Abstract
Background
This study aims to determine the predictors of acquired exon 20 T790M mutation in advanced non-small cell lung cancer (NSCLC) patients harbouring sensitizing epidermal growth factor receptor (EGFR) mutation following the failure of first- or second-generation EGFR-tyrosine kinase inhibitor (TKI).
Methods
This is a retrospective observational study of NSCLC patients with sensitising EGFR mutation experiencing disease progression (PD) whilst on first- or second-generation EGFR-TKIs with subsequent investigations to detect acquired T790M mutation at the University of Malaya Medical Centre from 1st January 2015 to 31st December 2017.
Results
A total of 87 patients were included. Upon PD, acquired T790M mutation was found in 55 (63.2%) patients and was significantly more common in patients who achieved partial response (PR) whilst on the EGFR-TKIs (p = 0.008) or had new lung metastasis upon PD (p = 0.048). It was less frequent in patients who developed new symptomatic brain lesions (p = 0.021). Patients with exon 19 deletion were more likely to acquire T790M mutation compared to those with exon 21 L858R point mutation (p = 0.077). Multivariate analysis revealed PR whilst on EGFR-TKI treatment was an independent predictor of acquiring T790M mutation (p = 0.021), whereas development of new symptomatic brain lesions (p = 0.034) or new lymph node metastases (p = 0.038) upon PD was independently against acquiring T790M mutation. Patients with exon 19 deletion were more likely to acquire T790M mutation compared to those with exon 21 L858R point mutation (odds ratio: 2.3, 95% confidence interval: 0.84–6.25, p = 0.104).
Conclusion
The best tumour response of PR to first- or second-generation EGFR-TKI treatment independently predicts acquired T790M mutation. Patients with exon 19 deletion are likely to acquire T790M mutation. This would prove useful for clinicians to prognosticate and plan subsequent treatments for patients with advanced NSCLC harbouring EGFR mutations.
Keywords: non-small cell lung cancer, epidermal growth factor receptor, acquired T790M mutation, independent predictor, tyrosine kinase inhibitor
Introduction
Lung cancer, 85% of which are non-small cell lung cancer (NSCLC), remains the leading cause of cancer mortality globally.1 Upon diagnosis, the majority of NSCLC patients have locally advanced or metastatic disease. Conventional first-line chemotherapy in these patients confers a dismal median overall survival of 8–10 months and a 2-year survival rate of 11%.2,3
The discovery of mutations of the epidermal growth factor receptor (EGFR) has completely revolutionized the management of patients with advanced NSCLC. EGFR (HER 1) is a transmembrane tyrosine kinase receptor belonging to the HER family.4 The binding of ligands consisting of transforming growth factor-α or epidermal growth factor to EGFR leads to auto-phosphorylation of key tyrosine residues.5 This activates downstream signalling involving and mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K)/Akt which promotes cellular proliferation and survival. The presence of EGFR mutation results in constitutive activation of the MAPK and PI3K/Akt pathway independent of ligand binding, subsequently leading to the development and progression of NSCLC.
EGFR-tyrosine kinase inhibitors (EGFR-TKIs) including gefitinib, erlotinib, afatinib, dacomitinib and osimertinib bind to the ATP-binding sites of EGFR, thereby inhibiting the activation of the EGFR, MAPK and PI3K/Akt pathway.6,7 This results in reduced cellular proliferation and increased apoptosis. Several clinical trials have reported an impressive median progression-free survival (mPFS) of 9–13 months in NSCLC patients harbouring EGFR-TKI sensitising mutations treated with first-line gefitinib, erlotinib, or afatinib.5
Despite the obvious efficacy of these EGFR-TKIs, the majority of these patients develop drug resistance after a median treatment period of one year mainly due to the acquisition of the exon 20 T790M resistant mutation. This study aims to determine the predictors of acquiring T790M mutation as a resistance mechanism among NSCLC patients who develop disease progression whilst taking first- or second-generation EGFR-TKI treatment.
