Supplemental Digital Content is available in the text.
Abstract
Introduction:
Annual influenza vaccination is recommended for all US children 6 months and older to prevent morbidity and mortality. Despite these recommendations, only ~50% of US children are vaccinated annually. Influenza vaccine administration in the pediatric emergency department (ED) is an innovative solution to improve vaccination rates. However, during the 2017–2018 influenza season, only 75 influenza vaccinations were given in this tertiary care ED. We aimed to increase the number of influenza vaccines administered to ED patients from 75 to 1,000 between August 2018 and March 2019.s
Methods:
Process mapping identified potential barriers and solutions. Key interventions included mandatory vaccine screening, creation of a vaccine administration protocol, education for family, provider, and nursing, a revised pharmacy workflow, and weekly staff feedback. Interventions were tested using plan-do-study-act cycles. The process measure was the percent of patients screened for vaccine status. The primary outcome was the number of influenza vaccines administered. The balancing measures were ED length of stay (LOS), wasted vaccines, and financial impact on the institution.
Results:
We included 33,311 children in this study. Screening for vaccine status improved from 0% to 90%. Of those screened, 58% were eligible for vaccination, and 8.5% of eligible patients were vaccinated in the ED. In total, 1,323 vaccines were administered with no significant change in ED LOS (139 min) and no lost revenue to the hospital.
Conclusions:
We implemented an efficient, cost-effective, influenza vaccination program in the pediatric ED and successfully increased vaccinations in a population that might not otherwise receive the vaccine.
INTRODUCTION
Influenza is a serious public health problem for the US children. Over 11 million children have been affected annually, and healthcare services for pediatric influenza reach $2.5 billion each season.1,2 On average, 113 children die annually from influenza-related illness, and 80% of these deaths occur in unvaccinated children.3–5 Universal administration of the seasonal influenza vaccine remains the best strategy for the prevention of illness and death, and immunization is recommended by the American Academy of Pediatrics (AAP) and Centers for Disease Control and Prevention (CDC) for everyone 6 months and older.6,7 The influenza vaccine decreases influenza-related death by 50% in pediatric patients with an underlying chronic medical condition and by 65% in otherwise healthy children.5
Despite current recommendations, influenza vaccine coverage of children remains poor. Only 57% of eligible US children were fully vaccinated against influenza in 2017.8 Vaccination rates vary by state, and in Wisconsin, only 38% of eligible children received influenza vaccines in 2017.9 Disparities in vaccination rates also exist based on age, race, ethnicity, and insurance status.10–13 Barriers to receiving vaccines include educational deficiencies, fear or mistrust, and poor healthcare access.14 To overcome these barriers, the CDC sponsors the Vaccines For Children (VFC) program, a federally funded US program that provides vaccines free of charge to qualified children: Medicaid-eligible, uninsured, under-insured, or American Indian and Alaskan Native.15 In addition, the AAP encourages the routine assessment of immunization status and administering vaccines during all visits, including sick visits.6
The emergency department (ED) presents a unique opportunity to reach children who may not otherwise be vaccinated against influenza. Rao et al. demonstrated that 42% of healthy children hospitalized for influenza had a “missed opportunity visit” defined as a tertiary pediatric inpatient, outpatient, or ED visit early in the flu season where the child did not receive the influenza vaccine, and 27% of these missed visits occurred in an ED or urgent care.16 Parent surveys suggest the majority of parents would accept influenza vaccines in a pediatric ED.14,17,18 Newcombe et al19 demonstrated that the strongest predictor of influenza vaccine uptake is having a healthcare provider recommend the vaccine.20 However, surveys from providers reveal that over half of providers forget to inquire about vaccination status or fear they do not have enough time to address parent concerns about vaccines while in the ED.14,21 Therefore, there is a need for a highly reliable, efficient way to offer and administer the vaccine in the pediatric ED.
We theorized that a protocolized, efficient, and cost-effective influenza vaccination program in the pediatric ED would increase vaccination rates, especially in pediatric populations with historically low vaccination rates.13,22,23 We aimed to increase the number of influenza vaccines administered to patients discharged from the ED from 75 to 1,000 between August 2018 and March 2019. This goal was chosen after the first 2 months of vaccine count data. It demonstrated that 1,000 vaccinations were a lofty yet achievable goal for nursing staff.
