Abstract
Previous preliminary work mapped the distribution of neck muscle fat infiltration (MFI) in the deep cervical extensor muscles (multifidus and semispinalis cervicis) in a small cohort of participants with chronic whiplash associated disorders (WAD), recovered, and healthy controls. While MFI was reported to be concentrated in the medial portion of the muscles in all participants, the magnitude was significantly greater in those with chronic WAD. This study aims to confirm these results in a prospective fashion with a larger cohort and compare the findings across a population of patients with varying levels of WAD-related disability one-year following the motor vehicle collision. Sixty-one participants enrolled in a longitudinal study: Recovered (n = 25), Mild (n = 26) and Severe WAD (n = 10) were studied using Fat/Water magnetic resonance imaging, 12-months post injury. Bilateral measures of MFI in four quartiles (Q1–Q4; medial to lateral) at cervical levels C4 through C7 were included. A linear mixed model was performed, controlling for covariates (age, sex, body mass index), examining interaction effects, and comparing MFI distribution between groups. The recovered group had significantly less MFI in Q1 compared to the two symptomatic groups. Group differences were not found in the more lateral quartiles. Results at 12 months are consistent with the preliminary study, indicating that MFI is spatially concentrated in the medial portions of the deep cervical extensors regardless of WAD recovery, but the magnitude of MFI in the medial portions of the muscles is significantly larger in those with severe chronic WAD.
Subject terms: Skeletal muscle, Anatomy, Musculoskeletal system, Skeleton, Prognostic markers
Introduction
Various investigations across three continents (United States1–3, Australia2,4–6, and Sweden7,8) have used similar manual segmentation methods with magnetic resonance imaging (MRI) to demonstrate widespread fatty infiltrates in the neck muscles of individuals with acute and chronic whiplash associated disorders (WAD). Comparably high levels of muscle fat infiltration (MFI) were not identified in individuals who (i) had recovered or reported lower levels of WAD-related disability1,3,4,7,8, (2) had chronic non-traumatic neck pain9, or (3) those without a history of neck disorders10. Knowledge of a precise mechanism for MFI expression and distribution (e.g. extra- or intra-fascicular MFI) would provide a target for clinical remediation of any adverse symptoms; but this is largely unknown.
There remains conjecture in the literature about what constitutes extra- or intra-fascicular MFI, which likely centres on our ability to reliably distinguish the epimysium/perimysium and associated muscle fascicles on MRI. Some have proposed standardised, anatomical definitions of regions of interest in quantifying spinal muscle composition11,12, which we concede can be difficult for the cervical spine (eg transversospinal muscles like multifidus).
With this in mind, total MFI has been observed and reported to occur throughout cervical spine levels with a greater magnitude of MFI occurring amongst the deepest muscular layer of the extensors (e.g. comprising the cervical multifidus and semispinalis cervicis) when compared with the more superficial musculature (e.g. including semispinalis capitis, splenius capitis and upper trapezius)10,13. In earlier preliminary work, Abbott et al.1 reported that, in a small cohort of participants with chronic WAD and healthy controls, the composition of the deep cervical muscles appeared dependent on location in the transverse plane where the most medial muscle tissues approximating the spinous processes have larger proportions of MFI.
It is noteworthy that while MFI was reported to be more concentrated in the medial portion of the muscles in all participants1,7, the magnitude was significantly greater in a small subgroup of participants with chronic WAD, suggesting a potential phenotypic expression of chronicity. Despite positive findings from both quantitative1 or qualitative7 measures, replication of these findings in a larger cohort with varying levels of chronic WAD is required before the pervasive finding of, and specific distribution for, MFI in this population can be confirmed. As such, MRI data from participants enrolled in a longitudinal study of WAD recovery were included from the 1-year post injury time point and used to (i) confirm previous results1 by quantifying the magnitude and spatial distribution of MFI in the deep cervical extensor muscles (multifidus and semispinalis cervicis) and (ii) compare these findings across a population of patients with varying levels of WAD-related disability one-year following the injury event.
Methods
Sub-study population
Sixty-one participants, out of a total 97, were enrolled in this sub-study of a prospective, longitudinal parent study investigating the neuromuscular mechanisms underlying poor recovery following whiplash injury from a motor vehicle collision (MVC) (ClinicalTrials.gov (Identifier: NCT02157038)). The study was approved by Northwestern University’s Institutional Review Board. All applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research according to the Declaration of Helsinki, and written informed consent was obtained from every participant.
