Abstract
Melastoma malabathricum is a well-known herb in Malaysia where it being used in various ways for treatment of different diseases and ailments including skin problems. The study aims to investigate acute and subacute dermal toxicity of ethanolic extract of M. malabathricum leaves following to a single or repeated doses exposure. A total of 30 female Sprague-Dawley rats were grouped into 5 groups (n = 6 per group) for both acute and subacute toxicity study. The duration for each study was determined at 14 days for acute toxicity and 28 days for subacute toxicity. The rats were topically applied with the plant extract at three different doses; 2.5%, 5.0% and 10.0% on the shaved area of dorsal skin. For acute toxicity study, rats in all three groups received single application of the extract on the first day of the experimental period, while rats in subacute toxicity study were topically applied with the extract once daily for 28 days. Throughout the respective 14-day and 28-day study periods, all rats were monitored for any changes in their physical appearance and behavioural patterns that might develop due to toxic effects of the plant. There were no mortality or abnormal physical appearance, and physiological and behavioural changes observed in all rats in both studies. Body weights, kidney and liver weights, and both haematology and serum biochemistry results showed no significant (p > 0.05) differences between all groups in both studies. All of the findings were supported by normal macroscopic and microscopic architectures of liver, kidneys and skin of all rats applied topically with the extract. This study suggests that topical application of M. malabathricum leaf ethanolic extract at 2.5%, 5% and 10% does not induce acute and subacute adverse effects on the skin or systemic toxic reactions in rats.
Keywords: Dermal toxicity, Haematology, Kidney parameters, Liver enzymes, Histology
Introduction
Melastoma malabathricum Linn is one of the plants within the Melastomataceae family that has gained popularity as a folk remedy among Malayo-Polynesians. The plant comprises of two subspecies, namely M. malabathricum L. sp. malabathricum and M. malabathricum Linn sp. normale [1]. Its leaf is used topically for treatment of skin ulcer, scar, pimple and black spot [2], while combination of the leaves and flowers is used orally for treatment of diarrhoea, prolonged fever, dysentery and complicated skin problems [3]. For both methods of treatment application, evaluation of acute and subacute toxicity effects of the plant is important to make sure its safety before it could be safely used for any medicinal purposes. The OECD guideline No. 402 [4] defines acute dermal toxicity as adverse effects observed within a short period after dermal application of a single-dose of a test substance. For subacute dermal toxicity, the OECD guideline No. 410 [5] considers a test substance is toxic when potential health hazards are likely to arise from repeated exposures to the test substance by the dermal route over a limited period. For both studies, observation for any physical and behavioural changes of the experimental animals are crucial, while evaluation of blood parameters alterations and histological examination of skin and selected vital organs are important particularly for subacute toxicity study; where assessment of toxic compounds present in the plant products applied on the skin for a long period could be thoroughly evaluated [4, 5]. A few studies reported certain herbal plants for examples kava, aloe vera, eucalyptus, camphor, henna, yohimbine (reviewed by Ernst [6]) and Pistacia lentiscus fruit fatty oil [7] induce allergic dermatitis [6, 7] and granulomatous dermatitis [7]. Hence, the present study was conducted to evaluate dermal toxicity of M. malabathricum leaf ethanolic extract in rats, which could be later used to establish a safe and optimal dose to treat skin problems.
Materials and methods
Animal management
A total number of 60 female Sprague-Dawley rats weighing between 180 and 220 g were used in this study. Upon arrival, the rats were weighed and randomly assigned in polypropylene plastic cages. All rats were placed in single cages, which were placed in the same animal room with controlled conditions; temperature (22 ± 2 °C), humidity (55 ± 10%) and lighting (12 h light/dark) at the Animal Metabolism, Toxicity and Reproductive Centre (AMTREC), Malaysian Agricultural and Research Development Institute (MARDI), Serdang, Selangor. The rats were provided with tap water and fed ad libitum with commercial chow. The rats were acclimatised for 2 weeks before the study was conducted. The experimental protocol was approved by Animal Ethic Committee (AEC), MARDI.
Experimental design
Rats in both acute and subacute toxicity studies were divided into 5 groups (n = 6), namely control, vehicle (applied topically with 20% white soft paraffin), 2.5%, 5.0% and 10.0% herbal extract (applied topically with different concentrations of herbal extract).