METHODS
Context
This 296-bed, tertiary care, pediatric academic center located in Milwaukee, WI, has a 36-bed, level 1 pediatric trauma center with 71,440 ED visits and 7,478 admissions in 2017. As the only children’s hospital in Southeastern Wisconsin, it serves as a primary referral site for surrounding urban and rural areas. It is an important safety net for the community and provides a medical home for families unable to attend primary care visits. The team defined the target population as ED patients ages 6 months to 18 years, with Emergency Severity Index (ESI) levels 2–5, who were discharged home from August 26, 2018 through March 31, 2019. We excluded patients if they had a prior influenza vaccine this season. Vaccines were deferred until hospital discharge in admitted patients due to concerns that vaccine side effects, especially fever, may misinform medical decision-making for inpatients. Reviewing immunization status was a hospital-wide priority. In early 2017, 97.5% of ED patients at this institution had their immunization status reviewed by a nurse as a part of the required ED nursing documentation. Influenza vaccine status was added to the immunizations’ tab in September 2017; however, it was not required for immunization documentation.
INTERVENTIONS
The multidisciplinary ED immunization workgroup was formed in May 2018 and included an ED nurse, ED clinical nurse specialist, ED physicians, a pharmacist, and hospital leadership. Technical support was provided by a data analyst and electronic health record (EHR) analysts. The 2015 version of Epic Systems, Corp. (Verona, Win.) was utilized as the EHR throughout this project. An electronic extract including process, outcome, and balancing measures for this refined population was available to the team for continuous result monitoring throughout the influenza season.
We performed process mapping to identify barriers to influenza vaccine screening and administration in the ED. Three key drivers arose: mandatory influenza vaccination screening, a standardized educational message for nurses to recommend and offer the vaccine, and an efficient workflow to order, prepare, and administer the vaccine. Five strategic interventions were implemented using plan-do-study-act cycles, including required influenza vaccine status screening by nurses, creation of an influenza vaccine administration nursing protocol, education for providers, nurses, and families; a modified pharmacy workflow, and weekly staff feedback.
Influenza Vaccine Screening
The influenza vaccine screening question became available in the EHR on October 1, and screening was performed during the initial nursing assessment in triage or at the bedside. The screening question asked, “Has the patient received the influenza vaccine this season?” The nurse then documented, “yes already received vaccine,” “no, requests vaccine,” or “no, declines vaccine.” Two weeks later, the screening question became required documentation for all nurses. Based on nursing feedback, the influenza screening process was revised to include scripted dialog to strongly recommend the vaccine to any unvaccinated child and address family concerns.
Influenza Vaccine Administration Protocol
ED nursing protocols are hospital policies sanctioned by a committee and reviewed annually. To facilitate efficient vaccine administration, we implemented a nursing protocol on October 15 to allow one-step influenza vaccine ordering by the nurse. If the screening nurse answered “no, requests vaccine,” a clinical decision support tool and best practice advisory (BPA) were triggered in the EHR. The BPA allowed the nurse to verify patient eligibility, review the influenza vaccine protocol, and order the vaccine. Vaccine contraindications included prior serious allergic reaction to the vaccine or any component of the vaccine, and precautions included moderate to severe illness with or without fever and history of Guillian–Barré syndrome within 6 weeks of the vaccine. These vaccine contraindications and precautions were listed in a table in the BPA. No hard stops existed to prevent nursing from ordering a vaccine; however, once a contraindication or precaution was identified, the nurse was instructed to click, “do not order” and “vaccine contraindicated.”
Before vaccine administration, the provider received a separate BPA to confirm eligibility for the influenza vaccine. If a precaution existed, provider discretion would determine if the patient could safely receive the vaccine. If the order was approved, the nurse administered the vaccine at the time of discharge. Figure 1 outlines this revised workflow in comparison to the baseline process.
Fig. 1.
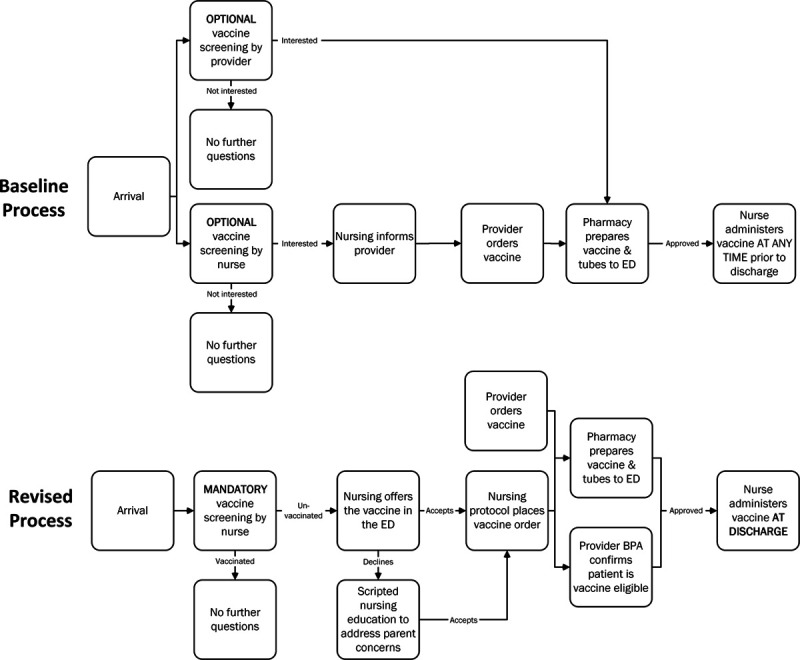
Process map highlighting the revised influenza vaccine administration process compared to the baseline ED process. Differences include mandatory influenza screening, nurse-initiated vaccine orders, scripted education, best practice advisories to confirm vaccine eligibility, and standardized vaccine administration.
