SUMMARY
The hypocretin system consists of two peptides hypocretin-1and hypocretin-2 (HCRT1 and HCRT2). Hypocretin-containing neurons are located in the posterior and lateral hypothalamus, and have widespread projections throughout the brain and spinal cord. In addition to its presence in the cerebrospinal fluid, peripheral HCRT1 has been detected in plasma. Robust experimental evidence demonstrates functions of hypothalamic-originated HCRT1 in regulation of multiple biological systems related to sleep-wake states, energy homeostasis and endocrine function. In contrast, HCRT1 studies with human participants are limited by the necessarily invasive assessment of CSF HCRT1 to patients with underlying morbidity.
Regulation by HCRT1 of energy homeostasis and reproduction in animals suggests similar regulation in humans and prompts these two systematic reviews. These reviews translate prior experimental findings from animal studies to humans and examine associations between HCRT1 and: 1) metabolic risk factors; 2) reproductive function in men, women and children. A total of 21 studies and 6 studies met the inclusion criteria for the two searches, respectively. Research question, study design, study population, assessments of HCRT1, reproductive, cardiometabolic data and main findings were extracted. Associations between HCRT1, metabolic and reproductive function are inconsistent. Limitations of studies and future research directions are outlined.
Keywords: Hypocretin-1, Orexin-A, narcolepsy, metabolic health, overweight, reproductive hormones, gonadotropin-releasing hormone
INTRODUCTION
The global obesity epidemic negatively impacts the health and quality of life of >700 million adults and children worldwide. Overweight and obesity (body mass index (BMI)>25) accounts for 4 million deaths annually.1 As a complex disease, obesity impairs endocrine function of metabolic and reproductive systems in men, women and children.2,3 Recently, poor sleep has emerged as a contributor to deleterious health outcomes such as cardiovascular morbidity,4,5 dyslipidemia, diabetes and obesity,6–8 and high mortality risk.9 Further, negative effects of sleep disturbances on reproductive systems have been shown in the general population,10,11 in pregnant women,12,13 shift workers14,15 and animals.11,16
In 1998, a new system of hypothalamic neuropeptides was independently discovered by two research groups, who named them respectively orexins17 and hypocretins.18 The hypocretin system consists of two peptides, hypocretin-1 and hypocretin-2 (HCRT1 and HCRT2; also called Orexin-A and Orexin-B), which act through two G-coupled receptors, HCRTR1 and HCRTR2.19 HCRTR1 has greater affinity for HCRT1, whereas HCRTR2 is non-selective and binds HCRT1 and HCRT2 with equal affinity.17 Hypocretin-containing neurons are located in the posterior and lateral hypothalamus, and have widespread projections throughout the brain and spinal cord.20,21 Differential distribution of HCRT1 and HCRT2-immunoreactive fibers, and the central expression of mRNA for each of these hypocretin receptors suggest that the two peptides serve different physiological roles.22 In addition to its presence in the cerebrospinal fluid (CSF), peripheral HCRT1 or the HCRTR1 have been detected in the blood, gastrointestinal tract, pancreas, kidney, thyroid, adrenal gland, adipose tissue, placenta, testis and reproductive tract.20,21,23 Peripheral HCRT2R expression has been shown in the adrenal glands and the lung.23
The ability of HCRT1 to cross the blood brain barrier (BBB) by simple diffusion has been shown in mice.24 While HCRT1 demonstrated a rapid entry rate to the brain from the blood, HCRT2 had an accelerated degradation in the blood that hinders its ability to cross the BBB.24 Despite the presence of HCRT1 outside the brain, its source in peripheral tissue and in the blood, whether synthesized in the brain or locally, remains unknown.21,25–27 The absence of a specific hypocretin transport system from brain to blood may support the notion of peripheral production of hypocretins.24 Clinical studies, i.e. involving human participants, revealed significantly lower peripheral HCRT1 than CSF levels.28,29
Robust experimental evidence from animal studies has demonstrated functions of hypothalamic-originated HCRT1 in regulation of multiple biological systems related to sleep-wake states, energy homeostasis, cognition, locomotion, reward, sensory modulation, stress, bone remodeling,30 and endocrine function.31,32
The hypothalamus is a key brain region that integrates metabolic and endocrine signals to regulate feeding and energy homeostasis. Hypocretin neurons respond to several metabolic cues including extracellular levels of glucose, the satiety hormone leptin, and the hunger hormone ghrelin.33,34 These neurons increase their firing rates in response to low levels of glucose and ghrelin, and are inhibited by leptin. Furthermore, central administration of HCRT1 produces a dose-dependent increase in food intake. The increase in food consumption is primarily mediated via the HCRTR1.35
Reproductive functions are controlled by the hypothalamic-pituitary-gonadal axis. Gonadotropin releasing hormones (GnRH) are produced in the hypothalamus and regulate secretion of reproductive hormones, i.e. follicle stimulating hormone (FSH) and luteinizing hormone (LH), from the pituitary gland. These hormones control gonadal function.36–38
The role of HCRT1 in modulation of GnRH has been shown in rodents.38–40 The presence of HCRT1 receptors in the gonads of chickens, rodents, swine and porcine as well as in human testis, further supports its putative effect at the hypothalamic-pituitary-gonadal level.41,42 As optimal energy balance and sleep-wake cycle support successful reproduction, these findings highlight the role of HCRT1, a sleep-wake and metabolic regulator, in reproduction.
In contrast to experimental data, clinical reports on HCRT1 are limited – by the invasive nature of assessment of HCRT1 in the CSF – to patients with underlying morbidity. Narcolepsy with cataplexy (NC) is a rare chronic neurological condition with impaired sleep-wake cycles. This sleep disorder impacts 1 in 2000 adults in the US and is marked by daytime sleepiness, sleep attacks, sleep paralysis and hypnagogic hallucinations. The dysregulation of sleep-wake cycles in narcoleptic patients is attributed to deficiency in HCRT1 neurons, observed in 90% of these patients.43 An additional barrier to clinical investigations on HCRT1 is the scarcity of reliable methodology sensitive to picomolar quantities.20
Reproduction and energy balance are intricately entwined as optimal reproductive processes are sensitive to fluctuating metabolic conditions.44 Reproductive functions are controlled by the hypothalamic-pituitary-gonadal axis and sensitive to metabolic cues. The metabolic hormones insulin and leptin are hypothesized to act as mediators or modulators the impact of energy metabolism on reproductive function. Numerous reports demonstrate the effects of sleep duration, timing and quality on metabolic and endocrine systems.45 Recent evidence highlights the role of sleep disruptions and circadian misalignment in poor reproductive outcomes.46
Hypocretin regulation of sleep-wake states, feeding, energy homeostasis and reproduction in animals,25,31,47 suggests similar interrelationships in humans,16,48–51 and therefore has prompted the present systematic reviews. These reviews will translate prior experimental findings from animal studies to humans and will examine the impact of HCRT1 on reproductive and metabolic systems in men, women and children.
METHODS
An experienced health sciences librarian (CS) conducted a systematic search of PubMed and EMBASE (Embase.com) to identify articles related to the effect HCRT1 (Orexin A) has on reproduction and on metabolism. Four sentinel articles on each topic were used as a means of harvesting search terms, including Medical Subject Headings (MeSH) and Emtree terms, and keywords (tagged as title/abstract). Cited reference searching using the eight sentinel articles was conducted in Scopus to find articles that might have been missed because they were not indexed.
Reference lists of included articles were also scanned to see if we had missed articles suitable for inclusion in this review. To reduce biases, no filters other than Human were used and both published (i.e., peer reviewed papers) as well as unpublished (i.e., abstract of posters or oral presentations) studies were considered through searches in Embase and Scopus. We did not include a language filter in our search to reduce language bias. Findings are reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement,52 elaboration, and explanation.53(Online Supplementary Appendix A)
Any publication prior to 9 February 2019 was included. A full description of the search strategy and complete list of search terms and limits used in each database are included in the Online Supplementary Appendix B.
Citations were imported into EndNote (Clarivate Analytics) for deduplication, then exported into Excel (Microsoft Office 2016) for analysis.
Hypocretin-1 and Metabolism
The complete search strategy for metabolism, created in PubMed and translated to Embase, was constructed by combining selected keywords and medical subject headings for HCRT1 and metabolism: (orexin[mh] OR orexin[tiab] OR orexin-A[tiab] OR hypocretin1[tiab]) AND (adipokines[mh] OR adipokines[tiab] OR adiposity[mh] OR adiposity[tiab] OR blood pressure[mh] OR blood pressure[tiab] OR c-reactive protein[mh] OR c-reactive protein[tiab] OR cardiometabolic health[tiab] OR cardiovascular health[tiab] OR cardiovascular disease[mh] OR cardiovascular diseases[tiab] OR cholesterol[mh] OR cholesterol[tiab] OR diabetes insipidus[mh] OR diabetes insipidus[tiab] OR diabetes mellitus[mh] OR diabetes mellitus[tiab] OR ghrelin[mh] OR ghrelin[tiab] OR glucose[mh] OR glucose[tiab] OR “glucose intolerance”[tiab] OR hypertension[mh] OR hypertension[tiab] OR insulin[mh] OR insulin[tiab] OR leptin[tiab] OR metabolic syndrome[mh] OR metabolic syndrome[tiab] OR overweight[mh] OR overweight[tiab] OR prediabetes[mh] OR prediabetes[tiab]) NOT (“Animals”[mh] NOT (“Animals”[mh] AND “Humans”[mh])
Exclusion criteria were 1) No cardiometabolic or HCRT1 measures; 2) manuscript language other than English; 3) partial HCRT1 measures; 4) letters, abstracts, case studies, review articles; and 5) did not test the relationship of interest. Inclusion criteria were 1) a study population of humans, 2) complete HCRT1 measures in plasma or CSF, 3) cardiometabolic measures, and 4) articles that examined the associations between HCRT1 and cardiometabolic health. The following information was extracted from all included articles: author’s name, year of publication and country, research question, study design, study population, HCRT1 and cardiometabolic assessment and main findings.
