Highlights
-
•
There was a large effect of BTI combined with other strategies on postural control at short-term.
-
•
Combining BTI with other intervention may enhance effects on postural control in young CP patients.
-
•
Due to the very low quality of the current evidence, caution is needed.
Keywords: Rehabilitation, Cerebral palsy, Postural control, Balance-training interventions, Systematic review, Meta-analysis
Abstract
Background
Improvement of postural control in children and adolescents with cerebral palsy is a primary goal in child rehabilitation.
Objective
A systematic review investigated whether combining balance-training interventions with other active interventions enhances the effects of the active intervention alone on postural control of children and adolescents with cerebral palsy.
Methods
Searches were performed in MEDLINE, PEDro, CINAHL, Cochrane and EMBASE databases without date or language restrictions. Randomized controlled trials investigating the combination of balance-training interventions with other active interventions on the postural control of children and adolescents with cerebral palsy were included. Two independent reviewers screened studies, extracted data, and assessed methodological quality of included studies. Meta-analysis was conducted, and quality of the evidence followed the GRADE methodology. Pooled data were presented using standardized mean difference and 95% confidence interval.
Results
Seven studies involving 194 participants were included in this review. A large additional effect on postural control was found when balance-training interventions were combined with Neurodevelopmental Treatment at short-term (standardized mean difference of 1.3; 95% confidence interval 0.5, 2.0, p = 0.001). The quality of the evidence was very low due to publication bias, imprecision and inconsistency.
Conclusion
Combining balance-training interventions with other active interventions may enhance effects on postural control of this population at short-term. As the estimated effect had only very low quality of evidence to support it, larger studies with low risk of bias are needed.
Introduction
Postural control is defined as the ability to control the body's center of mass in relation to the person's base of support for maintenance of stability.1, 2 Impairments on postural control were documented in children and adolescents with cerebral palsy (CP) during static (i.e. maintaining a posture) and dynamic (i.e. changing positions and moving through the environment) activities3, 4, 5 as a result of sensory deficits, muscular weakness and biomechanical misalignment,6, 7 adversely affecting their performance of daily activities. Improvement of postural control is one of the main goals in physical therapy interventions.1, 4, 5, 7, 8, 9 These treatments comprise activities that cause unpredicted perturbations (e.g. on unstable or mobile surfaces) in multiple training settings, described as generic term “balance-training interventions” (BTI).6, 8, 10 Studies testing the effects of BTI are accumulating and these interventions have been identified as potentially effective.11
Improvement of postural control in children and adolescents with CP has been achieved using balance-training platform, hippotherapy, virtual reality (VR) and treadmill training.6, 12, 13, 14 In 2005, Harris and Roxborough10 published a systematic review of studies that investigated the effectiveness of postural control strategies in children and adolescents with CP. This review included studies of interventions in which individuals were active (e.g. motor rehabilitation exercises) or passive (e.g. adaptive seating devices and ankle foot orthoses).10 Authors concluded that interventions comprising externally generated perturbations, as balance platforms, improved postural control responses in standing, whereas applying orthoses or whole-body garments demonstrated no effects on postural control outcomes. In addition, most of the reviewed studies provided lower levels of evidence. A decade later, Dewar et al.11 published a systematic review with similar aims and population, comparing exercise interventions. They concluded that there was moderate evidence to support the use of intervention modalities such as hippotherapy, treadmill training with no body weight support, trunk-targeted training, reactive balance training and gross motor task training to improve postural control in children with CP. This review also reported that neurodevelopmental therapy (NDT), when applied alone or combined with joint mobility, muscle strengthening, and mobility activities, had effects on postural control,11 although the level of evidence is weak. However, it is unclear whether comprehensive approaches, such as NDT or others, combined with BTI is more effective than isolated interventions. It is acknowledged that management of CP population claims therapeutic techniques to address body function/structure, activity and participation limitations. Therapists frequently choose a pool of techniques that encompass a variety of exercises that may have significant effects across different domains of the International Classification of Function, Disability and Health (ICF).15 Investigating the efficacy of combining comprehensive approaches with BTI would support clinical decision-making.
The aim of this systematic review with meta-analysis was to investigate whether combining balance-training interventions with other active interventions enhances the effects of other active interventions alone on postural control in children and adolescents with CP.
Methods
Search strategy and inclusion criteria
This study was a systematic review with meta-analysis. Review followed the PRISMA guidelines16 and was registered at PROSPERO (CRD42016043272). A systematic literature search from the earliest record to February 2019 was performed on MEDLINE, PEDro, CINAHL, Cochrane and EMBASE databases, without language restrictions. The search terms were related to “randomized controlled trial”, “cerebral palsy”, “balance training”, “motor rehabilitation” and “postural control” (see Appendix 1 on the eAddenda for detailed search strategy). Hand search on previously published systematic reviews in the area was also conducted.
