Abstract
T-type, low-voltage activated, calcium channels, now designated Cav3 channels, are involved in a wide variety of physiological functions, especially in nervous systems. Their unique electrophysiological properties allow them to finely regulate neuronal excitability and to contribute to sensory processing, sleep, and hormone and neurotransmitter release. In the last two decades, genetic studies, including exploration of knock-out mouse models, have greatly contributed to elucidate the role of Cav3 channels in normal physiology, their regulation, and their implication in diseases. Mutations in genes encoding Cav3 channels (CACNA1G, CACNA1H, and CACNA1I) have been linked to a variety of neurodevelopmental, neurological, and psychiatric diseases designated here as neuronal Cav3 channelopathies. In this review, we describe and discuss the clinical findings and supporting in vitro and in vivo studies of the mutant channels, with a focus on de novo, gain-of-function missense mutations recently discovered in CACNA1G and CACNA1H. Overall, the studies of the Cav3 channelopathies help deciphering the pathogenic mechanisms of corresponding diseases and better delineate the properties and physiological roles Cav3 channels.
Keywords: Calcium channels, T-type, Calcium channelopathies, Epilepsy, Ataxia, Autism, Schizophrenia, Primary aldosteronism
Introduction
In the early 1980s, Llinas and Yarom [91] reported that hyperpolarization of inferior olivary neurons of the cerebellum could reveal a low-threshold Ca2+ conductance, which was inactivated at their resting membrane potential. The concept of low-voltage activated (LVA) Ca2+ current then arose in the neuroscience community as this conductance was described in many different types of neurons, including thalamic [90], sensory [19, 152], and hippocampal [165] neurons. This LVA Ca2+ current, also typical for its fast inactivation (Transient) and small unitary conductance (Tiny), was soon after named “T-type” [110, 112]. The unique voltage sensitivity of T-type Ca2+ channels is particularly well suited to regulate neuronal excitability and their oscillatory behavior near the resting membrane potential. A transient membrane hyperpolarization arising from inhibitory post-synaptic potentials (IPSPs) or activation of potassium (K+) channels deinactivates T-type channels. A subsequent rebound in the membrane potential triggers opening of T-type channels and favors a low-threshold spike (LTS) that initiates rebound burst firing (Fig. 1a). The role of T-type channels in bursting behavior is physiologically relevant, especially in sleep [5, 84] with the generation of sleep spindles. In the last two decades, following the molecular cloning of the Cav3 (T-type) channels in the 2000s, genetic studies have greatly contributed to elucidate the role of T-type channels in normal physiology, as well as to identify their implication in diseases. Notably, mutations in the genes encoding the Cav3 channels have been linked essentially to neurodevelopmental, neurological, and psychiatric diseases designated here as neuronal Cav3 channelopathies.
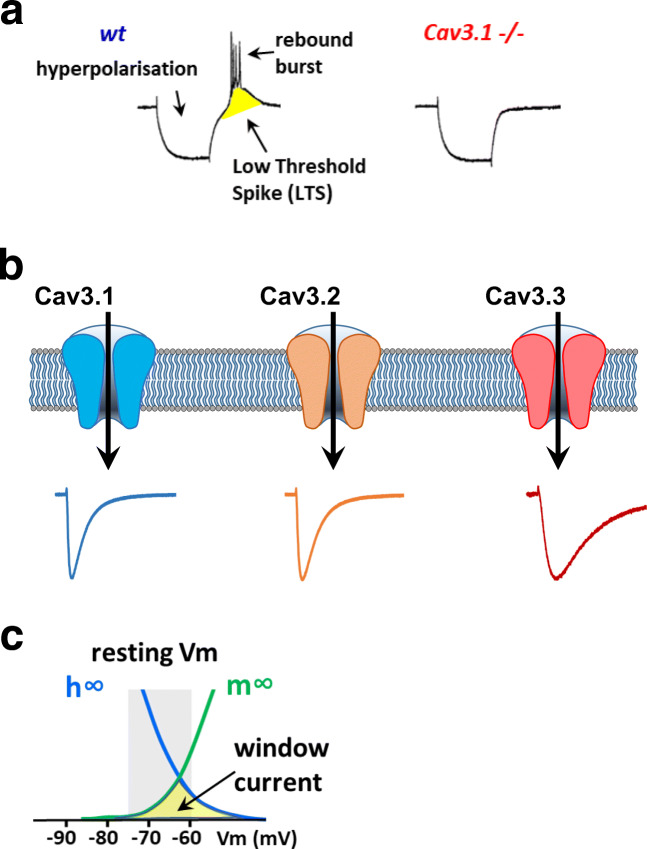
Fig. 1.
Electrophysiological properties of T-type/Ca3 channels. a Illustration of the implication of Cav3.1 channels in rebound burst firing in thalamocortical relay neurons, as reported in [81]. Hyperpolarization deinactivates T-type channels, which promotes low-threshold spike and rebound burst firing. This firing activity in completely lost in thalamocortical relay neurons from Cav3.1−/− mice (for details, see [81]). b Current traces for Cav3.1, Cav3.2, and Cav3.3 channels obtained in HEK-293 cells, illustrating their differences in inactivation kinetics (see [13, 28]). c Illustration of the Cav3 window current that occurs in the range of the resting membrane potential
Cav3 channels: from molecular to physiological diversity
Cav3 molecular diversity
Before the cloning era, a diversity within T-type channels was already proposed, based on observed differences in inactivation properties and sensitivity to blockers, such as nickel (Ni2+) ions [74]. The first cDNA coding for the pore-forming subunit of a T-type channel was cloned in 1998 by Perez-Reyes and colleagues [119]. This was followed by extensive molecular cloning of several paralogs (isoforms) and orthologs, mainly in vetebrates, leading to the actual landscape of three genes (CACNA1G, CACNA1H, and CACNA1I) encoding the α1 subunit of T-type channels, Cav3.1 (α1G), Cav3.2 (α1H), and Cav3.3 (α1I), respectively (Figs. 1, 2, and 3) (for representative reviews, see [117, 118, 158]). The distinctive features of T-type channels making them well suited to regulate excitability (low voltage range for activation, ion selectivity, fast kinetics for activation and inactivation) are conserved in the most early-diverging animals, such as in Trichoplax adhaerens, which expresses a single Cav3 channel [135]. Hence, genome survey in Salpingoeca rosetta indicates that Cav3 channels have emerged more than a billion years ago in an eukaryotic ancestor of choanoflagellates and metazoans [103].
Fig. 2.
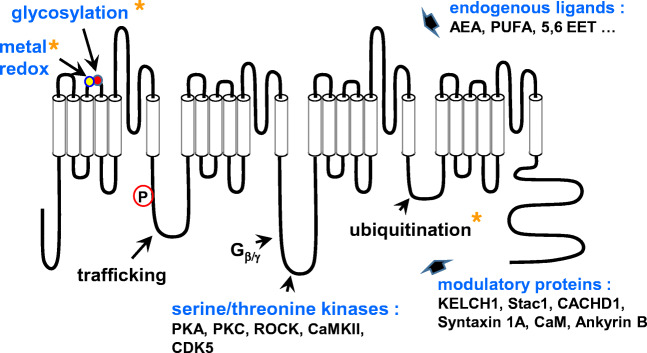
Schematic representation of the main Cav3 regulations (for previous reviews, see [29, 73, 75, 170]). The yellow asterisks point to the Cav3.2-selective regulations, including the metal/redox (His191, yellow circle) and glycosylation (Asn192, red circle) sites in S3–S4 extracellular linker of the domain I
Fig. 3.
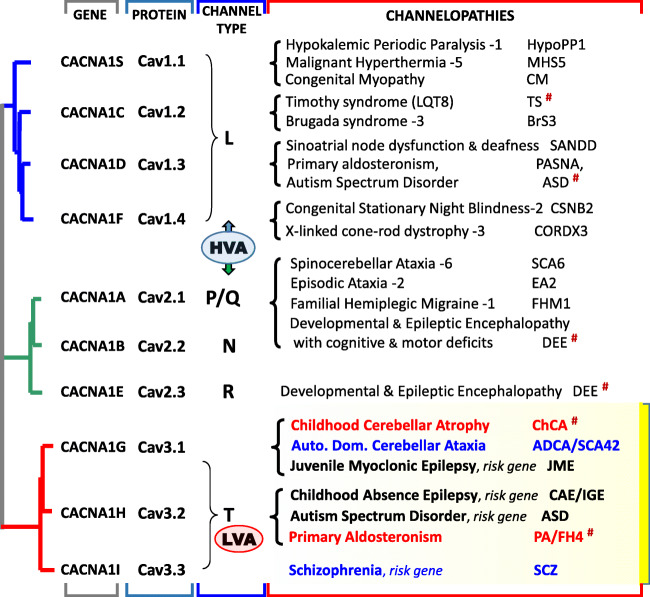
Cladogram representation of the Cav channel family including the gene names and the corresponding Cav subunits. HVA stands for high-voltage activated channels (L-, P/Q-, N-, and R-types) and LVA stands for low-voltage activated channels (T-type). The channelopathies column refers to the overall so-called Ca2+ channelopathies, with the detailed properties of the Cav3 channelopathies presented and discussed in the text. The diseases caused by mutations in the S6 segments of the corresponding Cav channels are indicated (#)
In mammals, the functional diversity in T-type channels arises not only from the three genes expressing Cav3 isoforms with distinct electrophysiological properties [13, 28] but also from several alternative splicing events [56, 98, 99, 118]. Alternative splicing can generate multiple variants from a single Cav3 isoform with significantly distinct electrophysiological properties and drug sensitivity [25, 26, 54, 83, 101, 105, 132, 172]. Also, alternative splicing can regulate the Cav3 channel expression at the plasma membrane [133]. Alternative splicing could contribute to the clinical severity of Cav3 channelopathies, as documented by in vitro studies showing that disease-associated mutations exhibit distinct electrophysiological properties when reproduced in different splice variants [66, 122].
The tissue-specific expression of the Cav3 channels is clearly important to consider when investigating their physiological roles, as well as their implication in disease phenotypes [131]. In mammals, all Cav3 channels are expressed early during development. In adult, the three Cav3 isoforms are expressed mainly in the central and peripheral nervous systems and also in neuroendocrine and cardiac tissues [101, 102]. Within the brain, in situ hybridization studies have shown that the three Cav3 isoforms display both specific and distinct patterns of expression [12, 144]. In addition, Cav3 splice variants can be expressed in a tissue/cell-specific manner and be developmentally regulated [118]. Until now, the lack of highly specific antibodies for any of the Cav3 isoforms/variants has hampered precise analysis of their tissue and cellular and subcellular distribution at the protein level [1, 100, 166], which was partly circumvented by the generation of knock-in (KI) animals carrying epitope-tagged Cav3 channels [8, 58].
Cav3 physiology
A hallmark of Cav3 channels is their unique ability to control neuronal excitability, requiring small membrane depolarizations to open (LVA), which distinguishes them from the high-voltage activated (HVA) channels [108, 168]. Their low threshold of voltage activation, coupled with their tonic inactivation near resting membrane potential, allows Cav3 channels to deinactivate and to underly the low-threshold spike/rebound bursting phenomenon seen in many types of neurons (Fig. 1a). The three Cav3 isoforms, which exhibit distinct electrophysiological properties [13, 28] (Fig. 1b), regulate differentially neuronal excitability [12, 39, 100]. In addition, the Ca2+ influx through Cav3 channels can also directly regulate intracellular Ca2+ concentrations [24, 51]. Indeed, all three Cav3 channels display an overlap of their steady-state inactivation and activation properties giving rise to a window current (Fig. 1c) that ressembles a “background” Ca2+ current [153]. It results from the activity of a small fraction of Cav3 channels remaining open in the voltage range near the resting membrane potential [34, 40]. The physiological role of this Cav3 window current is still poorly understood. It was shown to contribute to the slow oscillation in non-REM sleep [46].