Methodology
Study Design and Patients
This is a retrospective observational study of NSCLC patients with sensitising EGFR-mutation who progressed while on first- or second-generation EGFR-TKI treatment with subsequent investigations to determine the mode of resistance at the University Malaya Medical Center (UMMC) from 1st January 2015 to 31st December 2017. All patients included had demonstrated an objective clinical benefit from the initial EGFR-TKI treatment as evidenced by either a complete response (CR), partial response (PR) or a minimum of six months of stable disease (SD) according to the Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1.8 They were investigated for resistance mechanisms as soon as the monitoring computed tomography (CT)-scan detected a PD according to RECIST version 1.1 criteria,9 without interruption of their EGFR-TKI treatment. Patients who had prior chemotherapy, inadequate tissue sample for resistance mechanism analysis, or incomplete medical records were excluded from this study. The study was conducted after receiving approval from the hospital medical ethics committee.
Procedure
Patients who fulfilled the inclusion criteria were consecutively identified from the lung cancer registry of the Division of Respiratory Medicine, UMMC. Baseline demographic, clinical findings, treatment, pattern of PD, and investigation into resistance mechanisms were obtained from the electronic medical records.
At diagnosis, every patient underwent a baseline CT thorax, abdomen and pelvis (CT-TAP). CT-brain was only performed if there were neurological symptoms or signs. The initial tumor was staged according to the 7th edition of the American Joint Committee on Cancer system.10 All patients were tested for the presence of EGFR mutation in their pre-treatment biopsy specimens. Gefitinib or erlotinib was given in the first-line setting while afatinib was given either in the first-line setting or as a second-line treatment when patients failed to respond to gefitinib or erlotinib. Gefitinib and erlotinib are first-generation EGFR-TKI that bind reversibly to EGFR/ErbB1; while afatinib is second-generation EGFR-TKI that binds irreversibly to all the ErbB family (EGFR/ErbB1, HER2/ErbB2, ErbB3, and ErbB4).11,12 It was our standard practice to evaluate the tumor response by performing a repeat CT-TAP 4 weeks after initiation of EGFR-TKI and subsequently, once every three months. Tumor response was categorized according to RECIST version 1.1.9
Before 1st December 2016, tissue re-biopsy was the first-line investigation at PD unless the patient refused, was unfit or the procedure was not technically feasible, in which case the patient would be offered liquid biopsy (detection of T790M mutation from a blood sample) as an alternative. Starting 1st December 2016, liquid biopsy was the first-line investigation while tissue re-biopsy was offered if liquid biopsy failed to detect acquired T790M mutation. We did not repeat tissue biopsy for those already tested negative for acquired T790M mutation in their re-biopsy tissue sample to prevent delay in initiation of second-line treatment. Identification of c-MET amplification by fluorescent in-situ hybridisation (FISH) was only done in seven patients who were tested negative for acquired T790M mutation and histological transformation during PD, as part of a clinical trial.13 Investigations for other resistance mechanisms were not available in Malaysia outside of clinical research during the period of this study.
Tissue Re-Biopsy
Tissue was obtained by either image-guided biopsy, endobronchial biopsy or excisional biopsy as clinically indicated. The histologic confirmation of lung cancer subtypes was based on tumor morphology on haematoxylin and eosin staining, complemented by immunohistochemical staining as needed to distinguish adenocarcinoma from squamous cell carcinoma. T790M mutation was detected by cobas® EGFR Mutation Test v2 (Roche Molecular Systems, New Jersey, USA), an allele-specific real-time polymerase chain reaction (PCR) assay.
Liquid Biopsy
Detection of T790M mutation in circulating cell-free tumor DNA obtained in the plasma using the QIAamp® Circulating Nucleic Acid kit (Qiagen, Hilden, Germany) was by the peptic nucleic acid-locked nucleic acid PCR (PNA-LNA PCR) clamp method (PANAGEN, Daejon, Korea) before 1st December 2016 and by droplet digital PCR (ddPCR) (Sanomics, Hong Kong, China) after that.
Statistical Analysis
Categorical variables were expressed as percentages while continuous variables were expressed as mean ± standard deviation (SD) or median with range. Differences in clinical variables were examined between patients with acquired T790M mutation versus those without. Differences in categorical variables were compared using the Chi-Squared test or Fisher Exact test. Differences in continuous variables were compared using independent t-test or Mann–Whitney U-test. Multivariate analyses were performed using logistic regression. A two-sided p-value of <0.05 was considered as statistically significant. Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS for Windows version 23.0, SPSS Inc., Chicago, IL, USA).
