Abstract
The outbreak of SARS-CoV-2-associated pneumonia, a disease called COVID-19, has caused a pandemic worldwide. To investigate the immune responses after infection of SARS-CoV-2 in non-critical patients may help to better understand the disease progression. We collected 334 confirmed COVID-19 cases including 212 still in hospital with nucleic acid test positive on halfway for SARS-CoV-2 and 122 discharged from hospital, compared specific antibodies, immune cells, and cytokine changes between the hospitalized and discharged patients. The hospitalized patients had a longer illness time compared with discharged patients. Analysis of viral loads explained long-term or persistent infection of SARS-CoV-2, which existed with the median time of 18.5 days of the positive nucleic acid test. Serum analysis showed that the specific anti-N IgG antibody was positive in all detected patients after infection of two weeks. Neutrophils, Monocytes, NK cells, and CD4+ T cells significantly increased, while total lymphocytes and CD8+ T cells decreased from non-critical hospitalized patients after longer-term infection. Further analysis of the cytokines showed that IL-6, TNF-α, IFN-γ, IL-2, IL-4, and IL-10 from the hospitalized patients were significantly higher, indicating a potential of the increased CD4+ T cell differentiation.
Keywords: SARS-CoV-2, Long-term infection, Immune response
Highlights
-
•
Viral loads explained the long-term or persistent infection of SARS-CoV-2 in some non-critical and hospitalized patients.
-
•
After infection of two weeks, serum analysis showed that the specific anti-N IgG was positive in all detected patients.
-
•
Compared with the discharged patients, inpatients had different immune cells after longer-infection of SARS-CoV-2.
-
•
Compared with the discharged patients, hospitalized patients had an increase in some cytokines.
1. Introduction
The outbreak of SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) -associated pneumonia, a disease called COVID-19 (Coronavirus Disease 2019), had caused a pandemic worldwide [1,2]. Although the fatality rate of COVID-19 is lower, the virus has already caused more death than the other lethal cousin MERS-CoV (Middle East Respiratory Syndrome Coronavirus) and SARS-CoV (Severe Acute Respiratory Syndrome Coronavirus) outbreaks combined.
As an infectious disease, the pathogen, a betacoronaviruse, now called SARS-CoV-2 has been identified and the whole genome sequencing was reported and disclosure successively [3]. According to the analysis of the structure of spike protein and knockout strains of cell receptor-specific gene, the invading receptor was identified as ACE2 (Angiotensin-Converting Enzyme 2), the same with SARS-CoV [4,5]. As for clinical classification, most cases were classified as mild with non-pneumonia and mild pneumonia, 14% were severe involving serious pneumonia and shortness of breath, and 5% were the agency called “critical cases”, which were more likely to face death [6].
However, under the enormous pressure of virus fast transmission [7,8], it is crucial to diagnose the disease on the onset of the disease in the early stage. The chest CT (computed tomography) scans provided strong evidence for diagnose [9]. Due to the disclosure of whole genome sequencing results, methods for nucleic acid detection by RT-PCR (Reverse Transcription Polymerase Chain Reaction) were quickly established. SARS-CoV-2 viral load in upper respiratory specimens of infected patients within 15 days and 21 days had been reported, indicating the decline of the viral loads in these two individual studies [10,11]. These studies gave us the first glimpses from the aspect of nucleic acid to know the characteristics of the duration of infection of SARS-CoV-2. Nevertheless, surprisingly, the nucleic acid detection of SARS-CoV-2 became positive again in some patients who were discharged from hospital, which demonstrated the cunning faces of SARS-CoV-2 [12].
Indeed, these findings of SARS-CoV-2 were far from our knowledge. Understandings of the patients' recovery time and the undetectable time-points for virus are indispensable to judge how long the virus exists in the body. In this prospective study, we analyzed the disease duration from symptom onset to recovery, the time-points of undetectable viral nucleic acid from collected 122 cases who had been discharged from the hospital. Furthermore, we addressed the viral loads of the 212 patients who were still in the hospital to understand their changes in the long-time SARS-CoV-2 infected hospitalized patients. Comparison of the viral loads, immune cells, and cytokine changes will provide information to get some new insights into SARS-CoV-2 infections and better control the spread of disease.