Genetic manipulation of Cav3 expression in the mouse has provided significant information regarding the physiological roles of neuronal Cav3 channels and a quick overview of the findings obtained with Cav3 knock-out (KO) mouse models is provided here. In KO mice for Cacna1g (Cav3.1−/−), no LVA T-type current could be recorded in thalamocortical relay neurons and these neurons showed no burst firing activity [81] (Fig. 1a). In these animals, spike-and-wave discharges that occur in absence epilepsy models were prevented. The loss of thalamocortical oscillations was also observed in central medial nucleus, which reflects the overall importance of Cav3.1 channels in thalamic neurons [146]. Cav3.1−/− mice were less prone to develop tonic seizures in the maximal electroshock seizure test, compared with wt littermates and Cav3.2−/− mice, suggesting a prominent role of the Cav3.1 isoform in mediating tonic seizure [127]. Interestingly, overexpression of the Cav3.1 channel in a Cacna1g transgenic mouse line results in a pure absence epilepsy phenotype with no ataxia or other neurological disturbances [57], suggesting that an increase in Cav3.1 current is sufficient to the pathogenesis of spike-wave seizures. Cav3.1−/− animals display a deficit in motor performance and in cerebellar learning [23, 94] and are resistant to harmaline-induced tremor [115]. In these animals, the T-type current was also significantly reduced in the subiculum, which is involved in hippocampal-dependent cognitive processes [79].
The KO mice for Cacna1h (Cav3.2−/−) exhibit a variety of phenotypes including neurological deficits [36]. The nociceptive role of Cav3.2 channels, which are highly expressed in the dorsal root sensory neurons [10, 11, 136], was validated using these Cav3.2 KO animals [41, 147] and further established when Cav3.2 channels were selectively deleted in low-threshold mechanoreceptor primary afferent neurons [58]. In the brain, Cav3.2 is predominantly expressed in the dentate gyrus of the hippocampus [1, 8]. In Cav3.2−/− animals subjected to pilocarpine-induced status epilepticus, which models temporal lobe epilepsy, the appearance of epileptic seizures was strongly attenuated, validating the pro-epileptogenesis role of upregulated hippocampal Cav3.2 channels [7, 151]. In addition, Cav3.2−/− mice show elevated anxiety and impaired hippocampus-dependent contextual memory and learning [38, 59].
Inactivation of Cacna1i in the mouse (Cav3.3−/−) resulted in the loss of LVA Ca2+ currents in the thalamic reticular nucleus (nRT) neurons and revealed a role of Cav3.3 channels in sleep. Cav3.3 channels dominate nRT rhythmogenesis and play a role in sleep spindles, the electroencephalographic hallmark of non-rapid eye movement (NREM) sleep [5, 85]. Of note, experiments performed in double Cav3.3 and Cav3.2 KO mice revealed that the lack of Cav3.2 channels further aggravates neuronal, synaptic, and EEG deficits in the Cav3.3−/− background, indicating a role of Cav3.2 channels in regulating nRT excitability and rhythmogenesis [116, 145].
Cav3 modulation
There is no evidence for a requirement of protein-protein association to obtain “native-like” properties of T-type channels when the Cav3 proteins are expressed in heterologous systems, as the HEK-293 cell line. This is contrasting with HVA Ca2+ channels, the L-type Cav1.1 to Cav1.4, and neuronal Cav2.1 P/Q-type, Cav2.2 N-type and Cav2.3 R-type, which require the auxiliary α2/δ, β, and γ subunits for their proper expression and function (reviewed in [108, 168]). However, a regulatory role of several proteins was identified for Cav3 channels, including the HVA auxiliary subunits [52], Kelch-like 1 [3], Stac1 [126], or the putative “Ca2+ channel and chemotaxis receptor domain containing 1,” CACHD1 [43] (Fig. 2). Regulation of Cav3 channels by such endogenous proteins would more likely reflect the numerous signalling pathways targeting Cav3 channels, as reported for the G protein βγ-dimer [50, 162], calmodulin [33, 86], syntaxin-1A [159], and spectrin α/β and ankyrin B [61] (Fig. 2).
The fine tuning of the functional properties of Cav3 channels by a large variety of endogenous pathways and ligands is now well established [29, 73, 75, 170] (Fig. 2). One of the first endogenous modulations described for Cav3 channels was the inhibitory effect of the endocannabinoid anandamide [27]. Other bioactive lipids, including arachidonic acid [143], N-acyl ethanolamides and polyunsaturated fatty acids [31], or 5,6-EET [21], were shown to also inhibit Cav3 channels in the micromolar range through a direct interaction [32]. Cav3 channels are also modulated by phosphorylation pathways, including the serine/threonine kinases, PKA and PKC [30, 71], Ca2+/CaM-dependent protein kinase II (CaMKII) [4, 161], rho-associated kinase (ROCK) [76], CDK5 [63], exchange factor activated by cAMP (Epac) [111], and hypoxia-inducible factor (HIF) [18]. As demonstrated for Cav3.2 channels, the phosphorylation status greatly influences the gating properties [9]. Phosphorylation also regulates an activity-dependent Ca2+ inhibition recently discovered for Cav3 channels, especially Cav3.3 [22, 35]. Other post-translational modifications regulating Cav3 channels, here Cav3.2 channels, including ubiquitination [60] and glycosylation [114, 160] have also been described. These latter studies point out that Cav3 isoform-specific modulations exist and are important to investigate further as they represent physiologically relevant selective regulations (Fig. 2). Of interest, the metal/redox modulation of T-type channels is also Cav3.2-specific. Cav3.2 channels are selectively upregulated by reducing agents such as l-cysteine, while the oxidizing agent ascorbate produces Cav3.2 channel inhibition [148]. This redox regulation occurs through the metal-catalyzed oxidation of a histidine residue (His191 in the human isoform) localized in the extracellular S3–S4 linker of domain I of Cav3.2 channel [72, 80]. Cav3.2 channels are also preferentially inhibited by the trace metal zinc (Zn2+) with an IC50 in the submicromolar range (∼ 0.8 μM), which is 100- and 200-fold lower than Cav3.1 and Cav3.3 channels, respectively [149]. Further studies, using a KI mouse model, have demonstrated that His191 is important for fine tuning of neuronal excitability in dorsal root sensory neurons [156].
Cav3 channelopathies
CACNA1G/Cav3.1 in late-onset cerebellar ataxia ADCA/SCA42
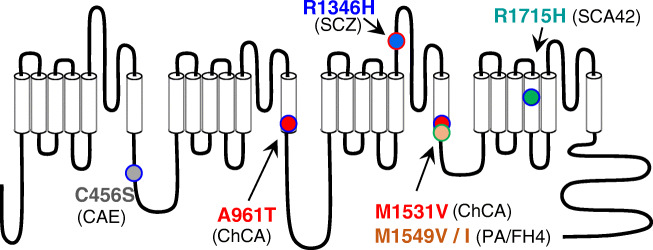
The Cav3.1 channel is highly expressed in the cerebellum, especially in Purkinje neurons [144], and was therefore a likely candidate for cerebellar disorders, especially ataxia. Hereditary cerebellar ataxias are rare neurodegenerative disorders, characterized by a cerebellar syndrome (gait alteration, limb incoordination, dysarthria, eye movement anomalies) with or without other neurological symptoms [45]. Using linkage analysis and whole-exome sequencing, CACNA1G was linked to an autosomal dominant cerebellar ataxia (ADCA) phenotype in three families, supporting its implication in spinocerebellar ataxia SCA42 [44] (Fig. 3). A recurrent missense mutation causing the p.Arg1715His substitution in the voltage sensor S4 segment of domain IV (IVS4, Fig. 4) of Cav3.1 was identified in these three unrelated pedigrees. This p.Arg1715His mutation affects the gating properties of the Cav3.1 channel with the steady-state activation properties shifted positively when expressed in HEK-293 cells. The expected reduction in channel activity was confirmed using computer modeling in deep cerebellar nuclei neurons that showed a decreased neuronal excitability. SCA42 is characterized by a slowly progressive ataxia with a variable onset but mainly in young adulthood. Athough the prevalence of SCA42 is very low, the association of this p.Arg1715His-Cav3.1 mutation with SCA42 was subsequently confirmed in Japanese and Chinese families [82, 88, 104, 109]. Additional CACNA1G missense mutations have been identified in other SCA42 patients, including p.Arg1068Cys, p.His1611Gln, and p.Pro2273His variants. However, they have shown no statistically significant electrophysiological effect in heterologous expression systems [44], while the variant p.Met1574Lys [88] has not been yet electrophysiologically explored. These additional CACNA1G variants clearly require further functional analysis to validate them as SCA42-causative mutations. Recently a KI mouse model of SCA42 was generated [65], harboring the above described mutation (p.Arg1723His in the mouse). Both heterozygous and homozygous KI mice demonstrated an adult-onset mild ataxia phenotype with comparable levels of motor impairment using rotarod and footprint tests, confirming the dominant inheritance of SCA42. Significant Purkinje neuron loss and degeneration of the molecular layer were also observed. This mouse model of SCA42 recapitulates well the observations made in SCA42 patients, as well as the electrophysiological analyses showing a positive shift of the voltage dependence of Cav3.1 channels [44]. Overall, this study confirms that SCA42 is caused by the p.Arg1715His mutation in Cav3.1 [65].
Fig. 4.
Schematic localization of the main Cav3 mutations described in the text, including (i) the Cav3.1 mutations: p.Arg1715His in SCA42 [44, 65], p.Arg961Thr and p.Met1531Val in ChCA [6, 34]; (ii) the Cav3.2 mutations: p.Arg1549Val and p.Arg1549Ile in PA/FH4 [47, 130], and p.Cys456Ser in CAE [53, 155]; and (iii) the Cav3.3 mutation: p.Arg1346His in SCZ [2, 62]
CACNA1G/Cav3.1 in childhood cerebellar atrophy
Another set of de novo CACNA1G mutations was identified in a cohort of infants with childhood cerebellar atrophy (ChCA), using a combination of candidate gene panel and whole-exome sequencing [34]. ChCA is a devastating infantile neurodevelopmental disorder, with severe motor and cognitive impairments, cerebellar atrophy, and variable features including facial dysmorphism, digital anomalies, microcephaly, hirsutism, and epilepsy. Two mutations were identified in four individuals, three patients carrying a p.Ala961Thr mutation (in the Cav3.1 IIS6 segment) and one patient carrying a p.Met1531Val mutation (in the Cav3.1 IIIS6 segment) [34] (Fig. 4). Both mutations drastically altered the channel gating properties, especially the inactivation properties, with a significant slowing of the inactivation kinetics (5 times) and a negative shift (− 10 mV) of the potential for half-inactivation. In addition, these two mutations promoted a much larger window current that was fully inhibited by TTA-P2, a selective Cav3 channel blocker [34]. Overall, this study has demonstrated that p.Ala961Thr and p.Met1531Val are gain-of-function mutations. Importantly, this first description of de novo dominant CACNA1G mutations causing ChCA was confirmed by a recent study reporting on the same mutations (p.Ala961Thr and p.Met1531Val), identified in three patients and in one patient, respectively [6], strengthening the recurrence of these mutations in ChCA. The devastating consequence of ChCA gain-of-function mutations in humans reveals that Cav3.1 channel has a critical role in setting up cerebellar physiology during development. Further studies are necessary to uncover the pathogenic mechanism underlying the ChCA condition and to establish more precisely the developmental and functional roles of Cav3.1 channels in the cerebellum.
