Abstract
Purpose
Our study is a retrospective observational study conducted in one of the largest clinical centers of neurosurgery in China. We aimed to investigate the antimicrobial susceptibility patterns of the Enterobacteriaceae isolates responsible for nosocomial meningitis/encephalitis in post-neurosurgical patients. Meanwhile, we tried to evaluate the risk factors for mortality following Enterobacteriaceae meningitis/encephalitis.
Patients and Methods
Medical data on clinical characteristics, antibiotic susceptibilities, and mortality were reviewed until patients’ discharge or death in the hospital. Data for a total of 164 cerebrospinal fluid (CSF) infection cases due to Enterobacteriaceae after neurosurgery were collected between January 2014 and November 2019 in order to identify risk factors affecting the outcome. Kaplan–Meier survival analysis and multivariable Cox proportional hazard models were applied.
Results
In this study, a total of 2416 neurosurgical meningitis/encephalitis cases were reported between 2014 and 2019. Enterobacteriaceae accounted for 7.3% (176/2416) of all the bacterial infections. Of them, 164 Enterobacteriaceae isolates were available to divide into two groups according to the final outcome of whether the patient died or survived. In total, 38 patients died (23.2%) and 126 patients survived (76.8%). The most frequent infecting species was Klebsiella pneumoniae (47.0%, 77/164). Fourteen-day and 30-day mortality rates were 7.9% (13/164) and 15.2% (25/164). Kaplan–Meier survival analysis revealed that the risk factors of Enterobacteriaceae meningitis/encephalitis that resulted in poor outcomes included comorbidities, Glasgow Coma Scale (GCS) score, sepsis, intensive care unit (ICU) admission, extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae, and ventilation. A GCS score of less than or equal to 8 (P=0.04, HR 2.562) was identified to be a significant risk factor for mortality according to the multivariable Cox proportional hazards model.
Conclusion
In-hospital mortality caused by Enterobacteriaceae meningitis/encephalitis in neurosurgery was high. A GCS score of ≤8 was an independent risk factor for mortality following Enterobacteriaceae meningitis/encephalitis in post-neurosurgical patients.
Keywords: Enterobacteriaceae, post-neurosurgical meningitis/encephalitis, antibiotic susceptibility, antibiotic resistance, in-hospital mortality
Introduction
Meningitis is one of the main complications occurring in post-neurosurgical patients, and poses a high mortality with prolonged hospital stays and costs.1,2 It has been reported that the incidence of post-neurosurgical bacterial meningitis/encephalitis is 0.3–8.6% and it is particularly high in developing countries.3,4 The etiology of post-neurosurgical meningitis/encephalitis includes a wide spectrum of microorganisms from gram-positive cocci to gram-negative bacilli. During the last decades, gram-negative organisms have seemed to be increasing as causative agents of post-neurosurgical meningitis/encephalitis.5 Among the gram-negative bacteria, Enterobacteriaceae is a large group of etiology resulting in post-neurosurgical meningitis/encephalitis with high morbidity and mortality rates.6
However, related research on the clinical risk factors of post-neurosurgical Enterobacteriaceae meningitis/encephalitis is limited. We retrospectively analyzed the epidemiology of post-neurosurgical Enterobacteriaceae meningitis/encephalitis patients in 2014–2019 from Beijing Tiantan hospital to evaluate the risk factors for predicting the survival of post-neurosurgical patients with Enterobacteriaceae meningitis/encephalitis. The results may be able to identify the factors that significantly affect post-neurosurgical Enterobacteriaceae meningitis/encephalitis and provide evidence for the prevention and early clinical treatment of post-neurosurgical Enterobacteriaceae meningitis/encephalitis.
To our knowledge, this is the largest case series on post-neurosurgical Enterobacteriaceae meningitis/encephalitis and is the first study of such crucial risk factors in predicting survival in China. We believe the data in this paper is of great clinical significance.
Patients and Methods
Study Setting
This study was performed at the Beijing Tiantan Hospital & Capital Medical University, a tertiary hospital with 1850 beds and more than 15,000 annual surgeries, between January 2014 to November 2019. The protocol for this study was approved by the clinical diagnosis department of the Beijing Tiantan Hospital & Capital Medical University. Neurosurgery operations included craniotomy, transsphenoidal and spinal surgeries. Patients with incomplete medical records were excluded from the study.
Clinical and Epidemiological Data
Post-neurosurgical patients’ medical records were reviewed to determine whether infections were nosocomial Enterobacteriaceae using cerebral spinal fluid (CSF) bacteria culture reports and antimicrobial susceptibility test results. All of the patients’ eligible daily progress notes were extracted from the standard database. We reviewed several factors including the patients’ routine information (age, gender), comorbidities (hypertension, diabetes mellitus), primary pathology, Glasgow Coma Scale (GCS) score, time from procedure to infection, length of hospital stay (LOS), sepsis, Intensive Care Unit (ICU) admission, producing Extended-Spectrum Beta-Lactamase (ESBLs), surgical wound classification, extra ventricular drainage (EVD), lumbar drainage (LD), ventilation and cerebrospinal fluid (CSF) leakage.7,9 The antibiotic susceptibility of Enterobacteriaceae isolations was determined by CLSI (Clinical and Laboratory Standards Institute) guidelines.10 Post-neurosurgical meningitis/encephalitis patients were followed up until discharge or death in the hospital to assess the clinical outcome.