Plant extraction
Melastoma malabathricum leaves (Fig. 1) were obtained from MARDI, Muadzam Shah, Pahang. The leaves were separated from the twigs and dried at room temperature between 25 °C and 30 °C for three days. The dried leaves were ground into powder and powdered leaves were macerated with ethanol in a flask. The mixture was left in a water bath at temperature between 55 °C and 60 °C for 1 day to allow the chemical compounds in the leaves dissolved in the ethanol solution at the ratio of 0.20 g sample to 400 mL of 100% ethanol (Sigma-Aldrich, USA). The ethanol was isolated from the mixture by filtration followed by evaporation using a rotary evaporator (R-215, Buchi Rotavaporator, Switzerland). The crude extract with extraction yield of 10% were kept in a refrigerator for later used. The leaf extract was prepared at 2.5%, 5.0% and 10.0% (% w/w) and mixed with white soft paraffin.
Fig. 1.

Melastoma malabathricum leaves
Skin preparation for dermal toxicity study
Skin preparation was conducted under general anaesthesia. The rats were anaesthetised with a mixture of ketamine (50 mg/kg) and xylazine (5 mg/kg) injected intramuscularly. Furs at the dorsal thoracic region were clipped with an electric clipper and shaved using a razor blade. The shaved area (Fig. 2) was applied either with herbal extract, vehicle (white paraffin) or no treatment.
Fig. 2.
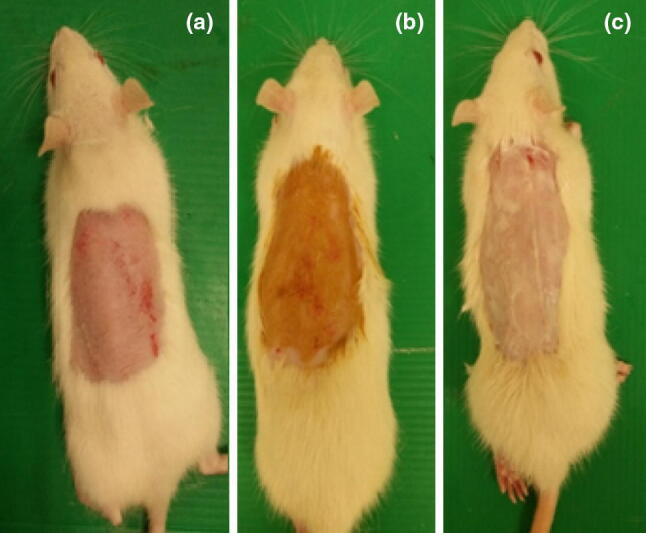
Clipped area of skin of a negative control rat (a), applied with herbal extract (b) and white soft paraffin (c)
Dosage preparation and mode of treatment
For each treatment group, approximately 3.0 g of herbal extract was applied topically on the shaved skin. For acute dermal toxicity study, the extract was applied only once on the first day of experiment, while rats in subacute dermal toxicity study received the treatment daily for 28 days.
General assessment on general signs and behavioural of rats
All rats were monitored daily for mortality, changes in their fur, eyes, mucous membranes, and behavioural (salivation, tremors, convulsions, diarrhoea and lethargy) and breathing patterns. Body weights of the rats were recorded weekly.
Euthanasia and necropsy
At the end of each experiment, all rats were humanely euthanised by using carbon dioxide inhalation method. Complete gross examination was conducted and any gross changes on the skin were recorded. The abdominal cavity was explored by performing a cut at the caudal part of the abdominal wall, followed by exposure of posterior vena cava by mobilisation of the abdominal viscera out from the abdominal cavity. Blood samples were then withdrawn from the posterior vena cava from each necropsied rat and preserved in ethylene diamine tetra acetic acid (EDTA) blood tubes for haematology analysis. Blood samples were also collected into blood tubes contain no anticoagulant for serum biochemical analysis. Sera were separated once the blood samples were allowed to clot for a minimum of 30 min at room temperature followed by centrifugation (Hettichzent-EBA20, Germany) at 5000 rpm for 5 min. The samples were then kept in a − 20 °C freezer until further analysis. Gross examination of vital organs was then performed, followed by excision of whole liver and kidneys out of the carcasses. Both organs were blotted dry, weighed and their relative weights recorded. Both organs and shaved and/or treated skin were then preserved in 10% buffered formalin for 48 h until further process and analysis.
Haematological and serum biochemical analysis
Haematology analysis was conducted using an automatic haematology analyser (Cell-Dyn®, 3700, Abbot, USA). Total numbers of red blood cell (RBC), white blood cell (WBC) and platelet, and concentration of haemoglobin were obtained for the analyser, while packed cell volume (PCV), icteric index and plasma protein values were manually determined using standard methods. The percentage of each WBC type was also determined manually from the blood smear that was stained using Wright’s stain, followed by determination of the absolute number of each WBC type by multiplying the percentage value with the total WBC number.
