Abstract
Exposure to cigarette smoke (CS) is associated with an increased risk of several neurological diseases such as stroke, Alzheimer’s disease, and dementia. At present, commercialization of E-cigarettes (ECs) is increasing, and they are advertised as a less harmful nicotine-delivery system. There are, however, limited studies regarding the neurotoxicity effects of ECs on the brain, which remains a subject of debate. In the present study, we aimed to evaluate the in vivo effects of short-term EC vapor exposure on the brain and compare them with the effects of cigarette smoke (CS). BALB/c mice were exposed to air, CS, and EC for 14 days. We then assessed the inflammatory responses, oxidative stress, and cognitive functions of the mice by using maze tests. Cognitive spatial tests showed that the mice exposed to CS and ECs had delayed time in finding food rewards. EC exposure demonstrated no improvement in spatial memory learning to find the food reward on the next day. This implies that CS and EC exposure possibly causes damage to the olfactory system. Notably, EC exposure potentially causes abnormalities in mice memory functions. Histological staining of the cerebral cortex of mice brain in the EC-exposed group demonstrated inflammatory responses such as necrosis and cytoplasm vacuolization. Immunohistochemical staining revealed high expression of proinflammatory cytokine TNF-α in both the EC- and CS-exposed groups. Hence, we conclude that ECs share similar toxicity profiles as CS, which potentially negatively impact brain function.
Keywords: Brain, Cigarette, Cognitive, Inflammation, Memory, Vape
Introduction
Cigarette smoking (CS) has been reported to be associated with numerous negative outcomes including possible effects on the brain [1–3]. Evidence suggests that smokers experience decreased functions in several cognitive domains such as cognitive flexibility and memory [4–6]. Several studies have also reported that smoking is associated with an increased risk of dementia, and it is estimated that nearly 14% of Alzheimer’s disease cases worldwide could be attributable to smoking [7–9]. Electronic cigarettes (ECs) are a recent innovation that are advertised as a safe way to help people quit smoking tobacco cigarettes [10]. However, studies regarding the effects of ECs on brain cognitive functions remain limited.
ECs are devices that effectively deliver vaporized liquid nicotine to the lungs. The user can choose nicotine concentration of the EC liquid that is loaded into the device’s cartridge [11]. When the user inhales, the e-liquid, which is primarily nicotine in propylene glycol (PG) and vegetable glycerin (VG), is heated to produce a vapor that is inhaled into the lungs. ECs are assumed to generate fewer noxious materials/toxicants, but they are not emission-free devices [12–14]. Hence, ECs could possibly share some of the toxicant profiles of CS and may expose users to similar health risks.
Although ECs are marketed as perfectly harmless nicotine-delivery devices, emerging evidence implies that ECs may cause damage to biological systems [15–17]. Recent in vitro studies demonstrated that although ECs are less cytotoxic than CS, they can promote cytotoxicity in human pulmonary fibroblasts cells, primary human bronchial epithelial cells, and human gingival fibroblasts (HGFs) [18–20]. In vivo studies in mice show that EC exposure stunts growth and induces an allergy-based asthma inflammatory response [21, 22]. Furthermore, ECs enhance oxidative stress and inflammation in mice and impair immune defenses against bacterial and viral infections [23]. To the best of our knowledge, studies on the effects of ECs on cognition remain lacking. Exposure to CS has reportedly been linked to neurodevelopmental delays and cognitive impairment, including attention deficit disorder and lower IQ [24].
The present study examined the effects of short-term EC exposure over 14 days on brain inflammatory responses associated with cognitive spatial and memory functions in mice. We found that inhaled EC vape triggered neurotoxicity that induces brain inflammatory effects similar to those observed in the CS-exposed group. We posit that these toxicological effects are associated with decreased cognitive spatial and memory functions of EC and CS as compared to control mice. The current data provide direct evidence of the potential harmful neurotoxicity effects of ECs on brain inflammation associated with cognitive memory functions.
Materials and methods
Chemicals
The e-liquid mixture brand Zero e-liquid (18 mg/mL nicotine, 30% PG, 70% VG, with a grape flavor) was obtained from Vape Clinic. Joyetech 510-T ECs were used for all the experiments with 510-T tank cartridges, atomizers, and batteries. Primary antibodies against TNF-α were acquired from Fine Biotech (Wuhan, China). All the other chemicals were purchased from Sigma Aldrich (St. Louis, MO, USA).