Microbiologic Methods
Identification and antimicrobial susceptibility were performed in the clinical diagnosis department of the Beijing Tiantan Hospital testing according to a standardized procedure. CSF culture positive specimens were placed onto Columbia blood agar and incubated in an incubator containing 5% CO2 for 18–24 hours. All of the isolates were identified by the Vitek-2 Compact or Vitek MS automated system (bioMerieux, Marcy l ‘etoile, France). Vitek AST-GN and AST-GP cards were used on the Vitek-2 instrument for broth microdilution to obtain the minimum inhibitory concentration (MIC) of all the isolates. Breakpoint values were used according to the 2019 guidelines as described in the CLSI document M100-S23.
Treatment Protocol and Outcomes
All of the patients were given antibiotic prophylaxis 0.5 hours before neurosurgery according to the department’s antibiotic policy. When meningitis/encephalitis was clinically suspected, the patients initially received empirical antibiotics until the culture sensitivity reports were awaited. Once the antimicrobial susceptibility results were available, the patients received definitive antibiotics.
The clinical outcomes of this study were defined as follows: (1) In-hospital mortality: Death in the hospital with clinical evidence of post-neurosurgical Enterobacteriaceae meningitis/encephalitis infection. (2) 30-day mortality: Death within 30 days after the first CSF bacteria culture was positive; mortality not attributable to infection: Patients who died after post-neurosurgical meningitis/encephalitis but as a result of causes independent of the infectious process were excluded from this study.
Statistical Analysis
Student’s t-test and Mann–Whitney U-test were used to compare the two groups for continuous variables depending on whether the data was of normal distribution. Categorical variables were performed using the χ2 test in mortality risk factor analysis. A value of P<0.05 was considered to have statistical significance. The number of days from the first CSF bacteria positive culture to death in the hospital within 30 days was displayed on Kaplan–Meier curves, and the rates of survival were compared using Log rank testing. In addition, values of P<0.10 were considered to be probable predictor variables for the multivariable Cox proportional hazards regression analysis and hazard ratios based on multivariate analysis were calculated. All tests were two-tailed. The data were analyzed by SPSS 21.0 software (IBM New York, US) and WHONET 5.5. The graph of antimicrobial susceptibility was performed using Prism 8.0 (Graphpad, San Diego, USA).
Results
Microbiology
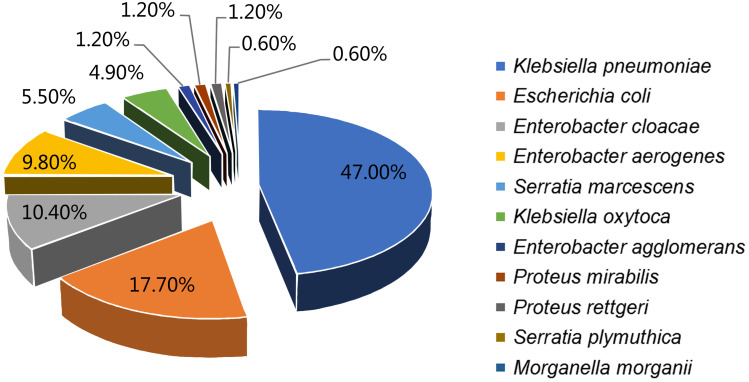
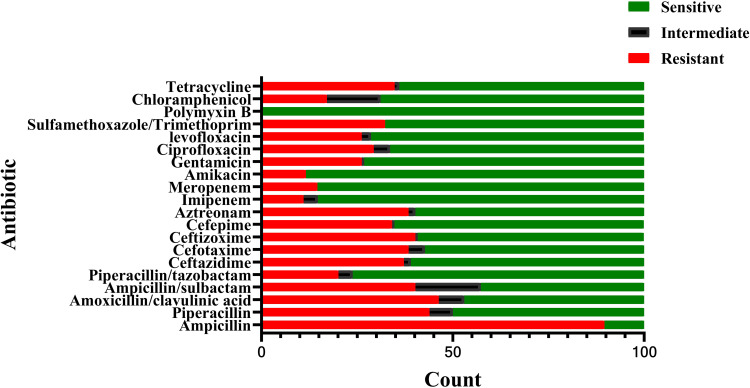
In total, 176 Enterobacteriaceae isolates from 2416 isolates collected from CSF bacteria positive cultures between January 2014 and November 2019 were collected in one of the largest centers for neurosurgery in China. The respective microorganisms are presented in Figure 1. No multiple organism infections were found and antimicrobial susceptibility test results are illustrated in Figure 2.
Figure 1.
Species found in Enterobacteriaceae related meningitis/encephalitis during 2014–2019.
Figure 2.
Antimicrobial susceptibility tests of species found in Enterobacteriaceae related meningitis/encephalitis during 2014–2019.
