Abstract
Purpose
To evaluate the efficacy and safety of drug-eluting beads transarterial chemoembolization (DEB-TACE) and conventional TACE (C-TACE) in treating hypovascular hepatocellular carcinoma (HCC).
Materials and Methods
The medical records based on HCC patients who underwent TACE from January 2016 to June 2019 were reviewed in the study. The diagnosis of hypovascular HCC was conducted by two senior radiologists according to imaging. We evaluated the adverse events (AEs), objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS) and overall survival (OS) in the study.
Results
A total of 98 patients with hypovascular HCC were included in the study. 46 patients underwent DEB-TACE treatment, and 52 patients underwent C-TACE treatment. The PFS of DEB-TACE group and C-TACE group was 12.0 months and 7.0 months (P < 0.001), and OS was 21.0 months and 14.0 months (P = 0.035), respectively. In addition, DEB-TACE group had better ORR (76.1% vs 40.4%, P < 0.001) and DCR (91.3% vs 75.0%, P = 0.033) compared to C-TACE group. The occurrence rate of AEs showed no difference between the two groups (67.3% vs 57.7%, P = 0.323). Furthermore, we found that DEB-TACE can be identified as a positive independent prognostic factor for improved PFS and OS.
Conclusion
DEB-TACE, as an effective treatment, can yield better objective response rate, similar safety profile and improved survival for hypovascular HCC patients compared to C-TACE.
Keywords: hypovascular hepatocellular carcinoma, drug-eluting beads, lipiodol, transarterial chemoembolization, survival
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignant tumor and the fourth leading cause of tumor-related death worldwide.1 According to the statistics, new cases of liver cancer exceeds 840 thousands and deaths more than 780 thousands every year.2 Early detection and screening is necessary to deal with such a high risk of new cases and deaths. Imaging, as an important technique, permits diagnosis and staging of HCC based mainly on assessment of vascularity.3 HCCs develop via multistep changes of drainage vessels, and range from hypovascular HCCs to typical hypervascular HCCs.4 As is known to all, the typical feature of hypervascular HCCs on contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) is the presence of marked arterial enhancement (wash-in) and then rapidly wash-out in the portal venous or delayed phase. Because of various differentiated degrees of HCCs, some hypovascular lesions present a low or medium enhancement on dynamic CT or MRI in the arterial and portal venous phases.5 Besides, faint tumor staining and limited tumor-feeding arteries can be detected on digital substraction angiography (DSA).6 In recent years, some studies have mainly focused on the treatment of hypervascular HCCs,7,8 whereas the optimal treatment of hypovascular HCC remains to be further considered.
Transarterial chemoembolization (TACE) is widely used in the treatment of HCC patients unsuitable for radical therapies.9,10 Chemotherapeutic agents like doxorubicin or epirubicin could be suspended in lipiodol are infused through tumor-feeding arteries and then followed by gelatin sponge particles to embolize, which can cause tumor hypoxia necrosis and toxic effects.11,12 In the past few decades, conventional TACE (C-TACE) with lipiodol has achieved a distinct survival benefit in the treatment of unresectable, large, or multinodular noninvasive HCCs.13 With technical advances in the performance of TACE, an alternative has been proposed and used for regional tumors. Drug-eluting beads TACE (DEB-TACE), which can achieve sustained drug delivery and permanent embolization, is considered an effective treatment for HCC.14,15 Some studies suggested DEB-TACE had a better short-term efficacy and lower complication rates in the treatment of Chinese patients with HCC.16,17 Besides, pharmacokinetic studies revealed that DEB-TACE resulted in higher intratumoral drug concentrations and lower systemic exposure.14,18 Despite promising results of these studies, few studies have focused on hypovascular HCC and evaluate the efficacy and safety after TACE.
Therefore, the present retrospective study was to analyze the efficacy and safety of DEB-TACE and C-TACE treatment for hypovascular HCC patients.
Materials and Methods
Study Design and Patients
This retrospective study was approved by the institutional review board. We reviewed the electronic medical record based on HCC patients who underwent TACE from January 2016 to June 2019 in the study. The diagnosis of hypovascular HCC was performed by two senior radiologists according to dynamic CT or MRI (Figure 1A).
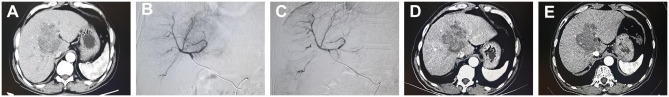
Figure 1.