CACNA1G/Cav3.1 in epilepsy
Because of their presence in cortical and thalamic structures and their role in modulating neuronal firing, T-type channels have always been considered candidates for idiopathic generalized epilepsies (IGEs). Interestingly, it was shown that Cacna1g is a genetic modifier of epilepsy in a mouse model of Dravet syndrome caused by mutations in the voltage-gated Na+ channel gene Scn1a [16], as well as a modifier in a Scn2a mouse model of focal epilepsy [15]. In humans, mutations in CACNA1G have been reported in juvenile myoclonic epilepsy patients [134]. However, the two reported missense mutations coding for p.Ala570Val and p.Ala1089Ser substitutions had no electrophysiological effect when explored in heterologous expression system, questioning their pathogenic status on a monogenic basis. A recent study has documented that IGEs have complex (oligogenic or multigenic) inheritance patterns with a likely combination of both common and rare genetic risk variants required to cause the disease. Among them, CACNA1G, carrying a high number of missense variants in IGEs samples, still represents a susceptibility gene [55].
CACNA1G/Cav3.1 in other neurological diseases
Potential disease-causing variants in CACNA1G have also been identified in intellectual disability/cognitive disorders [106] and monoallelic deletions of the CACNA1G gene have been associated with mild intellectual disability without cerebellum atrophy [123]. Also, CACNA1G was identified as a candidate gene for autism spectrum disorder (ASD) in a subset of cases [142] but the CACNA1G association with ASD has yet to be replicated in a larger study [48]. CACNA1G also appeared to be a candidate gene in essential tremor, one of the most common movement disorders, with CACNA1G variants identified in three families [113]. It is therefore likely that the clinical spectrum of diseases associated with CACNA1G mutations will increase in a near future.
CACNA1H/Cav3.2 in epilepsy
The CACNA1H gene, as CACNA1G, has received much attention regarding its potential implication in inherited epilepsy phenotypes. In some spontaneous mouse models of generalized epilepsy, the tottering (tg), lethargic (lh), and stargazer (stg) mouse strains, an increase in T-type current density was observed [169]. Strikingly, in the GAERS rat (Genetic Absence Epilepsy Rats from Strasbourg), a missense mutation, p.Arg1584Pro in Cacna1h, was found to co-segregate with the slow-wave discharge phenotype [150]. In heterologous expression system, this missense substitution in the intracellular loop linking the domains III to IV (LIII–IV) could induce a gain of channel activity when introduced in a Cav3.2 splice variant containing exon 25 [14, 122].
In humans, several studies have reported associations between CACNA1H single nucleotide polymorphisms (SNPs) and epileptic phenotypes, especially in idiopathic generalized epilepsy (IGE) [42, 69, 87, 138], reviewed in [158, 167], since the first report by Chen et al. [37] describing CACNA1H SNPs in childhood absence epilepsy (CAE) patients. Functional studies of several of these CACNA1H missense variants revealed that they could modify biophysical properties or protein trafficking of Cav3.2 in heterologous expression systems [69, 154, 155], in a loss- or gain-of-function manner. Many of these missense variants were found in the intracellular loop linking the domains I and II (LI–II) of Cav3.2 and one of them, p.Cys456Ser (Fig. 4), significantly increased spontaneous firing and reduced the threshold for rebound burst firing, when overexpressed in hippocampal neurons [53]. Yet, to date, none of these CACNA1H variants has been undoubtedly identified as causing seizure phenotypes. In other words, CACNA1H variants are not causing monogenic epilepsy [17]. As for CACNA1G, CACNA1H variants should be considered a risk factor for developing epilepsy, most likely implicating other genetic and/or environmental factors [67].
CACNA1H/Cav3.2 in other neurological diseases
Four missense variants in the CACNA1H gene were identified in six individuals with ASD and the corresponding Cav3.2 variants showed altered electrophysiological properties in heterologous expression [140]. Whether these CACNA1H variants segregate with the ASD phenotype remains to be validated as these variations have low penetrance and some of them were also found in unaffected individuals. Additional CACNA1H variants were reported in a patient with persistent pain [137] and in patients with amyotrophy lateral sclerosis [125, 141]. Again, further studies are needed to validate association of CACNA1H variation with these conditions.
CACNA1H/Cav3.2 in primary aldosteronism
Using whole-exome sequencing, Scholl et al. [130] identified a recurrent missense mutation, p.Met1549Val, in the CACNA1H gene in five unrelated patients from a cohort of patients diagnosed with primary aldosteronism (PA) in early childhood (Fig. 3). This point mutation resulted in a significant gain of Cav3.2 channel activity. Soon after, Daniil et al. [47] also performing whole-exome sequencing in PA patients reported another substitution at this residue, p.Met1549Val, with similar gain-of-function properties. Additional gain-of-function mutations, p.Ser196Leu, p.Pro2083Leu, and p.Val1951Glu, were also identified in this study [47]. If one patient was diagnosed with minor mental retardation and multiplex developmental disorder, other patients showed no apparent signs of seizures, cardiac arrhythmia, or muscular or neurological alterations. CACNA1H-related PA is now defined as familial hyperaldosteronism type 4 (FH4; [120]).
The Met1549 amino acid is located in highly conserved sequence of the IIIS6 segment of Cav3.2 that lines the inner part of the channel pore and is involved in channel inactivation [97] (Fig. 4), indeed at the same position as Met1531 in Cav3.1 (Fig. 3). The two pathogenic substitutions of Met1549 (Val and Ile) confer ultraslow inactivation kinetics, significant negative shift in the steady-state inactivation properties, and an increased window current. These data support an increase in channel activity and a rise in intracellular Ca2+ [47, 130]. Notably, all these mutants led to increased aldosterone production and increased expression of the genes coding for steroidogenic enzymes in the adrenocortical H295R cell line after K+ stimulation [47, 124]. Additional CACNA1H mutations causing PA/FH4 will likely be identified, as exemplified by the recent description of a p.Ile1430Thr substitution (IIIS5 segment), in an aldosterone-producing adenoma [107].
CACNA1I/Cav3.3 in neurological/psychiatric diseases
Genome-wide association studies (GWAS), as well as the identification of de novo variants in the CACNA1I gene, have contributed to implicate CACNA1I as a genetic risk factor in schizophrenia (SCZ) [64, 77, 129]. When expressed in the HEK-293 cell line, one of the two Cav3.3 missense variations identified in [64], p.Arg1346His (Fig. 4), resulted in a lower expression level of the Cav3.3 protein, a reduced N-glycosylation, and a reduced expression at the plasma membrane, reducing the Cav3.3 current but with no change in the electrophysiological properties [2]. A KI mouse model was generated using the CRISPR/Cas9 editing approach to introduce the p.Arg1305His orthologous mutation [62]. The homozygous animals show altered excitability in the nRT and deficits in sleep spindle occurrence and at NREM/REM transitions. This animal model will facilitate further investigations of the role of Cav3.3 channels in impaired sleep spindle and nRT function in SCZ. Additional CACNA1I variants have been identified in SCZ patients, confirming CACNA1I as a genetic risk factor in SCZ [95, 163, 164]. CACNA1I is also considered a risk gene in autism [93] and other complex neuropsychiatric disorders [128].
Conclusions and perspectives
De novo gain-of-function mutations in Cav3 channels: a wider group of S6-pathies?
These last years, many novel disease-related Cav3 channel variants have been reported and some of them are causing severe disorders. This is the case for the de novo gain-of-function mutations in Cav3.1 and Cav3.2 channels in childhood cerebellar atrophy (ChCA) and primary aldosteronism (PA/FH4), respectively. These deleterious missense mutations involve residues, Ala961 and Met1531 in Cav3.1 and Met1549 in Cav3.2, in the highly conserved S6 segments lining the inner part of the pore channel (Fig. 4). These residues were implicated in Cav3 channel inactivation in earlier structure-function studies [49, 97]. Notably, these “S6 mutations” in Cav3.1 and Cav3.2 are reminiscent to several de novo gain-of-function mutations recently described in other genes encoding Cav channels (Fig. 3). These other Cav “S6 mutations” also cause severe, mainly neurodevelopmental, clinical phenotypes. S6 mutations in CACNA1C, which encodes the Cav1.2 L-type channel, cause Timothy syndrome (TS), a congenital long-QT cardiac arrhythmia with or without severe neurological phenotypes, including autism and mental retardation [89, 139]. There are S6 mutations in CACNA1D, which encodes Cav1.3, another L-type channel, that causes a neurodevelopmental disorder including ASD, intellectual disability with or without neurological (hypotonia, epilepsy) and endocrine (primary aldosteronism or hyperinsulinemic hypoglycaemia) features (PASNA) [70, 121]. There are also S6 mutations in Cav2.3, the neuronal R-type channel encoded by CACNA1E, that causes developmental and epileptic encephalopathies (DEE) [68]. There is also recent evidence for de novo S6 mutations in CACNA1A, encoding the neuronal P/Q-type Cav2.1 channel, linked to severe DEE with intellectual disability and variable motor symptoms [78]. All these S6 missense mutations share functional features: they significantly impair the inactivation properties of the affected Cav channels, likely promoting increase in intracellular Ca2+ concentration and the subsequent cellular damages caused by abnormal Ca2+ homeostasis [96]. Considering the similarity in their pathogenic mechanism, we tentatively propose here to define this group of Ca2+ channelopathies as “S6-pathies.” Further studies, exploiting animal models of the corresponding channelopathies, will help to identify the pathogenic mechanisms underlying the diseases and better delineate the precise implication(s) of the corresponding Cav channels. Deciphering the role of Cav3.1 and Cav3.2 in ChCA and PA/FH4, respectively, should benefit from a combined effort of the “calcium channelopathy” community.
Structural studies of Cav3 channels: further deciphering of the disease mechanisms
High-resolution structural studies can provide atomic-level views of disease mechanisms [20]. Notably, the Cryo-EM structure of the Cav3.1 channel was recently reported [171], opening new opportunities to better understand the molecular and functional consequences of disease mutations in Cav3.1 channel, as well as in Cav3.2 and Cav3.3 channels by homology modeling. It is also anticipated that the pharmacology of Cav3 channels will benefit from the development of novel therapeutic approaches using structure-guided drug discovery. Cav3/T-type channels have always been considered promising pharmacological targets considering their implication in a wide variety of neurological conditions, including epilepsy and pain. However, until now, the clinical development of drugs targeting Cav3 channels has not been as successful as expected [92, 157], likely because of the wide tissue expression of the Cav3 channels, the lack of selective Cav3 channel blockers, and, beyond that, the lack of Cav3 isoform-specific blockers. Further studies should establish whether there is a therapeutic potential of Cav3 blockers in the treatment of ChCA and PA/FH4 diseases that are directly caused by increased activity of the Cav3.1 and Cav3.2 channels.
