Abstract
A growing body of evidence indicates that opioids regulate mechanisms activated during the stress response. This study was aimed to investigate the effect of methadone dependency on blood glucose, lipids and glucose-modulating hormones in male and female Wistar rats.This study was performed on 40 Wistar rats weighing 150–350 g, in four methadone exposure and control groups of both males and females. All rats were weighed at the beginning and end of the study and their fasting blood glucose was measured using a glucometer. In order to induce addiction, methadone was injected intraperitoneal for 10 consecutive days at 5 mg/kg dose. The control group received the same volume of only normal saline. At the end of the study, the rats were sacrificed and their blood serum collected to measure cortisol, glucagon, adrenaline and lipid profile levels.There was a significant decrease in the mean final blood glucose of methadone-treated versus control male rats (p = 0.02). There was no significant glucose difference, however, in female rats. Furthermore, a decrease in the mean serum levels of triglyceride, cortisol, and adrenaline occurred in male rats of methadone-dependent compared with control animals, but there was no significant difference in these values in female rats. Our results showed that methadone significantly reduced serum glucose as well as triglyceride levels only in male rats, this being associated with a reduction in the level of counter-regulating hormones of carbohydrate metabolism. Changes in lipid profiles, however, occurred independently of gender.
Keywords: Methadone, Glucose-modulating hormones, Wistar rats, Blood glucose
Introduction
Drug abuse is an important and costly social and health problem that leads to several physical, mental and other medical consequences [1]. Based on the Iranian Drug Control Headquarters, 2.8 million 15–64 year-olds in 2015 were involved in illicit drug abuse. The United Nations’ Office on Drugs and Crime (UNODC) in 2013 estimated that of the 32.4 million adult opioid users in the world, 16.5 million were users of opiates (opium and its derivatives, specifically heroin) [2, 3].
Due to the increasing rate of heroin addiction and its derivatives in developing countries and the third world, particularly in western Asia, also broad range of problems associated with the high failure rate of opioid withdrawal, the government adopted a treatment and harm reduction policy. In this regard, Methadone Maintenance Treatment (MMT) is widely considered by many national drug prevention programs in the treatment of opioid dependence and prevent its harmful consequences to the individual user, his or her family, and society [4].
Methadone is a long-acting narcotic drug which has been used as an alternative treatment to prevent relapse in addicts to opium, heroin and other opiates. MMT also decreases the risk of transmission of infectious diseases such as HIV/AIDS, Hepatitis C virus (HCV) and Hepatitis B virus (HBV) infections and improves physical and psychosocial health of the patient [4, 5]. Although the MMT program has shown positive outcome, overdose and poisoning by accidental and intentional methadone use may lead to high morbidity and mortality [6]. Heroin addicts on MMT and patients who take methadone for cancer pain control may show unexpected hypoglycaemia [7–10].
In response to stress, many stress-related neurotransmitters are released, including endogenous opioids, as well as hypothalamic-hypophysial-adrenal (HPA) axis hormones, all involved in survival of the organism and affecting both the physiological and pharmacological modulators of blood glucose regulation. There is widespread opioid receptor distribution with varying densities throughout the central, peripheral and autonomic nervous systems and in several endocrine tissues. These lead to a broad range of functions and behaviors including regulation of pain, neuroendocrine modulation, behavior reinforcement and reward [11].
The hypothalamic-hypophysial-adrenal (HPA) axis includes corticotrophin releasing hormone (CRH), adrenocorticotropic hormone (ACTH) and cortisol, which synergize with catecholamines and other counter regulatory hormones for blood glucose regulation during the stress response [12]. The effect of opiates on blood glucose has been reported with contradicting results in different studies. Some studies have indicated stimulation of hyperglycemia in humans and rats [13, 14] whereas another showed hypoglycemia in mice [15]. Several important questions therefore remain regarding the mechanism of these effects.