An illustrative case of 61-year-old man who went DEB-TACE treatment. (A) Contrast-enhanced CT showed a tumor with weak enhancement near the hepatic hilar region. (B) DSA showed that faint tumor staining before treatment. (C) After DEB-TACE treatment, the tumor staining and obvious tumor nodule disappeared on DSA. (D) One month after treatment, enhanced CT revealed that tumor density decreased and necrosis occurred. (E) Three months after treatment, tumor shrunk and no recurrence or intrahepatic metastasis occurred.
Abbreviations: DEB-TACE, drug-eluting beads transarterial chemoembolization; CT, computed tomography; DSA, digital subtraction angiography.
The inclusion criteria for this study population were as follows: (1) accord with the imaging diagnosis of hypovascular HCC, (2) no imaging evidence of vascular invasion or extrahepatic metastasis, (3) Child-Pugh class A or B, and (4) Barcelona clinic liver cancer (BCLC) stage A or B. Exclusion criteria were the patients who had (1) hepatic metastasis derived from another carcinoma, (2) Child-Pugh class C, or severe liver, renal and coagulation dysfunction, and (3) hepatic encephalopathy, refractory ascites, esophageal variceal bleeding or other serious complications.
Transarterial Chemoembolization Procedure
Hepatic arteriography was performed with 5-F Yashiro catheter (Cook Inc., Indiana, USA) and 3-F coaxial microcatheter (Terumo, Tokyo, Japan) to select the celiac lobar, segmental, or subsegmental arteries as sequentially as possible, which mainly depended on the location of tumor-feeding arteries (Figure 1B).
One group of patients were treated with CalliSpheres drug-eluting beads (Jiangsu Hengrui Medicine Co. Ltd., Jiangsu, China). Firstly, 80 mg epirubicin powders were dissolved to 20 mg/mL, and mixed with a certain size (100–300 μm or 300–500 μm) dehydrated beads for 30 min. Then, appropriate contrast agents were added before and the drug-eluting beads were infused slowly into the proper hepatic arteries at a rate of 1 mL/min. Postoperative angiography showed complete embolization and inconspicuous tumor staining (Figure 1C).
Conventional TACE with lipiodol was applied to the other group of patients. Initially, the emulsion of 5–20 mL lipiodol and 10–30 mg epirubicin hydrochloride was administered into the feeding arteries, and then followed by vascular stagnation achieved with embolization in absorbable gelatin sponge particles (300–500 μm or 500–700 μm; Alicon medical Co., Hangzhou, China).19
Efficacy and Safety Assessments
The tumor response, adverse events (AEs), progression-free survival (PFS) and overall survival (OS) were reviewed in the study. The assessment of tumor response was conducted by contrast-enhanced CT or MRI according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria.20 Complete response (CR) was defined as disappearance of all target lesions and maintained for at least 4 weeks after treatment. Partial response (PR) was considered as more than 30% decrease in the sum of diameters of target lesions. Stable disease (SD) and progressive disease (PD) were considered as range from 30% decrease to 20% increase, more than 20% increase in the sum of diameters of target lesions, respectively. In the study, objective response rate (ORR) = (CR + PR)/total number of cases x 100%, disease control rate (DCR) = (CR+PR+SD)/total number of cases x 100%. To evaluate the safety of treatment, all AEs were recorded and assessed within 1 week after treatment according to the Society of Interventional Radiology (SIR) Standards of Practice Committee.21
Follow-Up
All patients underwent regular follow-up after treatment in our institution. The evaluation system of patients included detailed clinical examination, laboratory tests, and abdominal three-phase dynamic spiral CT or MRI (Figure 1D-E). The above evaluation of follow-up was conducted at a 4-6-week interval after the previous TACE treatment. Repeated TACE treatment would be performed if residual lesion or intrahepatic recurrent tumor was found on contrast-enhanced CT or MRI.
Statistical Analysis
All data were processed and analyzed by IBM SPSS Statistics version 24.0 (IBM, Corp., Chicago, USA), and a P value < 0.05 indicated significant difference. The statistical methods of independent-samples t-test, Person χ2 test, and continuity correction were used to determine differences between the two groups. Survival curves of two groups were estimated by applying Kaplan-Meier method and using the Log-rank test to analyze progression-free survival and overall survival. Univariate and multivariate analyses were assessed by Cox proportional hazard regression model. A value of P < 0.100 in univariate analysis would be further considered for multivariate analysis of independent prognostic factors.
