Abstract
Organophosphate and carbamate (OPC) poisoning is a major global health hazard requiring immediate medical intervention. Atropine (ATR) is an essential antidote in organophosphate and carbamate poisoning, with the inclusion of cholinesterase reactivators and other anticholinergics, namely pralidoxime (PAM) and glycopyrrolate (GPR). This study aimed to compare the efficacy of various treatment regimens and identify the factors affecting mortality. The data of patients presented at the emergency unit with the consumption of OPC compounds between the years 2013 and 2017 were retrospectively reviewed. The study population was then categorized into four treatment patterns (1) ATR alone, (2) ATR and PAM, (3) ATR and GPR, (4) ATR, PAM and GPR. The outcome of the patients was assessed in terms of survival, intubation, ICU days, and days of ventilation and hospitalization. Univariate and multivariate analyses were performed to investigate the risk factors associated with mortality and odds ratio (OR). A total of 441 patients were included in the study, of which 69.16% were males, and 375 patients survived. Consumption of poison with a suicidal intention was reported in 98.19% of the patients, and the treatment with ATR and PAM (42.86%) was observed to have lower days of ventilation in comparison to the treatment with ATR and GPR (p = 0.003). Patients requiring intubation were also lowest in the group treated with ATR and PAM (27.51%). The age group of > 50 years (OR 4.275 [CI 2.179–8.387]), male gender (OR 2.608 [CI 1.258–5.406]), and the treatment pattern with ATR, PAM and GPR (OR 2.233 [CI 1.002–4.040]) were independently associated with mortality. In summary, male gender, elderly population, and treatment patterns followed adversely affected the outcome in patients with OPC poisoning.
Keywords: Atropine, Carbamate, Glycopyrrolate, Organophosphate, Older age, Pralidoxime
Introduction
Organophosphate and carbamate (OPC) poisoning occupy a major share of poisoning with the intention of self -harm. It is one of the major public health issues in Asian countries. In developing countries like India that rely mostly on agriculture, the ease of availability and an extensive use of pesticides in horticulture have made this issue portentous. Worldwide, the number of organophosphate pesticide intoxications alone are estimated to be as high as 3,000,000 per year, and the causalities and deaths are about 300,000 per year [1].
OPC compounds, being highly lipid soluble, easily penetrate the skin and gastric mucosa, and are thereby rapidly distributed to tissues and penetrate the blood–brain barrier to act on the CNS system [2]. In the case of OPC poisoning, the inhibition of the acetylcholinesterase activity by the phosphorylation of acetylcholinesterase renders the enzyme inactive, thereby causing the accumulation of acetylcholine at the synapse, leading to the cholinergic toxidrome. The symptoms such as miosis, bronchospasm, increased secretions, vomiting, arrhythmia, fasciculation, and respiratory failure are the results of enhanced muscarinic and nicotinic stimulation [3, 4]. The OPC poisoning is usually determined by verifying the acetylcholinesterase and pseudocholinesterase levels. However, acetylcholinesterase assay requires special precautions during the sample collection, such as rapid dilution and cooling, to avoid ex vivo reactions occurring overtime in acute poisoning [3]. Minor difficulties in the auto analyzer [5] also add up to the unavailability of the analysis in developing nations [6].
Atropine (ATR) remains the mainstay in the treatment of OPC poisoning with its anticholinergic actions and its ability to penetrate the CNS. It competitively inhibits muscarinic acetylcholine receptors and prevents further stimulation. The dose of atropine usually varies from patient to patient. The patients were treated until the suggestive end-points of atropinization such as having a clear chest on auscultations, the absence of secretions, heart rate > 100 bpm, dilation of the pinpointed pupil and dry axillae were achieved. A systematic review by Eddleston et al. reveals that there are more than 30 different dosing regimens in place, and the physicians had to administer a high dose of atropine to attain atropinization, which led to ATR toxicity [7]. ATR toxicity leads to extended hospitalization and associated complications [8].
Apart from atropine, antidotes such as oximes and glycopyrrolate (GPR) were considered based on the patient’s condition. Pralidoxime (PAM), belonging to the oxime group, was reported to reactivate acetylcholinesterase inhibited by the organophosphate compounds. The efficacy and addition of PAM to the treatment regime in organophosphate poisoning is still debated and unconvincing [3]. The usage of PAM is usually avoided in carbamate poisoning unless the poison is unknown and the patient is presented with cholinergic toxidrome [9]. Glycopyrrolate being a Quaternary Ammonium anticholinergic agent exerts its peripheral antimuscarinic actions and is considered to be more potent than ATR [10]. A retrospective study by Arendse et al. suggests that co-administration of GPR with ATR may improve the efficacy of the treatment in comparison to previous studies pertaining to the treatment with atropine alone at the same hospital [11, 12]. However, a previously similar retrospective study at our hospital suggested an increased need for ventilation and intubation with the combined usage of ATR and GPR [13].
We hereby analyzed the data obtained from Kasturba Hospital, Manipal, to determine the effectiveness of various treatment patterns and their outcomes.
Materials and methods
Study setting and data sources
The hospital is a tertiary care centre situated in South India catering to the admission of 78,000 rural population per year. Ethical clearance was obtained from the Institutional Ethics Committee of Kasturba Hospital, Manipal Academy of Higher Education, Manipal, for the disclosure of the records. (IEC: 590/2017). A retrospective descriptive analysis study was performed on the patients identified from January 2013 to September 2017.
The diagnosis was made on the history of poison consumption and clinical characteristics of the patients. The serum pseudocholinesterase levels were assessed to determine OPC poisoning. Though red blood cell cholinesterases are the gold standard in the estimation of OPC poisoning, the facilities to measure were not available at the hospital. The pseudocholinesterase levels were determined colorimetrically based on the assay published by Schmidt et al. using Hitachi Roche Cobas C702 [14]. The reference range followed at our hospital is 5300–12,000 U/L.
