Abstract
The purpose of this study was to investigate the protective effects of beet root (Beta vulgaris var. rubra) in lipopolysaccharide (LPS) and alcohol induced liver damage. Beta vulgaris ethanol extract (BVEE) showed good antioxidant activity in the contents of polyphenol and flavonoid compounds, and the electron-donating ability and ABTS+ radical scavenging activity. As for anti-inflammatory effect in RAW 264.7 cells, inhibition rate of nitric oxide production was increased in dose dependent manner. In hepatotoxicity model induced by LPS and alcohol in rat, BVEE significantly decreased serum AST, ALT and γ-GTP concentrations in a dose-dependent manner. The histopathological changes after H&E staining showed that fat accumulation and inflammatory cell infiltration were decreased by BVEE. The collagen fibers around the central lobule observed by Masson’s trichrome staining were also decreased by BVEE. In addition, as for the immunohistochemical staining and Transmission electron microscopy, BVEE improved morphological characteristics of damaged liver lesion. The increased mRNA expressions of NF-κB, MAPK1, MAPK3, CYP2E1, and α-SMA were significantly decreased in BVEE treated group. These results indicated that BVEE would have protective effects in hepatotoxicity by altering various indicators related to the liver damage induced by LPS or alcohol.
Keywords: Beet root, Beta vulgaris, Hepatotoxicity, Lipopolysaccharide (LPS), Alcohol
Introduction
Modern people often drink alcohol excessively and it can pose a serious health threat since it can lead to impaired immune function and hepatic damage. Particularly, drinking has been responsible for the liver disease. Various studies have been conducted to find out the cause and mechanism of alcohol-induced hepatic damage [1]. According to a recent study on alcohol pathogenesis, liver damage is caused by the intracellular signaling that is generated from hepatocytes [2]. The Kupffer cells and macrophases are another key cells in that crucial process [3]. After binge drinking, lipopolysaccharide (LPS) is recognized by toll-like receptor 4 (TLR4), which is present in macrophases or other cells in the liver, activating downstream signal transduction system. TLRs are pattern recognition receptors that recognize damage-associated molecular patterns and pathogen-associated molecular patterns, including LPS [4]. It activates transcription factors such as activator protein 1 (AP-1), nuclear factor kappa B (NF-κB) and increases the production of inflammatory cytokine [3]. In addition to mitogen-activated protein kinase (MAPK) such as extracellular signal-regulated kinase (ERK), p38 induced by LPS is also involved in liver damage [5]. Thus, the liver exposed to alcohol produces not only substances involved in signal transduction system, but also direct oxidative stress induced by inflammatory cytokines. TLR4, NF-κB, and MAPK, signalling molecules, are related to alcohol damage [6].
Beta vulgaris (beet root) is a plant which is included in Betoideae subfamily in the Amaranthaceae family. The consumption of Beta vulgaris is expected to give beneficial physiological effect and to be appropriate for protection of a preoperative patient’s liver. Also, a variety of components of Beta vulgaris can control many cell pathways. Beta vulgaris contains diverse beneficial components, such as flavonoid, polyphenol, betanin, betaxanthins, betaine, vitamins (thiamine, riboflavine, pyridoxine, and ascorbic acid), folic acid, biotin, soluble fiber and pectin, and especially, flavonoid, betanin and polyphenol have very effective antioxidant capacity [7]. The most important physiological role of betaine is that it may serve as an inhibitor of lipid peroxidation and heme decomposition, and which may attenuate liver damage [8].
This study aimed to verify the effect of the Beta vulgaris which is commercially available. At first, the anti-inflammatory and antioxidant ability of Beta vulgaris ethanol extract (BVEE) was evaluated for in vitro assays. And then, anti-hepatotoxicity effects of BVEE at various concentrations was evaluated in Sprague-Dawley rats treated with LPS and ethanol to induce hepatic damage. After necropsy, biochemical, histological, immunohistochemical, transmission electron microscopical and molecular biological analyses were carried out to confirm the preventive and improving effect of BVEE in damaged liver.
Materials and methods
Preparation of BVEE
Beta vulgaris powder (Radiance, New Zealand) was used in this experiment. The active components of Beta vulgaris were extracted with ethanol: (1) 70% ethanol was added to the specimen in 10:1 ratio (e.g., 10 g of ethanol:1 g of the specimen); (2) the mixture was stirred at room temperature for 24 h and (3) the mixture was filtered. These steps were repeated 3 times. Thereafter, the supernatant was collected and concentrated using a lyophilizer before the use.