Results
Incidence of Acquired T790M Mutation and Other Resistance Mechanisms
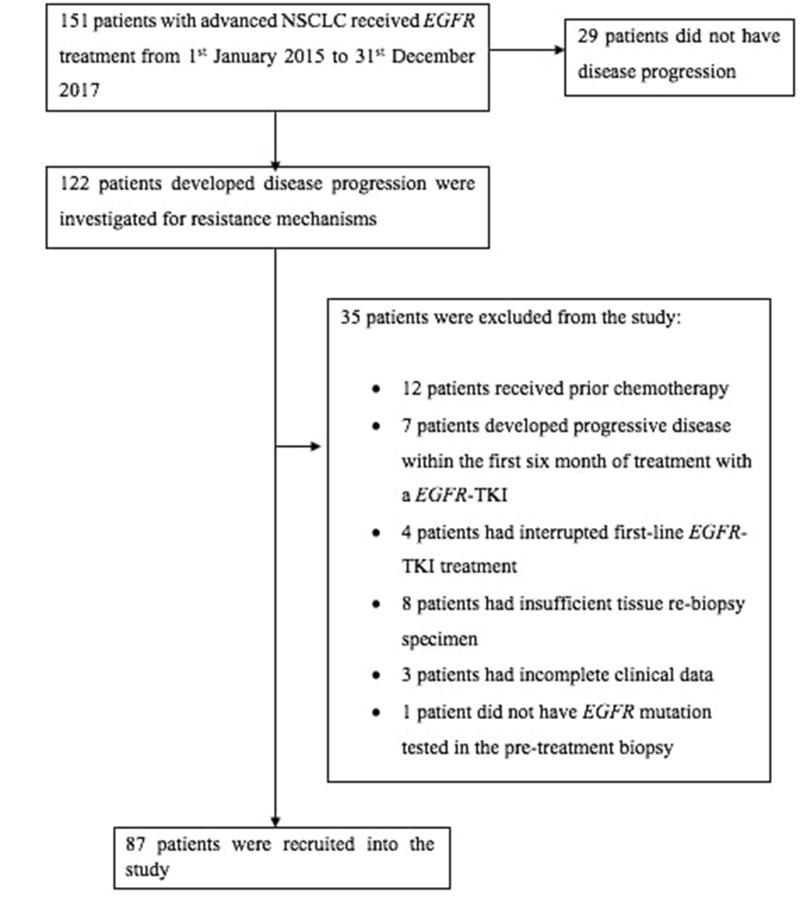
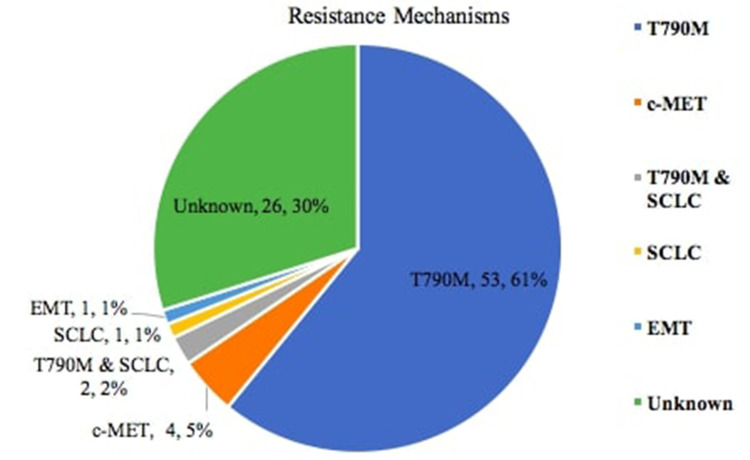
Of 122 patients with PD while on first- or second-generation EGFR-TKI, 87 patients who fulfilled the inclusion criteria were studied (Figure 1). At PD, acquired T790M mutation was found in 55 (63.2%) patients, two of whom (2.3%) also had concomitant small cell lung cancer (SCLC) transformation (Figure 2). Of the other patients, four (4.6%) had c-MET amplification, and one (1.1%) each had SCLC transformation and epithelial–mesenchymal transition (EMT), respectively. The resistance mechanism was unknown in 26 (29.9%) patients.
Figure 1.