Education for Nurses, Providers, and Families
During staff meetings in September, targeted nursing and provider education demonstrated the rationale for influenza vaccine administration, safety and efficacy of the vaccine, and screenshots of the new influenza vaccine BPAs. Nurse training addressed the new protocol, scripted dialog to promote vaccine acceptance, and strategies to overcome vaccine hesitancy. Formal CDC “flu fact sheets” and organizational posters, table-tents, and TV messages were available to families in the ED waiting room to promote influenza vaccine safety and efficacy.24
Studying the intervention
Two concerns arose after the multidisciplinary team reviewed weekly data, shared outcomes with the staff, and elicited staff feedback. In December, pharmacy noted an increase in wasted vaccines (vaccines ordered, not administered, and then discarded). Due to storage and temperature requirements, the vaccine could not be reused once distributed to the ED; therefore, vaccines were wasted if patients left before vaccination. In January, a significant decrease in vaccination rates occurred, and feedback from families and staff uncovered concerns over the potential for increased out-of-pocket costs to the patients. An audit demonstrated that the influenza vaccine did not increase out-of-pocket costs. Despite sharing these findings with nursing, vaccination rates continued to stagnate. To address these 2 issues, the team adjusted the pharmacy workflow to reduce wasted vaccines, and they developed a new motivational tool to provide weekly staff feedback.
Pharmacy Workflow
In the original process, the vaccine was sent from the pharmacy to the ED immediately after the nursing protocol order and before provider confirmation of the vaccine order. This process resulted in wasted vaccines. Therefore, the workflow was modified, so the vaccine was prepared and held in the pharmacy until the time of discharge. Once the provider confirmed vaccine eligibility, the discharging nurse called the pharmacy and requested vaccine delivery to the ED.
Weekly Staff Feedback
In January 2019, the team developed a visual thermometer graphic to illustrate the current count of vaccines given in the ED compared to the 1,000 vaccine goal. Nurses administering the most vaccines each week were recognized. A celebration and photoshoot were performed after the administration of the 1,000th influenza vaccine in the ED. This success was shared throughout the organization and on social media.
MEASURES
Process measures included the percent of included patients screened for influenza vaccine status, percent of screened patients eligible for the vaccine, and percent of eligible patients accepting the vaccine. The primary outcome measures were the total number of vaccines administered, and the percent of eligible patients vaccinated during this influenza season. The balancing measures included ED length of stay (LOS) for vaccine eligible patients, the number of wasted vaccines, and the financial impact of the vaccination program to the hospital.
Analysis
We used descriptive statistics to define the total vaccine eligible population and used chi-square analysis to compare demographic characteristics in vaccine eligible patients accepting the vaccine to those declining the vaccine. Statistical process control charts were utilized to measure the impact of the interventions in real time. The centerline and control limits were revised when a special cause was noted as defined by 8 consecutive points above or below the mean or a single data point above or below the upper or lower confidence interval.