Clinical Characteristics
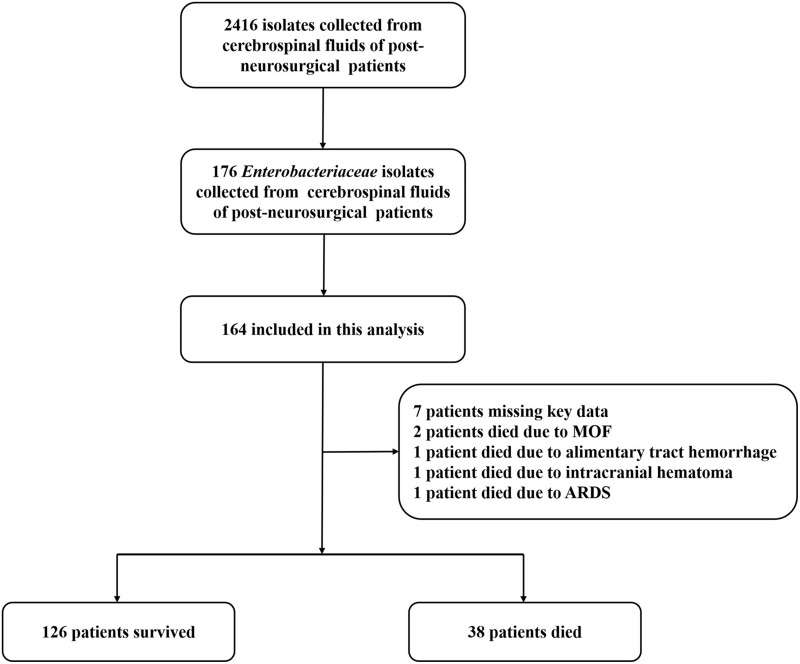
Between January 2014 and November 2019, 176 patients with post-neurosurgical Enterobacteriaceae meningitis/encephalitis were included in this study. There were seven patients missing key data and five patients who died due from other causes all of whom were excluded from this study (death occurred after an episode of meningitis/encephalitis but as a result of causes independent of the infectious process). Two of the five patients died from multiple organ failure (MOF), while another patient died from an alimentary tract hemorrhage. In addition, intracranial hematoma and acute respiratory distress syndrome (ARDS) resulted in another two patients’ death respectively. A flow chart is shown in Figure 3. All of the patients were divided into two groups according to the final outcomes. One was a survivor group containing 126 patients and the other was the non-survivor group containing 38 patients. The median age was 40.1±15.4 years. Among these patients, 94 of 164 patients (57.3%) were male. There were no significant differences in age and sex distributions between the two groups. During hospitalization the average GCS score was 7.7±3.8. The GCS scores in the non-survivor group (5.8±3.8) were significantly lower than those in the survivor group (8.6±3.5) (P=0.021). Patients LOS was 26.4 days (IQR 20.0–40.0 days) and the median time for a CSF positive culture was 6 days (IQR 2.5–13.5 days). In total, 72 of the 164 patients (43.9%) suffered from a tumor and three of the patients’ primary pathology was trauma. Other primary pathologies included tuberculosis 1.2% (2/164) and hepatitis B 2.4% (4/164). There were 68 patients with a clean surgical wound operation while the rest had a clean-contaminated surgical wound operation. Sixty-seven (40.9%) cases had extra ventricular drainage (EVD) and 92 (56.1%) had lumbar drainage (LD). Overall, 61 patients (37.2%) were admitted into the ICU, and there were statistically more patients admitted into ICU from the non-survivor group (71.1%) than the survivor group (27.0%) (P<0.001). The ESBL-producing Enterobacteriaceae isolates identified from the non-survivor group (52.6%) were significantly more than those of the survivor group (33.3%) (P=0.032). The rate of the non-survivor group (55.3%) for using ventilation was significantly higher than that in the survivor group (16.7%) (P<0.001). There were 89 patients who underwent a craniotomy. The incidence of CSF leakage was 22.0% (36/164). All of the factors are shown in Table 1.
Figure 3.
Flowchart of the included patients with post-neurosurgical Enterobacteriaceae meningitis/encephalitis.
Abbreviations: MOF, multiple organ failure; ARDS, acute respiratory distress syndrome.
Table 1.