Hypocretin-1 and Reproduction
The complete search strategy for reproduction, created in PubMed and translated to Embase, was constructed by combining selected keywords and medical subject headings for HCRT1 and reproduction: (orexin[mh] OR orexin[tiab] OR orexin-A[tiab] OR orexin receptors[mh] OR orexin receptors[tiab] OR hypocretin1[tiab]) AND (estradiol[mh] OR estradiol[tiab] OR estrogens[mh] OR estrogen[tiab] OR estrogens[tiab] OR fertility[mh] OR fertility[tiab] OR “follicle stimulating hormone*”[tiab] OR FSH[tiab] OR gonadotropins[mh] OR gonadotropin*[tiab] OR gonadotropin-releasing hormone[mh] OR gonadotropin-releasing hormone*[tiab] OR infertility[mh] OR infertility[tiab] OR “luteinizing hormone*”[tiab] OR ovulation[mh] OR ovulation[tiab] OR reproduction[mh] OR reproduction[tiab] OR testosterone[mh] OR testosterone[tiab]) NOT (“Animals”[mh] NOT (“Animals”[mh] AND “Humans”[mh]).Exclusion criteria were 1) No reproductive or HCRT1 measures; 2) manuscripts language other than English; 3) partial HCRT1 measures; 4) letters, abstracts, case studies, review articles; and 5) did not test the relationship of interest. Inclusion criteria were 1) a study population of humans, 2) complete HCRT1 measures in plasma or CSF, 3) reproductive measures, and 4) articles that examined the associations between HCRT1 and reproductive function. The following information was extracted from all included articles: author’s name, year of publication and country, research question, study design, study population, HCRT1 and reproductive assessment and main findings. All articles were screened by GLD and LMO. The number of articles was small, agreement was nearly unanimous, with an overall 98% agreement rate, representing an average of 96% and 100% agreement rates in the metabolic and reproductive reviews, respectively. All disagreements were resolved by discussion.
RESULTS
Hypocretin-1 and Metabolism
Selection and Characteristics of Studies
Figure 1 outlines the screening of 1,247 articles identified in this systematic search. We reviewed 535 studies, of which 75 full-text articles were assessed. After all exclusions, 21 articles were included in the final analyses. Eleven of these studies measured HCRT1 in plasma (Table 1) and 10 in the CSF (Table 2). Most studies had a small sample size, less than 100 participants. With the exception of one US-based investigation,54 all studies were conducted in Europe or Asia (Turkey, Poland, Finland, India, Japan, Spain, Italy, Germany, Holland, Norway and the Czech Republic). One study had enrolled participants in three countries.55 The vast majority of these studies were cross-sectional (n=19), and two had a case-control design. In all but two investigations, HCRT1 was considered as an exposure to metabolic outcomes; in the latter, HCRT1 was the outcome. Adiposity measures were frequently examined in relation to HCRT1, e.g. BMI, body fat and waist circumference.
Figure 1:
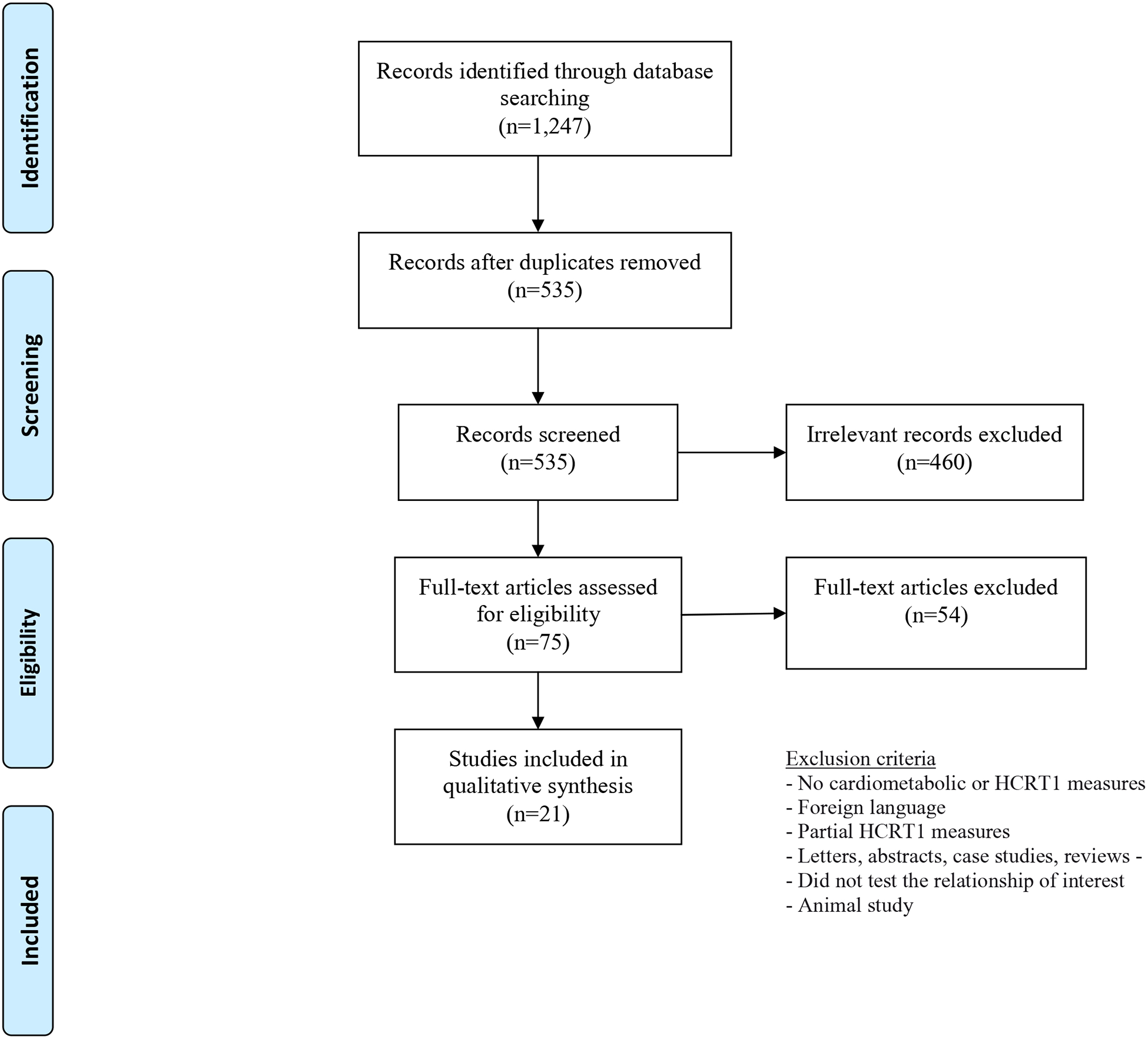
Flowchart of screening of records in the systematic review of hypocretin1 and metabolism
TABLE 1:
Summary of 11 studies on serum hypocretin-1 and metabolic function in children, women and men
| Author, Year Country | Research Question | Study Design | Study population | Exposure | Outcome (s) | HCRT1 Assessment | Main Findings |
|---|---|---|---|---|---|---|---|
| Adam 2002, Holland [56] | Association between HCRT1 and BMI | Cross-sectional | 15 morbidly obese adults 23 lean controls |
HCRT1 | BMI, leptin levels | Serum Radio-immunoassay |
HCRT1 inversely correlated with BMI; Leptin positively correlated with BMI; Study design matched for age and sex |
| Akbulut 2014, Turkey [64] | Correlations between HCRT1 and BMI | Cross-sectional | 40 adults with COPD No control group |
HCRT1 | BMI | Serum Enzyme-immunoassay |
HCRT 1 and BMI are not correlated; Study did not include control group |
| Baranowska 2005, Poland [57] | Association between HCRT1 and BMI | Cross-sectional Descriptive | 36 obese women 16 lean women |
BMI | HCRT1 | Serum Radio-immunoassay |
HCRT1 lower in obese vs. lean women; HCRT1 lower in women with BMI>40 vs. BMI<40; No information about control for confounding |
| Gupta 2015, India [58] | Association between HCRT1 and MET syndrome | Case-control | 172 women/MET syndrome 170 controls |
HCRT1 | MET syndrome | Serum Enzyme immunoassay |
HCRT1 was significantly lower in women with MET syndrome vs. controls; Study matched for age |
| Kawada 2004, Japan [61] | Associations between HCRT1, metabolic and anthropometric markers | Cross-sectional | 47 obese children 26 non-obese controls |
HCRT1 | BMI, WC, body fat, skinfold thickness, LDL | Serum Radio-immunoassay |
HCRT 1, leptin higher in obese vs. controls; in obese group, HCRT 1 positively correlated with leptin, waist-to-hip ratio; Study matched for age |
| Kolodziejski 2018, Poland [59] | Associations between BMI and SPX, KISS, insulin, glucagon, ghrelin, adiponectin, HCRT 1 and leptin | Cross-sectional | 15 obese women 15 non-obese women |
Body composition | SPX, KISS, insulin, glucagon, ghrelin, adiponectin, HCRT 1 and leptin | Serum Enzyme immunoassay |
Statistically insignificant lower HCRT1 in obese vs. non-obese women; HCRT 1 and SPX positively correlated, but not KISS; Analyses adjusted for sex |
| Mishra 2017, India [60] | Associations between leptin, adiponectin, insulin, HCRT 1 and obesity | Cross-sectional | 168 obese women BMI > 30 150 lean women BMI < 25 |
HCRT1 | BMI | Serum Enzyme immunoassay |
HCRT 1, adiponectin levels negatively associated with BMI; Leptin, insulin levels positively related to BMI; No information about control for confounding |
| Nishino 2001, Holland, US, Czech Republic [55] | Associations between HCRT1 and obesity | Cross-sectional | 38 patients NC 19 w/neurological symptoms 15 controls |
HCRT1 deficiency | BMI, CSF leptin | Serum and Cerebrospinal fluid Radio-immunoassay |
BMI, CSF leptin were significantly higher in NC patients vs controls; Study controlled for race/ethnicity |
| Sauchelli 2016, Spain [62] | Associations between HCRT 1, sleep quality and BMI | Cross-sectional | 40 morbid obese women 26 obese women 32 normal weight women |
HCRT1 | BMI, poor sleep quality | Serum Enzyme immunoassay |
HCRT1 was positively associated with BMI, poor sleep quality; Poor sleep quality mediates the association between HCRT1 and BMI; Analyses adjusted for age |
| Tabak 2012, Turkey [63] | Associations between HCRT1 and MET syndrome | Cross-sectional | 87 adults with MET 40 controls |
HCRT 1, metabolic markers | MET syndrome | Serum Enzyme immunoassay |
HCRT1 significantly higher in MET group vs. controls; HCRT1 positively correlated with ghrelin; Study matched for age, sex |
| Yilmaz 2013, Turkey [71] | Associations between GDM, maternal and fetal serum HCRT 1 | Cross-sectional | 70 pregnant with GDM 70 pregnant controls | GDM oral glucose test; Serum fasting glucose and insulin | HCRT1 maternal serum, cord blood | Serum Enzyme immunoassay |
GDM women had lower maternal HCRT 1 but higher fetal HCRT 1 levels vs. controls; maternal and fetal HCRT 1 positively correlated in GDM group; Study matched for age |
TABLE 2:
Summary of 10 studies on CSF hypocretin-1 and metabolic function in children, women and men
