Abstract
High and low hematocrit (Hct) and hemoglobin (Hb) levels are associated with the risk of cardiovascular disease. The purpose of this study was to determine the relationships of Hct, Hb and red blood cells (RBCs) with vascular function and structure. We measured flow-mediated vasodilation (FMD), nitroglycerin-induced vasodilation (NID), brachial intima media thickness (IMT), and brachial-ankle pulse wave velocity (baPWV) in 807 men. The subjects were divided into six groups according to the levels of Hct, Hb and RBCs. NID was highest in the 46.0–48.9% Hct group among the six groups according to Hct levels. Brachial IMT was lowest in the 46.0–48.9% Hct group among the six groups. There were no significant differences in FMD and baPWV among the six groups. We used 46.0–48.9% Hct as a reference to define the lower tertile. The adjusted odds ratio of being in the low tertile of NID was significantly higher in the < 42.9% and ≥ 49.0% Hct groups. Adjusted odds ratio of being in the low tertile of brachial IMT was significantly lower in the < 39.9% Hct groups. Similar results were obtained for Hb and RBCs. Low and high levels of Hct, Hb and RBCs were associated with vascular smooth muscle dysfunction, and low Hct levels were associated with abnormal vascular structure. Increases in the levels of Hct, Hb and RBCs within normal ranges may have beneficial effects on the vasculature.
Subject terms: Cardiology, Cardiovascular biology, Biomarkers
Introduction
Hematocrit (Hct), the volume percentage of red blood cells (RBCs) in total blood, and hemoglobin (Hb) are associated with a risk of cardiovascular disease. A high Hct level has been shown to be associated with an increased risk of cardiovascular disease1–3. On the other hand, J- or U-shaped relations between Hct and morbidity and mortality from cardiovascular events have been shown1. The relationship between a low Hct level and cardiovascular disease is controversial1,3,4. It is well known that Hct and Hb levels are major determinants of blood viscosity and oxygen delivery dynamics. It is thought that changes in blood viscosity and oxygen delivery dynamics alter vascular function and structure. Indeed, Lee et al. showed that high blood viscosity was associated with increased carotid intima-media thickness (IMT)5. However, there is no information on the associations of Hct, Hb and RBCs with vascular function and vascular structure.
Endothelial dysfunction is the initial step of atherosclerosis and leading to the development and progression of this condition6,7. Recently, flow-mediated vasodilation (FMD) as an index of endothelium-dependent vasodilation and nitroglycerin-induced vasodilation (NID) and an index of endothelium-independent vasodilation have been widely used as methods for assessment of endothelial function and vascular smooth muscle function, respectively8,9. Measurement of FMD reflects the response to the release of nitric oxide (NO). Moreover, growing evidence has shown that endothelial function assessed by FMD and vascular smooth muscle function assessed by NID can serve as independent predictors of cardiovascular events10–12. Measurement of brachial IMT in the artery as an index of structural change of the artery and measurement of brachial-ankle pulse wave velocity (baPWV) as an index of arterial stiffness have be shown to be significantly correlated with cardiovascular risk factors13,14.
The purpose of this study was to evaluate the relationships of levels of Hct, Hb and RBCs with vascular function and vascular structure and to evaluate the optimal cutoff levels of Hct, Hb and RBCs for maintenance of vascular function and vascular structure.
Results
Baseline clinical characteristics
The baseline clinical characteristics of the subjects are summarized in Table 1. Of the 807 subjects, 627 (77.7%) had hypertension, 496 (61.5%) had dyslipidemia, 269 (33.3%) had diabetes mellitus, 171 (21.2%) had previous coronary artery disease, 70 (8.7%) had previous stroke, and 188 (23.3%) were current smokers. Mean values were 3.5 ± 2.6% for FMD, 11.7 ± 5.8% for NID, 0.34 ± 0.08 mm for brachial IMT and 1683 ± 382 cm/s for baPWV.
Table 1.
Clinical characteristics of the subjects according to hematocrit levels.
| Variables | Total (n = 807) |
Hematocrit < 37.0% (n = 111) |
Hematocrit 37.0–39.9% (n = 138) |
Hematocrit 40.0–42.9% (n = 221) |
Hematocrit 43.0–45.9% (n = 214) |
Hematocrit 46.0–48.9% (n = 91) |
Hematocrit 49.0% ≤ (n = 32) |
P value |
|---|---|---|---|---|---|---|---|---|
| Age, year | 62 ± 14 | 71 ± 12 | 67 ± 11 | 62 ± 13 | 59 ± 14 | 55 ± 14 | 54 ± 12 | < 0.01 |