Comparison of the Factors of the Non-Survivor Group versus the Survivor Group. (Data are Presented as Number (%) of Patients, Mean ± SD, or Median [IQR] Unless Indicated Otherwise)
| Factors | Total (n=164) | Non-Survivor (n=38) | Survivor (n=126) | P |
|---|---|---|---|---|
| Age (years) | 40.1±15.4 | 42.0±16.9 | 39.5±15.0 | 0.518 |
| Male (%) | 94 (57.3%) | 20 (52.7%) | 74 (58.7%) | 0.505 |
| Comorbidities | ||||
| Diabetes mellitus | 4 (2.4%) | 3 (7.9%) | 1 (0.8%) | 0.013 |
| Hypertension | 16 (9.8%) | 8 (2.1%) | 8 (6.3%) | 0.007 |
| Primary pathology | ||||
| Tumor | 72 (43.9%) | 12 (31.6%) | 60 (47.6%) | 0.081 |
| Trauma | 3 (1.8%) | 0 (0.0%) | 3 (2.4%) | 0.337 |
| Tuberculosis | 2 (1.2%) | 0 (0.0%) | 2 (1.2%) | 0.435 |
| Hepatitis B | 4 (2.4%) | 1 (2.6%) | 3 (2.4%) | 0.93 |
| GCS Score | 7.7±3.8 | 5.8±3.8 | 8.6±3.5 | 0.021 |
| Time from procedure to infection, days | 6 [2.5–13.5] | 13.5 [8–16.8] | 6 [2.0–12.0] | 0.084 |
| LOS, days | 26.5 [20.0–40.0] | 29.5 [19.3–42.0] | 26 [20.0–39.8] | 0.665 |
| Sepsis | 31 (18.9%) | 17 (44.7%) | 14 (11.1%) | P<0.001 |
| ICU admission | 61 (37.2%) | 27 (71.1%) | 34 (27%) | P<0.001 |
| Producing ESBLs | 62 (37.8%) | 20 (52.6%) | 42 (33.3%) | 0.032 |
| Surgical wound classification | ||||
| Clean | 68 (41.5%) | 17 (44.7%) | 51 (40.5%) | 0.640 |
| Clean-contaminated | 96 (58.5%) | 21 (55.3%) | 75 (59.5%) | 0.640 |
| EVD | 67 (40.9%) | 19 (50.0%) | 48 (38.1%) | 0.191 |
| LD | 92 (56.1%) | 23 (60.5%) | 69 (42.1%) | 0.530 |
| Ventilator | 42 (25.6%) | 21 (55.3%) | 21(16.7%) | P<0.001 |
| CSF leakage | 36 (22.0%) | 8 (21.1%) | 28 (22.2%) | 0.879 |
| Craniotomy | 89 (54.3%) | 25 (65.8%) | 64 (50.8%) | 0.104 |
Abbreviations: GCS, Glasgow Coma Scale; LOS, length of hospital stay; ICU, intensive care unit; ESBLs, extended-spectrum beta-lactamase; EVD, extra ventricular drainage; LD, lumbar drainage; CSF, cerebrospinal fluid.
Treatments and Mortality
Antibiotic therapies and outcomes of the Enterobacteriaceae infections are illustrated in Table 2. In total, 90.2% of the patients were treated with antibiotic prophylaxis medication. Cefuroxime sodium and ceftriaxone were found to be the two most commonly used prophylactic antibiotics among post-neurosurgical Enterobacteriaceae meningitis/encephalitis patients. Vancomycin plus meropenem were the most commonly used empirical antibiotics. There were no significant differences in antibiotic therapy between the two groups. Total in-hospital mortality due to Enterobacteriaceae meningitis/encephalitis was 23.2% (38/164). The LOS in the patients of the non-survivor group was 29.5 days (IQR 19.3–42.0 days). Overall, the 126 patients who survived (76.8%) had a median length of hospital stay of 26.0 days (IQR 20.0–39.8 days).
Table 2.
Antibiotic Therapy and Clinical Outcomes of Patients with Post-Neurosurgical Enterobacteriaceae Meningitis/Encephalitis
| Factors | Total (n=164) | Non-Survivor (n=38) | Survivor (n=126) | P |
|---|---|---|---|---|
| Antibiotic prophylaxis | 148 (90.2%) | 32 (84.2%) | 116 (92.1%) | 0.153 |
| Ceftriaxone | 39 (23.8%) | 8 (21.1%) | 31 (24.6%) | 0.652 |
| Ceftazidime | 15 (9.1%) | 3 (7.9%) | 12 (9.5%) | 0.76 |
| Cefuroxime | 51 (31.1%) | 7 (18.4%) | 44 (34.9%) | 0.054 |
| Meropenem | 28 (17.1%) | 8 (21.1%) | 20 (15.9%) | 0.457 |
| Vancomycin | 15 (9.1%) | 6 (15.8%) | 9 (7.1%) | 0.105 |
| Cefoperazone/Sulbactam | 2 (1.2%) | 0 (0.0%) | 2 (1.6%) | 0.435 |
| Received empirical antibiotics | 131 (79.9%) | 32 (84.2%) | 99 (78.6%) | 0.447 |