Animals
Three-month-old male BALB/c mice were purchased from the Medical Faculty, University of Mataram (Mataram, Indonesia) and were housed in groups of 3–6 at room temperature (24 °C) under 12/12 h light/dark cycles with access to water and food ad libitum. Body weight was measured daily during the 14 days of CS and EC exposure. All the animal experimental protocols were approved by the ethics committee of the Faculty of Medicine, University of Mataram, Indonesia.
CS and EC exposure protocol
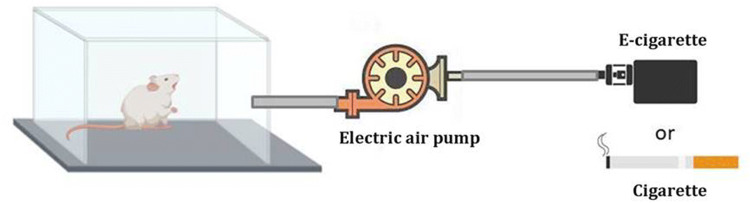
CS and EC exposure on the mice was conducted in a smoke/vapor whole body exposure chamber system (Fig. 1). A total of 18 rats exposed to CS and ECs were randomized into three groups of 6 animals each as follows: group 1: control group with no CS and EC treatment in which the mice were exposed to clean air; group 2: cigarette-treated group in which the mice were exposed to tobacco smoke of six simultaneously lit cigarettes; and group 3: EC-treated group in which the mice were exposed to a total of 150 puffs per day. The puff duration was 3 s, the puff interval was 1 min, and the puff volume was 50 mL, all of which mimic real-life exposure scenarios.
Fig. 1.
Schematic diagram of smoke and vapor from EC/CS pumped into the chamber by electric air pump (Agptek co., Ltd., USA)
Learning and memory assessment
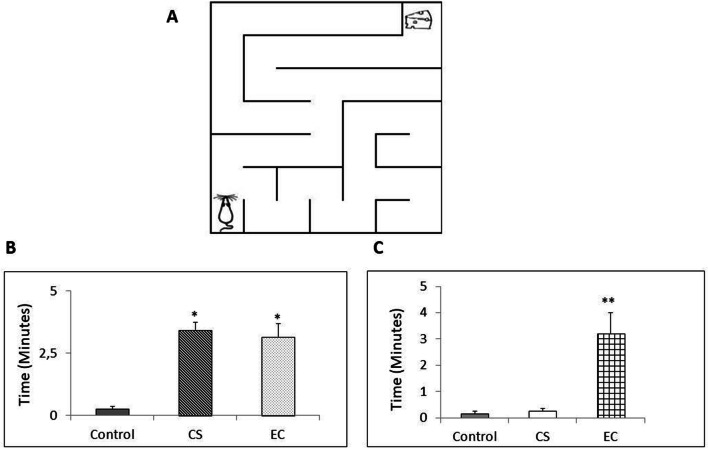
Learning and memory functions of the mice were evaluated with buried food as a reward in maze tests [25]. Prior to training, the mice were placed on a food-restricted schedule for 2 days to maintain them at 80–85% of their free feeding weight. The next day, the subjects were placed in the maze apparatus (50 × 50 cm) to find the location of the buried food as a reward. The time for the mice to find the reward was measured as the cognitive spatial learning ability. Before conducting each test, the maze apparatus was cleaned with alcohol. The next day (24 h) the mice were reintroduced into the maze apparatus to evaluate their cognitive memory functions. The time for the mice to find the reward was assessed as their short-term memory functions. This procedure was performed on the last day of treatment.
Hematoxylin and eosin (H&E) staining
Brain tissue specimens were fixed in 4% formaldehyde and embedded in paraffin. The brain sections were then deparaffinized in xylene, rehydrated through a graded alcohol series, and stained with H&E (Biosharp, Wuhan, China) according to the manufacturer’s protocol. Pathological changes were observed under an inverted microscope (Zeiss, Gottingen, Germany).
Immunohistochemistry (IHC)
The mouse brains were isolated, fixed in 4% paraformaldehyde (PFA) overnight at 4 °C, and embedded in paraffin [26]. The brain sections were then de-paraffinized in xylene, rehydrated through a graded ethanol series, and subjected to antigen retrieval by boiling the slices in citrate buffer (pH 6.0) with high heat for 15 min and medium heat for 15 min in a microwave oven. For the IHC analysis, the sections were treated with 3% H2O2 for 10 min to remove endogenous peroxidase, blocked with 1% bovine serum albumin (BSA) in PBS (blocking solution) at room temperature for 1 h, and incubated with anti-TNF-α antibodies (Fine Biotech, Wuhan, China) diluted (1:200) in a blocking solution at 4 °C overnight. After washing 3 times in PBS, the sections were incubated with HRP-conjugated secondary antibody (DAKO, Glostrup, Denmark) at room temperature for 30 min and then stained with 3ʹ-diaminobenzidine (DAB) for 15 s. Hematoxylin was used for cell nuclei detection. The stained sections were visualized and digitally scanned with an inverted light microscope (Zeiss).
