biosci (Elite Ed) 2013;5:706–19. doi: 10.2741/e651. [DOI] [PubMed] [Google Scholar]
- 110.Soltani A, Pourgheysari B, Shirzad H, Sourani Z. Antiproliferative and Apoptosis-Inducing Activities of Thymoquinone in Lymphoblastic Leukemia Cell Line. Indian J Hematol Blood Transfus. 2017;33:516–524. doi: 10.1007/s12288-016-0758-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Diab-Assaf M, Semaan J, El-Sabban M, Al Jaouni SK, Azar R, Kamal MA, et al. Inhibition of Proliferation and Induction of Apoptosis by Thymoquinone via Modulation of TGF Family, p53, p21 and Bcl-2α in Leukemic Cells. Anticancer Agents Med Chem. 2018;18:210–215. doi: 10.2174/1871520617666170912133054. [DOI] [PubMed] [Google Scholar]
- 112.Hussain AR, Ahmed M, Ahmed S, Manogaran P, Platanias LC, Alvi SN, et al. Thymoquinone suppresses growth and induces apoptosis via generation of reactive oxygen species in primary effusion lymphoma. Free Radic Biol Med. 2011;50:978–87. doi: 10.1016/j.freeradbiomed.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 113.Hussain AR, Uddin S, Ahmed M, Al-Dayel F, Bavi PP, Al-Kuraya KS. Phosphorylated IκBα predicts poor prognosis in activated B-cell lymphoma and its inhibition with thymoquinone induces apoptosis via ROS release. Climent JAM, editor. PLoS One. 2013;8:e60540. doi: 10.1371/journal.pone.0060540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zihlif MA, Mahmoud IS, Ghanim MT, Zreikat MS, Alrabadi N, Imraish A, et al. Thymoquinone efficiently inhibits the survival of EBV-infected B cells and alters EBV gene expression. Integr Cancer Ther. 2013;12:257–63. doi: 10.1177/1534735412458827. [DOI] [PubMed] [Google Scholar]
- 115.Li F, Rajendran P, Sethi G. Thymoquinone inhibits proliferation, induces apoptosis and chemosensitizes human multiple myeloma cells through suppression of signal transducer and activator of transcription 3 activation pathway. Br J Pharmacol. 2010;161:541–54. doi: 10.1111/j.1476-5381.2010.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Badr G, Mohany M, Abu-Tarboush F. Thymoquinone decreases F-actin polymerization and the proliferation of human multiple myeloma cells by suppressing STAT3 phosphorylation and Bcl2/Bcl-XL expression. Lipids Health Dis. 2011;10:236. doi: 10.1186/1476-511X-10-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Effenberger-Neidnicht K, Schobert R. Combinatorial effects of thymoquinone on the anti-cancer activity of doxorubicin. Cancer Chemother Pharmacol. 2011;67:867–74. doi: 10.1007/s00280-010-1386-x. [DOI] [PubMed] [Google Scholar]
- 118.Banerjee S, Kaseb AO, Wang Z, Kong D, Mohammad M, Padhye S, et al. Antitumor activity of gemcitabine and oxaliplatin is augmented by thymoquinone in pancreatic cancer. Cancer Res. 2009;69:5575–83. doi: 10.1158/0008-5472.CAN-08-4235. [DOI] [PubMed] [Google Scholar]
- 119.Jafri SH, Glass J, Shi R, Zhang S, Prince M, Kleiner-Hancock H. Thymoquinone and cisplatin as a therapeutic combination in lung cancer: In vitro and in vivo. J Exp Clin Cancer Res. 2010;29 doi: 10.1186/1756-9966-29-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Khalife R, El-Hayek S, Stephany E-H, Tarras O, Hodroj MH, Rizk S. Antiproliferative and proapoptotic effects of topotecan in combination with thymoquinone on acute myelogenous leukemia. Clin Lymphoma Myeloma Leuk. 2014;14 Suppl:S46–55. doi: 10.1016/j.clml.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 121.Siveen KS, Mustafa N, Li F, Kannaiyan R, Ahn KS, Kumar AP, et al. Thymoquinone overcomes chemoresistance and enhances the anticancer effects of bortezomib through abrogation of NF-κB regulated gene products in multiple myeloma xenograft mouse model. Oncotarget. 2014;5:634–48. doi: 10.18632/oncotarget.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pazhouhi M, Sariri R, Rabzia A, Khazaei M. Thymoquinone synergistically potentiates temozolomide cytotoxicity through the inhibition of autophagy in U87MG cell line. Iran J Basic Med Sci. 2016;19:890–898. [PMC free article] [PubMed] [Google Scholar]