| Author, Year Country | Research Question | Study Design | Study population | Exposure assessment | Outcome (s) assessment | HCRT1 Assessment | Main Findings |
|---|---|---|---|---|---|---|---|
| Arnulf, 2006, USA [54] | Association between HCRT 1 and leptin | Cross-sectional | 93 narcoleptic low HCRT1 72 narcoleptic normal HCRT1 89 w/other sleep disorders 111 controls |
HCRT1 deficiency | Serum, CSF leptin, CRP | Cerebrospinal fluid Radio-immunoassay |
Serum leptin similar in all groups; HCRT 1 was not associated with serum or CSF leptin; CRP levels similar across groups; Analyses adjusted for sex, age, BMI, CRP, sleep duration, meds use |
| Beitinger 2011, Germany [70] | Association between HCRT1 deficiency and glucose metabolism | Case-control | 17 adults with NC 17 controls |
HCRT1 deficiency | Glucose, insulin sensitivity following 4-hour oral glucose tolerance test | Cerebrospinal fluid Radio-immunoassay |
No differences in glucose metabolism between groups; Study matched for age, sex and BMI |
| Donadio 2014, Italy [74] | Association between HCRT 1, sympathetic and cardiovascular function | Cross-sectional | 19 adults with NC 19 controls |
HCRT1 deficiency | HR, BP | Cerebrospinal fluid Radio-immunoassay |
HCRT1 deficiency is associated with lower HR and BP; HCRT 1 correlated (positively) with HR, but not with BP; Study matched for age, sex |
| Fronczek 2008, Holland [72] | Association between HCRT1 and autonomic balance | Cross-sectional | 15 men with NC 15 controls |
HCRT1 deficiency | Resting MET rate, HR, BP | Cerebrospinal fluid Radio-immunoassay |
Similar resting MET rate, mean HR, systolic and diastolic BP among NC men and controls, but variability of HR and BP differed between groups; Study matched for sex, BMI, age |
| Heier 2011, Norway [67] | Association between HCRT 1 and metabolic risk factors | Cross-sectional | 26 adults with NC, deficient HCRT1 23 adults with NC, normal HCRT1 11 adults with IH 43 controls |
HCRT1 deficiency | BMI, HbA1C, serum and CSF leptin | Cerebrospinal fluid Radio-immunoassay |
Similar mean BMI in all groups; serum, CSF leptin not associated with HCRT 1 levels; similar HbA1C across all groups; Study matched for age, sex |
| Omokawa 2016, Japan [68] | Associations between HCRT1 and BMI | Cross-sectional | 14 patients with PWS 37 with NC 14 with IH |
HCRT1 | BMI | Cerebrospinal fluid Radio-immunoassay | Moderate HCRT1 in PWs significantly higher than NC patients, but lower than those with IH. HCRT 1 and BMI not correlated; Study controlled for race/ethnicity |
| Poli 2009, Italy [65] | Associations between HCRT 1, metabolic function and MET syndrome | Cross-sectional | 14 men with NC 14 men with IH |
HCRT1 deficiency | Adiposity measures, systolic, diastolic BP, HR, lipid profile, glucose, insulin | Cerebrospinal fluid Radio-immunoassay |
NC men had higher adiposity, BP, total cholesterol, triglycerides, insulin, leptin, but similar HR, lower HDL vs. IH men; MET syndrome only present in NC group (64%); Study controlled for age and sex; all patients were white and drug naive |
| Poli 2013, Italy [66] | Associations between HCRT 1deficiency, precocious puberty and BMI | Cross-sectional | 43 children with NC 52 obese controls |
HCRT1 deficiency | Adiposity measures, lipid profile, CRP, glucose, insulin, precocious puberty | Cerebrospinal fluid Radio-immunoassay |
HCRT1 lower in NC group with vs. without precocious puberty and with excessive vs. normal weight, statistically insignificant; Precocious puberty shown in 17% and 2% of NC patients and controls, respectively; Study matched for age |
| Santiago 2018, Germany [69] | Association between HCRT1 and body composition | Cross-sectional | 26 adults BMI≥25 15 adults normal weight |
HCRT1 | BMI, body weight, fat, fat-free mass, total water, WC | Cerebrospinal fluid Radio-immunoassay | Inverse associations between HCRT 1, body weight, WC, fat-free mass and total water; no association with BMI, fat mass, insulin, glucose and adiponectin; Study matched for race/ethnicity |
| Sieminski 2017, Finland [73] | Associations between HCRT1 deficiency and BP patterns | Cross-sectional | 11 adults with NC, HCRT1 depleted 10 adults with NC, HCRT1 deficient |
HCRT1 deficiency | BP patterns, daytime and nocturnal systolic, diastolic BP, Dip of systolic, diastolic BP | Cerebrospinal fluid Radio-immunoassay |
Nocturnal, daytime BP patterns similar in both groups; No information about control for confounding |
BMI=body mass index; BP=blood pressure; CSF=cerebrospinal fluid; HCRT1=hypocretin-1; HR=heart rate; IH=idiopathic hypersomnia; MS=metabolic syndrome; NC=narcolepsy and cataplexy; PWS=Prader-Willi Syndrome; WC=waist circumference;
Results from Studies
Clinical studies show relationships between CSF or plasma HCRT1 and energy balance in both adults and children. A cross-sectional study with 15 morbidly obese adults and 23 lean controls reported inverse correlation between plasma HCRT1 and BMI.56 Similar relationships have been observed in women with obesity or metabolic syndrome.57–60 Conversely, higher plasma HCRT1 levels in relation to increased adiposity measures were reported in 47 Japanese children,61 66 obese women,62 and 87 women with metabolic syndrome.63 Finally, one small, cross-sectional study, without a comparison group, found no correlation between BMI and plasma HCRT1 in 40 adults with chronic obstructive pulmonary disease.64
Reports on central HCRT1 (CSF) and adiposity are likewise inconsistent. One study of 14 narcoleptic men and 14 controls with idiopathic hypersomnia and a second study of 43 narcoleptic children and 52 obese age-matched controls, found inverse associations between HCRT1 and adiposity.65,66 Null associations between HCRT1 and adiposity were reported in a study of 26 obese adults vs. 15 normal weight controls as well as in 14 patients with Prader-Willi syndrome compared with 37 patients with NC and 14 controls with idiopathic hypersomnia.67,68 A small cross-sectional study of 41 adults that examined HCRT1 and body composition, found no association between CSF HCRT1, BMI and fat mass, but inverse association with body weight and waist circumference.69
Investigation of leptin in HCRT1-deficient adults has shown higher leptin levels in adults with NC compared with controls.55,65 In contrast, two studies conducted a comparison of central leptin levels in narcoleptic adults, with or without HCRT1 deficiency, and in controls; all groups showed similar leptin levels.54,67 In obese children, plasma HCRT1 has shown positive correlation with leptin.61 This study of 47 obese children (mean age 10.6) found increased levels of leptin and HCRT1 compared with 26 normal weight age-matched controls. Ghrelin and plasma HCRT1 were also positively correlated in adults. This study of 87 adults with metabolic syndrome and 40 healthy, age- and sex-matched controls found increased levels of plasma HCRT1 and ghrelin among cases in comparison to controls.63
Few studies evaluated the relationships between HCRT1 and glucose metabolism. A case-control study of 172 premenopausal women reported significantly lower plasma HCRT1 levels in women with metabolic syndrome in comparison to age-matched controls.58 However, null associations were found between CSF HCRT1 and glucose metabolism in a cross-sectional and a case-control study of adults with NC,67,70 or a small cross-sectional study with 26 overweight adults and 15 normal weight controls.69
