Abstract
Solanum aethiopicum is used in ethnomedicine for the treatment of overweight, constipation and anaemia. This study evaluated the ameliorative effect of aqueous leaf extract of S. aethiopicum on phenylhydrazine-induced anaemia in rats. Acute toxicity was determined in male and female rats (n = 5/group/sex) by oral administration of single dose of up to 5000 mg/kg of the S. aethiopicum extract. The experimental rats were randomly grouped into five (5) groups of 6 rats each. Group (i) served as normal control, group (ii) negative control, group (iii) standard drug-5 mg/kg ferrous sulphate, groups (iv) and (v), 200 and 400 mg/kg of S. aethiopicum extract respectively. Phenylhydrazine (PHZ) was administered intraperitoneally at the dose of 50 mg/kg body weight for two consecutive days to groups (ii–v). After 14 days, the rats were sacrificed; blood, liver and kidney were collected. The haematological, lipid profile, liver and kidney function parameters were determined and the histopathology of the liver and kidney were examined. In acute toxicity study, no signs of toxicity or death were recorded. The study shows an observable significant (P < 0.05) increase in packed cell volume, haemoglobin and red blood cell counts at 400 mg/kg S. aethiopicum extract in both the male and female rats when compared to other groups. Solanum aethiopicum extract at the dose of 400 mg/kg reduced aspartate aminotransferase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), urea, creatinine and chloride. The results of this study lent credence to the use of S. aethiopicum leaf as an anti-anaemic tonic with a wide margin of safety and hepato/reno-protective potentials.
Keywords: Solanum aethiopicum, Toxicity, Anaemia, Liver and kidney, Haematology
Introduction
The use of medicinal plant for the management and treatment of human diseases is as old as human existence [1]. Interestingly, there has been increased attention in herbal medicine worldwide because of its significant contribution to health care delivery and as an alternative to clinical practice [2]. These medicinal plants have been demonstrated to be a good source of bioactive compounds (phytochemicals) which are useful for the treatment of myriad of ailments such as nervous, respiratory, sexual, visual, renal and circulatory [3, 4]. Some of the phytochemicals present in medicinal plants include phenols, phytates, flavonoids, alkaloids and steroids [5]. Most of the medicinal plants are still being used in rural African communities as herbal drugs prepared and dispensed by herbalists without formal training or knowledge of the safety and efficacy of the herbs. Hence, proper evaluation of the acclaimed activities of these medicinal plants ascertains their level of safety and efficacy [6].
Anaemia is a haematological disorder characterized by a decrease in the amount of haemoglobin in human to less than 13 g/dL in males and 12 g/dL in females with decreased red blood cells count which leads to pallor, weakness, breathlessness and confusion [7]. Worldwide, anaemia affects about 800 million including children and women, while in sub-Saharan Africa, an estimate of 83.5 million people are affected by this haematological disorder [8, 9]. Various factors such as iron and micronutrient deficiencies, gastrointestinal tract parasitic infestation (hookworm) and HIV infections are known to cause anaemia [10]. It has also been reported that protozoan infections like malaria and haemoglobinopathies like sickle cell disease and thalassemia may also induce anaemia in human [11, 12].
The use of phenylhydrazine has been found promising as a widely used anaemic model in experimental animals. The administration of various doses of plant extract to animals in this condition may serve as a medium in unravelling the possible bioactive potentials and activities of such plants and drugs against haemolysis [13, 14]. Phenylhydrazine (PHZ) elicits anaemic activity due to its powerful oxidant nature. It releases phenyldiazene, phenylhydrazyl radical and benzenediazonium as reactive oxygen species when metabolized in vivo. Again, the free radical activity of PHZ can initiate red blood cell damage by oxidizing haemoglobin [13]. Siraki et al. [15] reported that free radical through the processes of electron theft; stimulation and triggering of undesirable biochemical reactions, have the potential to induce tissue and organ damage such as hepato-renal damage, depletion of antioxidants and alteration of biochemical activities of the cell with increased toxicities.
Solanum aethiopicum L. also known as garden egg or scarlet eggplant is a seasonal plant which belongs to the family Solanaceae. Solanaceae has over 1000 species worldwide with over 100 species found in Africa (e.g. S. aethiopicum, S. macrocarpon and S. muricatum.) Eggplant is widely distributed in Africa (Sierra Leone, Nigeria, Cameroon, Ethiopia and Zimbabwe), Asia, Brazil and Southern Europe [16, 17]. The leaves of S. aethiopicum are used to prepare delicacies like stew, soup and yam porridge. The leaves of S. aethiopicum are oval shaped, wavy margined and alternately arranged. They are about 10–30 cm long and 4–15 cm wide. The leaves have a high content of crude fibre, calcium, iron, zinc, protein, fat, vitamins and phytochemicals [18]. In traditional medicine, S. aethiopicum is used in treatment of various ailments such as overweight, constipation, asthma, allergic disease, dyspepsia, swollen joint pain and gastro-esophageal reflux disease [19]. Scientific reports have also demonstrated the potentials of using S. aethiopicum as purgative, anti-diabetic, weight reduction, sedative, anti-hyperlipidaemia, anti-inflammatory, anti-ulcerogenic and anti-constipation [19, 20]. In advanced countries of the world, herbal decoctions and products from medicinal plants are falsely considered to be safe without evaluating its health potentials [21, 22]. Although, S. aethiopicum leaves have been shown to have various health benefits, their anti-anaemic activity against PHZ have not been investigated. Therefore, the present study evaluated the haematopoietic potentials of S. aethiopicum on phenylhydrazine-induced anaemia and its influence on other biochemical parameters in rats.
Materials and methods
Collection and authentication of S. aethiopicum
The garden egg leaves were obtained in June 2018 from Ukabi farmland in Ideato South Local Government Area of Imo State, Nigeria and authenticated by a plant taxonomist at University of Nigeria Nsukka as S. aethiopicum with herbarium number UNH No 331.
Extract preparation
The leaves were destalked from the stem, sorted, washed with distilled water and macerated. The residue was separated from the filtrate using a clean sieve and then re-filtered using a Whatman No 1 filter paper (Fisher Scientific Loughborough, UK). The filtrate was freeze-dried and reconstituted in distilled water to appropriate concentrations.
Animal experiment
Healthy male and female Wistar rats weighing between 150 and 180 g were used in this study. The rats were kept under normal standard environmental conditions of temperature (25–28 °C), humidity (35–60%) and 12 h light/12 h dark condition. The rats were allowed free access to feed and water. Experimental procedures and animal handling were approved by the Abia State University, Research Ethical Clearance Committee (ABSU/REC/BMR/0017). Ethical principles of World Health Organization for good laboratory practice regulations of 1998 and United State guideline for experimental animal [23] were strictly adhered to throughout the study.