- 123.al-Shabanah OA, Badary OA, Nagi MN, al-Gharably NM, al-Rikabi AC, al-Bekairi AM. Thymoquinone protects against doxorubicin-induced cardiotoxicity without compromising its antitumor activity. J Exp Clin Cancer Res. 1998;17:193–8. [PubMed] [Google Scholar]
- 124.Nagi MN, Mansour MA. Protective effect of thymoquinone against doxorubicin-induced cardiotoxicity in rats: a possible mechanism of protection. Pharmacol Res. 2000;41:283–9. doi: 10.1006/phrs.1999.0585. [DOI] [PubMed] [Google Scholar]
- 125.Brown RK, Wilson G, Tucci MA, Benghuzzi HA. The effects of thymoquinone and Doxorubicin on leukemia and cardiomyocyte cell lines. Biomed Sci Instrum. 2014;50:391–6. [PubMed] [Google Scholar]
- 126.Matthaus B, Özcan MM. Fatty Acids, Tocopherol, and Sterol Contents of Some Nigella Species Seed Oil. Czech J Food Sci. 2011;29:145–150. [Google Scholar]
- 127.Hamrouni-Sellami I, Kchouk ME, Marzouk B. Lipid and aroma composition of black cumin (Nigella sativa L) seeds from Tunisia. J Food Biochem. 2008;32:335–352. [Google Scholar]
- 128.Benkaci-Ali F, Baaliouamer A, Wathelet JP, Marlier M. Chemical Composition and physiochemical characteristics of fixed oils from Algerian Nigella sativa seeds. Chem Nat Compd. 2012;47:811–815. [Google Scholar]
- 129.Juhaimi F Al, Matthäus B, Ghafoora K, ElBabiker EF, Özcan MM. Fatty acids, tocopherols, minerals contents of Nigella sativa and Trigonella foenum-graecum seed and seed oils. Riv Ital delle Sostanze Grasse. 2016;93:165–171. [Google Scholar]
- 130.Al-Jasass FM, Al-Jasser MS. Chemical composition and fatty acid content of some spices and herbs under Saudi Arabia conditions. ScientificWorldJournal. 2012;2012:859892. doi: 10.1100/2012/859892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cheikh-Rouhou S, Besbes S, Hentati B, Blecker C, Deroanne C, Attia H. Nigella sativa : Chemical composition and physicochemical characteristics of lipid fraction. Food Chem. 2007;101:673–681. [Google Scholar]
- 132.Toma CC, Simu GM, Hanganu D, Olah N, Vata FMG, Hammami C, et al. Chemical composition of the Tunisian Nigella sativa Note I Profile on essential oil. Farmacia. 2010;58:458–464. [Google Scholar]
- 133.Nickavar B, Mojab F, Javidnia K, Ali M, Amoli R. Chemical Composition of the Fixed and Volatile Oils of Nigella sativa L. from Iran. Z Naturforsch. 2003;58:629–631. doi: 10.1515/znc-2003-9-1004. [DOI] [PubMed] [Google Scholar]
- 134.Gokyer A. Determination of Physicochemical Properties of Nigella sativa Seed Oil from Balıkesir Region , Turkey. Chem Process Eng Res. 2016;41:43–46. [Google Scholar]
- 135.Akloul R, Benkaci-Ali F, Zerrouki M, Eppe G. Composition and biological activities of the essential oil of Nigella sativa seeds isolated by accelerated microwave steam distillation with cryogenic grinding. Am J Essent Oils Nat Prod AJEONP. 2014;1:23–33. [Google Scholar]