After an MVC, participants were asked to participate through the university-affiliated emergency medicine department. Inclusion criteria were: neck pain following an MVC, within the Quebec Task Force Classification category of WAD Grade II (primary neck pain complaint, reduced neck range of movement and point tenderness in the neck, with no radicular symptoms), and completed both the questionnaires and magnetic resonance imaging (< 1-week, 2-weeks, 3-months, and 12-months post MVC). Exclusion criteria were: < 18 or > 65 years of age at time of collision, prior motor vehicle collisions, treatment for neck pain disorders within the past 10 years, neurological or metabolic disorders (Multiple Sclerosis, Parkinson’s, Alzheimer’s, or diabetes), or at risk for poly-trauma (determined by emergency department protocols). A total of 61 participants were included having the available MRI data at 12-months post injury that was suitable for quartile segmentation in the transverse plane, with Q1 being most medial near the spinous process and Q4 most lateral, employing a method previously described1 and shown in Fig. 1. Neck Disability Index scores at one-year post injury were used to determine severity groups: (1) severe group (≥ 30%), (2) mild group (10–29%), and (3) recovered group (< 10%)3,4.
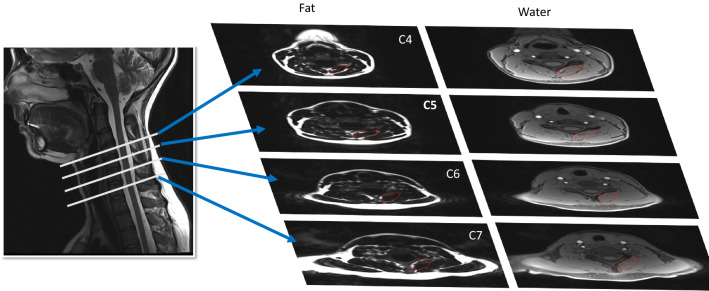
Figure 1.
Deep cervical extensor muscle segmentation was delineated into the four quartiles, with Q1 being most medial (near the spinous process) and Q4 most lateral.
Magnetic resonance imaging
Each participant received MRI of their deep cervical extensors from C4 through C7 (Fig. 2), using a 3.0 T scanner with a 64-channel head/neck coil (Siemens, Munich Germany). Specifics of the fat/water MRI were as follows: dual-echo gradient-echo sequence (2-point Dixon), TR = 7.05 ms, TE1 = 2.46 ms, TE2 = 3.69 ms, flip angle = 12°, bandwidth = 510 Hz/pixel, FOV = 190 × 320 mm2, slab oversampling of 20% with 40 partitions to prevent aliasing in the anterior–posterior direction, in-plane resolution = 0.7 × 0.7 mm2, slice thickness = 3.0 mm3, number of averages = 6, acquisition time = 4 min 5s14. This fat/water sequence has been validated in animal models15.
Figure 2.
Fat/Water MRI of the deep cervical extensors from C4 through C7.
The deep cervical extensors (multifidus and semispinalis cervicis) were manually segmented12 according to an established method previously described1. Briefly, this approach was shown to have high inter- and intra-rater reliability1. MFI was calculated in the same fashion as the previous cross-sectional study using an average of three slices per vertebral level: Muscle Fat Infiltration = IF/(IF + IW) × 100, where IF = fat signal intensity and IW = water signal intensity1. Deep cervical extensor muscle segmentation was delineated into the four quartiles, with Q1 being most medial (near the spinous process) and Q4 most lateral, using a custom MATLAB script (The MathWorks, Inc, Natick, MA) (see Fig. 1).
Statistical analysis
SPSS Version 23.0 was used to perform all statistical analyses (IBM Corporation, Armonk, NY). Baseline descriptive statistics were summarized and assessed for potentially important demographic differences. A repeated-measures, linear mixed-model approach was used to investigate MFI differences between recovery groups. The main and interactive effects of muscle quartile and group (severe, mild, and recovered) were modelled as fixed effects, and each cervical level was analyzed utilizing a separate mixed model. Inter-participant differences in the change in MFI across quartiles were modelled as random effects. Age, sex, and body mass index were entered as covariates into each model as per our primary objective of confirming previous work in which these covariates were used1. Pairwise comparisons were used to investigate between-group differences in intra-quartile MFI. For all analyses, the significance level was set to p < 0.05.