Acute toxicity studies of S. aethiopicum
The acute toxicity of aqueous extract of S. aethiopicum was studied following the guideline of Organization for Economic Cooperation and Development (OECD) [24] guideline 423 with little modifications. The rats were randomly divided into 6 groups of 10 rats comprising of 5 males and 5 females (kept in different cage) in each group. Then, graded single dose of 500, 1000, 2000, 3000, 4000 and 5000 mg/kg of aqueous extract of S. aethiopicum were orally administered. The rats were allowed free access to food and monitored for behavioural changes and signs of toxicity within and after 24 h.
Induction of anaemia
Prior to induction of anaemia, baseline haematological parameters in rats were determined. PHZ (BDH Poole England) was used to induce anaemia to the rats via intraperitoneal injection of 50 mg/kg for two consecutive days [25]. Anaemia was considered induced when RBC level as well as haemoglobin concentration of the blood reduced to 30% or less.
Sub-acute toxicity study
The sub-acute toxicity study was investigated following the guideline of Organization for Economic Cooperation and Development (OECD) [26] guideline 407 with little modifications. Rats were grouped into five groups of six each (Group I to V) for male and female rats respectively. All the rats were induced with 50 mg/kg PHZ for 2 consecutive days except the normal control. Group 1, non-induced rats served as normal control and received 0.25 mL/kg distilled water throughout the experiment, Group II, anaemia-induced rats served as negative control, Group III, anaemia-induced rats received 5 mg/kg ferrous sulphate (Reagans Remedies LTD, Owerri, Nigeria), Group IV, anaemia-induced rats received 200 mg/kg of S. aethiopicum and Group V, anaemia-induced rats received 400 mg/kg of S. aethiopicum daily for 14 days. The rats were allowed access to food and water ad libitum and were weighed daily throughout the duration of experiment.
Blood sample and organ collection
Exactly 14 days post treatment with aqueous extract of S. aethiopicum, the rats were fasted overnight, anaesthetized and sacrificed. Blood samples were collected by cardiac puncture and dispensed into plain bottles for clinical chemistry analysis and EDTA (Ethylenediaminetetraacetic acid) container for haematological tests. The organs including liver, kidneys, heart, lungs, and spleen were dissected and removed carefully and the relative organ weight calculated as follows: Relative organ weight = Absolute organ weight (g)/Body weight of rat on the sacrificed day (g) × 100.
Blood analysis
Packed cell volume (PCV), haemoglobin (Hb) level, red blood cell (RBC) indices, mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), mean corpuscular volume (MCV), white blood cell (WBC) count and platelets were estimated following the methods described previously by Bain et al. [27].
Clinical chemistry analysis
Liver enzymes [alkaline phosphatase (ALP), alanine aminotransferase (ALT) and aspartate aminotransferase (AST)], bilirubin and renal function parameters (urea, creatinine and electrolyte [sodium (Na+), potassium (K+), chloride (Cl−) and bicarbonate HCO3−)] levels were estimated spectrophotometrically using standard laboratory kits (Randox Laboratory Ltd., Co. Antrim, UK). Total cholesterol (TC), triacylglycerol (TAG), very low-density lipoprotein cholesterol (VLDL-C), low density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) level were also estimated spectrophotometrically with standard laboratory kits (Randox Laboratory Ltd., Co. Antrim, UK). The biochemical estimations were performed under strict adherence to manufacturer’s instructions.
Histological studies
The liver and kidney were fixed in 10% formalin immediately after the rats were sacrificed. They were routinely processed and embedded in paraffin wax. The tissues were thereafter sectioned to obtain 5 μm thickness, stained with haematoxylin and eosin dyes. The sectioned samples were examined using a light microscope and histological deviations from normal were carefully recorded.
Data analysis
Following normal distribution and homogeneity of variance check, the data were analysed using One-way analysis of variance (ANOVA) with the R™ Statistic software package, version 3.0.3 (https://cran.r-project.org/src/base/R-3/). Tukey test post hoc was used to identify statistical differences among groups and figures generated with GraphPad Prism version 5.0 (San Diego, USA). A P value of ≤ 0.05 was considered statistically significant.
Results
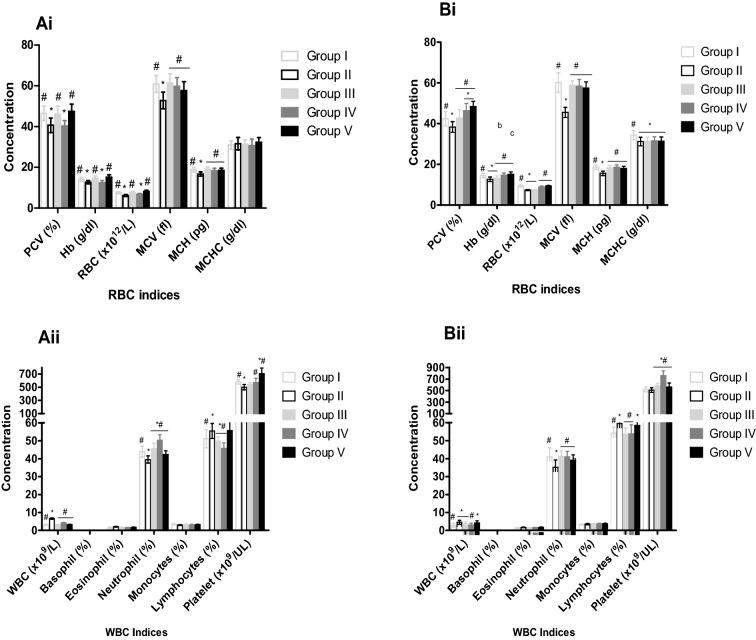
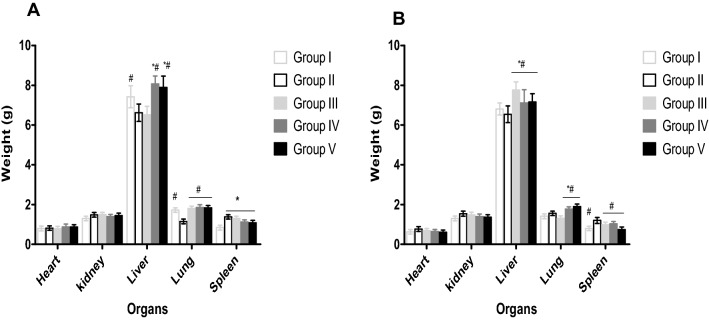
Table 1 shows the acute toxicity study of Wistar rats within and after 24 h administration of aqueous leaf extract of S. aethiopicum. No behavioural changes or lethal effects were observed within and after 24 h post oral administration of various graded doses of aqueous leaf extract of S aethiopicum (500, 1000, 2000 3000, 4000 and 5000 mg/kg body weight) in both the male and the female rats. Male and female rats treated with 200 and 400 mg/kg of the leaf extract of S. aethiopicum demonstrated a significant increase (P < 0.05) in PCV, Hb, RBC, MCV, MCH, WBC, neutrophils, lymphocytes and platelets when compared to negative control (Fig. 1). No significant changes were observed in MCHC, basophil, monocytes and eosinophils. The same trend of result was also observed for rats treated with 5 mg/kg ferrous sulphate compared negative control (Fig. 1). Generally, an increase in body weight was observed in all the rats (male and female) during the treatment period. Although significant increase (P < 0.05) was observed in the body weight of rats that received 5 mg/kg ferrous sulphate, 200 and 400 mg/kg of aqueous leaf extract compared to the group treated only with phenylhydrazine (Table 2). Increase in liver and lung weights were also observed in rats treated with 5 mg/kg ferrous sulphate, 200 and 400 mg/kg of aqueous leaf extract of S. aethiopicum after anaemia induction with PHZ (Fig. 2).
