Abstract
Background
The Paleolithic diet is promoted worldwide for improved gut health. However, there is little evidence available to support these claims, with existing literature examining anthropometric and cardiometabolic outcomes.
Objective
To determine the association between dietary intake, markers of colonic health, microbiota, and serum trimethylamine-N-oxide (TMAO), a gut-derived metabolite associated with cardiovascular disease.
Design
In a cross-sectional design, long-term (n = 44, > 1 year) self-reported followers of a Paleolithic diet (PD) and controls (n = 47) consuming a diet typical of national recommendations were recruited. Diets were assessed via 3-day weighed diet records; 48-h stool for short chain fatty acids using GC/MS, microbial composition via 16S rRNA sequencing of the V4 region using Illumina MiSeq. TMAO was quantified using LC–MS/MS.
Results
Participants were grouped according to PD adherence; namely excluding grains and dairy products. Strict Paleolithic (SP) (n = 22) and Pseudo-Paleolithic (PP) (n = 22) groups were formed. General linear modelling with age, gender, energy intake and body fat percentage as covariates assessed differences between groups. Intake of resistant starch was lower in both Paleolithic groups, compared to controls [2.62, 1.26 vs 4.48 g/day (P < 0.05)]; PERMANOVA analysis showed differences in microbiota composition (P < 0.05), with higher abundance of TMA-producer Hungatella in both Paleolithic groups (P < 0.001). TMAO was higher in SP compared to PP and control (P < 0.01), and inversely associated with whole grain intake (r = − 0.34, P < 0.01).
Conclusions
Although the PD is promoted for improved gut health, results indicate long-term adherence is associated with different gut microbiota and increased TMAO. A variety of fiber components, including whole grain sources may be required to maintain gut and cardiovascular health.
Clinical trial registrations
Australian and New Zealand Clinical Trial Registry (ANZCTRN12616001703493).
Electronic supplementary material
The online version of this article (10.1007/s00394-019-02036-y) contains supplementary material, which is available to authorized users.
Keywords: Paleolithic diet, Gut health, Resistant starch, TMAO, Whole grains
Introduction
The Paleolithic diet is a dietary pattern based on the hypothesis that the human genome has not adapted to consume products of agriculture, and thus is based on consumption of meat, fish, eggs, nuts, fruits and vegetables; with no processed foods, grains or dairy products included [1]. The diet is promoted worldwide for improved gut health [2]. However, it excludes grains and dairy, food groups that form part of the evidence-based national Australian and international dietary guidelines [3, 4].
While total dietary fiber intake can be maintained on a Paleolithic diet through fruit and vegetable consumption [5], the exclusion of whole grains and legume products alters the fiber profile consumed, and in particular, results in reductions of resistant starch (RS) intake [6]. RS consistently improves markers of bowel health, such as increased SCFA levels [7–12], and long-term the effect of reduced intake has not been previously explored, nor the implications for microbial diversity, metabolites, and other markers of gastrointestinal health. While the Paleolithic diet can be classed as a low carbohydrate diet [5], other studies of low carbohydrate diets and the impact on markers for gastrointestinal health have been very low in total dietary fiber [13–16], thus limiting comparability to the current Paleolithic dietary patterns and the impact on markers of gut health.
The elimination of grains, dairy and legume protein sources, means PDs are rich in animal-based protein, which may increase serum trimethylamine-N-oxide (TMAO) concentrations [17]. TMAO has been associated with CVD and atherosclerotic plaque in both animal and human models [18–21], however, there is little evidence around how TMAO concentrations vary with total dietary patterns in healthy individuals. Given the established mechanism for the production of TMA within the colon [18], modulations of the gut microbiome through dietary intervention and changes in fiber intake have the potential to alter circulating TMAO concentrations. We have previously shown that a short-term, 4-week intervention using the PD, did not significantly impact TMAO, but lowered RS intake, in a small cohort of healthy Australian women [6]. However, longer-term studies of a PD have not examined the relationship between RS intake and TMAO concentrations [22–24]. Our short-term, randomised, controlled intervention study, comprised a small sample size and the energy restricted diet may have limited our ability to detect significant differences in TMAO concentrations [6]; furthermore, we did not concurrently examine fecal microbiota. Given the identified link between TMAO concentrations and CVD [18–21], and the limited literature regarding long-term health implications of the PD, it is important to determine if the Paleolithic dietary pattern alters the ability of the gut microbiota to produce TMA. Therefore, the current cross sectional study compared subjects with long-term (> 1 year) adherence to a Paleolithic diet to those following a standard Australian diet to examine the impact of each diet on gastrointestinal health and potential downstream effects on cardiovascular health.
Methods
Trial design
The study was designed as a cross-sectional comparator study, and registered on the Australian and New Zealand Clinical Trial Register (ANZCTRN12616001703493) and approved by the Edith Cowan University, Human Research Ethics Committee (13402).
Participants
Recruitment for the study took place between August 2016 and June 2017 through online advertisements. Primary inclusion criteria for the Paleolithic diet group were adherence to the dietary pattern for > 1 year period and consumption of no more than 1 serve/day of grains and dairy products. For inclusion in the control group, participants needed to have made no changes to their diet in the previous year, and follow a relatively healthy diet which included grains, legumes and dairy or alternatives. Specific inclusion criteria for both groups were: men and women aged 18–70 years; willingness to complete a 3-day weighed diet records (3d WDR), provide blood, urine and stool samples; non-smoker, not participating in other studies and had BMI < 30 kg/m2. Subjects were excluded if they had taken antibiotics in the previous 6-month, had a past or present digestive disorder, surgery on the gastrointestinal tract, used anti-hypertensive or lipid or glucose-lowering medication, previous cardiovascular events or diagnosed CVD. Participants were screened via email or phone confirming exclusion/inclusion criteria were met and provided written informed consent prior to study commencement. Participants completed a diet history interview, followed by a 3d WDR, including 2 week days and 1 weekend day. Samples collected were a 24-h urine and fasted (overnight) blood sample. Portable freezers (Waeco-CF-40,Dometic, Australia) were supplied to collect all stool samples over a 48-h period. Physical activity was assessed by the International Physical Activity Questionnaire [25].
Validity of dietary intake data
Dietary data provided by the 3d WDR were validated by urine nitrogen analysed using a 1 in 50 dilution of urine sample on a Shimadzu Total Carbon and Nitrogen Analyser, TOC-Vcsh/TMN-1 (Shimadzu, Japan). Total nitrogen intake was determined by dividing protein intake by 6.25 [26], with an acceptable intake to excretion ratio set at 80% ± 24% [27]. Those with an intake to excretion ratio outside of this range were deemed to be protein intake under or over reporters. Potential energy under reporters were identified utilising the Goldberg cut point [28]. Those who were identified as under reporting both protein and energy were defined as unreliable dietary reporters. Confirmation that the 3d WDR was representative of usual dietary intake was achieved by statistical analysis of the energy and protein intake of both methods.
Paleolithic scoring protocol
Due to individual differences in interpretation of the Paleolithic dietary pattern noted during data-entry of the 3d WDR, a post hoc scoring protocol was developed to rank adherence to the Paleolithic diet principles, namely the exclusion of grain and dairy products. Those who consumed < 1 serve per day of grains and dairy, in-line with the inclusion criteria, were allocated to the Strict Paleolithic (SP), while those who consumed > 1 serve per day of grains and/or dairy were allocated to the Pseudo-Paleolithic group (PP).
Outcomes assessment
Dietary intake
The diet history and 3d WDR data were entered into FoodWorks v8.0 [29], by the same assessor, a registered nutritionist with advanced competencies in dietary analysis. All food records were checked for completeness. Minimum and maximum RS content of each food item, utilised in the 91 food items generated from the 3d WDR, were determined using methods described elsewhere [6].
Anthropometric measures
Subjects were fasted for 2-h prior to the clinic appointment and reported dressed in tightly fitting gym clothes. Blood pressure measurement was conducted, in duplicate, on the right arm with an Omron IA1B Automated Blood Pressure Device (Omron Health Care Ltd, Japan) at heart level, 1 min apart. The mean of the two systolic and diastolic measures respectively were recorded as per the protocol described by the American Heart Foundation [30]. Standing height to the nearest 0.1 cm was recorded using a SECA 763 digital stadiometer (SECA Ltd, USA). Waist circumference was measured to the nearest 0.1 cm at the narrowest part of the waist by a Lufkin steel tape measure, following standardised ISAK techniques [31]. The BodPod body composition chamber (Cosmed, USA) calculated both weight and body fat percentage (to the nearest 0.001 kg and 0.01%, respectively). As per the manufacturer’s protocol, hair was covered with a tightly fitted cap, with all jewellery and footwear removed.