Statistical analyses
Statistical analyses were conducted using KaleidaGraph 4.5.4 (Synergy Software, Reading, PA, USA) with a two-tailed unpaired Student’s t test and one-way analysis of variance (ANOVA) followed by the Tukey–Kramer test. The data are presented as mean ± standard deviation (SD). Differences among comparisons were considered statistically significant for p-values less than 0.05.
Results
CS and EC exposure causes weight loss of mice body and brain
Three-month-old BALB/c mice were exposed for 14 days to CS and ECs. On the first 6 days of treatment, the mean weight of mice in all the treatment groups was similar. Starting on day 7, the body weights of the CS- and EC-exposed mice slowly declined compared to that of the untreated controls. This condition persisted throughout the 14 days of CS and EC exposure (Fig. 2).
Fig. 2.
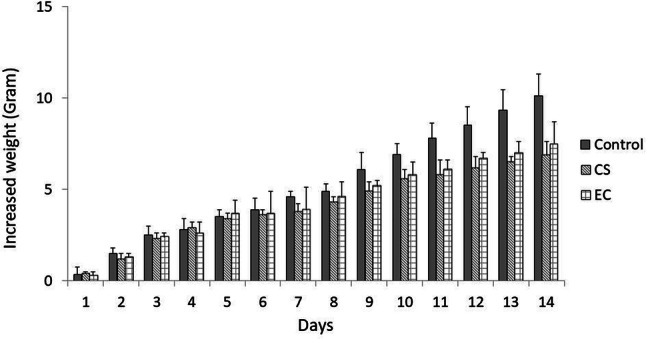
Body weight of mice during the experimental period (14 days). Body weight was measured every day (n = 6 per group)
Evaluation of cognitive memory functions
The evaluation of cognitive functions was performed on the last day of treatment in the maze apparatus (Fig. 3). Analysis of the spatial learning functions showed a statistically significant group effect (Fig. 3a). As expected, the control group exhibited a significantly shorter time to find the buried food reward than the CS- and EC-exposed groups. The CS- and EC-exposed groups required a significantly longer time to find the reward. CS is reported to cause nearly six-fold more olfactory deficits in smokers than in nonsmokers [27]. The current result implies that ECs possibly induce negative effects on olfactory functions similar to those by CS. However, the EC-exposed groups required a significantly longer time to find the reward the next day (Fig. 3b), indicating that ECs potentially cause reduced cognitive memory functions.
Fig. 3.
a Maze apparatus for assessment of cognitive learning and memory functions (50cm × 50cm). b CS and EC effects on cognitive learning test. Values are mean ± SEM, n = 6 animals per group. **p < 0.01 vs control. *p < 0.05 vs control. c CS and EC effects on short term memory test. Values are mean ± SEM, n = 6 animals per group. **p < 0.01 vs control. *p < 0.05 vs control
Histopathological injuries in the brains of mice exposed to CS and EC as determined by H&E staining
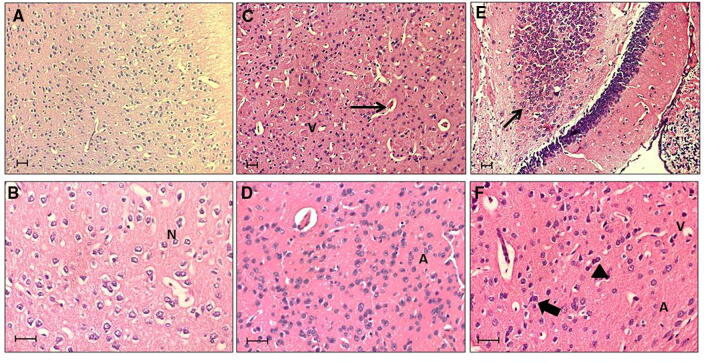
H&E staining reflected the histopathological injuries and inflammation of the mice brains exposed to CS and ECs. No histopathological injuries or inflammatory response were observed in the untreated group, as normal neurons were found to be abundant in the brains of the untreated mice. Inflammatory characteristics such as necrotic cells and cytoplasmic vacuolization were present in the brain tissues of both CS- and EC-exposed groups. A significant amount of apoptotic cells and dead neurons were also observed (Fig. 4).
Fig. 4.