Despite the high prevalence of methadone users in Iran, there are contradictory studies with respect to the effect of methadone on blood glucose. Since very few studies evaluated human and experimental rodent gender differences in blood glucose after methadone exposure, this study was aimed to investigate the effect of methadone on blood glucose, lipids and glucose-modulating hormones in methadone-dependent Wistar rats and evaluate any effect of gender differences in this regard.
Materials and methods
Animals
Forty Wistar rats (20 male, 20 female) weighing 150–350 g, were obtained from the Birjand University of Medical Sciences Research Center for Experimental Medicine. They were housed with a 12 h photoperiod at 25 °C with unlimited access to water and ad libitum food. The ethical committee of the Birjand University of Medical Sciences approved the study protocol with code number of IR.bums.REC.1396.240. After 7 days acclimatization to the laboratory conditions the rats were divided randomly into 4 groups: methadone addiction and the controls, each contained groups, 10 males and 10 females.
Chemicals
Methadone HCl in powder form was received from Faran Shimi Pharmaceutical Co., Iran, and methadone solution prepared by dissolving it in normal saline. Male and female rats were treated with methadone HCl solution for 10 consecutive days by intraperitoneal injection at the dose of 5 mg/kg for induction of addiction [16]. Control rats received only the same volume of normal saline. After the final methadone injection, 3 mg/kg naloxane HCl (Caspian Pharmaceutical Co., Iran) was injected intraperitoneally to one rat in each male and female addiction group for assessment of dependence [17]. Animals were placed in a perspex box and observed at 30 min for the presence or absence of behaviors indicating withdrawal sings consists of Counted signs and checked signs.
Counted signs Activity (Crossing of a quadrant mark) in male rat 3 times and in female rat 2 times during the first 10 min, Rearing (Lifting the forepaws off the ground) in male rat was repeated intermittently during 30 min (28 s during the first 10 min and 35 s during second 10 min and so…) and in female rat (23 s during the first 10 min and 30 s during second 10 min and so…), Teeth chattering (Teeth grinding) (in male rat 15 times during the last 10 min and in female rat 12 times during second 10 min) and Shake (Shaking of the head only or rest of body -wet-dog shake (20 times in male rat during the first 10 min and 17 times in female rat during the last 10 min) were observed in our study and checked sign: Abnormal posture (Lying on the side; writhing or hunching of body) in male rat 2 times and in female rat 3 times during the first 10 min.
It is necessary to mention that only one rat in each male and female methadone group were monitored for withdrawal signs to confirm the dependence on methadone in both groups male and female.
Experimental protocol
The animals in all groups were weighed at the beginning and end of the study and fasting blood sugar (FBS) determined by glucometer after 12 h of fasting.
Biochemical analysis
At the end of the treatment protocol blood samples were collected via cardiac puncture under general anesthesia with intraperitoneal injection of sodium thiopental at 20 mg/kg body mass. After separation, and by use of commercially available ELIZA kits, the serum cortisol (Rat Cortisol ELISA Kit, Hangzhou Eastbiopharm Co.,Ltd, USA), glucagon (Rat Glucagon (GC) ELISA Kit, Hangzhou Eastbiopharm Co.,Ltd, USA), adrenaline (Rat Adrenaline ELISA Kit, Hangzhou Eastbiopharm Co.,Ltd, USA), as well as for lipid profile (high density lipoprotein (HDL), low density lipoprotein (LDL), triglyceride, and total cholesterol) with Auto-analyzer Prestige 24i, Japan, were measured.
Statistical analysis
Data were reported as mean ± standard Error (SE). At first the normal distribution of the data was assessed through running the Kolmogrov–Smirnov test. One-way ANOVA and bonferroni post hoc was done among variables of methadone exposure and control groups of both male and female rats using SPSS version 16 statistical package and data were considered statistically significant if p < 0.05 for all statistical tests.