Results
Study Population
A total of 98 hypovascular HCC patients who underwent DEB-TACE or C-TACE were included according to the previous inclusion and exclusion criteria in the study (DEB-TACE, n = 46; C-TACE, n = 52). Detailed baseline data of patients are shown in Table 1. There are no significant differences in the baseline data between the two groups. The mean follow-up times of patients were 14.32 ± 8.98 months (range, 3.5–39.0) in the DEB-TACE group and 14.20 ± 9.22 months (range, 3.0–36.0) in the C-TACE group (P = 0.951). In addition, 36 (78.3%) of 46 patients in the DEB-TACE group and 45 (86.5%) of 52 patients in the C-TACE group underwent repeated TACE, with average times of 2.0 (range, 1–6) and 2.4 (range, 1–7), respectively.
Table 1.
Baseline Data of Patients in the Study
| Variables | DEB-TACE Group (n=46) | C-TACE Group (n=52) | P value |
|---|---|---|---|
| Age (years)* | 59.59 ± 9.74 | 58.10 ± 12.37 | 0.513 |
| Sex | 0.814 | ||
| Male | 38 (82.6%) | 42 (80.8%) | |
| Female | 8 (17.4%) | 10 (19.2%) | |
| Etiology | 0.383 | ||
| Hepatitis B or C virus | 33 (71.7%) | 33 (63.5%) | |
| Other etiology | 13 (28.3%) | 19 (36.5%) | |
| Previous treatment | 0.152 | ||
| Untreated | 19 (41.3%) | 16 (30.8%) | |
| Surgery | 8 (17.4%) | 20 (38.5%) | |
| C-TACE | 12 (26.1%) | 13 (25.0%) | |
| Ablation | 2 (4.3%) | 1 (1.9%) | |
| Systemic treatment | 5 (10.9%) | 2 (3.8%) | |
| Tumor size (cm) | 0.614 | ||
| ≤5 | 26 (56.5%) | 32 (61.5%) | |
| >5 | 20 (43.5%) | 20 (38.5%) | |
| Number of nodules | 0.856 | ||
| Single | 16 (34.8%) | 19 (36.5%) | |
| Multiple | 30 (65.2%) | 33 (63.5%) | |
| Type of selectivity | 0.979 | ||
| Lobar | 5 (10.9%) | 6 (11.5%) | |
| Segmental | 26 (56.5%) | 30 (57.7%) | |
| Subsegmental | 15 (32.6%) | 16 (30.8%) | |
| Child-Pugh class | 0.601 | ||
| A | 39 (84.8%) | 42 (80.8%) | |
| B | 7 (15.2%) | 10 (19.2%) | |
| BCLC stage | 0.845 | ||
| A | 15 (32.6%) | 16 (30.8%) | |
| B | 31 (67.4%) | 36 (69.2%) | |
| α-Fetoprotein (μg/L) | 0.845 | ||
| ≤400 | 31 (67.4%) | 36 (69.2%) | |
| >400 | 15 (32.6%) | 16 (30.8%) | |
| Follow-up period (months)* | 14.32 ± 8.98 | 14.20 ± 9.22 | 0.951 |
Note: Data are numbers of patients unless indicated. Data in parentheses are percentages. *Data are expressed as the means ± standard deviation.
Abbreviations: DEB-TACE, drug-eluting beads transarterial chemoembolization; C-TACE, conventional transarterial chemoembolization; BCLC, Barcelona clinic liver cancer.
Treatment Response
The evaluation of tumor response was performed 4–6 weeks after previous treatment on the enhanced CT or MRI. And tumor responses in the two groups are shown in Table 2. The objective response rate for the DEB-TACE group was 76.1%, which was significantly higher than the 40.4% observed in the C-TACE group (P < 0.001). The disease control rate for the DEB-TACE group was 91.3% and slightly higher than the 75.0% of C-TACE group (P = 0.033). In addition, 4 (8.7%) of 46 patients for DEB-TACE group resulted in lower progressive disease than 13 (25.0%) of 52 patients for C-TACE group. In the imaging evaluation of patients who achieved progressive disease for DEB-TACE group, one (25%) patient developed peritoneal metastasis, two (50%) developed new tumor lesions, one (25%) developed main portal vein tumor thrombus. And for C-TACE group, three (23%) patients developed extrahepatic metastases, two (15%) developed portal invasion, eight (62%) developed new or enlarged residual lesion.
Table 2.
Assessment of Tumor Response Between Two Treatment Groups
| Response | DEB-TACE Group (n=46) | C-TACE Group (n=52) | P value |
|---|---|---|---|
| CR | 7 (15.2%) | 2 (3.9%) | 0.111 |
| PR | 28 (60.9%) | 19 (36.5%) | 0.016 |
| SD | 7 (15.2%) | 18 (34.6%) | 0.028 |
| PD | 4 (8.7%) | 13 (25.0%) | 0.033 |
| ORR | 35 (76.1%) | 21 (40.4%) | <0.001 |
| DCR | 42 (91.3%) | 39 (75.0%) | 0.033 |
Abbreviations: DEB-TACE, drug-eluting beads transarterial chemoembolization; C-TACE, conventional transarterial chemoembolization; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate.