Table 1.
Acute (oral) toxicity study of Wistar rats after 24 h administration of S. aethiopicum extract
| Dose (mg/kg) | Death/number of animals | Death/total number of rats | |
|---|---|---|---|
| Male | Female | ||
| 0 | 0/5 | 0/5 | 0/10 |
| 500 | 0/5 | 0/5 | 0/10 |
| 1,000 | 0/5 | 0/5 | 0/10 |
| 2,000 | 0/5 | 0/5 | 0/10 |
| 3,000 | 0/5 | 0/5 | 0/10 |
| 4,000 | 0/5 | 0/5 | 0/10 |
| 5,000 | 0/5 | 0/5 | 0/10 |
Fig. 1.
Effect of aqueous leaf extract of S. aethiopicum on renal biomarkers of phenylhydrazine induced anaemia in male and female Wistar rats. Values represent the mean ± SD (N = 6). Statistical significance (P < 0.05) are indicated by asterisk (*) when compared to normal control and hash (#) when compared to negative control. PCV, Packed Cell Volume; HB, Haemoglobin; RBC, Red Blood Cells; MCV, Mean Corpuscular Volume; MCH, Mean Corpuscular Haemoglobin; MCHC, Mean Corpuscular Haemoglobin Concentration; WBC, White Blood Cell. A, male; B, female; Group I, Normal control; Group II, Negative control; Group III, Ferrous sulphate (5 mg/kg); Group IV, S. aethiopicum extract (200 mg/kg); Group V, S. aethiopicum extract (400 mg/kg)
Table 2.
Effect of S. aethiopicum extract on body weight of phenylhydrazine induced anaemia in male and female Wistar rats
| Days | Group I | Group II | Group III | Group IV | Group V |
|---|---|---|---|---|---|
| A | |||||
| 0 | 170.00 ± 2.00 | 175.00 ± 4.36 | 184.00 ± 4.00 | 192.00 ± 2.00 | 182.33 ± 5.65 |
| 7 | 182.00 ± 2.00 | 181.33 ± 3.06 | 192.33 ± 3.51 | 208.67 ± 2.31 | 195.00 ± .1.00 |
| 14 | 197.33 ± 4.73 | 189.33 ± 1.15 | 215.00 ± 2.65 | 225.33 ± 5.03 | 209.67 ± 4.51 |
| B | |||||
| 0 | 168.33 ± 2.89 | 162.33 ± 2.52 | 169.67 ± 5.03 | 167.00 ± 3.61 | 177.67 ± 3.21 |
| 7 | 176.67 ± 2.89 | 169.00 ± 2.65 | 180.67 ± 3.06 | 176.00 ± 1.73 | 179.67 ± 2.08 |
| 14 | 187.00 ± 2.65 | 182.00 ± 2.00 | 184.00 ± 4.58 | 178.67 ± 2.31 | 185.67 ± 4.04 |
Values represent the mean ± SD for N = 6. A, Male; B, Female; Group II, Negative control; Group III, Ferrous sulphate (5 mg/kg); Group IV, S. aethiopicum extract (200 mg/kg); Group V, S. aethiopicum extract (400 mg/kg)
Fig. 2.
Effect of 14 days treatment of S. aethiopicum on organ weights of phenylhydrazine induced anaemia in male and female wistar rats. Values represent the mean ± SD (N = 6). Statistical significance (P < 0.05) are indicated by * when compared to normal control and # when compared to negative control. A, male; B, female; Group I, Normal control; Group II, Negative control; Group III, Ferrous sulphate (5 mg/kg); Group IV, S. aethiopicum extract (200 mg/kg); Group V, S. aethiopicum extract (400 mg/kg)
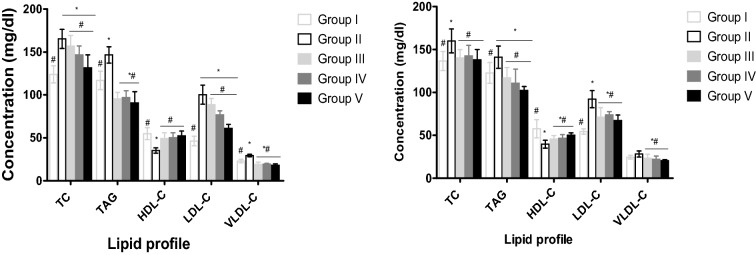
The effects of aqueous leaf extract of S. aethiopicum on lipid profile of male and female Wistar rats induced anaemia with PHZ are shown in Fig. 3. Treatment with 5 mg/kg of ferrous sulphate, 200 and 400 mg/kg of the leaf extract demonstrated a significant decrease (P < 0.05) in TC, TAG, LDL-C and VLDL-C serum concentrations in male and female compared to the normal and negative controls. However, a significant increase (P < 0.05) in HDL-C was observed in female and male rats treated with aqueous leaf extract and ferrous sulphate compared to negative and positive control (Fig. 3).
Fig. 3.