Published randomized controlled trials including children and adolescents with a medical diagnosis of CP, aged up to 18 years old, from primary, secondary or tertiary care settings were included. Any study whose participants used Botulinum toxin or underwent orthopedic surgery six months prior to the study was excluded. Included studies had to compare BTI combined with any other active intervention such as Neurodevelopmental Treatment (NDT) with the other active intervention alone. BTI was defined as any intervention that causes perturbations on body's center of mass and requires active body movement to maintain the dynamic stabilization. Our outcome of interest was postural control, and studies were included if they reported any valid measures of postural stability and orientation such as Berg Balance Scale, Pediatric Balance Scale (PBS), timed up and go (TUG), center of pressure and center of mass displacements.
Study selection
After searches, identified references were exported to an Endnote® file and duplicates were removed. Then, two independent reviewers (JS and PAA) screened all titles and abstracts, and selected potential full-texts. Potential full-texts were assessed against the inclusion criteria outlined above. Between-reviewer disagreements were resolved by consensus.
Assessment of methodological quality of included studies
Included studies were assessed by two independent reviewers (JS and PAA) for methodological quality (i.e. items 2–9) and statistical reporting (i.e. items 10 and 11) using the 0–10 PEDro scale.17 When the study was rated by PEDro database (https://www.pedro.org.au/), its score was used. Any between-reviewer disagreement was resolved by consensus in the cases where the trial was not available on PEDro.
Data extraction
Two independent reviewers (JS and PAA) extracted data on characteristics (i.e. age, sex, CP severity and type, intervention groups, outcome and timepoints) and outcome data (i.e. post-intervention means, standard deviations (SD) and sample sizes) from the included studies. Disagrements were resolved by consensus. Level of Gross Motor Function Classification System (GMFCS) was used to describe mobility18 groups of children and adolescents, when available. GMFCS identifies five levels of gross motor function of children and adolescents with CP on the basis of their self-initiated movement with focus on sitting, walking and wheeled mobility. Levels I and II include children and adolescents who walk independently, level III refers to those who walk with mobility devices, levels IV and V denote children and adolescents who use wheelchair.19
Outcome data were extracted for three timepoints, when available: short-term as follow-up ≤3 months after baseline; medium-term as follow-up >3 months and <12 months after baseline; and long-term as follow-up ≥12 months after baseline. If more than one timepoint was available within the same follow-up period, the one closer to the end of the intervention for any of the three timepoints was considered. Authors from one study were contacted to clarify information not provided in the published trial,20 but we have received no answer. Imputation following the Cochrane recommendations21 was conducted in this case20 and SD was imputed from other similar studies included in this systematic review.
Data analysis
It was not possible to transform outcome data into a common scale. Overall stability index, Berg and Pediatric Balance scale use dimension scores, and TUG is a time measure. Therefore, analysis was based on standardized mean differences (SMDs) with 95% confidence intervals (CIs). Meta-analysis was performed using random-effects model, considering clinical heterogeneity among included studies. Heterogeneity was identified with the I2 statistics,22 with values up to 50% considered low heterogeneity and over 50% moderate to high heterogeneity.22 The critical value for rejecting the null hypothesis was set at a level of 0.05 (two-tailed). Meta-analysis was performed using the Comprehensive Meta-Analysis software, Version 3, Biostat, Englewood NJ, USA. We followed Cohen's guidelines for interpretation of effect sizes: d = 0.2 for small; d = 0.5 for medium; and d = 0.8 for large effects.23
The Grading of Recommendations Assessment, Development and Evaluation system (GRADE) summarized the quality of the evidence of this review.16 Evidence started from high-quality, with possibility to downgrade this level according to five factors24: (a) risk of bias (i.e. average PEDro score <5 out of 10); (b) inconsistency of estimates (i.e. raw I2 > 50%); (c) indirectness of participants (i.e. self-reported diagnosis of CP); (d) imprecision (i.e. pooling <300 participants); and (e) publication bias or absence of its assessment due to small number of included studies, n < 10).25 Independent reviewers (JS and PAA) assessed the strength of the current evidence and disagreements were resolved by consensus.
A sensitivity analysis was planned to assess the impact of methodological quality by excluding poor-quality studies (i.e. removing those studies with PEDro score <5 out of 10). We also planned to assess whether the estimated effects were influenced by sample's characteristics, such as age group, GMFCS level and intervention type.
