Abstract
Cuticular drusen show some similarities to and differences from soft drusen in age-related macular degeneration (AMD) and might thus be a unique AMD subtype. Previous studies on cuticular drusen were performed mainly in white ethnic groups, but AMD shows ethnic differences. We investigated clinical manifestations of cuticular drusen in Korean patients to evaluate possible ethnic differences. Clinical records of Korean patients with cuticular drusen were retrospectively reviewed. Fundus distribution pattern, imaging features, and presence of large drusen, drusenoid pigment epithelial detachment (PED), and macular complications, including geographic atrophy (GA), choroidal neovascularization (CNV), and acquired vitelliform lesion (AVL), were assessed via multimodal imaging in 162 eyes with cuticular drusen (n = 81 patients; 67 females; mean age: 66.6 ± 9.1 years). Diffuse distribution was found in 61.7% and peripapillary involvement in 75.3% of eyes. Large drusen, drusenoid PED, GA, CNV, and AVL were observed in 59.3%, 26.5%, 18.5%, 3.7%, and 1.2% of eyes, respectively. The macular complication prevalence was similar between patients ≤ 60 and those > 60 years old. In Korean patients, cuticular drusen were less frequently associated with macular complications than in white patients, and the proportion of macular complications differed significantly, with AVL representing an uncommon complication.
Subject terms: Visual system, Retinal diseases, Macular degeneration, Vision disorders
Introduction
Cuticular drusen are multiple, small, round drusen that appear hyperfluorescent in fluorescein angiography (FA), giving a “stars-in-the-sky” appearance1,2. A histopathological study revealed that cuticular drusen are located between the basal lamina of the retinal pigment epithelium (RPE) and the inner collagenous layer of Bruch’s membrane, similar to the soft drusen in age-related macular degeneration (AMD)3,4. Imaging studies using spectral domain optical coherence tomography (SD-OCT) have also shown the similarity in location of cuticular drusen and soft drusen4,5.
Although cuticular drusen share many features in common with the soft drusen in AMD, there are also several differences between these two types of drusen. Cuticular drusen are more widely scattered on the fundus, with symmetrical distribution patterns in both eyes. In cuticular drusen, the age of onset is younger and genetic associations are stronger than in AMD6–8. In addition, smoking, one of the most important environmental factors in AMD, is weakly associated with cuticular drusen9. Cuticular drusen can cause vision loss as a result of vitelliform macular detachment, and it could be related to membranoproliferative glomerulonephritis type II and other renal diseases2,4,10,11. Thus, cuticular drusen might be a unique subtype of AMD, with particular characteristics.
Previous studies on cuticular drusen were performed mainly in white ethnic groups, and clinical features in other ethnic groups remain largely unknown4,6,10,12–14. Yet, there are known ethnic differences in AMD. The prevalence of AMD varies in different ethnic groups, and the prevalence of subtypes of neovascular AMD also varies; for instance, polypoidal choroidal vasculopathy (PCV) is more prevalent in Asians than in white ethnic groups15–18. Considering ethnic differences in AMD and proposed genetic associations in cuticular drusen, there might also be ethnic differences in the manifestation of cuticular drusen18.
Thus, this study aimed to broaden our understanding of cuticular drusen by examining multimodal imaging features, clinical phenotypes, and prevalence of macular complications in Korean patients. Additionally, we sought clues to the possible existence of ethnic differences in the manifestation of cuticular drusen.
Results
Of the 210 eyes of 105 patients previously diagnosed with cuticular drusen, 162 eyes of 81 Korean patients were included in this study. Forty-eight eyes were excluded for the following reasons: in 22 eyes, drusen were distributed only in the peripheral retina; in 4 eyes, multimodal imaging data were insufficient; in 2 eyes, there was a history of previous vitreoretinal surgery; 2 eyes had severe cataract, and 18 eyes had accompanying diabetic retinopathy.
Demographic and clinical features are summarized in Table 1. All patients were Koreans, and 67 patients were female (82.7%). Eleven (13.4%) patients had various types of kidney disease, including renal cyst, polycystic kidney disease, renal stone, and acute kidney injury associated with the ingestion of herbs. However, no patients had membranoproliferative glomerulonephritis type II, which was previously reported to be associated with cuticular drusen11.
Table 1.