Hematoxylin and Eosin (H&E)-stained brain sections from mice treated for 14 days with a, b. Clean air (Control) showing Normal Cells (N); c, d. Cigarette Smoking (CS) showing signs of early neuronal injury, vacuolated cells (V), Apoptotic cells (A), and vascular congestion (long arrow); e, f. E-Cigarette (EC) showing hyperchromatic cells (arrow head), cellular atrophy, shrinkage apoptotic cells (A), Vacuolated cells (V), cellular infiltration (long arrow), and Nuclear Swelling (thick arrow). Scale: 20 μM
TNF-α was highly expressed in the brain tissues of CS- and EC-exposed groups as detected by IHC
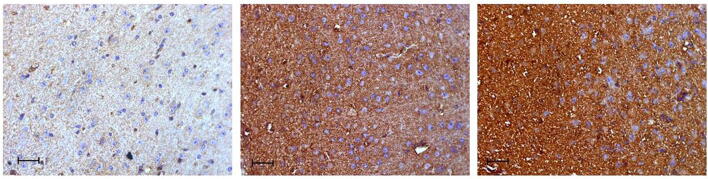
Brain inflammation is reportedly associated with loss of memory functions. Furthermore, proinflammatory cytokine TNF-α is reported to play a critical role in brain immune and inflammatory activities. Therefore, we used IHC to investigate the expression and localization of TNF-α in the cerebral cortex brain sections of the CS- and EC-exposed mice. As shown in Fig. 5, proinflammatory cytokine TNF-α immunoreactivity significantly increased in the CS- and EC-exposed groups compared to the control. Based on this result, ECs have similar toxicity profiles to CS. Hence, EC exposure could potentially correlate with a loss of learning ability and memory functions.
Fig. 5.
Immunohistochemical (IHC) staining of proinflammatory cytokine TNF-α in the cerebral cortex brain tissue of CS and EC exposed mice for 14 days. Brown indicates a positive result of TNF-α immunoreactivity. Scale: 20 μM
Discussion
CS is associated with the cause of several diseases and accounts for more than 18% of annual deaths worldwide [28]. Consequently, because ECs are perceived to have a higher safety profile, their use has markedly increased in the past decade [29]. These devices emit considerable levels of toxicants, some of which are common to tobacco smoking. Thus, their harm should not be underestimated [30]. However, in contrast to the well-known negative effects of tobacco smoking and despite the increase in EC research, the safety profile of ECs has not been fully studied in the context of their effects on brain cognitive functions and memory.
Based on these considerations, in the present study, we aimed to evaluate the potential toxicity effects of ECs on mice brain and the associated neurobehavioral features. Exposure to CS and ECs was adjusted to real-life smoking and puffing topography experimental conditions. At the end of 14 days of treatment, the body weights of the EC-exposed group showed similar profiles to those of the CS-exposed group. Numerous studies have examined the relationship between smoking and body weight [31, 32]. Current smokers tend to have lower BMIs and body weights than former or nonsmokers [33]. In addition, former smokers report gaining weight after quitting smoking [34]. In both humans and rats, nicotine administration or CS has been shown to induce weight loss [35]. Our present study found that exposure to ECs caused significant inhibition of body weight gain in these animals. This effect was clearly seen on day 7 of treatment. However, because our initial experiment was not designed to study body weight changes, we did not measure the food and water consumption of these animals. Therefore, it is unclear whether the effect of ECs on body weight loss is through decreased food intake or through other mechanisms.
Recent research indicates that CS is associated with an increased risk for many biomedical conditions that may directly or indirectly compromise brain neurobiology and neurocognition [8, 36, 37]. However, at present, the neurotoxicity effect of ECs remains a subject of debate. Wistar rats exposed to CS demonstrated a reduction of body and brain weight [38]. Changes in cortical thickness and structure have been reported to correlate with neurocognitive functioning [39]. Our findings show that the CS- and EC-exposed mice had reduced cognitive spatial learning abilities and memory functions. Previous studies assumed that CS is adversely associated with cognitive functioning, fine motor speed, flexibility, memory, and sleep quality [40, 41]. The possible mechanism may be the toxic effects of cigarette smoke components, including oxidative stress and inflammation of the human brain [42]. In the current study, the EC group demonstrated similar performance to the CS group in learning the spatial maze apparatus and finding the buried food as a reward. Hence, ECs could potentially share similar neurotoxicity effects as CS. This result also overlaps with previous findings that reported attention and working memory deficits in cigarette smoke-exposed mice [43]. In addition, EC exposure showed no improvement in finding the reward the next day after cognitive spatial learning. This implies that the short-term EC group potentially had reduced memory functions compared to the CS and control groups (Fig. 3b). Previous research reported that memory deficits in Alzheimer’s patients are often correlated with their history of smoking habits [44]. Other evidence suggests that smokers experience poorer global cognitive flexibility and memory [45]. However, the effects of CS on memory functions were not observed during a short-term exposure period. Our study showed the effects of ECs on memory functions during a 14-day exposure period. Previous research also reported that ECs may affect the central nervous system and alter brain functions, which affects the mood, learning abilities, and memory and can even induce drug dependence in both humans and animals [46].