Antioxidant activity of BVEE
DPPH (50 µL of 0.2 mM) was added to each sample (100 µL) and stirred for 30 min. The absorbance was then measured at a wavelength of 517 nm. The electron donating ability of BVEE could be estimated by measuring the absorbance reduction rate of the group to which the sample solution and the control group were added [9]. Free radical scavenging activity could be estimated by measuring ABTS + cationic decolorization assay [10]. Potassium sulfate (2.45 mM) and 2.2-azinobis (3-ethylbenzthiazoline -6-sulfonic acid) (7 mM) were mixed and then stored in a dark room at room temperature for 12 h. Then, ABTS + was prepared to have an absorbance value of 0.7 (± 0.02) at a wavelength of 734 nm and it was diluted with ethanol at 1:1 ratio. In detail, 100 µL of the sample was diluted with 100 µL of ABTS + solution for 7 min in a dark room. Therefore, the absorbance of the sample was measured at a wavelength of 734 nm. Radical scavenging activity of BVEE was measured without adding extracts for estimating the control absorbance.
Total flavonoid and polyphenol content of BVEE
The total flavonoid and total polyphenol contents of BVEE were measured using the colorimetric method [11].
Cell experiment
Mouse macrophage cell line RAW 264.7 (Korean Cell Line Bank, KCLB 40071, Seoul, Korea) were used. These cells were cultured in DMEM supplemented with 1% penicillin/streptomycin (P/S) and 10% FBS at 37 °C under the supply of 5% CO2. The cytotoxicity assay and nitric oxide (NO) inhibitory efficacy were measured using the method of Guzik [12].
Animal study
Thirty-six male Sprague–Dawley rats (weight: 180–200 g, age: 5 week old) were obtained from Samtako Co., Ltd. (Korea). All animal experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) (approval number, KM-2017-007) and all experiments of this study were conducted according to IACUC guidelines. The animals were given ad libitum access to water and food. The diets were obtained from Harlan Teklad (Madison, WI). Body weight and food intake of rats were measured once every 2 days. Standard experimental conditions were as follows: temperature, 22 ± 3 °C; humidity, 50 ± 5%; and a 12-h light/dark cycle. After 1 week of acclimatization, the rats were randomly divided into six groups (Table 1). Ethanol (35%, 10 ml/kg) was orally administered one times a day to make a liver damage, and LPS (2 mg/kg) was intraperitoneally injected 4 h before necropsy. The rats in the C1, E1 and E2 groups were orally administered BVEE (200 or 400 mg/kg, the ultrasound wave was applied to dissolved BVEE in 3 ml of distilled water) once a day for 4 weeks and rats in the N and C2 groups were administered the same amount of vehicle (distilled water). Blood samples were then collected from the inferior vena cava of the rats, and each liver was isolated and stored at − 80 °C until further analysis.
Table 1.
Experimental groups of animal study
| Groups | Ethanol and LPS treat | Test compound | No. of rat |
|---|---|---|---|
| Normal (N) | − | Distilled water | 6 |
| Control 1 (C1) | − | BVEE 400 mg/kg | 6 |
| Control 2 (C2) | + | Distilled water | 6 |
| Positive control (PC) | + | Silymarin 50 mg/kg | 6 |
| Experimental 1 (E1) | + | BVEE 200 mg/kg | 6 |
| Experimental 2 (E2) | + | BVEE 400 mg/kg | 6 |
Serum biochemistry analysis
To assess the liver damage in the rats, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) and γ-glutamyltransferase (γ-GPT) levels were determined using SPOTCHEMEZ reagent strips (Arkray, Inc., Kyoto, Japan). The blood was collected from tail vein and incubated at room temperature in vacuum tube for 1 h. The sample was centrifuged at 3000 rpm for 10 min to obtain serum using an automatic biochemical analyzer.
Histological analysis
The anterior portions of the left lateral lobe of the rat livers were sectioned, fixed in 10% neutral-buffered formalin, embedded in paraffin, and sliced into 4-μm sections. The sections were stained with hematoxylin and eosin (H&E), Masson’s trichrome (MT), transmission electron microscopy analysis (MET). Histological changes were evaluated in nonconsecutive histological fields, randomly chosen at a magnification of 100.
Immunohistochemistry observation
The immunohistochemical analysis of the rat livers was performed as previously described with modifications [13]. After necropsy, their livers were trimmed into a strip, soaked in 10% neutral-buffered formalin, embedded in paraffin, and then sectioned. The liver sections were dewaxed, hydrated, subjected to heat-induced antigen retrieval, blocked in blocking buffer, and then incubated overnight at 4 °C with an anti-alpha-smooth muscle actin (α-SMA) and S100 antibody (1:100; Dako, Denmark, Europe). The sections were then washed and further incubated with Super Enhancer and a poly-horseradish-conjugated reagent. The color was developed by incubating the sections with the 3,3′-diaminobenzidine and substrate reaction mixtures (1:38) as well as with hematoxylin. After washing the sections with water, the specific staining was visualized using light microscopy.
