Abstract
Objective
Vitamin D could prevent cognitive decline because of its neuroprotective, anti-inflammatory and antioxidant properties. This study aimed to evaluate the associations of plasma 25-hydroxyvitamin D (25(OH)D) concentrations with global cognitive function and incident dementia, including Alzheimer’s disease (AD).
Methods
The Canadian Study of Health and Aging is a 10-year cohort study of a representative sample of individuals aged 65 years or older. A total of 661 subjects initially without dementia with frozen blood samples and follow-up data were included. Global cognitive function was measured using the validated Modified Mini-Mental State (3MS) examination. A consensus diagnosis of all-cause dementia and AD was made between the physician and the neuropsychologist according to published criteria. Cognitive decline for a 5-year increase in age at specific 25(OH)D concentrations was obtained using linear mixed models with repeated measures. Hazard ratios of incident dementia and AD were obtained using semi-parametric proportional hazards models with age as time scale.
Results
Over a mean follow-up of 5.4 years, 141 subjects developed dementia of which 100 were AD. Overall, no significant association was found between 25(OH)D and cognitive decline, dementia or AD. Higher 25(OH)D concentrations were associated with an increased risk of dementia and AD in women, but not in men.
Conclusion
This study does not support a protective effect of vitamin D status on cognitive function. Further research is needed to clarify the relation by sex.
Electronic supplementary material
The online version of this article (10.17269/s41997-019-00290-5) contains supplementary material, which is available to authorized users.
Keywords: 25-hydroxyvitamin D, Cognitive impairment, Alzheimer disease, Prospective study
Résumé
Objectif
La vitamine D pourrait avoir un effet protecteur sur le déclin cognitif en raison de ses propriétés neuroprotectrices, anti-inflammatoires et antioxydantes. L’objectif de cette étude était d’évaluer les associations entre la concentration plasmatique de 25-hydroxyvitamine D (25(OH)D), la fonction cognitive globale et l’incidence de la démence incluant la maladie d’Alzheimer (MA).
Méthodes
L’Étude sur la santé et le vieillissement au Canada est une étude de cohorte de 10 ans réalisée dans un échantillon représentatif des Canadiens âgés de 65 ans et plus. Un total de 661 participants sans démence, pour lesquels un échantillon sanguin congelé et des données au suivi étaient disponibles, ont été inclus dans l’analyse. La fonction cognitive globale a été mesurée à l’aide d’un outil validé, le Modified Mini-Mental State (3MS) Examination. Les diagnostics de démence toutes causes et de MA ont été obtenus par consensus entre un médecin généraliste et un neuropsychologue selon des critères publiés. Le déclin cognitif pour chaque augmentation de 5 ans d’âge à des concentrations spécifiques de 25(OH)D a été mesuré à l’aide de modèles linéaires mixtes avec données répétées. Des rapports de risques de la démence et de la MA ont été obtenus à l’aide de modèles à risques proportionnels semi-paramétriques en utilisant l’âge comme échelle du temps.
Résultats
En cours de suivi (moyenne : 5,4 ans), 141 individus ont développé une démence dont 100 étaient la MA. Globalement, aucune association statistiquement significative n’a été observée entre le 25(OH)D et le déclin cognitif, la démence ou la MA. Des concentrations plus élevées de 25(OH)D étaient associées à une augmentation du risque de démence et de MA chez les femmes, mais pas chez les hommes.
Conclusion
Cette étude n’appuie pas l’hypothèse d’un effet protecteur de la vitamine D sur la fonction cognitive. D’autres études seraient nécessaires pour clarifier la relation selon le sexe.
Mots-clés: 25-hydroxyvitamine D, Trouble neurocognitif léger, Maladie d’Alzheimer, Étude prospective
Introduction
Fifty million people worldwide were living with dementia in 2018 and according to projections, this number will rise to an estimated 152 million by 2050 (Alzheimer’s Disease International 2018). Mild cognitive impairment (MCI) can affect up to 23% of older people, nearly three-fold the proportion of individuals with dementia (Luck et al. 2010). It has been estimated that 46% of people with MCI will develop dementia within 3 years (Tschanz et al. 2006). Considering the social and economic burden of cognitive decline, identification of preventive factors is greatly needed.
Vitamin D could maintain cognitive function because of its neuroprotective, anti-inflammatory and antioxidant properties (Aspell et al. 2018; Littlejohns et al. 2016). However, prospective studies on the role of 25-hydroxyvitamin D (25(OH)D), the main biomarker of vitamin D status, in cognitive function have shown inconsistent results. Protective and no association were equally reported (Overman et al. 2017; Llewellyn et al. 2010; Slinin et al. 2012; Kuzma et al. 2016; Toffanello et al. 2014; Wilson et al. 2014; Feart et al. 2017; Bartali et al. 2014; Licher et al. 2017; Knekt et al. 2014; Littlejohns et al. 2014). Pooled estimates from two meta-analyses found weak protective effects on dementia (Jayedi et al. 2018; Sommer et al. 2017) and Alzheimer’s disease (AD) (Jayedi et al. 2018). Furthermore, sex differences in this association have not been studied extensively. This difference is relevant as the prevalence of dementia and AD is higher among women (Prince et al. 2015) and they usually show lower concentrations of 25(OH)D (Greene-Finestone et al. 2011).
This study will fill this gap in the literature by contributing to the body of prospectively collected data on blood 25(OH)D concentrations and the risk of cognitive decline, all-cause dementia and AD among older individuals, and by evaluating the potential effect modification of risk associated with sex.