| Body mass index, kg/m2 | 24.7 ± 3.9 | 23.1 ± 3.6 | 24.1 ± 3.2 | 24.6 ± 3.5 | 25.1 ± 4.4 | 25.9 ± 3.5 | 26.9 ± 4.4 | < 0.01 |
| Systolic blood pressure, mmHg | 134 ± 19 | 135 ± 21 | 133 ± 19 | 132 ± 18 | 134 ± 19 | 135 ± 20 | 141 ± 20 | 0.17 |
| Diastolic blood pressure, mmHg | 80 ± 12 | 75 ± 12 | 78 ± 11 | 80 ± 12 | 81 ± 12 | 83 ± 12 | 85 ± 12 | < 0.01 |
| Heart rate, bpm | 70 ± 13 | 68 ± 13 | 70 ± 12 | 70 ± 13 | 70 ± 13 | 73 ± 13 | 72 ± 11 | 0.08 |
| Total cholesterol, mmol/L | 4.86 ± 0.96 | 4.58 ± 0.83 | 4.71 ± 0.88 | 4.84 ± 0.93 | 5.09 ± 0.96 | 5.12 ± 0.93 | 5.09 ± 1.16 | < 0.01 |
| Triglycerides, mmol/L | 1.70 ± 1.20 | 1.46 ± 1.10 | 1.47 ± 0.98 | 1.64 ± 1.04 | 1.76 ± 1.05 | 2.09 ± 1.64 | 2.59 ± 2.07 | < 0.01 |
| HDL cholesterol, mmol/L | 1.47 ± 0.44 | 1.50 ± 0.49 | 1.55 ± 0.49 | 1.42 ± 0.44 | 1.47 ± 0.39 | 1.37 ± 0.36 | 1.37 ± 0.34 | 0.01 |
| LDL cholesterol, mmol/L | 2.84 ± 0.85 | 2.46 ± 0.78 | 2.66 ± 0.75 | 2.84 ± 0.83 | 3.05 ± 0.91 | 3.05 ± 0.85 | 2.92 ± 0.75 | < 0.01 |
| Glucose, mmol/L | 6.77 ± 2.50 | 6.99 ± 2.89 | 6.94 ± 2.22 | 6.72 ± 2.33 | 6.49 ± 2.00 | 6.61 ± 2.39 | 7.99 ± 5.11 | 0.05 |
| Hemoglobin A1c, % | 5.8 ± 1.0 | 6.1 ± 1.5 | 5.8 ± 0.8 | 5.7 ± 0.8 | 5.8 ± 0.8 | 5.9 ± 1.1 | 6.3 ± 1.8 | 0.04 |
| BUN, mmol/L | 5.71 ± 1.93 | 7.50 ± 2.93 | 6.43 ± 2.03 | 5.36 ± 1.39 | 5.36 ± 1.39 | 5.36 ± 1.39 | 5.71 ± 1.93 | < 0.01 |
| Creatinine, mmol/L | 81.3 ± 25.6 | 100.8 ± 46.0 | 82.2 ± 27.4 | 78.7 ± 15.0 | 74.3 ± 17.7 | 76.9 ± 15.9 | 84.0 ± 18.6 | < 0.01 |
| eGFR, mL/min/1.73 m2 | 71 ± 20 | 59 ± 23 | 69 ± 21 | 71 ± 15 | 78 ± 19 | 77 ± 18 | 69 ± 16 | < 0.01 |
| Medical history, n (%) | ||||||||
| Hypertension | 627 (77.7) | 88 (79.3) | 118 (85.5) | 174 (78.7) | 151 (70.6) | 67 (73.6) | 29 (90.6) | < 0.01 |
| Dyslipidemia | 496 (61.5) | 69 (62.2) | 82 (59.4) | 135 (61.1) | 129 (60.3) | 58 (63.7) | 23 (71.9) | 0.84 |
| Diabetes mellitus | 269 (33.3) | 47 (42.3) | 43 (31.2) | 69 (31.2) | 69 (32.2) | 30 (33.0) | 11 (34.4) | 0.42 |
| Previous coronary heart disease | 171 (21.2) | 36 (32.4) | 44 (31.9) | 46 (20.8) | 21 (9.8) | 14 (15.4) | 10 (31.3) | < 0.01 |
| Previous stroke | 70 (8.7) | 19 (17.1) | 17 (12.3) | 14 (6.3) | 11 (5.1) | 6 (6.6) | 3 (9.4) | < 0.01 |
| Current smoker, n (%) | 188 (23.3) | 18 (16.2) | 17 (12.3) | 40 (18.1) | 66 (30.8) | 34 (37.4) | 13 (40.6) | < 0.01 |
| Medication, n (%) | ||||||||
| Antiplatelets | 225 (27.9) | 53 (47.8) | 51 (37.0) | 58 (26.2) | 41 (19.2) | 16 (17.6) | 6 (18.8) | < 0.01 |
| Calcium channel blockers | 377 (46.7) | 53 (47.8) | 71 (51.5) | 104 (47.1) | 92 (43.0) | 40 (44.0) | 17 (53.1) | 0.65 |
| ACEI or ARB | 319 (39.5) | 56 (50.5) | 71 (51.5) | 93 (42.1) | 56 (26.2) | 28 (30.8) | 15 (46.9) | < 0.01 |
| β-blockers | 194 (24.0) | 36 (32.4) | 42 (30.4) | 58 (26.2) | 32 (15.0) | 19 (20.9) | 7 (21.9) | < 0.01 |
| Diuretics | 105 (13.0) | 24 (21.6) | 23 (16.7) | 26 (11.8) | 13 (6.1) | 14 (15.4) | 5 (15.6) | < 0.01 |
| Statins | 287 (35.6) | 51 (46.0) | 56 (40.6) | 76 (34.4) | 57 (26.6) | 36 (39.6) | 11 (34.4) | 0.01 |
| Medically treated diabetes mellitus | ||||||||
| Any | 176 (21.8) | 31 (27.9) | 31 (22.5) | 43 (19.5) | 46 (21.5) | 22 (24.2) | 3 (9.4) | 0.23 |
| Insulin dependent | 24 (3.0) | 9 (8.1) | 6 (4.4) | 3 (1.4) | 2 (0.9) | 2 (2.2) | 2 (6.3) | < 0.01 |
HDL indicates high-density lipoprotein, LDL low-density lipoprotein, BUN blood urea nitrogen, eGFR estimated-glomerular filtration rate, ACEI angiotensin-converting enzyme inhibitor, ARB angiotensin II receptor blocker.
Results are presented as means ± SD for continuous variables and percentages for categorical variables.
We divided the subjects into six groups according to Hct levels. The baseline characteristics of subjects in the six groups are summarized in Table 1. There were significant differences among the six groups according to Hct levels in age, BMI, diastolic blood pressure, total cholesterol, triglycerides, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, HbA1c, estimated glomerular filtration rate (eGFR), prevalence of hypertension, prevalence of previous coronary heart disease, prevalence of previous stroke, current smokers, use of antiplatelets, use of an angiotensin-converting enzyme inhibitor or an angiotensin II receptor blocker, use of β-blockers, use of diuretics, use of statins, and use of insulin. There were no significant differences in other parameters among the six groups. Hematologic parameters are summarized in Table 2. There were significant differences among the six groups according to Hct levels in Hb, RBCs, mean corpuscular Hb concentration and platelets. There was no significant difference in other parameters among the six groups.
Table 2.