Results
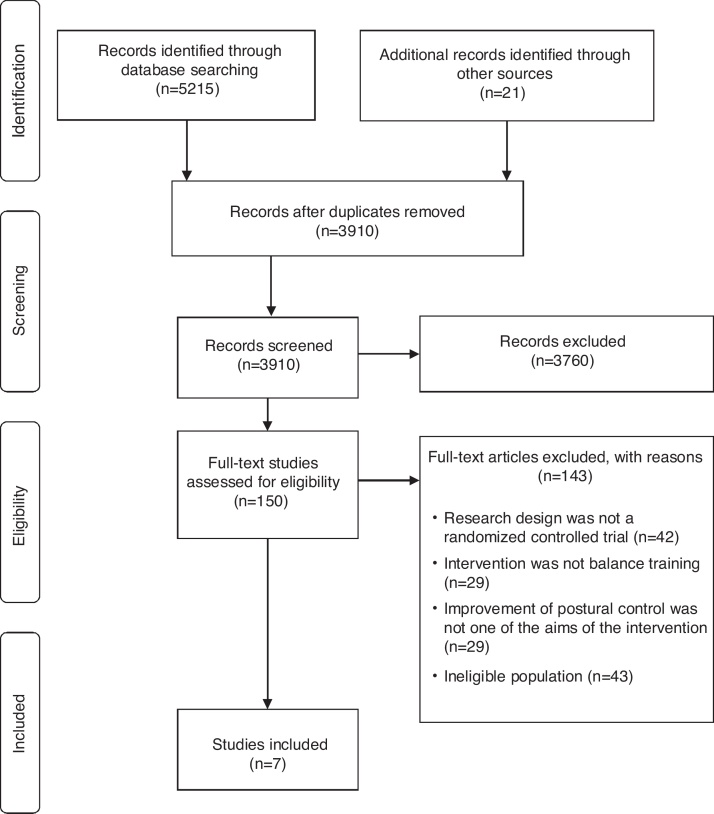
A total of 5236 records were identified: 5215 from search strategies, and 21 from hand search. After removing duplicates, titles and abstracts of 3910 records were screened and 3760 of them were excluded. The remaining 150 potential full-texts were assessed, and seven original studies fulfilled the inclusion criteria20, 26, 27, 28, 29, 30, 31 (Fig. 1).
Figure 1.
Flow of studies in the review.
Characteristics of included studies
Reviewed studies included 194 children and adolescents and sample sizes ranged from 20 to 30 in individual studies. Age ranged from 5 to 15 years old. One study did not report sex of children and adolescents.28 In the other six studies, 40.9% of children and adolescents were female. Two studies did not inform the GMFCS classification of the children and adolescents.20, 28 In the other five studies, 49.3% of children and adolescents were classified as level I and 50.7% as level II. Concerning topographic distribution of the motor involvement, 85.6% of children and adolescents had bilateral and 14.4% had unilateral impairment. All children and adolescents had spastic form of CP. Table 1 shows characteristics of the included studies.
Table 1.
Characteristics of included studies (n = 7).
| Study | Sample characteristics | Intervention | Outcome measures |
|---|---|---|---|
| Abd El-Kafy et al. (2014)29 |
n = 30 Source = pediatrics outpatient clinic of the Faculty of Physical Therapy & Al Kaser Al Eini Hospital, Cairo University, Cairo, Egypt. Age (yr) = 8.8 (SD 0.7) % Female = 56.7% Classification = spastic diplegia GMFCS Level I = 13; II = 17. |
Balance training Comparison: Additional effect EG (n = 15): Balance training plus NDT CG (n = 15): NDT 120 min × 3/wk × 8 wk |
Overall/anteroposterior/mediolateral stability index. Step length/velocity/cycle time/stance phase percentage/ swing phase percentage. Follow-up = 8 wk |
| El-Shamy (2014)27 |
n = 30 Source = pediatrics outpatient clinic at the Faculty of Physical Therapy, Cairo University, Cairo, Egypt. Age (yr) = 9.8 (SD 1.2) % Female = 23.3% Classification = spastic diplegia GMFCS Level I = 13; II = 17. |
Whole body vibration training Comparison: Additional effect EG (n = 15): Whole body vibration training plus NDT CG (n = 15): NDT 60 min × 5/wk × 12 wk |
Overall/ anteroposterior/mediolateral stability index. Follow-up = 12 wk |
| El-Shamy et al. (2014)26 |
n = 30 Source = physical therapy department, Al Noor Hospital, Mecca, Saudi Arabia. Age (yr) = 10.6 (SD 1.4) % Female = 33.3% Classification = spastic diplegia GMFCS Level I = 13; II = 17. |
Balance training Comparison: Additional effect EG (n = 15): Balance training plus NDT CG (n = 15): NDT 120 min × 3/wk × 12 wk |
Overall directional control/ PBS Follow-up = 12 wk |
| El-Shamy (2017)31 |
n = 30 Source = Maternity and Children Hospital, Makkah, Saudi Arabia. Age (yr) = 10.3 (SD 1.3) % Female = 40.0% Classification = spastic diplegia GMFCS Level I = 13; II = 17. |
Antigravity treadmill training Comparison: Additional effect EG (n = 15): Antigravity treadmill training plus NDT CG (n = 15): NDT 20 min × 3/wk × 12 wk |
Overall/ anteroposterior/ mediolateral stability index. Fall risk test Cadence/Stride length/velocity/time spent in double-limb support. Follow-up = 12 wk |
| Ibrahim et al. (2014)28 |
n = 30 Source = the outpatient clinic, college of Physical Therapy, Cairo University, Cairo, Egypt. Age (yr) = 9.6 (SD 1.4) % Female = not reported Classification = spastic diplegia GMFCS Level not reported. |
Whole body vibration training Comparison: Additional effect EG (n = 15): Whole body vibration training plus NDT CG (n = 15): NDT 60 min × 3/wk × 12 wk |
6MWT TUG Follow-up = 12 wk |
| Uysal and Baltaci (2016)30 |
n = 24 Source = Hacettepe University, Faculty of Physiotherapy and Rehabilitation, Department of Paediatric Neurological Clinic, Ankara, Turkey. Age (yr) = 9.6 (SD 2.6) % Female = 58.3% Classification = spastic hemiplegia GMFCS Level I = 19; II = 5. |
Wii therapy Comparison: Additional effect EG (n = 12): Wii therapy plus NDT CG (n = 12): NDT 30 min × 2/wk × 12 wk |
PBS Follow-up = 12 wk |
| Yildirim et al. (2012)20 |
n = 23 Source = Not reported. Age (yr) = 7 (SD not reported) % Female = 35.0% Classification = spastic diplegia (11), hemiplegia (4) and quadriplegia (5). GMFCS Level Not reported. |
Hippotherapy Comparison: Additional effect EG (n = 13): Hippotherapy plus NDT CG (n = 10): NDT 30–45 min × 7/wk × 10 wk. |
PBS Cadence/walking speed. Follow-up = 10 wk |
n, participants included in the baseline; Yr, year; min, minutes; wk, week; GMFCS, Gross Motor Function Classification System; EG, experimental group; CG, control group; PBS, Pediatric Balance Scale; TUG, Timed Up and Go test; NDT, neurodevelopmental treatment.