Method
Study population
The Canadian Study of Health and Aging (CSHA) is a longitudinal study of dementia conducted in a representative sample of people aged 65 years or older. More details about CSHA are provided elsewhere (Lindsay et al. 2004). In brief, 18 research centres participated at the baseline examination in 1991–1992 (CSHA-1) and two follow-ups in 1996–1997 (CSHA-2) and 2001–2002 (CSHA-3). Subjects were randomly selected from the Enumeration Composite in the province of Ontario and from Medicare lists in the other provinces (Lindsay et al. 2004). Institutionalized subjects were selected from random samples of institutions. The ethics review committees in each research centre and the coordinating centre approved each phase.
At baseline, 10,263 men and women were recruited, of whom 97% were Caucasian; 9008 were living in the community and 1255 in institutions. Community-dwelling subjects were screened for dementia with the 100-point Modified Mini-Mental State (3MS) examination (Teng and Chui 1987). All subjects with a 3MS score < 78 and a random sample of those who scored > 77 were invited to an extensive, standardized, clinical examination conducted by a nurse, a physician and a psychometrist (if 3MS score > 49). Institutionalized subjects were automatically invited to the clinical examination. A similar diagnostic process was used at each phase. In total, 2914 subjects in CSHA-1, 2305 in CSHA-2 and 1386 in CSHA-3 were clinically examined (Lindsay et al. 2004).
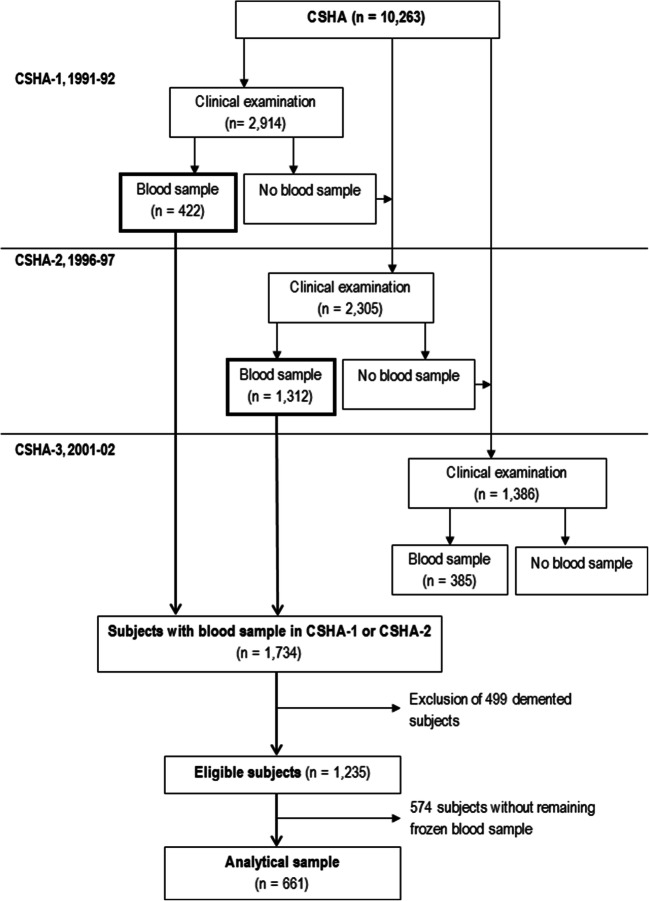
Nine of the 18 centres in CSHA-1 and all centres in CSHA-2 participated in a substudy which requested non-fasting blood samples for future research. In CSHA-1, all subjects with dementia and an equal number of subjects without dementia randomly chosen were invited to provide blood. In CSHA-2, all clinically assessed subjects were invited to provide blood. In total, 422 subjects in CSHA-1 and 1312 in CSHA-2 provided blood. After exclusion of 499 subjects with dementia, 1235 subjects without dementia were eligible for the present study. Frozen blood samples were still available for 661 of them and served for plasma 25(OH)D concentration measurement (Fig. 1).
Fig. 1.
Flowchart of the study sample from the Canadian Study of Health and Aging (CSHA)
Measures
Plasma 25-hydroxyvitamin D
Vitamin D status was evaluated with plasma 25(OH)D, the main biomarker of vitamin D status (Jones 2012). The half-life of 25(OH)D is about 14 days; 25(OH)D is a useful indicator to characterize the usual vitamin D status (Jones et al. 2014). Blood samples were stored at the National Microbiology Laboratory, Winnipeg, Canada. Total plasma 25(OH)D was measured with a competitive assay using electrochemiluminescence on an Elecsys 2010 system (Roche Diagnostics, Laval, Canada), which was standardized by the National Institute of Standards and Technology (Phinney 2008). Coefficients of variation were 11.0%, 4.3% and 8.3% at 12.0, 50.5 and 79.0 nmol/L, respectively.
Global cognitive function and dementia assessments
Global cognitive function was measured with the validated 3MS (Teng and Chui 1987). For all subjects who attended the clinical examination, preliminary diagnoses were made independently by a physician and a neuropsychologist, who subsequently reached a consensus diagnosis in a case conference. In CSHA-1, Diagnostic and Statistical Manual of Mental Disorders, 3rd edition, revised criteria (DSM-III-R) were used for all-cause dementia, and the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association criteria were used for AD. In CSHA-2 and CSHA-3, Diagnostic and Statistical Manual of Mental Disorders 4th edition criteria were used for all-cause dementia and AD. The diagnosis of cognitive impairment-no dementia (CIND) was made according to the DSM-III-R and the International Classification of Diseases, 10th revision criteria. CIND encompasses different subtypes of cognitive impairment including neurological disorder or those of vascular or psychiatric origin (Graham et al. 1997).