Quantitative PCR
Total RNA of dissected liver was extracted using Nucleo Spin RNA extraction kit (Macherey–Nagel, Germany), and quantified using a spectrophotometer (Nanodrop 2000). The cDNA was synthesized in the iScript cDNA synthesis kit using total RNA (1 µg). The reaction was carried out in a thermal cycler at 25 °C for 5 min, at 42 °C for 30 min, and at 85 °C for 5 min. For gene expression analysis, cDNA diluted with sterilized deionized water was mixed with primers (Table 2) and SYBR green supermix (Bio-Rad, CA, USA) and dispensed into a 0.2 mL PCR tube strip with volume of 20 µL at a time. Subsequently, the PCR tube strips were spin down. The PCR tube strips were added to the RNA amplification apparatus and reacted at 95 °C for 2 min, 10 s at 95 °C amplified 40 times at 72 °C for 20 s, and reacted at 95 °C for 20 s.
Table 2.
Primer sequences for qPCR
| Genes | Primers sequencea | Expected size (bp)b | |
|---|---|---|---|
| NF-κB | Forward | GGGCTGACCTGAGTCTTCTG | 119 |
| Reverse | GATAAGGAGTGCTGCCTTGC | ||
| MAPK1 | Forward | TCTCCCGCACAAAAATAAGG | 129 |
| Reverse | GCCAGAGCCTGTTCAACTTC | ||
| MAPK3 | Forward | TCCAAGGGCTACACCAAATC | 188 |
| Reverse | AGGTAGTTTCGGGCCTTCAT | ||
| CYP2E1 | Forward | AGGCTGTCAAGGAGGTGCTA | 114 |
| Reverse | ATGTGGGCCCATTATTGAAA | ||
| α-SMA | Forward | TGTGCTGGACTCTGGAGATG | 148 |
| Reverse | GAAGGAATAGCCACGCTCAG | ||
| β-actin | Forward | GTCGTACCACTGGCATTGTG | 291 |
| Reverse | CTCTCAGCTGTGGTGGTGAA | ||
aPrimers sequence (Bionics, Seoul, Korea)
bbp: basepair
Statistical analysis
The results of quantitative PCR were analyzed by a t test in Bio-Rad CFX Maestro System (Bio-Rad, CA, USA). One-way ANOVA was used to compare the results from all of the test groups and followed by Duncan’s multiple range post-hock test (SPSS 21.0, USA) to analyzed the difference between the groups. Statistical significance was determined at p < 0.05.
Results
Total contents of polyphenol and flavonoid in BVEE
The total polyphenol content of BVEE was 21.4 ± 0.3 mg/g. The total flavonoid content of BVEE was 24.6 ± 1.2 mg/g. It was relatively lower than the previously reported flavonoid and polyphenol contents of wild plants and herbal plants [14].
Antioxidant activity of BVEE
The electron donating ability and radical scavenging activity of BVEE were highly correlated with the concentration (Fig. 1). The electron donating ability of BVEE was up to 61.4 ± 3.7% of ascorbic acid at 1000 µg/mL. The ABTS+ radical scavenging activity of BVEE (1000 µg/mL) was up to 59.5 ± 3.1%.
Fig. 1.
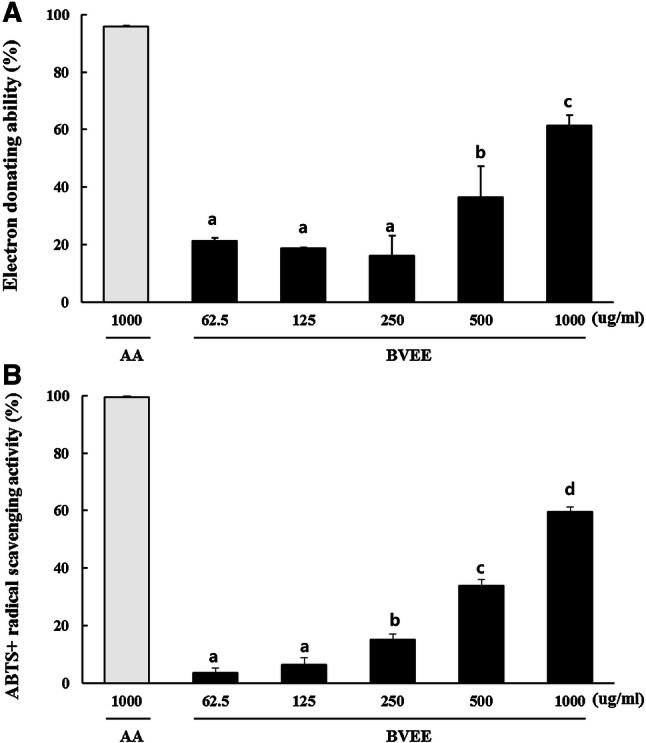
Electron donating ability (a) and ABTS + radical scavenging activity (b) of BVEE. Values represent the mean ± SD of 3 independent measurements. Values with different letters (a, b, c, d) are significantly different (p < 0.05) by ANOVA and Duncan’s multiple range test. AA: ascorbic acid, BVEE: Beta vulgaris ethanol extract
Cytotoxicity and NO inhibitory effect of BVEE in RAW 264.7 cells
BVEE did not show cytotoxicity up to 100 µg/mL to the RAW 264.7 cells. The cell viability dropped to 64.53% when the concentration of BVEE was 200 µg/mL, indicating cytotoxicity of BVEE at this concentration. In LPS treated RAW 264.7 cells, the inhibitory efficacy of BVEE on NO production was partially recovered in a concentration-dependent manner (Fig. 2).