Algorithm of patient selection.
Abbreviations: NSCLC, non-small cell lung cancer, EGFR-TKI epidermal growth factor receptor-tyrosine kinase inhibitor.
Figure 2.
Incidence of acquired T790M mutation and other resistance mechanisms.
Abbreviations: SCLC, small cell lung cancer; EMT, epithelial-mesenchymal transition.
Baseline Demographic, Clinical and Treatment History
The patients’ baseline demographic and clinical characteristics, as well as treatment history, are shown in Table 1. Of the initial sensitising EGFR mutations, 62.1% of patients had sensitizing EGFR exon 19 deletion and the remaining patients had EGFR exon 21 L858R point mutation. For treatment in the first-line setting, 65.5% of patients received gefitinib, 16.1% received erlotinib and 18.4% received afatinib. Afatinib was also given in 12.6% of these patients as a second-line treatment before work-up for resistance mechanisms.
Table 1.
Baseline Demographic, Clinical and Treatment Characteristics of Patients with Acquired T790M Mutation
| Characteristics | All Patients (n = 87) | Patients with T790M (n = 55) | p value* |
|---|---|---|---|
| Age, year | |||
| Mean (± SD) | 61.7 ± 9.7 | 61.5 ± 10.1 | 0.693 |
| Gender, No. (%) | |||
| Female | 50 | 33 (66.0) | 0.532 |
| Male | 37 | 22 (59.5) | |
| Ethnicity, No. (%) | |||
| Chinese | 68 | 41 (60.3) | 0.294 |
| Malay | 12 | 10 (83.3) | |
| India | 7 | 4 (57.1) | |
| Smoking history, No. (%) | |||
| Never smoker | 70 | 44 (62.9) | 0.887 |
| Former or current smoker | 17 | 11 (64.7) | |
| Baseline ECOG, No. (%) | |||
| 0–1 | 80 | 52 (65.0) | 0.244 |
| 2–4 | 7 | 3 (42.9) | |
| Tumour stage, No. (%) | |||
| IIIB | 8 | 5 (62.5) | 0.822 |
| IVA | 28 | 19 (67.9) | |
| IVB | 51 | 31 (60.9) | |
| Histology, No. (%) | |||
| Adenocarcinoma | 86 | 55 (64.0) | 0.187 |
| Large cell carcinoma | 1 | 0 (0) | |
| EGFR mutation subtype, No. (%) | |||
| Exon 19 deletion | 54 | 38 (70.4) | 0.077 |
| Exon 21 L858R point mutation | 33 | 17 (51.5) | |
| First-line EGFR-TKI treatment, No. (%) | |||
| Gefitinib | 57 | 40 (70.2) | 0.134 |
| Erlotinib | 14 | 6 (42.9) | |
| Afatinib | 16 | 9 (56.3) | |
| EGFR-TKI treatment before biopsy, No. (%) | |||
| 1st generation EGFR-TKI only | 60 | 38 (63.3) | 0.683 |
| 2nd generation EGFR-TKI only | 16 | 9 (56.3) | |
| 1st followed by 2nd generation EGFR-TKI | 11 | 8 (72.7) | |
| Best tumour response to EGFR-TKI, No. (%) | 0.008 | ||
| Partial response | 72 (82.8) | 49 (68.1) | |
| Stable disease | 15 (17.2) | 6 (40.0) | |
| Progression-free survival on EGFR-TKI, months | |||
| Median (95% CI) | 12.4 (10.9–13.9) | 12.6 (9.4–15.8) | 0.977 |
| Duration from EGFR-TKI initiation to re-biopsy, months | |||
| Median (95% CI) | 17.3 (12.2–22.4) | 18.5 (13.1–23.9) | 0.321 |