Hematologic parameters of the subjects according to hematocrit levels.
| Variables | Total (n = 807) |
Hematocrit < 37.0% (n = 111) |
Hematocrit 37.0–39.9% (n = 138) |
Hematocrit 40.0–42.9% (n = 221) |
Hematocrit 43.0–45.9% (n = 214) |
Hematocrit 46.0–48.9% (n = 91) |
Hematocrit 49.0% ≤ (n = 32) |
P value |
|---|---|---|---|---|---|---|---|---|
| Hemoglobin, g/dL | 14.3 ± 1.6 | 11.7 ± 0.8 | 13.2 ± 0.5 | 14.2 ± 0.5 | 15.3 ± 0.5 | 16.3 ± 0.5 | 17.2 ± 0.7 | < 0.01 |
| Hematocrit, % | 41.8 ± 4.3 | 34.3 ± 2.3 | 38.7 ± 0.9 | 41.4 ± 0.8 | 44.4 ± 0.8 | 47.2 ± 0.8 | 50.4 ± 1.2 | |
| Red blood cell, ×106/μL | 4.6 ± 0.5 | 3.8 ± 0.3 | 4.2 ± 0.2 | 4.6 ± 0.3 | 4.9 ± 0.3 | 5.2 ± 0.3 | 5.6 ± 0.2 | < 0.01 |
| Mean corpuscular volume, fL | 91.1 ± 4.8 | 91.5 ± 6.0 | 91.6 ± 4.4 | 90.6 ± 4.7 | 91.2 ± 4.7 | 90.8 ± 4.2 | 90.8 ± 4.1 | 0.35 |
| Mean corpuscular hemoglobin, pg | 31.2 ± 1.8 | 31.1 ± 2.2 | 31.3 ± 1.7 | 31.0 ± 1.8 | 31.3 ± 1.8 | 31.3 ± 1.5 | 31.0 ± 1.5 | 0.53 |
| Mean corpuscular hemoglobin concentration, g/dL | 34.3 ± 1.1 | 34.1 ± 1.1 | 34.1 ± 1.0 | 34.3 ± 1.0 | 34.4 ± 0.9 | 34.5 ± 1.0 | 34.4 ± 2.2 | 0.02 |
| Platelets, × 103/μL | 205.3 ± 52.8 | 197.4 ± 53.4 | 195.5 ± 51.6 | 207.5 ± 54.1 | 217.6 ± 48.1 | 201.7 ± 56.9 | 190.2 ± 47.2 | < 0.01 |
| Mean platelet volume, fL | 10.2 ± 0.9 | 10.2 ± 1.0 | 10.1 ± 0.8 | 10.1 ± 0.9 | 10.2 ± 0.8 | 10.3 ± 1.0 | 10.5 ± 0.7 | 0.14 |
The baseline characteristics of subjects in the six groups according to Hb and RBC levels are summarized in the Supplemental Tables S1–S4 and are presented in the Supplemental Results section.
Relationships of Hct, Hb, and RBCs with vascular function
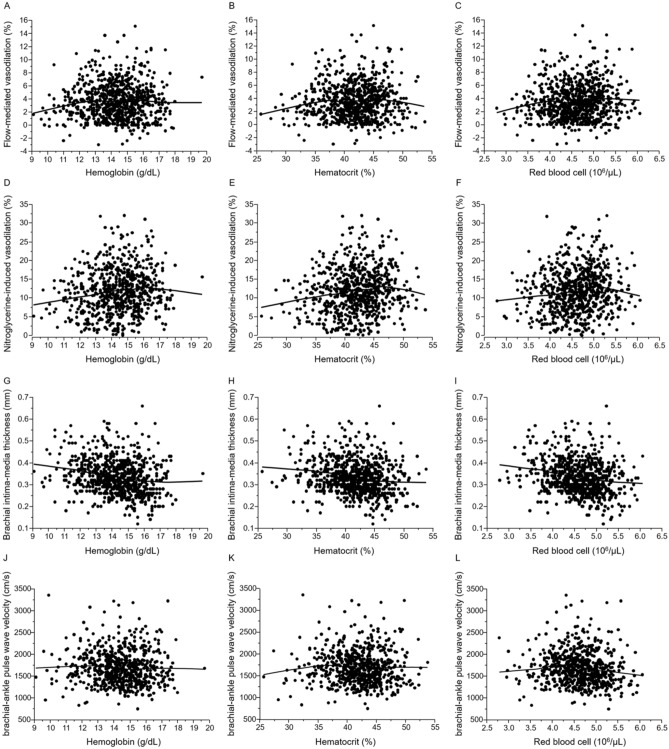
Scatter plots between vascular function and hematologic parameters with a Lowess smoothed curve are shown in Fig. 1. Both FMD and NID gradually increased up to Hct levels of about 46–48% and then decreased with further increase in Hct levels. NID was highest in the 46.0–48.9% Hct group among the six groups (10.0 ± 5.3% in the < 37.0% Hct group, 10.8 ± 5.9% in the 37.0–39.9% Hct group, 12.0 ± 6.1% in the 40.0–42.9% Hct group, 12.0 ± 5.7% in the 43.0–45.9% Hct group, 14.2 ± 5.6% in the 46.0–48.9% Hct group and 10.4 ± 4.5% in the ≥ 49.0% Hct group; P < 0.01; Supplemental Figure S1A). There were no significant differences in FMD among the six groups (3.3 ± 2.6% in the < 37.0% Hct group, 3.4 ± 2.6% in the 37.0–39.9% Hct group, 3.6 ± 2.6% in the 40.0–42.9% Hct group, 3.7 ± 2.6% in the 43.0–45.9% Hct group, 3.9 ± 3.1% in the 46.0–48.9% Hct group and 2.9 ± 2.7% in the ≥ 49.0% Hct group; P = 0.39; Supplemental Figure S1B). We used 46.0–48.9% Hct as a reference to define the lower tertile. After adjustment for age, BMI, current smoking and presence of hypertension, dyslipidemia, and diabetes mellitus, adjusted odds ratio of being in the low tertile of NID was significantly higher in the < 42.9% and ≥ 49.0% Hct groups (Table 3). There was no significant difference in low tertile of NID between the 43.0–45.9% Hct group and the 46.0–48.9% Hct group (Table 3).
Figure 1.
Scatter plots show the relationships of hemoglobin (A), hematocrit (B), and red blood cell (C) with flow-mediated vasodilation, the relationships of hemoglobin (D), hematocrit (E), and red blood cell (F) with nitroglycerine-induced vasodilation, the relationships of hemoglobin (G), hematocrit (H), and red blood cell (I) with brachial intima-media thickness and the relationships of hemoglobin (J), hematocrit (K), and red blood cell (L) with brachial-ankle pulse wave velocity.
Table 3.