Fig. 2.
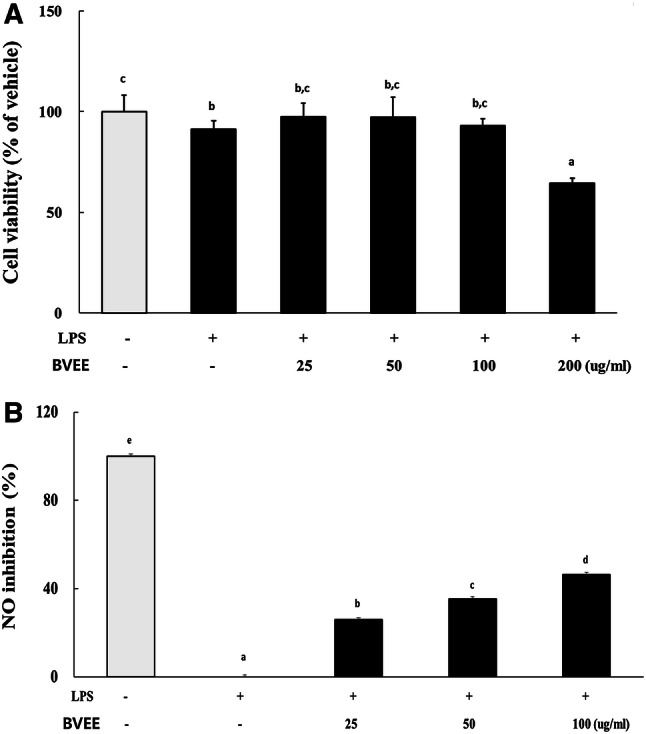
Cytotoxicity (a) and NO inhibitory efficacy (b) of BVEE in RAW 264.7 cells. The values represent mean ± SD of 3 independent measurements. Cell viability expressed as a percentage for the non-treatment control group. Values with different letters (a, b, c, d) are significantly different (p < 0.05) by ANOVA and Duncan’s multiple range test. BVEE: Beta vulgaris ethanol extract
Body weight and food consumption of SD rats
There was no significant difference in daily feed intake and body weight between 6 groups, although other groups tended to show a little bit lower than N group (data not shown).
Serum AST, ALT and γ-GTP levels of SD rat
The hepatotoxic factors such as serum ALT, AST and γ-GPT level of C2 were increased significantly, but silymarin treatment (PC group) decreased all hepatotoxicity factors. After 4 weeks of BVEE administration, the GPT level of E1 group (200 mg/kg) was significantly decreased, and the serum ALT, AST and γ-GPT levels in E2 group (400 mg/kg) were significantly decreased similar to those of PC group (Table 3).
Table 3.