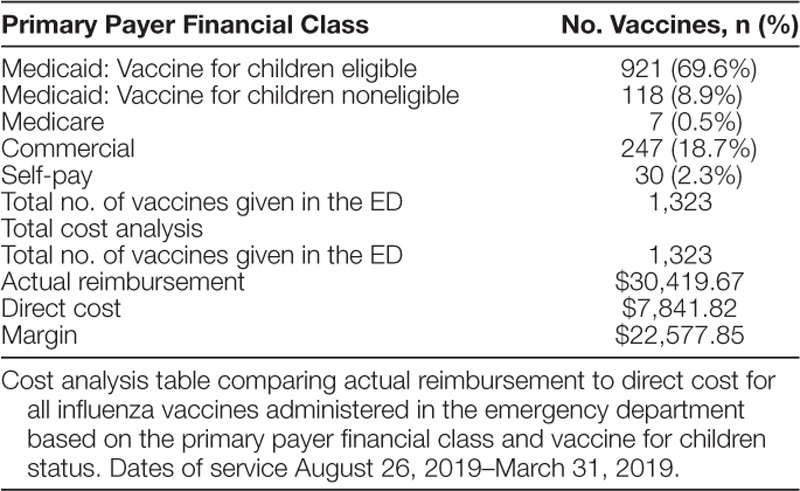
We performed a formal cost analysis by comparing the payments received from the patient’s insurance company to the direct variable costs for these charges. All payers are charged the same amount for the services; however, payments vary based on the insurance company’s contracted fee schedule. Direct variable costs include administration charge, determined by an internal cost accounting system, and vaccine charge, determined by the actual invoice cost. Any patients covered under the VFC program had a vaccine cost of zero.15
Ethical Considerations
The institutional review board determined the project to be quality improvement and thus exempt from informed consent. There were no identified conflicts of interest.
RESULTS
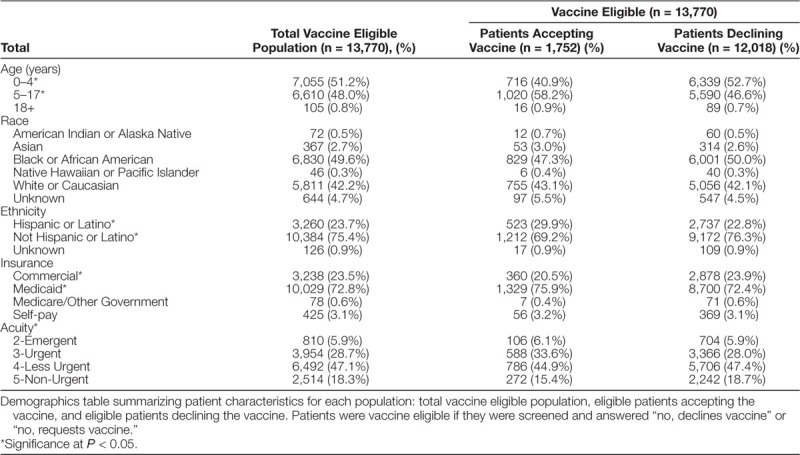
Thirty-three thousand three hundred eleven patients met the inclusion criteria for this quality improvement initiative. Table 1 describes the demographic variables for the total vaccine eligible population (n = 13,770) and compares these variables in eligible patients accepting the vaccine (n = 1,752) to those declining the vaccine (n = 12,018). Significantly higher acceptance rates were seen in patients 5–17 years of age, Hispanics/Latinos, patients with Medicaid Insurance, and those with level 3 acuity.
Table 1.
Demographics in Vaccine Eligible Patients
When comparing the preintervention to postintervention periods, screening for influenza vaccination status improved from 0% to 90% (Fig. 2). Figure 3 is a consort diagram detailing the vaccination process. Figure 3 illustrates that of the 33,311 included patients, 71% (n = 23,635) were screened for vaccine status, and 58% (n = 13,770) of those screened were unvaccinated and eligible to receive a vaccine. Of those identified as eligible to receive a vaccine, 13% (n = 1,752) accepted/asked to receive the vaccine in the ED. The most common reasons for declining included a plan to receive the vaccine at the primary care office or uncertainty about the vaccine. Additional documented refusal reasons included a contraindication/allergy, child too sick, no guardian present, the patient refused, or patient already vaccinated. Of those accepting the vaccine, 67% (n = 1,167) were vaccinated. Therefore, 8.5% (n = 1,167) of the eligible population was vaccinated via the vaccine administration protocol. Another (n = 156) vaccines were administered outside of the protocol. Of these 156 patients, 59 were not screened, yet, providers ordered the vaccine outside of the protocol. Twenty-seven patients required and received their second influenza vaccine of the season per CDC recommendations, and 70 initially declined the vaccine but agreed to receive it after additional provider counseling.
Fig. 2.