Results
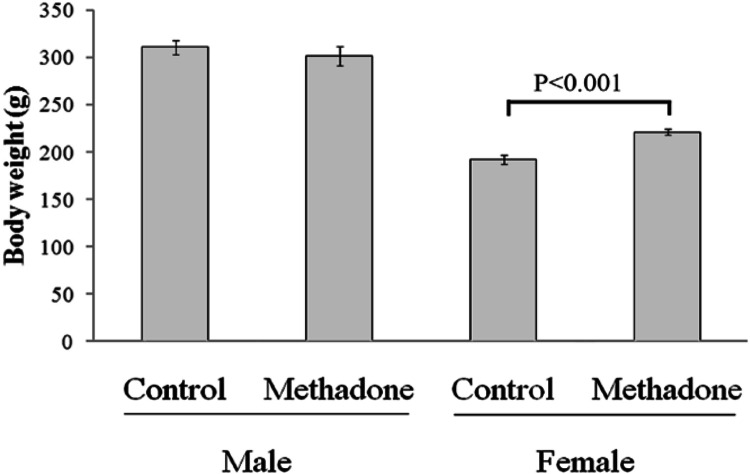
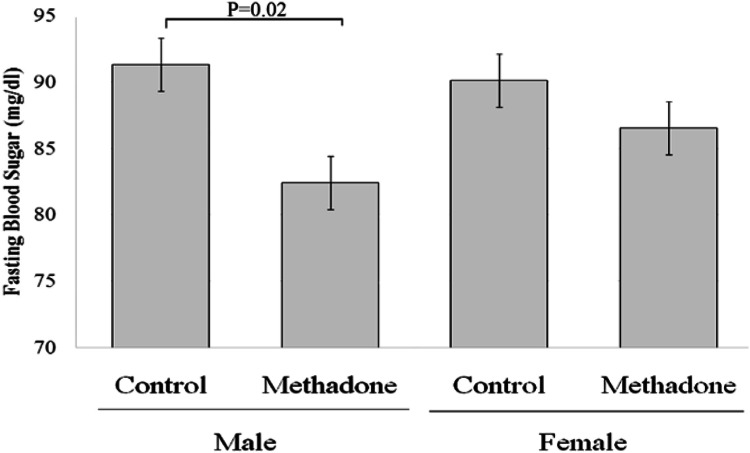
One-way ANOVA test showed that there was significant difference among four groups (p < 0.001). Bonferroni post hoc test indicated a significant increase in mean final mass of female rats but not male rats in the methadone receiving group versus the corresponding control group (Fig. 1). One-way ANOVA test cleared significant difference among four groups (P = 0.04). Bonferroni post hoc test demonstrated a significant decrease in the mean blood glucose level of methadone dependent male rats when compared with the control group but not in female rats (Fig. 2). Although blood glucose level did drop in the female methadone dependent rats as well, just it was not statistically significant. Similarly, a significant decrease in mean serum levels of cortisol and adrenaline was found in methadone dependent male rats when compared with the control group but not in female rats (Table 1).
Fig. 1.
Effect of methadone administration on final body mass in male and female Wistar rats when compared with their same sex control groups. Data are shown as mean ± SEM. p < 0.05 is significant
Fig. 2.
Effect of methadone administration on final blood glucose level in both female and male Wistar rats compared with their same-sex control groups. Data are shown as mean ± SEM. p < 0.05 is significant
Table 1.
Comparison of mean Adrenaline, Glucagon, Cortisol and Insulin in methadone treated and control group both female and male Wistar rats
| Variables | Groups | p value | |||
|---|---|---|---|---|---|
| Female rats (n = 10) | Male rats (n = 10) | ||||
| Methadone | Control | Methadone | Control | ||
| Adrenaline (ng/ml( | 30.2 ± 1.26 | 29.84 ± 1.8 | 25.76 ± 2.15 | 33.55 ± 2.58 | 0.03* |
| Glucagon (ng/ml) | 100.19 ± 5.06 | 112.03 ± 7.65 | 97.51 ± 11.57 | 126.51 ± 8.39 | 0.14 |
| Cortisol (ng/ml( | 85.42 ± 4.14 | 75.95 ± 3.52 | 69.97 ± 5.09 | 86.26 ± 4.41 | 0.02* |
| Insulin (mIU/L( | 5.41 ± 0.16 | 5.71 ± 0.36 | 4.84 ± 0.35 | 5.85 ± 0.44 | 0.24 |
Values are expressed as mean ± SD (One-way ANOVA and Bonferroni post hoc tests)
*p < 0.05 is significant
Table 2 shows a significant decrease in the mean of HDL (p ≤ 0.001), a very slight increase in LDL (p ≤ 0.001) between control groups and methadone treated groups in male and female rats. Also, there was a decrease in triglyceride in male rats of the methadone treatment versus the control group (p = 0.01), but this difference was not observed in mean of cholesterol serum level in male and female rats (p = 0.08) (Table 2). Bonferroni post hoc test showed that this difference in the mean serum level of HDL and LDL was observed between male and female methadone treated with controls (p ≤ 0.001; p = 0.001) and (p = 0.02; p ≤ 0.001) respectively.