Acknowledgments
This work is supported by the Labex “Ion Channel Science and Therapeutics” (ICST), Fondation Maladies Rares (FMR), Association Française contre les Myopathies (AFM), and Fondation pour la Recherche Médicale (FRM).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
This article is part of the special issue on Channelopathies: from mutation to diseases in Pflügers Archiv—European Journal of Physiology
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aguado C, Garcia-Madrona S, Gil-Minguez M, Lujan R. Ontogenic changes and differential localization of T-type Ca(2+) channel subunits Cav3.1 and Cav3.2 in mouse hippocampus and cerebellum. Front Neuroanat. 2016;10:83. doi: 10.3389/fnana.2016.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrade A, Hope J, Allen A, Yorgan V, Lipscombe D, Pan JQ. A rare schizophrenia risk variant of CACNA1I disrupts CaV3.3 channel activity. Sci Rep. 2016;6:34233. doi: 10.1038/srep34233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aromolaran KA, Benzow KA, Cribbs LL, Koob MD, Piedras-Renteria ES. T-type current modulation by the actin-binding protein Kelch-like 1. Am J Physiol Cell Physiol. 2010;298:C1353–C1362. doi: 10.1152/ajpcell.00235.2009. [DOI] [PubMed] [Google Scholar]
- 4.Asmara H, Micu I, Rizwan AP, Sahu G, Simms BA, Zhang FX, Engbers JDT, Stys PK, Zamponi GW, Turner RW. A T-type channel-calmodulin complex triggers alphaCaMKII activation. Mol Brain. 2017;10:37. doi: 10.1186/s13041-017-0317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Astori S, Wimmer RD, Prosser HM, Corti C, Corsi M, Liaudet N, Volterra A, Franken P, Adelman JP, Luthi A. The Ca(V)3.3 calcium channel is the major sleep spindle pacemaker in thalamus. Proc Natl Acad Sci U S A. 2011;108:13823–13828. doi: 10.1073/pnas.1105115108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barresi S, Dentici ML, Manzoni F, Bellacchio E, Agolini E, Pizzi S, Ciolfi A, Tarnopolsky M, Brady L, Garone G, Novelli A, Mei D, Guerrini R, Capuano A, Pantaleoni C, Tartaglia M. Infantile-onset syndromic cerebellar ataxia and CACNA1G mutations. Pediatr Neurol. 2020;104:40–45. doi: 10.1016/j.pediatrneurol.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Becker AJ, Pitsch J, Sochivko D, Opitz T, Staniek M, Chen CC, Campbell KP, Schoch S, Yaari Y, Beck H. Transcriptional upregulation of Cav3.2 mediates epileptogenesis in the pilocarpine model of epilepsy. J Neurosci. 2008;28:13341–13353. doi: 10.1523/JNEUROSCI.1421-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernal Sierra YA, Haseleu J, Kozlenkov A, Begay V, Lewin GR. Genetic tracing of Cav3.2 T-type calcium channel expression in the peripheral nervous system. Front Mol Neurosci. 2017;10:70. doi: 10.3389/fnmol.2017.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blesneac I, Chemin J, Bidaud I, Huc-Brandt S, Vandermoere F, Lory P. Phosphorylation of the Cav3.2 T-type calcium channel directly regulates its gating properties. Proc Natl Acad Sci U S A. 2015;112:13705–13710. doi: 10.1073/pnas.1511740112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourinet E, Alloui A, Monteil A, Barrere C, Couette B, Poirot O, Pages A, McRory J, Snutch TP, Eschalier A, Nargeot J. Silencing of the Cav3.2 T-type calcium channel gene in sensory neurons demonstrates its major role in nociception. EMBO J. 2005;24:315–324. doi: 10.1038/sj.emboj.7600515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bourinet E, Francois A, Laffray S. T-type calcium channels in neuropathic pain. Pain. 2016;157(Suppl 1):S15–S22. doi: 10.1097/j.pain.0000000000000469. [DOI] [PubMed] [Google Scholar]
- 12.Broicher T, Kanyshkova T, Meuth P, Pape HC, Budde T. Correlation of T-channel coding gene expression, IT, and the low threshold Ca2+ spike in the thalamus of a rat model of absence epilepsy. Mol Cell Neurosci. 2008;39:384–399. doi: 10.1016/j.mcn.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Cain SM, Snutch TP. Contributions of T-type calcium channel isoforms to neuronal firing. Channels (Austin) 2010;4:475–482. doi: 10.4161/chan.4.6.14106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cain SM, Tyson JR, Choi HB, Ko R, Lin PJC, LeDue JM, Powell KL, Bernier LP, Rungta RL, Yang Y, Cullis PR, O’Brien TJ, MacVicar BA, Snutch TP. CaV 3.2 drives sustained burst-firing, which is critical for absence seizure propagation in reticular thalamic neurons. Epilepsia. 2018;59:778–791. doi: 10.1111/epi.14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calhoun JD, Hawkins NA, Zachwieja NJ, Kearney JA. Cacna1g is a genetic modifier of epilepsy caused by mutation of voltage-gated sodium channel Scn2a. Epilepsia. 2016;57:e103–e107. doi: 10.1111/epi.13390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calhoun JD, Hawkins NA, Zachwieja NJ, Kearney JA. Cacna1g is a genetic modifier of epilepsy in a mouse model of Dravet syndrome. Epilepsia. 2017;58:e111–e115. doi: 10.1111/epi.13811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calhoun JD, Huffman AM, Bellinski I, Kinsley L, Bachman E, Gerard E, Kearney JA, Carvill GL. CACNA1H variants are not a cause of monogenic epilepsy. Hum Mutat. 2020;41:1138–1144. doi: 10.1002/humu.24017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carabelli V, Marcantoni A, Comunanza V, de Luca A, Diaz J, Borges R, Carbone E. Chronic hypoxia up-regulates alpha1H T-type channels and low-threshold catecholamine secretion in rat chromaffin cells. J Physiol. 2007;584:149–165. doi: 10.1113/jphysiol.2007.132274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carbone E, Lux HD. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature. 1984;310:501–502. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- 20.Catterall WA, Lenaeus MJ, Gamal El-Din TM. Structure and pharmacology of voltage-gated sodium and calcium channels. Annu Rev Pharmacol Toxicol. 2020;60:133–154. doi: 10.1146/annurev-pharmtox-010818-021757. [DOI] [PubMed] [Google Scholar]
- 21.Cazade M, Bidaud I, Hansen PB, Lory P, Chemin J. 5,6-EET potently inhibits T-type calcium channels: implication in the regulation of the vascular tone. Pflugers Arch. 2014;466:1759–1768. doi: 10.1007/s00424-013-1411-0. [DOI] [PubMed] [Google Scholar]
- 22.Cazade M, Bidaud I, Lory P, Chemin J (2017) Activity-dependent regulation of T-type calcium channels by submembrane calcium ions. Elife 6. 10.7554/eLife.22331 [DOI] [PMC free article] [PubMed]
- 23.Chang KY, Park YG, Park HY, Homanics GE, Kim J, Kim D. Lack of CaV3.1 channels causes severe motor coordination defects and an age-dependent cerebellar atrophy in a genetic model of essential tremor. Biochem Biophys Res Commun. 2011;410:19–23. doi: 10.1016/j.bbrc.2011.05.082. [DOI] [PubMed] [Google Scholar]
- 24.Chemin J, Monteil A, Briquaire C, Richard S, Perez-Reyes E, Nargeot J, Lory P. Overexpression of T-type calcium channels in HEK-293 cells increases intracellular calcium without affecting cellular proliferation. FEBS Lett. 2000;478:166–172. doi: 10.1016/s0014-5793(00)01832-9. [DOI] [PubMed] [Google Scholar]
- 25.Chemin J, Monteil A, Bourinet E, Nargeot J, Lory P. Alternatively spliced alpha(1G) (Ca(V)3.1) intracellular loops promote specific T-type Ca(2+) channel gating properties. Biophys J. 2001;80:1238–1250. doi: 10.1016/S0006-3495(01)76100-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chemin J, Monteil A, Dubel S, Nargeot J, Lory P. The alpha1I T-type calcium channel exhibits faster gating properties when overexpressed in neuroblastoma/glioma NG 108-15 cells. Eur J Neurosci. 2001;14:1678–1686. doi: 10.1046/j.0953-816x.2001.01796.x. [DOI] [PubMed] [Google Scholar]
- 27.Chemin J, Monteil A, Perez-Reyes E, Nargeot J, Lory P. Direct inhibition of T-type calcium channels by the endogenous cannabinoid anandamide. EMBO J. 2001;20:7033–7040. doi: 10.1093/emboj/20.24.7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chemin J, Monteil A, Perez-Reyes E, Bourinet E, Nargeot J, Lory P. Specific contribution of human T-type calcium channel isotypes (alpha(1G), alpha(1H) and alpha(1I)) to neuronal excitability. J Physiol. 2002;540:3–14. doi: 10.1113/jphysiol.2001.013269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chemin J, Traboulsie A, Lory P. Molecular pathways underlying the modulation of T-type calcium channels by neurotransmitters and hormones. Cell Calcium. 2006;40:121–134. doi: 10.1016/j.ceca.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Chemin J, Mezghrani A, Bidaud I, Dupasquier S, Marger F, Barrere C, Nargeot J, Lory P. Temperature-dependent modulation of CaV3 T-type calcium channels by protein kinases C and A in mammalian cells. J Biol Chem. 2007;282:32710–32718. doi: 10.1074/jbc.M702746200. [DOI] [PubMed] [Google Scholar]
- 31.Chemin J, Nargeot J, Lory P. Chemical determinants involved in anandamide-induced inhibition of T-type calcium channels. J Biol Chem. 2007;282:2314–2323. doi: 10.1074/jbc.M610033200. [DOI] [PubMed] [Google Scholar]
- 32.Chemin J, Cazade M, Lory P. Modulation of T-type calcium channels by bioactive lipids. Pflugers Arch. 2014;466:689–700. doi: 10.1007/s00424-014-1467-5. [DOI] [PubMed] [Google Scholar]
- 33.Chemin J, Taiakina V, Monteil A, Piazza M, Guan W, Stephens RF, Kitmitto A, Pang ZP, Dolphin AC, Perez-Reyes E, Dieckmann T, Guillemette JG, Spafford JD. Calmodulin regulates Cav3 T-type channels at their gating brake. J Biol Chem. 2017;292:20010–20031. doi: 10.1074/jbc.M117.807925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chemin J, Siquier-Pernet K, Nicouleau M, Barcia G, Ahmad A, Medina-Cano D, Hanein S, Altin N, Hubert L, Bole-Feysot C, Fourage C, Nitschke P, Thevenon J, Rio M, Blanc P, Vidal C, Bahi-Buisson N, Desguerre I, Munnich A, Lyonnet S, Boddaert N, Fassi E, Shinawi M, Zimmerman H, Amiel J, Faivre L, Colleaux L, Lory P, Cantagrel V. De novo mutation screening in childhood-onset cerebellar atrophy identifies gain-of-function mutations in the CACNA1G calcium channel gene. Brain. 2018;141:1998–2013. doi: 10.1093/brain/awy145. [DOI] [PubMed] [Google Scholar]