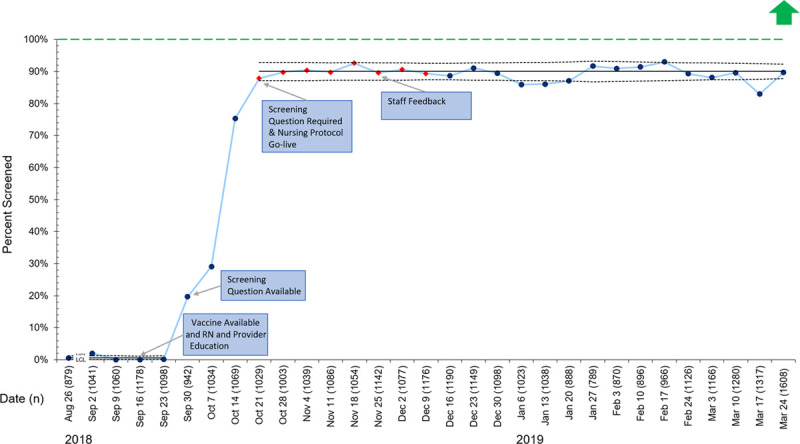
P chart representing the percentage of included patients screened for influenza vaccination each week. Red diamonds indicate a shift in the data. Green dashed line represents the goal line. LCL, lower control limit; UCL, upper control limit.
Fig. 3.
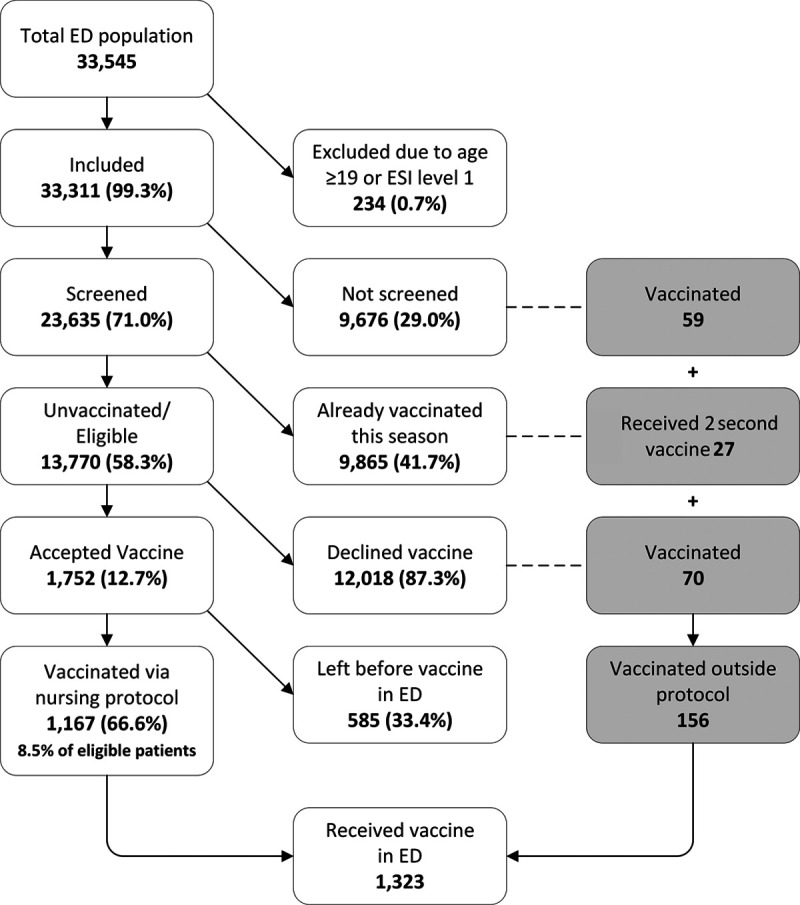
Consort diagram illustrating the vaccination process.
In total, 1,323 vaccines were administered in the ED during the project timeframe, surpassing the goal of administering 1000 vaccines. Figure 4 demonstrates the impact of each plan-do-study-act cycle on weekly vaccine counts. Vaccination rates peaked in the fall after the go-live of the screening questionnaire and the nursing vaccination protocol. In mid-December, the team noted a decrease in vaccines due to concerns over increased waste and the potential increased out-of-pocket cost to the patients. Despite addressing these concerns, vaccination rates did not improve. Therefore, in January, the team provided weekly feedback to staff and awarded the nurses ordering the most vaccines each week. This change led to the administration of the 1000th vaccine in early February. The hospital supply of influenza vaccines was exhausted by mid-February; however, we obtained additional vaccines from the hospital system’s outpatient primary care group, and the ED vaccination campaign continued. Vaccination rates in eligible patients mirror the pattern seen in the vaccine count data (see Fig. 1, Supplemental Digital Content 1, http://links.lww.com/PQ9/A198).
Fig. 4.