Table 2.
Comparison of mean HDL, LDL, TG and total cholesterol in methadone treated and control group both female and male Wistar rats
| Variables | Groups | p value | |||
|---|---|---|---|---|---|
| Female rats (n = 10) | Male rats (n = 10) | ||||
| Methadone | Control | Methadone | Control | ||
| HDL (mg/dL) | 49.50 ± 2.47 | 55.90 ± 2.29 | 43.62 ± 0.94 | 50.20 ± 1.25 | ≤ 0.001* |
| LDL (mg/dL) | 17.30 ± 0.21 | 16.50 ± 0.17 | 18.75 ± 0.29 | 18.10 ± 0.23 | ≤ 0.001* |
| TG (mg/dL) | 32.00 ± 2.51 | 32.03 ± 2.82 | 32.00 ± 1.38 | 40.04 ± 2.05 | 0.01* |
| Total cholesterol (mg/dL) | 66.70 ± 3.25 | 66.10 ± 3.11 | 66.75 ± 2.71 | 59.10 ± 2.05 | 0.8 |
Values are expressed as mean ± SD (One-way ANOVA and Bonferroni post hoc tests)
HDL high-density lipoprotein, LDL low-density lipoprotein, TG triglyceride
*p < 0.05 is significant
Discussion
According to the findings in this experiment there was a significant decrease in mean final mass of female rats but not in male rats between methadone treated and control groups.
These data are consistent with the study by Fenn et al. in 2015 on 96 human patients enrolled in an outpatient methadone clinic [18] and in contrast to the study by Kolarzyk et al. in 2005 who examined body weight in 30 Polish methadone-treated patients [19].
Although opiate dependent patients, especially heroin addicts, have unbalanced diets, malnutrition, loss of appetite and nutrient deficiencies, some studies have shown MMT is effective in improvement of nutritional habits, lifestyle and several hormonal disorders. Hormones derived from adipose tissue interconnect nutritional status with the immune system and metabolic regulation [20, 21]. Therefore, the increased weight of female rats in our study may be attributed to the MMT treatment effect on adipocytokines including leptin, which exhibits sexual dimorphism. The leptin level in humans is gender dependent [22]. In addition, opioids have a greater role in rewarding aspects of food intake and in rodents extensive research on opioids indicated that sweet and high fat diets were most preferred [23].
Our study showed a significant difference in the mean glucose level of male rats but not in females between methadone consumption and control groups; although glucose did drop in the female rats it was not found to be statistically significant. This finding supports the results of a study by Faskowitz et al. in 2013 on CD1 mice that received methadone and other opioids subcutaneously [9]. Our findings however, are inconsistent with the previous study by Sadava et al. in 1997 on female albino rats that received methadone dissolved in their daily water intake [24].
Although previous studies have shown that opiates are involved in glucose homeostasis, the exact mechanisms are still unclear. There are many complex effects of opioids on the various hormones of the endocrine system in man and animals, often differing depending on whether exposure is acute or chronic [12]. Based on our results a possible explanation of methadone-associated hypoglycemia may be suppression of counter regulatory mechanisms including adrenaline and cortisol but not glucagon. Additionally, the effect of counter regulatory hormones on blood glucose is time and hormone dependent. Other variables such as route of injection and dosage can also play a role in the different reported results [12, 14].