Clinical and demographic features.
| Feature | Data |
|---|---|
| No. of eyes | 162 |
| No. of patients | 81 |
| Women | 67 (82.7) |
| Age (years) | 66.6 ± 9.1 |
| BCVA (logMAR) | 0.15 ± 0.28 |
| Refraction (spherical equivalent) | 0.20 ± 1.43 |
| SFCT (µm) | 220.1 ± 85.3 |
| Ocular comorbidities | |
| Pseudophakia | 34 (20.1) |
| Epiretinal membrane | 7 (4.3) |
| Glaucoma/glaucoma suspect | 8 (4.9) |
| Medical history | |
| Diabetes | 16 (19.8) |
| Hypertension | 40 (49.4) |
| Thyroid disease | 11 (13.6) |
| Kidney disease | 11 (13.6) |
Data are total no. (%) or mean ± standard deviation, unless otherwise indicated.
BCVA best-corrected visual acuity, logMAR logarithm of the minimum angle of resolution, SFCT subfoveal choroidal thickness.
Multimodal imaging
All study subjects underwent color fundus photography (CFP), spectral-domain optical coherence tomography (SD-OCT), and FA. All individual imaging results met the diagnostic criteria. Red-free (RF), near-infrared reflectance (NIR), and fundus autofluorescence (FAF) imaging were performed in 50.6%, 97.5%, and 96.3% of patients, respectively. Ultrawide-field photographic images were acquired in 48.1% of patients. Imaging characteristics are summarized in Table 2. A macular-type distribution pattern was seen in 62 eyes (38.3%) and a diffuse-type in 100 eyes (61.7%).The average age of patients with each type of distribution pattern was 65.8 ± 8.4 and 67.1 ± 9.6 years, respectively, which was not statistically significantly different (P = 0.522, Student’s t test). Peripapillary involvement and occupation of the total retinal area by cuticular drusen in more than five disc-areas were observed in 122 eyes (75.3%) and in 134 eyes (82.7%), respectively.
Table 2.
Imaging characteristics of cuticular drusen.
| Characteristics | Data |
|---|---|
| Distribution pattern of drusen | |
| Macula | 62 (38.3) |
| Diffuse | 100 (61.7) |
| Involved fundus area | |
| Peripapillary involvement | 122 (75.3) |
| Occupied retinal area ≥ 5DA | 134 (82.7) |
| Accompanying macular lesions | |
| Large drusen | 96 (59.3) |
| Drusenoid PED | 43 (26.5) |
| Geographic atrophy | 30 (18.5) |
| CNV | 6 (3.7) |
| Vitelliform lesion | 2 (1.2) |
Data are total no. (%), unless otherwise indicated.
CNV choroidal neovascularization, DA disc-areas, PED pigment epithelial detachment.
Multimodal imaging features of cuticular drusen in Korean patients were similar to those reported in patients of white ethnicities. Cuticular drusen were seen as hyperchromic dots on RF images in all eyes. NIR imaging of cuticular drusen showed hyporeflective centers with a surrounding hyperreflective margin (63.3%), diffuse hyperreflectivity (19.0%), heterogeneous reflectivity (3.8%), or a combination of these patterns (13.9%). The characteristic FAF finding of cuticular drusen, i.e., central hypoautofluorescence and a rim of hyperautofluorescence, was observed in 65.4% of eyes. The remaining eyes showed nonspecific findings of FAF. The morphological findings of cuticular drusen on SD-OCT were categorized into three patterns: type 1 pattern (37.0%), type 2 pattern (43.2%), and type 3 pattern (19.8%). Indocyanine green angiography (ICGA) showed hyperfluorescence in 60% of eyes (18/30). Figure 1 shows the multimodal imaging characteristics of cuticular drusen.
Figure 1.
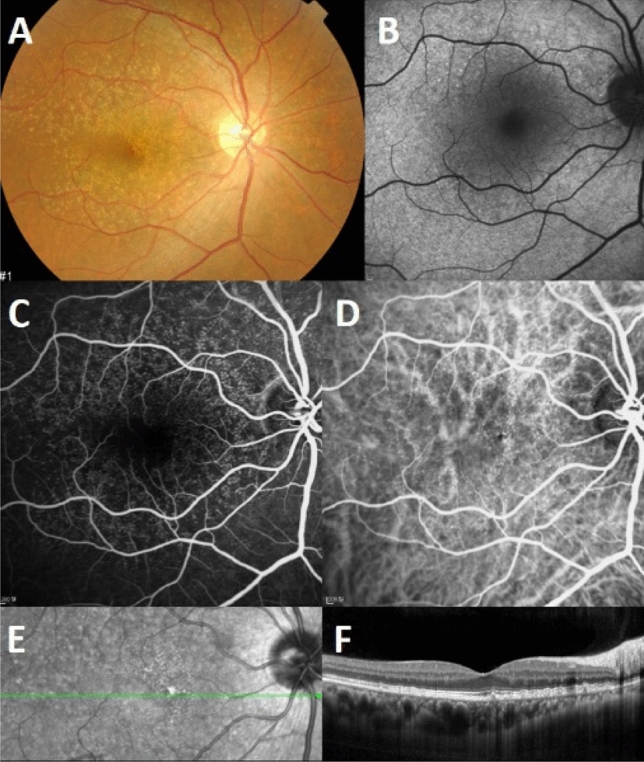
Multimodal imaging of cuticular drusen. CFP (A) shows multiple scattered pale or yellow spots. A hypoautofluorescent center with a mild hyperautofluorescent margin was observed on FAF image (B). “Stars-in-the-sky” hyperfluorescent lesions are observed in the early phase of FA (C) and ICGA (D). A combination of a hyporeflective center and hyperreflectivity is seen on the NIR image (E). A “sawtooth pattern” of small and confluent sub-RPE deposits is observed in OCT image (F). CFP color fungus photography, FA fluorescein angiography, FAF fundus autofluorescence, ICGA indocyanine green angiography, NIR near-infrared reflectance, OCT optical coherence tomography, RPE retinal pigment epithelium.