Associations between HCRT1 and diabetes have been also examined in pregnancy. In 2013 a study of 70 pregnant women with gestational diabetes mellitus (GDM) and 70 pregnant controls obtained umbilical and maternal plasma HCRT1.71 Venous fasting blood samples were obtained from all women after the second stage of delivery, i.e. following the delivery of the baby, but prior to the placenta delivery. Umbilical plasma samples were collected post placental delivery. Compared with controls, women with GDM had lower levels of plasma HCRT1, but higher umbilical HCRT1 levels. Among all women, maternal plasma HCRT1 was correlated with umbilical HCRT1, and maternal HCRT1 was inversely correlated with fasting glucose and insulin.71
Only one report examined the relationships between HCRT1 and lipid profile among men with NC in comparison to men with idiopathic hypersomnia. This study found that men with NC had higher levels of total cholesterol and triglycerides, but lower levels of HDL vs. controls. Further, the metabolic syndrome was present in 64% of narcoleptic men, but absent from the control group.65
Patterns of blood pressure (BP) in relation to HCRT1 were examined in four studies. An Italian study showed increased BP patterns in 14 HCRT1-deficient men with NC, compared with 14 controls.65 Similar mean systolic and diastolic BP, but different BP patterns have been observed in a small cross-sectional study with 15 men with NC and 15 healthy controls.72 Nocturnal and daytime BP patterns were also similar in narcoleptic men with HCRT1 depletion or HCRT1 deficiency.73 However, another study of 19 patients with NC found lower BP in comparison to 19 controls, though the two measures were not correlated.74
Hypocretin-1 and Reproduction
Selection and Characteristics of Studies
In this systematic search, we identified 191 articles, of which we screened 91 and assessed 11 full-text articles. (Figure 2) After these exclusion, 6 articles were included in the final analyses. Of these studies, one was conducted in the US,75, while others took place in Turkey, Egypt, Italy or South Korea. All studies included smaller samples <100 participants. (Table 3)
Figure 2:
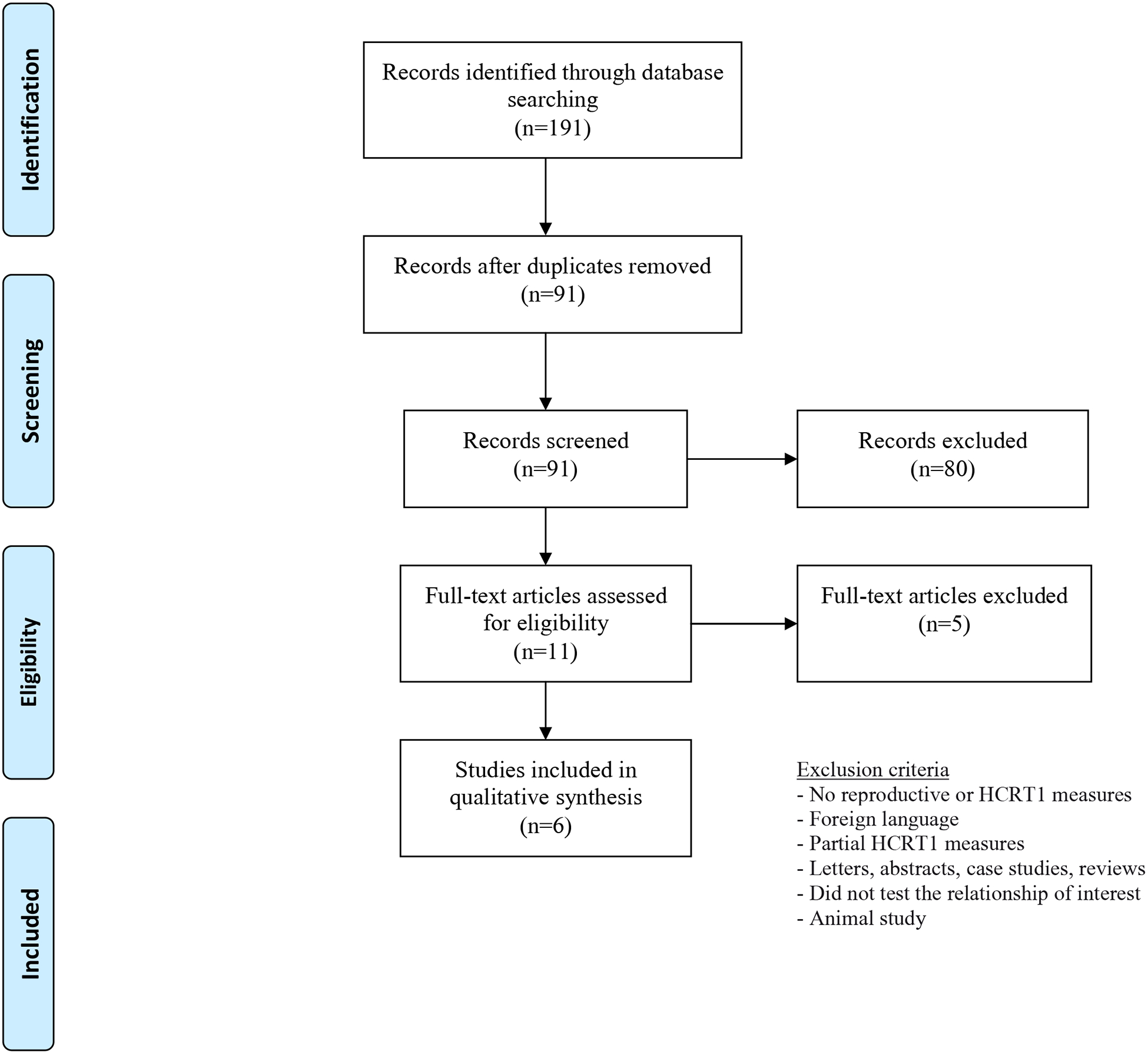
Flowchart of screening of records in the systematic review of hypocretin1 and reproduction
TABLE 3:
Summary of six studies on hypocretin-1 and reproductive function in children, women and men
| Author, Year Country | Research Question | Study Design | Study population | Exposure assessment | Outcome (s) assessment | HCRT1 Assessment | Main Findings |
|---|---|---|---|---|---|---|---|
| Cho 2015, South Korea [77] | Associations between HCRT 1 and idiopathic polyhydramnios | Cross-sectional | Pregnant women 10 with polyhydramnios 20 controls |
HCRT1 maternal se rum, cord blood | Amniotic fluid index | Serum Enzyme immunoassay |
Cord blood HCRT 1 levels lower in cases vs. controls; Cord blood and maternal serum HCRT 1 positively correlated; cord blood, maternal serum HCRT 1 negatively correlated with birth weight and placental weight; HCRT1 cord levels but not maternal serum, negatively correlated with amniotic fluid index; Study design controlled for singleton pregnancies |
| Cintron 2017, US [75] | Associations between HCRT1 and menopause hormone therapy | Randomized, double-blinded 4-year trial | Menopausal women 23 patch and placebo pill 20 pill and placebo patch 31 placebo pill, placebo patch |
Menopausal horm o ne therapy: oral pil l or T D patch | HCRT 1 at baseline, during and 3 years post intervention | Serum Enzyme immunoassay |
In oral therapy group, HCRT 1 levels significantly increased from baseline; During trial, HCRT 1 levels increased differentially between groups, highest increase in oral therapy group; HCRT 1 levels similar post intervention among and within groups; Randomized study design controlled for confounders |
| El-Sedeek 2010, Egypt [78] | Associations between estrogen and HCRT 1 | Cross-sectional | Pre- and menopausal women 30 menopausal ERT+ 30 menopausal ERT− 30 premenopausal controls |
Estrogen replacement therapy |
HCRT1 | Serum Radio-immunoassay |
Menopausal women not on ERT had increased HCRT 1 levels compared with those on ERT or controls; Analyses adjusted for BMI |
| Karakus 2012, Turkey [79] | Associations between hormone replacement therapy, adiposity and metabolic function | Cohort study 6 months | 20 postmenopausal women 20 premenopausal women |
Hormone replacement therapy | Adiposity, FSH, LH, lipid profile, HCRT1, metabolic hormones | Serum Enzyme immunoassay |
In postmenopausal women, HRT did not change BMI, HCRT1, ghrelin, but significantly increased leptin; HCRT 1 negatively correlated with BMI; Post- vs. pre-menopausal women had lower median HCRT 1; GLP-1, adiponectin positively correlated with HCRT 1 in premenopausal women; Within-group analyses controlled for BMI, age |
| Poli 2013, Italy [66] | Associations between HCRT1 deficiency, precocious puberty and BMI | Cross-sectional | 43 children with NC 52 obese controls |
HCRT1 deficiency | Adiposity measures, lipid profile, CRP, glucose, insulin, precocious puberty | Cerebrospinal fluid Radio-immunoassay |
HCRT1 lower in NC group with vs. without precocious puberty and with excessive vs. normal weight, but statistically insignificant; Precocious puberty shown in 17% and 2% of NC patients and controls, respectively; Study matched for age |
| Yilmaz 2013, Turkey [76] | Associations between PCOS, HCRT1 and metabolic biomarkers | Cross-sectional | Reproductive-age women 36 with PCOS 40 healthy controls |
PCOS diagnosis Rotterdam criteria | Plasma glucose, HDL, LDL, TG, LH, FSH, E2, SHBG, DHEAS; blood pressure | Serum Enzyme immunoassay |
PCOS women had lower HCRT1 levels; HCRT1 was negatively correlated to systolic blood pressure, LH and free testosterone; Women were age and BMI matched; |
BMI=body mass index; CRH=corticotropin-releasing hormone; CRP=c-reactive lipoprotein; DHEAS= Dehydroepiandrosterone sulfate; E2=estradiol; ERT=estrogen replacement therapy; FSH=follicle stimulating hormone; GDM=gestational diabetes mellitus; HCRT1=hypocretin-1; HDL=high-density lipoprotein cholesterol; HRT=hormone replacement therapy; LDL=low-density lipoprotein cholesterol; LH=luteinizing hormone; NC=narcolepsy and cataplexy; NPY=neuropeptide Y; PCOS=polycystic ovarian syndrome; PL=placebo; TD=transdermal; TG=triglyceride; SHBG=sex hormone binding globulin;
Results from Studies
Few clinical studies have examined associations between plasma HCRT1 on reproductive function among men, women and children. Deficiency in HCRT1 has been shown to alter gonadal function in adults, but pediatric evidence remains rare. One Italian cross-sectional study of 95 children (mean age 11.8 years) investigated the potential impact of HCRT1 on reproductive function in 43 children and adolescents with NC and 52 age-matched obese controls. This condition is marked by both HCRT1 deficiency and obesity.66 Its findings suggest a higher rate of precocious puberty (17% vs 2%) in the NC group compared with the obese group.