Covariates
Socio-demographic, lifestyle and medical history variables were obtained in subjects without dementia using a self-administered risk factor questionnaire at baseline or data from clinical examinations. These variables included age (continuous, years), education (continuous, years), sex, body mass index (BMI) (weight (kg)/height squared (m2), continuous), smoking status (ever been smoking regularly (almost every day) or not), alcohol intake (ever been drinking regularly (at least once a week) or not), history of stroke or myocardial infarction, and diabetes. Hypertension was defined as a blood pressure > 160 mmHg systolic or > 95 mmHg diastolic, or a physician’s diagnostic, or the use of medication for hypertension. The presence of allele e4 on the apolipoprotein E gene (ApoE4 (yes/no)) was based on a modified method of Zivelin et al. (McLeod et al. 1998).
Statistical analyses
The distribution of 25(OH)D was log-transformed because it was right skewed. The 3MS scores and plasma 25(OH)D concentrations were used as continuous variables. To determine the effect of 25(OH)D on the change of the 3MS in time, an interaction term was introduced between 25(OH)D and age at each phase in a linear mixed model with repeated measures. For a better understanding of the trend in cognitive decline over time according to 25(OH)D in continuous, results are presented for 3 specific concentrations corresponding to the median points of each tertile group of the distribution (i.e., 23.4, 42.4 and 69.4 nmol/L). A differential effect of age and 25(OH)D by sex was investigated by introducing a triple interaction following the hierarchical principle. Model 1 was fit with a minimal adjusted set including age, sex, education, phase and 25(OH)D concentration by age interaction. Potential confounders including ApoE4, BMI, institutionalization, alcohol intake and smoking status were added in model 2. Other potential confounders or intermediates including depression, hypertension, diabetes, and history of stroke or myocardial infarction were further entered in model 3.
Hazard ratios (HR) for all-cause dementia and AD according to the log-transformed 25(OH)D concentration in continuous were calculated using a semi-parametric proportional hazards model with delayed entry and age as the time scale. To facilitate interpretation of the results, the log-transformed 25(OH)D unit was set at 0.41, which corresponds to a 50% increase in 25(OH)D. Since the date of onset dementia was not known, time-to-event on the age scale was interval-censored between the examination before diagnosis and the examination at the diagnosis. Assuming that dementia could not be developed in a short period of time, subjects who remained cognitively normal, died or were lost to follow-up were right censored at 6 months after the last examination. Interaction terms between 25(OH)D and sex were included in the models where HRs were presented separately for men and women. The same sequence of adjustment as in the global cognitive function analysis was fit with the addition of CIND in models 2 and 3, while the interaction with age could not be included as age was already entered as the time scale. Seasonal trends in 25(OH)D were examined graphically and modeled using generalized additive models. Aside from a slight linear trend in time, there was no evidence of fluctuations in our sample and therefore, seasons were no longer considered in the models. Furthermore, adjustment for physical activity gave similar results. In this old cohort, the institutionalization status could be viewed as a better proxy of sun exposure than physical activity.
Among the 661 subjects with 25(OH)D data, 40% had at least one missing data on one of the covariates. Data were complete for 25(OH)D, age, sex, education and phase, which are the covariates included in model 1, but 32 and 36 events of dementia in models 2 and 3, respectively, were lost in the adjustment because of missing data. Multiple imputation employing chained equations was therefore performed. As recommended, the number of imputed datasets was equal to the percentage of incomplete cases (40%) (White et al. 2011). Binary and categorical variables were imputed using logistic regressions, whereas continuous variables were imputed using predictive mean matching based on linear regressions.
Sensitivity analyses comprised (1) multiple imputation on the 1235 eligible subjects; (2) adjustment for the current intake of vitamin D supplements (either vitamin D or multivitamin); (3) restriction to subjects with no CIND at baseline for the cognitive decline analysis; and (4) with 25(OH)D in three categories (< 25 nmol/L; 25–49 nmol/L and ≥ 50 nmol/L) for comparison with other studies on dementia (Jayedi et al. 2018). An a posteriori sensitivity analysis stratifying by age was done.
Results
Compared with non-deficient subjects, those with 25(OH)D concentrations < 50 nmol/L were, on average, older and more likely to be women and had a lower 3MS and a higher BMI (Table 1). They were also more likely to be institutionalized and less likely to ever been smoking or drinking alcohol and to be taking vitamin D or multivitamin supplements. They also showed a higher prevalence of diabetes, depression and CIND.
Table 1.