Results
A total of 97 acutely injured participants were enrolled in our parent study and followed for four time points (< 1-week, 2-weeks, 3-months, and 12-months post MVC). All participants were followed up for the first three time points, but a total of 19 were lost to attrition between 3-months and 12-months. Thus, a total of 78 returned for the 12-month follow-up. Of this 78, we had available quartile imaging data for each time point (< 1 week, 2 weeks, 3 months and 12 months) in a total of 61 participants, which leaves a total of 17 participants without such data; one of which was due to poor scan quality that was not amenable to quartile measures. The remaining 16 were not included (severe = 5; mild = 6; recovered = 5) on grounds they did not have imaging that was amenable for quartile segmentation in the transverse plane (see Fig. 3).
Figure 3.
Flowchart of participants involved in parent study, sub-study, and previous preliminary work.
A total of 10 participants were assigned to the severe group, 26 to the mild group, and 25 to the recovered group (N = 61 for all participants, see Table 1 including comparison to the Severe and Recovered participants from Abbott et al.1) based on NDI % scores at 12 months post-MVC. Table 2 details group descriptive data at 12-months post whiplash for those in, and those not in, this sub-study investigating quartile MFI.
Table 1.
Participant Demographics and comparison to Abbott et al.1 participants.
| Characteristic | Severe N = 10 |
Severe N = 5 Abbott et al.1 |
Mild N = 26 |
Recovered N = 25 |
Recovered N = 5 Abbott et al.1 |
|---|---|---|---|---|---|
| BMI, kg/m2 | 25.7 ± 2.8 | 29.6 ± 24.6 | 23.8 ± 3.7 | 23.6 ± 3.1 | 26.4 ± 1.1 |
| Age, y | 37.0 ± 12.5 | 30.6 ± 9.0 | 37.9 ± 12.5 | 31.5 ± 10.7 | 32.8 ± 7.5 |
| Sex (female), n (%) | 8 (80) | 3 (60) | 23 (88) | 14 (56) | 3 (60) |
Values are mean ± SD unless otherwise indicated.
BMI body mass index.
Table 2.
Additional participant demographics (from parent study, not included).
| Characteristic | Not included | Included | Not included | Included | Not included | Included |
|---|---|---|---|---|---|---|
| Severe N = 5 |
Severe N = 10 |
Mild N = 6 |
Mild N = 26 |
Recovered N = 5 |
Recovered N = 25 |
|
| BMI, kg/m2 | 28.0 ± 7.3 | 25.7 ± 2.8 | 25.7 ± 6.1 | 23.8 ± 3.7 | 26.6 ± 2.8 | 23.6 ± 3.1 |
| Age, y | 36.1 ± 9.1 | 37.0 ± 12.5 | 27.9 ± 9.3 | 37.9 ± 12.5 | 32.8 ± 7.3 | 31.5 ± 10.7 |
| Sex (female), n (%) | 4 (80) | 8 (80) | 5 (83) | 23 (88) | 3 (60) | 14 (56) |
| NDI | 29.2 ± 19.2 | 31.8 ± 11.6 | 17.6 ± 16.1 | 19.5 ± 8.7 | 2.8 ± 4.4 | 6.1 ± 6.7 |
Values are mean ± SD unless otherwise indicated.
BMI body mass index.
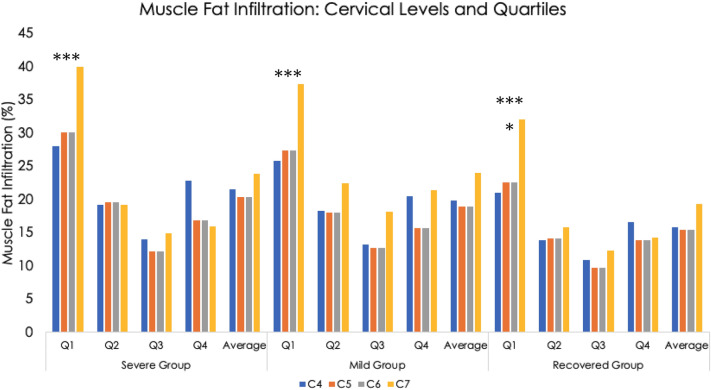
For all three groups, the most medial quartile, Q1, had the highest level of MFI (p < 0.001, see Fig. 4). For all four cervical levels (C4 through C7), the recovered group had significantly less MFI in Q1 compared to the symptomatic groups (p < 0.05). This was also found for the second-most medial quartile, Q2, at levels C4 and C5 (p < 0.05). Group differences were not found in the more lateral quartiles, Q3 and Q4 at any level (C4 through C7). There was a trend towards a significant group * quartile interaction effect at the cervical level, C5 (p = 0.05).