2. Methods
2.1. Patients
In this study, we collected 334 confirmed COVID-19 patients including 212 still in hospital with nucleic acid test positive on halfway for SARS-CoV-2 and 122 discharged from hospital from Feb 27, 2020, to Mar 1, 2020, in Wuhan Union hospital, a designated hospital for treating COVID-19. In the current situation, most patients in the hospital are with apparent infection and need supportive therapeutic efficacy to help to recover from the infection. The enrolled patients in this study were all diagnosed as COVID-19 according to WHO interim guidance. This case series was approved by the Institutional Ethics Committee of Wuhan Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. Written informed consent was waived by the Ethics Commission of the designated hospital for emerging infectious diseases.
2.2. Procedures
We extracted demographic data, medical history, symptoms, signs, laboratory findings, and the treatment measures from electronic medical records. Laboratory confirmation of SARS-CoV-2 was done in different hospitals with valid evidence and steroid treatment cases had been excluded from our collected cases. Followed the newly classification methods given by china CDC, mild patients are including non-pneumonia and mild pneumonia, severe are involving serious pneumonia with dyspnea, respiratory frequency ≥ 30/min, blood oxygen saturation ≤ 93%, partial pressure of arterial oxygen to fraction of inspired oxygen ratio < 300, and/or lung infiltrates >50% within 24 to 48 h and shortness of breath, the agency calls “critical” patients are with respiratory failure, septic shock, and/or multiple organ dysfunction or failure [6]. The collected cases were divided by whether the patients were still discharged from the hospital, and the data were analyzed according to the following flowchart.

Throat-swab specimens from the upper respiratory tract that were obtained from all patients at admission were maintained in a viral-transport medium. In particular, to ensure the accuracy of the analysis results, only the viral load data acquiring under the same kit and operating conditions were accepted.
2.3. Statistical analysis
Categorical variables were expressed as frequency rates and percentages (%), and continuous variables were expressed as mean (SD) if they are normally distributed or median (IQR) if not. Means for continuous variables were compared using independent group t-test when the data were normally distributed; otherwise, the Mann-Whitney test was used. Proportions for categorical variables were compared using the χ2 test, although the Fisher exact test was used when the data were limited. All statistical analyses were performed using SPSS (version 13.0).
3. Results
3.1. Demographic and clinical characteristics
In all collected cases the patients' median age was 62 years (IQR (interquartile range), 50–68), and the younger were more likely to discharge from the hospital (Table 1 ). Of the 334 patients, 57.82% had one or more coexisting medical comorbidities, including cardiovascular diseases, diabetes, pulmonary disease, and malignancy. The most common symptoms at the onset of illness were fever and cough, Other symptoms included anorexia, chest congestion. Fatigue, myalgia, agrypnia, diarrhea, dyspnea, hypoxemia, nausea, and pharyngalgia (Table 1), as well as ever reported [13,14].
Table 1.
Clinical characteristics of patients with non-critical COVID-19 pneumonia.a
| No.(%) |
|||||
|---|---|---|---|---|---|
| Total (n = 334) |
Hospitalized (n = 212) |
Discharged (n = 122) |
P Valuea |
||
| Age, median (IQR), y | 62(50,68) | 64(56,69) | 56(40,65) | <0.0001 | |
| ≤60 years | 144(43.11%) | 77(36.32%) | 67(54.92%) | 0.0010 | |
| >60 years | 190(56.89%) | 135(63.68%) | 55(45.08%) | ||
| Sex | |||||
| Male | 152(45.51%) | 100(47.17%) | 52(42.62%) | 0.4217 | |
| Female | 182(54.49%) | 112(52.83%) | 70(57.38%) | ||
| Comorbidities | |||||
| Cardiovascular disease | 106(31.74%) | 75(35.38%) | 31(25.41%) | 0.0595 | |
| Pulmonary disease | 20(5.99%) | 8(3.77%) | 12(9.84%) | 0.0245 | |
| Diabetes | 45(13.47%) | 31(14.62%) | 14(11.48%) | 0.4173 | |
| Cerebrovascular disease | 4(1.20%) | 2(0.94%) | 2(1.64%) | 0.6249 | |
| Malignancy | 8(2.40%) | 4(1.89%) | 4(3.28%) | 0.4699 | |
| Chronic liver disease | 15(4.49%) | 9(4.25%) | 6(4.92%) | 0.7750 | |
| Chronic kidney disease | 6(1.80%) | 2(0.94%) | 4(3.28%) | 0.1963 | |
| Signs and symptoms | |||||
| Fever | 237(70.96%) | 150(70.75%) | 87(71.31%) | 0.9141 | |
| Anorexia | 58(17.37%) | 34(16.04%) | 24(19.67) | 0.3985 | |
| Cough | 224(67.07%) | 140(66.04%) | 84(68.85%) | 0.5982 | |
| Fatigue | 106(31.74%) | 69(32.55%) | 37(30.33%) | 0.6748 | |
| Chest congestion | 126(37.72%) | 83(39.15%) | 43(35.25%) | 0.4783 | |
| Myalgia | 49(14.67%) | 36(16.98%) | 13(10.66%) | 0.1157 | |
| Agrypnia | 14(4.19%) | 14(6.60%) | 0(0.00%) | 0.0216 | |
| Diarrhea | 35(10.48%) | 34(16.04%) | 1(0.82%) | <0.0001 | |
| Dyspnea | 34(10.18%) | 24(11.32%) | 10(8.20%) | 0.3633 | |
| Nausea | 36(10.78%) | 20(9.43%) | 16(13.11%) | 0.2963 | |
| Pharyngalgia | 26(7.78%) | 18(8.49%) | 8(6.56%) | 0.5255 | |
| Percent of severity degrees | |||||
| Mild | 311(93.11%) | 193(91.04%) | 118(96.12%) | 0.0483 | |
| Severe | 23(6.89%) | 19(8.96%) | 4(3.28%) | ||
P values indicate differences between the hospitalized and the discharged patients. P < .05 was considered statistically significant. Means for continuous variables were compared using independent group t-test when the data were normally distributed; otherwise, the Mann-Whitney test was used.