Demographic details like age, gender, occupation, social history, and patient outcomes were collected. Information regarding the presence of any psychological illness, the typical route of exposure and estimated quantity of the pesticide ingested were retrieved from the files on a structured proforma. The intention of poisoning was categorized into suicidal, accidental, and homicidal as noted in the medical records of the patients. Most of the patients presented were referred cases from the local hospital, and hence, the prehospitalization period was categorized to more or less than 24 h after the consumption of poison. The patients were categorized into four categories based on the treatment regimen followed (1) atropine alone, (2) atropine and pralidoxime, (3) atropine and glycopyrrolate, (4) atropine, pralidoxime and glycopyrrolate. The majority of the severely ill and unresponsive patients were usually assigned the treatment with atropine, pralidoxime and glycopyrrolate at the discretion of the physician in a life-saving approach. Patients who usually required intubation, ventilation and large doses of atropine for achieving atropinization with declining clinical parameters were considered as severely ill and GPR was added as an additional antidote. The severity of poisoning was evaluated in accordance to the WHO International Programme on Chemical Safety [15]. The severity was assigned by considering the most severe signs and symptoms and graded between 0 and 4. Total ATR administered to the patient was calculated by adding all the doses from the day of admission to the day of discharge including the infusion and intermittent dosing. The effectiveness of each treatment pattern was assessed with primary and secondary outcomes.
Study population
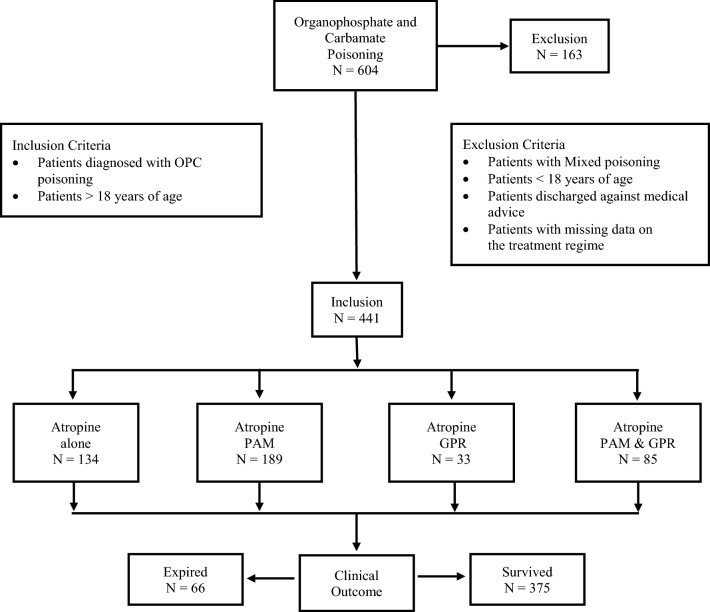
The records of 604 patients presented at the emergency ward with the diagnosis of OPC poisoning were identified electronically using the ICD code T60.0. The data was retrospectively retrieved from medical records manually. The majority of patients were referred from the local hospitals. The follow-up information after their discharge was not considered. A total of 441 adult patients were considered eligible for the study. An exclusion criteria comprised all patients less than 18 years of age, patients with missing data in their treatment regime, patients discharged against medical advice, and patients with mixed poisoning with varied chemicals such as phenol and rodenticides owing to their dissimilar treatment pattern and overall morbidity and mortality (Fig. 1).
Fig. 1.
Patient flow diagram. PAM pralidoxime, GPR glycopyrrolate
Outcomes
Patients diagnosed with OPC poisoning are usually admitted to the ICUs. Primary outcomes were assessed in terms of recovery status as either survived or expired. The survived category included the patients completely recovered and discharged. The secondary outcomes were assessed in terms of days of intubation, ICU days, days on ventilation and days of hospitalization as shown in Table 2.
Table 2.
Outcomes of the patients, serum pseudocholinesterase levels and atropine utilization
| Treatment pattern | |||||
|---|---|---|---|---|---|
| Parameter | ATR alone N = 134 n (%) |
ATR and PAM N = 189 n (%) |
ATR and GPR N = 33 n (%) |
ATR, PAM and GPR N = 85 n (%) |
p value |
| Primary outcome | |||||
| Survived | 115 (85.82%) | 167 (88.36%) | 29 (87.88%) | 64 (75.29%) | 0.041c |
| Expired | 19 (14.18%) | 22 (11.64%) | 4 (12.12%) | 21 (24.71%) | |
| Secondary outcomes | |||||
| Intubation | 55 (41.04) | 52 (27.51%) | 18 (54.55%) | 43 (50.59%) | < 0.001c |
| ICU days, mean (SD) | 9.49 (6.04) | 9.16 (5.72) | 11.63 (5.95) | 11.51 (7.80) | |
| ICU days, median (95% CI) | 8 (5–12) | 8 (6–11) | 12 (7–15) | 10 (6–15) | 0.022a |
| Days on ventilator, mean (SD) | 9.15 (6.72) | 8.80 (7.28) | 11.84 (4.18) | 10.71 (7.41) | |
| Days on ventilator, median (95% CI) | 8 (3.25–13) | 8 (4, 11) | 12 (7, 14) | 9.5 (5–14.75) | 0.030a |
| Hospitalization days, mean (SD) | 13.11 (8.33) | 12.39 (7.17) | 13.79 (7.68) | 15.16 (9.92) | |
| Hospitalization days, median (95% CI) | 11 (8.75–16) | 11 (8–15.5) | 13 (7–19) | 13 (8.5–19) | 0.168a |
| Poison Severity Score | |||||
| Minor | 78 (58.21) | 96 (50.79) | 21 (63.64) | 25 (29.41) | < 0.001c |
| Moderate | 25 (18.66) | 52 (27.51) | 7 (21.21) | 24 (28.24) | 0.237c |
| Severe | 12 (8.96) | 19 (10.05) | 1 (3.03) | 15 (17.65) | 0.077c |
| Fatal | 19 (14.18) | 22 (11.64) | 4 (12.12) | 21 (24.71) | 0.041c |
| Serum pseudo-cholinesterase levels | |||||
| Median (95% CI) (IU/L) | 601.5 (147.5–3316.75) | 258 (171–2767) | 742 (123–3184) | 242 (160–984) | 0.530a |
| Total dosage of ATR utilized | |||||
| Median (95% CI) (mg) | 454 (146.5–1362.54) | 491.3 (166.4–1281.58) | 687 (106.3–1971) | 864.55 (304.2–2010.4) | 0.016a |