Figure 4.
Muscle fat infiltration by group, Cervical level (C4–C7, Quartiles (Q1–Q4), and average of the all Quartiles*Level.
Discussion
Consistent with preliminary work1,7, MFI appears to be equally distributed between the more lateral quartiles at all levels analysed (C4-C7) and across all groups, but, once again, Q1 contains the highest MFI levels with significantly larger magnitudes in the two symptomatic groups compared to the recovered group. This provides supportive data mapping the spatial distribution and magnitude of MFI in the deep extensor muscles in a larger sample of individuals with varying levels of WAD-related disability at one-year post injury when compared to preliminary quantitative1 and qualitative7 reports.
As a reflection of the natural history of decline in muscle quality with age, it is plausible that the medial aspect of deep paraspinal muscles is susceptible to increased MFI, thereby raising mechanistic questions of whether (i) having higher pre-trauma MFI in Q1 or (ii) the acute trauma-related expressions of Q1 MFI contribute to the clinical course and further degeneration in total MFI over time. Questions also remain regarding the influence that age, sex, BMI, stress and/or an inflammatory response, and injury severity, have on muscle composition and if certain cervical levels are more mechanically disadvantaged than others. For example, the C5/C6 disc has been found to be at highest risk of injury during both frontal and rear impact MVC’s16. In addition to the C5/C6 disc, others have demonstrated the intervertebral foramen at C5/C6 narrowed by as much as 1.8 mm during simulated rear impacts using cadaveric tissue17,18. It is proposed that such narrowing could compress the nerve roots and ganglia in the lower cervical spine, particularly in individuals with congenitally narrow foramen or those with pre-collision osteophytes. Recent evidence also provides foundation that the number of degenerative pathologies seen on initial post MVC computed tomography19, and pre-collision medical diagnoses20 may be associated with the subsequent clinical course of whiplash, and this could align with our findings of significantly larger magnitudes of Q1 MFI, notably at C5, in the symptomatic participants at 12-months. Comparing quartile MFI to the number of degenerative pathologies at all cervical levels across injured male and female participants of varying age is warranted and underway.
It is important to also recognize these findings support previous preliminary cross-sectional work involving patients with varying levels of chronic WAD-related disability and healthy controls across two cultures (United States1 and Sweden7); where different insurance schemes and medical/rehabilitative options may influence recovery. While larger proportions of MFI in the multifidus muscles have been attributed to severe WAD, further prospective investigations, with larger sample sizes, are required to identify an element of pre-existing organic origins19–21, specific injury mechanisms underlying MFI22,23, biomechanical consequences24–28 and their relationships to the further development of MFI with a long-term goal of informing clinical trials; exercise in particular29.
The deep cervical extensors (e.g. multifidus and semispinalis cervicis muscles) are architecturally complex, with Q1 fascicles attaching to the spinous process of a superior vertebra (e.g. C5) and then traveling laterally (Q2-Q4) to attach to the transverse process of an inferior vertebra (e.g. C6). While the specific biomechanical consequences of MFI distribution remain speculative, several hypotheses arise. If increases in local MFI have the potential to influence motor function30, the magnitude and distribution of MFI may be clinically important in traumatic neck pain conditions31,32 and towards informing biomechanical models of the human head/neck33. Despite an emerging body of evidence examining muscle compositional change, the magnitude and spatial distribution of MFI has received little attention in informing current modelling efforts. Further work in this domain appears warranted as has been documented for the lumbar spine34,35.
The multifidus and semispinalis cervicis share a common nerve supply with, and attach directly to, the zygapophyseal joint capsules. The latter have been widely implicated in the generation and maintenance of neck pain following a traumatic MVC36–39, with nearly half of those injured treated for, and receiving benefit from their, facetogenic symptoms40–43. Specifically, radiofrequency neurotomy (RFN) interventions have shown to attenuate the pervasive psychophysical signs/symptoms (e.g. thermal/pressure pain thresholds) common to chronic WAD44. However, in considering the procedure is frequently repeated (once the pain returns) in a large proportion of patients, the long-term biomechanical consequences of RFN and its effects on muscle structure, function, and long-term outcomes, are largely unknown, but could be the result of chronic inhibition of muscle tissue. Further exploration with current quantitative methods are warranted to better understand the long-term biomechanical and functional outcomes with repeated RFN procedures45.