Adverse Events and Safety of Treatment
Adverse events related to treatment between two groups are shown in Table 3. No treatment-related death or grade 4 AEs occurred in the study. Only two patients (one experienced grade 3 diarrhea and other experienced grade 3 abdominal pain) in the DEB-TACE group appeared grade 3 AEs during hospitalization and gradually relieved after empirical treatment, while no patients occurred in the C-TACE group. The other patients experienced lower and tolerable grade AEs. 51 AEs occurred in 31 (67.4%) of 46 patients after DEB-TACE treatment, while 52 AEs occurred in 30 (57.7%) of 52 patients after C-TACE treatment. When the above AEs occurred in the patients, the associated treatment was performed and relieved soon. There was no significant difference in the occurrence rates of AEs between two treatment groups.
Table 3.
The Comparison of Adverse Events Between Two Treatment Groups
| AEs | DEB-TACE Group (n=46) | C-TACE Group (n=52) | P value |
|---|---|---|---|
| Acute liver function impairment | 3 (6.5%) | 3 (5.8%) | 0.877 |
| Acute renal insufficiency | 1 (2.2%) | 0 | 0.469 |
| Hypoalbuminemia | 5 (10.9%) | 5 (9.6%) | 0.838 |
| Anemia | 1 (2.2%) | 5 (10.9%) | 0.210 |
| Vomiting | 15 (32.6%) | 11 (21.2%) | 0.200 |
| Fever | 5 (10.9%) | 10 (19.2%) | 0.251 |
| Abdominal pain | 20 (43.5%) | 18 (34.6%) | 0.369 |
| Diarrhea | 1 (2.2%) | 0 | 0.469 |
| Total | 31 (67.4%) | 30 (57.7%) | 0.323 |
Abbreviations: AEs, adverse events; DEB-TACE, drug-eluting beads transarterial chemoembolization; C-TACE, conventional transarterial chemoembolization.
Progression-Free Survival and Overall Survival
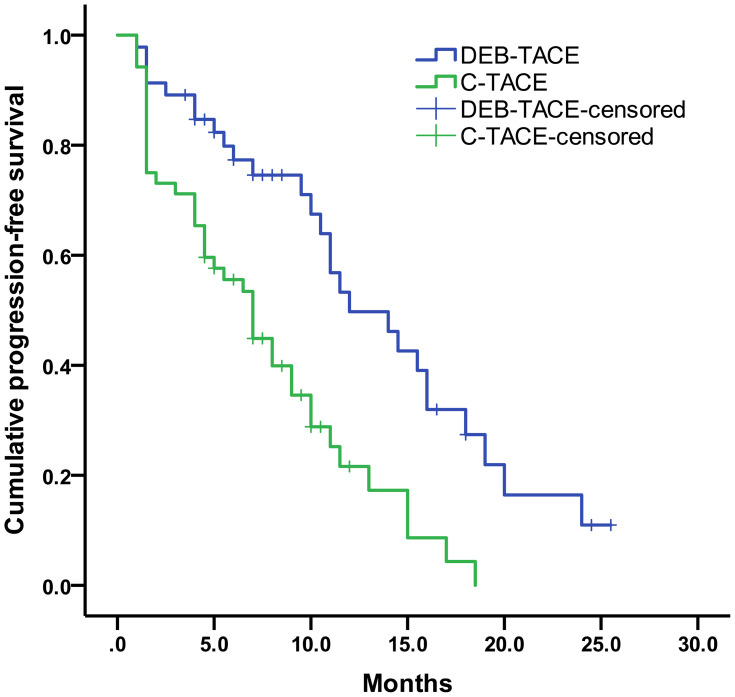
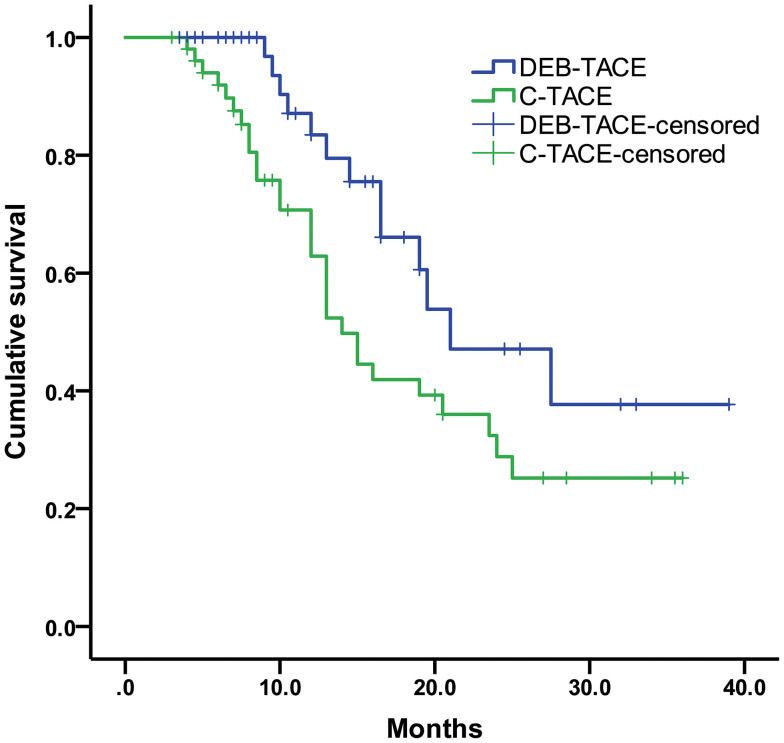
The median progression-free survival was 12.0 months (95% CI: 95% confidence interval, 7.72–16.29) for DEB-TACE group and 7.0 months (95% CI, 5.06–8.94) for C-TACE group (log-rank P < 0.001) (Figure 2). Moreover, our result showed that the median overall survival was 21.0 months (95% CI, 12.61–29.39) in the DEB-TACE group and 14.0 months (95% CI, 11.47–16.53) in the C-TACE group (log-rank P = 0.035) (Figure 3).
Figure 2.
Kaplan-Meier curves of PFS for DEB-TACE and C-TACE groups.
Figure 3.
Kaplan-Meier curves of OS for DEB-TACE and C-TACE groups.
Univariate and Multivariate Analysis