The majority of the confirmed patients were mild in enrolled cases (Table 1). We collected the detailed data of the discharged patients of the disease duration, the undetectable time-point of viral nucleic acid, and the illness time of the inpatients (Fig. 1A). The average disease duration from initial symptoms to discharged from hospital was 25.9 days, median time was 25 (IQR, 20–31). And the median time of undetectable time-point of viral nucleic acid was 19 (IQR,13–24.5) (Fig. 1B). The illness time is much longer than the viral undetectable time-point. It suggested that the immunopathological damage still happened in the patients infected with SARS-CoV-2, similar to the SARS-CoV infected patients [15]. Moreover, the time of illness of hospitalized patients was longer than the discharged patients. It also seemed reasonable for most of the simultaneous admission under the special quarantine condition in China with few new infection cases occurring.
Fig. 1.
The ill time of disease of COVID-19 patients.
(A) The scheme of COVID-19 patients from onset to discharge or be hospitalized. (B) The undetectable timepoint of viral nucleic acid in discharged patients (n = 122), and the time of illness in discharged patients (n = 122) and hospitalized patients(n = 212).
3.2. Viral loads suggested a long-term infection of SARS-CoV-2 in hospitalized non-critical patients
The executing discharge rules promoted us to follow up on the inpatient's nucleic acid tests. While it was easy to find that there were still many patients classified as mild ones who couldn't be discharged from hospital. We collected all positive nucleic acid tests in inpatients and recorded the time. Surprisingly, the result showed that the long-term infection of SARS-CoV-2 might really do exist, over 6 weeks some patients with mild pneumonia owned still positive nucleic test results (Fig. 2A). The median time of positive RT-PCR in inpatients was 18.5 (IQR, 12–25) days, which was much close to the undetectable time of nucleic acid of the discharged patients and approaching to the median duration of viral shedding time in critical patients, 20.0 days [16], which illustrated a long-term infection of SARS-CoV-2 in non-critical patients. Multiple positive nucleic acid tests in the same patient were even more telling the truth by excluding the false positives (Fig. 2B). The comparison of the viral loads between the mild (n = 16) and severe patients (n = 5) revealed that there was no significant difference in the nucleic acid positive patients on day 19 and day 20 since onset (Fig. 2C). These suggested the possibility of long-term carrying viruses in both mild and severe cases. It could be speculated that the virus could cause a long-term persistent infection in these nucleic acid positive inpatients with apparent pneumonia but not critical, confirmed by chest CT scans, long-term infection of SARS-CoV-2 occurred in hospitalized non-critical patients.
Fig. 2.

The viral loads in hospitalized patients.
(A) The Ct value of the positive test results of viral ORF-1 fragment and the corresponding detected time were recorded. And in this figure to exhibit the positive nucleic acid detection results the abscissa showed the time, and the ordinate showed the Ct value, the median time of the positive RT-PCR was 18.5 (IQR, 12–25) days. (B) Multiple positive nucleic acid tests of the same patient in different hospitalized patients were shown. (C) The comparison of the viral loads in mild (n = 16) and severe patients(n = 5) by comparison of the Ct value of ORF-1. Means for continuous variables were compared using independent group t-test when the data were normally distributed; otherwise, the Mann-Whitney test was used.