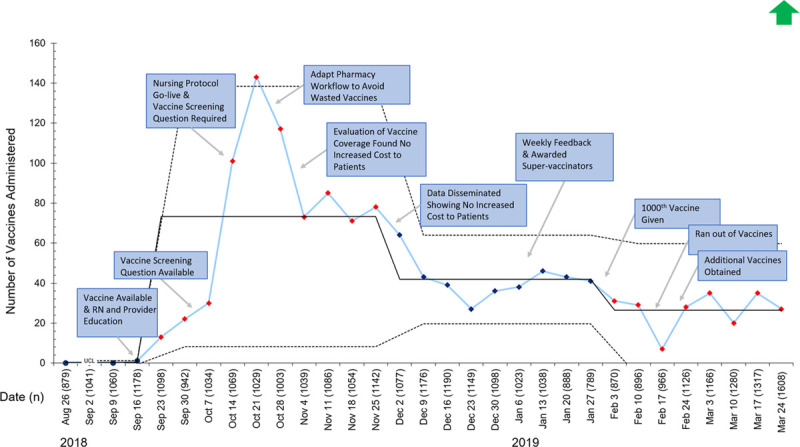
I chart representing a count of the number of influenza vaccines administered in the pediatric ED each week. Red and blue diamonds indicate a shift in the data. LCL, lower control limit; UCL, upper control limit.
We analyzed the impact of the ED census on vaccination efforts and found that the ED census did not inversely correlate with the number of patients receiving vaccines. (see Fig. 2, Supplemental Digital Content 2, http://links.lww.com/PQ9/A198) Instead, the uptake of pediatric influenza vaccines demonstrates seasonal variation where vaccination rates peak in early fall when demand is high then decline throughout the influenza season. Data on pediatric influenza administration from the WI Department of Health mimics the pattern seen in our ED, where 87% of patients were vaccinated before January 2019 (see Fig. 3, Supplemental Digital Content 3, http://links.lww.com/PQ9/A198). These findings have been corroborated (Danielle Sill, MSPH, WI Department of Health Services, Unpublished Data March 2019).
To balance these interventions, we observed no statistically significant increase in ED LOS in eligible patients. Wasted vaccines (20 per week) were noted within the first month of the project; however, revised nursing and pharmacy workflows nearly eliminated wasted vaccines. A formal cost analysis comparing hospital reimbursement and direct cost of vaccine administration revealed approximately $22,500 net revenue to the hospital (Table 2).
Table 2.
Cost Analysis
DISCUSSION
Summary
Following the implementation of this quality improvement effort, screening for influenza vaccine status improved from zero to 90% and was sustained. One thousand three hundred twenty-three children received an influenza vaccine in the pediatric ED compared to 75 children during the prior season. Influenza vaccination rates were improved by 8.5% in eligible patients. There was no significant increase in ED LOS. The project resulted in over $22,500 increase in net revenue to the hospital.
Interpretation
The pediatric ED population is unique because, unlike the primary care office, patients present for perceived medical emergencies and vaccinations are not the primary goal of the visit. Therefore, increasing influenza vaccination rates by 8.5% in patients who may not have otherwise received the vaccine corresponds to potentially preventing 264 cases of influenza and 110 cases of influenza-like-illness.25 One thousand three hundred twenty-three children now have a 50%–65% lower risk of death and a 74% lower risk of PICU admission from influenza-related illness.5,26
The success of this project was dependent on nursing leadership and enhancements in the EHR. The first-hand knowledge of ED nursing workflows resulted in streamlined revisions of the vaccine screening and administration process. Established relationships with nurses facilitated the promotion of the vaccination campaign and collection of honest, real-time feedback. Engineering the EHR with a clinical decision support tool and BPAs improved reliability by mandating screening and offering the vaccine. One prior study in a general ED demonstrated the feasibility of using a clinical decision support tool to increase influenza vaccination rates.27
This ED vaccination initiative provided a strategy to vaccinate children equitably. Lower vaccination rates exist nationally in children 5–17 years of age, minorities, and uninsured or patients with public insurance.13,22,23 This initiative resulted in higher vaccination rates in several of these undervaccinated populations. This result is likely due to the ED visit naturally overcoming vaccination barriers such as transportation, time, and cost limitations. The ED visit also capitalizes on the face-to-face opportunity for the provider/nurse to address the family’s fears or misunderstandings regarding the vaccine. Because the ED serves as a safety net for children without primary care providers and those with lower vaccination rates, it is ideally positioned to reach these populations and impact disparities in vaccination rates.
Challenges to the success of this initiative included a high vaccine refusal rate (87%; 12,018 patients) and patients leaving before administration (33%; 585 patients). Reasons for refusal aligned with prior studies, including a lack of vaccination information, underestimating the severity of influenza, and fear of side effects.14,19,28 Feedback from staff suggested families left before vaccination due to a perceived increased ED length of stay. To overcome these barriers and improve vaccine acceptance in the future, targeted efforts to address vaccine hesitancy and administration time delays are needed.