| Total dosage of PAM utilized | |||||
| Median (95% CI) (gm) | NA | 4 (3–6) | NA | 6 (4–7) | < 0.001b |
| Total dosage of GPR utilized | |||||
| Median (95% CI) (mg) | NA | NA | 10.28 (3.6–65.2) | 21.2 (7.8–63.3) | 0.276b |
n number, % percentage, SD standard deviation, CI confidence interval, mg milligram, gm gram, IU/L international units per litre, NA not applicable, ATR atropine, PAM pralidoxime, GPR glycopyrrolate
aKruskal–Wallis test
bMann–Whitney pair-wise comparison test
cChi-Square test
Statistical analysis
All statistical analyses were carried out using the SPSS 20.0 package. The values for continuous variables are expressed as mean and standard deviation (SD); values for categorical variables are expressed as frequency and percentages. The data of days in ICU, days of ventilation, days of hospitalization, pseudocholinesterase levels, and dose of atropine, pralidoxime, glycopyrrolate utilized is represented in the median and quartile values. Kruskal–Wallis’s test was applied for the comparison of the four treatment groups followed by the Mann–Whitney U test for pairwise comparison. With the Bonferroni correction for pair wise comparison, p < 0.05 was divided between six groups (0.05/6) and a value of p ≤ 0.0084 was considered statistically significant for the days of ventilation, days in ICU and the dose of atropine utilized (Table 4). Univariate followed by multivariate regression was used to identify risk factors associated with deterioration of medical conditions in patients poisoned with OPC, and odds ratio (OR) was calculated. Variables significantly associated in OPC poisoning in the univariate analysis were selected as independent variables in the multivariate analysis. The data of patients with unknown poisoning agents and poisoning agents having less than an adequate number of patients (< 10) were not included in the regression analysis. Age stratified into three groups to determine the risk, and p value < 0.05 was considered as statistically significant.
Table 4.
Comparison of days of ventilation, days in ICU and dosage of ATR utilized between various treatment groups
| Comparison of treatment regimena | Days of ventilation | ATR dosage utilized | Days in ICU |
|---|---|---|---|
|
p valueb ≤ 0.0084 |
p valueb ≤ 0.0084 |
p valueb ≤ 0.0084 |
|
| ATR Alone vs ATR and PAM | 0.741 | 0.462 | 0.775 |
| ATR Alone vs ATR and GPR | 0.029 | 0.615 | 0.053 |
| ATR Alone vs ATR and PAM and GPR | 0.223 | 0.002c | 0.056 |
| ATR and PAM vs ATR and GPR | 0.003c | 0.947 | 0.019 |
| ATR and PAM vs ATR, PAM and GPR | 0.126 | 0.006c | 0.018 |
| ATR and GPR vs ATR, PAM and GPR | 0.125 | 0.156 | 0.567 |
aMann–Whitney pair-wise comparison test
bBonferroni correction for pair wise comparison p < 0.05 was divided between six groups (0.05/6) and a value of p ≤ 0.0084 is considered statistically significant
cStatistically significant
Results
Demographics
A total of 441 eligible patients were admitted to the emergency ward with a history of OPC poisoning. The mean age of the study population was 36.81 ± 14.91 years. Gender distribution shows a substantial variation between the male and female population in the study, with males being 305 (69.16%) and females 136 (30.84%) (Table 1). Male population was strikingly high (95.28%) in the patients observed with a history of alcohol consumption.
Table 1.
Demographic data of patients
| Total number of patients | N = 441, n (%) |
|---|---|
| Age, mean (SD) | 36.81 (14.91) |
| Male | 305 (69.16) |
| Female | 136 (30.84) |
| Social history | |
| Alcoholism (% of males) | 106 (95.28) |
| Intention of poisoning | |
| Suicidal | 433 (98.19) |
| Accidental | 6 (1.36) |
| Homicidal | 2 (0.45) |
| Comorbidities | |
| Psychological illness | 73 (16.55) |
| No psychological illness | 368 (83.45) |
| Occupation | |
| Daily wagers | 120 (27.21) |
| Farmers | 102 (23.13) |
| Housewives | 94 (21.32) |
| Salaried | 49 (11.11) |
| Students | 37 (8.39) |
| Business | 15 (3.40) |
| Others | 24 (5.44) |
| Clinical outcome | |
| Survived | 375 (85.03) |
| Expired | 66 (14.97) |
n number, % percentage
There were no cases of poisoning by inhalational or dermal exposure. The consumption of poison with a suicidal intention was widely observed (98.19%), followed by accidental consumption (1.36%). The study population comprised a majority of daily wagers (120) (27.21%), followed by farmers (102) (23.13%), and housewives (94) (21.32%) (Table 1).
There were 13 OPC compounds found in our study. Chlorpyrifos was the most consumed poison in 108 (24.49%) of the total OPC admissions during the study, while in 136 (30.84%) patients the poison ingested was not specified. The patients poisoned with ethion 6, malathion 4, dichlorvos 3 and propoxur 3 were not considered for the univariate analysis (Table 5).
Table 5.