In some eyes, large drusen (59.3%, Fig. 2A) were scattered among cuticular drusen, and drusenoid pigment epithelial detachment (PED, 26.5%, Fig. 2C) accompanied the cuticular drusen. Some eyes were complicated by geographic atrophy (GA, 18.5%, Fig. 2A), choroidal neovascularization (CNV, 3.7%, Fig. 2B), and acquired vitelliform lesion (AVL, 1.2%, Fig. 2C). Six eyes with CNV were classified as follows: 1 eye (0.6%) with type 1, 2 eyes (1.2%) with type 2, 2 eyes (1.2%) with a mixture of type 1 and type 2, and 1 eye (0.6%) with type 3. There was no PCV. The previously reported prevalence rates of GA, CNV, and AVL in cuticular drusen in the white ethnic groups and Japanese patients are summarized in Table 3. Statistical analysis of the relationship between age and the prevalence macular complications revealed no statistically significant differences between patients ≤ 60 years and those > 60 years old (Table 4).
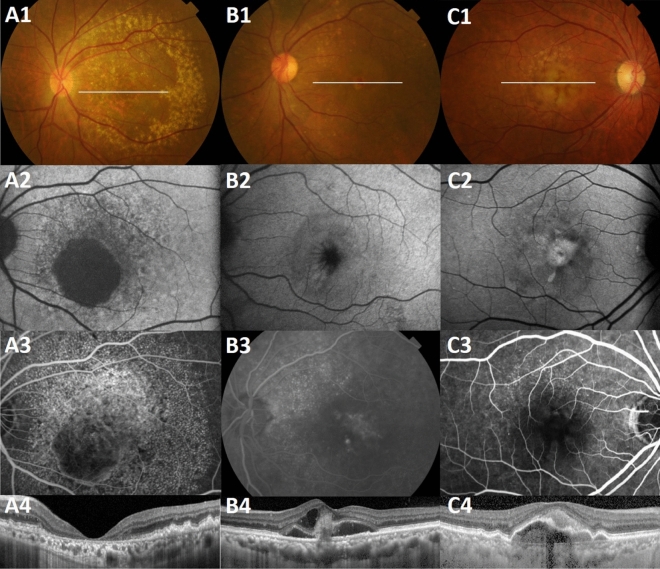
Figure 2.
Macular complications of cuticular drusen. (A) Geographic atrophy is seen on (A1) CFP, (A2) FAF, (A3) FA, and (A4) OCT in a 65-year-old man with cuticular drusen. Geographic atrophy appears in both eyes symmetrically. Large drusen are also visible on CFP and OCT. (B) Type 3 choroidal neovascularization is seen on (B1) CFP, (B2) FAF, (B3) FA, and (B4) OCT in a 67-year-old woman with cuticular drusen. (C) Drusenoid pigment epithelial detachment and acquired vitelliform lesion appear as (C1) round yellow lesions on CFP, (C2) intense hyperautofluorescence on FAF, (C3) hypofluorescence in the early phase of FA, and a (C4) subretinal location of vitelliform material with pigment epithelial detachment is seen on OCT, in a 74-year-old man. The position of the OCT line scans is shown in the CFP as a white transverse line. CFP color fundus photograph, FA fluorescein angiography, FAF fundus autofluorescence, OCT optical coherence tomography.
Table 3.