- 136.Botnick I, Xue W, Bar E, Ibdah M, Schwartz A, Joel DM, et al. Distribution of primary and specialized metabolites in Nigella sativa seeds, a spice with vast traditional and historical uses. Molecules. 2012;17:10159–77. doi: 10.3390/molecules170910159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Toma CC, Simu GM, Olah N, Georgiana FM. Chemical composition of the Tunisian Nigella sativa Note I Profile on essential oil. Farmacia. 2010;58:458–464. [Google Scholar]
- 138.Bourgou S, Ksouri R, Bellila A, Skandrani I, Falleh H, Marzouk B. Phenolic composition and biological activities of Tunisian Nigella sativa L shoots and roots. C R Biol. 2008;331:48–55. doi: 10.1016/j.crvi.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 139.Houghton P, Zarka R, de las Heras B, Hoult J. Fixed Oil of Nigella sativa and Derived Thymoquinone Inhibit Eicosanoid Generation in Leukocytes and Membrane Lipid Peroxidation. Planta Med. 1995;61:33–36. doi: 10.1055/s-2006-957994. [DOI] [PubMed] [Google Scholar]
- 140.El-Dakhakhny M, Madi N, Lembert N, Ammon HP. Nigella sativa oil, nigellone and derived thymoquinone inhibit synthesis of 5-lipoxygenase products in polymorphonuclear leukocytes from rats. J Ethnopharmacol. 2002;81:161–164. doi: 10.1016/s0378-8741(02)00051-x. [DOI] [PubMed] [Google Scholar]
- 141.Mansour M, Tornhamre S. Inhibition of 5-lipoxygenase and Leukotriene C4 Synthase in Human Blood Cells by Thymoquinone. J Enzyme Inhib Med Chem. 2004;19:431–436. doi: 10.1080/14756360400002072. [DOI] [PubMed] [Google Scholar]
- 142.Mohamed A, Shoker A, Bendjelloul F, Mare A, Alzrigh M, Benghuzzi H, et al. Improvement of experimental allergic encephalomyelitis (EAE) by thymoquinone; an oxidative stress inhibitor. Biomed Sci Instrum. 2003;39:440–445. [PubMed] [Google Scholar]
- 143.Mohamed A, Waris HM, Ramadan H, Qureshi M, Kalra J. Amelioration of Chronic Relapsing Experimental autoimmune encephalomyelitis (CR-EAE) using thymoquinone. Biomedical Sciences Instrumentation. 2009:274–279. [PubMed] [Google Scholar]
- 144.Mahgoub AA. Thymoquinone protects against experimental colitis in rats. Toxicol Lett. 2003;143:133–143. doi: 10.1016/s0378-4274(03)00173-5. [DOI] [PubMed] [Google Scholar]
- 145.Juhás S, Cikos S, Czikková S, Veselá J, Il’ková G, Hájek T, et al. Effects of borneol and thymoquinone on TNBS-induced colitis in mice. Folia Biol (Praha) 2008;54:1–7. [PubMed] [Google Scholar]
- 146.Emekli-Alturfan E, Yarat A, Tunali-Akbay T, Isik F, Yenidogan G, Sener G, et al. Effect of black cumin (Nigella sativa) seed oil on gastric tissue in experimental colitis. Adv Environ Biol. 2011;5:483–490. [Google Scholar]
- 147.Isik F, Tunali Akbay T, Yarat A, Genc Z, Pisiriciler R, Caliskan-Ak E, et al. Protective Effects of Black Cumin (Nigella sativa) Oil on TNBS-Induced Experimental Colitis in Rats. Dig Dis Sci. 2011;56:721–730. doi: 10.1007/s10620-010-1333-z. [DOI] [PubMed] [Google Scholar]
- 148.Suguna P, Geetha A, Aruna R, Siva GV. Effect of thymoquinone on ethanol and high fat diet induced chronic pancreatitis - a dose response study in rats. Indian J Exp Biol. 2013;51:292–302. [PubMed] [Google Scholar]
- 149.Mutabagani A, El-Mahdy SAM. A study of the anti-inflammatory activity of Nigella sativa L and thymoquinone in rats. Saudi Pharm J. 1997;5:110–113. [Google Scholar]
- 150.Hajhashemi V, Ghannadi A, Jafarabadi H. Black cumin seed essential oil, as a potent analgesic and antiinflammatory drug. Phyther Res. 2004;18:195–199. doi: 10.1002/ptr.1390. [DOI] [PubMed] [Google Scholar]
- 151.Al-Ghamdi MS. The anti-inflammatory, analgesic and antipyretic activity of Nigella sativa. J Ethnopharmacol. 2001;76:45–48. doi: 10.1016/s0378-8741(01)00216-1. [DOI] [PubMed] [Google Scholar]