Univariate analysis of factors affecting mortality in OPC poisoning patients
| Factor | Survived 375 n (%) |
Expired 66 n (%) |
OR (95% CI) | p value |
|---|---|---|---|---|
| Poisoning agent | ||||
| Chlorpyrifos (OP) | 91 (24.27) | 17 (25.76) | 1.083 (0.594–1.973) | 0.795 |
| Parathion methyl (OP) | 13 (3.47) | 5 (7.58) | 2.282 (0.786–6.631) | 0.129 |
| Phorate (OP) | 27 (7.20) | 5 (7.58) | 0.914 (0.392–2.850) | 0.914 |
| Monocrotophos (OP) | 22 (5.87) | 3 (4.55) | 0.764 (0.222–2.629) | 0.670 |
| Quinalphos (OP) | 26 (6.93) | 6 (9.09) | 1.342 (0.530–3.399) | 0.535 |
| Dimethoate (OP) | 17 (4.53) | 4 (6.06) | 0.592 (0.442–4.173) | 0.592 |
| Triazophos (OP) | 11 (2.93) | 1 (1.52) | 0.521 (0.065–4.011) | 0.521 |
| Profenofos (OP) | 18 (4.80) | 2 (3.03) | 2.736 (0.140–2.736) | 0.528 |
| Carbofuran (C) | 20 (5.33) | 1 (1.52) | 0.273 (0.036–2.070) | 0.209 |
| Pre-hospitalization period | ||||
| < 24 h | 280 (74.67) | 51 (77.27) | 1.154 (0.620–2.146) | 0.652 |
| > 24 h | 95 (25.33) | 15 (22.73) | 0.867 (0.466–1.613) | 0.652 |
| Type of decontamination | ||||
| Gastric lavage | 24 (6.40) | 3 (4.55) | 0.696 (0.204–2.382) | 0.564 |
| Activated charcoal | 19 (5.07) | 7 (10.61) | 2.217 (0.893–5.504) | 0.086 |
OP organophosphate, C carbamate, OR odds ratio
Patients admitted with OPC poisoning were grouped into one of the four treatment categories.
The four treatment groups were:
ATR alone treatment group: This group consisted of 134 (30.39%) subjects.
ATR and PAM treatment group: This group consisted of 189 (42.86%) subjects.
ATR and GPR treatment group: This group consisted of 33 (7.48%) subjects.
ATR, PAM and GPR: This group consisted of 85 (19.27%) subjects and the treatment included usage of all the three drugs.
Primary outcomes
Primary outcomes of various treatment regimen were assessed in terms of the patient’s condition at discharge (survived or expired). It was observed that the patients treated with ATR and PAM has a better clinical improvement 167 (88.36%) patients than the patients treated with other regimens. A significant difference (p = 0.041) was observed between the treatment groups, and the patient group treated with ATR, PAM and GPR had the least improvement 64 as the group consisted of a majority of severely ill patients (75.29%) (Table 2).
Secondary outcomes
The secondary outcomes of different treatment regimens were assessed in terms of intubation, ICU days, days on the ventilator, and hospitalization days. Hospitalization days were higher in the group treated with ATR, PAM and GPR, whereas the group treated with ATR and PAM had the least number of days of ICU and ventilator (Table 2). The group treated with ATR and PAM was also observed to have the least intubation (27.51%). A significant difference in the rate of intubation (p = 0.001) was observed between the treatment groups. Classification by Poison Severity Score revealed a significant difference in the allotment of minor (p < 0.001) and fatal categories (p = 0.041) (Table 2). The proportion of fatal patients was higher in the male population and in the patients over the age of 50 in the Poison Severity Score (Table 3).
Table 3.
Poison Severity Score across gender and age groups
| Factor | Poison Severity Score | |||
|---|---|---|---|---|
| Minor 220 n (%) |
Moderate 108 n (%) |
Severe 47 n (%) |
Fatal 66 n (%) |
|
| Age | ||||
| 18–30 years | 129 (58.64) | 44 (40.74) | 14 (29.79) | 18 (27.27) |
| 31–50 years | 72 (32.73) | 35 (32.41) | 19 (40.43) | 20 (30.30) |
| 50 years above | 19 (8.64) | 29 (26.85) | 14 (29.79) | 28 (42.42) |
| Gender | ||||
| Female | 78 (35.45) | 33 (30.56) | 15 (31.92) | 10 (15.15) |
| Male | 142 (64.55) | 75 (69.44) | 32 (68.08) | 56 (84.85) |
n number, % percentage
The days of ventilation were analyzed using the Kruskal–Wallis test and Mann–Whitney’s pair-wise comparison test to assess the effectiveness of various treatment patterns. The results of the Kruskal–Wallis test revealed that the median days of ventilation varied significantly among the various treatment regimen (p = 0.030) (Table 2).
Pair-wise comparison test reported a significant difference (p = 0.003) in the days of ventilation between patients treated with ATR and PAM versus ATR and GPR (Table 4).
Although the Kruskal–Wallis test reported the existence of a significant difference in the days of ICU (p = 0.022) (Table 2), and further analysis by the Mann–Whitney test revealed no significant difference in the pair-wise comparison (Table 4).
The results of the univariate regression analysis are summarised in Table 5. Variables of poison type, prehospitalization period, and the type of decontamination were analyzed to identify a possible association with the mortality of the patients. Variables, namely, chlorpyrifos, methyl parathion, phorate, patients presenting to the emergency unit 24 h post poisoning, and decontamination involving activated charcoal had an OR > 1. However, they were not statistically significant. Older age, gender and treatment pattern followed were independently associated with mortality in OPC poisoning (Table 6).
Table 6.
Adjusted OR and 95% CI for the factors associated with mortality in OPC poisoning patients
| Factor | Survived 375 n (%) |
Expired 66 n (%) |
Adjusted OR (95% CI) | p value |
|---|---|---|---|---|
| Age | ||||
| 18–30 years | 187 (49.87) | 18 (27.27) | Reference category | |
| 31–50 years | 126 (33.60) | 20 (30.30) | 1.455 (0.732–2.892) | 0.285 |
| 50 years above | 62 (16.53) | 28 (42.42) | 4.275 (2.179–8.387) | < 0.001a |
| Gender | ||||
| Female | 126 (33.60) | 10 (15.15) | Reference category | |
| Male | 249 (66.40) | 56 (84.85) | 2.608 (1.258–5.406) | 0.010a |
| Treatment pattern | ||||
| ATR and PAM | 167 (44.53) | 22 (33.33) | Reference category | |
| ATR alone | 115 (30.67) | 19 (28.79) | 1.123 (0.567–2.221 | 0.740 |
| ATR and GPR | 29 (7.73) | 4 (6.06) | 0.757 (0.232–2.468 | 0.644 |
| ATR, PAM and GPR | 64 (17.07) | 21 (31.82) | 2.233 (1.002–4.040) | 0.049a |
OR odds ratio, ATR atropine, PAM pralidoxime, GPR glycopyrrolate
aStatistically significant p < 0.05
Atropine utilization
ATR remains the cornerstone in the treatment of OPC poisoning. It was present in all the four treatment patterns of comparison and was administered to all the patients presented with OPC poisoning. The median ATR dose was found to be higher in the group treated with ATR, PAM and GPR and the least in the group treated with ATR alone (Table 2).