Multiple analysis of relationships between low nitroglycerine-induced vasodilation and variables.
| Variables | Hematocrit < 37.0% |
Hematocrit 37.0–39.9% |
Hematocrit 40.0–42.9% |
Hematocrit 43.0–45.9% |
Hematocrit 46.0–48.9% |
Hematocrit 49.0% ≤ |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Unadjusted model | 4.1 (2.10–7.87) | < 0.01 | 2.7 (1.44–5.07) | < 0.01 | 2.2 (1.24–4.01) | < 0.01 | 1.9 (1.04–3.38) | 0.04 | 1 (reference) | 4.6 (1.91–11.05) | < 0.01 | |
| Model 1 | 2.7 (1.36–5.42) | < 0.01 | 2.0 (1.04–3.81) | 0.04 | 1.9 (1.03–3.41) | 0.04 | 1.7 (0.92–3.04) | 0.09 | 1 (reference) | 4.8 (1.95–11.57) | < 0.01 | |
| Model 2 | 3.2 (1.57–6.46) | < 0.01 | 2.2 (1.11–4.19) | 0.02 | 2.0 (1.09–3.72) | 0.03 | 1.8 (0.96–3.24) | 0.06 | 1 (reference) | 4.3 (1.73–10.49) | < 0.01 | |
Low tertile of nitroglycerine-induced vasodilation indicates less than 10.4%. Model 1: adjusted for age. Model 2: adjusted for age, body mass index, current smoking, hypertension, dyslipidemia and diabetes mellitus.
Clinical characteristics and hematologic parameters of the subjects with Hct of < 48.9% are summarized in Supplemental Tables S5 and S6. Hct was positively correlated with FMD and NID (r = 0.08, P = 0.03 and r = 0.18, P < 0.01, respectively; Supplemental Table S7). Multivariate analysis revealed that Hct was an independent variable of NID (β = 0.11, P < 0.01; Supplemental Table S8). Hct was not an independent variable of FMD (β = − 0.01, P = 0.73; Supplemental Table S9). Hct of 42.0% was the optimal cut-off value for the low tertile of NID (sensitivity, 58.4%; specificity, 55.9%). Characteristics and hematologic parameters of the subjects with Hct of > 46.0% are summarized in Supplemental Tables S10 and S11. Hct was negatively correlated with FMD and NID (r = − 0.11, P = 0.24 and r = − 0.25, P < 0.01, respectively; Supplemental Table S12). Multivariate analysis revealed that Hct was an independent variable of NID in multivariate analysis (β = − 0.23, P = 0.01; Supplemental Table S13). Hct was not an independent variable of FMD (β = − 0.07, P = 0.39; Supplemental Table S14). Hct of 49.4% was the optimal cut-off value for the low tertile of NID (sensitivity, 46.0%; specificity, 88.2%).
The relationships of Hb and RBCs with vascular function are presented in the Supplemental Results section, Supplemental Figures S2 and S3 and Supplemental Tables S15–S35.
Relationships of Hct, Hb, and RBCs with vascular structure
Scatter plots between vascular structure and hematologic parameters with a Lowess smoothed curve are shown in Fig. 1. Brachial IMT significantly decreased in relation to an increase in the levels of Hct categories (0.36 ± 0.08 mm, 0.36 ± 0.07 mm, 0.34 ± 0.07 mm, 0.32 ± 0.08 mm, 0.31 ± 0.08 mm and 0.32 ± 0.08 mm; P < 0.01; Supplemental Figure S4A). There were no significant differences in baPWV among the six groups (1714 ± 410 cm/s, 1729 ± 374 cm/s, 1662 ± 384 cm/s, 1647 ± 385 cm/s, 1702 ± 388 cm/s and 1752 ± 494 cm/s; P = 0.35; Supplemental Figure S4B). We used 46.0–48.9% Hct as a reference to define the lower tertile. After adjustment for age, BMI, current smoking and presence of hypertension, dyslipidemia, and diabetes mellitus, adjusted odds ratio of being in the low tertile of brachial IMT was significantly lower in the < 39.9% Hct groups (Table 4). There were no significant differences in the low tertile of brachial IMT among the 40.0–42.9% Hct group, 43.0–45.9% Hct group, and ≥ 49.0% Hct group and 46.0–48.9% Hct group.
Table 4.
Multiple analysis of relationships between low brachial intima-media thickness and variables.
| Variables | Hematocrit < 37.0% |
Hematocrit 37.0–39.9% |
Hematocrit 40.0–42.9% |
Hematocrit 43.0–45.9% |
Hematocrit 46.0–48.9% |
Hematocrit 49.0% ≤ |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Unadjusted model | 0.2 (0.10–0.39) | < 0.01 | 0.2 (0.10–0.39) | < 0.01 | 0.4 (0.25–0.72) | < 0.01 | 0.7 (0.43–1.21) | 0.22 | 1 (reference) | 0.6 (0.26–1.50) | 0.29 | |
| Model 1 | 0.4 (0.20–0.83) | 0.01 | 0.3 (0.17–0.65) | < 0.01 | 0.6 (0.34–1.04) | 0.07 | 0.9 (0.52–1.57) | 0.72 | 1 (reference) | 0.6 (0.23–1.49) | 0.27 | |
| Model 2 | 0.4 (0.18–0.78) | < 0.01 | 0.3 (0.18–0.62) | < 0.01 | 0.6 (0.32–1.01) | 0.06 | 0.9 (0.49–1.52) | 0.61 | 1 (reference) | 0.6 (0.24–1.59) | 0.32 | |
Low tertile of brachial intima-media thickness indicates less than 0.30 mm. Model 1: adjusted for age. Model 2: adjusted for age, body mass index, current smoking, hypertension, dyslipidemia and diabetes mellitus.
The relationships of Hb and RBCs with vascular structure are presented in the Supplemental Results section, Supplemental Figures S5 and S6 and Supplemental Tables S36–S38.
Discussion
In the present study, we demonstrated for the first time that Hct, Hb and RBCs were associated with vascular function and vascular structure in men. Adjusted odds ratio of being in the low tertile of NID was significantly higher in the < 42.9% and ≥ 49.0% Hct groups. Adjusted odds ratio of being in the low tertile of NID was significantly higher in the < 13 g/dL Hb group, 14.0–14.9 g/dL Hb group and ≥ 17.0 g/dL Hb group. Adjusted odds ratio of being in the low tertile of NID was significantly higher in the < 4.19 × 106/μL and ≥ 5.40 × 106/μL RBCs groups. In addition, adjusted odds ratio of being in the low tertile of brachial IMT was significantly lower in the < 39.9% Hct groups than in the 46.0–48.9% Hct group. Adjusted odds ratio of being in the low tertile of brachial IMT was significantly lower in the < 14.9 g/dL Hb groups than in the 16.0–16.9 g/dL Hb group. Hct of 42.0–49.4%, Hb of 14.7–16.8 g/dL and RBCs of 4.82–5.24 × 106/μL may be the optimal target levels for maintenance of vascular function and vascular structure.