Biochemical analysis were done using a fully automated biochemistry analyser (TRX 7070, Biorex, Germany). A few parameters include urea, creatinine, alanine transaminase (ALT), alkaline phosphatase (ALP), aspartate transaminase (AST), creatine kinase (CK), total protein and albumin were selected. Globulin concentration was estimated by subtracting albumin concentration from total protein.
Formalin fixed samples preparation and histopathology examination
Formalin fixed skin, liver and kidney tissues were sliced to 0.5 cm thickness and placed in plastic cassettes. The tissues were processed automatically using an automated processor (Leica ASP300, Germany) followed by embedding with paraffin, trimming and sectioning at 4 µm thickness using a rotary microtome (Leica RM 2155, Germany). The tissue sections were mounted on glass slides, placed on a hot plate (Leica H11220, Germany) at 42 °C and allowed to dry for overnight. Subsequently, the tissue sections were deparaffinised using xylene for 5 min and rehydrated by two changes of different ethanol dilutions (100% and 80%) for 5 min each. The tissue sections were rinsed in tap water and stained with Haematoxylin and Eosin (H&E) staining. Lesions in all tissues were scored as 0 (no lesion observed), 1 (mild = 1–30%), 2 (moderate = 31–69%) and 3 (severe = 70–100%) [8].
Statistical analysis
Body weight, organ weight, haematology and serum biochemistry data were statistically analysed using Statistical Package for Social Science (SPSS) software version 23. The values were expressed as mean ± standard error mean (SEM). Analysis of variance (ANOVA) was done to compare the differences of data between and within groups. Post hoc analysis using Duncan test was used to determine the level of statistical significance which was set at p < 0.05. Histopathology scoring results were also expressed as mean ± SEM. Global comparison of histopathology lesions in all groups was analysed using Kruskal–Wallis test, and comparison of histopathology lesions between two groups was analysed using Mann–Whitney U test.
Results
General sign and behaviour of rats
During the 24 h observation period, all rats in both studies did not display any significant changes or impairment in their behaviour, breathing pattern, posture, skin, fur, feed intake and water consumption. Mortality and toxic effects were not observed in all rats for both studies throughout the study periods.
Body and relative organ weight
Body weights (Figs. 3, 4) and relative organ weights (Table 1) of treated rats in both studies were not significantly different (p > 0.05) compared to controls.
Fig. 3.
Weekly mean body weights (g) (mean ± SEM) of rats in all groups in acute dermal toxicity study of M. malabathricum leaf ethanolic extract. G1: M. malabathricum (2.5%), G2: M. malabathricum (5%), G3: M. malabathricum (10%), G4: negative control, G5: paraffin
Fig. 4.

Weekly mean body weights (g) of rats in all groups in sub-acute dermal toxicity study of M. malabathricum leaf ethanolic extract. G1: M. malabathricum (2.5%), G2: M. malabathricum (5%), G3: M. malabathricum (10%), G4: negative control, G5: paraffin. All values are not statistically significant (p > 0.05) between treated groups and negative control group
Table 1.
Relative organ weights of rats in dermal toxicity study of M. malabathricum leaf ethanolic extract
| Group | Liver (g) | Kidneys (g) | Spleen (g) |
|---|---|---|---|
| Acute toxicity study | |||
| G1 | 3.42 ± 0.06a | 0.83 ± 0.02a | 0.25 ± 0.02a |
| G2 | 3.33 ± 0.04a | 0.82 ± 0.01a | 0.26 ± 0.02a |
| G3 | 3.35 ± 0.04a | 0.85 ± 0.03a | 0.25 ± 0.01a |
| G4 | 3.47 ± 0.09a | 0.85 ± 0.02a | 0.25 ± 0.02a |
| G5 | 3.44 ± 0.06a | 0.88 ± 0.02a | 0.27 ± 0.01a |
| Subacute toxicity study | |||
| G1 | 3.32 ± 0.05a | 0.85 ± 0.02a | 0.26 ± 0.01a |
| G2 | 3.39 ± 0.06a | 0.87 ± 0.01a | 0.27 ± 0.01a |
| G3 | 3.35 ± 0.06a | 0.85 ± 0.01a | 0.26 ± 0.01a |
| G4 | 3.73 ± 0.07a | 0.82 ± 0.03a | 0.24 ± 0.02a |
| G5 | 3.73 ± 0.07a | 0.80 ± 0.02a | 0.23 ± 0.01a |
Values in the same column with similar superscripts are not significantly different at p > 0.05
G1: M. malabathricum (2.5%), G2: M. malabathricum (5%), G3: M. malabathricum (10%), G4: negative control, G5: paraffin
Haematology and serum biochemistry
Similarly, there were no significant changes (p > 0.05) observed in the erythron, thrombon, leukon and plasma protein values of all treated rats in both studies compared to controls (Tables 2, 3). Kidney parameters, liver and muscle enzymes, and total serum protein concentration of all treated rats were also showed no significant different (p > 0.05) compared to controls (Table 4). All haematology and serum biochemical results of all rats in both studies were indeed fall within normal ranges.