Inflammation is an immune response to injury, pathogens, irritants, or oxidative stress [47]. Accumulating evidence has linked inflammation to cognitive decline and risk of dementia [48]. Normally, inflammation is a protective response that facilitates the healing process [49]. However, prolonged inflammation can cause tissue damage. Several studies have reported evidence that cigarette smoke induces brain inflammation and oxidative stress [50]. Inflammation in specific regions of the brain can potentially induce several neurological diseases. ECs have been advertised as safe nicotine-delivery systems for those who want to quit cigarette smoking. Our current results show that necrotic cells were abundant in the EC brain section, similar to CS. Cells undergoing necrosis lose membrane integrity and leak their intracellular components, some of which serve as danger signals that stimulate inflammation [51]. Apoptotic cells may not stimulate inflammation if they are ingested by phagocytes before they release their intracellular contents [52]. Furthermore, EC brain injury is also revealed by the presence of vacuolization, which is also known to cause caspase-independent cell death including methuosis, paraptosis, oncosis, and necroptosis [53].
To confirm the specific events of inflammation, we examined the expression of inflammatory marker TNF-α in the brain tissue of the CS- and EC-exposed mice. Cigarette smoking is often associated with increased inflammatory cytokines such as TNF-α, IL-β, and IL-6 in the blood and organs [54, 55]. Our findings showed that TNF-α immunoreactivity increased in the brain sections of the EC-exposed mice. Hence, EC exposure could potentially induce brain inflammation, which leads to deficits in cognitive functions. In a previous clinical study, TNF-α did not emerge as a significant marker of cognitive function [56]. However, another study reported that proinflammatory cytokines IL-1 and TNF-α could stimulate the hypothalamic–pituitary–adrenal (HPA) axis, further contributing to stress-induced cognitive deficits [57]. Hence, it appears that the increased expression of TNF-α as a proinflammatory cytokine may also mediate the effects of stress, which alters cognitive function.
In conclusion, these findings are not only expected to increase awareness of the negative health consequences of increasingly popular e-cigarettes but also to add information for consideration of their safety profile. Overall, our current results provide evidence of an association of EC-induced inflammation in the brain, which potentially correlates with a deficit in cognitive and memory functions. Whether ECs are a safer substitute for tobacco needs further investigation and will be the subject of future studies.
Acknowledgments
This work was supported by an Indonesian Ministry of Research, Technology, and Higher Education grant in 2018.
Compliance with ethical standards
Conflict of interest
The authors have no conflicts of interest.
References
- 1.Chan YL, Saad S, Al-Odat I, Zaky AA, Oliver B, Pollock C, Li W, Jones NM, Chen H. Impact of maternal cigarette smoke exposure on brain and kidney health outcomes in female offspring. Clin Exp Pharmacol Physiol. 2016;43:1168–1176. doi: 10.1111/1440-1681.12659. [DOI] [PubMed] [Google Scholar]
- 2.Massarsky A, Prasad GL, Di Giulio RT. Total particulate matter from cigarette smoke disrupts vascular development in zebrafish brain (Danio rerio) Toxicol Appl Pharmacol. 2018;339:85–96. doi: 10.1016/j.taap.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Yuan M, Cross SJ, Loughlin SE, Leslie FM. Nicotine and the adolescent brain. J Physiol. 2015;593:3397–3412. doi: 10.1113/JP270492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendrek A, Monterosso J, Simon SL, Jarvik M, Brody A, Olmstead R, Domier CP, Cohen MS, Ernst M, London ED. Working memory in cigarette smokers: comparison to non-smokers and effects of abstinence. Addict Behav. 2006;31:833–844. doi: 10.1016/j.addbeh.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bashir S, Alghamd F, Alhussien A, Alohali M, Alatawi A, Almusned T, Habib SS. Effect of smoking on cognitive functioning in young saudi adults. Med Sci Monit Basic Res. 2017;23:31–35. doi: 10.12659/MSMBR.902385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong JS, Kim SM, Jung HY, Kang KD, Min KJ, Han DH. Cognitive avoidance and aversive cues related to tobacco in male smokers. Addict Behav. 