In the present study, Hct of 42.0–49.4% was best from the aspect of vascular smooth muscle function. Several studies have shown that high Hct levels were associated with an increased risk of cardiovascular disease1–3. On the other hand, the relationship between low Hct levels and cardiovascular disease is controversial. Gagnon et al. showed that there were J- or U-shaped relations between Hct and morbidity and mortality from cardiovascular events1. After risk factor adjustment, there was a significantly increased risk of cardiovascular disease in the high Hct group but not in the low Hct group in men. Gotoh et al. showed that low Hct levels were associated with hemorrhagic stroke3. The effects of Hct, Hb and RBCs on vascular function and vascular structure are unclear. In the present study, we demonstrated that both low and high levels of Hct, Hb and RBCs were associated with vascular smooth muscle dysfunction. Vosseler et al. showed that blood viscosity, which was calculated using account Hct and plasma proteins, was negatively correlated with FMD in patients without coronary artery disease, while there was no significant relationship between blood viscosity and FMD in patients with atheroclerosis15. The discrepancy in the result of our study and the results of previous studies regarding the relationship between vascular function and hematocrit is due to the different numbers of subjects and different characteristics of subjects. The number of subjects was larger in the present study than in the previous studies. Our study participants were enrolled from a general population including patients with cardiovascular disease. Interestingly, Giannattasio et al. showed that acute decreases in Hct from 39.9 ± 0.8% to 37.1 ± 0.4% and Hb from 13.3 ± 0.3 to 12.2 ± 0.4 g/dL, after removal of 500 mL of blood and infusion of 500 mL of saline, impaired vascular function in patients with hemochromatosis16. In subjects with Hct of < 48.9%, Hct was positively correlated with FMD and NID and Hct was an independent predictor of NID. These findings suggest that subjects with high or low levels of Hct, Hb and RBCs have a high risk of vascular dysfunction and prognostic atherosclerosis.
Some possible mechanisms underlying the association of low Hct with vascular smooth muscle function are postulated. It is possible that oxygen delivery dynamics at the levels of hemoglobin and hematocrit are associated with vascular function. Thorling et al. showed that Hct positively correlated with tissue tension of oxygen even within normal ranges of Hct levels, suggesting that a decrease in Hct leads to a decrease in oxygen supply to tissues17. Takemoto et al. showed that hypoxia decreased endothelial NO synthase (eNOS) expression via the activation of Rho-associated kinase18. Chronic hypoxia affects endothelial dysfunction via increases in inflammation and oxidative stress19,20. Several studies showed that Hct significantly correlated with viscosity21,22. Hct is one of the most important factors affecting blood viscosity. In addition, blood viscosity regulates shear stress, which is an inducer of NO production from the endothelium. Martini et al. showed that animals with increased Hct had increased plasma nitrate/nitrite concentrations compared with those in control animals and in eNOS knockout mice through an increase in blood viscosity22. These findings suggest that a low level of Hct is harmful for vascular function.
Some possible mechanisms underlying the association of excessively high Hct with vascular smooth muscle dysfunction are postulated. Lewis et al. showed that the patients with excessive erythrocytosis caused by chronic mountain sickness in Andean highlanders had endothelial dysfunction that was partially reversible during oxygen inhalation, suggesting that chronic hypoxia may induce endothelial dysfunction in patients with excessive erythrocytosis23. In addition, high blood viscosity caused by high levels of Hct as well as low blood viscosity caused by low levels of Hct induced low tissue tension of oxygen. According to the Hagen-Poiseuille law, blood flow depends on blood viscosity and vessel radius. Total peripheral vascular resistance is specified by blood viscosity and cardiac output. Fowler et al. showed that high viscosity caused low cardiac output24. These findings suggest that high levels of Hct may induce tissue tension of oxygen by high peripheral vascular resistance and low cardiac output. These findings also suggest that a high level of Hct may be one of the factors of vascular dysfunction.
It has been shown that RBCs directly affect endothelial function via the eNOS/NO pathway and NOS-like bioactivity and the production of reactive oxygen species25–27. Cortese-Krott et al. showed that RBCs contained eNOS and produced NO in healthy subjects as well as in patients with coronary artery disease and that FMD significantly correlated with the expression of eNOS and eNOS activity in RBCs in those subjects25. In addition, Zhou et al. showed new mechanisms by which endothelial function was impaired in type 2 diabetes mellitus through activation of RBC arginase 1 and increase in production of reactive oxygen species27. These findings suggest that RBC function per se plays an important role in the pathogenesis, maintenance, and development of atherosclerosis through the regulation of vascular function, leading to cardiovascular disease and cardiovascular events. Unfortunately, our study had no information on the function of RBCs, such as the eNOS/NO pathway, NOS like activity and oxidative stress. Assessment of RBC function would enable more specific conclusions concerning the role of RBCs other than the number of RBCs in vascular function to be drawn.
Simply, NID is assessed by brachial artery response to sublingual administration of nitroglycerine. However, we believe that vascular response to exogenous NO reflects vascular smooth muscle function since NO finally acts on vascular smooth muscle cells. Indeed, NID has been widely used as an indicator of vascular smooth muscle function. Several investigators have shown that vascular response to nitric acid including nitroglycerine reflects vascular smooth muscle function in the brachial artery and coronary artery of humans and in the isolated aorta artery of experimental animals28–30. It has been shown that NID is impaired in patients with multiple cardiovascular risk factors and that it serves as an independent predictor of cardiovascular events12,31. We believe that reduction in vascular smooth muscle response assessed by NID can also be defined vascular smooth muscle dysfunction.
Recently, some trials have shown that patients with type 2 diabetes mellitus who received an inhibitor of sodium-glucose cotransporter 2 in addition to conventional therapy had significantly lower rates of cardiovascular morbidity and mortality than did patients with type 2 diabetes mellitus who received a placebo in addition to conventional therapy32–34. The EMPA-REG OUTCOME trial showed that changes in Hct (increase by 5.0 ± 5.3% from baseline of 41.3 ± 5.7%) and Hb (increase by 0.8 ± 1.3 g/dL from baseline of 13.5 ± 1.5 g/dL) within normal ranges might be important mediators of the empagliflozin-induced reduction in incidence of cardiovascular events including cardiovascular mortality35. In the present study, Hct was positively correlated with FMD and NID in subjects with Hct < 48.9%, which was an independent variable of NID in multivariate analysis. In addition, Hct level of 42.0–49.4%, Hb level of 14.7–16.8 g/dL and RBC level of 4.82–5.24 × 106/μL may be the optimal target levels for maintenance of vascular function. An increase in the level of Hct up to 49.4% may reduce the incidence of cardiovascular events.