Baseline characteristics of the study sample by plasma vitamin D status (n = 661)
| Characteristics | Non-deficient (25(OH)D ≥ 50 nmol/L)a (n = 236) | Deficient/insufficient (25(OH)D < 50 nmol/L)a (n = 425) | Whole samplea (n = 661) |
|---|---|---|---|
| Age (years) | 80.4 ± 5.9 | 81.3 ± 6.5 | 81.0 ± 6.3 |
| Sex | |||
| Men | 114 (48.3) | 153 (36.0) | 267 (40.4) |
| Women | 122 (51.7) | 272 (64.0) | 394 (59.6) |
| Education (years) | 9.9 ± 4.3 | 9.7 ± 3.9 | 9.7 ± 4.0 |
| 3MS score | 83.1 ± 11.1 | 81.9 ± 12.1 | 82.3 ± 11.8 |
| CIND (yes) | 79 (33.5) | 165 (38.8) | 244 (36.9) |
| BMI (kg/m2) | 25.0 ± 4.3 | 25.8 ± 5.2 | 25.5 ± 4.9 |
| Carrier of ApoE4 (yes) | 46 (19.5) | 87 (20.5) | 133 (20.1) |
| Institutionalized (yes) | 26 (11.0) | 73 (17.2) | 99 (15.0) |
| Alcohol drinking (yes) | 95 (44.6) | 121 (32.0) | 216 (36.6) |
| Smoking (yes) | 113 (52.3) | 168 (44.4) | 281 (47.3) |
| Vitamin supplement intake (yes) | 62 (28.2) | 77 (19.0) | 139 (22.2) |
| Multivitamin (yes) | 57 (25.9) | 77 (19.0) | 134 (21.4) |
| Vitamin D (yes) | 7 (3.2) | 2 (0.5) | 9 (1.4) |
| Diabetes (yes) | 22 (9.5) | 82 (19.5) | 104 (16.0) |
| History of stroke or myocardial infarction (yes) | 77 (32.6) | 146 (34.4) | 223 (33.7) |
| Hypertension (yes) | 84 (35.6) | 152 (35.8) | 236 (35.7) |
| Depression (yes) | 13 (6.0) | 39 (9.6) | 52 (8.3) |
25(OH)D, 25-hydroxyvitamin D; 3MS, Modified Mini-Mental State examination; ApoE4, allele e4 on the apolipoprotein E gene; BMI, body mass index; CIND, cognitive impairment-no dementia. Missing values for 3MS: n = 5; alcohol drinking: n = 70; BMI: n = 40; depression: n = 36; diabetes: n = 10; smoking: n = 67; multivitamin: n = 36; vitamin D supplement: n = 36
aMean ± standard deviation or n (%)
Cognitive decline
Overall, 3MS scores showed mean declines ranging from 1.95 to 2.20 points (all p values < 0.01) for each 5-year increase in age, regardless of specific 25(OH)D concentrations and models (Table 2). These declines were not significantly associated with 25(OH)D concentrations. Declines were more pronounced among women than men (p values for 25(OH)D by sex interactions < 0.03 for all models). Results were similar with multiple imputations among eligible subjects, with additional adjustment for vitamin D supplementation, and when restricted to subjects with no CIND at baseline (Online Resource, Tables S1–S3).
Table 2.
Declines in 3MS scores for a 5-year increase in age at specific plasma 25(OH)D concentrations
| Model 1a | Model 2a | Model 3a | |
|---|---|---|---|
| Beta (95% CI) | Beta (95% CI) | Beta (95% CI) | |
| 25(OH)D | |||
| Global | |||
| At 23.4 nmol/L | − 2.20 (− 2.94; − 1.45) | − 2.17 (− 2.91; − 1.42) | − 2.19 (− 2.94; − 1.44) |
| At 42.4 nmol/L | − 2.11 (− 2.68; − 1.53) | − 2.05 (− 2.63; − 1.46) | − 2.08 (− 2.67; − 1.49) |
| At 69.4 nmol/L | − 2.03 (− 2.80; − 1.26) | − 1.95 (− 2.73; − 1.17) | − 1.98 (− 2.76; − 1.20) |
| p value for 25(OH)D | 0.7926 | 0.6879 | 0.6938 |
| p value for 25(OH)D by age interaction | 0.7412 | 0.6682 | 0.6731 |
| Men | |||
| At 23.4 nmol/L | − 1.52 (− 2.61; − 0.43) | − 1.47 (− 2.56; − 0.38) | − 1.48 (− 2.57; − 0.38) |
| At 42.4 nmol/L | − 1.43 (− 2.35; − 0.51) | − 1.35 (− 2.27; − 0.43) | − 1.36 (− 2.29; − 0.44) |
| At 69.4 nmol/L | − 1.35 (− 2.35; − 0.35) | − 1.25 (− 2.26; − 0.25) | − 1.26 (− 2.27; − 0.26) |
| Women | |||
| At 23.4 nmol/L | − 2.87 (− 3.64; − 2.11) | − 2.86 (− 3.64; − 2.09) | − 2.91 (− 3.69; − 2.13) |
| At 42.4 nmol/L | − 2.78 (− 3.48; − 2.09) | − 2.75 (− 3.45; − 2.04) | − 2.79 (− 3.51; − 2.08) |
| At 69.4 nmol/L | − 2.71 (− 3.63; − 1.79) | − 2.65 (− 3.58; − 1.72) | − 2.70 (− 3.63; − 1.76) |
25(OH)D, 25-hydroxyvitamin D; 3MS, Modified Mini-Mental State examination; ApoE4, allele e4 on apolipoprotein E gene; BMI, body mass index; CI, confidence interval
These specific 25(OH)D concentrations correspond to the median points of each tertile group of the distribution of the study sample
aBetas adjusted for age, education, sex and phase, with interaction term between 25(OH)D and age, and between sex and age in model 1; additionally adjusted for ApoE4, BMI, alcohol drinking, smoking and institutionalization in model 2; additionally adjusted for depression, hypertension, diabetes, and history of stroke or myocardial infarction in model 3
Dementia and Alzheimer’s disease
Over a mean follow-up of 5.4 years, 141 subjects developed all-cause dementia, including 100 incident cases of AD. No significant association was found between 25(OH)D and dementia or AD in models 1 and 2. A significantly increased risk with higher concentration of 25(OH)D was observed for AD only in model 3 (Table 3). An interaction was found between 25(OH)D and sex for AD (p = 0.0173), but not for all-cause dementia (p = 0.1261). Among men, no significant association was observed between 25(OH)D concentration and dementia or AD, but estimates showed protective HRs. In contrast, among women, a 50% increase of 25(OH)D concentration was marginally significantly associated with a higher risk of dementia in models 2 and 3 (HRs of 1.16 and 1.17, respectively), and AD in all models (HRs of 1.25, 1.28 and 1.31, respectively). Similar results were observed when 25(OH)D was treated in categories (Online Resource, Table S4). HRs were also similar in the sensitivity analyses with multiple imputations among all eligible subjects, and with additional adjustment for vitamin D supplementation (Online Resource, Tables S5–S6).