The experimental groups combined the following BTI with NDT: dynamic platform (two studies)26, 29; whole body vibration – WBV (two studies)27, 28; hippotherapy (one study)20; Wii therapy (one study)30; and antigravity training (one study).31 All the training protocols had at least 20 min of BTI for a period of 8–12 weeks. However, there were variations across studies on time of intervention, weekly frequency and treatment duration. In two studies,30, 31 the time of intervention was 20–30 min/day, with weekly frequency of 2–3 days/week, and treatment duration over 12 weeks. In the others five studies,20, 26, 27, 28, 29 intervention lasted 45–120 min/day, in 3–7 days/week, over 8–12 weeks.
Postural control of children and adolescents with CP was assessed in standing position in all studies.20, 26, 27, 28, 29, 30, 31 All studies used quantitative and valid outcome measures. Four studies used functional scales, and the others used the center of pressure displacement expressed by the stability index. One study focused on gait as the postural control outcome operationalized with the score of TUG test.28 Other studies chose specific measures of postural control: overall; anteroposterior and mediolateral stability indexes;26, 27, 29, 31 and PBS.20, 26, 30 All included studies investigated short-term effects only.
Methodological quality and statistical reporting of included studies
Table 2 shows the individual score on each item and the total scores of the studies on the PEDro scale.32 Quality ranged from 4 to 8, and the median PEDro score was 6. Randomization procedure was properly applied in 100% of studies, with only 57% reporting the allocation procedure. Four studies (57%) informed that examiners were blinded for outcome measurement, whereas no study blinded therapists or participants.
Table 2.
PEDro scores of included studies (n = 7).
| Study | Random allocation | Concealed allocation | Groups similar at baseline | Participant blinding | Therapist blinding | Examiner blinding | <15% dropouts | Intention-to-treat analysis | Between-group difference reported | Point estimate and variability reported | Total (0–10) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abd el-Kafy et al. (2014)29 | Y | Y | Y | N | N | N | N | N | Y | Y | 5 |
| El-Shamy (2014)27 | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8 |
| El-Shamy et al. (2014)26 | Y | N | Y | N | N | N | N | N | Y | Y | 4 |
| El-Shamy (2017)31 | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7 |
| Ibrahim et al. (2014)28 | Y | N | Y | N | N | N | N | N | Y | Y | 4 |
| Uysal and Baltaci (2016)30 | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7 |
| Yildirim et al. (2012)20 | Y | N | Y | N | N | Y | Y | N | Y | Y | 6 |
Y, yes; N, no.
Balance-training interventions combined with other active intervention versus the other active intervention alone
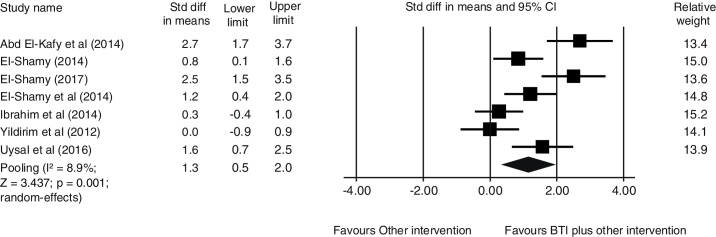
Data from seven studies were pooled to investigate whether BTI combined with other active intervention enhances the effect of the other active intervention alone. Data were pooled involving 194 participants. There is very-low quality evidence that BTI plus other active intervention has a large effect on postural control at short-term [SMD (95% CI) = 1.3 (0.5, 2.0), p = 0.001] when compared with the other active intervention alone (Fig. 2). The current quality of evidence was very low because of publication bias, imprecision and inconsistency.
Figure 2.