- 35.Chemin J, Stamenic TT, Cazade M, Llinares J, Blesneac I, Todorovic SM, Lory P. A novel phospho-modulatory mechanism contributes to the calcium-dependent regulation of T-type Ca(2+) channels. Sci Rep. 2019;9:15642. doi: 10.1038/s41598-019-52194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen CC, Lamping KG, Nuno DW, Barresi R, Prouty SJ, Lavoie JL, Cribbs LL, England SK, Sigmund CD, Weiss RM, Williamson RA, Hill JA, Campbell KP. Abnormal coronary function in mice deficient in alpha1H T-type Ca2+ channels. Science. 2003;302:1416–1418. doi: 10.1126/science.1089268. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, Lu J, Pan H, Zhang Y, Wu H, Xu K, Liu X, Jiang Y, Bao X, Yao Z, Ding K, Lo WH, Qiang B, Chan P, Shen Y, Wu X. Association between genetic variation of CACNA1H and childhood absence epilepsy. Ann Neurol. 2003;54:239–243. doi: 10.1002/ana.10607. [DOI] [PubMed] [Google Scholar]
- 38.Chen CC, Shen JW, Chung NC, Min MY, Cheng SJ, Liu IY. Retrieval of context-associated memory is dependent on the Ca(v)3.2 T-type calcium channel. PLoS One. 2012;7:e29384. doi: 10.1371/journal.pone.0029384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheong E, Shin HS. T-type Ca2+ channels in absence epilepsy. Pflugers Arch. 2014;466:719–734. doi: 10.1007/s00424-014-1461-y. [DOI] [PubMed] [Google Scholar]
- 40.Chevalier M, Lory P, Mironneau C, Macrez N, Quignard JF. T-type CaV3.3 calcium channels produce spontaneous low-threshold action potentials and intracellular calcium oscillations. Eur J Neurosci. 2006;23:2321–2329. doi: 10.1111/j.1460-9568.2006.04761.x. [DOI] [PubMed] [Google Scholar]
- 41.Choi S, Na HS, Kim J, Lee J, Lee S, Kim D, Park J, Chen CC, Campbell KP, Shin HS. Attenuated pain responses in mice lacking Ca(V)3.2 T-type channels. Genes Brain Behav. 2007;6:425–431. doi: 10.1111/j.1601-183X.2006.00268.x. [DOI] [PubMed] [Google Scholar]
- 42.Chourasia N, Osso-Rivera H, Ghosh A, Von Allmen G, Koenig MK. Expanding the phenotypic spectrum of CACNA1H mutations. Pediatr Neurol. 2019;93:50–55. doi: 10.1016/j.pediatrneurol.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 43.Cottrell GS, Soubrane CH, Hounshell JA, Lin H, Owenson V, Rigby M, Cox PJ, Barker BS, Ottolini M, Ince S, Bauer CC, Perez-Reyes E, Patel MK, Stevens EB, Stephens GJ. CACHD1 is an alpha2delta-like protein that modulates CaV3 voltage-gated calcium channel activity. J Neurosci. 2018;38:9186–9201. doi: 10.1523/JNEUROSCI.3572-15.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coutelier M, Blesneac I, Monteil A, Monin ML, Ando K, Mundwiller E, Brusco A, Le Ber I, Anheim M, Castrioto A, Duyckaerts C, Brice A, Durr A, Lory P, Stevanin G. A recurrent mutation in CACNA1G alters Cav3.1 T-type calcium-channel conduction and causes autosomal-dominant cerebellar ataxia. Am J Hum Genet. 2015;97:726–737. doi: 10.1016/j.ajhg.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coutelier M, Coarelli G, Monin ML, Konop J, Davoine CS, Tesson C, Valter R, Anheim M, Behin A, Castelnovo G, Charles P, David A, Ewenczyk C, Fradin M, Goizet C, Hannequin D, Labauge P, Riant F, Sarda P, Sznajer Y, Tison F, Ullmann U, Van Maldergem L, Mochel F, Brice A, Stevanin G, Durr A, network S A panel study on patients with dominant cerebellar ataxia highlights the frequency of channelopathies. Brain. 2017;140:1579–1594. doi: 10.1093/brain/awx081. [DOI] [PubMed] [Google Scholar]
- 46.Crunelli V, Lorincz ML, Connelly WM, David F, Hughes SW, Lambert RC, Leresche N, Errington AC. Dual function of thalamic low-vigilance state oscillations: rhythm-regulation and plasticity. Nat Rev Neurosci. 2018;19:107–118. doi: 10.1038/nrn.2017.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daniil G, Fernandes-Rosa FL, Chemin J, Blesneac I, Beltrand J, Polak M, Jeunemaitre X, Boulkroun S, Amar L, Strom TM, Lory P, Zennaro MC. CACNA1H mutations are associated with different forms of primary aldosteronism. EBioMedicine. 2016;13:225–236. doi: 10.1016/j.ebiom.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deciphering Developmental Disorders S. Large-scale discovery of novel genetic causes of developmental disorders. Nature. 2015;519:223–228. doi: 10.1038/nature14135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Demers-Giroux PO, Bourdin B, Sauve R, Parent L. Cooperative activation of the T-type CaV3.2 channel: interaction between domains II and III. J Biol Chem. 2013;288:29281–29293. doi: 10.1074/jbc.M113.500975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DePuy SD, Yao J, Hu C, McIntire W, Bidaud I, Lory P, Rastinejad F, Gonzalez C, Garrison JC, Barrett PQ. The molecular basis for T-type Ca2+ channel inhibition by G protein beta2gamma2 subunits. Proc Natl Acad Sci U S A. 2006;103:14590–14595. doi: 10.1073/pnas.0603945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dreyfus FM, Tscherter A, Errington AC, Renger JJ, Shin HS, Uebele VN, Crunelli V, Lambert RC, Leresche N. Selective T-type calcium channel block in thalamic neurons reveals channel redundancy and physiological impact of I(T)window. J Neurosci. 2010;30:99–109. doi: 10.1523/JNEUROSCI.4305-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dubel SJ, Altier C, Chaumont S, Lory P, Bourinet E, Nargeot J. Plasma membrane expression of T-type calcium channel alpha(1) subunits is modulated by high voltage-activated auxiliary subunits. J Biol Chem. 2004;279:29263–29269. doi: 10.1074/jbc.M313450200. [DOI] [PubMed] [Google Scholar]
- 53.Eckle VS, Shcheglovitov A, Vitko I, Dey D, Yap CC, Winckler B, Perez-Reyes E. Mechanisms by which a CACNA1H mutation in epilepsy patients increases seizure susceptibility. J Physiol. 2014;592:795–809. doi: 10.1113/jphysiol.2013.264176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Emerick MC, Stein R, Kunze R, McNulty MM, Regan MR, Hanck DA, Agnew WS. Profiling the array of Ca(v)3.1 variants from the human T-type calcium channel gene CACNA1G: alternative structures, developmental expression, and biophysical variations. Proteins. 2006;64:320–342. doi: 10.1002/prot.20877. [DOI] [PubMed] [Google Scholar]
- 55.Epi25 Collaborative. Electronic address sbuea. Epi C Ultra-rare genetic variation in the epilepsies: a whole-exome sequencing study of 17,606 individuals. Am J Hum Genet. 2019;105:267–282. doi: 10.1016/j.ajhg.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ernst WL, Noebels JL. Expanded alternative splice isoform profiling of the mouse Cav3.1/alpha1G T-type calcium channel. BMC Mol Biol. 2009;10:53. doi: 10.1186/1471-2199-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ernst WL, Zhang Y, Yoo JW, Ernst SJ, Noebels JL. Genetic enhancement of thalamocortical network activity by elevating alpha 1g-mediated low-voltage-activated calcium current induces pure absence epilepsy. J Neurosci. 2009;29:1615–1625. doi: 10.1523/JNEUROSCI.2081-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Francois A, Schuetter N, Laffray S, Sanguesa J, Pizzoccaro A, Dubel S, Mantilleri A, Nargeot J, Noel J, Wood JN, Moqrich A, Pongs O, Bourinet E. The low-threshold calcium channel Cav3.2 determines low-threshold mechanoreceptor function. Cell Rep. 2015;10:370–382. doi: 10.1016/j.celrep.2014.12.042. [DOI] [PubMed] [Google Scholar]
- 59.Gangarossa G, Laffray S, Bourinet E, Valjent E. T-type calcium channel Cav3.2 deficient mice show elevated anxiety, impaired memory and reduced sensitivity to psychostimulants. Front Behav Neurosci. 2014;8:92. doi: 10.3389/fnbeh.2014.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garcia-Caballero A, Gadotti VM, Stemkowski P, Weiss N, Souza IA, Hodgkinson V, Bladen C, Chen L, Hamid J, Pizzoccaro A, Deage M, Francois A, Bourinet E, Zamponi GW. The deubiquitinating enzyme USP5 modulates neuropathic and inflammatory pain by enhancing Cav3.2 channel activity. Neuron. 2014;83:1144–1158. doi: 10.1016/j.neuron.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 61.Garcia-Caballero A, Zhang FX, Hodgkinson V, Huang J, Chen L, Souza IA, Cain S, Kass J, Alles S, Snutch TP, Zamponi GW. T-type calcium channels functionally interact with spectrin (alpha/beta) and ankyrin B. Mol Brain. 2018;11:24. doi: 10.1186/s13041-018-0368-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghoshal A, Uygun DS, Yang L, McNally JM, Lopez-Huerta VG, Arias-Garcia MA, Baez-Nieto D, Allen A, Fitzgerald M, Choi S, Zhang Q, Hope JM, Yan K, Mao X, Nicholson TB, Imaizumi K, Fu Z, Feng G, Brown RE, Strecker RE, Purcell SM, Pan JQ. Effects of a patient-derived de novo coding alteration of CACNA1I in mice connect a schizophrenia risk gene with sleep spindle deficits. Transl Psychiatry. 2020;10:29. doi: 10.1038/s41398-020-0685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gomez K, Calderon-Rivera A, Sandoval A, Gonzalez-Ramirez R, Vargas-Parada A, Ojeda-Alonso J, Granados-Soto V, Delgado-Lezama R, Felix R. Cdk5-dependent phosphorylation of CaV3.2 T-type channels: possible role in nerve ligation-induced neuropathic allodynia and the compound action potential in primary afferent C fibers. J Neurosci. 2020;40:283–296. doi: 10.1523/JNEUROSCI.0181-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gulsuner S, Walsh T, Watts AC, Lee MK, Thornton AM, Casadei S, Rippey C, Shahin H, Consortium on the Genetics of S. Group PS. Nimgaonkar VL, Go RC, Savage RM, Swerdlow NR, Gur RE, Braff DL, King MC, McClellan JM. Spatial and temporal mapping of de novo mutations in schizophrenia to a fetal prefrontal cortical network. Cell. 2013;154:518–529. doi: 10.1016/j.cell.2013.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hashiguchi S, Doi H, Kunii M, Nakamura Y, Shimuta M, Suzuki E, Koyano S, Okubo M, Kishida H, Shiina M, Ogata K, Hirashima F, Inoue Y, Kubota S, Hayashi N, Nakamura H, Takahashi K, Katsumoto A, Tada M, Tanaka K, Sasaoka T, Miyatake S, Miyake N, Saitsu H, Sato N, Ozaki K, Ohta K, Yokota T, Mizusawa H, Mitsui J, Ishiura H, Yoshimura J, Morishita S, Tsuji S, Takeuchi H, Ishikawa K, Matsumoto N, Ishikawa T, Tanaka F. Ataxic phenotype with altered CaV3.1 channel property in a mouse model for spinocerebellar ataxia 42. Neurobiol Dis. 2019;130:104516. doi: 10.1016/j.nbd.2019.104516. [DOI] [PubMed] [Google Scholar]