Stool form and biochemistry
Upon arrival at the university, the Bristol stool number (as reported by the participant at collection time [32]) was recorded. Each individual sample was weighed and the total number of samples provided over the 48-h period allowed the calculation of stool frequency (bowel motions/day).
Individual stool samples were stored at − 80 °C until sample processing, where they were defrosted at 4 °C overnight. Samples from the same participant were combined and homogenised manually on ice for at least 1 min prior to weighing and aliquoting for each individual assay. SCFA analysis was undertaken using 1–1.5 g aliquots of stool using methods described by Bajka et al. [33], with the addition of a freeze–thaw distillation prior to GC analysis.
Moisture content was calculated by freeze-drying, in duplicate, 40 g of pre-aliquoted stool sample for 7 days using a Christ LD-alpha freeze drier (Martin Christ Ltd., Germany). Moisture content, expressed as a percentage, was calculated from the mean of the two individual moisture measurements.
Blood biochemistry
Participants reported to pathology for a blood test on the morning after completion of the 3WDR, after an overnight fast. Lipids were determined using standard enzymatic techniques (Abbott Architect c16000 assay) by Pathwest, a National Association of Testing Laboratories (NATA) accredited laboratory. Serum samples, stored at − 80 °C, were analysed for TMAO at the School of Science Analytical facility, Edith Cowan University, using the method described by Le et al. [34].
Microbiota analysis
The microbial analysis were conducted at the WA Human Microbiome Collaboration Centre, Curtin University, utilising the QIAmp PowerFecal DNA Kit for DNA extraction and Illumina MiSeq platform for sequencing. Full methods are available in the supplementary information.
Statistical methods
A priori power calculations were determined using G-Power software [35] and were based on reductions to our primary outcome variable, fecal butyrate excretion. Available literature suggested a medium to large Cohen’s effect size (d = 0.595) could be expected [13], providing sample size requirement of n = 72 at 80% power and α = 0.05, however, given fiber intake can be maintained on a Paleolithic diet, a more conservative estimate was determined using a medium effect size (d = 0.5). The actual sample requirement was therefore deemed to be between a total of n = 72 and n = 102, with n = 36 and n = 51 per group, respectively.
Data were analysed using SPSS v24.0 [36]. Non-normally distributed data were log10 transformed prior to analysis and back-transformed to allow calculation of the estimated marginal means and corresponding 95% confidence intervals. General linear modelling was used to compare the stratified Paleolithic vs control groups. Post hoc Bonferroni corrections were applied to P values from the three groups analysis. Amongst the entire cohort, exploration of associations between dietary intake, blood and stool biochemistry were conducted using linear regression. All models included age, gender, energy intake and body fat percentage as covariates, with additional covariates used where appropriate. Significance for the study was set at P < 0.05.
Microbiota analysis was conducted using Primer 7 (Quest Research, NZ) with permutation multivariate analysis of variance (PERMANOVA) [37]. At the phylum and genus level, relative abundance data were square or fourth root transformed, prior to the calculation of a Bray–Curtis similarity matrix. Principal coordinates analysis (PCO) was used to examine possible differences or separations among the groups visually at the 2-D level. This was followed by PERMANOVA to formally assess differences between groups. Dissimilarity percentage (SIMPER) analysis was used to determine the contribution of individual phyla and genera driving the average dissimilarities between groups based on the Bray–Curtis similarity matrix. Distance-based linear modelling (DistLM) was utilised to describe the patterns in the microbiota using the dietary intake variables. Measures of diversity were calculated using the Shannon and Simpson indices. The Bacteriodetes:Firmicutes ratio was calculated and exported to SPSS v24.0 (IBM Corporation, USA) [36] for analysis.
All analyses were conducted with and without the inclusion of participants identified as unreliable dietary reporters. Where the inclusion of these participants did not influence statistical significance of our findings, the reported results include all participants.
Results
Participants
As shown in Fig. 1, 90 subjects completed the study with partial data available for an additional control subject. Participant characteristics are presented in Table 1. No significant differences were found between groups for age, height, energy intake or physical activity. The average length of time Paleolithic participants had followed the diet was 2.38 ± 1.08 years. In terms of being representative of long-term dietary intake, bivariate correlations of energy and protein with the diet history showed good agreement (R = 0.596 and 0.763 respectively, P < 0.01) and a one sample t test showed the energy and protein intake determined from the 3d WDR and the diet history were not significantly different (P = 0.623 and 0.059 respectively). A Bland–Altman plot [38] confirmed acceptable agreement between urinary nitrogen and calculated nitrogen intake from protein intake (data not shown); and a one-sample t test showed no difference between these variables (P = 0.337). Five participants were identified as under-reporting both energy and protein intake, two from the SP group and three from the control group. The scoring protocol was used to rank participants according to adherence to the diet (Table 1). Body weight was significantly greater in the Pseudo-Paleolithic (PP) group, when compared to the controls, but there were no other differences between groups identified.
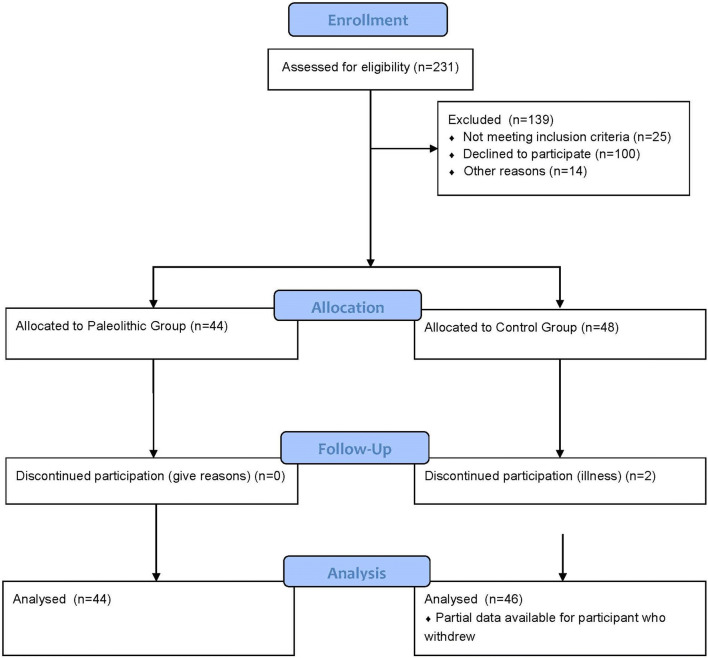
Fig. 1.
Flow diagram of the study. Of the 231 enquiries received, 92 participants were accepted into the study. Two participants in the control group withdrew due to illness, with partial data available for one subject. 44 Paleolithic and 46 controls completed the study data collection
Table 1.
Participant characteristics by dietary group
| Control Mean (95% CI) |
Strict Paleolithic (SP) Mean (95% CI) |
Pseudo-Paleolithic (PP) Mean (95% CI) |
|
|---|---|---|---|
| N | 47 | 22 | 22 |
| Age (years)a | 38.96 (35.02, 42.90) | 41.49 (35.46, 47.53) | 45.33 (39.69, 50.96) |
| Height (cm) | 169.6 (167.9, 171.4) | 169.8 (167.1, 172.5) | 172.8 (170.3, 175.3) |
| Weight (kg) | 69.19 (65.58, 72.79) | 70.23 (64.74, 75.72) | 79.08 (73.89, 84.27)** |
| BMI (kg/m2) | 23.94 (22.78, 25.10) | 24.33 (22.56, 26.10) | 26.39 (24.72, 28.07) |
| Systolic blood pressure (mmHg) | 120 (114, 125) | 116 (109, 124) | 114 (107, 125) |
| Diastolic blood pressure (mmHg) | 79 (75, 83) | 75 (70, 81) | 77 (72, 82) |
| Waist circumference (cm) | 81.3 (78.1, 84.5) | 83.0 (78.2, 87.8) | 86.1 (81.7, 90.6) |
| Body fat (%) | 22.68 (20.21, 25.14) | 22.38 (18.63, 26.13) | 25.96 (22.41, 29.50) |
| Energy intake (kJ) | 9384 (8800, 9968) | 9171 (8282, 10059) | 9812 (8972, 10652) |
| Physical activity (PAL)b | 1.82 (1.79, 1.86) | 1.87 (1.81, 1.93) | 1.83 (1.77, 1.86) |
Dietary intake
Total dietary fiber (TDF) intake in the SP group was not different from that of the control (Table 2), however the PP group had fiber intake below the recommended Australian adequate intake (AI) of 25 g/day. In line with the significantly lower TDF intake in the PP group, both soluble and insoluble fibre intake was also lower, when compared to the control group. RS intake was significantly lower in the SP and PP groups at both minimum and maximum estimated intakes (Table 2). Despite the low carbohydrate intake, vegetable intake in the SP group met the Australian recommendations of 5 serves per day [3], with a mean intake significantly higher than that of the control group. Across the whole cohort, total fiber intake was inversely associated with body weight [t(86) = − 4.22, partial r = − 0.414, P < 0.001), body fat percentage [t(86) = − 2.282, partial r = − 0.239, P = 0.025] and waist circumference [t(85) = − 3.397, partial r = − 0.346, P = 0.001]. Protein intake as percentage of energy and absolute intake in grams per day, was significantly higher in the SP group, when compared to the control and PP (Table 2). Fat intake was significantly higher in both Paleolithic groups, when compared to the control (Table 2). Saturated fat intake in all dietary groups exceeded the recommended intake of 10% of energy [3], with intakes in both of the Paleolithic groups more than double the recommended Australian and international levels [3, 39].