The total ATR utilized was analyzed using the Kruskal–Wallis test and the Pair-wise comparison test to assess the variation of ATR usage across the treatment patterns. The results of the Kruskal–Wallis test revealed that the median of the total ATR utilized varied significantly between various groups (p = 0.016). The Pair-wise comparison test revealed a significant difference in the total ATR administered between patient groups treated with ATR alone versus ATR, PAM and GPR, and ATR and PAM versus ATR, PAM and GPR at p = 0.002 and p = 0.006, respectively (Table 4).
Pralidoxime utilization
Pralidoxime was utilized in two treatment arms, namely, ATR and PAM and ATR, PAM and GPR. The median PAM utilized was found to be higher in the group treated with ATR, PAM and GPR. The Pair-wise comparison test revealed a significant difference in the total PAM administered between patient groups at p < 0.001 (Table 2).
Discussion
Consumption of poison with the intention of suicide was widely observed in the South Indian population [16, 17]. A male to female ratio of 2.2:1 was found to support male preponderance in poisoning by other studies [18]. The prevalence can be attributed to males, as they comprise the main working group in the farms, and the stress associated with crop failures, unpredictable monsoons and economic burden as well relies on them [19–21]. The mean age in our study population was about 36.81 ± 14.91, similar to the study by Coskun et al. with a mean age of 39 ± 16 years [22]. The self-poisoning was considerably observed in daily wagers and farmers who have easy access to the chemicals [23], and chlorpyrifos was the pesticide mostly consumed in our population owing to its wide usage in countries like India and Sri Lanka [24, 25]. The Insecticides Act, 1968, was enacted for the sale, transport and distribution of insecticide in order to prevent the risk to human beings. However, the provisions of the Act are restricted to the definition of ‘Insecticide’ and are insufficient to regulate substances being used as pesticides. The control measures should include the establishment of poison information centres, safe storage of pesticides at the community level, including investments to improve quality, affordability, and accessibility of health care in proximities of affected communities. More importantly, actions to improve the mental health of farmers and suggesting alternatives to pesticides should be taken up by the government [26]. A retrospective study by Al Mutairi et al. in the Saudi Arabian population reported that mental illness could be a driving force for the consumption of OPC poison, wherein 10.98% of the study population admitted with OPC poisoning was suffering from a psychiatric illness. A similar pattern was revealed in our study with 73 (16.55%) patients reporting a history of a psychiatric illness [27].
The treatment regimen of OPC varies according to the hospital setting. Our study observed four different treatment regimens, and is the first of its kind in the comparison of four different regimens, and it elucidates the effectiveness of each treatment pattern. Though atropine is predominantly used in the treatment of OPC poisoning with its wide antimuscarinic effects, its inability to act on nicotinic receptors [28] has led to the inclusion of oximes to the treatment regime.
Pralidoxime belongs to the class of oximes. Though it has proved its efficacy in the in vitro studies and is suggested in the treatment algorithms [29], its use in the clinical setting has always been a debate with varying outcomes as suggested by Syed S et al. in a randomized controlled trial [30]. The efficacious PAM usage as an adjunct therapy to atropine in OPC poisoning has been suggested by Worek et al. [31]. and Kurtz et al. [32]. An aggressive atropinization followed by a continuous PAM usage was found to decrease the overall mortality in a case series of 16 patients with severe OPC poisoning [33]. The delay in oxime therapy was associated to increased mortality in a retrospective study by Ahmed et al. [34]. Considering the evidence from such individual studies, the usage of PAM in high doses is still recommended in various guidelines for treating patients with moderate to severe OPC poisoning [29, 30, 34, 35]. A recent randomized control study on Indian population suggests the efficacy of PAM when used in high dosages [36]. In our study, the treatment with ATR and PAM was observed to have the highest rate of survival of 88.36%, minimal mortality of 11.64%, and the least hospital duration of 12.39 ± 7.17 compared to other treatment groups. An improvement in the overall outcome by the addition of PAM was reported in a prospective observational study by Baloch et al. [37]. Conversely, a sharp dissent in the usage of PAM was reported in a review by Eddleston et al. [3] and randomized clinical trials by Banerjee et al. [38] and Kamal et al. [39] stating increased mortality in the group treated with ATR and PAM. Theoretically, oximes play a supporting role to atropine by treating the nicotinic symptoms such as progressive muscle weakness leading to peripheral respiratory failure by reactivating the phosphorylated cholinesterase [40].The use of both ATR and PAM can alleviate the symptoms associated with the accumulation of acetylcholine in the CNS such as seizures, altered consciousness, and centrally mediated respiratory failure [40], hence, managing both peripheral and central respiratory symptoms at the time of acute cholinergic crisis. A randomized clinical trial by Cherian et al. [41] and metaanalysis by Rahimi et al. [42] suggest an increased need for ventilation in the patients treated with pralidoxime. However, our study results reported a decreased need for intubation and lower ventilator days in the group treated with ATR and PAM compared to ATR alone and ATR and GPR. The results of the group ATR and PAM could be biased as mentioned in the limitation, as the severely ill and unresponsive patients were started with an inclusion of GPR, thereby, the patients were moved to ATR, PAM and GPR group. There was also no statistically significant difference in the days of hospitalization and days in the ICU between the treatment groups.