- 66.He M, Xu Z, Zhang Y, Hu C. Splice-variant-specific effects of primary aldosteronism point mutations on human CaV3.2 calcium channels. Cell Calcium. 2019;84:102104. doi: 10.1016/j.ceca.2019.102104. [DOI] [PubMed] [Google Scholar]
- 67.Helbig I, Riggs ER, Barry CA, Klein KM, Dyment D, Thaxton C, Sadikovic B, Sands TT, Wagnon JL, Liaquat K, Cilio MR, Mirzaa G, Park K, Axeen E, Butler E, Bardakjian TM, Striano P, Poduri A, Siegert RK, Grant AR, Helbig KL, Mefford HC. The ClinGen Epilepsy Gene Curation Expert Panel-bridging the divide between clinical domain knowledge and formal gene curation criteria. Hum Mutat. 2018;39:1476–1484. doi: 10.1002/humu.23632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Helbig KL, Lauerer RJ, Bahr JC, Souza IA, Myers CT, Uysal B, Schwarz N, Gandini MA, Huang S, Keren B, Mignot C, Afenjar A, Billette de Villemeur T, Heron D, Nava C, Valence S, Buratti J, Fagerberg CR, Soerensen KP, Kibaek M, Kamsteeg EJ, Koolen DA, Gunning B, Schelhaas HJ, Kruer MC, Fox J, Bakhtiari S, Jarrar R, Padilla-Lopez S, Lindstrom K, Jin SC, Zeng X, Bilguvar K, Papavasileiou A, Xing Q, Zhu C, Boysen K, Vairo F, Lanpher BC, Klee EW, Tillema JM, Payne ET, Cousin MA, Kruisselbrink TM, Wick MJ, Baker J, Haan E, Smith N, Sadeghpour A, Davis EE, Katsanis N, Task Force for Neonatal G. Corbett MA, MacLennan AH, Gecz J, Biskup S, Goldmann E, Rodan LH, Kichula E, Segal E, Jackson KE, Asamoah A, Dimmock D, McCarrier J, Botto LD, Filloux F, Tvrdik T, Cascino GD, Klingerman S, Neumann C, Wang R, Jacobsen JC, Nolan MA, Snell RG, Lehnert K, Sadleir LG, Anderlid BM, Kvarnung M, Guerrini R, Friez MJ, Lyons MJ, Leonhard J, Kringlen G, Casas K, El Achkar CM, Smith LA, Rotenberg A, Poduri A, Sanchis-Juan A, Carss KJ, Rankin J, Zeman A, Raymond FL, Blyth M, Kerr B, Ruiz K, Urquhart J, Hughes I, Banka S, Deciphering Developmental Disorders S. UBS H, Scheffer IE, Helbig I, Zamponi GW, Lerche H, Mefford HC. De novo pathogenic variants in CACNA1E cause developmental and epileptic encephalopathy with contractures, macrocephaly, and dyskinesias. Am J Hum Genet. 2018;103:666–678. doi: 10.1016/j.ajhg.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heron SE, Khosravani H, Varela D, Bladen C, Williams TC, Newman MR, Scheffer IE, Berkovic SF, Mulley JC, Zamponi GW. Extended spectrum of idiopathic generalized epilepsies associated with CACNA1H functional variants. Ann Neurol. 2007;62:560–568. doi: 10.1002/ana.21169. [DOI] [PubMed] [Google Scholar]
- 70.Hofer NT, Tuluc P, Ortner NJ, Nikonishyna YV, Fernandes-Quintero ML, Liedl KR, Flucher BE, Cox H, Striessnig J. Biophysical classification of a CACNA1D de novo mutation as a high-risk mutation for a severe neurodevelopmental disorder. Mol Autism. 2020;11:4. doi: 10.1186/s13229-019-0310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hu C, Depuy SD, Yao J, McIntire WE, Barrett PQ. Protein kinase A activity controls the regulation of T-type CaV3.2 channels by Gbetagamma dimers. J Biol Chem. 2009;284:7465–7473. doi: 10.1074/jbc.M808049200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang D, Shi S, Liang C, Zhang X, Du X, An H, Peers C, Zhang H, Gamper N. Delineating an extracellular redox-sensitive module in T-type Ca(2+) channels. J Biol Chem. 2020;295:6177–6186. doi: 10.1074/jbc.RA120.012668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huc S, Monteil A, Bidaud I, Barbara G, Chemin J, Lory P. Regulation of T-type calcium channels: signalling pathways and functional implications. Biochim Biophys Acta. 2009;1793:947–952. doi: 10.1016/j.bbamcr.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 74.Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol. 1996;58:329–348. doi: 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
- 75.Iftinca MC, Zamponi GW. Regulation of neuronal T-type calcium channels. Trends Pharmacol Sci. 2009;30:32–40. doi: 10.1016/j.tips.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 76.Iftinca M, Hamid J, Chen L, Varela D, Tadayonnejad R, Altier C, Turner RW, Zamponi GW. Regulation of T-type calcium channels by rho-associated kinase. Nat Neurosci. 2007;10:854–860. doi: 10.1038/nn1921. [DOI] [PubMed] [Google Scholar]
- 77.Irish Schizophrenia Genomics C, the Wellcome Trust Case Control C Genome-wide association study implicates HLA-C*01:02 as a risk factor at the major histocompatibility complex locus in schizophrenia. Biol Psychiatry. 2012;72:620–628. doi: 10.1016/j.biopsych.2012.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jiang X, Raju PK, D’Avanzo N, Lachance M, Pepin J, Dubeau F, Mitchell WG, Bello-Espinosa LE, Pierson TM, Minassian BA, Lacaille JC, Rossignol E. Both gain-of-function and loss-of-function de novo CACNA1A mutations cause severe developmental epileptic encephalopathies in the spectrum of Lennox-Gastaut syndrome. Epilepsia. 2019;60:1881–1894. doi: 10.1111/epi.16316. [DOI] [PubMed] [Google Scholar]
- 79.Joksimovic SM, Eggan P, Izumi Y, Joksimovic SL, Tesic V, Dietz RM, Orfila JE, DiGruccio MR, Herson PS, Jevtovic-Todorovic V, Zorumski CF, Todorovic SM. The role of T-type calcium channels in the subiculum: to burst or not to burst? J Physiol. 2017;595:6327–6348. doi: 10.1113/JP274565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kang HW, Vitko I, Lee SS, Perez-Reyes E, Lee JH. Structural determinants of the high affinity extracellular zinc binding site on Cav3.2 T-type calcium channels. J Biol Chem. 2010;285:3271–3281. doi: 10.1074/jbc.M109.067660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim D, Song I, Keum S, Lee T, Jeong MJ, Kim SS, McEnery MW, Shin HS. Lack of the burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking alpha(1G) T-type Ca(2+) channels. Neuron. 2001;31:35–45. doi: 10.1016/s0896-6273(01)00343-9. [DOI] [PubMed] [Google Scholar]
- 82.Kimura M, Yabe I, Hama Y, Eguchi K, Ura S, Tsuzaka K, Tsuji S, Sasaki H. SCA42 mutation analysis in a case series of Japanese patients with spinocerebellar ataxia. J Hum Genet. 2017;62:857–859. doi: 10.1038/jhg.2017.51. [DOI] [PubMed] [Google Scholar]
- 83.Latour I, Louw DF, Beedle AM, Hamid J, Sutherland GR, Zamponi GW. Expression of T-type calcium channel splice variants in human glioma. Glia. 2004;48:112–119. doi: 10.1002/glia.20063. [DOI] [PubMed] [Google Scholar]
- 84.Lee J, Kim D, Shin HS. Lack of delta waves and sleep disturbances during non-rapid eye movement sleep in mice lacking alpha1G-subunit of T-type calcium channels. Proc Natl Acad Sci U S A. 2004;101:18195–18199. doi: 10.1073/pnas.0408089101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee SE, Lee J, Latchoumane C, Lee B, Oh SJ, Saud ZA, Park C, Sun N, Cheong E, Chen CC, Choi EJ, Lee CJ, Shin HS. Rebound burst firing in the reticular thalamus is not essential for pharmacological absence seizures in mice. Proc Natl Acad Sci U S A. 2014;111:11828–11833. doi: 10.1073/pnas.1408609111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee N, Jeong S, Kim KC, Kim JA, Park JY, Kang HW, Perez-Reyes E, Lee JH. Ca(2+) regulation of Cav3.3 T-type Ca(2+) channel is mediated by calmodulin. Mol Pharmacol. 2017;92:347–357. doi: 10.1124/mol.117.108530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee CG, Lee J, Lee M. Multi-gene panel testing in Korean patients with common genetic generalized epilepsy syndromes. PLoS One. 2018;13:e0199321. doi: 10.1371/journal.pone.0199321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li X, Zhou C, Cui L, Zhu L, Du H, Liu J, Wang C, Fang S. A case of a novel CACNA1G mutation from a Chinese family with SCA42: a case report and literature review. Medicine (Baltimore) 2018;97:e12148. doi: 10.1097/MD.0000000000012148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liao P, Soong TW. CaV1.2 channelopathies: from arrhythmias to autism, bipolar disorder, and immunodeficiency. Pflugers Arch. 2010;460:353–359. doi: 10.1007/s00424-009-0753-0. [DOI] [PubMed] [Google Scholar]
- 90.Llinas R, Jahnsen H. Electrophysiology of mammalian thalamic neurones in vitro. Nature. 1982;297:406–408. doi: 10.1038/297406a0. [DOI] [PubMed] [Google Scholar]
- 91.Llinas R, Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. J Physiol. 1981;315:549–567. doi: 10.1113/jphysiol.1981.sp013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lory P, Chemin J. Towards the discovery of novel T-type calcium channel blockers. Expert Opin Ther Targets. 2007;11:717–722. doi: 10.1517/14728222.11.5.717. [DOI] [PubMed] [Google Scholar]
- 93.Lu AT, Dai X, Martinez-Agosto JA, Cantor RM. Support for calcium channel gene defects in autism spectrum disorders. Mol Autism. 2012;3:18. doi: 10.1186/2040-2392-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ly R, Bouvier G, Schonewille M, Arabo A, Rondi-Reig L, Lena C, Casado M, De Zeeuw CI, Feltz A. T-type channel blockade impairs long-term potentiation at the parallel fiber-Purkinje cell synapse and cerebellar learning. Proc Natl Acad Sci U S A. 2013;110:20302–20307. doi: 10.1073/pnas.1311686110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Manoach DS, Pan JQ, Purcell SM, Stickgold R. Reduced sleep spindles in schizophrenia: a treatable endophenotype that links risk genes to impaired cognition? Biol Psychiatry. 2016;80:599–608. doi: 10.1016/j.biopsych.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mark MD, Schwitalla JC, Groemmke M, Herlitze S. Keeping our calcium in balance to maintain our balance. Biochem Biophys Res Commun. 2017;483:1040–1050. doi: 10.1016/j.bbrc.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 97.Marksteiner R, Schurr P, Berjukow S, Margreiter E, Perez-Reyes E, Hering S. Inactivation determinants in segment IIIS6 of Ca(v)3.1. J Physiol. 2001;537:27–34. doi: 10.1111/j.1469-7793.2001.0027k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mittman S, Guo J, Agnew WS. Structure and alternative splicing of the gene encoding alpha1G, a human brain T calcium channel alpha1 subunit. Neurosci Lett. 1999;274:143–146. doi: 10.1016/s0304-3940(99)00716-8. [DOI] [PubMed] [Google Scholar]
- 99.Mittman S, Guo J, Emerick MC, Agnew WS. Structure and alternative splicing of the gene encoding alpha1I, a human brain T calcium channel alpha1 subunit. Neurosci Lett. 1999;269:121–124. doi: 10.1016/s0304-3940(99)00319-5. [DOI] [PubMed] [Google Scholar]