Notes: *Chi-Square test for categorical variables; independent t-test for continuous variables. Bold p value was statistically significant.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
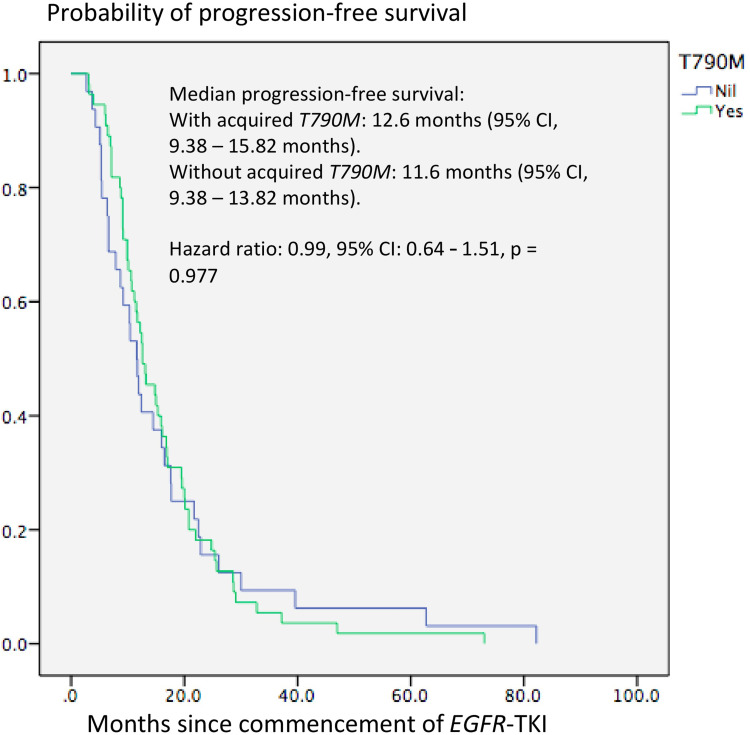
Acquired T790M mutation was found significantly more frequently in patients who achieved PR as the best response to the initial EGFR-TKI treatment compared to those who only had SD as the best response (68.1% versus 40.0%, p = 0.008). Patients with EGFR exon 19 deletion were more likely to acquire T790M mutation at PD compared to those with EGFR exon 21 L858R point mutation (70.4% versus 51.5%, p = 0.077). The mPFS was slightly longer in patients who acquired T790M mutation compared to those who did not acquire T790M mutation (12.6 months versus 11.6 months, p = 0.977) (Figure 3). The median duration from EGFR-TKI treatment initiation to re-biopsy was also longer in patients with acquired T790M mutation compared to those who did not (18.5 months versus 16.2 months, p = 0.321)
Figure 3.
Kaplan–Meier plot for progression-free survival of patients according to acquired T790M mutation status.
Pattern of Disease Progression
Upon PD, nearly half of the patients experienced enlargement of the lung primary, while 83.9% of the patients had new metastases (Table 2). The lung (48.3%) was the commonest site of new metastases, followed by intrathoracic lymph nodes (24.1%), bones (20.7%) and the liver (18.4%). The brain (11.5%) and adrenal glands (2.3%) were uncommon sites of new metastases.
Table 2.
Pattern of Disease Progression in Patients with Acquired T790M Mutation
| Characteristics | All Patients (n = 87) | Patients with T790M (n = 55) | p value* |
|---|---|---|---|
| Number of progression sites, No. (%) | |||
| 1 | 46 | 31 (67.4) | 0.260 |
| 2 | 21 | 13 (61.9) | |
| 3 | 17 | 8 (47.1) | |
| 4 | 3 | 3 (100) | |
| Progression site, No. (%) | |||
| Enlarged lung primary | 14 | 10 (71.4) | 0.242 |
| New metastases | 47 | 32 (68.1) | |
| Enlarged primary and new metastases | 26 | 13 (50.0) | |
| Enlarged lung primary, No. (%) | |||
| Yes | 40 | 23 (57.5) | 0.308 |
| No | 47 | 32 (68.1) | |
| New lung metastases, No. (%) | 0.048 | ||
| Yes | 42 | 31 (73.8) | |
| No | 45 | 24 (53.3) | |
| New symptomatic brain metastases, No. (%) | 0.021 | ||
| Yes | 10 | 3 (30.0) | |
| No | 77 | 52 (67.5) | |
| New liver metastases, No. (%) | |||
| Yes | 16 | 10 (62.5) | 0.947 |
| No | 71 | 45 (63.4) | |
| New adrenal metastases, No. (%) | |||
| Yes | 2 | 2 (100.0) | 0.275 |
| No | 85 | 53 (62.4) | |
| New intrathoracic lymph node metastases, No. (%) | |||
| Yes | 21 | 10 (47.6) | 0.089 |
| No | 66 | 45 (68.2) | |
| New bone metastases, No. (%) | |||
| Yes | 18 | 13 (72.2) | 0.374 |
| No | 69 | 42 (60.9) |
Notes: *Chi-square test for categorical variables. Bold p-value was statistically significant.