Univariate and multivariate analysis of prognostic factors affecting PFS and OS in all patients are shown in Table 4. The results of univariate analysis showed that treatment (P < 0.001) and tumor size (P < 0.052) were factors associated with PFS. Based on the findings, treatment and tumor size were further included in the multivariate analysis. The results revealed that DEB-TACE was identified as a positive factor for better PFS, while tumor size > 5 cm was hazard factor of PFS. As for OS, univariate and multivariate analyses showed that DEB-TACE and repeated TACE were important prognostic factors for OS in the study. Consequently, DEB-TACE was the only independent prognostic factor for improved PFS and OS.
Table 4.
Univariate and Multivariate Analysis of Prognostic Factors Affecting PFS and OS
| Parameters | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| PFS | ||||||
| DEB-TACE | 0.387 | 0.229–0.652 | <0.001 | 0.384 | 0.228–0.647 | <0.001 |
| Male | 1.195 | 0.657–2.172 | 0.559 | — | — | — |
| Previous treatment | 1.128 | 0.676–1.881 | 0.646 | — | — | — |
| Hepatitis B or C virus | 1.018 | 0.614–1.688 | 0.945 | — | — | — |
| Tumor size >5 cm | 1.637 | 0.996–2.691 | 0.052 | 1.665 | 1.013–2.737 | 0.044 |
| Child-Pugh B class | 0.918 | 0.451–1.868 | 0.813 | — | — | — |
| BCLC B stage | 1.100 | 0.661–1.831 | 0.713 | — | — | — |
| AFP >400 μg/L | 1.053 | 0.626–1.770 | 0.846 | — | — | — |
| Repeated TACE | 0.698 | 0.372–1.309 | 0.263 | — | — | — |
| AEs | 1.005 | 0.617–1.638 | 0.983 | — | — | — |
| OS | ||||||
| DEB-TACE | 0.505 | 0.263–0.973 | 0.041 | 0.246 | 0.106–0.568 | <0.001 |
| Male | 1.080 | 0.516–2.260 | 0.838 | — | — | — |
| Previous treatment | 0.801 | 0.429–1.496 | 0.486 | — | — | — |
| Hepatitis B or C virus | 0.709 | 0.379–1.328 | 0.283 | — | — | — |
| Tumor size >5 cm | 1.317 | 0.713–2.433 | 0.379 | — | — | — |
| Child-Pugh B class | 1.615 | 0.710–3.672 | 0.253 | — | — | — |
| BCLC B stage | 1.320 | 0.663–2.630 | 0.430 | — | — | — |
| AFP >400 μg/L | 0.590 | 0.289–1.202 | 0.146 | — | — | — |
| Repeated TACE | 0.270 | 0.132–0.555 | <0.001 | 0.113 | 0.044–0.291 | <0.001 |
| AEs | 0.994 | 0.539–1.834 | 0.985 | — | — | — |
Abbreviations: HR, hazard ratio; CI, confidence interval; PFS, progression-free survival; DEB-TACE, drug-eluting beads transarterial chemoembolization; BCLC, Barcelona clinic liver cancer; AFP, α-fetoprotein; TACE, transarterial chemoembolization; AEs, adverse events; OS, overall survival.