| Single antibiotics | 26 (15.9%) | 5 (13.2%) | 21 (16.7%) | 0.689 |
| Ceftazidime | 3 (1.8%) | 0 (0.0%) | 3 (2.4%) | 0.337 |
| Meropenem | 17 (10.4%) | 3 (7.9%) | 14 (11.1%) | 0.569 |
| Cefuroxime | 2 (1.2%) | 0 (0.0%) | 2 (1.6%) | 0.435 |
| Vancomycin | 3 (1.8%) | 1 (2.6%) | 2 (1.6%) | 0.674 |
| Polymyxin | 1 (0.6%) | 1 (2.6%) | 0 (0.0%) | 0.068 |
| Combination two antibiotics | 90 (54.9%) | 20 (12.2%) | 60 (47.6%) | 0.588 |
| Vancomycin+Meropenem | 75 (45.7%) | 14 (8.5%) | 61 (48.4%) | 0.209 |
| Meropenem+Ceftazidime | 3 (1.8%) | 1 (2.6%) | 2 (1.6%) | 0.674 |
| Others | 12 (7.3%) | 5 (13.2%) | 7 (5.6%) | 0.115 |
| Combination three antibiotics | 15 (9.1%) | 5 (13.2%) | 10 (7.9%) | 0.328 |
| Vancomycin+Meropenem+Ceftazidime | 12 (7.3%) | 3 (7.9%) | 9 (7.1%) | 0.876 |
| Others | 3 (1.8%) | 2 (5.3%) | 1 (0.8%) | 0.072 |
| Received definitive therapy | 137 (83.5%) | 31 (81.6%) | 106 (84.1%) | 0.710 |
| Single antibiotics | 21 (12.8%) | 2 (5.3%) | 19 (15.1%) | 0.112 |
| Meropenem | 19 (11.6%) | 2 (5.3%) | 17 (13.5%) | 0.165 |
| Vancomycin | 2 (1.2%) | 0 (0.0%) | 2 (1.6%) | 0.435 |
| Combination two antibiotics | 88 (53.7%) | 15 (39.5%) | 73 (57.9%) | 0.673 |
| Vancomycin+Meropenem | 77 (47.0%) | 14 (8.5%) | 63 (50.0%) | 0.154 |
| Vancomycin+Ceftazidime | 8 (4.9%) | 1 (2.6%) | 7 (5.6%) | 0.463 |
| Meropenem+Cefoperazone/Sulbactam | 3 (1.8%) | 0 (0.0%) | 3 (2.4%) | 0.337 |
| Others | 28 (17.1%) | 8 (21.1%) | 20 (15.9%) | 0.457 |
| Combination three antibiotics | 14 (8.5%) | 4 (10.5%) | 10 (7.9%) | 0.617 |
| Meropenem+Vancomycin+Ceftazidime | 6 (3.7%) | 3 (7.9%) | 3 (2.4%) | 0.113 |
| Meropenem+Ceftazidime+Tigecycline | 5 (3.0%) | 1 (2.6%) | 4 (2.4%) | 0.864 |
| Others | 3 (1.8%) | 0 (0.0%) | 3 (2.4%) | 0.337 |
Factors Affecting Survival
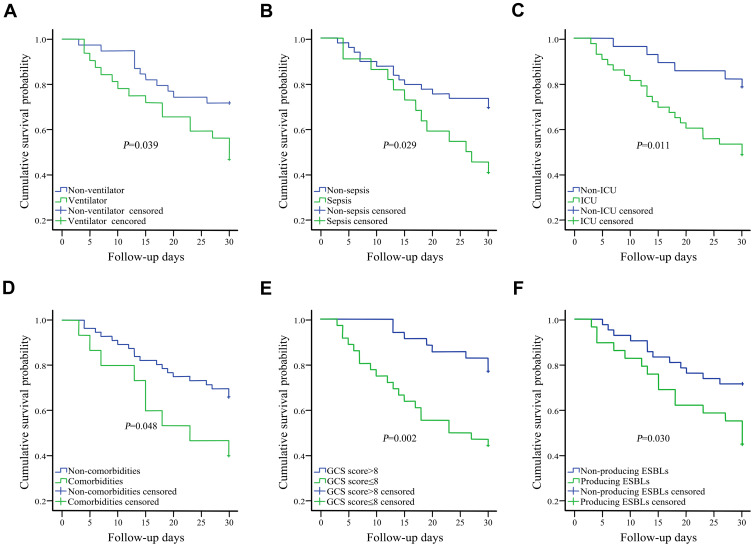
Seventy-one of the 164 cases were in hospital for more than 30 days after their first positive CSF bacteria culture. Finally, in this study, the overall 30-day mortality that was attributable to infection rate was 35.2% (25/71). On univariate analysis, comorbidities (diabetes mellitus and hypertension), GCS score ≤8, sepsis, ICU admission, producing ESBLs, and ventilation were related to poorer outcomes (P<0.05). Kaplan-Meier survival analysis for the above mentioned six risk factors can be seen in Figure 4. We included all factors with a P<0.10 on univariate analysis to make further evaluation using a Cox proportional hazards model, and hazard ratios were calculated. As shown in Table 3, on multivariate analysis, GCS scores ≤8 were found to be an independent risk factor for mortality.
Figure 4.
Kaplan–Meier survival analyses for ventilation (A), sepsis (B), ICU (C), comorbidities (D), GCS score (≤8) (E), and producing ESBLs (F).
Abbreviations: ICU, intensive care unit; GCS, Glasgow Coma Scale; ESBL, extended-spectrum beta-lactamase.
Table 3.
Prognostic Risk Factors of Post-Neurosurgical Enterobacteriaceae Meningitis/Encephalitis Screened by Multivariate Cox Proportional Hazards Model
| Factors | HR (95% CI) | P |
|---|---|---|
| Ventilation | 1.209 (0.519–2.816) | 0.660 |
| Sepsis | 1.281 (0.560–2.931) | 0.557 |
| Producing ESBLs | 1.119 (0.483–2.592) | 0.793 |
| ICU admission | 2.274 (0.832–6.215) | 0.109 |
| Comorbidities | 1.594 (0.679–3.739) | 0.284 |
| GCS score≤8 | 2.588 (1.056–6.346) | 0.038 |
Abbreviations: HR, hazard ratio; CI, confidence interval; ESBL, extended-spectrum beta-lactamase; ICU, intensive care unit; GCS, Glasgow Coma Scale.