Our study is not without limitations. Neck muscle composition may be influenced by an individual’s general activity and/or neck-specific exercise levels, particularly in people with persistent WAD where symptoms may limit participation46,47. Accordingly, future work should aim to capture general and task-specific activity data across a larger population of participants with varying levels of WAD recovery.
Conclusions
These results provide confirmation for unique patterns of MFI distribution within the deep extensor muscles of participants with varying levels of WAD-related disability one year following the injury event. This confirms previous findings highlighting that the distribution of deep neck muscle MFI is not uniquely featured in those with poor recovery one-year following a whiplash injury from an MVC, but rather the total magnitude of MFI is greater, especially regarding the medial portions at Q1, in those with poor recovery.
Advanced MRI sequencing methods are showing promise in the lumbar spine in identifying fibre type48, which has previously only been possible with biopsied tissues. Muscle fibre ‘transformation’ or ‘replacement’ models remain controversial and certainly warrant further study. Future mechanistic work towards identifying an element of pre-existing organic origins to the natural history of MFI is required before definitive conclusions can be drawn. Such work will help in understanding if certain individuals have a propensity for higher MFI and if the magnitude and distribution of MFI results in biomechanical deficits and persistent WAD-related disability. The revelation of different recovery phenotypes and mechanistic/physiologic processes would likely inform clinical trials.
Acknowledgements
The authors wish to thank the Emergency Medicine Department at Northwestern Memorial Hospital, Chicago Illinois, USA for their assistance in the recruitment of acutely injured participants. We also wish to thank all participants for their involvement.
Author contributions
Conceptualization: J.M.E., A.C.S., S.R.A., R.J.C., M.A.H., R.A. Data curation: J.M.E., A.C.S., M.A.H., R.A., M.W. Formal analysis: J.M.E., A.C.S., S.R.A. Funding acquisition: J.M.E. Investigation: J.M.E., A.C.S., S.R.A., R.J.C., M.A.H., R.A. Methodology: J.M.E., A.C.S., S.R.A., R.J.C., M.A.H., R.A., M.W. Project administration: J.M.E., A.C.S., M.W. Resources: J.M.E., A.C.S., S.R.A., R.J.C., M.A.H., R.A., M.W. Writing—original draft: J.M.E., A.C.S., S.R.A., R.J.C., M.A.H., R.A., M.W. All authors reviewed the final manuscript: J.M.E., A.C.S., S.R.A., R.J.C., M.A.H., R.A., M.W.
Competing interests
The Project described originated at Northwestern University, Feinberg School of Medicine, Department of Physical Therapy and Human Movement Sciences and supported by the National Institutes of Health (NIH) through Grant Number R01HD079076: Eunice Kennedy Shriver National Institute of Child Health & Human Development; National Center for Medical Rehabilitation Research. JME, ACS, and SRA are currently supported by NIH Grant Number R03HD094577. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Elliott, Smith, Hoggarth, Abbott, Wasielewski report funding from NIH; Elliott reports Editorial board member for JOSPT, Musculoskeletal Science & Practice; Advisory Board member the journal, Spine. Honoraria, conference travel, and accommodation for speaking engagements. Crawford and Albin report no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abbott R, Pedler A, Sterling M, et al. The geography of fatty infiltrates within the cervical multifidus and semispinalis cervicis in individuals with chronic whiplash-associated disorders. J. Orthop. Sports Phys. Ther. 2015;45:281–288. doi: 10.2519/jospt.2015.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elliott J, Jull G, Noteboom JT, Darnell R, Galloway G, Gibbon WW. Fatty infiltration in the cervical extensor muscles in persistent whiplash-associated disorders: a magnetic resonance imaging analysis. Spine. 2006;31:E847–855. doi: 10.1097/01.brs.0000240841.07050.34. [DOI] [PubMed] [Google Scholar]