Discussion
Due to occult and persistent angiogenesis of HCC, most patients present hypervascular nodules at the time of imaging diagnosis, that is, intense contrast uptake in the arterial phase and followed by contrast washout in the delayed venous phase on dynamic CT or MRI.3,22 Previously, a study revealed that TACE treatment can achieve definite efficacy and improved survival for hypervascular HCC patients.23 And the Ogasawara et al study analyzed the prognosis of hypervascular HCC patients with or without hypovascular nodules.24 However, few evidences focused on TACE treatment of hypovascular HCCs.
In the present study, we explained the definition of hypervascular and hypovascular HCC on imaging including dynamic CT or MRI, and retrospectively compared clinical outcomes of hypovascular HCC patients who underwent DEB-TACE and C-TACE. The results indicated DEB-TACE can be effectively used for hypovascular HCC. Compared to C-TACE, patients who underwent DEB-TACE can obtain satisfactory clinical benefits, which might be mainly attributed to a longer progression-free survival (12.0 months vs 7.0 months, P < 0.001) and overall survival (21.0 months vs 14.0 months, P = 0.035). And DEB-TACE achieved higher objective response rate (76.1% vs 40.4%, P < 0.001) and disease control rate (91.3% vs 75.0%, P = 0.033) than C-TACE. Referring to safety profile, DEB-TACE was associated with a similar result compared to C-TACE in our study (67.3% vs 57.7%, P = 0.323). Only two patients in the DEB-TACE group developed grade 3 treatment-related AEs and relieved during hospitalization. The other mild AEs such as vomiting, fever, and abdominal pain were no significant difference between two groups, the P values of which were 0.200, 0.251 and 0.369, respectively. Similar to our findings, several meta-analyses found DEB-TACE showed better treatment benefit and similar safety profile for HCC compared to C-TACE.25,26 And a multi-center study reported that DEB-TACE had a better treatment response than C-TACE as the first-line therapy in treating Chinese HCC patients.27
Importantly, we found that treatment was the positive prognostic factor for progression-free survival (HR: hazard ratio = 0.384, 95% CI = 0.228–0.647, P < 0.001) and overall survival (HR = 0.246, 95% CI = 0.106–0.568, P < 0.001) in our multivariate analysis. Besides, tumor size was the important independent prognostic factor for PFS (HR = 1.665, 95% CI = 1.013–2.737, P = 0.044), regardless of any treatment (DEB-TACE or C-TACE) was used. Our analysis showed that patients with tumor size (larger than 5 cm) seems to be related to a poor PFS. That may be because chemotherapy drugs are difficult to kill a wide range of tumor through the originally limited tumor-feeding arteries. Similarly, the results of Nakano et al study indicated that large tumor size and residual supplying vessels were significant risk factors associated with local recurrence after DEB-TACE treatment.28
Besides treatment, repeated TACE was the meaningful prognostic factor for OS (HR = 0.113, 95% CI = 0.044–0.291, P < 0.001). According to the guidance, repeated TACE, as a supplemental treatment, was necessary when residual lesion recurrence and new lesions were found on imaging.5,29 And repeated TACE can contribute to remove residual lesions and prolong overall survival if hepatic function reserves and patient tolerance permits. In addition, the Terzi et al study30 and Choi et al study31 reported intermediate-stage HCC patients can achieve better clinical outcomes during repeated TACE than the initial TACE.
There were several limitations in the study. Firstly, the study was a retrospective treatment strategy in patients with hypovascular HCC based on the preference of radiologists, or the patients, which may lead to a selection bias in the study population. But the bias may be low from our baseline data of patients in the study included. Secondly, the samples were not enough to evaluate all patients with hypovascular liver cancer. For example, patients with hepatic metastasis derived from another carcinoma and patients with BCLC stage C were not included in the study population. Last, it was regrettable that the subgroup analysis including tumor size and repeated treatment had not been performed in the study. Substratification of tumor size, as a promising prognostic factor, remains to be further studied.