Discussion
Meningitis/encephalitis after neurosurgical procedures is gradually increasing and has caused a formidable challenge, resulting in high mortality rates.11 In this study, we retrospectively analyzed 2416 isolates of neurosurgery in one of the largest neurosurgical centers of China. Among these isolates, there were 176 cases that resulted in neurosurgical bacterial meningitis/encephalitis. The incidence of meningitis/encephalitis infection was 7.3%, which is roughly the same as that reported in current literature.12
Enterobacteriaceae is the most important pathogenic bacteria in clinical bacterial infectious diseases. In some previous reports, staphylococcus aureus, coagulase negative staphylococcus, and enterococcus gram-positive bacteria were common pathogenic bacteria of post-neurosurgical meningitis/encephalitis. However, during the last decades, gram-negative bacilli, especially Enterobacteriaceae has become the most common pathogenic bacteria in post-neurosurgical meningitis/encephalitis patients.13,14 Carbapenem-resistant Enterobacteriaceae and ESBL-producing Enterobacteriaceae were one of the most urgent antibiotic-resistant bacteria assigned by the World Health Organization (WHO) in 2017.15 Haifa analyzed 185 cases of adult post-neurosurgical meningitis/encephalitis cases, of which, 117 (63.2%) were infected by gram-negative bacilli, with Enterobacteriaceae accounting for 55.16 Diagnosis and early treatment of infections caused by ESBL-producing Enterobacteriaceae bacteria has become an important problem that needs to be solved urgently due to its resistance to a variety of biological drugs, including three generations of cephalosporins. ESBL, which is common in Enterobacteriaceae, hydrolyzes super broad-spectrum cephalosporins and clavulanic acid inhibits their hydrolysis.17 In this study, the top three species of Enterobacteriaceae were K. pneumoniae (77, 47%), E. coli (29, 17.7%), and E. cloacae (17, 10.4%) (Figure 1), which is consistent with the results of our previous study.18
From this study, 37.8% of Enterobacteriaceae were found to produce ESBLs. Overall, 89.6% of Enterobacteriaceae were found to be resistant to ampicillin. It has been reported that the most effective and reliable antibiotics to treat infections caused by ESBL-producing Enterobacteriaceae are Carbapenems.19 Similarly, in our study, more than 80% of the isolates were sensitive to imipenem and meropenem indicating that this combination of antibiotics can be used for antibiotic prophylaxis of suspected meningitis after neurosurgery. All of the isolates were sensitive to polymyxin B.
According to the present study, preoperative antibiotic prophylaxis and intraoperative antibiotic administration are routine procedures currently used in neurosurgery. In our study, the combination of two antibiotics was more common in definitive therapy. In total, 77 of the 164 patients were given vancomycin plus meropenem. Although our results were not statistically significant, antibiotic combinations seemed to be more effective than single therapy antibiotics for Enterobacteriaceae meningitis/encephalitis in post-neurosurgical patients.
As previously reported, the mortality rate of meningitis/encephalitis in post-neurosurgical patients ranges from 15% to 35%,20,21 and consistent with these prior studies, the total in-hospital mortality rate for the diagnosis of Enterobacteriaceae meningitis/encephalitis in our study was 23.2%. Many previous studies have only shown the risk factors of neurosurgical infection, which include percentage of males, CSF leakage, surgical diagnosis, surgeon, early reoperation, surgical duration, EVD, LD, diabetes, and so on.3,22 However, few reports have focused on risk factors for predicting survival in post-neurosurgical patients. As we know, our study is the first to analyze death risk factors associated with Enterobacteriaceae meningitis/encephalitis in post-neurosurgical patients. Ravi Sharma’s recent study predicted survival following Acinetobacter meningitis/ventriculitis and suggested that an age >40 years, GCS score ≤8, presence of EVD, CSF WBC count >200 cells/mm3, and the presence of comorbidities were risk factors for mortality.23 While the impact of the surgical wound, ICU admission, ventilation, producing ESBLs, CSF leakage, and craniotomy were not evaluated in his report. In univariate analysis of the risk factors for predicting Enterobacteriaceae meningitis/encephalitis survival, we found that ICU admission, ventilation, producing ESBLs, sepsis, GCS score ≤8, and comorbidities were significant risk factors for mortality. GCS scores greater than 8 and without comorbidities (diabetes mellitus or hypertension) predicted better outcomes, which was in line with the Ravi Sharma’s study. In addition, patients with ventilation, sepsis, ICU admission, and producing ESBLs were found to have lower survival rates during the follow-up days.
GCS scores are widely used as a useful predictor for investigating patients with head injuries due to its ease of application, simplicity, and quickness.24,26 GCS scores for all of the patients were recorded on admission. There was a significant difference in the GCS scores between the dead (5.8±3.8) and alive (8.6±3.5) patients (P=0.021). According to our study, in multivariate Cox regression analysis it was also associated with 30-day mortality. A GCS score ≤8 was an independent risk factor for in-hospital death (HR=3.144, P=0.006). This finding highlights that patients with a low GCS score are more likely to have a poor outcome and antibiotic prophylaxis strategies could be developed in advance.