Comparison of recent cuticular drusen studies.
| Author(s), year | No. of patients | Sex (female:male) | Mean Age (years) |
Region | Imaging tools for diagnosis of CD | GA (%) | Neovascular AMD (%) | AVL (%) |
|---|---|---|---|---|---|---|---|---|
| Cohen et al., 199414 | 19 | 13:6 | 61.4 | France | CFP, FAa | 0 | 31.6c | 100d,e |
| Barbazetto et al., 20076 | 28 | 17:11 | 65.9 | United States | CFP, AF, FAb | 3.6d | 3.6d | 100d,e |
| Boon et al., 200810 | 30 | 20:10 | 53.3 | Netherlands | CFP, NIR, OCT, AF, FAa | 33.3d | 16.7d | 26.7d |
| Boon et al., 201312 | 198 | – | – | Netherlands | CFP, RF, NIR, OCT, AF, FAa, ICGA | – | 56d | – |
| Balaratnasingam et al., 20184 | 120 | 72:48 | 57.9 | United States, Australia, France | CFP, RF, NIR, OCT, AF, FAb, ICGA | 25.0c | 12.5c | 24.2c |
| Sakurada et al., 201913 | 12 | 8:4 | 60.8 | Japan | CFP, NIR, OCT, AF, FAa, ICGA | 8.3c | 4.2c | 0 |
| Sakurada et al., 201919,f | 38 | 35:3 | 59.9 | United States | CFP, FAF, OCT, FAa | 14.5f | 2.6f | – |
| Current study, 2018 | 81 | 67:14 | 66.6 | Korea | CFP, RF, NIR, OCT, AF, FAa, ICGA | 18.5c | 3.7c | 1.2c |
– unknown, AF fundus autofluorescence, AMD age-related macular degeneration, AVL acquired vitelliform lesion, CD cuticular drusen, CFP color fundus photography, FA fluorescein angiography, GA geographic atrophy, ICGA indocyanine green angiography, NIR near-infrared, OCT optical coherence tomography, RF red-free imaging.
aFA was performed in all patients.
bFA was performed in selected patients.
c% of eyes.
d% of patients.
ePatients who had both vitelliform or pseudovitelliform detachment and cuticular drusen were included.
fBaseline cross-sectional data before exclusion.
Table 4.
Age-related variations in macular complications of cuticular drusen.
| Entire cohort (n = 162) | Age ≤ 60 years (n = 36) | Age > 60 years (n = 126) | P-valuea | |
|---|---|---|---|---|
| GA | 30 (18.5) | 5 (13.9) | 25 (19.8) | 0.417b |
| CNV | 6 (3.7) | 1 (2.8) | 5 (4.0) | 1.000c |
| AVL | 2 (1.2) | 0 (0) | 2 (1.6) | 1.000c |
Data are number (%) or mean ± standard deviation, unless otherwise indicated.
AVL acquired vitelliform lesion, CNV choroidal neovascularization, GA geographic atrophy, PED pigment epithelial detachment.
aComparison between eyes in patients ≤ 60 years and those > 60 years of age.
bChi-square test.
cFisher’s exact test.
Peripheral reticular pigmentary degeneration was observed in 68 eyes of 34 patients (42.0%), and all patients had a symmetric degeneration pattern in both eyes. Peripheral reticular pigmentary degeneration was present in 6 of 31 patients (19.4%) with the macular-type and 28 of 50 patients (56.0%) with the diffuse-type distribution pattern. The diffuse-type distribution of cuticular drusen was more strongly associated with the presence of peripheral reticular pigmentary degeneration than the macular-type (P = 0.001; Chi-square test).
Discussion
There have been only a limited number of reports on cuticular drusen in Asian ethnic groups, and one recent study reported on about 12 Japanese patients13. In the present study, we report on a sizeable number of Korean patients (81) with cuticular drusen.
We report several similarities in demographic characteristics and clinical findings associated with cuticular drusen between Koreans and white ethnic groups. There was a trend toward female sex being a risk factor for cuticular drusen in white ethnic groups, with a female preponderance of proportion of 60.0–92.1%4,6,9,10,12–14,19. A similar female preponderance was observed in our Korean patients (82.7%) as well as in the recent Japanese study (66.6%)13. There were also similarities in the fundus distribution pattern between the white ethnic groups and Korean patients. For the diffuse-type distribution pattern, previous studies on white ethnic groups reported a prevalence of 67.1–81%, while the prevalence in Korean patients in this study was 61.7%4,12.
Most multimodal imaging features of cuticular drusen in Korean patients revealed no significant differences from those reported in patients of white ethnicities. For example, types 1, 2, and 3 OCT patterns were seen in 37.0%, 43.2%, and 19.8% of Korean patients, and in 33%, 49%, and 18% of patients of white ethnicities4. The FAF and ICGA findings were also similar between these ethnic groups.