Serum AST, ALT and γ-GTP levels of SD rat
| Item | Group | |||||
|---|---|---|---|---|---|---|
| N | C1 | C2 | PC | E1 | E2 | |
|
AST (IU/L) |
123.26 ± 7.24a | 116.25 ± 13.71a | 189.01 ± 6.99c | 142.86 ± 18.62a,b | 186.20 ± 34.60c | 136.05 ± 7.65a,b |
|
ALT (IU/L) |
36.65 ± 3.44a | 37.77 ± 3.33a | 90.29 ± 28.23b | 55.73 ± 11.54c | 87.16 ± 20.61b | 52.81 ± 5.42c |
|
γ-GPT (IU/L) |
1.07 ± 0.53a | 0.53 ± 0.02a | 6.72 ± 3.34b | 1.40 ± 0.91a | 1.09 ± 0.26a | 0.66 ± 0.42a |
The values represent mean ± SD. Values with different letters (a, b, c) are significantly different by ANOVA and Duncan’s multiple rage test. N: Normal, C1: BVEE 400 mg/kg, C2: EtOH (35%) + LPS (2 mg/kg), PC: EtOH + LPS + Silymarin (50 mg/kg), E1: EtOH + LPS + BVEE (200 mg/kg), E2: EtOH + LPS + BVEE (400 mg/kg)
Histopathologic changes in liver of SD rat
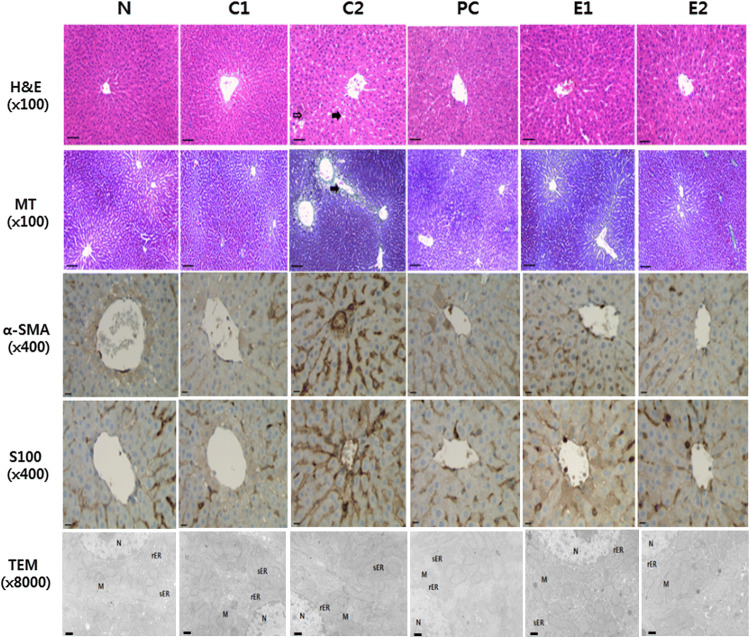
In H&E staining, N group showed no histological change, but the C2 group revealed severe fat accumulation, inflammation, and necrosis. In contrast, the liver morphology of the E1, E2, and PC groups was much improved than that of the C2 group. As a result of Masson’s trichrome staining, in the N group, the central vein and the collagen fibers (blue staining) around the central lobule were well observed. In the C2 group, the central vein and the collagen fibers around the central lobule showed irregular shapes. In contrast, toxic signs in the hepatic central vein and central lobule were significantly improved in the E1, E2, and PC groups after hepatotoxicity. As for immunohistochemical staining for α-SMA and S100, the C2 group showed intense immunoreactive staining of dark brown around the central vein and the lobule. In the E1 and E2 groups, which are BVEE treatment groups, less intense staining was found around the central vein and the lobule. The ultrastructural changes of hepatocytes by test substances were observed using a transmission electron microscope (TEM). The nucleus and mitochondrial structure of the N group maintained a regular shape, and the rough endoplasmic reticulum (rER) and smooth endoplasmic reticulum (sER) also showed normal structures. In the C2 group, the destruction of rER and sER and mitochondrial swelling were observed. In the PC, E1 and E2 groups, the destruction of rER and sER reduced and mitochondria edema was weakly observed (Fig. 3).
Fig. 3.
Histopathologic changes in liver of SD rat. N: Normal, C1: BVEE 400 mg/kg, C2: EtOH (35%) + LPS (2 mg/kg), PC: EtOH + LPS + Silymarin (50 mg/kg), E1: EtOH + LPS + BVEE (200 mg/kg), E2: EtOH + LPS + BVEE (400 mg/kg), H&E: hematoxylin and eosin stain (bar: 100 µm, open arrow: inflammation and necrosis, closed arrow: fat accumulation), MT: Masson’s trichrome stain (bar: 100 µm, close arrow: collagen fiber), bar for α-SMA and S100: 400 µm. TEM: Transmission electron microscope analysis (bar: 100 µm, M: mitochondria, N: nucleus, rER: rough endoplasmic reticulum, sER: smooth endoplasmic reticulum)
Changes of mRNA expression in liver of SD rat
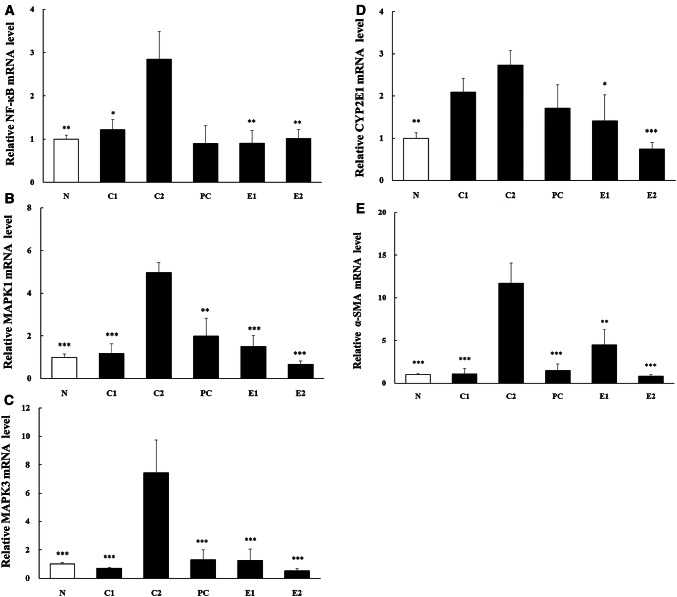
The NF-κB (p50), MAPK1, 3 and α-SMA mRNA expression levels of the E1 and E2 groups were significantly decreased compared with C2 group. The CYP2E1 mRNA expression levels of the E1 and E2 groups were significantly lower than C2 group. The mRNA expression level of C1 group was not significantly different from C2 group (Fig. 4).