3.3. Specific SARS-CoV-2 antibodies were produced in all patients for two weeks of onset
The question of whether the long-term carrying or infection of SARS-CoV-2 could arouse the specific antibody awaited an answer. The detection of specific IgG (Immunoglobulin G) or IgM (Immunoglobulin G) against nucleocapsid protein (N) was performed on February 29 and March 01. The detection values and time were recorded. Most of the collected detection values were between two and six weeks. And fortunately all the RT-PCR positive patients were antibody positive according to the prescribed standards of judgment. Analysis of the detailed data we found the value of IgG was much stable in inpatients but the values of IgM were in a wide range of variation (Fig. 3A and B), and there was no significant difference in both the IgG and IgM values between the time within 15 to 30 days (n = 173) or after 30 days (n = 104) (Fig. 3C). Also, little difference was seen in the discharged (n = 8) and hospitalized patients (n = 127) (Fig. 3D). Additionally, the same batch of antibody detection was less performed in the discharged patients, thus the discharged patients had less antibody detection numbers than the hospitalized patients. In conclusion, whatever, these results illustrated that the virus could arouse the acquired immune response of the infected bodies to produce specific antibodies both in inpatients and discharged patients. Specific SARS-CoV-2 antibodies were produced for two weeks of onset.
Fig. 3.

Specific antibodies were positive in patients after infection of 2 weeks of onset.
(A) The figure showed an overview of the COVID-19 patients' specific IgG antibodies test results. The detection values of the specific IgG antibodies of N protein and the corresponding detection time were recorded. After 2 weeks of onset, all the patients' IgG antibodies were positive. (B) The figure showed an overview of the COVID-19 patients' specific IgM antibodies test results. The detection values of the specific IgM antibodies of N protein and the corresponding detection time were recorded. The values of IgM were in a wide range of variations. (C) Comparison of the IgG and IgM detection values between the time within 15 to 30 days (n = 173) or after 30 days (n = 104) in hospitalized patients. (D) Comparison of anti-N IgG value between discharged (n = 8) and hospitalized patients (n = 127). Means for continuous variables were compared using independent group t-test when the data were normally distributed; otherwise, the Mann-Whitney test was used.
3.4. The long-term infection of SARS-CoV-2 influence immune cell responses
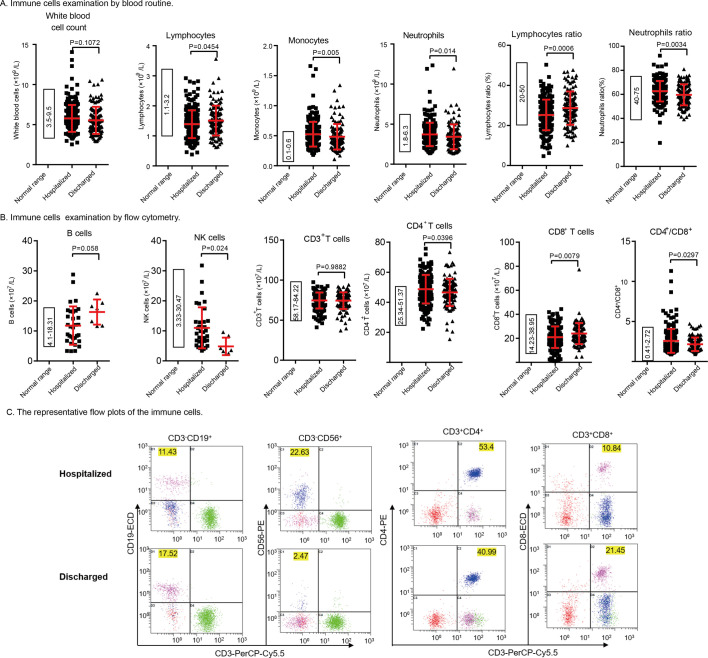
The production of the specific antibodies provided the clues about specific immune responses to SARS-CoV-2, and ample evidence had illustrated the decreased CD8+ T cells counts in critical patients, indicating the influence on cellular immunity. Collected data from blood routine and flow cytometry examinations of peripheral blood help us analyze the immune cell changes after longer-infection of SARS-CoV-2. Based on the results of these two examinations, we found the long-term infection of SARS-CoV-2 increased the account of monocytes, neutrophils, NK cells, and CD4+ T cells, but also declined the total lymphocytes and CD8+ T cells in the hospitalized patients (Fig. 4 , Table 2 ). Lymphocytopenia is one of the most prominent markers of COVID-19, it's also one of the diagnostic criteria for COVID-19 in China. And we found in longer infection inpatients lymphocytopenia deteriorated further. But the increases of the NK cells, neutrophils, and CD4+ T cells in the non-critical patients were much different from the characteristics of severe or critical patients. It might imply the differences between the non-critical or critical patients. The counts of CD4+ T cells was increased, as well as the ratio of CD4+ T cells against CD8+ T cells in the hospitalized non-critical patients, with the unchanged total CD3+ T cells (Fig. 4B, C). It should be noted that CD4+ T cells elevation was also present in discharged patients, but the increased proportion was not as obvious as in the patients with long-term but non-critical infection. The data also showed that the proportion of the increased and out of normal range CD4+ T cells were respectively 43.56% (n = 191) and 26.55% (n = 113) in the inpatients and discharged patients according to the clinical examining reports. Anyway, all these results revealed that the long-term SARS-CoV-2 infection caused changes in the immune responses in non-critical patients, different from the critical patients.