Despite these obstacles, the program successfully increased vaccination rates without placing a financial burden on the hospital. Hart et al29 estimated that the net societal cost of offering the vaccine was $103.69 per patient. Our study is unique as our formal cost analysis demonstrated a $22,500 net profit due in part to the zero cost vaccines from the VFC program. This analysis was limited as it did not estimate cost savings from influenza cases averted ($33.51 per case prevented), the cost of possibly wasted vaccines, or the cost of personnel to run the project.29 This hospital sanctioned influenza vaccination project included an estimated provider and nursing leadership team commitment of 275 staff-hours for planning, collecting, analyzing, and disseminating data, and providing education. This self-sustaining program can be initiated annually by turning on the EHR workflow and re-educating nurses and providers. Also, the electronic infrastructure and workflow created for this project could be replicated in the future to vaccinate against other life-threatening illnesses.
Limitations
This quality improvement effort was performed at a single tertiary care center where the institution valued influenza vaccine administration. There is an established electronic medical record and access to EHR analysts capable of creating BPAs and clinical decision support tools; therefore, these interventions may not be generalizable to institutions without them. This institution participates in the VFC program, which contributed to the net positive revenue seen in our cost analysis. The team closely monitored the vaccination program for adverse patient outcomes via nurse, provider, and pharmacy feedback. Although no adverse outcomes were reported, follow-up was limited as patients were discharged immediately after vaccination. Finally, due to the disparate systems used by other health systems and retail pharmacies, we were unable to confirm whether patients refusing the vaccine in the ED received the vaccination later in the flu season.
Conclusions
We demonstrated that the ED is a suitable location to provide the influenza vaccine to children. Utilizing strong nursing leadership and an engineered EHR, we overcame barriers to administering and receiving the vaccine in the ED without increasing LOS. Our results suggest that ED vaccination programs may be a strategy to equitably increase vaccination rates regardless of age, race, ethnicity, and insurance status without increased cost to the hospital. Future efforts to improve influenza vaccination rates will focus on overcoming vaccine hesitancy and spreading the vaccination effort to regional EDs and urgent care facilities.
DISCLOSURES
The authors have no financial interest to declare in relation to the content of this article.
Supplementary Material
Footnotes
Published online 8 July 2020
To cite: Baumer-Mouradian SH, Kleinschmidt A, Servi A, Jaworski B, Lazarevic K, Kopetsky M, Nimmer M, Hanson T, Gray MP, Drendel AL. Vaccinating in the Emergency Department, a Novel Approach to Improve Influenza Vaccination Rates via a Quality Improvement Initiative. Pediatr Qual Saf 2020;4:e322.
Presented at the Scientific Symposium on Improving the Quality and Value of Health Care, Institute for Healthcare Improvement Meeting, Orlando, FL; and at the Pediatric Emergency Medicine Quality Improvement Section, Pediatric Academic Society Meeting, Baltimore, MD (S.H.B.-M.).
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Putri WCWS, Muscatello DJ, Stockwell MS, et al. Economic burden of seasonal influenza in the United States. Vaccine. 2018; 36:3960–3966 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Estimated influenza illnesses, medical visits, hospitalizations, and deaths in the United States - 2017-2018 influenza season. https://www.cdc.gov/flu/about/burden/2017-2018.htm. Accessed June 11, 2019
- 3.Shang M, Blanton L, Brammer L, et al. Influenza-associated pediatric deaths in the United States, 2010-2016. Pediatrics. 2018; 141:e20172918. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Summary of the 2017-2018 influenza season. https://www.cdc.gov/flu/about/season/flu-season-2017-2018.htm. Accessed June 11, 2019
- 5.Flannery B, Reynolds SB, Blanton L, et al. Influenza vaccine effectiveness against pediatric deaths: 2010-2014. Pediatrics. 2017; 139:e20164244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Committee on Infectious Diseases. Recommendations for prevention and control of influenza in children, 2018-2019. Pediatrics. 2018; 142:e20182367. [DOI] [PubMed] [Google Scholar]
- 7.Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices-United States, 2018-19 influenza season. MMWR Recomm Rep. 2018; 67:1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estimates of Flu Vaccination Coverage among Children -United States, 2017-2018 Flu Season. Influenza [Report]. 2018. Webpage Report. Available at: https://www.cdc.gov/flu/fluvaxview/coverage-1718estimates-children.htm. Accessed November 6, 2019.