Several limitations exist in this study. Firstly, this is a single center, with a single source of patients, which may restrict the general applicability of the findings worldwide. In addition, the sample size of the survival analysis group was relatively small, because many patients stayed in the hospital less than 30 days. Therefore, the power of the outcome studies was limited. Secondly, post-neurosurgical aseptic meningitis was not concerned in this study. Among post-neurosurgical patients, aseptic meningitis accounts for a large proportion. Post-neurosurgical aseptic meningitis is induced by exfoliated tumor cells, hemolysis products, bone dust and implants, which are usually produced during surgery.27 Both aseptic meningitis and bacterial meningitis patients have similar clinical symptoms whereas patients with aseptic meningitis have milder clinical symptoms and require no antibiotic treatment. However, the main task of this study is to evaluate the clinical outcome of Enterobacteriaceae meningitis/encephalitis, so aseptic meningitis was not embedded in this article. Thirdly, the patients’ surgical exposure time is not fully recorded in our database, and the complexity of surgery is difficult to quantify, so we have not included these two indicators in our study. As previously reported, the surgery duration, major craniotomy and transsphenoidal surgery as associated with the development of meningitis after neurosurgical procedures. Patients undergoing long and complicated operations always mean an increased incidence of meningitis. Lastly, laboratory indicators were not involved in our study, we will assess the impact of laboratory indicators on post-neurosurgical meningitis outcome in the future. Considering all of these limitations, we intend to confirm the present results using a larger observational study or even a prospective cohort study.
Conclusion
In the current study, we classified the largest database of post-neurosurgical Enterobacteriaceae meningitis/encephalitis. More than 85% of the isolates were sensitive to imipenem and meropenem, and all of the isolates were sensitive to polymyxin B. We obtained a high mortality rate in the post-neurosurgical Enterobacteriaceae meningitis/encephalitis. GCS scores ≤8 were found to be an independent predictor for in-hospital mortality in post-neurosurgical Enterobacteriaceae meningitis/encephalitis. Appropriate definitive treatment for Enterobacteriaceae meningitis/encephalitis is essential.
Acknowledgment
We would like to thank Yu-meng Cai for helping the authors in collecting the data.
Ethics and Consent Statement
Our study has been conducted in accordance with the Declaration of Helsinki and been approved by the Beijing Tiantan Hospital of Capital Medical University. We performed a retrospective analysis of patient clinical information and did not conduct experiments on patient specimens. The data of the patients were maintained with confidentiality. In this study, only the medical records obtained from previous clinical treatment were used for retrospective analysis, and there was almost no risk to the patients. Considering of these reasons, after consultation with the Ethics Committee of Beijing Tiantan Hospital of Capital Medical University, written patient consent was not required.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Iwasaki Y, Inokuchi R, Harada S, Aoki K, Ishii Y, Shinohara K. Bacterial meningitis caused by hypervirulent Klebsiella pneumoniae capsular genotype K54 with development of granuloma-like nodal enhancement in the brain during the subacute phase. Intern Med. 2017;56(3):373–376. doi: 10.2169/internalmedicine.56.7384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Z, Wu X, Yu J, et al. Empirical combination antibiotic therapy improves the outcome of nosocomial meningitis or ventriculitis in neuro-critical care unit patients. Surg Infect (Larchmt). 2016;17(4):465–472. doi: 10.1089/sur.2015.060 [DOI] [PubMed] [Google Scholar]
- 3.Chen C, Zhang B, Yu S, et al. The incidence and risk factors of meningitis after major craniotomy in China: a retrospective cohort study. PLoS One. 2014;9(7):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma YF, Wen L, Zhu Y. Prospective study evaluating post-operative central nervous system infections following cranial surgery. Br J Neurosurg. 2019;33(1):80–83. doi: 10.1080/02688697.2018.1519112 [DOI] [PubMed] [Google Scholar]
- 5.Bi W, Liu H, Dunstan RA, et al. Extensively drug-resistant klebsiella pneumoniae causing nosocomial bloodstream infections in China: molecular investigation of antibiotic resistance determinants, Informing therapy, and clinical outcomes. Front Microbiol. 2017;8. doi: 10.3389/fmicb.2017.01230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Veen KEB, Brouwer MC, van der Ende A, van de Beek D. Bacterial meningitis in solid organ transplant recipients: a population-based prospective study. Transpl Infect Dis. 2016;18(5):674–680. doi: 10.1111/tid.12570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh TL, Querry AM, McCool S, et al. Risk factors for surgical site infections following neurosurgical spinal fusion operations: a case control study. Infect Control Hosp Epidemiol. 2017;38(3):340–347. doi: 10.1017/ice.2016.307 [DOI] [PubMed] [Google Scholar]