Pooling data showing a short-term additional effect of balance-training interventions (BTI) to other active intervention (i.e. NDT) (n = 194 participants).
Sensitivity analysis
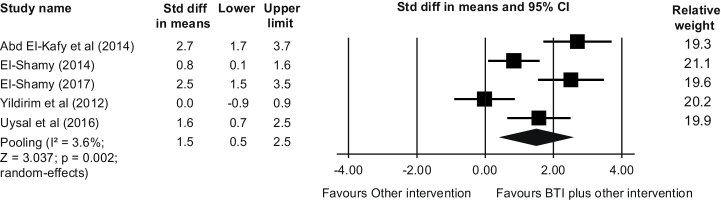
We performed sensitivity analysis to investigate the impact of poor methodological quality of studies26, 28 (PEDro scores <5) on the estimated effect. Findings did not suggest impact of methodological quality on our estimated additional effect of BTI on postural control at short-term [SMD (95% CI) = 1.5 (0.5, 2.5), p = 0.002] (Fig. 3). Other planned sensitivity analyses were not possible because of small number of studies with distinct sample characteristics.
Figure 3.
Sensitivity analysis (removing poor-quality studies, PEDro <5 out of 10) showing a short-term additional effect of balance-training interventions (BTI) to other active intervention (i.e. NDT) (n = 134 participants).
Discussion
The aim of this review was to investigate whether BTI combined with other active intervention enhances effects of the other active intervention alone on postural control in children and adolescents with CP. The pooled estimate showed that there is a large short-term effect of BTI combined with other active intervention when compared with the other active intervention alone on postural control. Nevertheless, findings should be interpreted with caution because current evidence is still very limited and future high-quality studies are likely to change the estimate.
There are few studies that have investigated whether the effect of the combination of conventional interventions with BTI is greater than the conventional treatments alone, although postural control in cerebral palsy is a relevant clinical and research topic. Actually, there are already several studies investigating the effect of conventional treatment or BTI on postural control.10, 11 However, many of these previous studies were not designed to answer our question and were excluded from the current review. In addition, the specific criteria established in this review might explain why only seven studies were included.
Findings suggest that BTI (i.e. hippotherapy, dynamic platform, antigravity training, Wii therapy or WBV) produces a large positive effect (i.e. effect size of 1.3, 95% CI 0.5, 2.0) on postural control when combined with NDT in children and adolescents with CP. Although, there was a lack of information detailing NDT techniques in the included studies, it did not impact on our findings because both groups (i.e. experimental and control groups, BTI plus other active intervention or other active intervention only) received the same NDT intervention.
Dynamic platform and WBV are motor activities that require the active movements of children and adolescents to recover stability after specific postural challenges. It is possible that the devices used in such interventions moved children and adolescents’ center of mass within their zone of postural reversibility and may have increased muscular recruitment to maintain or adjust postural control during tested activities.33
Similarly, during hippotherapy and Wii therapy, the children and adolescents’ center of gravity is displaced, facilitating dynamic postural responses to unexpected perturbations.34, 35 The rationale for testing the effect of hippotherapy on postural control is that the smooth and rhythmic horse's movements provide displacements of children and adolescents’ pelvis in such a way that resembles the pelvic displacement during gait.36 Thus, when on the horse's back, children and adolescents can experience controlled body displacements, leading to practices of weight shifting and muscle activations of children and adolescents’ trunk, head/neck, hip and lower limbs. With the tutoring of a therapist who is trained to certify that each child is given the “just right” postural challenge, it is assumed that these active ingredients of the intervention will contribute to improve children and adolescents’ performance on balance scales and/or walking tests. Previous systematic review considered hippotherapy an effective modality to improve postural control in our group of interest13 and our findings pointed to the same conclusion. The challenges proposed by Wii therapy are similar to those described in hippotherapy. It requires repetitive and active body movement of children and adolescents with weight bearing forward, backwards and sideways during virtual basketball, tennis and boxing.
In turn, antigravity treadmill training provides a safe environment for repetitive stepping since it reduces the effects of gravity and offers postural support, helping children and adolescents with CP in high intensity exercises. The improvement on overall strength caused by this program might facilitate the postural control.
When we excluded two studies with PEDro score lower than 5, the subgroup analysis revealed a similar effect (i.e. effect size of 1.5, 95% CI 0.5, 2.5), with statistical significance. This result suggested that inclusion of studies of low methodological quality did not influence the estimated short-term additional effect of BTI.
The lack of consensus on postural control's theoretical approach and implementation, as highlighted by Dewar et al.,2 was demonstrated by the diversity of outcome measures. Although standardized scales are available in the literature with well-established psychometrics properties, only three out of the seven studies used specific instruments for postural control assessment (i.e. PBS). This test, in addition to showing good validity and reliability, is easy to handle, not too long (i.e. administration lasts maximum of 15 min), includes materials available in clinical settings and assesses the performance of functional task that are part of the children and adolescents’ daily activities.