Fig. 4.
Changes of mRNA expression in liver of SD rat. Values are relative to the non-treatment control and mean ± SEM of 6 animals. Asterisk indicates a significant difference from C2 group by a t-test (*p < 0.05, **p < 0.01, ***p < 0.001). N: Normal, C1: BVEE 400 mg/kg, C2: EtOH (35%) + LPS (2 mg/kg), PC: EtOH + LPS + Silymarin (50 mg/kg), E1: EtOH + LPS + BVEE (200 mg/kg), E2: EtOH + LPS + BVEE (400 mg/kg)
Discussion
The purpose of this study was to identify the prevention and improvement mechanism of hepatic damage by administering BVEE after inducing a hepatotoxicity model by applying LPS and alcohol to SD rats. The content of flavonoids and polyphenols, antioxidant indicator, was not too high, but the electron-donating ability and ABTS + radical scavenging activity increased dose-dependently, which indicated BVEE has sufficient antioxidant effects. In anti-inflammatory experiments in RAW 264.7 cells, cell survival rate was decreased dose-dependently by BVEE. BVEE showed some cytotoxicity at 200 µg/mL. Consequently, it was possible to prove the excellent anti-hepatotoxic effect of BVEE through the anti-inflammatory effects [15].
In hepatotoxicity model induced by LPS and alcohol in rat, the serum AST, ALT and γ-GTP levels which are indicators for hepatic injury were significantly decreased in dose-dependent manner after treatment with BVEE. These results were similar to those of previous report [16] which showed the protective effects of caffeine after alcohol-induced hepatic injury. The histopathological changes in liver were recovered to normal level in PC, E1, E2 groups. The results suggested that BVEE was effective for hepatic injury induced by alcohol and LPS. The results agreed with those of Koo et al. [17] where showed the effects of ginger extracts after inducing hepatotoxicity by carbon tetrachloride.
As for Masson’s trichrome staining to identify the degree of collagen deposition due to hepatic injury, the C2 showed collagen fibers around the central vein and the lobule irregularly. But, in the E1 and E2 groups, the stain around the central vein and the lobule was similar with the normal group, indicating that the tissue was recovered to the normal status. The results implied that BVEE might reduce the collagen accumulation by alcohol as similar mechanism shown in Li et al. [18]. It would be desirable to confirm the degree of the reticulum fiber and the glycogen accumulation using Gomori’s staining method and Periodic acid-schiff (PAS) for more precise histological observations in the future.
S100 protein was used to evaluate the differences in the innervation of hepatic cells [19], and α-SMA was used to evaluate the hepatocirrhosis [20]. E1 and E2 groups, which are BVEE treatment groups, showed less intense staining around the central vein and the lobule compared with the C2 group. These results indicated that BVEE influenced the expression of α-SMA due to hepatic stellate cell activation and the reduction of S100 protein in hepatic neurons, commonly observed in patients with hepatic damage [21].
The characteristics of hepatic injury by the transmission electron microscopic observation have been known as intracytoplasmic granule, the changes in mitochondria, the increase of lysosome, the emergence of fat, and a large quantity of peroxisome and multi-vesicular body [22]. In the C2, the destruction of rER and sER and the expansion of a mitochondrion were observed. The lesion tended to decrease in the P group and the BVEE treatment group. The results suggested that BVEE was effective in improving the morphological characteristics of the liver.
The high levels of mRNA expression of NF-κB, MAPK1, MAPK3, CYP2E1, and α-SMA were observed in the hepatic injury group (C2) induced by alcohol and LPS [23–25]. However, NF-κB expression was significantly lower in the E1 and E2 group than in the C2 group. NF-κB is a transcription factor involved in the activation of many genes associated with alcoholic hepatic injury [26]. The activated NF-κB induces the expression of inflammatory mediators including cytokines and iNOS [26]. It is believed that the significant decrease of NF-κB expression would decrease the expression of cytokines related to liver damage [27]. It is possible that MAPKs may play a role in promoting the expression of pro-inflammatory mediators because the activation of NF-κB transcription factors is regulated by MAPKs [28]. Consequently, the anti-inflammatory effect of BVEE is thought to be due to the inhibition of MAPK signaling pathway through decreased mRNA expression of MAPK1 and MAPK3.