Though, GPR was addressed to portray its efficacy when added to the treatment regime according to the case reports and retrospective studies by Choi et al. [43], Arendse et al. [11] and Unnikrishnan et al. [44]. A significant difference (p = 0.003) in the median days on ventilation was found between treatments of ATR and PAM versus ATR and GPR with the latter having longer days of ventilation. Overall, there was an increased need for intubation (54.55%) and a longer duration of ICU. Ventilation, ICU, and hospital stay was observed to be longer in comparison with the group treated with ATR alone. These results were corroborated in the retrospective study by Bhandarkar et al. [13] and prospective observational study by Anju N et al. [45]. The results suggest that the inclusion of glycopyrrolate is not advantageous in alleviating the condition of patients with OP poisoning.
The dose of atropine was not reduced when used in combination with pralidoxime and glycopyrrolate, probably due to varying mechanism of actions. A similar observation was noticed in a prospective study by Chaudhary et al. [46] and a non-randomized clinico-therapeutic trial by Chugh et al. [47] wherein, the addition of PAM did not reduce the dosage of atropine. There are various factors such as poison load, delayed hospital admission, declining laboratory parametres and initial treatment that affect the dose of ATR. An individual case series observed an increased utilization of ATR and a longer duration of hospitalization in the severely ill patients unresponsive to the conventional dosage of atropine [48].
The risk factors significantly associated with mortality were older age > 50 years, male gender and the treatment with the combination of ATR, PAM and GPR (Table 6). In older patients, the decreased muscle mass and increased body fat enable rapid distribution and storage of pesticides in the body. Furthermore, the reduced blood flow and decreased renal and hepatic functions delay the elimination of pesticides from the body contributing to poor outcomes [49]. The increased mortality in males can be attributed to the social habits of the male population such as alcoholism, a comorbidity that might have led to poor prognosis. Chronic alcohol consumption can cause difficulties in the prognosis [50],; delirium and tachycardia ascribable to withdrawal further complicates the administration of atropine [51]. The treatment pattern of ATR, PAM and GPR was followed at our study centre on the unresponsive and severely ill patients. The results of this pattern were unpropitious with the highest rate of mortality of 24.71%, decreased survival rate of 75.29%, longer duration of ICU of 11.51 ± 7.80, and the highest mean hospitalization days of 15.16 ± 9.92. This could be as a result of compromising clinical conditions of the patients, and the addition of PAM and GPR was considered as an additional antidote in severely ill patients. Although there was a greater utilization of ATR and PAM with the inclusion of three antidotes in a life-saving approach, the treatment pattern was not beneficial in improving the condition of the severely ill patients and it reported greater mortality.
In summary, pesticide self-poisoning is a major health hazard in India, mostly affecting farmers and daily wagers. Suicidal intention was the predominant reason for the consumption of poison. The majority of patients were males belonging to the reproductive age. ATR is the well-known antidote used for the management of OPC poisoning. The treatment analysis revealed the treatment group with ATR and PAM had reduced intubation and lower days of ICU and ventilation. The study describes the on-going treatment patterns followed, and identifies older age, gender and treatment pattern as independent risk factors for mortality.
Limitations
Our study did not represent an equal number of patients in each treatment pattern, and the use of ATR, PAM and GPR in a majority of severely ill and unresponsive patients could lead to bias. The information on the poison type, GCS and APACHE scoring, serum OPC concentrations, serial pseudocholinesterase measurement, co-morbidities present, and the treatment provided at the local hospital was compromised, as this was a retrospective study.
Acknowledgements
We would like to thank Manipal Academy of Higher Education, Department of Medicine, Kasturba Medical College, Department of Pharmacy practice, Manipal College of Pharmaceutical Sciences, Centre for Toxicovigilance and Drug Safety, and the staff for supporting us during the study. We specially thank Ms.Pooja Poojari for helping us with the submission process.
Funding
The authors declare that they have no funding.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interests.
Contributor Information
B. Shrikar Reddy, Email: reddyshrikar@gmail.com.
Teny Grace Skaria, Email: teny18gs@gmail.com.
Girish Thunga, Email: girish.thunga@manipal.edu.
References
- 1.Palaniappen V. Current concepts in the management of organophosphorus compound poisoning Medicine update. Mumbai: The Association of Physician of India; 2013. pp. 427–433. [Google Scholar]
- 2.Singh S, Sharma N. Neurological syndromes following organophosphate poisoning. Neurol India. 2000;48:308–313. [PubMed] [Google Scholar]
- 3.Eddleston M, Buckley NA, Eyer P, Dawson AH. Management of acute organophosphorus pesticide poisoning. Lancet. 2008;371:597–607. doi: 10.1016/S0140-6736(07)61202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karalliedde L, Senanayake N. Organophosphorous insecticide poisoning. Br J Anaesth. 1989;63:736–750. doi: 10.1093/bja/63.6.736. [DOI] [PubMed] [Google Scholar]