- 8.Sherrod BA, Arynchyna AA, Johnston JM, et al. Risk factors for surgical site infection following nonshunt pediatric neurosurgery: a review of 9296 procedures from a national database and comparison with a single-center experience. J Neurosurg Pediatr. 2017;19(4):407–420. doi: 10.3171/2016.11.PEDS16454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang C, Zhu T, Zhang P, Xia L, Sun C. Risk factors of neurosurgical site infection after craniotomy: a systematic review and meta-analysis. Am J Infect Control. 2017;45(11):e123–e134. doi: 10.1016/j.ajic.2017.06.009 [DOI] [PubMed] [Google Scholar]
- 10.Weinstein MP, Lewis JS. The clinical and laboratory standards institute subcommittee on antimicrobial susceptibility testing: background, organization, functions, and processes. Kraft CS, ed. J Clin Microbiol. 2020;58(3):e01864–19. doi: 10.1128/JCM.01864-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortiz OHH, García HIG, Ramírez FM, et al. Development of a prediction rule for diagnosing postoperative meningitis: a cross-sectional study. J Neurosurg. 2018;128(1):262–271. doi: 10.3171/2016.10.JNS16379 [DOI] [PubMed] [Google Scholar]
- 12.Xiao X, Zhang Y, Zhang L, Kang P, Ji N. The diagnostic value of cerebrospinal fluid lactate for post-neurosurgical bacterial meningitis: a meta-analysis. BMC Infect Dis. 2016;16(1):1–9. doi: 10.1186/s12879-016-1818-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussein K, Rabino G, Feder O, et al. Risk factors for meningitis in neurosurgical patients with cerebrospinal fluid drains: prospective observational cohort study. Acta Neurochir (Wien). 2019;161(3):517–524. doi: 10.1007/s00701-019-03801-y [DOI] [PubMed] [Google Scholar]
- 14.van de Beek D, Cabellos C, Dzupova O, et al. ESCMID guideline: diagnosis and treatment of acute bacterial meningitis. Clin Microbiol Infect. 2016;22:S37–S62. doi: 10.1016/j.cmi.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 15.Willyard C. The drug-resistant bacteria that pose the greatest health threats. Nature. 2017;543(7643):15. doi: 10.1038/nature.2017.21550 [DOI] [PubMed] [Google Scholar]
- 16.Neuberger A, Shofty B, Bishop B, et al. Risk factors associated with death or neurological deterioration among patients with Gram-negative postneurosurgical meningitis. Clin Microbiol Infect. 2016;22(6):573.e1–573.e4. doi: 10.1016/j.cmi.2016.03.016 [DOI] [PubMed] [Google Scholar]
- 17.Peirano G, Pitout JDD. Extended-spectrum β-lactamase-producing enterobacteriaceae: update on molecular epidemiology and treatment options. Drugs. 2019;79(14):1529–1541. doi: 10.1007/s40265-019-01180-3 [DOI] [PubMed] [Google Scholar]
- 18.Guanghui Z, Jing L, Guojun Z, Hong L. Epidemiology and risk factors of neurosurgical bacterial meningitis/encephalitis induced by carbapenem resistant Enterobacteriaceae. J Infect Chemother. 2020;26(1):101–106. doi: 10.1016/j.jiac.2019.07.023 [DOI] [PubMed] [Google Scholar]
- 19.King M, Heil E, Kuriakose S, et al. Multicenter study of outcomes with ceftazidime-avibactam in patients with carbapenem-resistant Enterobacteriaceae infections. Antimicrob Agents Chemother. 2017;61(7):1–4. doi: 10.1128/AAC.00449-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sachdeva D, Singh D, Loomba P, Kaur A, Tandon M, Bishnoi I. Assessment of surgical risk factors in the development of ventilator-associated pneumonia in neurosurgical intensive care unit patients: alarming observations. Neurol India. 2017;65(4):779–784.d1. doi: 10.4103/neuroindia.NI_814_16 [DOI] [PubMed] [Google Scholar]
- 21.Kourbeti IS, Vakis AF, Ziakas P, et al. Infections in patients undergoing craniotomy: risk factors associated with post-craniotomy meningitis. J Neurosurg. 2015;122(5):1113–1119. doi: 10.3171/2014.8.JNS132557 [DOI] [PubMed] [Google Scholar]
- 22.Jiang L, Guo L, Li R, Wang S. Targeted surveillance and infection-related risk factors of nosocomial infection in patients after neurosurgical operation. Pak J Pharm Sci. 2017;30(3):1053–1056. [PubMed] [Google Scholar]
- 23.Sharma R, Goda R, Borkar SA, et al. Outcome following postneurosurgical Acinetobacter meningitis: an institutional experience of 72 cases. Neurosurg Focus. 2019;47(2):1–7. doi: 10.3171/2019.5.FOCUS19278 [DOI] [PubMed] [Google Scholar]
- 24.Nik A, Sheikh Andalibi MS, Ehsaei MR, Zarifian A, Ghayour Karimiani E, Bahadoorkhan G. The efficacy of glasgow coma scale (GCS) score and acute physiology and chronic health evaluation (APACHE) II for predicting hospital mortality of ICU patients with acute traumatic brain injury. Bull Emerg Trauma. 2018;6(2):141–145. doi: 10.29252/beat-060208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majdan M, Brazinova A, Rusnak M, Leitgeb J. Outcome prediction after traumatic brain injury: comparison of the performance of routinely used severity scores and multivariable prognostic models. J Neurosci Rural Pract. 2017;8(1):20–29. doi: 10.4103/0976-3147.193543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maserati M, Fetzick A, Puccio A. The glasgow coma scale (GCS): deciphering the motor component of the GCS. J Neurosci Nurs. 2016;48(6):311–314. doi: 10.1097/JNN.0000000000000242 [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Xiao X, Zhang J, Gao Z, Ji N, Zhang L. Diagnostic accuracy of routine blood examinations and CSF lactate level for post-neurosurgical bacterial meningitis. Int J Infect Dis. 2017;59:50–54. doi: 10.1016/j.ijid.2017.03.026 [DOI] [PubMed] [Google Scholar]