- 3.Elliott JM, Courtney DM, Rademaker A, Pinto D, Sterling MM, Parrish TB. The rapid and progressive degeneration of the cervical multifidus in whiplash: an MRI study of fatty infiltration. Spine (Phila Pa 1976) 2015;40:E694–E700. doi: 10.1097/BRS.0000000000000891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elliott J, Pedler A, Kenardy J, Galloway G, Jull G, Sterling M. The temporal development of fatty infiltrates in the neck muscles following whiplash injury: an association with pain and posttraumatic stress. PLoS ONE. 2011;6:e21194. doi: 10.1371/journal.pone.0021194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elliott J, Sterling M, Noteboom JT, Darnell R, Galloway G, Jull G. Fatty infiltrate in the cervical extensor muscles is not a feature of chronic, insidious-onset neck pain. Clin. Radiol. 2008;63:681–687. doi: 10.1016/j.crad.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Elliott JM, O'Leary S, Sterling M, Hendrikz J, Pedler A, Jull G. Magnetic resonance imaging findings of fatty infiltrate in the cervical flexors in chronic whiplash. Spine (Phila Pa 1976) 2010;35:948–954. doi: 10.1097/BRS.0b013e3181bb0e55. [DOI] [PubMed] [Google Scholar]
- 7.Abbott R, Peolsson A, West J, et al. The qualitative grading of muscle fat infiltration in whiplash using fat and water magnetic resonance imaging. Spine J. 2018;18:717–725. doi: 10.1016/j.spinee.2017.08.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karlsson A, Leinhard OD, Aslund U, et al. An investigation of fat infiltration of the multifidus muscle in patients with severe neck symptoms associated with chronic whiplash-associated disorder. J. Orthop. Sports Phys. Ther. 2016;46:886–893. doi: 10.2519/jospt.2016.6553. [DOI] [PubMed] [Google Scholar]
- 9.Elliott J, Sterling M, Noteboom JT, Darnell R, Galloway G, Jull G. Fatty infiltrate in the cervical extensor muscles is not a feature of chronic, insidious-onset neck pain. Clin. Radiol. 2008;63:681–687. doi: 10.1016/j.crad.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Elliott J, Jull G, Noteboom JT, Darnell R, Galloway G, Gibbon WW. Fatty infiltration in the cervical extensor muscles in persistent whiplash-associated disorders: a magnetic resonance imaging analysis. Spine (Phila Pa 1976) 2006;31:E847–E855. doi: 10.1097/01.brs.0000240841.07050.34. [DOI] [PubMed] [Google Scholar]
- 11.Crawford RJ, Cornwall JC, Abbott R, Elliott J. Manually defining regions of interest when quantifying paravertebral muscles fatty infiltration from axial magnetic resonance imaging: a proposed method for the lumbar spine with anatomical cross-reference. BMC Musculoskelet. Disord. 2017;18:25. doi: 10.1186/s12891-016-1378-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliott JM, Cornwall J, Kennedy E, Abbott R, Crawford RJ. Towards defining muscular regions of interest from axial magnetic resonance imaging with anatomical cross-reference: part II—cervical spine musculature. BMC Musculoskelet. Disord. 2018;19:171. doi: 10.1186/s12891-018-2074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elliott JM, Pedler AR, Ga Jull, Van Wyk L, Galloway GG, O’Leary SP. Differential changes in muscle composition exist in traumatic and nontraumatic neck pain. Spine. 2014;39:39–47. doi: 10.1097/BRS.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 14.Dixon W. Simple proton spectroscopic imaging. Radiology. 1984;153:189–194. doi: 10.1148/radiology.153.1.6089263. [DOI] [PubMed] [Google Scholar]
- 15.Smith AC, Parrish TB, Abbott R, et al. Muscle-fat MRI: 1.5 Tesla and 3.0 Tesla versus histology. Muscle Nerve. 2014;50:170–176. doi: 10.1002/mus.24255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito S, Ivancic PC, Pearson AM, et al. Cervical intervertebral disc injury during simulated frontal impact. Eur. Spine J. 2005;14:356–365. doi: 10.1007/s00586-004-0783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panjabi MM, Maak TG, Ivancic PC, Ito S. Dynamic intervertebral foramen narrowing during simulated rear impact. Spine. 2006;31:E128–E134. doi: 10.1097/01.brs.0000201243.81745.ba. [DOI] [PubMed] [Google Scholar]
- 18.Tominaga Y, Maak TG, Ivancic PC, Panjabi MM, Cunningham B. Head-turned rear impact causing dynamic cervical intervertebralforamen narrowing: implications for ganglion and nerve root injury. J. Neurosurg. Spine. 2006;4:380–387. doi: 10.3171/spi.2006.4.5.380. [DOI] [PubMed] [Google Scholar]