Acquired T790M mutation was significantly more frequent in patients who developed new lung metastases than those who did not (73.8% versus 53.3%, p = 0.048) and significantly less frequent in patients who had new symptomatic brain metastases than those who did not (30.0% versus 67.5%, p = 0.021). Patients who developed new intrathoracic lymph node metastases tended to be less likely to acquire T790M mutation than those who did not (47.6 versus 68.2%, p = 0.089).
Investigations for Resistance Mechanisms
Equal proportions of patients underwent liquid biopsy (40.2%) and tissue re-biopsy (40.2%) as the initial investigation to detect acquired T790M mutation, with a positive detection rate of 57.1% and 74.3%, respectively (Table 3). Following an initial negative liquid biopsy, 19.6% of patients underwent a tissue re-biopsy, which detected T790M mutation in 52.9% of the cases.
Table 3.
Investigations for Resistance Mechanisms in Patients with Acquired EGFR Exon 20 T790M Mutation
| Resistance Mechanism Investigation | All Patients (n = 87) | Patients with T790M (n = 55) | p value* |
|---|---|---|---|
| Type of biopsy, No. (%) | |||
| Liquid biopsy | 35 | 20 (57.1) | 0.205 |
| Tissue re-biopsy | 35 | 26 (74.3) | |
| Liquid followed by tissue re-biopsy | 17 | 9 (52.9) | |
| Type of liquid biopsy | All patients (n = 52) | Patients with T790M (n = 29) | p value* |
| Liquid biopsy platform, No. (%) | |||
| PNA-LNA PCR | 28 | 15 (53.6) | 0.730 |
| ddPCR | 24 | 14 (58.3) | |
| Tissue re-biopsy | All patients (n = 52) | Patients with T790M (n = 35) | p value* |
| Site of re-biopsy, No. (%) | |||
| Lung primary | 28 | 17 (60.7) | 0.323 |
| Lung metastasis | 11 | 9 (81.8) | |
| Brain metastasis | 1 | 0 | |
| Liver metastasis | 10 | 8 (80.0) | |
| Lymph node metastasis | 2 | 1 (50.0) |
Note: *Chi-square for categorical variables.
Abbreviations: PNA-LNA PCR, peptic nucleic acid-locked nucleic acid polymerase chain reaction; ddPCR, droplet digital polymerase chain reaction.
Among patients who underwent liquid biopsy, PNA-LNA PCR was performed in 46.2% of them with a T790M mutation detection rate of 58.3%, while ddPCR was performed in the remaining patients with a T790M mutation detection rate of 53.6%. The detection rate of acquired T790M mutation from tissue biopsy was 81.8% in biopsies of lung metastatic lesions, 80.0% in biopsies of liver metastatic lesions, 60.7% in lung primary tumor biopsies and 50.0% in intrathoracic lymph node biopsies. The investigation methods and sites of tissue biopsy did not have a significant effect on the T790M mutation detection rate.
Independent Predictors of Acquired T790M Mutation
In multivariate analysis, compared to SD, PR with EGFR-TKI treatment was associated with a significantly higher rate of acquiring T790M mutation at PD (OR: 4.1, 95% CI: 1.24–13.50, p = 0.021) (Table 4); while the development of new symptomatic brain metastases or new intrathoracic lymph node metastases at PD was associated with significantly lower rates of acquired T790M mutation (OR: 0.2, 95% CI: 0.04–0.88, p = 0.034 and OR: 0.3, 95% CI: 0.11–0.94, p = 0.038, respectively). Patients with sensitizing EGFR exon 19 deletion were more likely to acquire T790M mutation compared to those with sensitizing EGFR exon 21 L858R point mutation (OR: 2.3, 95% CI: 0.84–6.25, p = 0.104). Otherwise, the type of first-line EGFR-TKI received and having new lung metastases during PD did not have a significant effect on the T790M mutation rate.
Table 4.