Table 2.
Energy, macronutrient and food group intake by dietary group
| Dietary variable | Control Mean (95% CI)a,b (n = 47) |
Strict Paleolithic Mean (95% CI)b (n = 22) |
Effect size (SP vs control) | Pseudo-Paleolithic Mean (95% CI)b (n = 22) |
Effect size (PP vs control) | Overall dietary effect P valuec |
|---|---|---|---|---|---|---|
| Energy intake (kJ) | 9187 (8599, 9774) | 8955 (8076, 9834) | − 0.13 | 9820 (9005, 10635) | 0.33 | 0.285 |
| Protein (g) | 92.16 (83.69, 100.63) | 118.07 (105.69, 130.44)** | 1.02 | 102.7 (90.62, 114.77) | 0.39 | 0.001 |
| Protein (% of energy) | 18.49 (16.87, 20.12) | 23.63 (21.25, 26.00)** | 1.02 | 20.13 (17.81, 22.45) | 0.39 | 0.001 |
| Carbohydrate (g) | 202.62 (187.84, 217.41) | 99.19 (77.60, 120.79)** | − 2.33 | 81.40 (60.34, 102.47)** | − 2.59 | < 0.001 |
| Carbohydrate (% of energy) | 38.03 (35.49, 40.57) | 16.75 (13.03, 20.46)** | − 2.79 | 16.41 (12.79, 20.03)** | − 2.69 | < 0.001 |
| Sugars (g) | 75.58 (66.05, 85.11) | 51.77 (37.85, 65.68)** | − 0.83 | 44.34 (30.76, 57.91)** | − 1.04 | < 0.001 |
| Starch (g) | 124.56 (112.87, 136.26) | 45.86 (28.78, 62.95)** | − 2.24 | 34.74 (18.07, 51.4)** | − 2.43 | < 0.001 |
| Total fat (g) | 82.53 (76.33, 88.72) | 117.91 (108.86, 126.96)** | 1.90 | 133.16 (124.34, 141.99)**
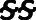
|
2.58 | < 0.001 |
| Fat (% of energy) | 36.45 (33.9, 39) | 52.95 (49.22, 56.67)** | 2.15 | 56.96 (53.33, 60.6)** | 2.54 | < 0.001 |
| Saturated fat (g) | 27.34 (22.77, 31.9) | 45.51 (38.84, 52.18)** | 1.33 | 52.47 (45.97, 58.98)** | 1.74 | < 0.001 |
| Saturated fat (% of energy) | 12.02 (10.05, 13.99) | 20.59 (17.71, 23.47)** | 1.45 | 22.09 (19.28, 24.9)** | 1.61 | < 0.001 |
| Polyunsaturated fat (g) | 15.17 (13.21, 17.14) | 16.86 (13.98, 19.73) | 0.29 | 17.47 (14.67, 20.28) | 0.37 | 0.295 |
| Monounsaturated fat (g) | 32.96 (29.63, 36.28) | 45.01 (40.16, 49.86)** | 1.21 | 52.28 (47.55, 57.02)**
|
1.84 | < 0.001 |
| Cholesterol (mg) | 324.19 (225.29, 423.08) | 708.28 (563.84, 852.73)** | 1.29 | 685.00 (544.10, 825.90)** | 1.15 | < 0.001 |
| Total dietary fiber (g) | 29.66 (26.36, 32.96) | 27.41 (22.59, 32.23) | − 0.23 | 20.79 (16.09, 25.49)* | − 0.85 | 0.006 |
| Soluble dietary fiber (g) | 11.99 (10.45, 13.53) | 10.78 (8.54, 13.03) | − 0.26 | 7.62 (5.43, 9.81)**
|
− 0.90 | 0.004 |
| Insoluble dietary fiber (g) | 17.07 (15.11, 19.03) | 16.17 (13.3, 19.04) | − 0.15 | 12.87 (10.07, 15.66)* | − 0.68 | 0.036 |
| Resistant starch minimum (g) | 4.48 (3.89, 5.06) | 2.62 (1.77, 3.47)** | − 1.03 | 1.26 (0.43, 2.09)**
|
− 1.75 | < 0.001 |
| Resistant starch maximum (g) | 14.16 (12.24, 16.07) | 6.13 (3.34, 8.93)** | − 1.35 | 2.95 (0.22, 5.68)** | − 1.85 | < 0.001 |
| Alcohol (g) | 7.07 (3.73, 10.4) | 8.31 (3.44, 13.19) | 0.12 | 10.34 (5.59, 15.09) | 0.31 | 0.488 |
| Vegetables serves/day (fresh equivalent 150–350 kJ) | 3.83 (2.99, 4.66) | 6.68 (5.51, 7.84)** | 1.12 | 4.31 (3.17, 5.44) | 0.16 | < 0.001 |
| Dark green vegetable serves/day (fresh equivalent 100 kJ) | 0.39 (0.17, 0.61) | 1.28 (0.97, 1.59)** | 1.34 | 0.70 (0.41, 1.00) | 0.45 | < 0.001 |
| Nuts and seeds (30 g protein equivalent) | 0.82 (0.48, 1.15) | 1.07 (0.59, 1.56) | 0.26 | 1.32 (0.84, 1.79) | 0.48 | 0.167 |
| Red meat serves/day (20 g protein equivalent) | 0.44 (0.21, 0.66) | 0.80 (0.47, 1.13) | 0.52 | 0.77 (0.45, 1.09) | 0.46 | 0.066 |
| Eggs serves/day (13 g protein equivalent) | 0.20 (0.09, 0.30) | 1.05 (0.72, 1.38)** | 0.87 | 0.89 (0.57, 1.21)* | 0.63 | < 0.001 |
| Total grains serves/day (14–16 g starch equivalent) | 6.14 (5.29, 7.04) | 0.19 (0.03, 0.47)** | − 3.80 | 0.63 (0.30, 1.08)** | − 3.07 | < 0.001 |
| Whole grain serves/day (14 g starch equivalent) | 2.91 (2.28, 3.61) | 0.09 (0.00, 0.34)** | − 2.32 | 0.16 (0.01, 0.46)** | − 2.09 | < 0.001 |
| Total dairy serves/day (300 mg calcium equivalent) | 1.60 (1.25, 2.0) | 0.17 (0.04, 0.37)* | − 1.26 | 0.87 (0.54, 1.27)*
|
− 0.73 | < 0.001 |
P values for stratified Paleolithic groups were adjusted using post hoc Bonferroni correction [43]
Significant P values are in bold
aGeneral linear modelling used to determine estimated marginal means and the difference between control and the overall Paleolithic groups after adjustment for age, gender, energy intake and body fat percentage.**P ≤ 0.01 different from control group, *P ≤ 0.05 different to control group
bGeneral linear modelling used to determine estimated marginal means and the difference between control, strict Paleolithic and Pseudo-Paleolithic groups after adjustment for age, gender, energy intake and body fat percentage.**P ≤ 0.01 different from control group, *P ≤ 0.05 different to control group.