In conclusion, DEB-TACE is an effective treatment to improve clinical outcomes of hypovascular HCC patients. Furthermore, patients who underwent DEB-TACE treatment had better ORR and DCR, similar AEs, improved PFS and OS compared to C-TACE treatment. On the basis of our findings, DEB-TACE is likely to be a promising therapeutic option for hypovascular HCC patients.
Funding Statement
The study was supported by the National Natural Science Foundation of China (81873917).
Ethics and Consent Statement
This study protocol was approved by the institutional review board of the Union Hospital, Tongji Medical college, Huazhong University of Science and Technology. The study was conducted in accordance with the ethical principles laid down in the Declaration of Helsinki. Written informed consent was obtained from all patients before treatment.
Disclosure
All authors declare that they have no conflicts of interest in this work.
References
- 1.Villanueva A, Longo DL. Hepatocellular Carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi: 10.1056/NEJMra1713263 [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 3.Lee JM, Trevisani F, Vilgrain V, Wald C. Imaging diagnosis and staging of hepatocellular carcinoma. Liver Transpl. 2011;17(Suppl):2S34–S43. doi: 10.1002/lt.22369 [DOI] [PubMed] [Google Scholar]
- 4.Maesaka K, Sakamori R, Yamada R, et al. Hypovascular hepatic nodules as a predictive factor for transcatheter arterial chemoembolization refractoriness in hepatocellular carcinoma. Hepatol Res. 2020;50(3):365–373. doi: 10.1111/hepr.13446 [DOI] [PubMed] [Google Scholar]
- 5.Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–370. doi: 10.1007/s12072-017-9799-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwazawa J, Ohue S, Mitani T, et al. Identifying feeding arteries during TACE of hepatic tumors: comparison of C-arm CT and digital subtraction angiography. AJR Am J Roentgenol. 2009;192(4):1057–1063. doi: 10.2214/AJR.08.1285 [DOI] [PubMed] [Google Scholar]
- 7.Yang W, Yan K, Chen MH, et al. Preliminary experience with direct percutaneous arterial embolisation combined with radiofrequency ablation for hypervascular HCC. Int J Hyperthermia. 2017;33(7):836–845. doi: 10.1080/02656736.2017.1305126 [DOI] [PubMed] [Google Scholar]
- 8.Zhang ZY, Lee JC, Yang W, et al. Percutaneous ablation of the tumor feeding artery for hypervascular hepatocellular carcinoma before tumor ablation. Int J Hyperthermia. 2018;35(1):133–139. doi: 10.1080/02656736.2018.1484525 [DOI] [PubMed] [Google Scholar]
- 9.Grandhi MS, Kim AK, Ronnekleiv-Kelly SM, Kamel IR, Ghasebeh MA, Pawlik TM. Hepatocellular carcinoma: from diagnosis to treatment. Surg Oncol. 2016;25(2):74–85. doi: 10.1016/j.suronc.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 10.Ha Y, Lee JB, Shim JH, et al. Validation and reappraisal of the assessment for retreatment with transarterial chemoembolization score for unresectable non-metastatic hepatocellular carcinoma in a hepatitis b virus-endemic region. Eur Radiol. 2016;26(10):3510–3518. doi: 10.1007/s00330-015-4185-2 [DOI] [PubMed] [Google Scholar]
- 11.Aramaki T, Moriguchi M, Bekku E, Asakura K, Sawada A, Endo M. Comparison of epirubicin hydrochloride and miriplatin hydrate as anticancer agents for transcatheter arterial chemoembolization of hepatocellular carcinoma. Hepatol Res. 2013;43(5):475–480. doi: 10.1111/j.1872-034X.2012.01100.x [DOI] [PubMed] [Google Scholar]
- 12.Shirono T, Iwamoto H, Niizeki T, et al. Epirubicin is more effective than miriplatin in balloon-occluded transcatheter arterial chemoembolization for hepatocellular carcinoma. Oncology. 2019;96(2):79–86. doi: 10.1159/000492822 [DOI] [PubMed] [Google Scholar]
- 13.Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016;64(1):106–116. doi: 10.1002/hep.28453 [DOI] [PubMed] [Google Scholar]
- 14.Zhang S, Huang C, Li Z, et al. Comparison of pharmacokinetics and drug release in tissues after transarterial chemoembolization with doxorubicin using diverse lipiodol emulsions and CalliSpheres Beads in rabbit livers. Drug Deliv. 2017;24(1):1011–1017. doi: 10.1080/10717544.2017.1344336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aliberti C, Carandina R, Lonardi S, et al. Transarterial chemoembolization with small drug-eluting beads in patients with hepatocellular carcinoma: experience from a cohort of 421 patients at an Italian center. J Vasc Interv Radiol. 2017;28(11):1495–1502. doi: 10.1016/j.jvir.2017.07.020 [DOI] [PubMed] [Google Scholar]