Effect of aqueous leaf extract of S. aethiopicum on lipid profile of phenylhydrazine induced anaemia in male and female Wistar rats. Values represent the mean ± SD (N = 6). Statistical significance (P < 0.05) are indicated by * when compared to normal control and # when compared to negative control. TC, Total Cholesterol; TAG, Triglyceride; HDL-C, High-Density Lipoprotein Cholesterol; LDL-C, Low-Density Lipoprotein Cholesterol; VLDL-C, Very Low-Density Lipoprotein Cholesterol. A, male; B, female; Group I, Normal control; Group II, Negative control; Group III, Ferrous sulphate (5 mg/kg); Group IV, S. aethiopicum extract (200 mg/kg); Group V, S. aethiopicum extract (400 mg/kg)
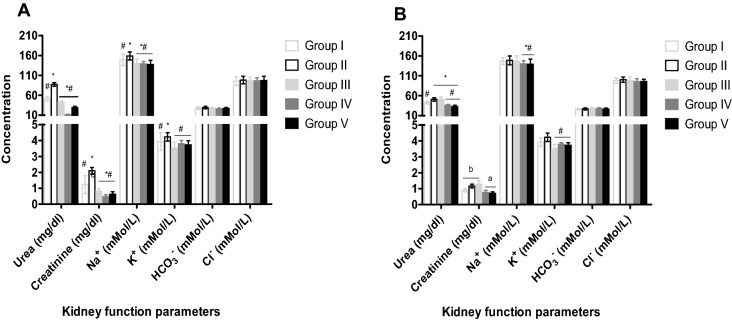
Male and female rats treated with 200 and 400 mg/kg of leaf extract after induction of anaemia showed a significant decrease (P < 0.05) in serum urea, creatinine, sodium (Na+) and potassium (K+) level compared to the normal and negative control (Fig. 4). No impact was observed in bicarbonate ion (HCO3−) and chloride ion (Cl−). Rats exposed to ferrous sulphate only showed a significant decrease (P < 0.05) in serum urea, creatinine, sodium (Na+) and potassium (K+) level compared to the normal control (Fig. 4).
Fig. 4.
Effect of aqueous leaf extract of S. aethiopicum on renal biomarkers of phenylhydrazine induced anaemia in male and female Wistar rats. Values represent the mean ± SD (N = 6). Statistical significance (P < 0.05) are indicated by * when compared to normal control and # when compared to negative control. Na+, Sodium ion; K+, Potassium ion; HCO3−, Bicarbonate; Cl−, Chloride ion. A, male; B, female; Group I, Normal control; Group II, Negative control; Group III, Ferrous sulphate (5 mg/kg); Group IV, S. aethiopicum extract (200 mg/kg); Group V, S. aethiopicum extract (400 mg/kg)
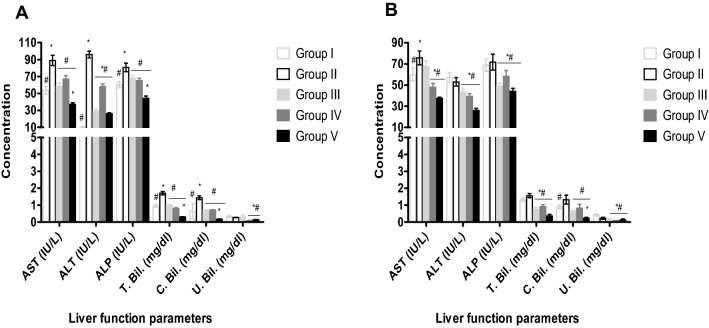
The effects of aqueous leaf extract of S. aethiopicum on liver biomarkers of male and female Wistar rats induced anaemia with phenylhydrazine are shown in Fig. 5. Treatment with 200 and 400 mg/kg of the extract demonstrated significant decrease (P < 0.05) in AST, ALT, ALP, total and conjugated bilirubin compared to the negative control in the male and female rats (Fig. 5). AST, ALT, ALP, total, conjugated and unconjugated bilirubin significantly (P < 0.05) increased in rats exposed to phenylhydrazine without treatment compared to the normal control (Fig. 5).
Fig. 5.
Effect of aqueous leaf extract of S. aethiopicum on liver biomarkers of phenylhydrazine induced anaemia in male and female Wistar rats. Values represent the mean ± SD (N = 6). Statistical significance (P < 0.05) are indicated by * when compared to normal control and # when compared to negative control. AST, Aspartate Aminotransferase; ALT, Alanine Transaminase; ALP, Alkaline Phosphatase; T. Bil., Total bilirubin; C. Bil., conjugated bilirubin, U.Bil., unconjugated bilirubin. A, male; B, female; Group I, Normal control; Group II, Negative control; Group III, Ferrous sulphate (5 mg/kg); Group IV, S. aethiopicum extract (200 mg/kg); Group V, S. aethiopicum extract (400 mg/kg)
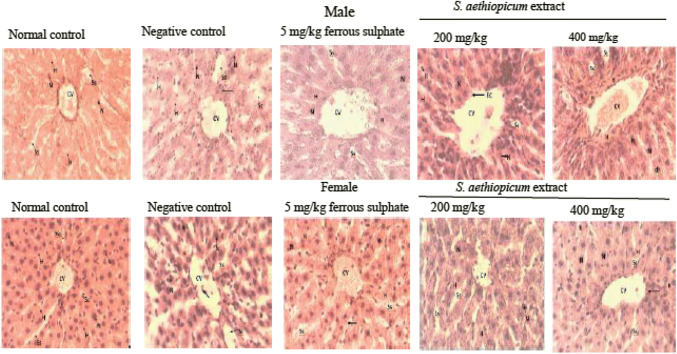
Figure 6 shows the hepatic micrograph of rats treated with aqueous leaf extract of S. aethiopicum after anaemia induction with phenylhydrazine. The hepatic lobes of the normal control group of both male and female rates were intact, the hepatocytes were also distinct, and sinusoid radiates out of the central vessels in its normal hexagonal pattern. The negative control micrograph showed a dilated central vessel and sinusoid, enlarged nuclei, occluded hepatocytes, depleted cells and connective tissues. The rats treated with ferrous sulphate (5 mg/kg) showed severe sclerotic activity with extension into the sinusoids. The sinusoid appeared dilated, the hepatocyte appears multi nucleated and cells infiltrated into the vessel and sinusoids. The rats treated with 200 mg/kg of S. aethiopicum extract showed a constricted central vessel, thick nuclei of hepatocyte, non-radiating sinusoids in its hexagonal pattern and presence of Kupffer cells. Eroded inner lining of central vessel, clusters of cells around the vessels, enlarged nuclei and presence of Kupffer cells were observed in group V (400 mg/kg of S. aethiopicum extract).
Fig. 6.