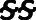 P < 0.01 different from Strict Paleolithic group,
P < 0.01 different from Strict Paleolithic group,
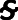 P < 0.05 different from the SP group
P < 0.05 different from the SP group
cSignificance of the overall general linear model for the three group analysis. Effect sizes reported using Cohen’s d, with a value of 0.2 representing a small effect, 0.5 a medium effect and 0.8 a large effect
Blood biochemistry
Total serum cholesterol was higher in the PP, when compared to the control group (Table 3). High-density lipoprotein (HDL) cholesterol was significantly higher in the SP and PP groups, when compared to the control group and led to non-significant differences among the groups in the total cholesterol:HDL cholesterol ratio (Table 3). Total serum cholesterol concentrations were inversely associated with carbohydrate intake, both expressed as g/day [t(77) = − 2.47, partial r = − 0.271, P = 0.016] and as a percentage of energy [t(77) = − 2.54, partial r = − 0.280, P = 0.013]. Further exploration showed significant negative association with the minimum [t(77) = − 3.329, partial r = − 0.355, P = 0.001], and maximum RS intakes [t(77) = − 2.922, partial r = − 0.316, P = 0.005] and whole grain intake [t(77) = − 2.032, r = − 0.226, P = 0.046].
Table 3.
Biochemistry results by dietary group
| Biomarker | Control Mean (95% CI)a,b (n = 46) |
Strict Paleolithic Mean (95% CI)b (n = 22) |
Effect size (strict Paleolithic vs control) | Pseudo-Paleolithic Mean (95% CI)b (n = 22) |
Effect size (Pseudo-Paleolithic vs control) | Overall dietary effect P valuec |
|---|---|---|---|---|---|---|
| Blood biochemistry | ||||||
| Total Cholesterol (mmol/L) | 4.88 (4.51, 5.26) | 5.02 (4.48, 5.56) | 0.13 | 5.60 (5.10, 6.11)* | 0.65 | 0.050 |
| LDL Cholesterold (mmol/L) | 3.00 (2.70, 3.30) | 3.10 (2.66, 3.53) | 0.12 | 3.49 (3.09, 3.90) | 0.55 | 0.113 |
| HDL Cholesterol (mmol/L) | 1.43 (1.29, 1.57) | 1.69 (1.49, 1.89)** | 0.64 | 1.79 (1.61, 1.98)* | 0.88 | 0.003 |
| Total Cholesterol/HDL Ratio | 3.49 (3.21, 3.77) | 3.14 (2.73, 3.55) | − 0.41 | 3.25 (2.87, 3.64) | − 0.28 | 0.122 |
| Triglycerides (mmol/L) | 0.94 (0.88, 1.0) | 0.86 (0.79, 0.94) | − 0.49 | 0.84 (0.77, 0.91) | − 0.60 | 0.049 |
| Serum TMAO (µM)e | 3.93 (2.79, 5.55) | 9.53 (5.86, 15.49)** | 0.86 | 5.47 (3.42, 8.75) | 0.32 | 0.008 |
| Stool markers | ||||||
| Stool weight (g/day wet weight) | 194.57 (161.7, 227.43) | 248.81 (200.55, 297.06) | 0.55 | 233.04 (186.03, 280.05) | 0.37 | 0.099 |
| Frequency (motions per day) | 1.37 (1.19, 1.54) | 1.60 (1.35, 1.85) | 0.46 | 1.91 (1.67, 2.16)** | 1.03 | 0.001 |
| Bristol number [31] | 4.12 (3.75, 4.48) | 4.3 (3.76, 4.84) | 0.18 | 4.54 (4.02, 5.07) | 0.38 | 0.338 |
| Moisture (%) | 73.96 (72.04, 75.89) | 74.69 (71.86, 77.52) | 0.12 | 74.5 (71.74, 77.26) | 0.09 | 0.880 |
| pHe | 7.19 (7.05, 7.36) | 7.19 (6.97, 7.41) | − 0.33 | 7.18 (6.97, 7.4) | − 0.06 | 0.974 |
| Residual fecal fat (%)e | 1.09 (0.69, 1.5) | 1.16 (0.57, 1.75) | 0.07 | 1.70 (1.13, 2.27) | 0.49 | 0.165 |
| Acetate excretion (mmol/day)e | 7.80 (6.18, 9.86) | 10.52 (7.46, 14.86) | 0.41 | 9.06 (6.49, 12.68) | 0.19 | 0.288 |
| Propionate excretion (mmol/day)e | 2.36 (1.87, 2.98) | 2.76 (1.96, 3.89) | 0.80 | 2.82 (2.02, 3.94) | 0.70 | 0.546 |
| Isobutyrate excretion (mmol/day)e | 0.27 (0.23, 0.32) | 0.34 (0.26, 0.44) | 0.42 | 0.34 (0.27, 0.44) | 0.41 | 0.152 |
| Butyrate excretion (mmol/day)e | 2.21 (1.72, 2.84) | 2.86 (1.98, 4.13) | 0.33 | 2.85 (1.99, 4.07) | 0.32 | 0.315 |
| Isovalerate excretion (mmol/day)e | 0.39 (0.33, 0.46) | 0.45 (0.36, 0.58) | 0.29 | 0.48 (0.38, 0.61) | 0.39 | 0.255 |
| Valerate excretion (mmol/day)e | 0.30 (0.25, 0.36) | 0.37 (0.29, 0.49) | 0.40 | 0.38 (0.29, 0.49) | 0.41 | 0.136 |
| Caproate excretion (µmol/day)e | 0.05 (0.04, 0.08) | 0.06 (0.03, 0.11) | 0.05 | 0.08 (0.04, 0.15) | 0.28 | 0.618 |
| Total SCFA excretion (mmol/day)e | 13.74 (11.02, 17.14) | 17.91 (12.94, 24.83) | 0.38 | 16.41 (11.94, 22.54) | 0.25 | 0.299 |
Significant P values are in bold
aGeneral linear modelling used to determine estimated marginal means and the difference between control and the overall Paleolithic groups after adjustment for age, gender, energy intake and body fat percentage.**P ≤ 0.01 different from control group, *P ≤ 0.05 different from control group
bGeneral linear modelling used to determine estimated marginal means and the difference between control, strict Paleolithic and Pseudo Paleolithic groups after adjustment for age, gender, energy intake and body fat percentage.**P ≤ 0.01 different from control group, *P ≤ 0.05 different from control group
cSignificance of the overall general linear model for the three group analysis. Effect sizes reported using Cohen’s d, with a value of 0.2 representing a small effect, 0.5 a medium effect and 0.8 a large effect
dLDL Cholesterol reported is calculated LDL. P values for stratified Paleolithic groups adjusted using post hoc Bonferroni correction [43]
eLog transformation conducted prior to analysis
TMAO was significantly higher in the SP group, when compared to the control group (Table 3). TMAO concentrations were associated with servings of red meat [t(67) = 3.372, partial r = 0.357, P = 0.001], and inversely associated with total grain [t(83) = − 3.143, partial r = − 0.335, P = 0.002] and whole grain intake [t(83) = − 2.973, partial r = − 0.319, P = 0.004], respectively.
Stool biochemistry
There was no difference in daily stool weight or form but stool frequency was greater in the PP group when compared to the control group (Table 3). Regression analysis across the whole cohort showed stool frequency was significantly associated with nut and seed consumption after adjustment for energy intake [t(87) = 2.14, partial r = 0.223, P = 0.036], with each serve of nuts increasing motions/day by 0.12. There were no significant differences in SCFA excretion between groups, however, total SCFA excretion was associated with fiber intake [t(84) = 2.331, partial r = 0.247, P = 0.022], but not with carbohydrate (expressed as a percentage of energy and g/day), grains, whole grains, or RS intake. Total dietary fibre predicted butyrate excretion [t(84) = 2.232, partial r = 0.237, P = 0.028] but no relationships with wholegrains, grains and resistant starch were noted. Vegetable intake significantly predicted total SCFA excretion [t(84) = 2.27, partial r = 0.240, P = 0.026] and further analysis showed the association was due intakes of the dark green vegetable group [t(84) = 2.047, partial r = 0.218, P = 0.044], with similar associations seen for acetate excretion; vegetables were associated with acetate excretion [t(84) = 2.69, partial r = 0.281, P = 0.009], and subgroup analysis showed the association was due to the dark green vegetables [t(5,84) = 2.52, partial r = 0.265, P = 0.014].