- 19.Elliott JM, Parrish TB, Walton DM, et al. Does overall cervical spine pathology relate to the clinical heterogeneity of chronic whiplash? Am. J. Emerg. Med. 2019;38:869–873. doi: 10.1016/j.ajem.2019.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osterland TB, Kasch H, Frostholm L, et al. Pre-collision medical diagnoses predict chronic neck pain following acute whiplash-trauma. Clin. J. Pain. 2018;35:304–314. doi: 10.1097/AJP.0000000000000683. [DOI] [PubMed] [Google Scholar]
- 21.Sjaastad O, Fredriksen TA, Batnes J, Petersen HC, Bakketeig LS. Whiplash in individuals with known pre-accident, clinical neck status. J. Headache Pain. 2006;7:9–20. doi: 10.1007/s10194-006-0270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elliott JM, Dewald JP, Hornby TG, Walton DM, Parrish TB. Mechanisms underlying chronic whiplash: contributions from an incomplete spinal cord injury? Pain Med. 2014;15:1938–1944. doi: 10.1111/pme.12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith AC, Parrish TB, Hoggarth M, et al. Potential associations between chronic whiplash and incomplete spinal cord injury. Spinal Cord Ser. Cases. 2015;1:15024. doi: 10.1038/scsandc.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee KE, Davis MB, Winkelstein BA. Capsular ligament involvement in the development of mechanical hyperalgesia after facet joint loading: behavioral and inflammatory outcomes in a rodent model of pain. J. Neurotrauma. 2008;25:1383–1393. doi: 10.1089/neu.2008.0700. [DOI] [PubMed] [Google Scholar]
- 25.Pearson AM, Ivancic PC, Ito S, Panjabi MM. Facet joint kinematics and injury mechanisms during simulated whiplash. Spine (Phila Pa 1976) 2004;29:390–397. doi: 10.1097/01.brs.0000090836.50508.f7. [DOI] [PubMed] [Google Scholar]
- 26.Siegmund GP, Winkelstein BA, Ivancic PC, Svensson MY, Vasavada A. The anatomy and biomechanics of acute and chronic whiplash injury. Traffic Inj. Prev. 2009;10:101–112. doi: 10.1080/15389580802593269. [DOI] [PubMed] [Google Scholar]
- 27.Elkin BS, Elliott JM, Siegmund GP. Whiplash injury or concussion? A possible biomechanical explanation for concussion symptoms in some individuals following a rear-end collision. J. Orthop. Sports Phys. Ther. 2016;46:874–885. doi: 10.2519/jospt.2016.7049. [DOI] [PubMed] [Google Scholar]
- 28.Yao HD, Svensson MY, Nilsson H. Transient pressure changes in the vertebral canal during whiplash motion—a hydrodynamic modeling approach. J. Biomech. 2016;49:416–422. doi: 10.1016/j.jbiomech.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Landén Ludvigsson M, Peterson G, Peolsson A. The effect of three exercise approaches on health-related quality of life, and factors associated with its improvement in chronic whiplash-associated disorders: analysis of a randomized controlled trial. Qual. Life Res. 2019;28:357–368. doi: 10.1007/s11136-018-2004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schomacher J, Farina D, Lindstroem R, Falla D. Chronic trauma-induced neck pain impairs the neural control of the deep semispinalis cervicis muscle. Clin. Neurophysiol. 2012;123:1403–1408. doi: 10.1016/j.clinph.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 31.Elliott JM, O'Leary SP, Cagnie B, Durbridge G, Danneels L, Jull G. Craniocervical orientation affects muscle activation when exercising the cervical extensors in healthy subjects. Arch. Phys. Med. Rehabil. 2010;91:1418–1422. doi: 10.1016/j.apmr.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 32.O'Leary S, Cagnie B, Reeve A, Jull G, Elliott J. Is there altered activity of the extensor muscles in chronic mechanical neck pain? A functional magnetic resonance imaging study. Arch. Phys. Med. Rehabil. 2011;92:929–934. doi: 10.1016/j.apmr.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 33.Zheng L, Siegmund G, Ozyigit G, Vasavada A. Sex-specific prediction of neck muscle volumes. J. Biomech. 2013;46:899–904. doi: 10.1016/j.jbiomech.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crawford RJ, Volken T, Ni Mhuiris Á, et al. Geography of lumbar paravertebral muscle fatty infiltration: the influence of demographics, low back pain, and disability. Spine. 2019;44:1294–1302. doi: 10.1097/BRS.0000000000003060. [DOI] [PubMed] [Google Scholar]