Multivariate Analysis of Predictors of Acquiring T790M Mutation
| Characteristics | All Patients (n = 87) | Patients with T790M (n = 55) | Multivariate Analysis, OR (95% CI), p value |
|---|---|---|---|
| EGFR mutation subtypes, No. (%) | |||
| Exon 19 deletion | 54 | 38 (70.4) | 2.3 (0.84–6.25), 0.104 |
| Exon 21 L858R point mutation# | 33 | 17 (51.5) | |
| First-line EGFR-TKI, No. (%) | |||
| Gefitinib | 57 | 40 (70.2) | 1.6 (0.41–6.38), 0.491 |
| Erlotinib | 14 | 6 (42.9) | 0.5 (0.09–2.70), 0.424 |
| Afatinib# | 16 | 9 (56.3) | |
| Best tumour response, No (%) | 4.1 (1.24–13.50), 0.021 | ||
| Partial response | 72 | 49 (68.1) | |
| Stable disease# | 15 | 6 (40.0) | |
| New lung metastases, No. (%) | |||
| Yes | 42 | 31 (73.8) | 1.6 (0.54–4.55), 0.404 |
| No# | 45 | 24 (53.3) | |
| New symptomatic brain metastases, No. (%) | 0.2 (0.04–0.88), 0.034 | ||
| Yes | 10 | 3 (30.0) | |
| No# | 77 | 52 (67.5) | |
| New intrathoracic lymph nodes metastases, No. (%) | 0.3 (0.11–0.94), 0.038 | ||
| Yes | 21 | 10 (47.6) | |
| No# | 66 | 45 (68.2) |
Notes: Bold p values are statistically significant. #Parameters as the reference group.
Abbreviations: EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; OR, odds ratio; 95% CI, 95% confidence interval.
Clinical and Treatment Characteristics of Patients Without PD
At the time of data cut-off, 29 patients continued to receive first- or second-generation EGFR-TKI without PD. 88.4% of these patients had EGFR exon 19 deletion and the remaining patients had EGFR exon 21 L858R point mutation as the original sensitizing mutation. 48.2% of patients received gefitinib, 27.6% received erlotinib and 24.2% received afatinib as the choice of EGFR-TKI selected in the first-line setting. 82.7% of patients had PR and the remaining had SD as the best tumour response. The median follow-up period for this group of patients was 17.1 months (95% CI: 6.38–27.82).
Osimertinib After Failure of First- or Second-Generation EGFR-TKI
Nineteen (34.5%) patients with acquired T790M mutation received osimertinib soon after first- or second-generation EGFR-TKI failure. The care of three of these patients were transferred to another center and further details about their response to osimertinib were not available. The second mPFS for the remaining 16 patients treated with osimertinib was 8.0 months (95% CI: 1.75–14.25).
Discussion
The present study identified acquired T790M mutation as the commonest resistance mechanism causing treatment failure to first- or second-generation EGFR-TKI in Malaysian patients with sensitising EGFR-mutant NSCLC. Having the best tumour response of PR while on first-line EGFR-TKI was the only significant independent predictor of acquiring T790M mutation causing PD. Acquired T790M mutation was more common in patients with tumors harbouring exon 19 deletion than those with exon 21 L858R point mutation as their original sensitizing mutation. On the other hand, the presence of new symptomatic brain metastases or intrathoracic lymph node metastases upon PD was significant independent predictors against acquiring T790M mutation. The yield of detecting T790M mutation from either tissue or liquid biopsy in the present study was high. Performing reflex tissue re-biopsy after the failure of liquid biopsy to detect T790M mutation could detect this resistance mutation in half of the cases.