Table 3.
Hazard ratios (HR) for all-cause dementia and Alzheimer’s disease associated with 25(OH)D status (n = 661)
| Dementia | Alzheimer’s disease | |||
|---|---|---|---|---|
| Events (n, %) | HR (95% CI)a | Events (n, %) | HR (95% CI)a | |
| 25(OH)D (per 50% increase) | ||||
| Global | 141 (21.3) | 100 (15.1) | ||
| Model 1 | 1.04 (0.92–1.17) | 1.12 (0.97–1.30) | ||
| Model 2 | 1.09 (0.97–1.23) | 1.15 (0.99–1.33) | ||
| Model 3 | 1.11 (0.98–1.26) | 1.18 (1.02–1.37) | ||
| Men | 47 (17.6) | 34 (12.7) | ||
| Model 1 | 0.92 (0.75–1.14) | 0.87 (0.69–1.11) | ||
| Model 2 | 0.95 (0.77–1.18) | 0.89 (0.70–1.14) | ||
| Model 3 | 0.98 (0.79–1.21) | 0.93 (0.72–1.19) | ||
| Women | 94 (23.9) | 66 (16.8) | ||
| Model 1 | 1.10 (0.95–1.27) | 1.25 (1.05–1.50) | ||
| Model 2 | 1.16 (1.01–1.35) | 1.28 (1.08–1.53) | ||
| Model 3 | 1.17 (1.01–1.36) | 1.31 (1.10–1.56) | ||
25(OH)D, 25-hydroxyvitamin D; 3MS, Modified Mini-Mental State examination; ApoE4, allele e4 on apolipoprotein E gene; BMI, body mass index; CI, confidence interval; HR, hazard ratio; CIND, cognitive impairment no dementia
aHRs and 95% CIs adjusted for age (as time scale), sex, education and phase in model 1; additionally adjusted for ApoE4, BMI, alcohol drinking, smoking, CIND and institutionalization in model 2; additionally adjusted for depression, hypertension, diabetes, and history of stroke and myocardial infarction in model 3. An interaction term between 25(OH)D and sex was introduced in global models to obtain HRs separately for men and women
Italicized values are statistically significant
Discussion
Our study suggests that vitamin D status is not associated with global cognitive function or its decline. Moreover, there is no association between plasma 25(OH)D concentrations and the incidence of all-cause dementia or AD, except for women, where higher 25(OH)D concentrations seemed to increase the incidence of dementia and AD.
Prospective studies on vitamin D status and global cognitive function have shown conflicting results. Among the 15 studies retrieved from the literature, 7 found no association between 25(OH)D and cognitive performance (Kilpatrick et al. 2018; Olsson et al. 2017; Graf et al. 2014; van Schoor et al. 2016; Slinin et al. 2010; Breitling et al. 2012; Perna et al. 2014). In contrast, 7 studies found a positive association between low 25(OH)D and cognitive decline (Llewellyn et al. 2010; Slinin et al. 2012; Kuzma et al. 2016; Toffanello et al. 2014) or lower cognitive score (Wilson et al. 2014; Feart et al. 2017; Bartali et al. 2014) compared with higher concentrations. Finally, one study among subjects aged ≥ 85 years found a positive association with both low and high 25(OH)D concentrations and cognitive decline when compared with the middle category (Granic et al. 2015).
Prospective studies on 25(OH)D and the risk of dementia and AD also showed conflicting results. Five (Olsson et al. 2017; Karakis et al. 2016; Graf et al. 2014; Afzal et al. 2014; Schneider et al. 2014) out of 9 retrieved studies found no association between low 25(OH)D and the risk of dementia or AD, while the other 4 (Licher et al. 2017; Feart et al. 2017; Knekt et al. 2014; Littlejohns et al. 2014) found a positive association. However, pooled estimates from two recent systematic reviews and meta-analyses, including respectively seven and five of these studies, reported significant associations of deficient 25(OH)D status (< 25 nmol/L) with the risk of dementia (Jayedi et al. 2018; Sommer et al. 2017) and a marginally significant association with the risk of AD (Jayedi et al. 2018) compared with 25(OH)D concentrations ≥ 50 nmol/L.
Methodological issues could explain some of the discrepancies noted between studies in the literature. Regarding the characteristics of the populations, our population included older subjects at baseline, with a mean age of 81 years compared with other studies where mean ages ranged from 56 to 78 years (Licher et al. 2017; Feart et al. 2017; Knekt et al. 2014; Littlejohns et al. 2014; Olsson et al. 2017; Karakis et al. 2016; Afzal et al. 2014; Schneider et al. 2014; Wilson et al. 2014; Bartali et al. 2014; Llewellyn et al. 2010; Slinin et al. 2012; Kuzma et al. 2016; Toffanello et al. 2014; Kilpatrick et al. 2018; van Schoor et al. 2016; Slinin et al. 2010; Breitling et al. 2012; Perna et al. 2014). Two studies were conducted among older people than ours (Graf et al. 2014; Granic et al. 2015). Like us, no association between low vitamin D status (25(OH)D < 25 nmol/L) and the development of dementia compared with subjects with high status (25(OH)D ≥ 75 nmol/L) was found in one of these studies (Graf et al. 2014). A U-shaped association between three categories of 25(OH)D concentrations made from season-standardized quartiles of the study population was observed in another study (Granic et al. 2015). Both low and high categories of 25(OH)D concentrations showed higher odds of cognitive decline compared with subjects in the middle category. This study was the only one that reported a counterintuitive association as we found in women. Among previous studies on global cognitive function, the length of follow-ups varied from 2 to 12 years, and from 2 to 21 years among studies on dementia. The vast majority of the studies considered several important potential confounders, including age, sex, education, season, lifestyle habits, and co-morbidities including depression. Only three studies adjusted for ApoE4 (Feart et al. 2017; Graf et al. 2014; Licher et al. 2017) and only one used multiple imputation to address missing data as we did (Licher et al. 2017).