Only one study examined associations between HCRT1 levels and polycystic ovarian syndrome (PCOS), an endocrine disorder affecting 15% of reproductive age women and characterized by hyperandrogenism, ovulatory dysfunction and polycystic ovaries. This Turkish study of 36 women with PCOS and 40 healthy reproductive-age matched controls found lower HCRT1 levels in women with PCOS relative to those without this condition. Further, in the presence of PCOS, HCRT1 was inversely correlated with LH.76
Limited data exist on HCRT1 in pregnancy. A 2015 study from South Korea examined the associations between maternal plasma and umbilical HCRT1 levels, and excessive amniotic fluid (polyhydramnios), a disorder linked to GDM or fetal anomalies. This small study enrolled women in their third trimester with a singleton pregnancy, 10 women had polyhydramnios and 20 pregnant controls had normal amount of amniotic fluid. Polyhydramnios has been associated with lower umbilical HCRT1 levels, but no relationship was found between amniotic fluid quantity and maternal plasma HCRT1 in 10 pregnancies with polyhydramnios.77 However, umbilical HCRT1 was correlated with maternal HCRT1 in plasma, similar to a prior report in pregnant women with gestational diabetes.71 Further, both plasma and cord HCRT1 measures were inversely correlated with placental and birth weight.
The impact of hormone replacement therapy (HRT) on plasma HCRT1 have been shown in a recent randomized controlled trial with menopausal women.75 Plasma HCRT1 levels were compared at baseline, during and after intervention in menopausal women randomly assigned to placebo (n=31), transdermal patch (n=23) or oral HRT (n=20) groups. While similar plasma HCRT1 levels were observed at baseline and 3 years later across all groups, during the intervention period a significant increase in HCRT1 levels was shown among women in the oral HRT group, but not in the patch or placebo groups.75 Inverse associations between HRT and HCRT1 have been reported in an observational study of 90 women. Plasma HCRT1 levels were compared in these women evenly divided in three groups – premenopausal, postmenopausal taking HRT for 6 months, and postmenopausal not on HRT – showed highest levels of plasma HCRT1 in postmenopausal women not receiving HRT.78 A second observational study examined the relationships between HRT and HCRT1 in a cohort of 20 postmenopausal women, pre- and post-therapy, and in comparison to 20 premenopausal controls.79 This study reported similar HCRT1 levels in postmenopausal women pre- and post-HRT, insignificantly lower levels in comparison to premenopausal women.
DISCUSSION
Hypocretin-1 and Metabolism
In this systematic review of the literature, we found mixed associations between HCRT1 and metabolic risk factors in adults and children. Most studies have inversely correlated plasma HCRT1 with body composition, metabolic syndrome or impaired metabolism. Associations between CSF HCRT1 and metabolic function were reported by few studies with small sample sizes (n<50).
Intervention studies also provide mixed results on the associations between plasma HCRT1 and BMI. Five weeks of weight-reduction therapy was associated with increase in HCRT1 levels among obese adolescents aged 7–18,80 while bariatric surgery for weight loss in morbidly obese adults resulted in decreased, unchanged or increased plasma HCRT1 levels post-surgery.81–83 Additional evidence for the impact of HCRT1 on excessive body weight has been obtained from studies among patients with NC or Prader-Willi syndrome. These conditions, marked with mild or deficient HCRT1 levels, are linked to elevated BMI and abdominal adiposity.55,68,84–88 On the other side of the BMI spectrum, it has been reported that girls with anorexia nervosa had decreased HCRT1 levels following interventions for weight gain.89,90
Ghrelin and leptin are two hormones that regulate energy balance. Ghrelin stimulates food intake while leptin inhibits feeding. Incongruent correlations between central HCRT1 and leptin have been reported. An intervention trial of 10 normal weight women inversely correlated HCRT1 and leptin. During a 10-day fasting period, leptin decreased but HCRT1 levels increased in comparison to pre-fasting levels.91 Inverse correlation between leptin and HCRT1 has also been observed in girls with restrictive anorexia nervosa treated for weight gain. Before treatment initiation, lower levels of leptin and HCRT1 were recorded in a sample of 30 anorexic girls compared with 20 age-matched healthy weight controls. However, HCRT1 level decreased while leptin concentration increased during the 6-month treatment.90 Another small study of 36 anorexic girls and 14 healthy controls found no association between HCRT1 and leptin before or after an 8-weeks weight gain therapy.89 While both studies examined associations of HCRT1 with leptin in a small sample of anorexic adolescent girls, they differed in treatment duration, age distribution and characteristics of controls. Changes in HCRT1 levels in relation to adiposity were examined in a study with 58 obese children aged 7–18.5 during a five-week weight-reduction intervention. While weight loss was associated with HCRT1 increase and leptin decrease, no correlation was found between them before or after initiation of therapy.80
Experimental data have linked ghrelin to sleep and metabolic neuronal circuits, with a direct influence on HCRT1 activity.92 However, clinical reports on the association between ghrelin and HCRT1 are lacking. Two studies examined ghrelin levels in eight narcoleptic men and eight narcoleptic women and men, respectively. These studies reported similar ghrelin levels as in healthy controls in these HCRT1-deficient adults.93,94 Among adults with metabolic syndrome, increased levels HCRT1 and ghrelin were observed in comparison to age- and sex-matched controls.63
A part of the metabolic syndrome, impaired insulin function, increases the risk for type 2 diabetes and cardiovascular morbidity. Plasma HCRT1 was inversely correlated with insulin and glucose in 10 non-obese women aged 14–49 years prior to and during fasting,91 and in men with NC compared with controls.65 A randomized clinical trial of 60 women with type 2 diabetes reported an inverse relationship between HCRT1 and insulin resistance, but positive correlation with insulin sensitivity at baseline.95 After initiation of treatment for glycemic control, levels of HCRT1 increased while insulin resistance decreased.
Despite inconsistent clinical findings, relationships between HCRT1 levels and metabolic function are plausible and supported by experimental evidence from animal studies and intervention studies for weight loss or weight gain.
Hypocretin-1 and Reproduction
This systematic review of the literature found limited and inconsistent evidence on the relationship between reproductive hormones and HCRT1 in postmenopausal women. The three studies that examined the impact of HRT on HCRT1 reported positive, negative or null associations. Comparisons of HCRT1 levels between premenopausal and postmenopausal women were similarly inconclusive. However, a negative relationship between HCRT1 and reproductive hormones was suggested by a report on higher rates of precocious puberty in children with NC compared with obese controls and among women with PCOS versus age- and BMI-matched controls.
During normal puberty the activation of the hypothalamus-pituitary-gonadal axis increases LH, FSH and sex steroids.96 As reproductive function is controlled by GnRH, itself regulated by HCRT1, lack of HCRT1 could affect the timing of puberty onset. Further, precocious puberty has been attributed in part to excessive weight, a common comorbidity of NC. To control for the influence of obesity on precocious puberty, this study examined rates of precocious puberty among children with NC in comparison to obese controls. The higher proportion of precocious puberty (17% vs 2%) observed in the NC group compared with the obese group, suggested a significant role for HCRT1 in the onset of puberty.66
One study reported lower HCRT1 levels in women with PCOS, a condition associated with obesity, insulin resistance, compensatory hyperinsulinema,97 and increased levels of LH.98 The lower levels of HCRT1 linked to higher LH levels reported in this study are in agreement with the metabolic profile of increased obesity and hyperinsulinema in women with PCOS.76
Measurements of HCRT1 in pregnancy are limited. A 2015 study from South Korea associated polyhydramnios with lower umbilical HCRT1 levels.77 This study also correlated umbilical HCRT1 with maternal HCRT1 in plasma, similar to a prior report in pregnant women with gestational diabetes.71 Plasma and cord HCRT1 were inversely correlated with placental and birth weight, similar to inverse relationships reported between HCRT1 and BMI.