Table 2.
The values of erythron, thrombon parameters and plasma protein concentration (mean ± SEM) of rats in dermal toxicity studies of M. malabathricum leaf ethanolic extract
| Parameter | G1 | G2 | G3 | G4 | G5 |
|---|---|---|---|---|---|
| Acute toxicity study | |||||
| RBC (× 1012/L) | 7.54 ± 0.18a | 7.38 ± 0.32a | 7.39 ± 0.45a | 7.76 ± 0.40a | 7.64 ± 0.41a |
| Hb (g/L) | 150.2 ± 1.68a | 156.03 ± 1.83a | 150.54 ± 1.37a | 154.1 ± 3.20a | 156.36 ± 2.06a |
| PCV (L/L) | 42.17 ± 0.72a | 42.18 ± 0.90a | 41.74 ± 0.79a | 39.0 ± 1.40a | 42.16 ± 1.38a |
| PP (g/L) | 74.54 ± 1.18a | 76.47 ± 0.61a | 74.61 ± 1.65a | 72.80 ± 1.62a | 74.73 ± 0.81a |
| MCV (fL) | 63.14 ± 1.49a | 61.15 ± 0.96a | 62.70 ± 0.80a | 62.87 ± 1.05a | 63.54 ± 0.61a |
| MCHC (g/L) | 302.26 ± 10.14a | 301.07 ± 12.30a | 307.35 ± 8.34a | 341.23 ± 4.34a | 308.2 ± 5.77a |
| Icterus Index (Unit) | 2.00 ± 0.00a | 2.00 ± 0.00a | 2.00 ± 0.00a | 2.00 ± 0.00a | 2.00 ± 0.00a |
| Platelets (109/L) | 1432.49 ± 61.77a | 1416.48 ± 32.38a | 1489.87 ± 68.37a | 1414.44 ± 45.07a | 1450.98 ± 17.03a |
| Subacute toxicity study | |||||
| RBC (× 1012/L) | 7.51 ± 0.18a | 7.61 ± 0.32a a | 7.70 ± 0.42a | 7.23 ± 0.15a | 7.45 ± 0.08a |
| Hb (g/L) | 152.71 ± 2.00a | 151.91 ± 1.43a | 149.27 ± 2.59a | 145.29 ± 1.97a | 146.45 ± 2.31a |
| PCV (L/L) | 40.77 ± 0.73a | 41.57 ± 2.22a | 40.67 ± 1.45a | 43.0 ± 1.47a | 44.11 ± 0.92a |
| PP (g/L) | 73.12 ± 1.32a | 73.72 ± 0.71a | 72.18 ± 1.12a | 60.60 ± 15.21a | 76.29 ± 2.85a |
| MCV (fL) | 63.95 ± 0.39a | 63.80 ± 0.37a | 63.55 ± 0.75a | 60.76 ± 1.22a | 59.87 ± 0.79a |
| MCHC (g/L) | 312.36 ± 11.87a | 304.89 ± 4.08 | 301.20 ± 7.88a | 326.80 ± 4.63a | 325.80 ± 1.53a |
| Icterus Index (Unit) | 2.00 ± 0.00a | 2.00 ± 0.00a | 2.00 ± 0.00a | 2.00 ± 0.00a | 2.00 ± 0.00a |
| Platelets (109/L) | 1432.60 ± 22.23a | 1407.54 ± 62.72a | 1481.83 ± 48.34a | 1319.17 ± 134.46a | 1478.91 ± 67.76a |
Values in the same row with similar superscripts are not significantly different at p > 0.05
G1: M. malabathricum (2.5%), G2: M. malabathricum (5%), G3: M. malabathricum (10%), G4: negative control, G5: paraffin
Table 3.