Microbiota profiles
The sequence data yielded 929 denoised operational taxonomic units (OTU) with an average 38,899 reads per sample (ranging from 2647 to 107,597 reads). Two participants from the control group were inadvertently tagged with identical forward and reverse barcodes and were excluded from the analysis. OTU comprising less than 0.01% of the total reads were eliminated (514 OTU), leaving 415 available for analysis. Five sequence controls comprised total reads between 3409 and 11,560. Five OTU not related to the gut microbiota, were present primarily in the blank samples, totalling 11,210 reads and were removed from the analysis. One OTU (432 reads) comprised human mitochondrial DNA and were also excluded from the analysis. The final dataset included 410 total OTU’s, which was converted to relative abundance before importing to PRIMER 7 [37] for analysis.
The microbial composition was analysed both at the phylum and at the genus level to examine major and more specific shifts in the gut microbiota between the three dietary groups. At the phylum level, there was no difference between groups for measures of diversity (Shannon and Simpson indices) or species richness between the three groups (Table S1). However, PERMANOVA analysis at the phylum level showed significant differences in microbiota composition between the control and PP groups (P = 0.048) and the SP and PP groups (P = 0.001). No difference was observed between the SP and control groups. SIMPER analysis indicated that group differences were driven by the relative abundances of Bacteriodetes, Firmicutes and Verrucomicrobia phyla. The relationship between Bacteriodetes and Firmicutes has been linked with increased body fat [40, 41], therefore, the overall Bacteriodetes:Firmicutes ratio was calculated, and between-group differences were evaluated using general linear modelling, with the addition of age, gender, energy intake and body fat percentage as covariates in the model. No differences were observed between the three groups. Although, in the entire cohort body fat percentage (partial R = − 0.33, P = 0.032) and energy intake (partial R = − 0.23, P = 0.033) were significant predictors of the ratio in a linear regression model.
At the genus level, there were no significant differences between the three groups, in measures of Shannon or Simpson diversities, and species richness. PERMANOVA analysis with covariates age, gender, energy intake and body fat percentage, showed there were significant differences between the SP and control (P = 0.004) and PP and control (P = 0.04), with no significant difference between the two Paleolithic groups.
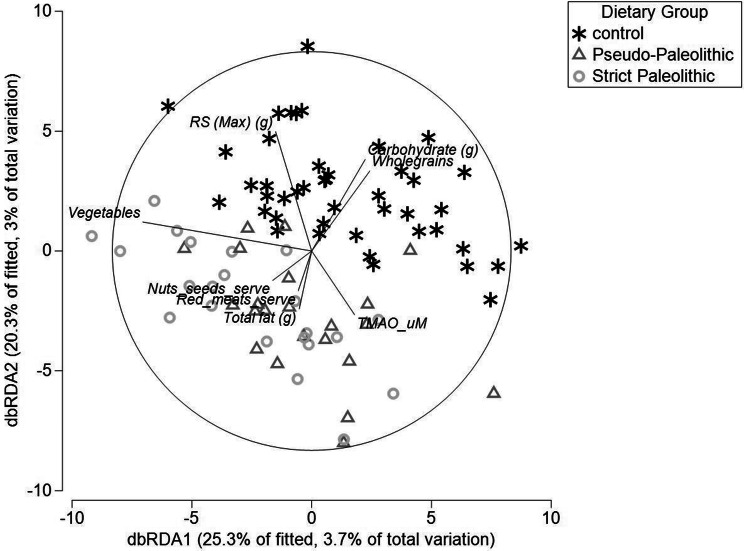
DistLM showed a significant influence of vegetable intake (P < 0.01), in addition to significant influences of whole grains (P < 0.01), RS maximum estimation (P < 0.01), total carbohydrate (P < 0.01) and total fat (P = 0.04) on the microbial composition (Fig. 2).
Fig. 2.
Distance-based redundancy plot, showing the effect of dietary intake, overlaid with serum TMAO, and microbiota composition. The added dietary variable vectors show the correlation between the dietary factor and the microbiota composition. The length of the line is indicative of the strength of the association. Whole grains (P < 0.01), carbohydrate (P < 0.01) and RS (P < 0.01) are associated with a shift in composition towards the top of the plot, consistent with microbiota composition of the control group. However, dietary fat (P = 0.04) was associated with a shift in the opposite direction, with vegetables (P < 0.01) falling in between the two groups. Consistent with the serum TMAO increase in the SP group, TMAO is closely aligned with microbiota composition, total fat and red meat consumption (P trend = 0.09)
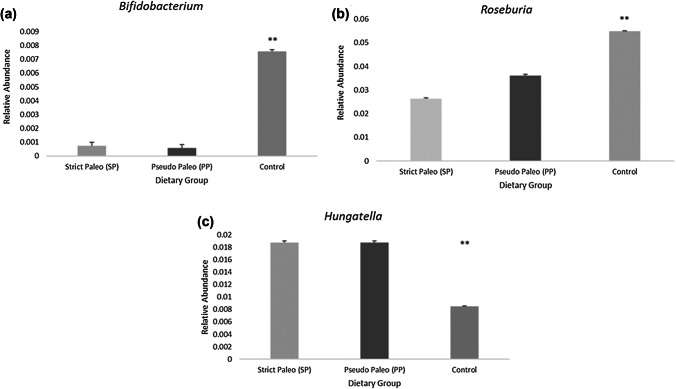
Having established significant differences at the genera level, SIMPER analysis was used to determine the species which contributed to the differences identified between groups. The average dissimilarity between SP and control groups was 27.25%, SP and PP 26.27% and PP and control 27.13%. Abundant genera driving dissimilarities between groups were Bacteriodes, Faecalibacterium, Blatia, Ruminoclostridium, Alistipes, Roseburia, Ruminococcus, Lachnospiracea Incertae sedis, Ruminoclostridium, Anaerostipes, Gemmiger, Irregularibacter, Akkermansia. After adjustment for the study covariates, age, gender, energy intake and body fat percentage, three of these genera were identified as being significantly different between groups, Bifidobacteria, Roseburia, and Hungatella (Fig. 3a–c).
Fig. 3.
a–c Significant contributors to genera differences between groups. Bar graphs show relative abundances by dietary group. **P < 0.01 difference between control and PP/SP groups
Relative abundance of Hungatella was significantly and inversely associated with grain consumption in a linear regression model after adjustment for age, gender, body fat and stool frequency (partial r = − 0.298, P = 0.006). Relative abundance of Roseburia was significantly related to total grain (partial r = 0.315, P = 0.002), whole grain (partial r = 0.298, P = 0.004) and carbohydrate consumption (partial r = 0.297, P = 0.004), but not with dietary fiber, or RS minimum or maximum estimates. Similar to Roseburia, Bifidobacteria abundance was related to total grain consumption (partial r = 0.266, P = 0.008) and the percentage of energy derived from carbohydrate (partial r = 0.221, P = 0.028), but not with RSi, whole grains or total dietary fiber. In a linear regression model, relative abundances of Roseburia and Bifidobacteria were significantly and inversely correlated with the abundance of Hungatella [t(87) = − 2.1, partial r = − 0.2, P = 0.03]. In line with the TMAO findings, relative abundance of Hungatella was also significantly associated with servings of protein foods per day [t(87) = − 2.84, partial r = 0.27, P = 0.006].
Discussion
This study evaluated the gastrointestinal implications of low carbohydrate, high fat, Paleolithic style diets through comparison with a cohort of healthy Australians in a cross-sectional study design. Consumption of a long-term Paleolithic diet was associated with markedly higher serum TMAO concentrations, but only in those who adhered to the diet strictly. Romano et al. [42], identified six species of bacteria associated with choline consumption and production of TMA, of which, one was identified in our cohort, Clostridium hathewayi, originating from the genera Hungatella. We did not identify the other species reported by Romano et al. [42], although our methods of short amplicon sequencing are not typically used to identify individual species, but rather provide robust data at the genus level. It is therefore possible the other species were present, but not identified at the species level in our cohort. Nonetheless, the relative abundance of the Hungatella genus was significantly higher in both the SP and PP groups. Our results show that serum TMAO concentrations and Hungatella abundance were inversely associated with total and whole grain consumption, indicating these food groups may downregulate the ability of Hungatella to dominate or interfere with the production of TMA. Notably, TMAO concentrations in the PP group were not statistically different from the controls, despite the increased Hungatella abundance and small to medium effect size noted. The stratification of the Paleolithic group into two groups may have reduced our ability to detect significance of this outcome variable. Furthermore, the lower overall fiber and higher fat content of the PP diet may have influenced the fermentative capacity of the microbiota to produce TMA, given high fat diets may attenuate the fermentation response [43].