Two hypotheses could partly explain the association between 25(OH)D concentrations and AD among women. First, a selection survival bias might have occurred which in our case could partly explain the spurious risk effect observed with the increase of vitamin D concentrations, most notably in women. Subjects had to survive without dementia until the entry into the study. Subjects with low vitamin D status, who did not die or develop dementia before the beginning of the study, might have shown some characteristics that prevented the development of the disease. This type of bias is frequent in studies of the determinants of dementia and, according to a simulation study, tends to show a deleterious effect where no real effect is present (Mayeda et al. 2016). This may be especially true among the older subjects. In our study, women were older than men, and more likely to be institutionalized. In a sensitivity analysis stratified by age (< 80, ≥ 80 years), the risk for dementia and AD among women was higher in the older age group (HRs 1.36 (95% CI 1.13–1.65) and 1.31 (95% CI 1.07–1.60), respectively) than in the younger age group (0.89 (95% CI 0.70–1.13) and 1.07 (95% CI 0.71–1.61)).
Second, our association could be confounded by vitamin D supplementation. In a study (Granic et al. 2015), the positive association of high 25(OH)D with cognitive impairment was non-significant when analysis was restricted to non-users of vitamin D supplements. People who take vitamin D supplements may have presented a vitamin D deficiency long before beginning to take the supplement, and older women are usually prescribed vitamin D supplements for the prevention of osteoporosis (Greene-Finestone et al. 2011). Vitamin D supplementation seems to be an important determinant of 25(OH)D concentrations among older women, but of less importance among men (Greene-Finestone et al. 2011). In our study, few subjects reported taking vitamin D supplements, but the information may be biased because subjects may have forgotten to mention over-the-counter, natural products. However, adjustment for vitamin D supplementation gave similar results.
Finally, it is plausible that high 25(OH)D concentrations have an adverse effect with aging. In a large-scale longitudinal study, a reverse J-shaped association between 25(OH)D concentrations and mortality was found (Durup et al. 2012). Lower mortality risks were found at 25(OH)D concentrations of 50–60 nmol/L. Compared with 50 nmol/L, there was a 1.5- and 2-fold increased risk of mortality in the high- and low-concentration groups, respectively. On the other hand, our results on cognitive decline did not support the findings observed in women when AD was the outcome.
Strengths and limitations
This analysis has strengths. The population-based study sample included both community-dwelling and institutionalized subjects, and dementia and AD were diagnosed according to a rigorous clinical process using published criteria.
There are a number of limitations. First, a selection bias may have occurred. Subjects with a 25(OH)D measurement and subjects who participated in the substudy with blood sampling were younger and had better cognitive performance than those without 25(OH)D measurement or without blood sampling. Thus, subjects included in our analysis could be less at risk of dementia and could have higher 25(OH)D concentrations than those not included. However, sensitivity analysis with multiple imputations among all eligible subjects showed similar results. Second, reverse causation is possible. Poorer cognitive function could influence the quality of diet and thus 25(OH)D concentrations at baseline, but the exclusion of CIND subjects at baseline yielded similar results. Third, vitamin D status was only assessed once and could not be representative of vitamin D status over the course of the follow-up. Thus, non-differential misclassification could have occurred in the measure of vitamin D which would generally lead to an underestimation of the true effect. However, it was demonstrated that vitamin D status was relatively stable over a 5-year period (Hofmann et al. 2010) and a 14-year period (Jorde et al. 2010). Finally, the possibility of residual confounding remains.
Conclusion
Overall, this study does not support the protective effect of vitamin D status on cognitive decline or dementia. A significant positive association with incident dementia and AD was found among women which seems more pronounced in the very old. This result must be taken with caution considering the possibility of a survival bias in this old cohort. Moreover, these results were not replicated in the analysis on cognitive decline. The role of vitamin D in maintaining cognitive function and preventing dementia is still supported by a plausible biological mechanism (Aspell et al. 2018). Despite the non-conclusive results of this study, further research is needed to clarify this relation, particularly in women.
Electronic supplementary material
(DOCX 38 kb)
Acknowledgements
The authors want to thank the “Institut sur le vieillissement et la participation sociale des aînés” for providing financial support for the redaction of this manuscript. CSD was supported by a doctoral training award from the Fonds de recherche du Québec-Santé (FRQS) and the CIHR. DL and YG were supported by a scientist award from the FRQS.