- 35.Mhuiris ÁN, Volken T, Elliott JM, Hoggarth MA, Samartzis D, Crawford R. Reliability of quantifying the spatial distribution of fatty infiltration in lumbar paravertebral muscles using a new segmentation method for T1-weighted MRI. BMC Musculoskeletal. Disord. 2016;17:234. doi: 10.1186/s12891-016-1090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lord SM, Barnsley L, Wallis BJ, Bogduk N. Chronic cervical zygapophysial joint pain after whiplash. A placebo-controlled prevalence study. Spine (Phila Pa 1976) 1996;21:1737–1744. doi: 10.1097/00007632-199608010-00005. [DOI] [PubMed] [Google Scholar]
- 37.Panjabi MM, Cholewicki J, Nibu K, Babat LB, Dvorak J. Simulation of whiplash trauma using whole cervical spine specimens. Spine (Phila Pa 1976) 1998;23:17–24. doi: 10.1097/00007632-199801010-00005. [DOI] [PubMed] [Google Scholar]
- 38.Siegmund GP, Myers BS, Davis MB, Bohnet HF, Winkelstein BA. Mechanical evidence of cervical facet capsule injury during whiplash: a cadaveric study using combined shear, compression, and extension loading. Spine (Phila Pa 1976) 2001;26:2095–2101. doi: 10.1097/00007632-200110010-00010. [DOI] [PubMed] [Google Scholar]
- 39.Winkelstein BA, Nightingale RW, Richardson WJ, Myers BS. The cervical facet capsule and its role in whiplash injury: a biomechanical investigation. Spine (Phila Pa 1976) 2000;25:1238–1246. doi: 10.1097/00007632-200005150-00007. [DOI] [PubMed] [Google Scholar]
- 40.Smith AD, Jull G, Schneider GM, Frizzell B, Hooper RA, Sterling M. Modulation of cervical facet joint nociception and pain attenuates physical and psychological features of chronic whiplash: a prospective study. PM R. 2015;7:913–921. doi: 10.1016/j.pmrj.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 41.Lord SM, Barnsley L, Bogduk N. Percutaneous radiofrequency neurotomy in the treatment of cervical zygapophysial joint pain: a caution. Neurosurgery. 1995;36:732–739. doi: 10.1227/00006123-199504000-00014. [DOI] [PubMed] [Google Scholar]
- 42.Lord SM, Barnsley L, Wallis BJ, Bogduk N. Chronic cervical zygapophysial joint pain after whiplash. A placebo-controlled prevalence study. Spine. 1996;21:1737–1744. doi: 10.1097/00007632-199608010-00005. [DOI] [PubMed] [Google Scholar]
- 43.Lord SM, Barnsley L, Wallis BJ, McDonald GJ, Bogduk N. Percutaneous radio-frequency neurotomy for chronic cervical zygapophyseal-joint pain. N. Engl. J. Med. 1996;335:1721–1726. doi: 10.1056/NEJM199612053352302. [DOI] [PubMed] [Google Scholar]
- 44.Smith AD, Jull G, Schneider G, Frizzell B, Hooper RA, Sterling M. Cervical radiofrequency neurotomy reduces central hyperexcitability and improves neck movement in individuals with chronic whiplash. Pain Med. 2014;15:128–141. doi: 10.1111/pme.12262. [DOI] [PubMed] [Google Scholar]
- 45.Abbott RE, Parrish TB, Hoggarth MA, Smith AC, Elliott JM, Smuck M, Cristostomo RA, Demirjian R, et al. Morphologic change in the lumbar spine after lumbar medial branch radiofrequency neurotomy: a quantitative radiological study. Spine J. 2014;14:1088–1089. doi: 10.1016/j.spinee.2014.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peterson G, Nilsson D, Trygg J, Peolsson A. Neck-specific exercise improves impaired interactions between ventral neck muscles in chronic whiplash: a randomized controlled ultrasound study. Sci. Rep. 2018;8:9649. doi: 10.1038/s41598-018-27685-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Leary S, Jull G, Van Wyk L, Pedler A, Elliott J. Morphological changes in the cervical muscles of women with chronic whiplash can be modified with exercise—a pilot study. Muscle Nerve. 2015;52:772–779. doi: 10.1002/mus.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shahidi B, Fisch KM, Gibbons MC, Ward SR. Increased fibrogenic gene expression in multifidus muscles of patients with chronic versus acute lumbar spine pathology. Spine (Phila Pa 1976) 2020;45:E189–E195. doi: 10.1097/BRS.0000000000003243. [DOI] [PMC free article] [PubMed] [Google Scholar]