Peripheral cuticular drusen were reported to be associated with senile reticular pigmentary degeneration in white ethnic groups10,12. In Korean patients, peripheral reticular pigmentary degeneration was present in 42% of patients, and the diffuse distribution-type was more strongly associated with peripheral reticular pigmentary degeneration than the macular-type (56% vs 19.4%). However, we could not compare the finding in Korean patients to those in white ethnic groups, because previous studies in white ethnic groups used different diagnostic criteria and classification methods.
However, the current data also revealed several noticeable differences in the clinical manifestation of cuticular drusen between Koreans and white ethnic groups (Table 3). According to the present study, Korean patients have GA far more frequently than CNV (18.5% vs 3.7%). One recent, small study reported similar findings in a white ethnic group; however, most previous studies on patients of white ethnicity reported smaller differences between the prevalence of GA and CNV. These studies reported that CNV could develop in 3.6–56% of patients in white ethnic groups4,6,10,12,14. In Korean patients, CNV was observed in only 6 eyes (3.7%) of 5 patients (6.2%); thus, the frequency of CNV in Koreans appeared to be relatively lower than the frequencies reported in white ethnic groups.
A similar ethnic difference was reported for exudative AMD. A previous study reported that Asian Americans had a lower risk of exudative AMD than in white Americans15. Interestingly, none of the eyes with CNV had PCV lesions, which is the preponderant neovascularization subtype in Asians20. A single hospital-based study reported that the prevalence of PCV in Korean exudative AMD patients was 24.6%21.
Around one-quarter (24.2–26.7%) of eyes with cuticular drusen in white ethnic groups reportedly develop vitelliform lesions4,10. However, only 1.2% of eyes among our Korean patients had vitelliform lesions. A recent Japanese study found no AVL in 12 patients, supporting the finding of a low frequency of AVL in Korean patients13. Thus, it appears that Korean patients might have AVL less frequently than white ethnic groups; however, prospective, longitudinal studies are needed to verify these findings.
In our analysis of the relationship between aging and the prevalence of macular complications, we found that, unlike in patients of white ethnicities, there were no statistically significant differences in macular complications between patients ≤ 60 years and those > 60 years old4. We found a similar result when we changed the age cut-off to 65 or 70 years (data not shown).
Recently, Sakurada et al. reported that drusenoid PED was the most frequent AMD-associated macular pathology in eyes of Japanese patients (33.3%)13. In Korean patients, drusenoid PED was seen in 26.5% of eyes. The frequency of drusenoid PED in white ethnic groups has not been reported. Further studies are therefore needed to compare drusenoid PED between Asians and white ethnic groups.
Previous studies of cuticular drusen have used various diagnostic criteria (Table 3). Cohen et al. used the simple criteria based on the characteristic FA finding, while Van de Ven et al. used more complex criteria based on size, location, symmetry, and number of drusen, in addition to the characteristic FA finding9,14. In a recent large observational cohort study by Balaratnasingam et al., cuticular drusen were diagnosed by a combination of multiple imaging modalities, and FA was not mandatory4. In the present study, strict diagnostic criteria were used with multimodal imaging. CFP, OCT, and FA were used in the diagnosis of all patients, and NIR and FAF images were also assessed in more than 96% of patients.
A previous study suggested that cuticular drusen might initially develop in the peripheral fundus during the early phase of the disease8,10,12. Of 210 eyes in 105 Korean patients previously diagnosed with cuticular drusen, 22 eyes had drusen distributed only in the peripheral retina. However, they were excluded from analysis in this study, because we consider that there is currently insufficient evidence that these lesions are the same as the cuticular drusen observed at the posterior fundus. Further studies including longitudinal studies are needed to understand the nature of peripheral drusen showing hyperfluorescence in FA.
The current study had several limitations. The study was retrospective and cross-sectional. The results from Korean patients were indirectly compared to those from white ethnic groups reported in previous studies, which used slightly different diagnostic criteria and assessment methods. Eyes having only peripheral lesions were excluded from analysis in this study; further studies are needed to verify the nature of these peripheral lesions. The number of patients ≤ 60 years old was markedly smaller than the number of patients > 60 years old, which may have affected statistical analyses.
In conclusion, Korean patients with cuticular drusen showed features similar to those previously reported in white ethnic groups with regard to female preponderance, distribution pattern, and most multimodal imaging characteristics. However, the cuticular drusen were less frequently associated with macular complications in Korean patients than in white ethnic groups, and AVL was found rarely. Thus, we thought that this study provides the preliminary evidences for future prospective studies to investigate ethnic differences in cuticular drusen.