- 100.Molineux ML, McRory JE, McKay BE, Hamid J, Mehaffey WH, Rehak R, Snutch TP, Zamponi GW, Turner RW. Specific T-type calcium channel isoforms are associated with distinct burst phenotypes in deep cerebellar nuclear neurons. Proc Natl Acad Sci U S A. 2006;103:5555–5560. doi: 10.1073/pnas.0601261103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Monteil A, Chemin J, Bourinet E, Mennessier G, Lory P, Nargeot J. Molecular and functional properties of the human alpha(1G) subunit that forms T-type calcium channels. J Biol Chem. 2000;275:6090–6100. doi: 10.1074/jbc.275.9.6090. [DOI] [PubMed] [Google Scholar]
- 102.Monteil A, Chemin J, Leuranguer V, Altier C, Mennessier G, Bourinet E, Lory P, Nargeot J. Specific properties of T-type calcium channels generated by the human alpha 1I subunit. J Biol Chem. 2000;275:16530–16535. doi: 10.1074/jbc.C000090200. [DOI] [PubMed] [Google Scholar]
- 103.Moran Y, Zakon HH. The evolution of the four subunits of voltage-gated calcium channels: ancient roots, increasing complexity, and multiple losses. Genome Biol Evol. 2014;6:2210–2217. doi: 10.1093/gbe/evu177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morino H, Matsuda Y, Muguruma K, Miyamoto R, Ohsawa R, Ohtake T, Otobe R, Watanabe M, Maruyama H, Hashimoto K, Kawakami H. A mutation in the low voltage-gated calcium channel CACNA1G alters the physiological properties of the channel, causing spinocerebellar ataxia. Mol Brain. 2015;8:89. doi: 10.1186/s13041-015-0180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Murbartian J, Arias JM, Lee JH, Gomora JC, Perez-Reyes E. Alternative splicing of the rat Ca(v)3.3 T-type calcium channel gene produces variants with distinct functional properties(1) FEBS Lett. 2002;528:272–278. doi: 10.1016/s0014-5793(02)03341-0. [DOI] [PubMed] [Google Scholar]
- 106.Najmabadi H, Hu H, Garshasbi M, Zemojtel T, Abedini SS, Chen W, Hosseini M, Behjati F, Haas S, Jamali P, Zecha A, Mohseni M, Puttmann L, Vahid LN, Jensen C, Moheb LA, Bienek M, Larti F, Mueller I, Weissmann R, Darvish H, Wrogemann K, Hadavi V, Lipkowitz B, Esmaeeli-Nieh S, Wieczorek D, Kariminejad R, Firouzabadi SG, Cohen M, Fattahi Z, Rost I, Mojahedi F, Hertzberg C, Dehghan A, Rajab A, Banavandi MJ, Hoffer J, Falah M, Musante L, Kalscheuer V, Ullmann R, Kuss AW, Tzschach A, Kahrizi K, Ropers HH. Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature. 2011;478:57–63. doi: 10.1038/nature10423. [DOI] [PubMed] [Google Scholar]
- 107.Nanba K, Blinder AR, Rege J, Hattangady NG, Else T, Liu CJ, Tomlins SA, Vats P, Kumar-Sinha C, Giordano TJ, Rainey WE. Somatic CACNA1H mutation as a cause of aldosterone-producing adenoma. Hypertension. 2020;75:645–649. doi: 10.1161/HYPERTENSIONAHA.119.14349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nanou E, Catterall WA. Calcium channels, synaptic plasticity, and neuropsychiatric disease. Neuron. 2018;98:466–481. doi: 10.1016/j.neuron.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 109.Ngo K, Aker M, Petty LE, Chen J, Cavalcanti F, Nelson AB, Hassin-Baer S, Geschwind MD, Perlman S, Italiano D, Lagana A, Cavallaro S, Coppola G, Below JE, Fogel BL. Expanding the global prevalence of spinocerebellar ataxia type 42. Neurol Genet. 2018;4:e232. doi: 10.1212/NXG.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nilius B, Hess P, Lansman JB, Tsien RW. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985;316:443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- 111.Novara M, Baldelli P, Cavallari D, Carabelli V, Giancippoli A, Carbone E. Exposure to cAMP and beta-adrenergic stimulation recruits Ca(V)3 T-type channels in rat chromaffin cells through Epac cAMP-receptor proteins. J Physiol. 2004;558:433–449. doi: 10.1113/jphysiol.2004.061184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nowycky MC, Fox AP, Tsien RW. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985;316:440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- 113.Odgerel Z, Sonti S, Hernandez N, Park J, Ottman R, Louis ED, Clark LN. Whole genome sequencing and rare variant analysis in essential tremor families. PLoS One. 2019;14:e0220512. doi: 10.1371/journal.pone.0220512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Orestes P, Osuru HP, McIntire WE, Jacus MO, Salajegheh R, Jagodic MM, Choe W, Lee J, Lee SS, Rose KE, Poiro N, Digruccio MR, Krishnan K, Covey DF, Lee JH, Barrett PQ, Jevtovic-Todorovic V, Todorovic SM. Reversal of neuropathic pain in diabetes by targeting glycosylation of Ca(V)3.2 T-type calcium channels. Diabetes. 2013;62:3828–3838. doi: 10.2337/db13-0813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Park YG, Park HY, Lee CJ, Choi S, Jo S, Choi H, Kim YH, Shin HS, Llinas RR, Kim D. Ca(V)3.1 is a tremor rhythm pacemaker in the inferior olive. Proc Natl Acad Sci U S A. 2010;107:10731–10736. doi: 10.1073/pnas.1002995107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pellegrini C, Lecci S, Luthi A, Astori S. Suppression of sleep spindle rhythmogenesis in mice with deletion of CaV3.2 and CaV3.3 T-type Ca(2+) channels. Sleep. 2016;39:875–885. doi: 10.5665/sleep.5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- 118.Perez-Reyes E, Lory P. Molecular biology of T-type calcium channels. CNS Neurol Disord Drug Targets. 2006;5:605–609. doi: 10.2174/187152706779025508. [DOI] [PubMed] [Google Scholar]
- 119.Perez-Reyes E, Cribbs LL, Daud A, Lacerda AE, Barclay J, Williamson MP, Fox M, Rees M, Lee JH. Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature. 1998;391:896–900. doi: 10.1038/36110. [DOI] [PubMed] [Google Scholar]
- 120.Perez-Rivas LG, Williams TA, Reincke M. Inherited forms of primary hyperaldosteronism: new genes, new phenotypes and proposition of a new classification. Exp Clin Endocrinol Diabetes. 2019;127:93–99. doi: 10.1055/a-0713-0629. [DOI] [PubMed] [Google Scholar]
- 121.Pinggera A, Lieb A, Benedetti B, Lampert M, Monteleone S, Liedl KR, Tuluc P, Striessnig J. CACNA1D de novo mutations in autism spectrum disorders activate Cav1.3 L-type calcium channels. Biol Psychiatry. 2015;77:816–822. doi: 10.1016/j.biopsych.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Powell KL, Cain SM, Ng C, Sirdesai S, David LS, Kyi M, Garcia E, Tyson JR, Reid CA, Bahlo M, Foote SJ, Snutch TP, O’Brien TJ. A Cav3.2 T-type calcium channel point mutation has splice-variant-specific effects on function and segregates with seizure expression in a polygenic rat model of absence epilepsy. J Neurosci. 2009;29:371–380. doi: 10.1523/JNEUROSCI.5295-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Preiksaitiene E, Mannik K, Dirse V, Utkus A, Ciuladaite Z, Kasnauskiene J, Kurg A, Kucinskas V. A novel de novo 1.8 Mb microdeletion of 17q21.33 associated with intellectual disability and dysmorphic features. Eur J Med Genet. 2012;55:656–659. doi: 10.1016/j.ejmg.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 124.Reimer EN, Walenda G, Seidel E, Scholl UI. CACNA1H(M1549V) mutant calcium channel causes autonomous aldosterone production in HAC15 cells and is inhibited by mibefradil. Endocrinology. 2016;157:3016–3022. doi: 10.1210/en.2016-1170. [DOI] [PubMed] [Google Scholar]
- 125.Rzhepetskyy Y, Lazniewska J, Blesneac I, Pamphlett R, Weiss N. CACNA1H missense mutations associated with amyotrophic lateral sclerosis alter Cav3.2 T-type calcium channel activity and reticular thalamic neuron firing. Channels (Austin) 2016;10:466–477. doi: 10.1080/19336950.2016.1204497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rzhepetskyy Y, Lazniewska J, Proft J, Campiglio M, Flucher BE, Weiss N. A Cav3.2/Stac1 molecular complex controls T-type channel expression at the plasma membrane. Channels (Austin) 2016;10:346–354. doi: 10.1080/19336950.2016.1186318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sakkaki S, Gangarossa G, Lerat B, Francon D, Forichon L, Chemin J, Valjent E, Lerner-Natoli M, Lory P. Blockade of T-type calcium channels prevents tonic-clonic seizures in a maximal electroshock seizure model. Neuropharmacology. 2016;101:320–329. doi: 10.1016/j.neuropharm.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 128.Sanchez-Roige S, Fontanillas P, Elson SL, Gray JC, de Wit H, MacKillop J, Palmer AA. Genome-wide association studies of impulsive personality traits (BIS-11 and UPPS-P) and drug experimentation in up to 22,861 adult research participants identify loci in the CACNA1I and CADM2 genes. J Neurosci. 2019;39:2562–2572. doi: 10.1523/JNEUROSCI.2662-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schizophrenia Working Group of the Psychiatric Genomics C Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Scholl UI, Stolting G, Nelson-Williams C, Vichot AA, Choi M, Loring E, Prasad ML, Goh G, Carling T, Juhlin CC, Quack I, Rump LC, Thiel A, Lande M, Frazier BG, Rasoulpour M, Bowlin DL, Sethna CB, Trachtman H, Fahlke C, Lifton RP. Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. Elife. 2015;4:e06315. doi: 10.7554/eLife.06315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Seitter H, Koschak A. Relevance of tissue specific subunit expression in channelopathies. Neuropharmacology. 2018;132:58–70. doi: 10.1016/j.neuropharm.2017.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Senatore A, Spafford JD. Gene transcription and splicing of T-type channels are evolutionarily-conserved strategies for regulating channel expression and gating. PLoS One. 2012;7:e37409. doi: 10.1371/journal.pone.0037409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shcheglovitov A, Vitko I, Bidaud I, Baumgart JP, Navarro-Gonzalez MF, Grayson TH, Lory P, Hill CE, Perez-Reyes E. Alternative splicing within the I-II loop controls surface expression of T-type Ca(v)3.1 calcium channels. FEBS Lett. 2008;582:3765–3770. doi: 10.1016/j.febslet.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Singh B, Monteil A, Bidaud I, Sugimoto Y, Suzuki T, Hamano S, Oguni H, Osawa M, Alonso ME, Delgado-Escueta AV, Inoue Y, Yasui-Furukori N, Kaneko S, Lory P, Yamakawa K (2007) Mutational analysis of CACNA1G in idiopathic generalized epilepsy. Mutation in brief #962. Online. Hum Mutat 28:524–525. doi:10.1002/humu.9491 [DOI] [PubMed]