In regards to characteristics of the included studies that could impact on the estimated effect, the dosage (i.e. type, intensity, frequency and duration) varied across them and severity distribution of participants was somewhat restricted. To the best of our knowledge and based on the analyses conducted in this systematic review, there was no obvious link between intervention doses and effect sizes. In this review, 100% of participants ambulated independently, with GMFCS levels I–II. Further research is required for children and adolescents with moderate-to-severe CP.
It is important to consider some limitations when analyzing results of this systematic review. Firstly, there was high levels of statistical heterogeneity (i.e. raw I2 > 50%). We used random-effects model to pool data in the current review to control the statistical heterogeneity. Secondly, despite the inclusion of randomized controlled trials, the quality of evidence for the comparison was very low because of absence of assessment of publication bias, imprecision and inconsistency. Furthermore, the small number of studies did not allow sensitivity analyses to evaluate the influence of sample's characteristics such as age group, GMFCS level and intervention type on the estimated effect. At last, four of the seven included studies were conducted by the same research group. It is not always clear whether and to what degree there was overlap in the samples. This may have introduced a bias in analysis. Further randomized controlled trials are required to confirm the estimated effect of BTI, mainly in groups with different characteristics, such as topography, age and GMFCS level. Moreover, future studies should also focus on examining the impact of different dosages of BTI.
Conclusion
In conclusion, very-low quality evidence suggests that BTI combined with other intervention enhances the effect of the other intervention alone on postural control at short-term in children and adolescents with CP.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
Funding was provided by the following agencies: National Council for Scientific and Technological Development (CNPq), Minas Gerais State Agency for Research and Development (FAPEMIG) and Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES).
Appendix 1.
Search strategy conducted on February 22nd 2019. OVID (MEDLINE, COCHRANE)
| 1. | Clinical Trial as Topic/ or Random Allocation/ or Randomized Controlled Trial as Topic/ or randomized controlled trial$.mp. |
| 2. | randomized controlled trial$.mp. or Randomized Controlled Trial/ |
| 3. | Controlled clinical trial$.mp. or Controlled Clinical Trial/ |
| 4. | random allocation$.mp. |
| 5. | single-blind method$.mp. or Single-Blind Method/ |
| 6. | double-blind method$.mp. or Double-Blind Method/ |
| 7. | clinical trial$.mp. or Clinical Trial/ |
| 8. | random$.mp. |
| 9. | comparative stud$.mp |
| 10. | evaluation stud$.mp. |
| 11. | follow-up stud$.mp. or Follow-Up Studies/ |
| 12. | prospective stud$.mp. or Prospective Studies/ |
| 13. | cross-over studies.mp. or Cross-Over Studies/ |
| 14. | control$.mp. |
| 15. | prospective$.mp. or Prospective Studies/ |
| 16. | Child, Preschool/ or child$.mp. or Child Care/ or Child/ |
| 17. | Infant Care/ or Infant, Premature/ or infant$.mp. or Infant, Newborn/ or Infant/ |
| 18. | newborn$.mp. |
| 19. | adolescent$.mp. or Adolescent/ |
| 20. | teenager$.mp. |
| 21. | preschool$.mp. |
| 22. | cerebral palsy.mp. or Cerebral Palsy/ |
| 23. | Nervous System Diseases/ or neurodisability.mp. or Developmental Disabilities/ |
| 24. | spastic.mp. or Muscle Spasticity/ |
| 25. | diplegic$.mp. or Gait Disorders, Neurologic/ |
| 26. | Hemiplegia/ or hemiplegic$.mp. or Movement Disorders/ |
| 27. | motor disorder$.mp. |
| 28. | Paraplegia/ or Quadriplegia/ or quadriplegic$.mp. |
| 29. | brain injur$.mp. or Brain Injuries/ |
| 30. | athetosis.mp or Athetosis/ |
| 31. | Exercise Therapy/ or motor rehabilitation$.mp. |
| 32. | rehabilitation$.mp. or “Physical and Rehabilitation Medicine”/ or Rehabilitation/ |
| 33. | Physical Therapy Modalities/ or physiotherapy.mp. |
| 34. | Exercise/ or exercise$.mp. or Exercise Movement Techniques |
| 35. | therap$.mp |
| 36. | physical therap$.mp. |
| 37. | occupational therapy.mp. or Occupational Therapy/ |
| 38. | “Early Intervention (Education)”/ or Intervention Studies/ or Early Medical Intervention/ or intervention$.mp. |
| 39. | treatment$.mp. or Therapeutics/ |
| 40. | activit$.mp. |
| 41. | kinesiotherapy.mp. |
| 42. | movement therapy.mp. |
| 43. | Eletric Stimulation Therapy/ or Eletric Stimulation/ or functional electrical stimulation |
| 44. | task training.mp. |
| 45. | hippotherapy.mp. or Equine-Assisted Therapy/ |
| 46. | neurodevelopmental therapy.mp. |
| 47. | Resistance Training/ or training.mp. |