In the present study, adjusted odds ratio of being in the low tertile of brachial IMT was significantly lower in the < 37.0% Hct group and 37.0–39.9% Hct group than in the 46.0–48.9% Hct group and was significantly lower in the < 13.9 g/dL Hb groups and 14.0–14.9 g/dL Hb group than in the 16.0–16.9 g/dL Hb group. Adjusted odds ratio of being in the low tertile of baPWV was significantly lower in the level < 3.80 × 106/μL RBCs group and 4.60–4.99 × 106/μL RBCs group than in the 5.00–5.39 × 106/μL RBCs group. Lee et al. showed that carotid IMT positively correlated with blood viscosity and Hct. In their study, blood viscosity was an independent variable of carotid IMT in multivariate analysis, while Hct was not an independent variable of carotid IMT5. Kawamoto et al. showed that Hb levels were not associated with baPWV in men36. Unfortunately, the relationships of Hct, Hb and RBCs with vascular structure are also controversial. The roles of Hct, Hb and RBCs in vascular structure need to be confirmed in future in large clinical trials.
In the present study, the groups with high levels of Hct, Hb and RBCs had vascular smooth muscle dysfunction but not abnormal vascular structure. It is well known that alteration of vascular function occurs before changes in vascular structure. Unfortunately, we had no information on the duration of high levels of Hct, Hb and RBCs. Cohort studies have shown that a high Hct level per se was associated with an increased risk of cardiovascular disease1–3. NID may be a more sensitive marker than brachial IMT or baPWV of cardiovascular disease in subjects with high levels of Hct and Hb.
Our study has a number of limitations. First, this study is a cross-sectional design. Therefore, we cannot define causal relationships of Hct, Hb and RBCs with vascular dysfunction and abnormal vascular structure. Further studies are needed to confirm the effects of changes in levels of Hct, Hb and RBCs on vascular function and structure in long-term follow-up periods using a prospective study design. Second, we evaluated the relationships of Hct, Hb and RBCs with vascular function and structure only in men. It is well known that menstrual bleeding affects the levels of Hct, Hb and RBCs. We had no information on menstrual cycle when measuring vascular function and structure. Therefore, we excluded women as study subjects. Further studies are needed to confirm the relationships of levels of Hct, Hb and RBCs with vascular function and structure in women including premenopausal women as well as men after adjustment of the menstrual cycle. Third, we defined vascular dysfunction assessed by FMD and that assessed by NID as low tertiles of FMD and NID. The use of criteria for vascular dysfunction is a better way to calculate the odds ratio. However, diagnostic criteria for endothelial dysfunction assessed by FMD and vascular smooth muscle dysfunction assessed by NID have not been established. Therefore, we used low tertiles of FMD and NID as vascular dysfunction for calculation of the odds ratio.
Conclusion
Low and high levels of Hct, Hb and RBCs were associated with vascular smooth muscle dysfunction, and low Hct levels were associated with abnormal vascular structure. Increases in the levels of Hct, Hb and RBCs within normal ranges may decrease the risk of cardiovascular disease. Hct level of 43.0–48.9%, Hb level of 14.7–16.8 g/dL and RBCs level of 4.82–5.24 × 106/μL may be the optimal target levels for maintenance of vascular function and vascular structure. Therefore, attention should be given to levels of Hct, Hb and RBCs when caring for patients with low or high levels of Hct, Hb and RBCs.
Methods
Subjects
Between September 2010 and June 2017, a total of 993 men were recruited for measurement of vascular function from subjects who underwent health-screening examinations or who visited the outpatient clinic at Hiroshima University Hospital. One hundred eighty-six of the 993 men, including 59 patients with infection, 50 patients with advanced cancer, 11 patients with bleeding, 35 patients with end-stage renal disease, 16 patients who had received prednisolone treatment, and 15 patients with hematologic disease, were excluded. Finally, 807 men were enrolled in this study. Hypertension was defined as systolic blood pressure of more than 140 mm Hg or diastolic blood pressure of more than 90 mm Hg in a sitting position, on at least three different occasions. DM was defined according to the American Diabetes Association or a previous diagnosis of diabetes37,38. Dyslipidemia was defined according to the third report of the National Cholesterol Education Program39.
We divided the subjects into six groups according to Hct levels (< 37.0% group, 37.0–39.9% group, 40.0–42.9% group, 43.0–45.9% group, 46.0–48.9% group and ≥ 49.0% group), six groups according to Hb levels (< 13 g/dL group, 13.0–39.9 g/dL group, 14.0–14.9 g/dL group, 15.0–15.9 g/dL group, 16.0–16.9 g/dL group and ≥ 17.0 g/dL group), and six groups according to RBCs levels (< 3.80 × 106/μL group, 3.80–4.19 × 106/μL group, 4.20–4.59 × 106/μL group, 4.60–4.99 × 106/μL group, 5.00–5.39 × 106/μL group and ≥ 5.40 × 106/μL group).
All methods were carried out in accordance with relevant guidelines and regulations. The Ethics Review Board of Hiroshima University approved the study protocol. Written informed consent for participation in the study was obtained from all of the subjects. All methods were performed in accordance with the relevant guidelines and regulations overseen by the Ethical Committee in Hiroshima University.
Study protocol
We measured vascular function using measurement of FMD and NID and vascular structure using measurement of IMT in the brachial artery and baPWV. Subjects fasted the previous night for at least 12 h and the study began at 8:30 a.m. The subjects were kept in the supine position in a quiet, dark, and air-conditioned room (constant temperature of 22–25°C) throughout the study. A 23-gauge polyethylene catheter was inserted into the left deep antecubital vein to obtain blood samples. After thirty minutes of maintaining the supine position, we measured FMD, NID, brachial IMT and baPWV. The observers were blind to the form of examination40. Clinical trial registration information: URL for Clinical Trial: https://www.umin.ac.jp Registration Number for Clinical Trial: UMIN000003409.