Bergeron et al. [44] detected changes in TMAO concentrations after a 2-week dietary intervention with 52 subjects and found low carbohydrate diets, high in RS, were associated with increased plasma TMAO. Conversely, in the current study TMAO was not associated with RS, but inversely associated with grain intake. This may indicate that other components of the grain carbohydrate and fiber are responsible for modulating abundances of Hungatella. Bergeron et al. [44] did not report food group intake for the intervention diets, which limits comparability with our study. In support of the findings presented here is the identification of the genera associated with producing TMA in the fecal microbiota [42], in addition to the statistical association found with red meat intake, a known TMA precursor food [45].
While there were no observed differences in measures of fecal microbiota diversity (α-diversity), a significant group difference (β-diversity) at both the phylum and genus levels were reported. An inverse association was found between the Bacteriodetes:Firmicutes ratio and body fat, supporting previous research showing a reduced ratio was associated with obesity [40, 41, 46]. At the genus level, different community structures were associated with intakes of vegetables, dietary fat, RS, whole grain and dietary fiber. The direction of the shift in microbiota composition was similar for vegetable intake, whole grains, RS and dietary fiber and is likely to be beneficial, given the large body of evidence associated with health benefits from consumption of these food groups [3, 47–54]. Conversely the direction of the shift in microbiota composition associated with fat consumption was in the opposite direction and suggests a more deleterious profile. Reductions to core bacteria including Roseburia, as seen in the current study, have been associated with inflammatory bowel diseases [55]. In animal models, high-fat diets have been shown to drive obesity independently of the composition of the microbiota [56]. There were reductions in the Paleolithic dietary groups to genera Roseburia and Bifidobacterium, known to metabolise carbohydrate and produce butyrate. Moreover, low abundances of known beneficial genera such as Bifidobacterium in the Paleolithic groups support the findings of Brinkworth et al. [13], who reported low abundances of this genera after an 8 week low-carbohydrate diet, comprising 58% fat [13]. A decrease in relative abundance of Bifidobacterium has been previously associated with irritable bowel syndrome, and obesity [57], however, these disorders have also been associated with lower fecal excretion of acetate and butyrate, which was not found in the current cohort. Given that total fat intake was associated with microbiota composition at both the phylum and genus level, and positively correlated with body weight, the differences observed in microbiota composition are unlikely to be beneficial.
Supporting our previous findings from a short-term intervention using the Paleolithic diet [6], the elimination of the grains food group on a Paleolithic diet resulted in a significantly lower intake of RS than the control group, despite no significant differences found in total soluble or insoluble fiber intakes. Intakes of RS in the control group were slightly higher than previous estimates of Australian intakes of 3.4–9.4 g/day [58] and may be due to higher consumption of whole grains than the average Australian intake of 1.5 serves/day [59]. Both groups consumed less than the proposed 20 g RS/day required for bowel health [60] and may be an area for focus in future interventions.
Despite the differences in RS intake, we did not observe any differences between groups in SCFA excretion. While this was an unexpected finding, the high intake of saturated fat in the Paleolithic groups may have influenced the results, given Fava et al. [61], showed fecal SCFA concentrations were higher following a 4-week diet comprising 17% saturated fat in 88 participants at risk of metabolic syndrome. It is not yet understood whether saturated fat leads to changes in fermentation patterns or alterations to colonic uptake of SCFA. Differences in microbiota composition attributed to the intervention diets used by Fava et al. [61] were assessed using fluorescence in situ hybridisation (FISH), which limits comparability with the current study. In addition, the lack of difference we observed for SCFA excretion may have been due to the differences in the sites of fermentation for grains as opposed to vegetables, with the latter likely to be fermented in the distal colon due to the difference in fiber structure, although there is little literature in this area. Even though fecal acetate concentration has been inversely associated with acetate absorption [62] fecal SCFA may not provide a sensitive enough marker to estimate differences in total fermentation along the colon. [63].
Previous short-term interventions using the Paleolithic diet have found increases to HDL cholesterol in diabetics [64], but not in healthy populations [5, 65–67], however, the latter also reported reductions to total and LDL cholesterol, in addition to significant weight loss, which may have influenced HDL concentrations. Further, a 2-year intervention showed no significant change in total or HDL cholesterol, despite significant reductions in triglycerides over the intervention period [23]. The current data showed small, but significantly greater HDL concentrations in the Paleolithic groups, which were associated with the reduction in carbohydrate intake, and increase in saturated fat consumption. The findings reported here support previous work showing that saturated fat consumption increases concentrations of calculated HDL cholesterol [68]. The current study also shows a significant relationship between saturated fat consumption and total cholesterol levels. A systematic review and meta-analysis showed that for every 1 mmol/L increase in total cholesterol, the relative risk of CVD was 1.20 for women (95% CI 1.16, 1.24) and 1.24 in men (1.20, 1.28) [69]. Therefore, while there may be conflicting evidence surrounding the specific effect of saturated fat consumption on CVD risk, the positive association noted in the current data between saturated fat and HDL concentrations must be interpreted with caution, as saturated fat intake was also associated with total cholesterol concentrations in the Paleolithic groups and may result in increased CVD risk over a longer-term period. Furthermore, we found a positive association between saturated fat intake, body weight, and BMI, which are known to increase CVD risk [70]. Taken together with the greater observed serum TMAO concentrations, it cannot be concluded that the Paleolithic diet is associated with improved gut health and a reduction in risk of CVD as promoted [71, 72].
Conclusion
The cross-sectional data collected suggests that long-term adherence to the Paleolithic diet may not be beneficial for gut health, due to the association with lower relative abundances of known beneficial bacterial genera, and the increased relative abundance of TMA producing genera Hungatella. Our findings highlight that further research is required to understand the role of vegetables and saturated fat and how they influence colonic uptake of SCFA and subsequent excretion. The rationale to exclude whole grains is not supported by data presented here; being inversely associated with body weight and TMAO concentrations. Despite the maintenance of SCFA excretion, and stool frequency and form, the differences noted in microbiota composition associated with the high fat and low carbohydrate intake may not be beneficial for long-term health.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to acknowledge the valuable contribution of the study participants, who donated their time and effort to complete the research study. We also acknowledge and thank the CSIRO for providing laboratory expertise and support, namely from Bruce May and Julie Dallimore, who completed the analysis of stool biochemistry parameters. In addition, we would like to thank Paul Greenfield, from CSIRO, for his assistance and training in analysing the microbiota sequence files using the GHAP pipeline. The authors would also like to thank the Western Australian Department of Health for their funding of the Western Australian Human Microbiome Collaboration Centre (WAHMCC) as part of their support to the Telethon—Perth Children’s Hospital Research Fund (Round 4, Stream 2).
Abbreviations
- TMAO
Trimethylamine-N-oxide
- SP
Strict Paleolithic group
- PP
Psuedo-Paleolithic group
- PD
Paleolithic diet
- RS
Resistant starch
Author contributions
AG, CC, JL, MB, PL-W and AD designed the research study, developed research plan and scope. AG conducted the research study and collated data. MB and ML provided laboratory expertise and assistance, AG conducted statistical analysis, with input from JL. AB provided dietary fiber and RS expertise. AG, CC, JL, MB, PL-W, AB and AD wrote the paper, AG had responsibility for final content.
Compliance with ethical standards
Conflict of interest
The Authors declare no conflict of interest.
Data sharing
Data described in the manuscript, code book and analytic code will not be made available at the current time due to ongoing metabolomics analysis of samples.
Sources of support
The first author was the recipient of a postgraduate research scholarship from Edith Cowan University and Commonwealth Scientific and Industrial Research Organisation (CSIRO).
Contributor Information
Angela Genoni, Email: a.genoni@ecu.edu.au.
Claus T. Christophersen, Email: c.christophersen@ecu.edu.au
Johnny Lo, Email: j.lo@ecu.edu.au.
Mary C. Boyce, Email: m.boyce@ecu.edu.au
Anthony R. Bird, Email: Tony.Bird@csiro.au
Philippa Lyons-Wall, Email: p.lyons-wall@ecu.edu.au.
Amanda Devine, Email: a.devine@ecu.edu.au.