- 135.Smith CL, Abdallah S, Wong YY, Le P, Harracksingh AN, Artinian L, Tamvacakis AN, Rehder V, Reese TS, Senatore A. Evolutionary insights into T-type Ca(2+) channel structure, function, and ion selectivity from the Trichoplax adhaerens homologue. J Gen Physiol. 2017;149:483–510. doi: 10.1085/jgp.201611683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Snutch TP, Zamponi GW. Recent advances in the development of T-type calcium channel blockers for pain intervention. Br J Pharmacol. 2018;175:2375–2383. doi: 10.1111/bph.13906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Souza IA, Gandini MA, Wan MM, Zamponi GW. Two heterozygous Cav3.2 channel mutations in a pediatric chronic pain patient: recording condition-dependent biophysical effects. Pflugers Arch. 2016;468:635–642. doi: 10.1007/s00424-015-1776-3. [DOI] [PubMed] [Google Scholar]
- 138.Souza IA, Gandini MA, Zhang FX, Mitchell WG, Matsumoto J, Lerner J, Pierson TM, Zamponi GW. Pathogenic Cav3.2 channel mutation in a child with primary generalized epilepsy. Mol Brain. 2019;12:86. doi: 10.1186/s13041-019-0509-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H, Priori SG, Sanguinetti MC, Keating MT. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 140.Splawski I, Yoo DS, Stotz SC, Cherry A, Clapham DE, Keating MT. CACNA1H mutations in autism spectrum disorders. J Biol Chem. 2006;281:22085–22091. doi: 10.1074/jbc.M603316200. [DOI] [PubMed] [Google Scholar]
- 141.Stringer RN, Jurkovicova-Tarabova B, Huang S, Haji-Ghassemi O, Idoux R, Liashenko A, Souza IA, Rzhepetskyy Y, Lacinova L, Van Petegem F, Zamponi GW, Pamphlett R, Weiss N. A rare CACNA1H variant associated with amyotrophic lateral sclerosis causes complete loss of Cav3.2 T-type channel activity. Mol Brain. 2020;13:33. doi: 10.1186/s13041-020-00577-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Strom SP, Stone JL, Ten Bosch JR, Merriman B, Cantor RM, Geschwind DH, Nelson SF. High-density SNP association study of the 17q21 chromosomal region linked to autism identifies CACNA1G as a novel candidate gene. Mol Psychiatry. 2010;15:996–1005. doi: 10.1038/mp.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Talavera K, Staes M, Janssens A, Droogmans G, Nilius B. Mechanism of arachidonic acid modulation of the T-type Ca2+ channel alpha1G. J Gen Physiol. 2004;124:225–238. doi: 10.1085/jgp.200409050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Talley EM, Cribbs LL, Lee JH, Daud A, Perez-Reyes E, Bayliss DA. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci. 1999;19:1895–1911. doi: 10.1523/JNEUROSCI.19-06-01895.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tatsuki F, Sunagawa GA, Shi S, Susaki EA, Yukinaga H, Perrin D, Sumiyama K, Ukai-Tadenuma M, Fujishima H, Ohno R, Tone D, Ode KL, Matsumoto K, Ueda HR. Involvement of Ca(2+)-dependent hyperpolarization in sleep duration in mammals. Neuron. 2016;90:70–85. doi: 10.1016/j.neuron.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 146.Timic Stamenic T, Feseha S, Valdez R, Zhao W, Klawitter J, Todorovic SM. Alterations in oscillatory behavior of central medial thalamic neurons demonstrate a key role of CaV3.1 isoform of T-channels during isoflurane-induced anesthesia. Cereb Cortex. 2019;29:4679–4696. doi: 10.1093/cercor/bhz002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Todorovic SM, Jevtovic-Todorovic V. Neuropathic pain: role for presynaptic T-type channels in nociceptive signaling. Pflugers Arch. 2013;465:921–927. doi: 10.1007/s00424-012-1211-y. [DOI] [PubMed] [Google Scholar]
- 148.Todorovic SM, Jevtovic-Todorovic V. Redox regulation of neuronal voltage-gated calcium channels. Antioxid Redox Signal. 2014;21:880–891. doi: 10.1089/ars.2013.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Traboulsie A, Chemin J, Chevalier M, Quignard JF, Nargeot J, Lory P. Subunit-specific modulation of T-type calcium channels by zinc. J Physiol. 2007;578:159–171. doi: 10.1113/jphysiol.2006.114496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tsakiridou E, Bertollini L, de Curtis M, Avanzini G, Pape HC. Selective increase in T-type calcium conductance of reticular thalamic neurons in a rat model of absence epilepsy. J Neurosci. 1995;15:3110–3117. doi: 10.1523/JNEUROSCI.15-04-03110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.van Loo KM, Schaub C, Pitsch J, Kulbida R, Opitz T, Ekstein D, Dalal A, Urbach H, Beck H, Yaari Y, Schoch S, Becker AJ. Zinc regulates a key transcriptional pathway for epileptogenesis via metal-regulatory transcription factor 1. Nat Commun. 2015;6:8688. doi: 10.1038/ncomms9688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Veselovskii NS, Fedulova SA. 2 types of calcium channels in the somatic membrane of spinal ganglion neurons in the rat. Dokl Akad Nauk SSSR. 1983;268:747–750. [PubMed] [Google Scholar]
- 153.Visa A, Shaikh S, Alza L, Herreros J, Canti C. The hard-to-close window of T-type calcium channels. Trends Mol Med. 2019;25:571–584. doi: 10.1016/j.molmed.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 154.Vitko I, Chen Y, Arias JM, Shen Y, Wu XR, Perez-Reyes E. Functional characterization and neuronal modeling of the effects of childhood absence epilepsy variants of CACNA1H, a T-type calcium channel. J Neurosci. 2005;25:4844–4855. doi: 10.1523/JNEUROSCI.0847-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vitko I, Bidaud I, Arias JM, Mezghrani A, Lory P, Perez-Reyes E. The I-II loop controls plasma membrane expression and gating of Ca(v)3.2 T-type Ca2+ channels: a paradigm for childhood absence epilepsy mutations. J Neurosci. 2007;27:322–330. doi: 10.1523/JNEUROSCI.1817-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Voisin T, Bourinet E, Lory P. Genetic alteration of the metal/redox modulation of Cav3.2 T-type calcium channel reveals its role in neuronal excitability. J Physiol. 2016;594:3561–3574. doi: 10.1113/JP271925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Weiss N, Zamponi GW. T-type channel druggability at a crossroads. ACS Chem Neurosci. 2019;10:1124–1126. doi: 10.1021/acschemneuro.9b00031. [DOI] [PubMed] [Google Scholar]
- 158.Weiss N, Zamponi GW. Genetic T-type calcium channelopathies. J Med Genet. 2020;57:1–10. doi: 10.1136/jmedgenet-2019-106163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Weiss N, Hameed S, Fernandez-Fernandez JM, Fablet K, Karmazinova M, Poillot C, Proft J, Chen L, Bidaud I, Monteil A, Huc-Brandt S, Lacinova L, Lory P, Zamponi GW, De Waard M. A Ca(v)3.2/syntaxin-1A signaling complex controls T-type channel activity and low-threshold exocytosis. J Biol Chem. 2012;287:2810–2818. doi: 10.1074/jbc.M111.290882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Weiss N, Black SA, Bladen C, Chen L, Zamponi GW. Surface expression and function of Cav3.2 T-type calcium channels are controlled by asparagine-linked glycosylation. Pflugers Arch. 2013;465:1159–1170. doi: 10.1007/s00424-013-1259-3. [DOI] [PubMed] [Google Scholar]
- 161.Welsby PJ, Wang H, Wolfe JT, Colbran RJ, Johnson ML, Barrett PQ. A mechanism for the direct regulation of T-type calcium channels by Ca2+/calmodulin-dependent kinase II. J Neurosci. 2003;23:10116–10121. doi: 10.1523/JNEUROSCI.23-31-10116.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wolfe JT, Wang H, Howard J, Garrison JC, Barrett PQ. T-type calcium channel regulation by specific G-protein betagamma subunits. Nature. 2003;424:209–213. doi: 10.1038/nature01772. [DOI] [PubMed] [Google Scholar]
- 163.Xie Y, Huang D, Wei L, Luo XJ. Further evidence for the genetic association between CACNA1I and schizophrenia. Hereditas. 2018;155:16. doi: 10.1186/s41065-017-0054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xu W, Liu Y, Chen J, Guo Q, Liu K, Wen Z, Zhou Z, Song Z, Zhou J, He L, Yi Q, Shi Y. Genetic risk between the CACNA1I gene and schizophrenia in Chinese Uygur population. Hereditas. 2018;155:5. doi: 10.1186/s41065-017-0037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yaari Y, Hamon B, Lux HD. Development of two types of calcium channels in cultured mammalian hippocampal neurons. Science. 1987;235:680–682. doi: 10.1126/science.2433765. [DOI] [PubMed] [Google Scholar]
- 166.Yunker AM, Sharp AH, Sundarraj S, Ranganathan V, Copeland TD, McEnery MW. Immunological characterization of T-type voltage-dependent calcium channel CaV3.1 (alpha 1G) and CaV3.3 (alpha 1I) isoforms reveal differences in their localization, expression, and neural development. Neuroscience. 2003;117:321–335. doi: 10.1016/s0306-4522(02)00936-3. [DOI] [PubMed] [Google Scholar]
- 167.Zamponi GW, Lory P, Perez-Reyes E. Role of voltage-gated calcium channels in epilepsy. Pflugers Arch. 2010;460:395–403. doi: 10.1007/s00424-009-0772-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zamponi GW, Striessnig J, Koschak A, Dolphin AC. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol Rev. 2015;67:821–870. doi: 10.1124/pr.114.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang Y, Mori M, Burgess DL, Noebels JL. Mutations in high-voltage-activated calcium channel genes stimulate low-voltage-activated currents in mouse thalamic relay neurons. J Neurosci. 2002;22:6362–6371. doi: 10.1523/JNEUROSCI.22-15-06362.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang Y, Jiang X, Snutch TP, Tao J. Modulation of low-voltage-activated T-type Ca(2)(+) channels. Biochim Biophys Acta. 2013;1828:1550–1559. doi: 10.1016/j.bbamem.2012.08.032. [DOI] [PubMed] [Google Scholar]
- 171.Zhao Y, Huang G, Wu Q, Wu K, Li R, Lei J, Pan X, Yan N. Cryo-EM structures of apo and antagonist-bound human Cav3.1. Nature. 2019;576:492–497. doi: 10.1038/s41586-019-1801-3. [DOI] [PubMed] [Google Scholar]
- 172.Zhong X, Liu JR, Kyle JW, Hanck DA, Agnew WS. A profile of alternative RNA splicing and transcript variation of CACNA1H, a human T-channel gene candidate for idiopathic generalized epilepsies. Hum Mol Genet. 2006;15:1497–1512. doi: 10.1093/hmg/ddl068. [DOI] [PubMed] [Google Scholar]