| 48. | balance training.mp. |
| 49. | treadmill training.mp. |
| 50. | Therapy, Computer-Assisted/ or virtual reality.mp. |
| 51. | biofeedback.mp. |
| 52. | posture.mp. or Posture/ |
| 53. | balance.mp. or Postural Balance/ |
| 54. | postural control.mp. |
| 55. | body posture.mp. |
| 56. | body alignment.mp. |
| 57. | postural stability.mp. |
| 58. | stability.mp. |
| 59. | postural sway.mp. |
| 60. | trunk control.mp. |
| 61. | motor skill$.mp. or Motor Skills/ |
| 62. | pediatric balance scale.mp. |
| 63. | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 |
| 64. | 16 or 17 or 18 or 19 or 20 or 21 |
| 65. | 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 |
| 66. | 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 |
| 67. | 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 |
| 68. | 63 and 64 and 65 and 66 and 67 |
References
- 1.Westcott S.L., Burtner P. Postural Control in Children: implications for pediatric practice. Phys Occup Ther Pediatr. 2004;24(1–2):5–55. doi: 10.1300/j006v24n01_02. [DOI] [PubMed] [Google Scholar]
- 2.Dewar R., Claus A.P., Tucker K., Johnston L.M. Perspectives on postural control dysfunction to inform future research: a delphi study for children with cerebral palsy. Arch Phys Med Rehabil. 2017;98(3):463–479. doi: 10.1016/j.apmr.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 3.Woollacott M.H., Shumway-Cook A. Postural dysfunction during standing and walking in children with cerebral palsy: what are the underlying problems and what new therapies might improve balance? Neural Plast. 2005;12(2–3):211–219. doi: 10.1155/NP.2005.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu W.-Y., Zaino C.A., McCoy S.W. Anticipatory postural adjustments in children with cerebral palsy and children with typical development. Pediatr Phys Ther. 2007;19(3):188–195. doi: 10.1097/PEP.0b013e31812574a9. [DOI] [PubMed] [Google Scholar]
- 5.McCoy S.W., Bartlett D.J., Yocum A. Development and validity of the early clinical assessment of balance for young children with cerebral palsy. Dev Neurorehabil. 2014;17(6):375–383. doi: 10.3109/17518423.2013.827755. [DOI] [PubMed] [Google Scholar]
- 6.Shumway-Cook A., Hutchinson S., Kartin D., MSME R.P., Woollacott M. Effect of balance training on recovery of stability in children with cerebral palsy. Dev Med Child Neurol. 2007;45(9):591–602. doi: 10.1017/s0012162203001099. [DOI] [PubMed] [Google Scholar]
- 7.Pavão S.L., Barbosa K.A.F., Sato T de O., Rocha N.A.C.F. Functional balance and gross motor function in children with cerebral palsy. Res Dev Disabil. 2014;35(10):2278–2283. doi: 10.1016/j.ridd.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 8.Woollacott M., Shumway-Cook A., Hutchinson S., Ciol M., Price R., Kartin D. Effect of balance training on muscle activity used in recovery of stability in children with cerebral palsy: a pilot study. Dev Med Child Neurol. 2005;47(7):455–461. doi: 10.1017/s0012162205000885. http://ezproxy.library.usyd.edu.au/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=cin20&AN=2009034725&site=ehost-live. [DOI] [PubMed] [Google Scholar]
- 9.Pavão S.L., Santos A.N., Oliveira A.B., Rocha N.A.C.F. Postural control during sit-to-stand movement and its relationship with upright position in children with hemiplegic spastic cerebral palsy and in typically developing children. Brazilian J Phys Ther. 2015 doi: 10.1590/bjpt-rbf.2014.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris S.R., Roxborough L. Efficacy and effectiveness of physical therapy in enhancing postural control in children with cerebral palsy. Neural Plast. 2005;12(2–3):229–243. doi: 10.1155/NP.2005.229. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L41122524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dewar R., Love S., Johnston L.M. Exercise interventions improve postural control in children with cerebral palsy: a systematic review. Dev Med Child Neurol. 2015;57(6):504–520. doi: 10.1111/dmcn.12660. [DOI] [PubMed] [Google Scholar]
- 12.Gómez-Regueira N., Viñas-Diz S. Mejora del control postural y equilibrio en la parálisis cerebral infantil: revisión sistemática. Fisioterapia. 2016;38(4):196–214. [Google Scholar]
- 13.Zadnikar M., Kastrin A. Effects of hippotherapy and therapeutic horseback riding on postural control or balance in children with cerebral palsy: a meta-analysis. Dev Med Child Neurol. 2011;53(8):684–691. doi: 10.1111/j.1469-8749.2011.03951.x. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y., Fanchiang H.D., Howard A. Effectiveness of virtual reality in children with cerebral palsy: a systematic review and meta-analysis of randomized controlled trials. Phys Ther. 2018;98(1):63–77. doi: 10.1093/ptj/pzx107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franki I., Desloovere K., De Cat J. The evidence-base for conceptual approaches and additional therapies targeting lower limb function in children with cerebral palsy: a systematic review using the international clasification of functioning, disability and health as a framework. J Rehabil Med. 2012;44(5):396–405. doi: 10.2340/16501977-0984. [DOI] [PubMed] [Google Scholar]