2017;73:158–164. doi: 10.1016/j.addbeh.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Zhong G, Wang Y, Zhang Y, Guo JJ, Zhao Y. Smoking is associated with an increased risk of dementia: a meta-analysis of prospective cohort studies with investigation of potential effect modifiers. PLoS ONE. 2015;10:e0118333. doi: 10.1371/journal.pone.0118333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durazzo TC, Korecka M, Trojanowski JQ, Weiner MW, O’Hara R, Ashford JW, Shaw LM, Alzheimer’s Disease Neuroimaging Initiative Active cigarette smoking in cognitively-normal elders and probable alzheimer’s disease is associated with elevated cerebrospinal fluid oxidative stress biomarkers. J Alzheimers Dis. 2016;54:99–107. doi: 10.3233/JAD-160413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toda N, Okamura T. Cigarette smoking impairs nitric oxide-mediated cerebral blood flow increase: Implications for Alzheimer’s disease. J Pharmacol Sci. 2016;131:223–232. doi: 10.1016/j.jphs.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Levy DT, Yuan Z, Li Y. The prevalence and characteristics of E-cigarette users in the U.S. Int J Environ Res Public Health. 2017;14:E1200. doi: 10.3390/ijerph14101200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bold KW, Kong G, Cavallo DA, Camenga DR, Krishnan-Sarin S. E-cigarette susceptibility as a predictor of youth initiation of E-cigarettes. Nicotine Tob Res. 2017;20:140–144. doi: 10.1093/ntr/ntw393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sears CG, Hart JL, Walker KL, Robertson RM (2017) Generally recognized as safe: uncertainty surrounding E-cigarette flavoring safety. Int J Environ Res Public Health 14 [DOI] [PMC free article] [PubMed]
- 13.Rom O, Pecorelli A, Valacchi G, Reznick AZ. Are E-cigarettes a safe and good alternative to cigarette smoking? Ann N Y Acad Sci. 2015;1340:65–74. doi: 10.1111/nyas.12609. [DOI] [PubMed] [Google Scholar]
- 14.Simmons VN, Quinn GP, Harrell PT, Meltzer LR, Correa JB, Unrod M, Brandon TH. E-cigarette use in adults: a qualitative study of users’ perceptions and future use intentions. Addict Res Theory. 2016;24:313–321. doi: 10.3109/16066359.2016.1139700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes A, Hendrickson RG. An epidemiologic and clinical description of e-cigarette toxicity. Clin Toxicol (Phila) 2019;57:287–293. doi: 10.1080/15563650.2018.1510503. [DOI] [PubMed] [Google Scholar]
- 16.Bengalli R, Ferri E, Labra M, Mantecca P (2017) Lung toxicity of condensed aerosol from E-CIG liquids: influence of the flavor and the in vitro model used. Int J Environ Res Public Health 14 [DOI] [PMC free article] [PubMed]
- 17.Payne JD, Michaels D, Orellana-Barrios M, Nugent K. Electronic cigarette Toxicity. J Prim Care Community Health. 2017;8:100–102. doi: 10.1177/2150131916668645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sancilio S, Gallorini M, Cataldi A, Sancillo L, Rana RA, di Giacomo V. Modifications in human oral fibroblast ultrastructure, collagen production, and lysosomal compartment in response to electronic cigarette fluids. J Periodontol. 2017;88:673–680. doi: 10.1902/jop.2017.160629. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh A, Coakley RC, Mascenik T, Rowell TR, Davis ES, Rogers K, Webster MJ, Dang H, Herring LE, Sassano MF, et al. Chronic E-cigarette exposure alters the human bronchial epithelial proteome. Am J Respir Crit Care Med. 2018;198:67–76. doi: 10.1164/rccm.201710-2033OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aug A, Altraja S, Kilk K, Porosk R, Soomets U, Altraja A. E-cigarette affects the metabolome of primary normal human bronchial epithelial cells. PLoS ONE. 2015;10:e0142053. doi: 10.1371/journal.pone.0142053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lappas AS, Tzortzi AS, Konstantinidi EM, Teloniatis SI, Tzavara CK, Gennimata SA, Koulouris NG, Behrakis PK. Short-term respiratory effects of e-cigarettes in healthy individuals and smokers with asthma. Respirology. 2018;23:291–297. doi: 10.1111/resp.13180. [DOI] [PubMed] [Google Scholar]