The values of leukon parameters (mean ± SEM) of rats in dermal toxicity study of M. malabathricum leaf ethanolic extract
| Parameter | G1 | G2 | G3 | G4 | G5 |
|---|---|---|---|---|---|
| Acute toxicity study | |||||
| WBC (× 109/L) | 6.58 ± 0.24a | 6.69 ± 0.44a | 6.80 ± 0.30a | 6.94 ± 0.51a | 6.84 ± 0.45a |
| Neutrophils (× 109/L) | 1.27 ± 0.09a | 1.33 ± 0.07a | 1.23 ± 0.10a | 1.24 ± 0.12a | 1.27 ± 0.16a |
| Lymphocytes (× 109/L) | 4.60 ± 0.20a | 4.63 ± 0.36a | 4.88 ± 0.22a | 4.93 ± 0.31a | 5.02 ± 0.38a |
| Monocyctes (× 109/L) | 0.49 ± 0.03a | 0.46 ± 0.03a | 0.44 ± 0.03a | 0.42 ± 0.05a | 0.47 ± 0.07a |
| Eosinophils (× 109/L) | 0.19 ± 0.04a | 0.15 ± 0.02a | 0.17 ± 0.07a | 0.14 ± 0.01a | 0.15 ± 0.02a |
| Basophils (× 109/L) | 0.08 ± 0.03a | 0.08 ± 0.02a | 0.08 ± 0.02a | 0.06 ± 0.02a | 0.07 ± 0.02a |
| Subacute toxicity study | |||||
| WBC (× 109/L) | 6.51 ± 0.55a | 6.25 ± 0.17a | 6.60 ± 0.37a | 6.70 ± 0.92a | 6.87 ± 0.33a |
| Neutrophils (× 109/L) | 1.22 ± 0.08a | 1.15 ± 0.08a | 1.22 ± 0.07a | 1.17 ± 0.09a | 1.09 ± 0.07a |
| Lymphocytes (× 109/L) | 4.47 ± 0.46a | 4.40 ± 0.09a | 4.53 ± 0.31a | 4.70 ± 0.61a | 4.95 ± 0.11a |
| Monocyctes (× 109/L) | 0.50 ± 0.04a | 0.44 ± 0.04a | 0.42 ± 0.04a | 0.43 ± 0.04a | 0.43 ± 0.05a |
| Eosinophils (× 109/L) | 0.14 ± 0.04a | 0.20 ± 0.02a | 0.12 ± 0.02a | 0.14 ± 0.02a | 0.15 ± 0.01a |
| Basophils (× 109/L) | 0.10 ± 0.01a | 0.10 ± 0.02a | 0.10 ± 0.02a | 0.07 ± 0.01a | 0.07 ± 0.00a |
Values in the same row with similar superscripts are not significantly different at p > 0.05
G1: M. malabathricum (2.5%), G2: M. malabathricum (5%), G3: M. malabathricum (10%), G4: negative control, G5: paraffin
Table 4.
Serum biochemical parameters (mean ± SEM) of rats in dermal toxicity study of M. malabathricum leaf ethanolic extract
| Parameter | G1 | G2 | G3 | G4 | G5 |
|---|---|---|---|---|---|
| Acute toxicity study | |||||
| Urea (mmol/L) | 6.0 ± 0.11a | 5.92 ± 0.19a | 6.14 ± 0.44a | 6.02 ± 0.25a | 5.90 ± 0.26a |
| Creatinine (µmol/L) | 63.20 ± 1.15a | 60.93 ± 0.45a | 61.50 ± 1.04a | 63.0 ± 1.92a | 63.0 ± 2.78a |
| ALT (U/L) | 71.33 ± 0.95a | 71.64 ± 1.56a | 71.10 ± 1.61a | 72.00 ± 7.76a | 73.48 ± 4.79a |
| ALP (U/L) | 207.12 ± 10.96a | 207.54 ± 10.82a | 222.29 ± 12.52a | 214.0 ± 22.26a | 226.40 ± 13.32a |
| AST (U/L) | 173.32 ± 2.79a | 177.11 ± 2.83a | 181.74 ± 3.30a | 179.82 ± 19.88a | 176.17 ± 9.75a |
| CK (U/L) | 1386.36 ± 130.33a | 1312.69 ± 98.29a | 1323.83 ± 102.14a | 1298.69 ± 183.41a | 1389.56 ± 342.11a |
| TP (g/L) | 72.80 ± 0.93a | 72.35 ± 0.67a | 73.69 ± 0.86a | 74.6 ± 2.17a | 71.64 ± 1.13a |
| Albumin (g/L) | 43.39 ± 1.29a | 42.26 ± 1.39a | 42.84 ± 1.68a | 44.16 ± 1.37a | 42.80 ± 2.58a |
| Globulin (g/L) | 29.41 ± 1.58a | 30.09 ± 1.46a | 30.85 ± 2.44a | 30.84 ± 1.33a | 29.26 ± 3.04a |
| Subacute toxicity study | |||||
| Urea (mmol/L) | 7.10 ± 0.09a | 7.07 ± 0.17a | 7.05 ± 0.16a | 7.62 ± 0.60a | 6.23 ± 0.21a |
| Creatinine (µmol/L) | 70.53 ± 0.64a | 71.76 ± 0.78a | 69.96 ± 2.35a | 68.60 ± 1.60a | 71.50 ± 2.60a |
| ALT (U/L) | 59.97 ± 2.02a | 60.05 ± 0.99a | 57.44 ± 3.07a | 62.91 ± 4.59a | 56.53 ± 3.39a |
| ALP (U/L) | 152.61 ± 6.01a | 154.02 ± 6.42a | 153.58 ± 4.04a | 152.89 ± 8.59a | 154.09 ± 14.87a |