Fig. 4.
The differences in immune cells between hospitalized and discharged patients. The normal ranges were shown in the left panel.
(A) Immune cells examination by blood routine. Comparison of the counts of white blood cells, lymphocytes, monocytes and neutrophils between hospitalized and discharged patients. (B) Immune cells examination by flow cytometry. Comparison of the counts of B cells and NK cells between hospitalized (n = 34) and discharged patients (n = 7). The number of patients was less, for the detection of B cells and NK cells performed much less. Comparison of the counts of CD3+ T cells, CD4+ T cells, CD8+ T cells, and the ratio of CD4+ and CD8+ cells between the hospitalized (n = 190) and discharged patients (n = 113). Means for continuous variables were compared using independent group t-test when the data were normally distributed; otherwise, the Mann-Whitney test was used. (C) The representative flow plots of the immune cells. The cells were first gated by CD45-SSC. The number(x107/L) of different types of cells was displayed in the corresponding place.
Table 2.
Laboratory findings of patients with COVID-19.
| Laboratory Findings |
Median (IQR) |
P Valuea |
|||
|---|---|---|---|---|---|
| Blood routine (white blood cells) | Normal range | Total (n = 334) | Hospitalized (n = 212) | Discharged (n = 122) | |
| White blood cells (× 109/L) | 3.5–9.5 | 5.40(4.53, 6.69) | 5.44(4.68, 6.69) | 5.20(4.33, 6.62) | 0.1072 |
| Neutrophils (× 109/L) | 1.8–6.3 | 3.40(2.27, 4.30) | 3.45(2.81, 4.35) | 3.14(2.40, 4.04) | 0.0142 |
| Neutrophil percentage % | 40–75 | 61.67(56.18, 66.75) | 62.49(57.63, 67.32) | 59.10(53.68, 66.53) | 0.0033 |
| Lymphocytes (× 109/L) | 1.1–3.2 | 1.38(1.08, 1.71) | 1.37(1.04, 1.63) | 1.43(1.20, 1.77) | 0.0454 |
| Lymphocytes percentage | 20–50 | 26.2(20.9, 31.55) | 25.3(20, 30.15) | 28.43(23.38, 33.13) | 0.0006 |
| Monocytes (× 109/L) | 0.1–0.6 | 0.47(0.38, 0.59) | 0.49(0.41, 0.61) | 0.43(0.36, 0.56) | 0.0050 |
| Monocyte percentage % | 3–10 | 8.85(7.27, 10.68) | 9.05(7.42, 10.73) | 8.565(7.23, 10.58) | 0.3076 |
| Eosinophils (× 109/L) | 0.02–0.52 | 0.09(0.05, 0.17) | 0.10(0.06, 0.17) | 0.09(0.05, 0.13) | 0.0063 |
| Eosinophil percentage % | 0.4–8.0 | 1.64(0.95, 2.77) | 1.79(1.03, 2.98) | 1.5(0.91, 2.48) | 0.1032 |
| Basophils (× 109/L) | <0.06 | 0.02(0.01, 0.03) | 0.02(0.02, 0.03) | 0.02(0.01, 0.03) | 0.1250 |
| Basophil percentage % | 0–1 | 0.38(0.29, 0.51) | 0.39(0.31, 0.52) | 0.36(0.26, 0.48) | 0.9043 |
| Lymphocyte Subsets | Normal range | Total (n = 303) | Hospitalized (n = 190)1 | Discharged (n = 113)1 | |
| B cells (CD3−CD19+) (×107/L) | 4.1–18.31 | 12.01(7.89, 16.80) | 11.30(7.31, 16.41) | 17.52(12.46, 18.76) | 0.0398 |
| NK cells (CD3−CD16+CD56+) (×107/L) | 3.33–30.47 | 8.26(4.99, 12.82) | 9.33(6.46, 12.98) | 3.53(2.84, 6.27) | 0.0027 |
| CD 3+ T cells (CD3+CD19−) (×107/L) | 58–17-84.22 | 76.41(68.91, 81.04) | 76.32(68.90, 81.17) | 76.43(69.23, 80.89) | 0.9882 |
| CD8+ T cells (CD3+CD8+) (×107/L) | 14.32–38.95 | 21.64(16.97, 28.05) | 20.67(15.99, 27.21) | 23.57(18.32, 29.41) | 0.0079 |
| CD4+ T cells (CD3+CD4+) (×107/L) | 25.34–51.37 | 47.69(42.70, 53.88) | 48.99(42.66, 55.68) | 47.08(42.82, 51.58) | 0.0396 |
| CD4+ /CD8+ | 0.41–2.72 | 2.01(1.56, 2.93) | 2.25(1.59, 3.06) | 1.90(1.53, 2.66) | 0.0297 |