Whether HCRT1 is associated with adverse pregnancy outcomes is unclear. One study with 249 narcoleptic women, of which 216 had NC, a HCRT1-deficiency condition, and 33 had narcolepsy without cataplexy, suggest that women with NC were more likely to experience perinatal complications, e.g. anemia, impaired glucose metabolism, and increased cesarean section deliveries.99 In experimental study of 85 mice pregnancies, a significant increase in fetal death was observed in mice without HCRT1, in comparison to those of wild-type pregnancies.100
As women transition from their childbearing years to menopause, they are likely to experience disruption in sleep and metabolism101,102 in response to altered levels of reproductive hormones.103 Indeed, hormone replacement therapy (HRT) has been linked to improved sleep in menopausal women with vasomotor symptoms.104 A sleep-wake regulator, HCRT1 shows association with reproductive hormones and plausibly contributes to post-menopause poor sleep. During menopause, estrogen depletion parallels an increase in HCRT1 levels.105 Three studies examined associations between HRT and plasma HCRT1 in postmenopausal women produced inconsistent findings. A randomized controlled trial showed an increase in HCRT1 levels following a 6-month oral HRT, but not transdermal patch HRT.75 However, an observational study with premenopausal and postmenopausal women suggested an increase in HCRT1 levels during menopause and a decrease following HRT to similar levels of premenopausal women.78 This study did not report differences in HCRT1 prior and post HRT. Conversely, HCRT1 levels pre- and post HRT were similar in a small study of 20 menopausal women, but statistically insignificant, lower HCRT1 levels in comparison to premenopausal women.79 As expected, inverse associations between HRT and HCRT1 were present in the two observational studies, 78,79 in contrast to a positive association reported in the only randomized controlled trial.75 While these results suggest a direct impact of HRT on HCRT1 regulation, the reported inhibition or simulation of HCRT1 by HRT is attributed to mechanisms involving distinct HRT metabolites.75
Reproductive function in men has been linked to HCRT1 in two small studies. Among seven men with NC, a condition marked by HCRT1 deficiency, LH plasma levels were significantly lower than in the seven non-narcoleptic matched controls; both groups had a normal pituitary response to GnRH administration.106 The presence of HCRT1 has also been evaluated in testicular tissue of 70 men with non-obstructive azoospermia, a fertility disorder. In these men, increased testicular HCRT1 was associated with the presence of spermatozoa, and positively correlated with gonadotropin.107 Although insufficient and limited, these data suggest that endogenous HCRT1 could be involved in regulation of GnRH release.40
Risk of Bias Assessment
Selection of studies
In these systematic reviews of the literature, we conducted a comprehensive assessment of studies that examined associations between HCRT1 and metabolic risk factors, as well as HCRT1 and reproductive function. Only one filter was applied to exclude animal experiments. To reduce bias, this review included published, peer-reviewed articles, unpublished literature, non-English publications and cited references in Scopus. These strategies have resulted in minimal biases toward selection of studies. Further bias reduction was implemented by exclusion of studies with partial measurements of HCRT1, or lack the associations of interest.
Individual Studies
The role of HCRT1 in metabolic and reproductive functions has been examined by a small number of studies with limited sample sizes. With the exception of one randomized controlled trial, all studies were observational, of which the vast majority were of cross-sectional design. While cross-sectional investigations universally lack temporal assessments, they can generate causal insight on associations of interest. The heterogeneity of methods utilized for HCRT1 assessments could also introduce bias, though central HCRT1 has been correlated with plasma HCRT1. Finally, HCRT1 has been evaluated among highly select patient groups, i.e. Prader-Willi Syndrome, NC, menopausal women or obese adults who are not comparable to healthy populations.
SUMMARY AND FUTURE DIRECTIONS
These systematic reviews of the literature identified clinical reports on the associations between HCRT1 and metabolic function as well as HCRT1 and reproductive function. Metabolic disruptions have been observed by 15 of the 21 included studies among children and adults, in agreement with animal data. However, inconsistent associations were noted, particularly between HCRT1 and body weight. Such relationships are likely complex and could involve pathways through sleep disturbances, severity of metabolic insults on energy balance, and sedentary or eating behaviors. Similarly, despite the observed impact of HCRT1 on reproductive function in rodents, clinical reports are limited. Only six articles were identified, with one study in children, two in reproductive age women, and three among postmenopausal women. These three latter studies provided mixed findings on the presence of associations between HCRT1 and HRT in post-menopausal women and the direction of association. Discrepancies may be attributed to differential bioavailability of hormone therapies, variability of HRT metabolites, compliance to therapy, and unaccounted heterogeneity. Further, the timing of the therapy initiation in relation to menopausal transition could explain the variation of effect on HCRT1. Nonetheless, in light of consistent data from animal experiments, HCRT1 is plausibly involved in metabolic and reproductive functions in humans.
The lack of clinical investigations on HCRT1 in relation to metabolic and reproductive functions may be attributed to methodological barriers in HCRT1 measurement in plasma, on one hand, and the invasive assessment of HCRT1 in the CSF, on the other hand. The inconsistent findings could be explained by heterogeneity in sample sizes, age and comorbidities of study populations, analytic strategies, and methods to assay HCRT1. These correlational studies lack the ability to examine temporal, unbiased associations and rely on insufficient statistical power to detect a true effect. Longitudinal investigations with reliable assays, large, well-characterized cohorts and intensive measurements would provide opportunities to elucidate the temporal role of HCRT1 in metabolic and reproductive health and suggest potential causal pathways. As regulators of feeding and sleep, HCRT1 may link reproductive function and cardiometabolic health. Development of new methods for accurate plasma assessment of HCRT1 would extend investigations of HCRT1 functions to large and healthy cohorts.
Supplementary Material
PRACTICE POINTS.
Experimental animal reports associate HCRT1 deficiency with obesity and metabolic disruptions.
A limited number of clinical studies have suggested HCRT1 role in metabolic and reproductive functions, but their findings are mixed.
Current clinical studies are cross-sectional by design with small sample sizes and heterogeneity in assay methods for HCRT1.
RESEARCH AGENDA.
Prospective, larger cohorts with intensive biomarker assessment to examine temporal associations between HCRT1, metabolic and reproductive functions across the lifespan.
Development of a sensitive assay to reliably measure HCRT1 in plasma would allow large scale investigations.
Acknowledgements
The authors thank Drs. Carol F. Elias, Carrie R. Ferrario and Shelley Hershner for reviewing the manuscript, and April Gallant for providing technical support.
Funding
GLD was supported by a National Research Service Award from the National Institute of Child Health and Development (F32 HD091938) and by a Mentored Research Scientist Development Award from the National Heart, Lung, and Blood Institute (K01 HL144914)
Abbreviations
- BBB
blood brain barrier
- BP
blood pressure
- CSF
cerebrospinal fluid
- FSH
follicle stimulating hormone
- GDM
gestational diabetes mellitus
- GnRH
Gonadotropin releasing hormones
- HCRT
hypocretin
- HRT
hormone replacement therapy
- LH
luteinizing hormone
- NC
narcolepsy with cataplexy
- PCOS
polycystic ovarian syndrome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors report no conflicts of interests to declare
REFERENCES
- 1.Collaborators GO. Health effects of overweight and obesity in 195 countries over 25 years. New England Journal of Medicine. 2017;377(1):13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray GA. Medical consequences of obesity. The Journal of Clinical Endocrinology & Metabolism. 2004;89(6):2583–2589. [DOI] [PubMed] [Google Scholar]
- 3.Kahn BE, Brannigan RE. Obesity and male infertility. Curr Opin Urol. 2017;27(5):441–445. [DOI] [PubMed] [Google Scholar]
- 4.Nagai M, Hoshide S, Kario K. Sleep duration as a risk factor for cardiovascular disease-a review of the recent literature. Current cardiology reviews. 2010;6(1):54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palagini L, Maria Bruno R, Gemignani A, Baglioni C, Ghiadoni L, Riemann D. Sleep loss and hypertension: a systematic review. Current pharmaceutical design. 2013;19(13):2409–2419. [DOI] [PubMed] [Google Scholar]
- 6.Cappuccio FP, Taggart FM, Kandala N-B, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31(5):619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grandner MA, Chakravorty S, Perlis ML, Oliver L, Gurubhagavatula I. Habitual sleep duration associated with self-reported and objectively determined cardiometabolic risk factors. Sleep Med. 2014;15(1):42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jansen EC, Dunietz GL, Tsimpanouli ME, et al. Sleep, Diet, and Cardiometabolic Health Investigations: a Systematic Review of Analytic Strategies. Current nutrition reports. 2018;7(4):235–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alvarenga TA, Hirotsu C, Mazaro-Costa R, Tufik S, Andersen ML. Impairment of male reproductive function after sleep deprivation. Fertil Steril. 2015;103(5):1355–1362. e1351. [DOI] [PubMed] [Google Scholar]