| AST (U/L) | 203.32 ± 5.53a | 200.60 ± 7.60a | 194.60 ± 8.49a | 213.88 ± 12.23a | 214.96 ± 11.80a |
| CK (U/L) | 1467.64 ± 36.96a | 1143.8 ± 294.96a | 1448.38 ± 75.14a | 1499.31 ± 96.72a | 1567.58 ± 178.11a |
| TP (g/L) | 64.02 ± 0.41a | 61.73 ± 0.66a | 63.16 ± 0.90a | 62.20 ± 1.81a | 60.87 ± 1.56a |
| Albumin (g/L) | 48.93 ± 0.47a | 50.44 ± 1.89a | 50.44 ± 2.25a | 47.90 ± 2.30a | 49.44 ± 0.42a |
| Globulin (g/L) | 12.09 ± 0.70a | 12.92 ± 0.52a | 12.72 ± 2.08a | 11.32 ± 1.95a | 12.98 ± 0.99a |
Values in the same row with similar superscripts are not significantly different at p > 0.05
G1: M. malabathricum (2.5%), G2: M. malabathricum (5%), G3: M. malabathricum (10%), G4: negative control, G5: paraffin
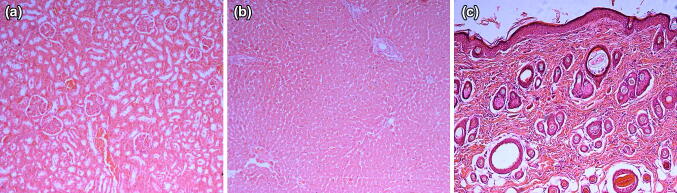
Macroscopic and microscopic examination findings
Grossly, skin, liver and kidneys of all rats were normal or showed no lesions related to toxicity. The findings were similar to the findings observed in the histological sections of the respective tissues (Fig. 5).
Fig. 5.
Representative photomicrographs of histopathological sections of kidney (a), liver (b) and skin (c) of rats for both toxicity studies in all groups. There are no significant pathological lesions observed in all group in dermal toxicity study of ethanolic extract of M. malabathricum leaf extract (H & E staining 100 × magnification)
Discussion
A simple thought that all medicinal plants are safe for consumption is risky, as researchers have discovered compounds of certain plants could result in toxicity [7, 9–11]. Herbal medicine possibly presents a greater risk of adverse effects and interactions than other complementary therapy. Moderate to severe adverse effects after consumption of herbal products have been reported, and in most cases, the herb products were self-prescribed, bought over the counter or obtained from unreliable sources [12]. Nevertheless, the incidence of adverse effects resulted from herbal products is less frequent when they are used appropriately as compared with commercially synthetic drugs [13].
As dermal care products getting greater interest among individuals nowadays, in vivo experimental study is compulsory to provide proven scientific evidence particularly on dermal toxicity study. Apart from that, toxicity study offers data on the doses or concentrations range for concocting of safe products [8, 14, 15]. In the present study, acute and subacute dermal toxicity of ethanolic extract of M. malabathricum leaves was evaluated for preceding-evaluation of its potential as a wound healing agent.
Both acute and subacute dermal toxicity of ethanolic extract of M. malabathricum leaves divulged no adverse skin or systemic toxic reactions at concentrations of 2.5%, 5.0% and 10.0% w/w. The findings are in accordance with our previous dermal toxicity studies using the similar extract that were topically applied on the skin of rats at concentrations of 2000 and 5000 mg/kg of body weight for acute dermal toxicity study and at concentrations of 500, 1000 and 2000 mg/kg of body weight/day for subacute dermal toxicity study [15]; concentrations of the extract used in our previous study were lower than the doses used in this study, as 1% (w/w) of the extract is equivalent to 10,000 mg/kg.