It was notable in our study that fasting blood glucose levels in methadone addicted Wistar rats was gender dependent, with males exhibiting a statistically significant decrease; females also underwent a drop in blood glucose but it was not statistically significant.
A recent study in rats [25] demonstrated gender difference in regional brain glucose metabolism following methadone administration that may be a candidate explanation for the findings of our study. Male animals treated with methadone had increased caudate/putamen glucose metabolism whereas female rats exhibited increased cingulate cortex metabolism. Overally, they indicated that the response to hypoglycemia in the two sexes is due to the alteration in mechanisms existing in the central nervous system (CNS) and in our study we showed it peripherally as alterations in counter-regulatory hormone levels in male and female rats’ blood. Thus, it seems that both central and peripheral mechanisms may be involved in the different pathogeneses of hypoglycemia in methadone addicted male and female rats. These gender specific differences, if extrapolated to humans, should therefore be considered in treatment strategies for reducing numbers of human clinical treatment failures [25].
The present study showed a significant difference in mean HDL and LDL level in both male and female rats between methadone consumption and control groups. This finding is in accordance with the study by Lin et al. in 2012 on 70 heroin users [26], but contrary to the findings of Montazerifar et al. in 2014 on 25 patients before and after MMT when compared with a control group [27].
In recent years our perception of adipose tissue has been significantly transformed due to the discovery that besides being a fat reservoir, it is an endocrine organ secreting many different metabolically active compounds, including leptin, adiponectin, resistin and etc. [28]. A previous study in heroin addicts reported that MMT treatment is accompanied by changes in the level of adipose tissue derived hormones such as leptin and adiponectin [29]. A low level of adiponectin is associated with a high level of LDL and triglycerides and adiponectin is positively correlated with HDL concentration [30]. Therefore hypoadiponectinemia may be a possible explanation for our results in Wistar rats.
The present study has a number of strengths. To our knowledge, reports regarding the association of methadone and glucose regulation in both genders in human clinical and preclinical studies are scarce. Although there are species differences in the effects of opioids on the endocrine system, specifically on those hormones regulating blood glucose levels, as well as many variations in response depending on factors including route, time and dose of administration [12], the present study was designed to evaluate the methadone effect on blood glucose in male and female Wistar rats.
Our study was limited partly due to lack of funding, and by us not being able to measure testosterone, estradiol, follicular stimulating hormone (FSH) and luteinizing hormone (LH).
Methadone maintenance treatment (MMT) in Iran is widely used for opioid dependence and also as an effective medication for severe pain such as in cancer. Statistics in Iran, however, shows a high rate of unsuccessful opioid withdrawal in outpatient clinics across the country. Our findings showed that blood glucose concentration alteration appeared statistically different between male and female methadone dependent rats; hence addressing this in humans, which are known to have a similar response, should be considered as a possible approach for reducing clinical treatment failures. Further research is necessary to elucidate the involved mechanisms.
Acknowledgements
The authors would like to thank the Vice Chancellor for the Research and Technology of the Birjand University of Medical Sciences for funding this study (the research Project No. 455381.)
Abbreviations
- ACTH
Adrenocorticotropic hormone
- CNS
Central nervous system
- CRH
Corticotrophin releasing hormone
- FSH
Follicular stimulating hormone
- HCV
Hepatitis C virus
- HBV
Hepatitis B virus
- HPA
Hypothalamic-hypophysial-adrenal
- HDL
High density lipoprotein
- LDL
Low density lipoprotein
- LH
Luteinizing hormone
- MMT
Methadone maintenance treatment
- SD
Standard deviation
- UNODC
The United Nations’ Office on Drugs and Crime
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest to disclose.
Contributor Information
Zoya Tahergorabi, Email: z.tahergorabi@yahoo.com.
Hadiseh Rahmani, Email: hadisra92@gmail.com.