References
- 1.Eaton SB, Konner M. Paleolithic nutrition: a consideration of its nature and current implications. N Engl J Med. 1985;312(5):283–289. doi: 10.1056/NEJM198501313120505. [DOI] [PubMed] [Google Scholar]
- 2.Evans P. The complete gut health cookbook. Sydney: Pan Macmillan Australia Pty Ltd; 2016. [Google Scholar]
- 3.National Health and Medical Research Council (2013) Australian Dietary Guidelines. National Health and Medical Research Council, Canberra. https://www.nhmrc.gov.au/guidelines-publications/n55. Accessed 15 June 2018
- 4.US Department of Health and Human Services, & US Department of Agriculture (2015) 2015-2020 Dietary Guidelines for Americans, 8th edn. https://health.gov/dietaryguidelines/2015/resources/2015-2020_Dietary_Guidelines.pdf. Accessed 15 June 2018
- 5.Genoni A, Lyons-Wall P, Lo J, Devine A. Cardiovascular, metabolic effects and dietary composition of ad libitum Paleolithic vs. Australian guide to healthy eating diets: a 4-week randomised trial. Nutrients. 2016 doi: 10.3390/nu8050314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Genoni A, Lo J, Lyons-Wall P, Boyce MC, Christophersen CT, Bird A, et al. A Paleolithic diet lowers resistant starch intake but does not affect serum trimethylamine-N-oxide concentrations in healthy women. Br J Nutr. 2018 doi: 10.1017/s000711451800329x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shortt C, Hasselwander O, Meynier A, Nauta A, Fernandez EN, Putz P, et al. Systematic review of the effects of the intestinal microbiota on selected nutrients and non-nutrients. Eur J Nutr. 2017;57(1):25–49. doi: 10.1007/s00394-017-1546-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lockyer S, Nugent AP. Health effects of resistant starch. Nutr Bull. 2017;42(1):10–41. doi: 10.1111/nbu.12244. [DOI] [Google Scholar]
- 9.Sajilata MG, Singhal RS, Kulkarni PR. Resistant starch: a review. Compr Rev Food Sci Food Saf. 2006;5(1):1–17. doi: 10.1111/j.1541-4337.2006.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 10.Schwiertz A, Lehmann U, Jacobasch G, Blaut M. Influence of resistant starch on the SCFA production and cell counts of butyrate-producing Eubacterium spp. in the human intestine. J Appl Microbiol. 2002;93(1):157–162. doi: 10.1046/j.1365-2672.2002.01679.x. [DOI] [PubMed] [Google Scholar]
- 11.Topping DL, Fukushima M, Bird AR. Resistant starch as a prebiotic and synbiotic: state of the art. Proc Nutr Soc. 2003;62(1):171–176. doi: 10.1079/PNS2002224. [DOI] [PubMed] [Google Scholar]
- 12.Topping DL, Morell MK, King RA, Li Z, Bird AR, Noakes M. Resistant starch and health—Himalaya 292 a novel barley cultivar to deliver benefits to consumers. Starch Stärke. 2003;55(12):539–545. doi: 10.1002/star.200300221. [DOI] [Google Scholar]
- 13.Brinkworth GD, Noakes M, Clifton PM, Bird AR. Comparative effects of very low-carbohydrate, high-fat and high-carbohydrate, low-fat weight-loss diets on bowel habit and faecal short-chain fatty acids and bacterial populations. Br J Nutr. 2009;101(10):1493–1502. doi: 10.1017/s0007114508094658. [DOI] [PubMed] [Google Scholar]
- 14.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Keefe SJ, Li JV, Lahti L, Ou J, Carbonero F, Mohammed K, et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun. 2015;6:6342. doi: 10.1038/ncomms7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell WR, Gratz SW, Duncan SH, Holtrop G, Ince J, Scobbie L, et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am J Clin Nutr. 2011;93(5):1062–1072. doi: 10.3945/ajcn.110.002188. [DOI] [PubMed] [Google Scholar]
- 17.Cho CE, Taesuwan S, Malysheva OV, Bender E, Tulchinsky NF, Yan J, et al. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: a randomized controlled trial. Mol Nutr Food Res. 2017 doi: 10.1002/mnfr.201600324. [DOI] [PubMed] [Google Scholar]
- 18.Al-Waiz M, Mikov M, Mitchell SC, Smith RL. The exogenous origin of trimethylamine in the mouse. Metab Clin Exp. 1992;41(2):135–136. doi: 10.1016/0026-0495(92)90140-6. [DOI] [PubMed] [Google Scholar]
- 19.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang WH, Wang Z, Li XS, Fan Y, Li DS, Wu Y, et al. Increased Trimethylamine N-oxide portends high mortality risk independent of glycemic control in patients with type 2 diabetes mellitus. Clin Chem. 2017;63(1):297–306. doi: 10.1373/clinchem.2016.263640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang WHW, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64(18):1908–1914. doi: 10.1016/j.jacc.2014.02.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manousou S, Stal M, Larsson C, Mellberg C, Lindahl B, Eggertsen R, et al. A Paleolithic-type diet results in iodine deficiency: a 2-year randomized trial in postmenopausal obese women. Eur J Clin Nutr. 2018;72(1):124–129. doi: 10.1038/ejcn.2017.134. [DOI] [PubMed] [Google Scholar]
- 23.Mellberg C, Sandberg S, Ryberg M, Eriksson M, Brage S, Larsson C, et al. Long-term effects of a Palaeolithic-type diet in obese postmenopausal women: a 2-year randomized trial. Eur J Clin Nutr. 2014;68(3):350–357. doi: 10.1038/ejcn.2013.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otten J, Mellberg C, Ryberg M, Sandberg S, Kullberg J, Lindahl B, et al. Strong and persistent effect on liver fat with a Paleolithic diet during a 2-year intervention. Int J Obes (Lond) 2016;40(5):747–753. doi: 10.1038/ijo.2016.4. [DOI] [PubMed] [Google Scholar]
- 25.International Physical Activity Questionnaire (2002) International Physical Activity Questionnaires: IPAQ: Short Form. https://sites.google.com/site/theipaq/. Accessed 1 Feb 2016
- 26.Mariotti F, Tomé D, Mirand PP. Converting nitrogen into protein—beyond 6.25 and Jones’ factors. Crit Rev Food Sci Nutr. 2008;48(2):177–184. doi: 10.1080/10408390701279749. [DOI] [PubMed] [Google Scholar]
- 27.Bingham SA. Urine nitrogen as a biomarker for the validation of dietary protein intake. J Nutr. 2003;133(3):921S–924S. doi: 10.1093/jn/133.3.921S. [DOI] [PubMed] [Google Scholar]
- 28.Goldberg G, Black A, Jebb S, Cole T, Murgatroyd P, Coward W, et al. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur J Clin Nutr. 1991;45(12):569–581. [PubMed] [Google Scholar]
- 29.Xyris Software (2015) FoodWorks Professional (Version 8.0). QLD, Australia
- 30.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on high blood pressure research. Circulation. 2005;111(5):697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 31.Marfell-Jones M, Stewart A, Olds T (2006) Kinanthropometry IX: Proceedings of the 9th International Conference of the International Society for the Advancement of Kinanthropometry: Routledge
- 32.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32(9):920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 33.Bajka BH, Topping DL, Cobiac L, Clarke JM. Butyrylated starch is less susceptible to enzymic hydrolysis and increases large-bowel butyrate more than high-amylose maize starch in the rat. Br J Nutr. 2006;96(2):276–282. doi: 10.1079/BJN20061807. [DOI] [PubMed] [Google Scholar]
- 34.Le TT, Shafaei A, Genoni A, Christophersen C, Devine A, Lo J, et al. Development and validation of a simple LC-MS/MS method for the simultaneous quantitative determination of trimethylamine-N-oxide and branched chain amino acids in human serum. Anal Bioanal Chem. 2018 doi: 10.1007/s00216-018-1522-8. [DOI] [PubMed] [Google Scholar]
- 35.Faul F, Buchner A, Erdfelder E, Lang A. G*Power. Kiel: University of Kiel; 2014. [Google Scholar]
- 36.IBM Corporation (2016) SPSS for Windows (Version 24.0.0.0). Chicago
- 37.Quest Research Limited (2017) Primer 7, Version 7.0.13 (Version 7.0.13). Auckland, New Zealand
- 38.Giavarina D. Understanding Bland Altman analysis. Biochem Med (Zagreb) 2015;25(2):141–151. doi: 10.11613/BM.2015.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization (2018) Draft guidelines: saturated fatty acid and trans-fatty acid intake for adults and children. https://extranet.who.int/dataform/upload/surveys/666752/files/Draft%20WHO%20SFA-TFA%20guidelines_04052018%20Public%20Consultation(1).pdf. Accessed 15 May 2018 [PubMed]