Determination of feed intake and water consumption of experimental animals are important to evaluate the safety use of herbal products. In the present study, the body weight, feed intake and water consumption of rats in all groups were normal, which provides considerable evidence to support topical application of the extract is safe. Similar findings were reported by Pednekar et al. [11] who demonstrated topical application of essential oil on rat’s skin once daily for 28 consecutive days showed no changes in the feed intake, water consumption, body weight, blood results, and also gross and microscopic architectures of the selected organs. In contrast to a study by Etuk et al. [16], they demonstrated that rats administered orally with high dose of a polyherbal product showed evidence of reduction in feed intake, water consumption and body weight. The rats also showed evidence of microscopic lesions in the heart, liver and kidneys.
At the systemic level, liver and kidneys are the most exposed organs to toxicants as their main function involves filtering toxins out of the blood into faeces and urine. Gross appearance of these organs and their weights on necropsy provides important measure to assess the toxic effects of medicinal plants. In this study, the gross appearance and relative weight of the selected organs were normal in all treated groups of both studies. Contrarily, a study by Soufane et al. [17] has shown reduction in body weights and relative organ weights of rats administered orally with Citrullus colocynthis fruit methanolic extract daily for 4 consecutive weeks. The rats also had abnormal values for ALT, AST, ALP, total protein, urea, uric acid, creatinine and electrolytes levels, and lesions in the organs.
Other important measures that should be included in the evaluation of toxic effects of medicinal plants are blood analyses. Blood serves as a major medium of transport and carrier for many drugs and xenobiotics in the body. Its components include erythrocytes, leukocytes and platelets are first exposed to the toxicants. The toxicants could directly damage matured cells in the blood circulation or indirectly damage cell precursors in the bone marrow, which result in reduction of cell numbers in the haemopoietic system. Apart from haematology, serum biochemical indices are equally useful panels in evaluating the toxicity of medicinal plants [18, 19]. In this study, M. malabathricum leaf ethanolic extract did not induce any significant alterations in the haematological and serum biochemical indices, suggesting that the herbal extract did not induce systemic toxicity in rats. The findings are similar to our previous study on acute and subacute dermal toxicity study of the similar extract that was applied topically onto rats’ skins at lower concentrations; 2000 mg/kg and 5000 mg/kg [15].
Histopathology is used as a possible confirmatory testing for diagnosis of systemic toxicity. In the present study, histopathology revealed no liver and renal lesions. The findings were consistent with normal serum biochemistry of all treated rats in both toxicity studies. Collectively, all the findings suggested that ethanolic extract of M. malabathricum leaves did not induce toxicity in the organs. Contrarily, Pednekar et al. [11] observed mild hepatic congestion in rabbits following application of Blumea eriantha essential oil on their skins at concentration of 15%, although normal liver architecture was noted in rabbits that were applied with the oil at 3% and 9% concentrations. They considered mild hepatic congestion has no clinical important which can affect liver function as changes in the total and conjugated bilirubin were not observed. For skin samples, microscopic examination of skin sections of rats applied with M. malabathricum leaf extract once or daily for 28 days at 2.5%, 5.0% and 10.0% revealed no changes in the epidermis, dermis and hypodermis. Decrease in the thickness of epidermis and dermis with numerous Langerhans and inflammatory cells were observed in guinea pig skins applied with colloidal nano silver at doses of 100, 1000 and 10,000 μg/mL [20].
In conclusion, results in this study suggest that topical application of M. malabathricum leaf ethanolic extract at 2.5%, 5.0% and 10.0% once or daily for 28 consecutive days is safe as it does not cause allergic skin and systemic toxic reactions in rats.
Acknowledgements
The authors would like to thank Universiti Putra Malaysia (UPM) for providing IPS Research Grant (GP-IPS/2016/9489700) and MARDI for providing Animal Research Facilities. The authors would also like to thank staff at the Veterinary Haematology and Clinical Biochemistry Laboratory, UPM and Animal Science Research Centre, MARDI for their contribution and cooperation towards completion of this study.
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest to disclose.