- 22.Clapp PW, Jaspers I. Electronic cigarettes: their constituents and potential links to asthma. Curr Allergy Asthma Rep. 2017;17:79. doi: 10.1007/s11882-017-0747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sussan TE, Gajghate S, Thimmulappa RK, Ma J, Kim JH, Sudini K, Consolini N, Cormier SA, Lomnicki S, Hasan F et al (2015) Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS ONE 10 [DOI] [PMC free article] [PubMed]
- 24.Neal RE, Jagadapillai R, Chen J, Webb C, Stocke K, Greene RM, Pisano MM. Developmental cigarette smoke exposure II: hippocampus proteome and metabolome profiles in adult offspring. Reprod Toxicol. 2016;65:436–447. doi: 10.1016/j.reprotox.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quiroz-Padilla MF, Guillazo-Blanch G, Vale-Martínez A, Martí-Nicolovius M. Excitotoxic lesions of the parafascicular nucleus produce deficits in a socially transmitted food preference. Neurobiol Learn Mem. 2006;86:256–263. doi: 10.1016/j.nlm.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Cardiff RD, Miller CH, Munn RJ. Manual immunohistochemistry staining of mouse tissues using the avidin-biotin complex (ABC) technique. Cold Spring Harb Protoc. 2014;2014:659–662. doi: 10.1101/pdb.prot073429. [DOI] [PubMed] [Google Scholar]
- 27.Katotomichelakis M, Balatsouras D, Tripsianis G, Davris S, Maroudias N, Danielides V, Simopoulos C. The effect of smoking on the olfactory function. Rhinology. 2007;45:273–280. [PubMed] [Google Scholar]
- 28.Onor IO, Stirling DL, Williams SR, Bediako D, Borghol A, Harris MB, Darensburg TB, Clay SD, Okpechi SC, Sarpong DF (2017) Clinical effects of cigarette smoking: epidemiologic impact and review of pharmacotherapy options. Int J Environ Res Public Health 14 [DOI] [PMC free article] [PubMed]
- 29.Glasser AM, Katz L, Pearson JL, Abudayyeh H, Niaura RS, Abrams DB, Villanti AC. Overview of electronic nicotine delivery systems: a systematic review. Am J Prev Med. 2017;52:e33–e66. doi: 10.1016/j.amepre.2016.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salamanca JC, Meehan-Atrash J, Vreeke S, Escobedo JO, Peyton DH, Strongin RM (2018) E-cigarettes can emit formaldehyde at high levels under conditions that have been reported to be non-averse to users. Sci Rep 8 [DOI] [PMC free article] [PubMed]
- 31.Jitnarin N, Kosulwat V, Rojroongwasinkul N, Boonpraderm A, Haddock CK, Poston WSC. The relationship between smoking, body weight, body mass index, and dietary intake among Thai adults: results of the national Thai food consumption survey. Asia Pac J Public Health. 2014;26:481–493. doi: 10.1177/1010539511426473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Audrain-McGovern J, Benowitz N. Cigarette smoking, nicotine, and body weight. Clin Pharmacol Ther. 2011;90:164–168. doi: 10.1038/clpt.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dare S, Mackay DF, Pell JP (2015) Relationship between smoking and obesity: a cross-sectional study of 499,504 middle-aged adults in the UK general population. PLoS ONE 10 [DOI] [PMC free article] [PubMed]
- 34.Aubin HJ, Farley A, Lycett D, Lahmek P, Aveyard P (2012) Weight gain in smokers after quitting cigarettes: meta-analysis. BMJ 345 [DOI] [PMC free article] [PubMed]
- 35.Mangubat M, Lutfy K, Lee ML, Pulido L, Stout D, Davis R, Shin C-S, Shahbazian M, Seasholtz S, Sinha-Hikim A, et al. Effect of nicotine on body composition in mice. J Endocrinol. 2012;212:317–326. doi: 10.1530/JOE-11-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalmijn S, van Boxtel MPJ, Verschuren MWM, Jolles J, Launer LJ. Cigarette smoking and alcohol consumption in relation to cognitive performance in middle age. Am J Epidemiol. 2002;156:936–944. doi: 10.1093/aje/kwf135. [DOI] [PubMed] [Google Scholar]
- 37.Starr JM, Deary IJ, Fox HC, Whalley LJ. Smoking and cognitive change from age 11 to 66 years: a confirmatory investigation. Addict Behav. 2007;32:63–68. doi: 10.1016/j.addbeh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 38.Omotoso GO, Babalola FA. Histological changes in the cerebelli of adult wistar rats exposed to cigarette smoke. Niger J Physiol Sci. 2014;29:43–46. [PubMed] [Google Scholar]