CYP2E1 enzyme has been used as the main index in various hepatotoxicity animal models, and excessive expression of CYP2E1 can increase ROS and it can be a major reason for hepatic injury [29]. In this study, CYP2E1 mRNA level was significantly lower in the E1 and E2 than in C2 group. It means BVEE can improve the liver functions. However, the C1 group (BVEE only treatment) increased the expression of CYP2E1, suggesting that the high dose of BVEE may cause toxicity to the liver.
α-SMA has been used as a key index to prove the improvement of liver fibrosis [30]. Particularly, Park et al. [31] reported that the certified Korean traditional fermented soybean paste inhibited the expression of α-SMA and it effectively protected the liver fibrosis. The results of this study showed that the BVEE treatment groups had significantly less mRNA expression of α-SMA than C2 group, which indicates that BVEE effectively improved the liver injury.
To summarize the results of the study, BVEE seems to be a preventive and therapeutic effect for liver damage by altering various indices related to the liver damage by alcohol or LPS (Fig. 5). However, further studies are needed to understand which components of BVEE were directly responsible for the improvement of the injured liver, and to confirm the efficacy in clinical studies.
Fig. 5.
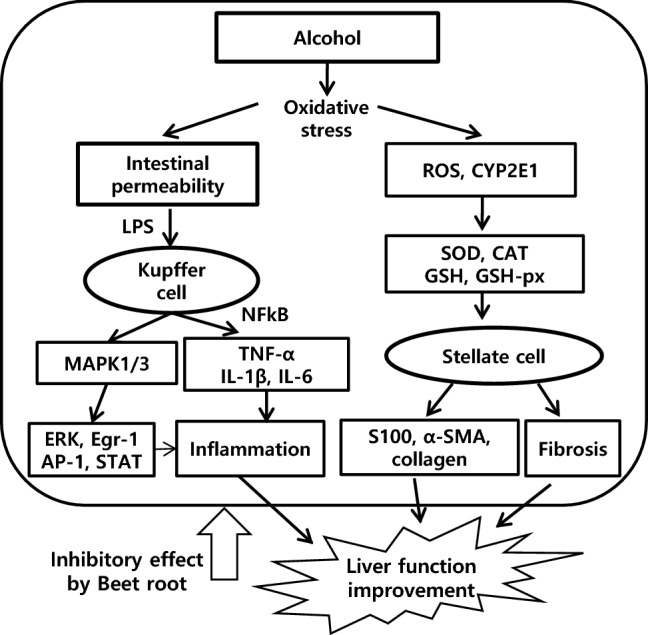
The schematic illustration for the mechanism of BVEE protection in LPS and alcohol-induced hepatotoxicity. Beet root improved liver function by inhibition of LPS and alcohol-induced hepatotoxicity
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest to disclose.
References
- 1.O’shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51:307–328. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 2.Friedman SL. Liver fibrosis from bench to bedside. J Hepatol. 2003;38:38–53. doi: 10.1016/S0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 3.Jeong WI, Park O, Gao B. Abrogation of the antifibrotic effects of natural killer cells/interferon-gamma contributes to alcohol acceleration of liver fibrosis. Gastroenterology. 2008;134:248–258. doi: 10.1053/j.gastro.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucas K, Maes M. Role of the Toll Like receptor (TLR) radical cycle in chronic inflammation: possible treatments targeting the TLR4 pathway. Mol Neurobiol. 2013;48:190–204. doi: 10.1007/s12035-013-8425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50:1258–1266. doi: 10.1016/j.jhep.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee HJ. Update and perspectives on alcoholic liver disease in Korea 2010. Clin Mol Hepatol. 2010;16:23–44. [Google Scholar]
- 7.Lichtenthaler R, Marx F. Total oxidant scavenging capacities of common European fruit and vegetable juices. Food Chem. 2005;53:103–110. doi: 10.1021/jf0307550. [DOI] [PubMed] [Google Scholar]
- 8.Junyan Han J, Zhang Z, Yang S, Wang J, Yang X, Tan D. Betanin attenuates paraquat-induced liver toxicity through a mitochondrial pathway. Food Chem Toxicol. 2014;70:100–106. doi: 10.1016/j.fct.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 9.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- 10.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 11.Folin O, Denis W. On phosphotungstic-phosphomolybdic compounds as color regents. J Biol Chem. 1912;12:239–243. [Google Scholar]
- 12.Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide superoxide in inflammation and immune regulation. J Physiol Phamacol. 2003;43:469–487. [PubMed] [Google Scholar]
- 13.Yang X, Kang MC, Lis Y, Kim EA, Kang SM, Jeon YJ. Anti-inflammatory activity of questinol isolated from marine-derived fungus eurotium amstelodami in lipopolysaccharide-stimulated raw 264.7 macrophages. J Korean Med Sci. 2014;8:367–373. doi: 10.4014/jmb.1405.05035. [DOI] [PubMed] [Google Scholar]
- 14.Kim EJ, Choi JY, Yu MR, Kim YM, Lee SH, Lee BH. Total polyphenols, total flavonoid contents, and antioxidant activity of Korean natural and medicinal plants. J Food Sci Technol. 2012;44:337–342. [Google Scholar]