- 5.Sanz P, Rodriguez-vicente MC, Diaz D, Repetto J, Repetto M. Red blood cell and total blood acetylcholinesterase and plasma pseudocholinesterase in humans: observed variances. J Toxicol Clin Toxicol. 1991;29:81–90. doi: 10.3109/15563659109038600. [DOI] [PubMed] [Google Scholar]
- 6.Rajapakse BN, Thiermann H, Eyer P, Worek F, Bowe SJ, Dawson AH, Buckley NA. Evaluation of the test-mate ChE (cholinesterase) field kit in acute organophosphorus poisoning. Ann Emerg Med. 2011;58:559–564. doi: 10.1016/j.annemergmed.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Eddleston M, Buckley NA, Checketts H, Senarathna L, Mohamed F, Sheriff MHR, Dawson A. Speed of initial atropinisation in significant organophosphorus pesticide poisoning—a systematic comparison of recommended regimens. J Toxicol Clin Toxicol. 2004;42:865–875. doi: 10.1081/clt-200035223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumaraswamy RC, Madhavi KMS, Basavanthappa SP, Gowda MRN. Open-label randomized controlled study comparing continuous infusion versus intermittent bolus dose of atropine with or without pralidoxime in the treatment of organophosphorus poisoning in a teaching hospital. SMJ. 2014;17:3–6. [Google Scholar]
- 9.Rosman Y, Makarovsky I, Bentur Y, Shrot S, Dushnistky T, Krivoy A. Carbamate poisoning: treatment recommendations in the setting of a mass casualties event. Am J Emerg Med. 2009;27:1117–1124. doi: 10.1016/j.ajem.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 10.Mirakhur RK, Dundee JW. Glycopyrrolate: pharmacology and clinical use. Anaesthesia. 1983;38:1195–1204. doi: 10.1111/j.1365-2044.1983.tb12525.x. [DOI] [PubMed] [Google Scholar]
- 11.Arendse R, Irusen E. An atropine and glycopyrrolate combination reduces mortality in organophosphate poisoning. Hum Exp Toxicol. 2009;28:715–720. doi: 10.1177/0960327109350666. [DOI] [PubMed] [Google Scholar]
- 12.Bardin PG, Van Eeden SF, Joubert JR. Intensive care management of acute organophosphate poisoning. A 7-year experience in the Western Cape. South Afr Med J. 1987;72:593–597. [PubMed] [Google Scholar]
- 13.Bhandarkar AA. Efficacy of atropine alone and with glycopyrrolate combination in organophosphate poisoning. Value Health. 2014;17:A750–A751. doi: 10.1016/j.jval.2014.08.197. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt E, Gerhardt W, Henkel E, Klauke R, Liese W, Lorentz K, Sonntag O, Stein W, Weidemann G. Proposal of standard methods for the determination of enzyme catalytic concentrations in serum and plasma at 37 °C. Clin Chem Lab Med. 1992;30:247–256. [PubMed] [Google Scholar]
- 15.Poisoning Severity Score (PSS) IPCS/EAPCCT. https://www.who.int/ipcs/poisons/pss.pdf/. Accessed 30 Mar 2019
- 16.Banday TH, Tathineni B, Desai MS, Naik V. Predictors of morbidity and mortality in organophosphorus poisoning: a case study in rural hospital in Karnataka, India. N Am J Med Sci. 2015;7:259–265. doi: 10.4103/1947-2714.159331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar Mr, Vignan Kumar G, Kumar Ss, Subrahmanyam B, Veeraprasad M, Rammohan P, Srinivas M, Babu Pr, Agrawal A. A retrospective analysis of acute organophosphorus poisoning cases admitted to the tertiary care teaching hospital in South India. Ann Afr Med. 2014;13:71. doi: 10.4103/1596-3519.129876. [DOI] [PubMed] [Google Scholar]
- 18.Kar N. Lethality of suicidal organophosphorus poisoning in an Indian population: exploring preventability. Ann Gen Psychiatry. 2006;5:1–5. doi: 10.1186/1744-859X-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Somasundaram KV, Patil A, Shukla S. Epidemiological Profile of OP poisoning cases Treated at Pravara Hospital, Loni, India. Indian J Prev Soc Med. 2009;40:184–188. [Google Scholar]
- 20.Gupta P, Honnungar RS, Pujar SS. Trend of male poisoning at North Karnataka from 2008–2013. J Indian Acad Forensic Med. 2015;37:278–280. [Google Scholar]
- 21.Chintale KN, Patne SV, Chavan SS. Clinical profile of organophosphorus poisoning patients at rural tertiary health care centre. Ind J Adv Med. 2016;3:268–272. [Google Scholar]
- 22.Coskun R, Gundogan K, Sezgin GC, Topaloglu US, Hebbar G, Guven M, Sungur M. A retrospective review of intensive care management of organophosphate insecticide poisoning: single center experience. Niger J Clin Pract. 2015;18:644–650. doi: 10.4103/1119-3077.158962. [DOI] [PubMed] [Google Scholar]
- 23.Selvaraj T, Sudharson T. Demographic and clinical profile of organophosphorus poisoning cases in a Medical College Hospital, Tamil Nadu. Indian J Forensic Community Med. 2016;3:124. [Google Scholar]
- 24.Eddleston M, Mohamed F, Davies JOJ, Eyer P, Worek F, Sheriff MR, Buckley NA. Respiratory failure in acute organophosphorus pesticide self-poisoning. QJM. 2007;99:513–522. doi: 10.1093/qjmed/hcl065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rathod AL, Garg RK. Chlorpyrifos poisoning and its implications in human fatal cases: a forensic perspective with reference to Indian scenario. J Forensic Leg Med. 2017;47:29–34. doi: 10.1016/j.jflm.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Gunnell D, Eddleston M, Phillips MR, Konradsen F. The global distribution of fatal pesticide self-poisoning: systematic review. BMC Public Health. 2007;7:1–15. doi: 10.1186/1471-2458-7-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almutairi MK, Almoaiqel FA, Alanazi A, Aljerian N, Alqahtani A, Harthy N Al, Qureshi S, Kumar S. A report on the incidence of organophosphate poisoning among patients admitted to King Abdul-Aziz Medical City, Riyadh, Saudi Arabia over a Period of 12 years. Br J Med Med Res. 2016;13:1–8. [Google Scholar]
- 28.Jokanović M. Medical treatment of acute poisoning with organophosphorus and carbamate pesticides. Toxicol Lett. 2009;190:107–115. doi: 10.1016/j.toxlet.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 29.Eyer P. The Role of Oximes in the management of organophosphorus pesticide poisoning. Toxicol Rev. 2003;22:165–190. doi: 10.2165/00139709-200322030-00004. [DOI] [PubMed] [Google Scholar]