Histology of liver section showing the effect of leaf extract of S. aethiopicum on phenylhydrazine induced anaemia in male and female Wistar rats. A, male; B, female; Group I, Normal control; Group II, Negative control; Group III, Ferrous sulphate (5 mg/kg); Group IV, S. aethiopicum extract (200 mg/kg); Group V, S. aethiopicum extract (400 mg/kg); CV, central vessel; Ss, sinusoid; H, hepatocytes; N, nuclei; K, Kupffer cells
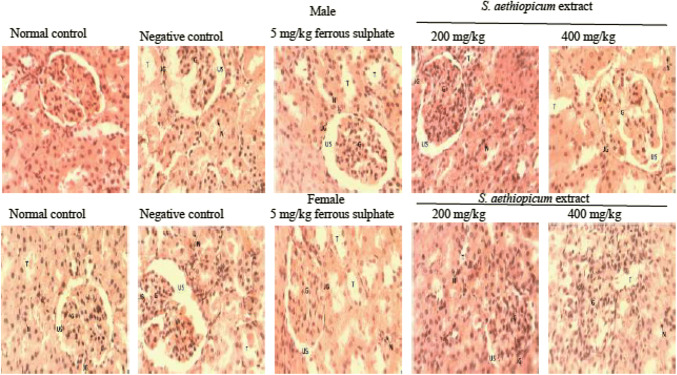
The kidney micrographs of rats treated with aqueous leaf extract of S. aethiopicum after anaemia induction with PHZ are shown in Fig. 7. The cells of the male rats were intact, but the cells were not distinguished. The female rats showed normal cyto-architecture of the kidney with the presence of glomerulus possessing its normal endothelium intact. The urinary space, blood capillaries, renal tubules were intact and appeared normal. Male and female rats exposed to phenylhydrazine without treatment (negative control) showed eroded endothelium which widened the urinary space and eroded its lining. Degenerated cells of urinary tubules and lack of distinct nuclei were also observed in negative control. Rats treated with ferrous sulphate showed constricted glomerulus and endothelium, dilated urinary space, intact Bowman’s capsule and Juxta-glomerular apparatus with depleted lining of urinary tubules and enlarged nuclei. The cortex of rats treated with S. aethiopicum showed an intact glomerulus with endothelium, urinary capsule and Juxta-glomerular apparatus appearing normal. The urinary space was small, but not constricted and aggregations of cells were observed in some part of the section.
Fig. 7.
Histology of kidney sections showing the effect of aqueous extract of S. aethiopicum on phenylhydrazine induced anaemia in male and female Wistar rats. A, male; B, female; Group I, Normal control; Group II, Negative control; Group III, Ferrous sulphate (5 mg/kg); Group IV, S. aethiopicum extract (200 mg/kg); Group V, S. aethiopicum extract (400 mg/kg); G, glomerulus; US, urinary space; T, urinary tubules; JG, Juxta glomerular apparatus; N, nuclei
Discussion
Various parts of eggplant including the leaves, fruits, roots and stems are used pharmaceutically in folklore medicine in the management of various ailments and diseases such as diabetes, hypertension, pile, rheumatism and dysentery [28]. The leaves are recommended for people with anaemia and low iron especially during pregnancy and post-partum period by traditional healers in Nigeria. The medicinal properties of egg plants may be as a result of phenolic compounds like flavonoids, saponins and tannins in the extracts. Although these metabolites have important therapeutic activities, they can also cause some degree of toxic effects. For example, phenolic compounds can be nephrotoxic, hepatotoxic, haematotoxic, and can also provoke mutagenesis and carcinogenesis [29]. Therefore, the evaluation of the toxicity/safety of S. aethiopicum in addition to haematopoietic properties is essential.
Anaemia was induced in the rats with PHZ except the normal control. Anaemia was diagnosed when a 30% decrease in PCV from the base line was observed in the experimental Wistar rats. The potential of PHZ to induce anaemia in animal models is attributable to its oxidative stress on erythrocytes, ATP depletion, cation imbalances, reduced membrane deformability and lipid peroxidation [30]. Blood parameters such as RBC, Hb, PCV, MCH, MCHC, MCV and iron levels are frequently used to assess the level of anaemia and the efficacy of treatment in human and experimental animals induced with anaemia [14]. In this study, significant increase in PCV, RBC, Hb, MCV and MCH values were observed in both male and female rats treated with ferrous sulphate and aqueous extract of S. aethiopicum compared to the normal and negative control. This suggests that S. aethiopicum may have haematopoietic potentials. Previously another specie of eggplant (S. macrocarpon) has been shown to increase RBC level in Wistar rats after induction of anaemia by exposure to urban air pollution [31]. This ameliorative property of S. aethiopicum may be as a result of their bio-constituents. For example, egg plants have been demonstrated to constitute high level of minerals (Fe2+, Zn2+ and Cu2+), vitamins (A, C, E, B) and phytochemicals such as polyphenols and flavonoids which are potent antioxidants [16]. These antioxidants may have moped up free radicals and inhibited the anaemia induced by PHZ [32] and the minerals and vitamins helped to increase haematopoiesis and erythropoiesis in the bone marrow [31]. Iron is known as an important integral part of haemoglobin, myoglobin and cytochrome, while zinc is RBC-SOD cofactor which plays essential role in the synthesis of haemoglobin, protects the integrity of erythrocytes and reduces oxidative stress [33]. The significant increase in platelet count in all animals fed with S. aethiopicum extract may be due to elevated secretion of thrombopoeitin, a hormone responsible for thrombopoeisis (synthesis of platelets). This may be as result of vitamins and polyphenols present in the plant extracts [31].
The elevated level of total WBC count in the rats induced with anaemia without treatment compared to the treated and normal control rats may be due to the injury caused by PHZ. WBC counts increase rapidly following exposure to foreign attack on the system by toxins and pathogens. Physiologically, the system will boost the body’s defensive attack and mechanisms in toxicity state [6, 34]. Onyeabo et al. [35] reported that rats treated with only PHZ had a lower WBC count compared to those treated with ethanolic leaf extract of Justicia carnea. Our present study revealed a significant increase (P < 0.05) in WBC in the group treated with PHZ when compared to the extract treated groups. Dornfest et al. [36] had earlier stated that PHZ is a mitogen and activator of lymphoid cells. Again, PHZ has shown to exhibit a marked level of leukocytosis in rats, due to increased circulating lymphoid cells [36]. The increased WBC in the groups treated with PHZ suggested that PHZ may have stimulated the immune system in rats due to its increased free radical activity [13].