| Inflammatory cytokines | Normal range | Total (n = 312) | Hospitalized (n = 212) | Discharged (n = 100) | |
| Tumor necrosis factor-α (pg/mL) | 0.1–23 | 3.18(2.25, 5.14) | 3.60(2.50, 5.39) | 2.57(2.12, 4.31) | 0.0011 |
| Interleukin-2 (pg/mL) | 0.1–4.1 | 2.56(2.31, 2.88) | 2.60(2.34, 3.02) | 2.47(2.22, 2.70) | 0.0007 |
| Interleukin-4 (pg/mL) | 0.1–3.2 | 2.11(1.58, 2.63) | 2.26(1.66, 2.80) | 1.91(1.53, 2.32) | 0.0013 |
| Interleukin-6 (pg/mL) | 0.1–2.9 | 14.70(5.29, 54.88) | 18.29(6.23, 57.32) | 9.18(4.07, 50.03) | 0.0057 |
| Interleukin-10 (pg/mL) | 0.1–5 | 3.18(2.59, 4.10) | 3.37(2.73, 4.50) | 2.82(2.37, 3.36) | <0.0001 |
| Interferon-γ (pg/mL) | 0.1–18 | 2.03(1.60, 2.58) | 2.13(1.71, 2.79) | 1.84(1.44, 2.23) | 0.0006 |
P values indicate differences between the hospitalized and the discharged patients. P < .05 was considered statistically significant. Means for continuous variables were compared using independent group t-test when the data were normally distributed; otherwise, the Mann-Whitney test was used. 1. The tests of B and NK cells were only performed for 34 inpatients and 7 discharged patients.
3.5. The long-term infection of SARS-CoV-2 increased cytokines secretion in non-critical patients
The secretion of cytokines could also reflect the body's immunity to viruses and they play important roles in the regulation of immune responses. We were also very curious about the changes in the body's cytokines secretion after a longer-term infection with SARS-CoV-2. The tests of the cytokines containing IL-6, TNF-α, IFN-γ, IL-2, IL-4, and IL-10 from the hospitalized and discharged patients give evidence. The results showed that after the longer-infection of SARS-CoV-2, all of the cytokines was upregulated (Fig. 5 , Table 2). It suggested compared with the discharged patients, in hospitalized patients, the body's immune system could present a significantly different immune status for that the different cytokines have different sources and different functions.
Fig. 5.
The differences of cytokines in hospitalized patients (n = 212) and discharged patients(n = 100). Comparison of IL-6, TNF-α, IFN-γ, IL-2, IL-4, and IL-10 between hospitalized patients and discharged patients were showed and the normal ranges were shown in the left panel. Means for continuous variables were compared using independent group t-test when the data were normally distributed; otherwise, the Mann-Whitney test was used.
4. Discussion
Recently many papers reported the immunological changes in patients with COVID-19, and a very reliable summary of the immunological changes after viral infection [17]. Nevertheless, there was no concern about the effects of longer-term infection of SARS-CoV-2 in the non-fatal cases, what were the immune changes between the patients who had recovered and the inpatients who were still with supporting treatment. As widely acknowledged, the immune system plays an important role in clearing the virus and the adaptive immune protects humans from re-infection, thus, it was important to figure these changes out. In this retrospective study, to investigate the difference of immune responses we analyzed the changes of antibodies, immune cells, and cytokines in hospitalized with positive nucleic acid test and discharged with negative nucleic acid test patients.