References
- 1.Meyer K. Revision of the Southeast Asian genus Melastoma. Blumea. 2001;46:351–398. [Google Scholar]
- 2.Lohézic-Le Dévéhat F, Bakhtiar A, Bezivin C, Amoros M, Boustie J. Antiviral and cytotoxic activities of some Indonesian plants. Fitoterapia. 2002;73:400–405. doi: 10.1016/S0367-326X(02)00125-9. [DOI] [PubMed] [Google Scholar]
- 3.Sharma HK, Chhangte L, Dolui AK. Traditional medicinal plants in Mizoram, India. Fitoterapia. 2001;72:146–161. doi: 10.1016/S0367-326X(00)00278-1. [DOI] [PubMed] [Google Scholar]
- 4.OECD . Test no. 402: acute dermal toxicity. OECD guidelines for the testing of chemicals, section 4. Paris: OECD Publishing; 2017. [Google Scholar]
- 5.OECD . Test no. 410: repeated dose dermal toxicity: 21/28-day study, OECD guidelines for the testing of chemicals, section 4. Paris: OECD Publishing; 1981. [Google Scholar]
- 6.Ernst E. Adverse effects of herbal drugs in dermatology. BJD. 2000;143:923–929. doi: 10.1046/j.1365-2133.2000.03822.x. [DOI] [PubMed] [Google Scholar]
- 7.Djerrou Z, Djaalab H, Riachi F, Serakta M, Chettoum A, Maameri Z, Hamdi-Pacha Y. Irritantcy potential and sub acute dermal toxicity study of Pistacia lentiscus fatty oil as a topical traditional remedy. Afr J Tradit Complement Altern Med. 2013;10:480–489. doi: 10.4314/ajtcam.v10i3.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nurul SAS, Hazilawati H, Rosly MS, Mohd FHR, Noordin MM, Norhaizan ME. Subacute oral toxicity assesment of ethanol extract of Mariposa christia vespertilionis leaves in male Sprague Dawley rats. Toxicol Res. 2018;34:85–95. doi: 10.5487/TR.2018.34.2.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saleem U, Amin S, Ahmad B, Azeem H, Anwar F, Mary S. Acute oral toxicity evaluation of aqueous ethanolic extract of Saccharum munja Roxb. roots in albino mice as per OECD 425 TG. Toxicol Rep. 2017;4:580–585. doi: 10.1016/j.toxrep.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azubike NC, Okwuosa CN, Achukwu PU, Maduka TC, Chike O. Acute toxicity and histopathological effects of crude aqueous extract of Jatropha curcas leaves in mice. Res J Med Plants. 2015;9:340–346. doi: 10.3923/rjmp.2015.340.346. [DOI] [Google Scholar]
- 11.Pednekar PP, Dhumal RV, Datar AG, Vanage GR. In vivo dermal absorption and subacute toxicity studies of essential oil from Blumea eriantha DC. Int J Pharm Pharm Sci. 2013;5:351–358. [Google Scholar]
- 12.Ajose FOA. Some Nigerian plants of dermatologic importance. Int J Dermatol. 2007;46:48–55. doi: 10.1111/j.1365-4632.2007.03466.x. [DOI] [PubMed] [Google Scholar]
- 13.Karimi A, Majlesi M, Rafieian-Kopaei M. Herbal versus synthetic drugs; belief and facts. J Nephropharmacol. 2015;4:27–30. [PMC free article] [PubMed] [Google Scholar]
- 14.Asyura SNN, Hamzah H, Shaari RM, Sithambaram S, Mustapha NM. Blood profiles and histopathological changes of liver and kidney tissues from male Sprague Dawley rats treated with ethanol extract of Clinacanthus nutans leaves. J Clin Toxicol. 2016;6:329. doi: 10.4172/2161-0495.1000329. [DOI] [Google Scholar]
- 15.Zahi AK, Hamzah H, Shaari MR, Widodo RT, Johnny L, Noordin MM. Investigation and evaluation of acute and subacute dermal toxicity studies of ethanolic leaves extract of Melastoma malabathricum in Sprague Dawley rats. AJPCR. 2017;3:84–99. [Google Scholar]
- 16.Etuk EU, Igbokwe V, Ajagbonna OP, Egua MO. Toxicological studies of a nigerian commercial polyherbal product in Albino rats. JMPR. 2009;3:52–60. [Google Scholar]
- 17.Soufane S, Bouzidi A, Mahdeb N, Krache S. Evaluation of acute and subacute toxicity of fruit methanolic extract from Citrullus colocynthis in male Albino rats. Int J Pharmacogn Phytochem Res. 2017;9:567–580. [Google Scholar]
- 18.Siti SA, Norhaizan ME, Hazilawati H. Histopathologic changes in liver and kidney tissues from male Sprague Dawley rats treated with Rhaphidophora decursiva (Roxb.) schott extract. J Cytol Histol. 2014;S4:001. [Google Scholar]
- 19.Fazliana MS, Muhajir H, Hazilawati H, Shafii K, Mazleha M. Effects of Ficus deltoidea aqueous extract on hematological and biochemical parameters in rats. Med J Malaysia. 2008;63:103–104. [PubMed] [Google Scholar]
- 20.Korani M, Rezayat SM, Gilani K, Bidgoli SA, Adeli S. Acute and subchronic dermal toxicity of nanosilver in guinea pig. Int J Nanomed. 2011;6:855–862. doi: 10.2147/IJN.S17065. [DOI] [PMC free article] [PubMed] [Google Scholar]