- 16.Atkins D., Best D., Briss P.A. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Morton N.A. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55(2):129–133. doi: 10.1016/s0004-9514(09)70043-1. [DOI] [PubMed] [Google Scholar]
- 18.Palisano R., Rosenbaum P., Walter S., Russell D., Wood E., Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. http://www.ncbi.nlm.nih.gov/pubmed/9183258. Accessed 13.08.16. [DOI] [PubMed] [Google Scholar]
- 19.Palisano R., Rosenbaum P., Bartlett D.J., Livingston M. CanChild Centre for Childhood Disability Research, McMaster University; 2007. Gross Motor Function Classification System—Expanded & Revised. http://motorgrowth.canchild.ca/en/GMFCS/resources/GMFCS-ER.pdf. Accessed 29.03.10. [Google Scholar]
- 20.Yildirim Sik B., Cekmece C., Dursun N. Is hyppotherapy beneficial for rehabilitation of children with cerebral palsy? Turkiye Klin J Med Sci. 2012;32(3):601–608. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L364386898. [Google Scholar]
- 21.Higgins J.P., Green S. Cochrane Book Series; 2011. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. https://pdfs.semanticscholar.org/4b43/91c08c45ebfcd046a53106c97ca09fcdf9fa.pdf. Accessed 07.08.18. [Google Scholar]
- 22.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Lawrence Erlbaum Associates; New York: 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- 24.Balshem H., Helfand M., Schünemann H.J. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 25.Ioannidis J.P.A., Trikalinos T.A. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. Can Med Assoc J. 2007;176(8):1091–1096. doi: 10.1503/cmaj.060410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.el-Shamy S.M., Abd el Kafy E.M. Effect of balance training on postural balance control and risk of fall in children with diplegic cerebral palsy [with consumer summary] Disabil Rehabil. 2014;36(September 14):1176–1183. doi: 10.3109/09638288.2013.833312. [DOI] [PubMed] [Google Scholar]
- 27.el-Shamy S.M. Effect of whole-body vibration on muscle strength and balance in diplegic cerebral palsy: a randomized controlled trial. Am J Phys Med Rehabil. 2014;93(February (2)):114–121. doi: 10.1097/PHM.0b013e3182a541a4. [DOI] [PubMed] [Google Scholar]
- 28.Ibrahim M.M., Eid M.A., Moawd S.A. Effect of whole-body vibration on muscle strength, spasticity, and motor performance in spastic diplegic cerebral palsy children. Egypt J Med Hum Genet. 2014;15(April (2)):173–179. [Google Scholar]
- 29.Abd el-Kafy E.M., el-Basatiny H. Effect of postural balance training on gait parameters in children with cerebral palsy. Am J Phys Med Rehabil. 2014;93(November (11)):938–947. doi: 10.1097/PHM.0000000000000109. [DOI] [PubMed] [Google Scholar]
- 30.Uysal A.S., Baltaci G. Effects of Nintendo Wii™ training on occupational performance, balance, and daily living activities in children with spastic hemiplegic cerebral palsy: a single-blind and randomized trial. Games Health J. 2016;5(5):311–317. doi: 10.1089/g4h.2015.0102. [DOI] [PubMed] [Google Scholar]
- 31.El-Shamy S.M. Effects of antigravity treadmill training on gait, balance, and fall risk in children with diplegic cerebral palsy. Am J Phys Med Rehabil. 2017;96(11):809–815. doi: 10.1097/PHM.0000000000000752. [DOI] [PubMed] [Google Scholar]
- 32.Maher C.G., Sherrington C., Herbert R.D., Moseley A.M., Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8):713–721. http://www.ncbi.nlm.nih.gov/pubmed/12882612. Accessed 07.08.18. [PubMed] [Google Scholar]
- 33.Rittweger J. Vibration as an exercise modality: how it may work, and what its potential might be. Eur J Appl Physiol. 2010;108(5):877–904. doi: 10.1007/s00421-009-1303-3. [DOI] [PubMed] [Google Scholar]
- 34.Shurtleff T.L., Engsberg J.R. Changes in trunk and head stability in children with cerebral palsy after hippotherapy: a pilot study. Phys Occup Ther Pediatr. 2010;30(2):150–163. doi: 10.3109/01942630903517223. [DOI] [PubMed] [Google Scholar]
- 35.Gatica-Rojas V., Méndez-Rebolledo G., Guzman-Muñoz E. Does Nintendo Wii Balance Board improve standing balance?. A randomized controlled trial in children with cerebral palsy. Eur J Phys Rehabil Med. 2017;53(4):535–544. doi: 10.23736/S1973-9087.16.04447-6. [DOI] [PubMed] [Google Scholar]
- 36.McGee M.C., Reese N.B. Immediate effects of a hippotherapy session on gait parameters in children with spastic cerebral palsy. Pediatr Phys Ther. 2009;21(2):212–218. doi: 10.1097/PEP.0b013e3181a39532. [DOI] [PubMed] [Google Scholar]