Methods
Participants
Clinical records of patients diagnosed with cuticular drusen at the retina clinic of Samsung Medical Center, Seoul, Korea, between January 2010 and July 2018 were retrospectively reviewed. Patients who had other retinal disorders that interfered with the assessment of angiography images, including diabetic retinopathy, hypertensive retinopathy, and retinal vein occlusion, were excluded. Patients who had ocular conditions that might affect the evaluation of cuticular drusen, such as severe cataract, previous trauma history, and previous vitreoretinal surgery history, were also excluded.
Demographic information, including age, sex, ocular and systemic comorbidities, and medical history were obtained for each patient. All patients underwent ophthalmic examinations including measurement of best-corrected visual acuity (BCVA), refractive error by manifest refraction, slit-lamp biomicroscopy, and dilated fundus examination. Patients underwent multimodal imaging, including CFP (TRC 50 IX, Topcon, Tokyo, Japan), RF, NIR, FAF imaging, and SD-OCT (Spectralis HRA + OCT, Heidelberg Engineering, Heidelberg, Germany). FA, ICGA (Spectralis HRA + OCT or Optos 200Tx, Optos PLC, Dunfermline, United Kingdom), and ultrawide-field photography (Optos 200Tx) were also performed.
Definition of cuticular drusen and AMD
The current study used strict diagnostic criteria for cuticular drusen, mainly based on the definition originally proposed by Gass and the criteria proposed by Van de Ven et al. and by Balaratnasingam et al.1,4,9. In brief, cuticular drusen were defined as multiple, yellow or pale, small, round lesions observed in fundus examination and CFP, showing a symmetric distributed pattern between bilateral eyes. There should be at least 50 scattered, uniformly-sized, small (25–75 μm) hyperfluorescent drusen with a typical “stars-in-the-sky” appearance on FA images in each eye4,9. The lesions had to be located beneath the RPE, with RPE elevation on OCT images4,24. NIR and FAF imaging features were used to assist the diagnosis, although neither of these imaging tests were considered as mandatory for diagnosis4,23.
Fundus topographic distribution patterns of cuticular drusen were classified as either the macular- or the diffuse-type, based on the results of CFP and FA4. Briefly, a macular distribution-type was defined as drusen distributed only within the major vascular arcades, whereas the diffuse-type was defined as drusen involving the macula, but also extending beyond the vascular arcades. Involvement of the peripapillary region and the total retinal area occupied by cuticular drusen, exceeding 5 disc-areas, were also analyzed4.
Multimodal imaging data were assessed for RF findings, FAF findings, NIR patterns, morphologic features on OCT b-scan, and ICGA findings. NIR patterns were categorized as hyporeflective centers with a surrounding hyperreflective margin, diffuse hyperreflectivity, heterogeneous reflectivity, or a combination of these patterns4. Morphological features on OCT b-scans were divided into a type 1 pattern, showing shallow elevation of the RPE-basal laminar band with difficult to discern internal drusen contents; a type 2 pattern, showing a sawtooth appearance and hyporeflective internal contents; and a type 3 pattern, showing broad, mound-shaped elevations of the RPE-basal laminar band with hyporeflective internal contents4.
The presence of large drusen, drusenoid PED, and macular complications, including GA, CNV, and AVL, was also assessed4,19. Large drusen was defined as at least 3 times the greatest height or diameter of typical cuticular drusen4. Drusenoid PED was defined as one-half disc diameter of confluent soft drusen under the center of the macula25. GA was defined as unifocal or multifocal sharply demarcated hypopigmented areas with large choroidal vessels visible due to RPE atrophy, observed on CFP images and hypoautofluorescence with a diameter of at least 175 μm observed on FAF images26–28. CNV was considered to be present if subretinal hemorrhage or fluid with subretinal tissue was observed on CFP and OCT images. A disciform scar was also included as evidence of old CNV. By using OCT, FA, and ICGA, CNV was classified into types 1, 2, and 3 using the Gass–Freund classification1,29. AVL was considered to be present if a yellowish subretinal material was observed in CFP images and hyperautofluorescent collections within the macular area, not secondary to vitelliform macular dystrophy, were observed on FAF images. These corresponded to hyperreflective lesions between the RPE-Bruch’s membrane band and the external limiting membrane seen on OCT images30,31.
Peripheral cuticular drusen are reported to be associated with senile reticular pigmentary degeneration10,12. Peripheral reticular pigmentary degeneration was defined as a reticular pattern of degenerated lesions that appeared hypofluorescent in the peripheral fundus areas on FA images32,33. The presence of peripheral pigmentary degeneration was evaluated and its prevalence according to the distribution pattern was compared.