- 9.Respiratory Virus Surveillance Report, Week 20: Ending May 18, 2019. Influenza Weekly Report [Report]. 2019. Webpage Report. Available at: https://www.dhs.wisconsin.gov/publications/p02346.pdf.
- 10.Lin CJ, Nowalk MP, Zimmerman RK, et al. Reducing racial disparities in influenza vaccination among children with Asthma. J Pediatr Health Care. 2016; 30:208–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anandappa M, Adjei Boakye E, Li W, et al. Racial disparities in vaccination for seasonal influenza in early childhood. Public Health. 2018; 158:1–8 [DOI] [PubMed] [Google Scholar]
- 12.Webb NS, Dowd-Arrow B, Taylor MG, et al. Racial/ethnic disparities in influenza vaccination coverage among US adolescents, 2010-2016. Public Health Rep. 2018; 133:667–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Flu vaccination coverage, United States, 2016-17 influenza season. https://www.cdc.gov/flu/fluvaxview/coverage-1617estimates.htm. Accessed July 19, 2019
- 14.Rao S, Fischman V, Moss A, et al. Exploring provider and parental perceptions to influenza vaccination in the inpatient setting. Influenza Other Respir Viruses. 2018; 12:416–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Vaccines for children program (VFC). https://www.cdc.gov/vaccines/programs/vfc/index.html. Accessed August 7, 2019
- 16.Rao S, Williams JT, Torok MR, et al. Missed opportunities for influenza vaccination among hospitalized children with influenza at a tertiary care facility. Hosp Pediatr. 2016; 6:513–519 [DOI] [PubMed] [Google Scholar]
- 17.Clevenger LM, Pyrzanowski J, Curtis CR, et al. Parents’ acceptance of adolescent immunizations outside of the traditional medical home. J Adolesc Health. 2011; 49:133–140 [DOI] [PubMed] [Google Scholar]
- 18.Strelitz B, Gritton J, Klein EJ, et al. Parental vaccine hesitancy and acceptance of seasonal influenza vaccine in the pediatric emergency department. Vaccine. 2015; 33:1802–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newcombe J, Kaur R, Wood N, et al. Prevalence and determinants of influenza vaccine coverage at tertiary pediatric hospitals. Vaccine. 2014; 32:6364–6368 [DOI] [PubMed] [Google Scholar]
- 20.Smith PJ, Kennedy AM, Wooten K, et al. Association between health care providers’ influence on parents who have concerns about vaccine safety and vaccination coverage. Pediatrics. 2006; 118:e1287–e1292 [DOI] [PubMed] [Google Scholar]
- 21.Davis TC, Fredrickson DD, Arnold CL, et al. Childhood vaccine risk/benefit communication in private practice office settings: a national survey. Pediatrics. 2001; 107:E17. [DOI] [PubMed] [Google Scholar]
- 22.Santoli JM, Huet NJ, Smith PJ, et al. Insurance status and vaccination coverage among US preschool children. Pediatrics. 2004; 1136 Suppl1959–1964 [PubMed] [Google Scholar]
- 23.Srivastav A, Zhai Y, Santibanez TA, et al. Influenza vaccination coverage of Vaccine for Children (VFC)-entitled versus privately insured children, United States, 2011-2013. Vaccine. 2015; 33:3114–3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Family & children. Education and prevention messages for parents. https://www.cdc.gov/flu/resource-center/freeresources/print/print-family.htm#Vaccination. Accessed July 31, 2019
- 25.Jefferson T, Rivetti A, Di Pietrantonj C, et al. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev. 2018; 2:CD004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferdinands JM, Olsho LE, Agan AA, et al. ; Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. Effectiveness of influenza vaccine against life-threatening RT-PCR-confirmed influenza illness in US children, 2010-2012. J Infect Dis. 2014; 210:674–683 [DOI] [PubMed] [Google Scholar]
- 27.Venkat A, Chan-Tompkins NH, Hegde GG, et al. Feasibility of integrating a clinical decision support tool into an existing computerized physician order entry system to increase seasonal influenza vaccination in the emergency department. Vaccine. 2010; 28:6058–6064 [DOI] [PubMed] [Google Scholar]
- 28.Bhat-Schelbert K, Lin CJ, Matambanadzo A, et al. Barriers to and facilitators of child influenza vaccine - perspectives from parents, teens, marketing and healthcare professionals. Vaccine. 2012; 30:2448–2452 [DOI] [PubMed] [Google Scholar]
- 29.Hart RJ, Stevenson MD, Smith MJ, et al. Cost-effectiveness of strategies for offering influenza vaccine in the pediatric emergency department. JAMA Pediatr. 2018; 172:e173879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