References
- 1.Chen C-Y, Lin K-M. Health consequences of illegal drug use. Curr Opin Psychiatry. 2009;22:287–292. doi: 10.1097/YCO.0b013e32832a2349. [DOI] [PubMed] [Google Scholar]
- 2.Ghane T, Zamani N, Hassanian-Moghaddam H, Beyrami A, Noroozi A. Lead poisoning outbreak among opium users in the Islamic Republic of Iran, 2016–2017. Bull World Health Organ. 2018;96:165. doi: 10.2471/BLT.17.196287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Najafipour H, Beik A. The impact of opium consumption on blood glucose, serum lipids and blood pressure, and related mechanisms. Front Physiol. 2016;7:436. doi: 10.3389/fphys.2016.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bozchelou S, Delirrad M. Effects of methadone maintenance therapy on thyroid function of adult men. Toxicol Res. 2019;35:9–12. doi: 10.5487/TR.2019.35.1.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heimer R, Bray S, Burris S, Khoshnood K, Blankenship KM. Structural interventions to improve opiate maintenance. Int J Drug Policy. 2002;13:101–111. doi: 10.1016/S0955-3959(02)00009-9. [DOI] [Google Scholar]
- 6.Soltaninejad K, Hassanian-Moghaddam H, Shadnia S. Methadone related poisoning on the rise in Tehran. Iran. Asia Pac J Med Toxicol. 2014;3:104–109. [Google Scholar]
- 7.Flory JH, Wiesenthal AC, Thaler HT, Koranteng L, Moryl N. Methadone use and the risk of hypoglycemia for inpatients with cancer pain. J Pain Symptom Manag. 2016;51:79–87. doi: 10.1016/j.jpainsymman.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toce MS, Stefater MA, Breault DT, Burns MM. A case report of methadone-associated hypoglycemia in an 11-month-old male. Clin Toxicol. 2018;56:74–76. doi: 10.1080/15563650.2017.1338347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faskowitz AJ, Kramskiy VN, Pasternak GW. Methadone-induced hypoglycemia. Cell Mol Neurobiol. 2013;33:537–542. doi: 10.1007/s10571-013-9919-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moryl N, Pope J, Obbens E. Hypoglycemia during rapid methadone dose escalation. J Opioid Manag. 2013;9:29–34. doi: 10.5055/jom.2013.0144. [DOI] [PubMed] [Google Scholar]
- 11.Droletm G, Dumont EC, Gosselin I, Kinkead R, Laforest S, Trottier JF. Role of endogenous opioid system in the regulation of the stress response. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:729–741. doi: 10.1016/S0278-5846(01)00161-0. [DOI] [PubMed] [Google Scholar]
- 12.Vuong C, Van Uum SH, Odell LE, Lutfy K, Friedman TC. The effects of opioids and opioid analogs on animal and human endocrine systems. Endocr Rev. 2009;31:98–132. doi: 10.1210/er.2009-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karam GA, Reisi M, Kaseb AA, Khaksari M, Mohammadi A, Mahmoodi M. Effects of opium addiction on some serum factors in addicts with non-insulin-dependent diabetes mellitus. Addict Biol. 2004;9:53–58. doi: 10.1080/13556210410001674095. [DOI] [PubMed] [Google Scholar]
- 14.Karam GA, Rashidinejad HR, Aghaee MM, Ahmadi J, Rahmani MR, Mahmoodi M, Azin H, Mirzaee MR, Khaksari M. Opium can differently alter blood glucose, sodium and potassium in male and female rats. Pak J Pharm Sci. 2008;21:180–184. [PubMed] [Google Scholar]
- 15.Park SH, Sim YB, Kang YJ, Kim SM, Lee JK, Jung JS, Suh HW. Characterization of blood glucose level regulation in mouse opioid withdrawal models. Neurosci Lett. 2010;476:119–122. doi: 10.1016/j.neulet.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Pačesová D, Novotný J, Bendová Z. The effect of chronic morphine or methadone exposure and withdrawal on clock gene expression in the rat suprachiasmatic nucleus and AA-NAT activity in the pineal gland. Physiol Res. 2016;65:517–525. doi: 10.33549/physiolres.933183. [DOI] [PubMed] [Google Scholar]