Toxicity of chemicals and medicinal plants are frequently studied with animal models because of their usefulness in early identification of undesirable effect [6, 22]. It is also well established that mortality is used to determine the acute toxicity of plants extracts and other chemicals [37]. In addition to mortality, behavioural changes, changes in organs and body weight are also essential when investigating toxicity using animal models. The acute toxicity studied via administration of aqueous leaf extract of S. aethiopicum at graded doses of 500, 1000, 2000, 3000, 4000 and 5000 mg/kg to rats did not cause any mortality, toxicity signs or behavioural changes. This suggests that the aqueous leaf extract of S. aethiopicum has an LD50 that is higher than 5000 mg/kg and LD50 ≥ 5000 mg/kg is considered non-toxic [24]. Reports have shown that reduction in body and organ weight may be employed in the assessment of the degree of toxicity of medicinal plants [38]. Investigation of body and organ weights in toxicity studies involving animal models serves as a tool for assessing the animal sensitivity to toxicity, physiologic disturbances, enzyme induction as well as acute organ damage [39, 40].
A significant increase (P < 0.05) in the body weight of male rats treated with 5 mg/kg ferrous sulphate, 200 mg/kg and 400 mg/kg of the aqueous extract for 14 days was observed compared to the control in the sub-acute toxicity study. From our study, the weight of the spleen increased in the phenylhydrazine treated groups indicating splenomegaly, while the extract treated groups revealed a restoration effect by reducing the weight of the spleen closed to the normal control. The extract treated groups (III to V) post 14 days produced a significant increase in the weights of liver and lungs in both male and female Wistar rats compared to the controls. Gain in body weights and organ weights irrespective of dosage (200 mg/kg or 400 mg/kg) of the animals showed that the plant extract is non-toxic. According to Teo et al. [38], exposure of animal models for some time to potentially toxic substances leads to a reduction in body weights and internal organ weights of such animals.
Changes in lifestyle and dietary modifications are among the efficient ways of managing hyperlipidaemia. Atherosclerosis, hypertension and cardiovascular diseases are associated complications of hyperlipidaemia [41]. Hyperlipidaemia/dyslipidaemia is characterized by elevated levels of cholesterol, phospholipids, triacylglycerol and variations in lipoprotein levels [42]. The variation in lipoprotein leads to a lower level of high-density lipoprotein (HDL) compared to other species and approximately 20% cholesterol ester and very little triglyceride [42]. While plasma LDL level, VLDL and cholesterol levels are directly related to coronary heart disease (CHD), high HDL level protects against coronary heart diseases [41]. The results of this study clearly indicated that the administration of aqueous leaf extract of S. aethiopicum stimulated hypolipidaemic effect in experimental animals irrespective of sex. While there were significant decrease in the levels of TC, TAG, LDL-C, and VLDL-C in the experimental animals fed with aqueous leaf extract of S. aethiopicum compared to the control, the treated animals showed a significant increase (P < 0.05) in the levels of high-density lipoprotein compared to the negative control. This suggests that S. aethiopicum has anti-hyperlipidaemic properties. This finding may be as a result of the extract constituents such as phytochemicals and fibre. S. aethiopicum has been shown to contain flavonoids, tannins, alkaloids, saponins, steroids as well as dietary fibre [5]. Previous studies have demonstrated that phytochemicals may act wholly or partly as anti-hyperlipidaemic agents [43]. For example, saponins may inhibit the absorption of lipid (cholesterol), absorption of bound bile acids leading to increase bile acid excretion. Saponins may also cause decrease in fatty acid synthesis, enhance LDL receptors, activation of lipase acetyl-CoA carboxylase as well as lecithin-cholesterol acetyl transferase (LCAT) [44]. Flavonoids and saponins may also inhibit synthesis of HMG-CoA both at the mRNA and protein levels thereby leading to decrease in endogenous cholesterol synthesis and the mobilization of cholesterol from the extra-hepatic tissues to the liver for the synthesis of bile acid [44]. In addition, dietary fibre may also lower plasma cholesterol and triacylglycerol levels by production of short chain fatty acids when fermented in the colon [45].The findings of this present study agree with the results of other researchers [20, 46], who reported a lipid-lowering effect of the extracts of S. aethiopicum. The finding of this study is similar with the result reported by [35] on the effect of phenylhydrazine-induced anaemia in rats treated with leaf extracts of Justicia carnea.
Changes in biochemical parameters of the liver and kidney are relevant risk assessment tools in ascertaining extract’s efficacy in animal model [47]. Serum urea, creatinine and electrolytes such as sodium, chloride, bicarbonate, potassium and inorganic phosphate level elevation are pivotal indicators for kidney dysfunction [48]. This study showed a significant decrease in the levels of urea, creatinine, sodium and potassium in the male and female animals treated with 200 mg/kg and 400 mg/kg of the extract. This suggests that S. aethiopicum does not have a negative effect but rather can protect the kidneys against toxic substances. The extracts enhanced the ability of the kidneys to excrete these toxic wastes (urea, creatinine) and did not cause renal damage or impairment in both males and females.
The liver is essential for xenobiotic metabolism/detoxification, secretion of bile, bilirubin metabolism, synthesis and storage of biomolecules [49]. ALP, AST, ALT and bilirubin levels are known to increase following cellular damage and tissue necrosis of the liver [49]. In this study, a dose dependent significant (P < 0.05) decrease in serum levels of AST, ALT, ALP, total bilirubin, conjugated and unconjugated bilirubin were observed in rats treated with aqueous leaf extract of S. aethiopicum in both male and female rats compared to the control. This suggests that the extract at the doses given did not cause any liver damage, but rather possesses hepato-protective properties. Histopathological examinations of the liver and kidney of rats induced with PHZ showed necrotic changes which was caused by mild toxicity of PHZ on both the kidneys and livers of both male and female rats. However, reduction of the impact was observed after treatment with S. aethiopicum which revealed the presence of Kupffer cells in the liver micrograph. Kupffer cells are essential for healing of liver inflammation, fibrosis and injury [50]. The histopathology results corroborate the finding of biochemical analysis in this study. Therefore, it could be suggested that the S. aethiopicum have both hepatoprotective and renal protective properties.
In conclusion, this study revealed that the LD50 of aqueous leaf extract of S. aethiopicum is well above 5000 mg/kg and that the oral administration of this extract up to the concentration of 400 mg/kg is non-toxic and safe for nutritional and therapeutic uses. In addition, aqueous leaf extract of S. aethiopicum is haematopoietic, hepato-protective, anti-hyperlipidaemic and reno-protective. Therefore, S. aethiopicum may be recommended as an adjunct in the management of anaemia as well as to boost the immune system.
Compliance with ethical standards
Conflict of interest
Authors declare that they have no conflict of interest and that they are responsible for the manuscript content.