- 16.Wu B, Zhou J, Ling G, Zhu D, Long Q. CalliSpheres drug-eluting beads versus lipiodol transarterial chemoembolization in the treatment of hepatocellular carcinoma: a short-term efficacy and safety study. World J Surg Oncol. 2018;16(1):69. doi: 10.1186/s12957-018-1368-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou GH, Han J, Sun JH, et al. Efficacy and safety profile of drug-eluting beads transarterial chemoembolization by CalliSpheres® beads in Chinese hepatocellular carcinoma patients. BMC Cancer. 2018;18(1):644. doi: 10.1186/s12885-018-4566-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46(3):474–481. doi: 10.1016/j.jhep.2006.10.020 [DOI] [PubMed] [Google Scholar]
- 19.Galle PR, Forner A, Llovet JM, European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 20.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi: 10.1055/s-0030-1247132 [DOI] [PubMed] [Google Scholar]
- 21.Khalilzadeh O, Baerlocher MO, Shyn PB, et al. Proposal of a new adverse event classification by the society of interventional radiology standards of practice committee. J Vasc Interv Radiol. 2017;28(10):1432–1437 e1433. doi: 10.1016/j.jvir.2017.06.019 [DOI] [PubMed] [Google Scholar]
- 22.Kudo M. Multistep human hepatocarcinogenesis: correlation of imaging with pathology. J Gastroenterol. 2009;44(Suppl):19112–19118. doi: 10.1007/s00535-008-2274-6 [DOI] [PubMed] [Google Scholar]
- 23.Murakami T, Ishimaru H, Sakamoto I, et al. Percutaneous radiofrequency ablation and transcatheter arterial chemoembolization for hypervascular hepatocellular carcinoma: rate and risk factors for local recurrence. Cardiovasc Intervent Radiol. 2007;30(4):696–704. doi: 10.1007/s00270-007-9003-z [DOI] [PubMed] [Google Scholar]
- 24.Ogasawara S, Chiba T, Motoyama T, et al. Prognostic significance of concurrent hypovascular and hypervascular nodules in patients with hepatocellular carcinoma. PLoS One. 2016;11(9):e0163119. doi: 10.1371/journal.pone.0163119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang K, Zhou Q, Wang R, Cheng D, Ma Y. Doxorubicin-eluting beads versus conventional transarterial chemoembolization for the treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29(5):920–925. doi: 10.1111/jgh.12439 [DOI] [PubMed] [Google Scholar]
- 26.Zhou X, Tang Z, Wang J, et al. Doxorubicin-eluting beads versus conventional transarterial chemoembolization for the treatment of hepatocellular carcinoma: a meta-analysis. Int J Clin Exp Med. 2014;7(11):3892–3903. [PMC free article] [PubMed] [Google Scholar]
- 27.Ma Y, Zhao C, Zhao H, et al. Comparison of treatment efficacy and safety between drug-eluting bead transarterial chemoembolization with CalliSpheres microspheres and conventional transarterial chemoembolization as first-line treatment in hepatocellular carcinoma patients. Am J Transl Res. 2019;11(12):7456–7470. [PMC free article] [PubMed] [Google Scholar]
- 28.Nakano MM, Yamamoto A, Nishida N, et al. Risk factors for local recurrence of hepatocellular carcinoma after transcatheter arterial chemoembolization with drug-eluting beads (DEB-TACE). Jpn J Radiol. 2019;37(7):543–548. doi: 10.1007/s11604-019-00840-4 [DOI] [PubMed] [Google Scholar]
- 29.Kudo M, Matsui O, Izumi N, et al. JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the liver cancer study group of Japan. Liver Cancer. 2014;3(3–4):458–468. doi: 10.1159/000343875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terzi E, Golfieri R, Piscaglia F, et al. Response rate and clinical outcome of HCC after first and repeated cTACE performed “on demand”. J Hepatol. 2012;57(6):1258–1267. doi: 10.1016/j.jhep.2012.07.025 [DOI] [PubMed] [Google Scholar]
- 31.Choi J, Shim JH, Shin YM, Kim KM, Lim YS, Lee HC. Clinical significance of the best response during repeated transarterial chemoembolization in the treatment of hepatocellular carcinoma. J Hepatol. 2014;60(6):1212–1218. doi: 10.1016/j.jhep.2014.01.014 [DOI] [PubMed] [Google Scholar]