- 39.Jacobus J, Squeglia LM, Sorg SF, Nguyen-Louie TT, Tapert SF. Cortical thickness and neurocognition in adolescent marijuana and alcohol users following 28 days of monitored abstinence. J. Stud. Alcohol Drugs. 2014;75:729–743. doi: 10.15288/jsad.2014.75.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bryant VE, Kahler CW, Devlin KN, Monti PM, Cohen RA. The effects of cigarette smoking on learning and memory performance among people living with HIV/AIDS. AIDS Care. 2013;25:1308–1316. doi: 10.1080/09540121.2013.764965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J-T, Lee I-H, Wang C-H, Chen K-C, Lee C-I, Yang Y-K. Cigarette smoking might impair memory and sleep quality. J Formos Med Assoc. 2013;112:287–290. doi: 10.1016/j.jfma.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Sartori AC, Vance DE, Slater LZ, Crowe M. The impact of inflammation on cognitive function in older adults: implications for health care practice and research. J Neurosci Nurs. 2012;44:206–217. doi: 10.1097/JNN.0b013e3182527690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang L, Spencer TJ, Biederman J, Bhide PG. Attention and working memory deficits in a perinatal nicotine exposure mouse model. PLoS ONE. 2018;13:e0198064. doi: 10.1371/journal.pone.0198064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kukull WA. The association between smoking and Alzheimer’s disease: effects of study design and bias. Biol Psychiat. 2001;49:194–199. doi: 10.1016/s0006-3223(00)01077-5. [DOI] [PubMed] [Google Scholar]
- 45.Corley J, Gow AJ, Starr JM, Deary IJ. Smoking, childhood IQ, and cognitive function in old age. J Psychosom Res. 2012;73:132–138. doi: 10.1016/j.jpsychores.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 46.Ponzoni L, Moretti M, Sala M, Fasoli F, Mucchietto V, Lucini V, Cannazza G, Gallesi G, Castellana CN, Clementi F, Zoli M, Gotti C, Braida D. Different physiological and behavioural effects of e-cigarette vapour and cigarette smoke in mice. Eur Neuropsychopharmacol. 2015;25:1775–1786. doi: 10.1016/j.euroneuro.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 47.Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2017;9:7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peila R, Launer LJ. Inflammation and dementia: epidemiologic evidence. Acta Neurol Scand Suppl. 2006;185:102–106. doi: 10.1111/j.1600-0404.2006.00693.x. [DOI] [PubMed] [Google Scholar]
- 49.Hart J. Inflammation. 1: its role in the healing of acute wounds. J Wound Care. 2002;11:205–209. doi: 10.12968/jowc.2002.11.6.26411. [DOI] [PubMed] [Google Scholar]
- 50.Wilson CB, McLaughlin LD, Nair A, Ebenezer PJ, Dange R, Francis J. Inflammation and oxidative stress are elevated in the brain, blood, and adrenal glands during the progression of post-traumatic stress disorder in a predator exposure animal model. PLoS ONE. 2013;8:e76146. doi: 10.1371/journal.pone.0076146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rock KL, Kono H. The inflammatory response to cell death. Annu Rev Pathol. 2008;3:99–126. doi: 10.1146/annurev.pathmechdis.3.121806.151456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang Y, Jiang G, Zhang P, Fan J (2015) Programmed cell death and its role in inflammation. Mil Med Res 2 [DOI] [PMC free article] [PubMed]
- 53.Shubin AV, Demidyuk IV, Komissarov AA, Rafieva LM, Kostrov SV. Cytoplasmic vacuolization in cell death and survival. Oncotarget. 2016;7:55863–55889. doi: 10.18632/oncotarget.10150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petrescu F, Voican SC, Silosi I. Tumor necrosis factor-α serum levels in healthy smokers and nonsmokers . Int J Chron Obstruct Pulmon Dis. 2010;5:217–222. doi: 10.2147/copd.s8330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanni SE, Pelegrino NR, Angeleli AY, Correa C, Godoy I. Smoking status and tumor necrosis factor-alpha mediated systemic inflammation in COPD patients. J Inflamm (Lond) 2010;7:29. doi: 10.1186/1476-9255-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, Launer L, Kuller L, Rubin S, Harris T. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61:76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- 57.Abramson JL, Vaccarino V. Relationship between physical activity and inflammation among apparently healthy middle-aged and older US adults. Arch Intern Med. 2002;162:1286–1292. doi: 10.1001/archinte.162.11.1286. [DOI] [PubMed] [Google Scholar]