References
- 1.Petrovska BB. Historical review of medicinal plants’ usage. Pharmacogn Rev. 2012;6:1. doi: 10.4103/0973-7847.95849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sushruta K, Satyanarayana S, Srinivas S, Sekhar JR. Evaluation of the blood-glucose reducing effects of aqueous extracts of the selected umbelliferous fruits used in culinary practices. Trop J Pharm Res. 2006;5:613–617. [Google Scholar]
- 3.Fasuyi AO. Nutritional potentials of some tropical vegetable leaf meals. Chemical characterization and functional properties. Afr J Biotechnol. 2006;5:49–53. [Google Scholar]
- 4.Kumar A, Iavarasan RI, Jayachandran T, Decaraman M, Aravindhan P, Padmanabhan N, Krishnan MRV. Investigation on a tropical plant Syzygium cumini from Kattuppalayam, Erode District, Tamil Nadu, South India. Pak J Nutr. 2009;8:83–85. [Google Scholar]
- 5.Edeoga HO, Okwu DE, Mbaebie BO. Phytochemical constituents of some Nigeria medicinal plants. Afr J Biotechnol. 2005;4:685–688. [Google Scholar]
- 6.Ugbogu AE, Akubugwo EI, Ude VC, Gilbert J, Ekeanyanwu B. Toxicological evaluation of phytochemical characterized aqueous extract of wild dried Lentinus squarrosulus (Mont.) mushroom in rats. Toxicol Res. 2019;35:181–190. doi: 10.5487/TR.2019.35.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanfer EJ, Nicol BA. Haemoglobin concentration and erythrocyte sedimentation rate in primary care patients. J R Soc Med. 1997;90:16–18. doi: 10.1177/014107689709000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO . The Global Prevalence of Anaemia in 2011. Geneva: World Health Organization; 2017. p. 2015. [Google Scholar]
- 9.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993–2005. Public Health Nutr. 1993;12:444–454. doi: 10.1017/S1368980008002401. [DOI] [PubMed] [Google Scholar]
- 10.WHO (2008) Worldwide prevalence of anaemia 1993–2005: WHO global database on anaemia
- 11.Crawley J. Reducing the burden of anemia in infants and young children in malaria-endemic countries of Africa: from evidence to action. Am J Trop Med Hyg. 2004;71:25–34. [PubMed] [Google Scholar]
- 12.de Benoist B, McLean E, Egli I, Cogswell M. Worldwide prevalence of anaemia 1993–2005—WHO Global Database on Anaemia. WHO-CDC. 2008;2008:48. [Google Scholar]
- 13.Berger J. Phenylhydrazine haematotoxicity. J Appl Biomed. 2007;5:125–130. [Google Scholar]
- 14.Lee HW, Kim H, Ryuk JA, Kil KJ, Ko BS. Hemopoietic effect of extracts from constituent herbal medicines of Samul-tang on phenylhydrazine-induced hemolytic anemia in rats. Int J Clin Exp Pathol. 2014;7:6179. [PMC free article] [PubMed] [Google Scholar]
- 15.Siraki AG, Klotz LO, Kehrer JP. Free radicals and reactive oxygen species. Compr Toxicol. 2018;1:262–294. [Google Scholar]
- 16.Anosike CA, Abonyi O, Ubaka C. Does the African garden egg offer protection against experimentally induced ulcers? Asian Pac J Trop Med. 2011;4:163–166. doi: 10.1016/S1995-7645(11)60061-8. [DOI] [PubMed] [Google Scholar]
- 17.Oboh G, Ekperigin MM, Kazeem MI. Nutritional and haemolytic properties of eggplants (Solanum macrocarpon) leaves. J Food Compos Anal. 2005;18:153–160. [Google Scholar]
- 18.Komlaga G, Sam GH, Dickson RA, Mensah MLK, Fleischer TC. Pharmacognostic studies and antioxidant properties of the leaves of Solanum macrocarpon. J Pharm Sci Res. 2014;6:1–4. [Google Scholar]
- 19.Anosike CA, Obidoa O, Ezeanyika LU. The anti-inflammatory activity of garden egg (Solanum aethiopicum) on egg albumin-induced oedema and granuloma tissue formation in rats. Asian Pac J Trop Med. 2012;5:62–66. doi: 10.1016/S1995-7645(11)60247-2. [DOI] [PubMed] [Google Scholar]
- 20.Edijala JK, Asagba SO, Eriyamremu GE, Atomatofa U. Comparative effect of garden egg fruit, oat and apple on serum lipid profile in rats fed a high cholesterol diet. Pak J Nutr. 2005;4:245–249. [Google Scholar]
- 21.Harizal SN, Mansor SM, Hasnan J, Tharakan JKJ, Abdullah J. Acute toxicity study of the standardized methanolic extract of Mitragyna speciosa Korth in rodent. J Ethnopharmacol. 2010;131:404–409. doi: 10.1016/j.jep.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Nurul SAS, Hazilawati H, Mohd RS, Mohd FHR, Noordin MM, Norhaizan E. Subacute oral toxicity assessment of ethanol extract of Mariposa christia vespertilionis leaves in male Sprague Dawley rats. Toxicol. Res. 2018;34:85–95. doi: 10.5487/TR.2018.34.2.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Research Council (NRC) Guide for the care and use of laboratory animals. Washington: National Academies Press; 2010. [Google Scholar]
- 24.Organization for economic cooperation and development (OECD) (2001) Guideline 423: acute oral toxicity—acute toxic class method. 470 adopted by the council on 17th, December 2001
- 25.Roque M, Danna C, Gatti C, Veuthey T. Hematological and morphological analysis of the erythropoietic regenerative response in phenylhydrazine-induced hemolytic anemia in mice. Scand J Lab Anim Sci. 2008;35:181–190. [Google Scholar]
- 26.Organization for economic cooperation and development (OECD) (1995) Guideline 407: repeated-dose 28-day oral toxicity study in rodents. 468 adopted by the council on 27th, July 1995
- 27.Bain BJ, Bates I, Laffan MA. Dacie and Lewis practical haematology. Amsterdam: Elsevier; 2016. [Google Scholar]
- 28.Seneff S, Wainwright G, Mascitelli L. Nutrition and Alzheimer’s disease: the detrimental role of a high carbohydrate diet. Eur J Int Med. 2011;22:134–140. doi: 10.1016/j.ejim.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 29.Michałowicz J, Duda W. Phenols–sources and toxicity. Pol J Environ Stud. 2007;16:347–362. [Google Scholar]