- 11.Kloss JD, Perlis ML, Zamzow JA, Culnan EJ, Gracia CR. Sleep, sleep disturbance, and fertility in women. Sleep medicine reviews. 2015;22:78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunietz GL, Shedden K, Schisterman EF, Lisabeth LD, Treadwell MC, O’Brien LM. Associations of snoring frequency and intensity in pregnancy with time-to-delivery. Paediatr Perinat Epidemiol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pamidi S, Pinto LM, Marc I, Benedetti A, Schwartzman K, Kimoff RJ. Maternal sleep-disordered breathing and adverse pregnancy outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;210(1):52.e51–52.e14. [DOI] [PubMed] [Google Scholar]
- 14.Gamble KL, Resuehr D, Johnson C. Shift work and circadian dysregulation of reproduction. Frontiers in endocrinology. 2013;4:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahoney MM. Shift work, jet lag, and female reproduction. International journal of endocrinology. 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein CA, Smith YR. Sleep, Circadian Rhythms, and Fertility. Current Sleep Medicine Reports. 2016;2(4):206–217. [Google Scholar]
- 17. *.Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–585. [DOI] [PubMed] [Google Scholar]
- 18. *.de Lecea L, Kilduff TS, Peyron C, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(1):322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebrahim IO, Semra YK, De Lacy S, et al. CSF hypocretin (Orexin) in neurological and psychiatric conditions. Journal of sleep research. 2003;12(1):83–84. [DOI] [PubMed] [Google Scholar]
- 20.Heinonen MV, Purhonen AK, Makela KA, Herzig KH. Functions of orexins in peripheral tissues. Acta physiologica (Oxford, England). 2008;192(4):471–485. [DOI] [PubMed] [Google Scholar]
- 21.Nakabayashi M, Suzuki T, Takahashi K, et al. Orexin-A expression in human peripheral tissues. Molecular and cellular endocrinology. 2003;205(1–2):43–50. [DOI] [PubMed] [Google Scholar]
- 22.Cutler DJ, Morris R, Sheridhar V, et al. Differential distribution of orexin-A and orexin-B immunoreactivity in the rat brain and spinal cord. Peptides. 1999;20(12):1455–1470. [DOI] [PubMed] [Google Scholar]
- 23.Johren O, Neidert SJ, Kummer M, Dendorfer A, Dominiak P. Prepro-orexin and orexin receptor mRNAs are differentially expressed in peripheral tissues of male and female rats. Endocrinology. 2001;142(8):3324–3331. [DOI] [PubMed] [Google Scholar]
- 24.Kastin AJ, Akerstrom V. Orexin A but not orexin B rapidly enters brain from blood by simple diffusion. Journal of Pharmacology and Experimental Therapeutics. 1999;289(1):219–223. [PubMed] [Google Scholar]
- 25.Tsunematsu T, Yamanaka A. The role of orexin/hypocretin in the central nervous system and peripheral tissues. Vitamins and hormones. 2012;89:19–33. [DOI] [PubMed] [Google Scholar]
- 26.Nilaweera KN, Barrett P, Mercer JG, Morgan PJ. Precursor-protein convertase 1 gene expression in the mouse hypothalamus: differential regulation by ob gene mutation, energy deficit and administration of leptin, and coexpression with prepro-orexin. Neuroscience. 2003;119(3):713–720. [DOI] [PubMed] [Google Scholar]
- 27.Kirchgessner AL, Liu M. Orexin synthesis and response in the gut. Neuron. 1999;24(4):941–951. [DOI] [PubMed] [Google Scholar]
- 28.Strawn JR, Pyne-Geithman GJ, Ekhator NN, et al. Low cerebrospinal fluid and plasma orexin-A (hypocretin-1) concentrations in combat-related posttraumatic stress disorder. Psychoneuroendocrinology. 2010;35(7):1001–1007. [DOI] [PubMed] [Google Scholar]
- 29.Dalal MA, Schuld A, Haack M, et al. Normal plasma levels of orexin A (hypocretin-1) in narcoleptic patients. Neurology. 2001;56(12):1749–1751. [DOI] [PubMed] [Google Scholar]
- 30.Wei W, Motoike T, Krzeszinski JY, et al. Orexin regulates bone remodeling via a dominant positive central action and a subordinate negative peripheral action. Cell Metab. 2014;19(6):927–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, Hu Z, Lecea L. The hypocretins/orexins: integrators of multiple physiological functions. British journal of pharmacology. 2014;171(2):332–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chieffi S, Carotenuto M, Monda V, et al. Orexin System: The Key for a Healthy Life. Frontiers in physiology. 2017;8:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. *.Yamanaka A, Beuckmann CT, Willie JT, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38(5):701–713. [DOI] [PubMed] [Google Scholar]
- 34.Jain MR, Horvath TL, Kalra PS, Kalra SP. Evidence that NPY Y1 receptors are involved in stimulation of feeding by orexins (hypocretins) in sated rats. Regulatory peptides. 2000;87(1–3):19–24. [DOI] [PubMed] [Google Scholar]
- 35. *.Haynes AC, Jackson B, Chapman H, et al. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regulatory peptides. 2000;96(1–2):45–51. [DOI] [PubMed] [Google Scholar]
- 36.Silveyra P, Cataldi NI, Lux-Lantos VA, Libertun C. Role of orexins in the hypothalamic-pituitary-ovarian relationships. Acta physiologica (Oxford, England). 2010;198(3):355–360. [DOI] [PubMed] [Google Scholar]
- 37.Martyńska L, Polkowska J, Wolińska-Witort E, et al. Orexin A and its role in the regulation of the hypothalamo-pituitary axes in the rat. Reprod Biol. 2006;2:29–35. [PubMed] [Google Scholar]
- 38.Nurmio M, Tena-Sempere M, Toppari J. Orexins and the regulation of the hypothalamic-pituitary-testicular axis. Acta physiologica (Oxford, England). 2010;198(3):349–354. [DOI] [PubMed] [Google Scholar]
- 39.Russell SH, Small CJ, Kennedy AR, et al. Orexin A interactions in the hypothalamo-pituitary gonadal axis. Endocrinology. 2001;142(12):5294–5302. [DOI] [PubMed] [Google Scholar]
- 40.Gaskins GT, Moenter SM. Orexin a suppresses gonadotropin-releasing hormone (GnRH) neuron activity in the mouse. Endocrinology. 2012;153(8):3850–3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Celik O, Aydin S, Celik N, Yilmaz M. Peptides: Basic determinants of reproductive functions. Peptides. 2015;72:34–43. [DOI] [PubMed] [Google Scholar]
- 42.Ciccimarra R, Bussolati S, Grasselli F, et al. Orexin system in swine ovarian follicles. Domestic animal endocrinology. 2018;62:49–59. [DOI] [PubMed] [Google Scholar]
- 43. *.Sakurai T. Roles of orexin/hypocretin in regulation of sleep/wakefulness and energy homeostasis. Sleep medicine reviews. 2005;9(4):231–241. [DOI] [PubMed] [Google Scholar]
- 44.Schneider JE. Energy balance and reproduction. Physiology & behavior. 2004;81(2):289–317. [DOI] [PubMed] [Google Scholar]
- 45.Morselli LL, Guyon A, Spiegel K. Sleep and metabolic function. Pflugers Archiv : European journal of physiology. 2012;463(1):139–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mills J, Kuohung W. Impact of circadian rhythms on female reproduction and infertility treatment success. Current opinion in endocrinology, diabetes, and obesity. 2019;26(6):317–321. [DOI] [PubMed] [Google Scholar]
- 47.Goforth PB, Myers MG. Roles for Orexin/Hypocretin in the Control of Energy Balance and Metabolism. Current topics in behavioral neurosciences. 2017;33:137–156. [DOI] [PubMed] [Google Scholar]
- 48.Ding XX, Wu YL, Xu SJ, et al. A systematic review and quantitative assessment of sleep-disordered breathing during pregnancy and perinatal outcomes. Sleep & breathing = Schlaf & Atmung. 2014;18(4):703–713. [DOI] [PubMed] [Google Scholar]
- 49.Cappuccio FP, D’elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes care. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fontana R, Torre SD. The deep correlation between energy metabolism and reproduction: a view on the effects of nutrition for women fertility. Nutrients. 2016;8(2):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Michalakis K, Mintziori G, Kaprara A, Tarlatzis BC, Goulis DG. The complex interaction between obesity, metabolic syndrome and reproductive axis: a narrative review. Metabolism-Clinical and Experimental. 2013;62(4):457–478. [DOI] [PubMed] [Google Scholar]
- 52.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269, w264. [DOI] [PubMed] [Google Scholar]
- 53.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–94. [DOI] [PubMed] [Google Scholar]
- 54.Arnulf I, Lin L, Zhang J, et al. CSF versus serum leptin in narcolepsy: is there an effect of hypocretin deficiency? Sleep. 2006;29(8):1017–1024. [DOI] [PubMed] [Google Scholar]
- 55. *.Nishino S, Ripley B, Overeem S, et al. Low cerebrospinal fluid hypocretin (Orexin) and altered energy homeostasis in human narcolepsy. Annals of neurology. 2001;50(3):381–388. [DOI] [PubMed] [Google Scholar]
- 56.Adam JA, Menheere PP, van Dielen FM, Soeters PB, Buurman WA, Greve JW. Decreased plasma orexin-A levels in obese individuals. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2002;26(2):274–276. [DOI] [PubMed] [Google Scholar]
- 57.Baranowska B, Wolinska-Witort E, Martynska L, Chmielowska M, Baranowska-Bik A. Plasma orexin A, orexin B, leptin, neuropeptide Y (NPY) and insulin in obese women. Neuro endocrinology letters. 2005;26(4):293–296. [PubMed] [Google Scholar]
- 58.Gupta V, Mishra S, Kumar S, Mishra S. Association of Circulating Orexin-A Level With Metabolic Risk Factors in North Indian Pre Menopausal Women. Indian journal of physiology and pharmacology. 2015;59(4):422–427. [PubMed] [Google Scholar]
- 59.Kolodziejski PA, Pruszynska-Oszmalek E, Korek E, et al. Serum levels of spexin and kisspeptin negatively correlate with obesity and insulin resistance in women. Physiological research. 2018;67(1):45–56. [DOI] [PubMed] [Google Scholar]
- 60.Mishra S, Gupta V, Mishra S, Sachan R, Asthana A. Serum level of orexin-A, leptin, adiponectin and insulin in north Indian obese women. Diabetes & metabolic syndrome. 2017;11 Suppl 2:S1041–s1043. [DOI] [PubMed] [Google Scholar]
- 61.Kawada Y, Hayashibe H, Asayama K, et al. Plasma levels of orexin-a and leptin in obese children. Clinical pediatric endocrinology : case reports and clinical investigations : official journal of the Japanese Society for Pediatric Endocrinology. 2004;13(1):47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sauchelli S, Jimenez-Murcia S, Fernandez-Garcia JC, et al. Interaction Between Orexin-A and Sleep Quality in Females in Extreme Weight Conditions. European eating disorders review : the journal of the Eating Disorders Association. 2016;24(6):510–517. [DOI] [PubMed] [Google Scholar]