- 40.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 41.Koliada A, Syzenko G, Moseiko V, Budovska L, Puchkov K, Perederiy V, et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017;17(1):120. doi: 10.1186/s12866-017-1027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romano KA, Vivas EI, Amador-Noguez D, Rey FE. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. MBio. 2015;6(2):e02481. doi: 10.1128/mBio.02481-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Charrier JA, Martin RJ, McCutcheon KL, Raggio AM, Goldsmith F, Goita M, et al. High fat diet partially attenuates fermentation responses in rats fed resistant starch from high-amylose maize. Obesity (Silver Spring) 2013;21(11):2350–2355. doi: 10.1002/oby.20362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bergeron N, Williams PT, Lamendella R, Faghihnia N, Grube A, Li X, et al. Diets high in resistant starch increase plasma levels of trimethylamine-N-oxide, a gut microbiome metabolite associated with CVD risk. Br J Nutr. 2016;116(12):2020–2029. doi: 10.1017/S0007114516004165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang ZN, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–U82. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley R, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–485. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang B, Zhao Q, Guo W, Bao W, Wang X. Association of whole grain intake with all-cause, cardiovascular, and cancer mortality: a systematic review and dose-response meta-analysis from prospective cohort studies. Eur J Clin Nutr. 2018;72(1):57–65. doi: 10.1038/ejcn.2017.149. [DOI] [PubMed] [Google Scholar]
- 48.Topping DL, Segal I, Regina A, Conlon M, Bajka B, Toden S, et al. Resistant starch and human health. In: van der Kamp JW, et al., editors. Dietary fibre: new frontiers for food and health. Wageningen: Wageningen Academic Publishers; 2010. [Google Scholar]
- 49.Ranhotra G (2008) Resistant starch: health aspects and food uses. In: Advanced dietary fibre technology. Blackwell Science Ltd, pp 424–429
- 50.Champagne CM, Bray G, Sacks F, Miketinas D. Fiber intake, dietary energy density, and diet-type predict 6-month weight-loss in free-living adults who adhered to prescribed macronutrient and energy composition of varying diets. FASEB J. 2017;31(1 Supplement):796. [Google Scholar]
- 51.Cummings JH, Bingham SA, Heaton KW, Eastwood MA. Fecal weight, colon cancer risk, and dietary intake of nonstarch polysaccharides (dietary fiber) Gastroenterology. 1992;103(6):1783–1789. doi: 10.1016/0016-5085(92)91435-7. [DOI] [PubMed] [Google Scholar]
- 52.de Vries J, Birkett A, Hulshof T, Verbeke K, Gibes K. Effects of cereal, fruit and vegetable fibers on human fecal weight and transit time: a comprehensive review of intervention trials. Nutrients. 2016;8(3):130. doi: 10.3390/nu8030130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang T, Xu M, Lee A, Cho S, Qi L. Consumption of whole grains and cereal fiber and total and cause-specific mortality: prospective analysis of 367,442 individuals. BMC Med. 2015;13:59. doi: 10.1186/s12916-015-0294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jenkins DJ, Kendall CW, Popovich DG, Vidgen E, Mehling CC, Vuksan V, et al. Effect of a very-high-fiber vegetable, fruit, and nut diet on serum lipids and colonic function. Metab Clin Exp. 2001;50(4):494–503. doi: 10.1053/meta.2001.21037. [DOI] [PubMed] [Google Scholar]
- 55.Willing BP, Dicksved J, Halfvarson J, Andersson AF, Lucio M, Zheng Z, et al. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology. 2010;139(6):1844–1854.e1841. doi: 10.1053/j.gastro.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 56.Rabot S, Membrez M, Blancher F, Berger B, Moine D, Krause L, et al. High fat diet drives obesity regardless the composition of gut microbiota in mice. Sci Rep. 2016;6:32484. doi: 10.1038/srep32484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simpson HL, Campbell BJ. Review article: dietary fibre–microbiota interactions. Aliment Pharmacol Ther. 2015;42(2):158–179. doi: 10.1111/apt.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roberts J, Jones GP, Rutishauser IHE, Birkett A, Gibbons C. Resistant starch in the Australian diet. Nutr Diet. 2004;61:69–104. [Google Scholar]
- 59.Galea LM, Beck EJ, Probst YC, Cashman CJ. Whole grain intake of Australians estimated from a cross-sectional analysis of dietary intake data from the 2011–13 Australian Health Survey. Public Health Nutr. 2017;20(12):2166–2172. doi: 10.1017/S1368980017001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baghurst PA, Record SJ, Baghurst K. Dietary fibre, non-starch polysaccharides and resistant starch: a review. Sydney: Australian Institute of Food Science and Technology; 1996. [Google Scholar]
- 61.Fava F, Gitau R, Griffin BA, Gibson GR, Tuohy KM, Lovegrove JA. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome ‘at-risk’ population. Int J Obes (Lond) 2013;37(2):216–223. doi: 10.1038/ijo.2012.33. [DOI] [PubMed] [Google Scholar]
- 62.Vogt JA, Wolever TMS. Fecal acetate is inversely related to acetate absorption from the human rectum and distal colon. J Nutr. 2003;133(10):3145–3148. doi: 10.1093/jn/133.10.3145. [DOI] [PubMed] [Google Scholar]
- 63.Ringel-Kulka T, Choi CH, Temas D, Kim A, Maier DM, Scott K, et al. Altered colonic bacterial fermentation as a potential pathophysiological factor in irritable bowel syndrome. Am J Gastroenterol. 2015;110:1339. doi: 10.1038/ajg.2015.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jönsson T, Granfeldt Y, Ahrén B, Branell U-C, Pålsson G, Hansson A, et al. Beneficial effects of a Paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol. 2009;8(1):35. doi: 10.1186/1475-2840-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frassetto LA, Schloetter M, Mietus-Synder M, Morris RC, Sebastian A. Metabolic and physiologic improvements from consuming a paleolithic, hunter-gatherer type diet. Eur J Clin Nutr. 2009;63(8):947–955. doi: 10.1038/ejcn.2009.4. [DOI] [PubMed] [Google Scholar]
- 66.Osterdahl M, Kocturk T, Koochek A, Wändell PE. Effects of a short-term intervention with a paleolithic diet in healthy volunteers. Eur J Clin Nutr. 2008;62(5):682–685. doi: 10.1038/sj.ejcn.1602790. [DOI] [PubMed] [Google Scholar]
- 67.Ryberg M, Sandberg S, Mellberg C, Stegle O, Lindahl B, Larsson C, et al. A Palaeolithic-type diet causes strong tissue-specific effects on ectopic fat deposition in obese postmenopausal women. J Intern Med. 2013;274:67–76. doi: 10.1111/joim.12048. [DOI] [PubMed] [Google Scholar]
- 68.Micha R, Mozaffarian D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: a fresh look at the evidence. Lipids. 2010;45(10):893–905. doi: 10.1007/s11745-010-3393-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peters SAE, Singhateh Y, Mackay D, Huxley RR, Woodward M. Total cholesterol as a risk factor for coronary heart disease and stroke in women compared with men: a systematic review and meta-analysis. Atherosclerosis. 2016;248:123–131. doi: 10.1016/j.atherosclerosis.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 70.Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, Danaei G. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1·8 million participants. Lancet (London, England) 2014;383(9921):970–983. doi: 10.1016/S0140-6736(13)61836-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cordain L. The Paleo diet. Hoboken: Wiley; 2011. [Google Scholar]
- 72.Evans P. Low carb, healthy fat. Sydney: Pan Macmillan Australia; 2017. [Google Scholar]
- 73.Edgar RC (2017). SEARCH_16S: a new algorithm for identifying 16S ribosomal RNA genes in contigs and chromosomes. https://www.biorxiv.org/content/biorxiv/early/2017/04/04/124131.full.pdf. Accessed 1 Apr 2018
- 74.Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, et al. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2008;37(suppl):D141–D145. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sedgwick P. Multiple significance tests: the Bonferroni correction. BMJ. 2012;344:e509. doi: 10.1136/bmj.e509. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.