- 30.Syed S, Gurcoo SA, Farooqui AK, Nisa W, Sofi K. Is the World Health Organization-recommended dose of pralidoxime effective in the treatment of organophosphorus poisoning? A randomized, double-blinded and placebo-controlled trial. Saudi J Anaesth. 2015;9:49–54. doi: 10.4103/1658-354X.146306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Worek F, Bäcker M, Thiermann H, Szinicz L, Mast U, Klimmek R, Eyer P. Reappraisal of indications and limitations of oxime therapy in organophosphate poisoning. Hum Exp Toxicol. 1997;16:466–472. doi: 10.1177/096032719701600808. [DOI] [PubMed] [Google Scholar]
- 32.Kurtz PH. Pralidoxime in the treatment of carbamate intoxication. Am J Emerg Med. 1990;8:68–70. doi: 10.1016/0735-6757(90)90299-f. [DOI] [PubMed] [Google Scholar]
- 33.Singh S, Chaudhry D, Behera D, Gupta D, Jindal S. Aggressive atropinisation and continuous pralidoxime (2-PAM) infusion in patients with severe organophosphate. Hum Exp Toxicol. 2001;20:15–18. doi: 10.1191/096032701671437581. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed SM, Das B, Nadeem A, Samal RK. Survival pattern in patients with acute organophosphate poisoning on mechanical ventilation: a retrospective intensive care unit-based study in a tertiary care teaching hospital. Indian J Anaesth. 2014;58:11–17. doi: 10.4103/0019-5049.126780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahesh M, Gowdar M, Venkatesh CR. A study on two dose regimens of pralidoxime in the management of organophosphate poisoning. Asia Pac J Med Toxicol. 2013;2:121–125. [Google Scholar]
- 36.Pawar KS, Bhoite RR, Pillay CP, Chavan SC, Malshikare DS, Garad SG. Continuous pralidoxime infusion versus repeated bolus injection to treat organophosphorus pesticide poisoning: a randomised controlled trial. Lancet. 2006;368:2136–2141. doi: 10.1016/S0140-6736(06)69862-0. [DOI] [PubMed] [Google Scholar]
- 37.Baloch GH, Khan AH, Madhudas C, Devrajani BR, Shah SZ, Das T, Shah SF. Outcome of acute organophosphorus poisoning at Liaquat University Hospital Hyderabad. World Appl Sci J. 2018;13:266–268. [Google Scholar]
- 38.Banerjee I, Roy As, Tripathi S. Efficacy of pralidoxime in organophosphorus poisoning: revisiting the controversy in Indian setting. J Postgrad Med. 2014;60:27. doi: 10.4103/0022-3859.128803. [DOI] [PubMed] [Google Scholar]
- 39.Kamal SM, Akhter S, Ahmed SFU, Chowdhury PK, Rashid A. Outcome of treatment of OPC poisoning patients with atropine or atropine plus pralidoxime. Bang Med J Khulna. 2017;50:3–7. [Google Scholar]
- 40.Roberts DM, Aaron CK. Managing acute organophosphorus pesticide poisoning. Br Med J. 2008;334:629–634. doi: 10.1136/bmj.39134.566979.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cherian AM, Peter JV, Samuel J, Jaydevan R, Peter S, Joel S, Jeyasselan L, Thomas K. Effectiveness of P2AM (PAM -pralidoxime) in the treatment of organophosphorus poisoning. A randomised, double blind placebo controlled trial. J Assoc Physicians India. 1997;45:22–24. [Google Scholar]
- 42.Rahimi R, Nikfar S, Abdollahi M. Increased morbidity and mortality in acute human organophosphate-poisoned patients treated by oximes: a meta-analysis of clinical trials. Hum Exp Toxicol. 2006;25:157–162. doi: 10.1191/0960327106ht602oa. [DOI] [PubMed] [Google Scholar]
- 43.Choi PT, Quinonez LG, Cook DJ, Baxter F, Whitehead L. The use of glycopyrrolate in a case of intermediate syndrome following acute organophosphate poisoning. Can J Anaesth. 1998;45:337–340. doi: 10.1007/BF03012025. [DOI] [PubMed] [Google Scholar]
- 44.Ramesh Unnikrishnan AS. Clinical outcomes of patients admitted with organophosphorus poisoning in a tertiary hospital in Udupi district Indian. J Respir Care. 2012;1:59–65. [Google Scholar]
- 45.Anju N, Nalini P. Comparison of efficacy and safety of atropine sulphate and glycopyrrolate in the treatment of organophosphorus poisoning at St. Martha’s Hospital, Bangalore. Indian J Pharm Pract. 2010;3:43–46. [Google Scholar]
- 46.Chaudhary S, Jain N, Atam V, Agarwal A, Singh K, Sawlani K, Vaish A, Patel M. Prognostic significance of estimation of pseudocholinesterase activity and role of pralidoxime therapy in organophosphorous poisoning. Toxicol Int. 2013;20:214. doi: 10.4103/0971-6580.121669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chugh SN, Aggarwal N, Dabla S, Chhabra B. Comparative evaluation of “atropine alone” and “atropine with pralidoxime (PAM)” in the management of organophosphorus poisoning. J Indian Acad Clin Med. 2005;6:33–37. [Google Scholar]
- 48.Karakus A, Celik MM, Karcioglu M, Tuzcu K, Erden ES, Zeren C. Cases of organophosphate poisoning treated with high-dose of atropine in an intensive care unit and the novel treatment approaches. Toxicol Ind Health. 2014;30:421–425. doi: 10.1177/0748233712462478. [DOI] [PubMed] [Google Scholar]
- 49.Ginsberg G, Hattis D, Russ A, Sonawane B. Pharmacokinetic and pharmacodynamic factors that can affect sensitivity to neurotoxic sequelae in elderly individuals. Environ Health Perspect. 2005;113:1243–1249. doi: 10.1289/ehp.7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moss M, Burnham EL. Alcohol abuse in the critically ill patient. Lancet. 2006;368:2231–2242. doi: 10.1016/S0140-6736(06)69490-7. [DOI] [PubMed] [Google Scholar]
- 51.Eddleston M, Gunnell D, Von Meyer L, Eyer P. Relationship between blood alcohol concentration on admission and outcome in dimethoate organophosphorus self-poisoning. Br J Clin Pharmacol. 2009;68:916–919. doi: 10.1111/j.1365-2125.2009.03533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]