Measurements of FMD and NID
Vascular response to reactive hyperemia in the brachial artery was used for assessment of endothelium-dependent FMD. A high-resolution linear artery transducer was coupled to computer-assisted analysis software (UNEXEF18G, UNEX Co, Nagoya, Japan) that used an automated edge detection system for measurement of brachial artery diameter12. The response to nitroglycerine was used for assessment of endothelium-independent vasodilation. NID was measured as described previously12. Additional details are available in the online-only Data Supplement.
Measurement of brachial IMT
Before FMD measurement, baseline longitudinal ultrasonographic images of the brachial artery, obtained at the end of diastole from each of 10 cardiac cycles, were automatically stored on a hard disk for off-line assessment of IMT with a linear, phased-array high-frequency (10-MHz) transducer using an UNEXEF18G ultrasound unit (UNEX Co)13. Additional details are available in the online-only Data Supplement.
Measurement of baPWV
Aortic compliance was assessed noninvasively on the basis of Doppler ultrasound measurements of PWV along the descending thoracoabdominal aorta, as previously published and validated41. Additional details are available in the online-only Data Supplement.
Statistical analysis
Results are presented as means ± SD for continuous variables and as percentages for categorical variables. Statistical significance was set at a level of P < 0.05. Categorical variables were compared by means of the χ2 test. Continuous variables were compared by ANOVA. Associations between variables were determined by Spearman rank correlation analysis. Associations of FMD, NID, brachial IMT and baPWV with hematologic parameters were examined visually using locally weighted regression smoothing (Lowess) plots. Cut-off values of Hct, Hb and RBCs were evaluated on the basis of receiver-operating characteristic curve analysis using the Youden index. Multivariate regression analysis was performed to identify independent variables associated with low tertiles of FMD (< 2.2%), NID (< 10.4%), brachial IMT (< 0.30 mm) and baPWV (< 1501 cm/s). A reverse U-shaped relation was showed between Hct and NID. We performed formal tests of linearity for the relation between Hct and NID. The R2 value of the quadratic model of Hct was better than that of the linear model of Hct (0.023 and 0.019, respectively). Thus, the model with Hct as a quadratic function gives a better fit. Peak Hct of 46.64% was calculated by the delta method. Peak Hct was used to determine the Hct range of 46.0–48.9% at which NID was the highest among the six groups, and the Hct range of 46.0–48.9% was used as the reference group in the multiple logistic regression analysis. Similar reverse U-shaped relations were found between Hb and NID and RBCs and NID. The peaks of Hb and RBCs were used to determine the ranges of 16.0–16.9 g/dL for Hb and 5.00–5.39 × 106/μL for RBCs at which NID values were the highest among the six groups, and the ranges of 16.0–16.9 g/dL for Hb and 5.00–5.39 × 106/μL for RBCs were used as reference groups in the multiple logistic regression analysis. Age, body mass index (BMI), current smoking and presence of hypertension, dyslipidemia, and diabetes mellitus were entered into the multiple logistic regression analysis. The data were processed using JMP pro version 13 (SAS institute. Cary, NC).
Supplementary information
Acknowledgements
We thank Miki Kumiji, Megumi Wakisaka, Ki-ichiro Kawano and Satoko Michiyama for their excellent secretarial assistance. This study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (18590815 and 21590898 to Higashi).
Author contributions
S.K. and Y.H. contributed to the study design. S.K., T.M., M.K., S.M., H.H., Y.T., T.H., T.Y., Y.H., C.G., F.M.Y., and A.N. performed the date collection. S.K. performed statistical analyses after discussion with all authors. S.K., Y.H. contributed to the writing of the manuscript. Y.K. and K.C. revised the article critically for important intellectual content. All authors contributed to interpretation of date and review of the manuscript, and approved this manuscript for submission.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-68319-1.
References
- 1.Gagnon DR, Zhang TJ, Brand FN, Kannel WB. Hematocrit and the risk of cardiovascular disease—the Framingham study: A 34-year follow-up. Am. Heart J. 1994;127:674–682. doi: 10.1016/0002-8703(94)90679-3. [DOI] [PubMed] [Google Scholar]
- 2.Toss F, Nordstrom A, Nordstrom P. Association between hematocrit in late adolescence and subsequent myocardial infarction in Swedish men. Int. J. Cardiol. 2013;168:3588–3593. doi: 10.1016/j.ijcard.2013.05.065. [DOI] [PubMed] [Google Scholar]
- 3.Gotoh S, et al. Hematocrit and the risk of cardiovascular disease in a Japanese community: The Hisayama Study. Atherosclerosis. 2015;242:199–204. doi: 10.1016/j.atherosclerosis.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 4.Brown DW, Giles WH, Croft JB. Hematocrit and the risk of coronary heart disease mortality. Am. Heart J. 2001;142:657–663. doi: 10.1067/mhj.2001.118467. [DOI] [PubMed] [Google Scholar]
- 5.Lee AJ, et al. Blood viscosity and elevated carotid intima-media thickness in men and women: The Edinburgh Artery Study. Circulation. 1998;97:1467–1473. doi: 10.1161/01.cir.97.15.1467. [DOI] [PubMed] [Google Scholar]
- 6.Ross R. Atherosclerosis–an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/nejm199901143400207. [DOI] [PubMed] [Google Scholar]
- 7.Higashi Y, Noma K, Yoshizumi M, Kihara Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ. J. 2009;73:411–418. doi: 10.1253/circj.CJ-08-1102. [DOI] [PubMed] [Google Scholar]
- 8.Celermajer DS, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-F. [DOI] [PubMed] [Google Scholar]
- 9.Soga J, et al. Rho-associated kinase activity, endothelial function, and cardiovascular risk factors. Arterioscler. Thromb. Vasc. Biol. 2011;31:2353–2359. doi: 10.1161/atvbaha.111.227892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gokce N, et al. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: A prospective study. Circulation. 2002;105:1567–1572. doi: 10.1161/01.CIR.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- 11.Lerman A, Zeiher AM. Endothelial function: Cardiac events. Circulation. 2005;111:363–368. doi: 10.1161/01.cir.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 12.Maruhashi T, et al. Nitroglycerine-induced vasodilation for assessment of vascular function: A comparison with flow-mediated vasodilation. Arterioscler. Thromb. Vasc. Biol. 2013;33:1401–1408. doi: 10.1161/atvbaha.112.300934. [DOI] [PubMed] [Google Scholar]