- 63.Tabak O, Gelisgen R, Cicekci H, et al. Circulating levels of adiponectin, orexin-A, ghrelin and the antioxidant paraoxonase-1 in metabolic syndrome. Minerva medica. 2012;103(4):323–329. [PubMed] [Google Scholar]
- 64.Akbulut G, Gezmen-Karadag M, Ertas Y, et al. Plasma orexin-A and ghrelin levels in patients with chronic obstructive pulmonary disease: Interaction with nutritional status and body composition. Experimental and therapeutic medicine. 2014;7(6):1617–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Poli F, Plazzi G, Di Dalmazi G, et al. Body mass index-independent metabolic alterations in narcolepsy with cataplexy. Sleep. 2009;32(11):1491–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. *.Poli F, Pizza F, Mignot E, et al. High prevalence of precocious puberty and obesity in childhood narcolepsy with cataplexy. Sleep. 2013;36(2):175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heier MS, Jansson TS, Gautvik KM. Cerebrospinal fluid hypocretin 1 deficiency, overweight, and metabolic dysregulation in patients with narcolepsy. J Clin Sleep Med. 2011;7(6):653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Omokawa M, Ayabe T, Nagai T, et al. Decline of CSF orexin (hypocretin) levels in Prader-Willi syndrome. American journal of medical genetics Part A. 2016;170a(5):1181–1186. [DOI] [PubMed] [Google Scholar]
- 69.Santiago JCP, Otto M, Kern W, Baier PC, Hallschmid M. Relationship between cerebrospinal fluid concentrations of orexin A/hypocretin-1 and body composition in humans. Peptides. 2018;102:26–30. [DOI] [PubMed] [Google Scholar]
- 70.Beitinger PA, Fulda S, Dalal MA, et al. Glucose tolerance in patients with narcolepsy. Sleep. 2012;35(2):231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yilmaz E, Celik O, Celik N, et al. Maternal and fetal serum orexin-A levels in gestational diabetes mellitus. The journal of obstetrics and gynaecology research. 2013;39(1):139–145. [DOI] [PubMed] [Google Scholar]
- 72.Fronczek R, Overeem S, Reijntjes R, Lammers GJ, van Dijk JG, Pijl H. Increased heart rate variability but normal resting metabolic rate in hypocretin/orexin-deficient human narcolepsy. J Clin Sleep Med. 2008;4(3):248–254. [PMC free article] [PubMed] [Google Scholar]
- 73.Sieminski M, Chwojnicki K, Sarkanen T, Partinen M. The relationship between orexin levels and blood pressure changes in patients with narcolepsy. PloS one. 2017;12(10):e0185975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Donadio V, Liguori R, Vandi S, et al. Lower wake resting sympathetic and cardiovascular activities in narcolepsy with cataplexy. Neurology. 2014;83(12):1080–1086. [DOI] [PubMed] [Google Scholar]
- 75. *.Cintron D, Beckman JP, Bailey KR, Lahr BD, Jayachandran M, Miller VM. Plasma orexin A levels in recently menopausal women during and 3 years following use of hormone therapy. Maturitas. 2017;99:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yilmaz E, Celik O, Celik N, Simsek Y, Celik E, Yildirim E. Serum orexin-A (OXA) level decreases in polycystic ovarian syndrome. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2013;29(4):388–390. [DOI] [PubMed] [Google Scholar]
- 77.Cho GJ, Hong HR, Kim SW, Hong SC, Oh MJ, Kim HJ. Decreased umbilical orexin-A level is associated with idiopathic polyhydramnios. Acta Obstet Gynecol Scand. 2015;94(3):295–300. [DOI] [PubMed] [Google Scholar]
- 78.El‐Sedeek M, Korish A, Deef M. Plasma orexin‐A levels in postmenopausal women: possible interaction with estrogen and correlation with cardiovascular risk status. BJOG: An International Journal of Obstetrics & Gynaecology. 2010;117(4):488–492. [DOI] [PubMed] [Google Scholar]
- 79.Karakus M, Gelisgen R, Topcuoglu A, et al. The effects of 17beta-estradiol plus drospirenone on anthropometric and biochemical measures of adiposity in menopausal women. Arch Gynecol Obstet. 2012;286(5):1233–1239. [DOI] [PubMed] [Google Scholar]
- 80.Bronsky J, Nedvidkova J, Zamrazilova H, et al. Dynamic changes of orexin A and leptin in obese children during body weight reduction. Physiological research. 2007;56(1):89–96. [DOI] [PubMed] [Google Scholar]
- 81.Cigdem Arica P, Kocael A, Tabak O, Taskin M, Zengin K, Uzun H. Plasma ghrelin, leptin, and orexin-A levels and insulin resistance after laparoscopic gastric band applications in morbidly obese patients. Minerva medica. 2013;104(3):309–316. [PubMed] [Google Scholar]
- 82.Gupta A, Miegueu P, Lapointe M, et al. Acute post-bariatric surgery increase in orexin levels associates with preferential lipid profile improvement. PloS one. 2014;9(1):e84803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heinonen MV, Purhonen AK, Miettinen P, et al. Apelin, orexin-A and leptin plasma levels in morbid obesity and effect of gastric banding. Regulatory peptides. 2005;130(1–2):7–13. [DOI] [PubMed] [Google Scholar]
- 84.Nixon JP, Mavanji V, Butterick TA, Billington CJ, Kotz CM, Teske JA. Sleep disorders, obesity, and aging: the role of orexin. Ageing research reviews. 2015;20:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dahmen N, Bierbrauer J, Kasten M. Increased prevalence of obesity in narcoleptic patients and relatives. European archives of psychiatry and clinical neuroscience. 2001;251(2):85–89. [DOI] [PubMed] [Google Scholar]
- 86.Kok SW, Overeem S, Visscher TL, et al. Hypocretin deficiency in narcoleptic humans is associated with abdominal obesity. Obesity research. 2003;11(9):1147–1154. [DOI] [PubMed] [Google Scholar]
- 87.Schuld A, Hebebrand J, Geller F, Pollmacher T. Increased body-mass index in patients with narcolepsy. Lancet. 2000;355(9211):1274–1275. [DOI] [PubMed] [Google Scholar]
- 88.Morales Drissi N, Romu T, Landtblom AM, et al. Unexpected Fat Distribution in Adolescents With Narcolepsy. Front Endocrinol (Lausanne). 2018;9:728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bronsky J, Nedvidkova J, Krasnicanova H, et al. Changes of orexin A plasma levels in girls with anorexia nervosa during eight weeks of realimentation. The International journal of eating disorders. 2011;44(6):547–552. [DOI] [PubMed] [Google Scholar]
- 90.Janas-Kozik M, Stachowicz M, Krupka-Matuszczyk I, et al. Plasma levels of leptin and orexin A in the restrictive type of anorexia nervosa. Regulatory peptides. 2011;168(1–3):5–9. [DOI] [PubMed] [Google Scholar]
- 91.Komaki G, Matsumoto Y, Nishikata H, et al. Orexin-A and leptin change inversely in fasting non-obese subjects. European journal of endocrinology. 2001;144(6):645–651. [DOI] [PubMed] [Google Scholar]
- 92.Garcia-Garcia F, Juarez-Aguilar E, Santiago-Garcia J, Cardinali DP. Ghrelin and its interactions with growth hormone, leptin and orexins: implications for the sleep-wake cycle and metabolism. Sleep medicine reviews. 2014;18(1):89–97. [DOI] [PubMed] [Google Scholar]
- 93.Huda MS, Mani H, Durham BH, et al. Plasma obestatin and autonomic function are altered in orexin-deficient narcolepsy, but ghrelin is unchanged. Endocrine. 2013;43(3):696–704. [DOI] [PubMed] [Google Scholar]
- 94.Donjacour CE, Pardi D, Aziz NA, et al. Plasma total ghrelin and leptin levels in human narcolepsy and matched healthy controls: basal concentrations and response to sodium oxybate. J Clin Sleep Med. 2013;9(8):797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zarifkar M, Noshad S, Shahriari M, et al. Inverse Association of Peripheral Orexin-A with Insulin Resistance in Type 2 Diabetes Mellitus: A Randomized Clinical Trial. The review of diabetic studies : RDS. 2017;14(2–3):301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peper JS, Brouwer RM, van Leeuwen M, et al. HPG-axis hormones during puberty: a study on the association with hypothalamic and pituitary volumes. Psychoneuroendocrinology. 2010;35(1):133–140. [DOI] [PubMed] [Google Scholar]
- 97.Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clinical epidemiology. 2014;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kumar P, Sait SF. Luteinizing hormone and its dilemma in ovulation induction. Journal of human reproductive sciences. 2011;4(1):2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Maurovich-Horvat E, Kemlink D, Hogl B, et al. Narcolepsy and pregnancy: a retrospective European evaluation of 249 pregnancies. J Sleep Res. 2013;22(5):496–512. [DOI] [PubMed] [Google Scholar]
- 100.Bastianini S, Berteotti C, Lo Martire V, Silvani A, Zoccoli G. A critical role of hypocretin deficiency in pregnancy. J Sleep Res. 2014;23(2):186–188. [DOI] [PubMed] [Google Scholar]
- 101.Guidozzi F. Sleep and sleep disorders in menopausal women. Climacteric. 2013;16(2):214–219. [DOI] [PubMed] [Google Scholar]
- 102.Polotsky HN, Polotsky AJ. Metabolic implications of menopause. Paper presented at: Seminars in reproductive medicine 2010. [DOI] [PubMed] [Google Scholar]
- 103.Murphy PJ, Campbell SS. Sex hormones, sleep, and core body temperature in older postmenopausal women. Sleep. 2007;30(12):1788–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cintron D, Lipford M, Larrea-Mantilla L, et al. Efficacy of menopausal hormone therapy on sleep quality: systematic review and meta-analysis. In: Springer; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Messina G, Viggiano A, De Luca V, Messina A, Chieffi S, Monda M. Hormonal changes in menopause and orexin-a action. Obstetrics and gynecology international. 2013;2013:209812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kok S, Roelfsema F, Overeem S, et al. Pulsatile LH release is diminished, whereas FSH secretion is normal, in hypocretin-deficient narcoleptic men. American Journal of Physiology-Endocrinology and Metabolism. 2004;287(4):E630–E636. [DOI] [PubMed] [Google Scholar]
- 107.Ozkanli S, Basar MM, Selimoglu S, et al. The ghrelin and orexin activity in testicular tissues of patients with idiopathic non-obstructive azoospermia. The Kaohsiung journal of medical sciences. 2018;34(10):564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