Funding information
This work was supported by the Canadian Institutes of Health Research (CIHR, grant number 133536).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Afzal S, Bojesen SE, Nordestgaard BG. Reduced 25-hydroxyvitamin D and risk of Alzheimer’s disease and vascular dementia. Alzheimers Dement. 2014;10(3):296–302. doi: 10.1016/j.jalz.2013.05.1765. [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Disease International . World Alzheimer report 2018. The state of the art of dementia research: new frontiers. London: Alzheimer’s Disease International; 2018. p. 48. [Google Scholar]
- Aspell N, Lawlor B, O’Sullivan M. Is there a role for vitamin D in supporting cognitive function as we age? Proc Nutr Soc. 2018;77(2):124–134. doi: 10.1017/s0029665117004153. [DOI] [PubMed] [Google Scholar]
- Bartali B, Devore E, Grodstein F, Kang JH. Plasma vitamin D levels and cognitive function in aging women: the nurses’ health study. J Nutr Health Aging. 2014;18(4):400–406. doi: 10.1007/s12603-013-0409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitling LP, Perna L, Muller H, Raum E, Kliegel M, Brenner H. Vitamin D and cognitive functioning in the elderly population in Germany. Exp Gerontol. 2012;47(1):122–127. doi: 10.1016/j.exger.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Durup D, Jorgensen HL, Christensen J, Schwarz P, Heegaard AM, Lind B. A reverse J-shaped association of all-cause mortality with serum 25-hydroxyvitamin D in general practice: the CopD study. J Clin Endocrinol Metab. 2012;97(8):2644–2652. doi: 10.1210/jc.2012-1176. [DOI] [PubMed] [Google Scholar]
- Feart C, Helmer C, Merle B, Herrmann FR, Annweiler C, Dartigues JF, et al. Associations of lower vitamin D concentrations with cognitive decline and long-term risk of dementia and Alzheimer’s disease in older adults. Alzheimers Dement. 2017;13(11):1207–1216. doi: 10.1016/j.jalz.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Graf CE, Rossi C, Giannelli SV, Nobari BH, Gold G, Herrmann FR, et al. Vitamin D is not associated with cognitive status in a cohort of very old hospitalized patients. J Alzheimers Dis. 2014;42(Suppl 3):S53–S61. doi: 10.3233/jad-132612. [DOI] [PubMed] [Google Scholar]
- Graham JE, Rockwood K, Beattie BL, Eastwood R, Gauthier S, Tuokko H, et al. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet. 1997;349(9068):1793–1796. doi: 10.1016/s0140-6736(97)01007-6. [DOI] [PubMed] [Google Scholar]
- Granic A, Hill TR, Kirkwood TB, Davies K, Collerton J, Martin-Ruiz C, et al. Serum 25-hydroxyvitamin D and cognitive decline in the very old: the Newcastle 85+ Study. Eur J Neurol. 2015;22(1):106–115. doi: 10.1111/ene.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene-Finestone LS, Berger C, de Groh M, Hanley DA, Hidiroglou N, Sarafin K, et al. 25-Hydroxyvitamin D in Canadian adults: biological, environmental, and behavioral correlates. Osteoporos Int. 2011;22(5):1389–1399. doi: 10.1007/s00198-010-1362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann JN, Yu K, Horst RL, Hayes RB, Purdue MP. Long-term variation in serum 25-hydroxyvitamin D concentration among participants in the Prostate, Lung, Colorectal, and Ovarian cancer Screening Trial. Cancer Epidemiol Biomarkers Prev. 2010;19(4):927–931. doi: 10.1158/1055-9965.epi-09-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayedi, A., Rashidy-Pour, A., & Shab-Bidar, S. (2018). Vitamin D status and risk of dementia and Alzheimer’s disease: a meta-analysis of dose-response. Nutr Neurosci, 1–10. 10.1080/1028415x.2018.1436639. [DOI] [PubMed]
- Jones G. Metabolism and biomarkers of vitamin D. Scand J Clin Lab Investig Suppl. 2012;243:7–13. doi: 10.3109/00365513.2012.681892. [DOI] [PubMed] [Google Scholar]
- Jones KS, Assar S, Harnpanich D, Bouillon R, Lambrechts D, Prentice A, et al. 25(OH)D2 half-life is shorter than 25(OH)D3 half-life and is influenced by DBP concentration and genotype. J Clin Endocrinol Metab. 2014;99(9):3373–3381. doi: 10.1210/jc.2014-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorde, R., Sneve, M., Hutchinson, M., Emaus, N., Figenschau, Y., & Grimnes, G. (2010). Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. American Journal of Epidemiology, 171(8), 903-908, 10.1093/aje/kwq005. [DOI] [PubMed]
- Karakis I, Pase MP, Beiser A, Booth SL, Jacques PF, Rogers G, et al. Association of serum Vitamin D with the risk of incident dementia and subclinical indices of brain aging: the Framingham Heart Study. J Alzheimers Dis. 2016;51(2):451–461. doi: 10.3233/jad-150991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick L, Houston DK, Wilson VK, Lovato J, Ayonayon HN, Cauley JA, et al. Low 25-hydroxyvitamin D concentrations and risk of incident cognitive impairment in Black and White older adults: the Health ABC Study. J Nutr Gerontol Geriatr. 2018;37(1):1–13. doi: 10.1080/21551197.2017.1419899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knekt P, Saaksjarvi K, Jarvinen R, Marniemi J, Mannisto S, Kanerva N, et al. Serum 25-hydroxyvitamin d concentration and risk of dementia. Epidemiology. 2014;25(6):799–804. doi: 10.1097/ede.0000000000000175. [DOI] [PubMed] [Google Scholar]
- Kuzma E, Soni M, Littlejohns TJ, Ranson JM, van Schoor NM, Deeg DJ, et al. Vitamin D and memory decline: two population-based prospective studies. J Alzheimers Dis. 2016;50(4):1099–1108. doi: 10.3233/jad-150811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licher S, de Bruijn R, Wolters FJ, Zillikens MC, Ikram MA, Ikram MK. Vitamin D and the risk of dementia: the Rotterdam Study. J Alzheimers Dis. 2017;60(3):989–997. doi: 10.3233/jad-170407. [DOI] [PubMed] [Google Scholar]