Imaging features of cuticular drusen, including involvement of the peripapillary region and the total retinal area occupied by cuticular drusen exceeding 5 disc-areas, were also analyzed4. To investigate the relationship between aging and the prevalence of macular complications, patients were divided into age groups ≤ 60 years and > 60 years. The prevalence of macular complications was statistically compared between these two groups.
Morphological and topographic characteristics of cuticular drusen were assessed by two investigators (D.H.S. and M.K.), and in case of disagreement, a senior interpreter (D.I.H.) made the final decision. Statistical analyses were performed with SPSS software version 25.0 (SPSS, Inc, Chicago, IL, USA). A P-value less than 0.05 was considered statistically significant.
Ethics approval and patient consent
This study was performed in accordance with the principles of the Declaration of Helsinki. It was approved by the institutional review board at Samsung Medical Center. Given the retrospective nature of the study and the use of anonymized data, requirements for informed consent were waived by the institutional review board.
Author contributions
D.H.S. and D.I.H. designed the research and wrote the main manuscript. D.H.S., M.K., G.H., and J.C.H. performed the data collection and image analysis and prepared figures. D.I.H. reviewed the design, the results, and the final paper. D.H.S. contributed to the manuscript as first author and D.I.H. contributed to the manuscript as corresponding author. All authors read and approved the final manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gass JD. Stereoscopic Atlas of Macular Diseases. 4. Mosby: St. Louis; 1997. pp. 26–30. [Google Scholar]
- 2.Gass JD, Jallow S, Davis B. Adult vitelliform macular detachment occurring in patients with basal laminar drusen. Am. J. Ophthalmol. 1985;99:445–459. doi: 10.1016/0002-9394(85)90012-1. [DOI] [PubMed] [Google Scholar]
- 3.Russell SR, Mullins RF, Schneider BL, Hageman GS. Location, substructure, and composition of basal laminar drusen compared with drusen associated with aging and age-related macular degeneration. Am. J. Ophthalmol. 2000;129:205–214. doi: 10.1016/S0002-9394(99)00345-1. [DOI] [PubMed] [Google Scholar]
- 4.Balaratnasingam C, et al. Cuticular drusen: clinical phenotypes and natural history defined using multimodal imaging. Ophthalmology. 2018;125:100–118. doi: 10.1016/j.ophtha.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 5.van de Ven JP, et al. Short-term changes of basal laminar drusen on spectral-domain optical coherence tomography. Am. J. Ophthalmol. 2012;154:560–567. doi: 10.1016/j.ajo.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Barbazetto IA, et al. Pseudo-vitelliform macular detachment and cuticular drusen: exclusion of 6 candidate genes. Ophthalmic Genet. 2007;28:192–197. doi: 10.1080/13816810701538596. [DOI] [PubMed] [Google Scholar]
- 7.Grassi MA, et al. Complement factor H polymorphism p.Tyr402His and cuticular Drusen. Arch. Ophthalmol. 2007;125:93–97. doi: 10.1001/archopht.125.1.93. [DOI] [PubMed] [Google Scholar]
- 8.van de Ven JP, et al. Clinical evaluation of 3 families with basal laminar drusen caused by novel mutations in the complement factor H gene. Arch. Ophthalmol. 2012;130:1038–1047. doi: 10.1001/archophthalmol.2012.265. [DOI] [PubMed] [Google Scholar]
- 9.van de Ven JP, et al. Association analysis of genetic and environmental risk factors in the cuticular drusen subtype of age-related macular degeneration. Mol. Vis. 2012;18:2271–2278. [PMC free article] [PubMed] [Google Scholar]
- 10.Boon CJ, et al. Basal laminar drusen caused by compound heterozygous variants in the CFH gene. Am. J. Hum. Genet. 2008;82:516–523. doi: 10.1016/j.ajhg.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan KN, et al. Differentiating drusen: drusen and drusen-like appearances associated with ageing, age-related macular degeneration, inherited eye disease and other pathological processes. Prog. Retin. Eye Res. 2016;53:70–106. doi: 10.1016/j.preteyeres.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Boon CJ, van de Ven JP, Hoyng CB, den Hollander AI, Klevering BJ. Cuticular drusen: stars in the sky. Prog. Retin. Eye Res. 2013;37:90–113. doi: 10.1016/j.preteyeres.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Sakurada Y, et al. Clinical characteristics of cuticular drusen in the Japanese population. Jpn. J. Ophthalmol. 2019;63:448–456. doi: 10.1007/s10384-019-00692-5. [DOI] [PubMed] [Google Scholar]