For the cases of the hospitalized and discharged non-critical cases, we found that SARS-CoV-2 had a persistent infection in non-critical patients and it could change the body's immune responses, both in innate and adaptive immune responses. We found after infection of two weeks, the majority of patients could produce specific antibodies. With the persistent infection of SARS-CoV-2 in non-critical patents, the immune cells including neutrophils, monocytes, NK cells, and CD4 + T cells were increased, but lymphocytopenia aggravated and CD8+ T cells were decreased (Fig. 6 ).
Fig. 6.

Long-term infection of SARS-CoV-2 evoked immune response changes in non-critical pneumonia patients. After infection of two weeks, the majority of the patients could produce specific antibodies. With the extension of the infection of SARS-CoV-2, the immune cells including neutrophils, monocytes, NK cells, and CD4 + T cells was increased, but the total lymphocytes and CD8+ T cells were decreased. And the secretion of IL-6, TNF-α, IFN-γ, IL-2, IL-4, and IL-10 was upregulated. Means for continuous variables were compared using independent group t-test when the data were normally distributed; otherwise, the Mann-Whitney test was used.
But as to the different duration of hospitalization between discharged patients and those still in the hospital, of course, it was a long and complicated story. First, viral mutation and the possible alteration of virulence is a factor to be considered, but until now it is hard to speculate whether the mutations influence the course of COVID-19. Second, differences in ACE2 receptor expression and individual immune status determined the susceptibility. The different expressions of ACE2 caused by gender, age or smoking [18] had been reported. The individual immune status was effected by age, comorbidities and drug administration, such as diabetes and steroids. Furthermore, co-infection of bacteria, fungus, and other viruses, locally or systemically, made a big difference. In our analysis, we found that most patients with diarrhea remained in hospitalization (Table 1), and diarrhea suggested that the systemic infection potentially co-occurred. Apart from the direct damage caused by a virus infection, the immunopathological injury caused by necrotic tissue and outraged immune response also should be responsible for the prolonged recovery time.
At last, the clearance of the virus played a crucial role. For such pathogen invaders, the body will initiate the immune responses. In the absence of specific drugs, the immune responses also became the key aspect to clear the virus. The virus activates non-specific immunity that is innate immunity in the early stages of virus infection, following up with the specific adaptive immune response formation. After longer-term infection of SARS-CoV-2 in the hospitalized patients, the cells containing neutrophils, monocytes, NK cells were increased. It suggested the body might be performing a stronger virus removal mission.
Benefited by the close surveillance, the production of antibodies and the specific T-cell immune responses were recorded during the development of this disease. Antibody production serves as an important protection for the body, partly by the interfering virus to bind to receptors and mediating the killing of infected cells. The current antibody test results for N protein showed no significant difference between the discharged patients and hospitalized patients. And we could not observe the antibodies rise in our study, the probable reason was that the titer of antibody might have reached a platform stage, for the most illness time of these patients were over two weeks in the same batch detection.
However, we observed that the persistent infection of the virus in non-critical patients caused the exacerbated lymphopenia and decrease of CD8+ T cells, as well as in the critical patients [19]. And more and more evidence supported that the exhaustion might be responsible for the decrease of CD8+ T cells for the long-term consistent activation in non-critical long-term infection of SARS-CoV-2 due to the characteristics of cytotoxic T cells [[20], [21], [22]]. But the increase of neutrophils, monocytes, NK cells, and CD4+ T cells were much different from the critical patients, and we speculated these increased cells might play a role in fighting against virus and made a big difference in prognosis.
The new finding of the increase of CD4+ T cells in inpatients led us to pay more attention to the functions of CD4+ T cells for its central roles in immune regulation. As we had known that the cytokines mediate the differentiation of CD4+ T cells. Based on our present knowledge, IFN-γ was associated with TH1 cells medicated immunity, and CD4+ T cell differentiated into TH1 cells, IL-4 and IL-2 were associated with CD4+ T cells differentiated into TH2 and Treg cells respectively, while IL-6 implied the possibility of CD4+ T cells differentiation into TFH or TH17 involved with other cytokines [[23], [24], [25]]. Detection of the cytokines, IL-6, TNF-α, IFN-γ, IL-2, IL-4, and IL-10 in these patients, gave us the chance to explore what had happened to the increased CD4+ T cells. However, the results showed that all six cytokines were elevated in hospitalized patients with long-term infection of SARS-CoV-2 (Fig. 5). In other words, in patients with longer-term SARS-CoV-2 infection, the increased CD4+ T cells had the potential to differentiate into TH1, TH2, Treg, or TH17/TFH cells, and the differentiated into TH1, TH2, or TH17/TFH had been reported [[26], [27], [28]]. Just for the moment, it was impossible to tell which immune response of CD4+ T cells would be dominant. It was difficult to judge whether these changes were more conducive to virus clearance given the complexity of the immune system, but a very informative and valuable article reported that CD4+ T cells were important in the control of SARS-CoV infection functioning cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice [29]. The increased CD4 could have a role in the fight with SARS-CoV-2, and it would be the focus of our next work.