- Lindsay J, Sykes E, McDowell I, Verreault R, Laurin D. More than the epidemiology of Alzheimer’s disease: contributions of the Canadian Study of Health and Aging. Can J Psychiatr Rev Can Psychiatr. 2004;49(2):83–91. doi: 10.1177/070674370404900202. [DOI] [PubMed] [Google Scholar]
- Littlejohns TJ, Henley WE, Lang IA, Annweiler C, Beauchet O, Chaves PH, et al. Vitamin D and the risk of dementia and Alzheimer disease. Neurology. 2014;83(10):920–928. doi: 10.1212/wnl.0000000000000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlejohns TJ, Kos K, Henley WE, Kuźma E, Llewellyn DJ. Vitamin D and dementia. J Prev Alzheimer’s Dis. 2016;3(1):43–52. doi: 10.14283/jpad.2015.68. [DOI] [PubMed] [Google Scholar]
- Llewellyn DJ, Lang IA, Langa KM, Muniz-Terrera G, Phillips CL, Cherubini A, et al. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med. 2010;170(13):1135–1141. doi: 10.1001/archinternmed.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck T, Luppa M, Briel S, Riedel-Heller SG. Incidence of mild cognitive impairment: a systematic review. Dement Geriatr Cogn Disord. 2010;29(2):164–175. doi: 10.1159/000272424. [DOI] [PubMed] [Google Scholar]
- Mayeda ER, Tchetgen Tchetgen EJ, Power MC, Weuve J, Jacqmin-Gadda H, Marden JR, et al. A simulation platform for quantifying survival bias: an application to research on determinants of cognitive decline. Am J Epidemiol. 2016;184(5):378–387. doi: 10.1093/aje/kwv451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod D, Arnott B, Gaudreault N, Boudreau S, Sévigny P. A comparison of two methods for routine, accurate determination of apolipoprotein E genotypes. Alzheimer’s Rep. 1998;1:211–215. [Google Scholar]
- Olsson E, Byberg L, Karlstrom B, Cederholm T, Melhus H, Sjogren P, et al. Vitamin D is not associated with incident dementia or cognitive impairment: an 18-y follow-up study in community-living old men. Am J Clin Nutr. 2017;105(4):936–943. doi: 10.3945/ajcn.116.141531. [DOI] [PubMed] [Google Scholar]
- Overman MJ, Pendleton N, O’Neill TW, Bartfai G, Casanueva FF, Finn JD, et al. Evaluation of cognitive subdomains, 25-hydroxyvitamin D, and 1,25-dihydroxyvitamin D in the European Male Ageing Study. Eur J Nutr. 2017;56(6):2093–2103. doi: 10.1007/s00394-016-1247-4. [DOI] [PubMed] [Google Scholar]
- Perna L, Mons U, Kliegel M, Brenner H. Serum 25-hydroxyvitamin D and cognitive decline: a longitudinal study among non-demented older adults. Dement Geriatr Cogn Disord. 2014;38(3–4):254–263. doi: 10.1159/000362870. [DOI] [PubMed] [Google Scholar]
- Phinney KW. Development of a standard reference material for vitamin D in serum. Am J Clin Nutr. 2008;88(2):511S–512S. doi: 10.1093/ajcn/88.2.511S. [DOI] [PubMed] [Google Scholar]
- Prince M, Wimo A, Guerchet M, Ali G, Wu Y, Prina M. World Alzheimer report 2015—the global impact of dementia: an analysis of prevalence, incidence, cost and trends. London: Alzheimer’s Disease International; 2015. [Google Scholar]
- Schneider AL, Lutsey PL, Alonso A, Gottesman RF, Sharrett AR, Carson KA, et al. Vitamin D and cognitive function and dementia risk in a biracial cohort: the ARIC brain MRI study. Eur J Neurol. 2014;21(9):1211–1218. doi: 10.1111/ene.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slinin Y, Paudel M, Taylor BC, Ishani A, Rossom R, Yaffe K, et al. Association between serum 25(OH) vitamin D and the risk of cognitive decline in older women. J Gerontol Ser A Biol Sci Med Sci. 2012;67(10):1092–1098. doi: 10.1093/gerona/gls075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slinin Y, Paudel ML, Taylor BC, Fink HA, Ishani A, Canales MT, et al. 25-Hydroxyvitamin D levels and cognitive performance and decline in elderly men. Neurology. 2010;74(1):33–41. doi: 10.1212/WNL.0b013e3181c7197b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer I, Griebler U, Kien C, Auer S, Klerings I, Hammer R, et al. Vitamin D deficiency as a risk factor for dementia: a systematic review and meta-analysis. BMC Geriatr. 2017;17(1):16. doi: 10.1186/s12877-016-0405-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- Toffanello ED, Coin A, Perissinotto E, Zambon S, Sarti S, Veronese N, et al. Vitamin D deficiency predicts cognitive decline in older men and women: the Pro.V.A. Study. Neurology. 2014;83(24):2292–2298. doi: 10.1212/wnl.0000000000001080. [DOI] [PubMed] [Google Scholar]
- Tschanz JT, Welsh-Bohmer KA, Lyketsos CG, Corcoran C, Green RC, Hayden K, et al. Conversion to dementia from mild cognitive disorder: the Cache County Study. Neurology. 2006;67(2):229–234. doi: 10.1212/01.wnl.0000224748.48011.84. [DOI] [PubMed] [Google Scholar]
- van Schoor NM, Comijs HC, Llewellyn DJ, Lips P. Cross-sectional and longitudinal associations between serum 25-hydroxyvitamin D and cognitive functioning. Int Psychogeriatr. 2016;28(5):759–768. doi: 10.1017/s1041610215002252. [DOI] [PubMed] [Google Scholar]
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- Wilson VK, Houston DK, Kilpatrick L, Lovato J, Yaffe K, Cauley JA, et al. Relationship between 25-hydroxyvitamin D and cognitive function in older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2014;62(4):636–641. doi: 10.1111/jgs.12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 38 kb)