- 30.Pandey S, Ganeshpurkar A, Bansal D, Dubey N. Hematopoietic effect of Amaranthus cruentus extract on phenylhydrazine-induced toxicity in rats. J Diet Suppl. 2016;13:607–615. doi: 10.3109/19390211.2016.1155685. [DOI] [PubMed] [Google Scholar]
- 31.Olajire AA, Azeez L. Effects of Solanum macrocarpon (African eggplant) on haematological parameters of Wistar rats exposed to urban air pollution. Adv Environ Res. 2012;1:109–123. [Google Scholar]
- 32.Aduwamai UH, Abimbola MM, Ahmed ZH. Effect of Solanum nigrum methanol leaf extract on phenylhydrazine induced anemia in rats. Jordan J Biol Sci. 2018;11:65–71. [Google Scholar]
- 33.Fukushima T, Horike H, Fujiki S, Kitada S, Sasaki T, Kashihara N. Zinc deficiency anemia and effects of zinc therapy in maintenance hemodialysis patients. Ther Apher Dial. 2009;13:213–219. doi: 10.1111/j.1744-9987.2009.00656.x. [DOI] [PubMed] [Google Scholar]
- 34.Han CT, Kim MJ, Moon SH, Jeon YR, Hwang JS, Nam C, Park CW, Lee SH, Na JB, Park CS, Park HW, Lee JM, Jang HS, Park SH, Han KG, Choi YW, Lee HY, Kang JK. Acute and 28-day subacute toxicity studies of hexane extracts of the roots of Lithospermum erythrorhizon in Sprague–Dawley rats. Toxicol Res. 2015;31:403–414. doi: 10.5487/TR.2015.31.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Onyeabo C, Achi NK, Ekeleme-Egedigwe CA, Ebere CU, Okoro CK. Haematological and biochemical studies on Justicia carnea leaves extract in phenylhydrazine induced-anemia in albino rats. Acta Sci Pol Technol Aliment. 2017;16:217–230. doi: 10.17306/J.AFS.0492. [DOI] [PubMed] [Google Scholar]
- 36.Dornfest BS, Lapin DM, Naughton BA, Adu S, Korn L, Gordon AS. Phenylhydrazine-induced leukocytosis in the rat. J Leukoc Biol. 1986;39:37–48. doi: 10.1002/jlb.39.1.37. [DOI] [PubMed] [Google Scholar]
- 37.Asare GA, Gyan B, Bugyei K, Adjei S, Mahama R, Addo P, Otu-Nyarko L, Wiredu E, Knyarko A. Toxicity potentials of the nutraceutical Moringa oleifera at supra-supplementation levels. J Ethnopharmacol. 2012;139:265–272. doi: 10.1016/j.jep.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Teo S, Stirling D, Thomas S, Hoberman A, Kiorpes A, Khetani V. A 90-day oral gavage toxicity study of d-methylphenidate and d, l-methylphenidate in Sprague–Dawley rats. Toxicology. 2002;179:183–196. doi: 10.1016/s0300-483x(02)00338-4. [DOI] [PubMed] [Google Scholar]
- 39.Michael B, Yano B, Sellers RS, Perry R, Morton D, Roome N, Johnson JK, Schafer K. Evaluation of organ weights for rodent and non-rodent toxicity studies: a review of regulatory guidelines and a survey of current practices. Toxicol Pathol. 2007;35:742–750. doi: 10.1080/01926230701595292. [DOI] [PubMed] [Google Scholar]
- 40.Ugbogu EA, Akubugwo EI, Ude VC, Emmanuel O, Nduka OO, Ibeh C, Onyero O. Safety evaluation of aqueous extract of Termitomyces robustus (Agaricomycetes) in Wistar rats. Int J Med Mushroom. 2019;21:193–201. doi: 10.1615/IntJMedMushrooms.2018029737. [DOI] [PubMed] [Google Scholar]
- 41.Enechi OC, Ozougwu VEO. Effects of ethanol extract of Mucuna pruriens leaves on the lipid profile and serum electrolytes of rats. J Pharm Biol Sci. 2014;9:18–23. [Google Scholar]
- 42.Bagdade JD, Helve E, Taskinen MR. Effects of continuous insulin infusion therapy on lipoprotein surface and core lipid composition in insulin-dependent diabetes mellitus. Metabolism. 1991;40:445–449. doi: 10.1016/0026-0495(91)90222-i. [DOI] [PubMed] [Google Scholar]
- 43.Gaamoussi F, Israili ZH, Lyoussi B. Hypoglycemic and hypolipidemic effects of an aqueous extract of Chamaerops humilis leaves in obese, hyperglycemic and hyperlipidemic Meriones shawi rats. Pak J Pharm Sci. 2010;23:212–219. [PubMed] [Google Scholar]
- 44.Sharmila BG, Kumar G, Rajasekara PM. Cholesterol lowering activity of the aqueous fruit extract of Trichosanthes dioica Roxb. in normal and streptozotocin diabetic rats. J Clin Diagn Res. 2007;1:561–569. [Google Scholar]
- 45.Wong JM, De Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 46.Ogunka-Nnoka CU, Onyegeme-Okerenta BM, Omeje HC. Effects of ethanol extract of S. aethiopicum stalks on lipid profile and haematological parameters of Wistar albino rats. Int J Sci Res Methods Hum. 2018;10:215–229. [Google Scholar]
- 47.Olson H, Betton G, Robinson D, Thomas K, Monro A, Kolaja G, Lilly P, Sanders J, Sipes G, Bracken W, Dorato M. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul Toxicol Pharmacol. 2000;32:56–67. doi: 10.1006/rtph.2000.1399. [DOI] [PubMed] [Google Scholar]
- 48.Kong BH, Tan NH, Fung SY, Pailoor J. Sub-acute toxicity study of tiger milk mushroom Lignosus tigris Chon S. Tan Cultivar E. sclerotium in Sprague Dawley rats. Front Pharmacol. 2016;7:246. doi: 10.3389/fphar.2016.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adeyemi OT, Osilesi O, Adebawo OO, Onajobi FD, Oyedemi SO, Afolayan AJ. Alkaline phosphatase (ALP), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities in selected tissues of rats fed on processed atlantic horse mackerel (Trachurus trachurus) Adv Biosci Biotechnol. 2015;6:139. [Google Scholar]
- 50.Kawada N, Seki S, Inoue M, Kuroki T. Effect of antioxidants, resveratrol, quercetin, and N-acetylcysteine, on the functions of cultured rat hepatic stellate cells and Kupffer cells. Hepatology. 1998;27:1265–1274. doi: 10.1002/hep.510270512. [DOI] [PubMed] [Google Scholar]