- 14.Cohen SY, Meunier I, Soubrane G, Glacet-Bernard A, Coscas GJ. Visual function and course of basal laminar drusen combined with vitelliform macular detachment. Br. J. Ophthalmol. 1994;78:437–440. doi: 10.1136/bjo.78.6.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanderbeek BL, et al. Racial differences in age-related macular degeneration rates in the United States: a longitudinal analysis of a managed care network. Am. J. Ophthalmol. 2011;152:273–282.e273. doi: 10.1016/j.ajo.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee MY, Yoon J, Ham DI. Clinical characteristics of reticular pseudodrusen in Korean patients. Am. J. Ophthalmol. 2012;153:530–535. doi: 10.1016/j.ajo.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Wong WL, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob. Health. 2014;2:e106–116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 18.Fisher DE, et al. Incidence of age-related macular degeneration in a multi-ethnic United States population: the multi-ethnic study of atherosclerosis. Ophthalmology. 2016;123:1297–1308. doi: 10.1016/j.ophtha.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakurada Y, et al. Cuticular drusen: risk of geographic atrophy and macular neovascularization. Retina. 2020;40:257–265. doi: 10.1097/IAE.0000000000002399. [DOI] [PubMed] [Google Scholar]
- 20.Cheung CMG, et al. Polypoidal choroidal vasculopathy: definition, pathogenesis, diagnosis, and management. Ophthalmology. 2018;125:708–724. doi: 10.1016/j.ophtha.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 21.Byeon SH, et al. Incidence and clinical patterns of polypoidal choroidal vasculopathy in Korean patients. Jpn. J. Ophthalmol. 2008;52:57–62. doi: 10.1007/s10384-007-0498-2. [DOI] [PubMed] [Google Scholar]
- 22.Hoeg TB, et al. An evaluation of fundus photography and fundus autofluorescence in the diagnosis of cuticular drusen. Br. J. Ophthalmol. 2016;100:378–382. doi: 10.1136/bjophthalmol-2015-307197. [DOI] [PubMed] [Google Scholar]
- 23.Meyerle CB, Smith RT, Barbazetto IA, Yannuzzi LA. Autofluorescence of basal laminar drusen. Retina. 2007;27:1101–1106. doi: 10.1097/IAE.0b013e3181451617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leng T, Rosenfeld PJ, Gregori G, Puliafito CA, Punjabi OS. Spectral domain optical coherence tomography characteristics of cuticular drusen. Retina. 2009;29:988–993. doi: 10.1097/IAE.0b013e3181ae7113. [DOI] [PubMed] [Google Scholar]
- 25.Roquet W, Roudot-Thoraval F, Coscas G, Soubrane G. Clinical features of drusenoid pigment epithelial detachment in age related macular degeneration. Br. J. Ophthalmol. 2004;88:638–642. doi: 10.1136/bjo.2003.017632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bird AC, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv. Ophthalmol. 1995;39:367–374. doi: 10.1016/S0039-6257(05)80092-X. [DOI] [PubMed] [Google Scholar]
- 27.Holz FG, Bellman C, Staudt S, Schutt F, Volcker HE. Fundus autofluorescence and development of geographic atrophy in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2001;42:1051–1056. [PubMed] [Google Scholar]
- 28.Holz FG, Strauss EC, Schmitz-Valckenberg S, van Lookeren Campagne M. Geographic atrophy: clinical features and potential therapeutic approaches. Ophthalmology. 2014;121:1079–1091. doi: 10.1016/j.ophtha.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 29.Freund KB, Zweifel SA, Engelbert M. Do we need a new classification for choroidal neovascularization in age-related macular degeneration? Retina. 2010;30:1333–1349. doi: 10.1097/IAE.0b013e3181e7976b. [DOI] [PubMed] [Google Scholar]
- 30.Chen KC, et al. Intraretinal hyperreflective foci in acquired vitelliform lesions of the macula: clinical and histologic study. Am J Ophthalmol. 2016;164:89–98. doi: 10.1016/j.ajo.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Freund KB, et al. Acquired vitelliform lesions: correlation of clinical findings and multiple imaging analyses. Retina. 2011;31:13–25. doi: 10.1097/IAE.0b013e3181ea48ba. [DOI] [PubMed] [Google Scholar]
- 32.Deutman AF, Rumke AM. Reticular dystrophy of the retinal pigment epithelium. Dystrophia reticularis laminae pigmentosa retinae of H. Sjogren. Arch Ophthalmol. 1969;82:4–9. doi: 10.1001/archopht.1969.00990020006002. [DOI] [PubMed] [Google Scholar]
- 33.Shuler RK, Jr, et al. Peripheral reticular pigmentary change is associated with complement factor H polymorphism (Y402H) in age-related macular degeneration. Ophthalmology. 2008;115:520–524. doi: 10.1016/j.ophtha.2007.06.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.