- 15.Stewart S, Jones D, Day CP. Alcoholic liver disease: new insights into mechanisms and preventative strategies. Trends Mol Med. 2001;7:408–413. doi: 10.1016/S1471-4914(01)02096-2. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, Dai X, Yang W, Wang H, Zhao H, Yang F, Yang Y, Li J, Lv X. Caffeine protects against alcohol-induced liver fibrosis by dampening the cAMP/PKA/CREB pathwayin rat hepatic stellate cells. Int Immunopharmacol. 2015;25:340–352. doi: 10.1016/j.intimp.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Koo SW, Lee KW. The protective effect of ginger aqueous extracts on CCl4-induced hepatic damage in mice. J Vet Clin. 2012;29:441–446. [Google Scholar]
- 18.Li W, Zhu C, Li Y, Wu Q, Gao R. MEST attenuates CCl4-induced liver fibrosis in rats by inhibiting the wnt/β-catenin signaling pathway. Gut Liver. 2014;8:282–291. doi: 10.5009/gnl.2014.8.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duarte Rojo A, Ruiz Margain A, Macias Rodriguez RU, Cubero FJ, Estradas Trujillo J, Munoz Fuentes RM, Torre A. Clinical scenarios for the use of S100β as a marker of hepatic encephalopathy. World J Gstroenterol. 2016;22:4397–4402. doi: 10.3748/wjg.v22.i17.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li CH, Piao DM, Xu WX, Yin ZR, Jin JS, Shen ZS. Morphological and serum hyaluronic acid, laminin and type IV collagen changes in dimethylnitrosamine-induced hepatic fibrosis of rats. World J Gastroenterol. 2005;11:7620–7624. doi: 10.3748/wjg.v11.i48.7620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyazawa Y, Fukuda Y, Imoto M, Koyama Y, Nagura H. Immunohistochemical studies on the distribution of nerve fibers in chronic liver diseases. Am J Gastroenterol. 1988;83:1108–1114. [PubMed] [Google Scholar]
- 22.Sternlieb I, Quintana N. The peroxisomes of human hepatocytes. Lab Investig. 1977;36:140–149. [PubMed] [Google Scholar]
- 23.Zhao M, Howard EW, Parris AB, Guo Z, Zhao Q, Yang X. Alcohol promotes migration and invasion of triple-negative breast cancer cells through activation of p38 MAPK and JNK. Mol Carcinog. 2017;56:849–862. doi: 10.1002/mc.22538. [DOI] [PubMed] [Google Scholar]
- 24.Chang X, Luo F, Jiang W, Zhu L, Gao J, He H, Wei T, Gong S, Yan T. Protective activity of salidroside against ethanol-induced gastric ulcer via the MAPK/NF-κB pathway in vivo and in vitro. Int Immunopharmacol. 2015;28:604–615. doi: 10.1016/j.intimp.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 25.Xu J, Zhao Y, Aisa HA. Anti-inflammatory effect of pomegranate flower in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages. Pharm Biol. 2017;55:2095–2101. doi: 10.1080/13880209.2017.1357737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnes PJ, Karin M. Nuclear factor κB-a pivotal factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 27.Dela Pena A, Leclercq I, Field J, George J, Jones B, Farrell G. NFkappaB activation, rather than TNF, mediates hepatic inflammation in a murine dietary model of steatohepatitis. Gastroenterology. 2005;129:1663–1674. doi: 10.1053/j.gastro.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Majdalawieh A, Ro HS. Regulation of IkappaBalpha function and NF-kappaB signaling: AEBP1 is a novel proinflammatory mediator in macrophages. Mediat Inflamm. 2010;2010:823821. doi: 10.1155/2010/823821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ha KT, Yoon SJ, Choi DY, Kim DW, Kim JK, Kim CH. Protective effect of lycium chinense fruit on carbon tetrachloride-induced hepatotoxicity. J Ethnopharmacol. 2005;96:529–535. doi: 10.1016/j.jep.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 30.Yu ES, Choe GY, Gong GG, Lee IC. Expression of alpha-smooth muscle actin in liver disease. J Korean Med Sci. 1993;8:367–373. doi: 10.3346/jkms.1993.8.5.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park SL, Lee SY, Kim IS, Lim SI, Choi JS, Choi SY. Hepatoprotective activity of quality certificated traditional doenjang in korea. Food Eng Prog. 2013;17:401–406. doi: 10.13050/foodengprog.2013.17.4.401. [DOI] [Google Scholar]