- 17.Pierce T, Hope W, Raper C. The induction and quantitation of methadone dependence in the rat. J Pharmacol Toxicol Methods. 1996;36:137–146. doi: 10.1016/S1056-8719(96)00108-6. [DOI] [PubMed] [Google Scholar]
- 18.Fenn JM, Laurent JS, Sigmon SC. Increases in body mass index following initiation of methadone treatment. J Subst Abuse Treat. 2015;51:59–63. doi: 10.1016/j.jsat.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolarzyk E, Pach D, Wojtowicz B, Szpanowska-Wohn A, Szurkowska M. Nutritional status of the opiate dependent persons after 4 years of methadone maintenance treatment. Przeglad lekarski. 2005;62:373–377. [PubMed] [Google Scholar]
- 20.Kheradmand A, Banazadeh N, Abedi H. Physical effects of methadone maintenance treatment from the standpoint of clients. Addict Health. 2010;2:66–73. [PMC free article] [PubMed] [Google Scholar]
- 21.Kolarzyk E, Chrostek Maj J, Pach D, Janik A, Kwiatkowski J, Szurkowska M. Assessment of daily nutrition ratios of opiate-dependent persons before and after 4 years of methadone maintenance treatment. Przegl Lek. 2005;62:368–372. [PubMed] [Google Scholar]
- 22.Noor S, Alam F, Fatima SS, Khan M, Rehman R. Role of Leptin and dyslipidemia in chronic kidney disease. Pak J Pharm Sci. 2018;31:893. [PubMed] [Google Scholar]
- 23.Olszewski PK, Levine AS. Central opioids and consumption of sweet tastants: when reward outweighs homeostasis. Physiol Behav. 2007;91:506–512. doi: 10.1016/j.physbeh.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Sadava D, Alonso D, Hong H, Pettit-Barrett D. Effect of methadone addiction on glucose metabolism in rats. Gen Pharm Vasc Syst. 1997;28:27–29. doi: 10.1016/S0306-3623(96)00165-6. [DOI] [PubMed] [Google Scholar]
- 25.Santoro GC, Carrion J, Patel K, Vilchez C, Veith J, Brodie JD, Dewey SL. Sex differences in regional brain glucose metabolism following opioid withdrawal and replacement. Neuropsychopharmacol. 2017;42:1841–1849. doi: 10.1038/npp.2017.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin SH, Yang YK, Lee SY, Hsieh PC, Chen PS, Lu RB, Chen KC. Association between cholesterol plasma levels and craving among heroin users. J Addict Med. 2012;6:287–291. doi: 10.1097/ADM.0b013e318262a9a1. [DOI] [PubMed] [Google Scholar]
- 27.Montazerifar F, Karajibani M, Lashkaripour K, Yousefi M. Effects of methadone maintenance therapy (MMT) on serum leptin, lipid profile and anthropometric parameters in opioid addicts. Heroin Addict Relat Clin Probl. 2014;16(1):9–16. [Google Scholar]
- 28.Berndt J, Klöting N, Kralisch S, Kovacs P, Fasshauer M, Schön M. Plasma visfatiDn concentrations and fat depot-specific mRNA expression in humans. Diabetes. 2005;54:2911–2916. doi: 10.2337/diabetes.54.10.2911. [DOI] [PubMed] [Google Scholar]
- 29.Housova J, Wilczek H, Haluzik MM, Kremen J, Krizova J, Haluzik M. Adipocyte-derived hormones in heroin addicts: the influence of methadone maintenance treatment. Physiol Res. 2005;54:73–78. doi: 10.33549/physiolres.930568. [DOI] [PubMed] [Google Scholar]
- 30.Tschritter O, Fritsche A, Thamer C, Haap M, Shirkavand F, Rahe S, Staiger H, Maerker E, Häring H, Stumvoll M. Plasma adiponectin concentrations predict insulin sensitivity of both glucose and lipid metabolism. Diabetes. 2003;52:239–243. doi: 10.2337/diabetes.52.2.239. [DOI] [PubMed] [Google Scholar]