5. Conclusions
In the end, we must emphasize the persistent infection of SARS-CoV2 in non-critical patients and it must attract enough attention. In conclusion, in this study we portrayed the long-term infection of SARS-CoV-2 changed the body's immune status. The analysis of viral loads explained the long-term or persistent infection of SARS-CoV-2 in some non-critical and hospitalized patients. After infection of two weeks, serum analysis showed that the specific anti-N IgG was positive in all detected patients. And compared with the discharged patients, hospitalized patients had the increases of Neutrophils, Monocytes, NK cells, and CD4+ T cells but with the decrease of CD8+ T cells after longer-infection of SARS-CoV-2; had an increase of cytokines of IL-6, TNF-α, IFN-γ, IL-2, IL-4, and IL-10. These differences might suggest that the longer-term infection of SARS-CoV-2 could bring stronger immune responses.
Acknowledgments
Acknowledgements
This study was funded by the grants from the project of Thousand Youth Talents for DH; and from the China National Natural Science Foundation (Nos. 31770983 and 81974249 to DH, No. 81601747 to SL, No. 81201026 to HW, No. 81974530 to LZ). We thank all sites who participated in this survey and the patients whose data were used in this study. We also would like to thank the department of Clinical laboratory of Wuhan Union Hospital, who also contributed to the data collection of the study.
Declaration of Competing Interest
The authors have declared that no conflict of interest exists.
Contributor Information
Yu Hu, Email: dr_huyu@126.com.
Lin Wang, Email: lin_wang@hust.edu.cn.
Desheng Hu, Email: desheng.hu@hust.edu.cn.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C., Horby P.W., Hayden F.G., Gao G. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5(4):562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 7.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang Y., Zhang H., Xie J., Lin M., Ying L., Pang P. Sensitivity of Chest CT for COVID-19: Comparison to RT-PCR. Radiology. 2020 doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N. Engl. J. Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q.. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020;20(4):411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lan L., Xu D., Ye G., Xia C., Wang S., Li Y. Positive RT-PCR Test Results in Patients Recovered From COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Hung I.F., Poon L.L. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin. Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brake SJB, K.; Lu, W.; McAlinden, K.D.; Eapen, M.S.; Sohal, S.S. Smoking Upregulates Angiotensin-Converting Enzyme-2 Receptor: A Potential Adhesion Site for Novel Coronavirus SARS-CoV-2 (Covid-19). J Clin Med. 2020; 9:841. [DOI] [PMC free article] [PubMed]
- 19.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y. Dysregulation of immune response in patients with COVID-19 in Wuhan. China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pauken K.E., Wherry E.J. SnapShot: T Cell Exhaustion. Cell. 2015;163(4):1038–e1. doi: 10.1016/j.cell.2015.10.054. [DOI] [PubMed] [Google Scholar]
- 21.Wherry E.J., Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015;15(8):486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J., Li S., Liu J., Liang B., Wang X., Wang H. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy K., Weaver C. 2016. Janeway's immunobiology. New York: Garland science. [Google Scholar]
- 24.Zhou L., Chong M.M., Littman D.R. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30(5):646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Luckheeram R.V., Zhou R., Verma A.D., Xia B. CD4⁺T cells: differentiation and functions. Clin. Dev. Immunol. 2012;2012:925135. doi: 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thevarajan I., Nguyen T.H.O., Koutsakos M., Druce J., Caly L., van de Sandt C.E. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat. Med. 2020;26(4):453–455. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng H.Y., Zhang M., Yang C.X., Zhang N., Wang X.C., Yang X.P. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell. Mol. Immunol. 2020;17(5):541–543. doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J., Lau Y.F., Lamirande E.W., Paddock C.D., Bartlett J.H., Zaki S.R. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J. Virol. 2010;84(3):1289–1301. doi: 10.1128/JVI.01281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]