- 13.Iwamoto Y, et al. Intima-media thickness of brachial artery, vascular function, and cardiovascular risk factors. Arterioscler. Thromb. Vasc. Biol. 2012;32:2295–2303. doi: 10.1161/atvbaha.112.249680. [DOI] [PubMed] [Google Scholar]
- 14.Maruhashi T, et al. Endothelial dysfunction, increased arterial stiffness, and cardiovascular risk prediction in patients with coronary artery disease: FMD-J (Flow-Mediated Dilation Japan) Study A. J. Am. Heart Assoc. 2018 doi: 10.1161/jaha.118.008588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vosseler M, et al. Parameters of blood viscosity do not correlate with the extent of coronary and carotid atherosclerosis and with endothelial function in patients undergoing coronary angiography. Clin. Hemorheol. Microcirc. 2012;52:245–254. doi: 10.3233/ch-2012-1602. [DOI] [PubMed] [Google Scholar]
- 16.Giannattasio C, Piperno A, Failla M, Vergani A, Mancia G. Effects of hematocrit changes on flow-mediated and metabolic vasodilation in humans. Hypertension. 2002;40:74–77. doi: 10.1161/01.HYP.0000022571.86090.F3. [DOI] [PubMed] [Google Scholar]
- 17.Thorling EB, Erslev AJ. The, "tissue" tension of oxygen and its relation to hematocrit and erythropoiesis. Blood. 1968;31:332–343. doi: 10.1182/blood.V31.3.332.332. [DOI] [PubMed] [Google Scholar]
- 18.Takemoto M, Sun J, Hiroki J, Shimokawa H, Liao JK. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation. 2002;106:57–62. doi: 10.1161/01.cir.0000020682.73694.ab. [DOI] [PubMed] [Google Scholar]
- 19.Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am. J. Respir. Crit. Care Med. 2002;165:934–939. doi: 10.1164/ajrccm.165.7.2104126. [DOI] [PubMed] [Google Scholar]
- 20.El-Solh AA, et al. Adhesion molecules in patients with coronary artery disease and moderate-to-severe obstructive sleep apnea. Chest. 2002;121:1541–1547. doi: 10.1378/chest.121.5.1541. [DOI] [PubMed] [Google Scholar]
- 21.de Simone G, et al. Relation of blood viscosity to demographic and physiologic variables and to cardiovascular risk factors in apparently normal adults. Circulation. 1990;81:107–117. doi: 10.1161/01.CIR.81.1.107. [DOI] [PubMed] [Google Scholar]
- 22.Martini J, Carpentier B, Negrete AC, Frangos JA, Intaglietta M. Paradoxical hypotension following increased hematocrit and blood viscosity. Am. J. Physiol. Heart. Circ. Physiol. 2005;289:H2136–2143. doi: 10.1152/ajpheart.00490.2005. [DOI] [PubMed] [Google Scholar]
- 23.Lewis NC, et al. Conduit artery structure and function in lowlanders and native highlanders: Relationships with oxidative stress and role of sympathoexcitation. J. Physiol. 2014;592:1009–1024. doi: 10.1113/jphysiol.2013.268615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fowler NO, Holmes JC. Blood viscosity and cardiac output in acute experimental anemia. J. Appl. Physiol. 1975;39:453–456. doi: 10.1152/jappl.1975.39.3.453. [DOI] [PubMed] [Google Scholar]
- 25.Cortese-Krott MM, et al. Human red blood cells at work: Identification and visualization of erythrocytic eNOS activity in health and disease. Blood. 2012;120:4229–4237. doi: 10.1182/blood-2012-07-442277. [DOI] [PubMed] [Google Scholar]
- 26.Pernow J, Mahdi A, Yang J, Zhou Z. Red blood cell dysfunction: A new player in cardiovascular disease. Cardiovasc. Res. 2019;115:1596–1605. doi: 10.1093/cvr/cvz156. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Z, et al. Erythrocytes from patients with type 2 diabetes induce endothelial dysfunction via arginase I. J. Am. Coll. Cardiol. 2018;72:769–780. doi: 10.1016/j.jacc.2018.05.052. [DOI] [PubMed] [Google Scholar]
- 28.Higashi Y, et al. Regular aerobic exercise augments endothelium-dependent vascular relaxation in normotensive as well as hypertensive subjects: Role of endothelium-derived nitric oxide. Circulation. 1999;100:1194–1202. doi: 10.1161/01.CIR.100.11.1194. [DOI] [PubMed] [Google Scholar]
- 29.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 30.Verbeuren TJ, et al. Effect of hypercholesterolemia on vascular reactivity in the rabbit. II. Influence of treatment with dipyridamole on endothelium-dependent and endothelium-independent responses in isolated aortas of control and hypercholesterolemic rabbits. Circ. Res. 1986;59:496–504. doi: 10.1161/01.res.59.5.496. [DOI] [PubMed] [Google Scholar]
- 31.Kajikawa M, et al. Combination of flow-mediated vasodilation and nitroglycerine-induced vasodilation is more effective for prediction of cardiovascular events. Hypertension. 2016;67:1045–1052. doi: 10.1161/hypertensionaha.115.06839. [DOI] [PubMed] [Google Scholar]
- 32.Zinman B, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 33.Neal B, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 34.Wiviott SD, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 35.Inzucchi SE, et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME Trial. Diabetes Care. 2018;41:356–363. doi: 10.2337/dc17-1096. [DOI] [PubMed] [Google Scholar]
- 36.Kawamoto R, et al. A slightly low hemoglobin level is beneficially associated with arterial stiffness in Japanese community-dwelling women. Clin. Exp. Hypertens. 2012;34:92–98. doi: 10.3109/10641963.2011.618202. [DOI] [PubMed] [Google Scholar]
- 37.American Diabetes Association Clinical practice recommendations 1999. Diabetes Care. 1999;22(Suppl 1):S1–114. [PubMed] [Google Scholar]
- 38.American Diabetes Association 2. Classification and diagnosis of diabetes. Diabetes Care. 2017;40:S11–S24. doi: 10.2337/dc17-S005. [DOI] [PubMed] [Google Scholar]
- 39.Expert Panel on Detection EExpert Panel on Detection E Executive summary of the third report of The National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 40.Kishimoto S, et al. Endothelial dysfunction and abnormal vascular structure are simultaneously present in patients with heart failure with preserved ejection fraction. Int. J. Cardiol. 2017;231:181–187. doi: 10.1016/j.ijcard.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 41.Kimoto E, et al. Preferential stiffening of central over peripheral arteries in type 2 diabetes. Diabetes. 2003;52:448–452. doi: 10.2337/diabetes.52.2.448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.