The incidence of acquired T790M mutation in the present study corresponds to that of 45.1% to 62.0% reported in the literature.14–22 Several studies have highlighted a longer mPFS with EGFR-TKI treatment as the most important independent predictor of acquired T790M mutation.18,19,21-24 Even though the mPFS of our patients who acquired T790M mutation was slightly longer than the mPFS of those who did not acquire T790M mutation, the difference was not statistically significant because of the small number of patients. The exclusion of a number of prolonged EGFR-TKI responders who had not experienced PD in the present study could be a confounding factor because previous studies suggested these patients are more likely to acquire T790M mutation at PD.18,19,21-23
A few studies have shown that initial exon 19 deletion mutation is another predictor of acquiring T790M mutation as a resistance mechanism.19–21,25 The present study too showed a trend favoring acquired T790M mutation among patients with initial sensitizing EGFR exon 19 deletion compared to those with EGFR exon 21 L858R point mutation. The lack of statistical significance could be attributed to the small number of patients in our study. Matsuo et al reported a significant association of better tumour response to EGFR-TKI with acquired T790M mutation (objective response rate: 84.7% versus 60.0%, p = 0.001) which is also shown by the present study.19 Oxnard et al and Hata et al, respectively, reported extra-thoracic disease progression (p = 0.014) and new brain metastases (p = 0.042) as features against acquiring T790M mutation as a resistance mechanism.15,23 Our finding is in agreement with the latter observation. A recent study by Del Re et al reported a higher incidence of T790M mutation detected by plasma biopsy at PD among patients receiving first-line gefitinib or erlotinib compared to afatinib.26 Otherwise, the present study and other studies consistently observe that gender, smoking status, proportion of stage IV disease and the site of re-biopsy do not have a significant effect on the frequency of acquired T790M mutation.21,23,27,28
The substitution of methionine by threonine at the gatekeeper residue in position 790 of the EGFR kinase domain leads to T790M mutation which confers resistance to first- or second-generation EGFR-TKI by steric hindrance to TKI, increased ATP-binding affinity and increased in auto-phosphorylation levels.29–31 The emergence of T790M mutation during EGFR-TKI treatment could be by selection or by acquisition.32–34 In the selection hypothesis, a small proportion of T790M resistant clone is already present even before EGFR-TKI therapy. This T790M clone proliferates when the sensitive clones are successfully eradicated by EGFR-TKI therapy. This hypothesis is supported by the discovery of de novo T790M mutation in 31.5–38.0% of patients with sensitising EGFR-mutant NSCLC prior to treatment.35–37 On the other hand, the acquisition hypothesis suggests that the tumor cells develop novel genetic or epigenetic defects as a consequence of prolonged EGFR-TKI treatment.38–40 In the light of these two hypotheses, the higher incidence of acquired T790M mutation among patients who achieve PR with EGFR-TKI can be explained by the selection model while the higher incidence of acquired T790M mutation among patients with longer mPFS and longer duration on EGFR-TKI treatment before biopsy can be explained by the acquisition model.
Compared to patients with exon 21 L858R point mutation, better tumour response and longer mPFS among patients with exon 19 deletion may reflect the higher incidence of acquired T790M mutation in patients with the latter mutation.41 Secondary T790M mutation is less likely in patients who experience new brain metastases because first- or second-generation EGFR-TKI do not cross the blood-brain barrier well to promote T790M mutation resistance by selection or acquisition mechanisms.
The findings of our study further complement the result of existing literature. While ethnicity, smoking status and histologic subtypes of lung cancer are associated with initial EGFR mutation, the present study and other studies show that initial EGFR mutation subtypes, tumor response to EGFR-TKI treatment and sites of PD are predictors of acquired T790M mutation.18,19,21-23,25,42,43 The initial EGFR mutation subtypes and best tumor response while on EGFR-TKI treatment are the clinically more relevant predictors of acquired T790M mutation compared to PFS and sites of PD. This is because these information enable the treating clinicians to predict the likelihood of their patients developing T790M mutation before the actual PD, and therefore allows early prognostication and management planning. Patients with tumors harbouring exon 19 deletion and patients who achieve PR as the best tumor response while on EGFR-TKI shall have a better overall survival because they are more likely to acquire T790M mutation as a cause of PD. This is supported by several studies that report a significantly longer post-progression survival in patients with acquired T790M mutation compared to those without.15,20,23
The importance of planning tissue sampling carefully when patients fail EGFR-TKI is also highlighted in the current study. First, acquired T790M mutation could be detected in tissue biopsy in half of the patients who were initially tested negative for T790M mutation in their plasma. Second, the yield of acquired T790M mutation from different sites of tissue biopsied was not the same. Third, even though PR with EGFR-TKI treatment was a predictor for acquiring T790M mutation, this resistance mutation was detected in two-fifths of patients with SD as the best tumor response.
The strength of this study lies in the fact that only patients who were treated with first- or second-generation EGFR-TKI were included, therefore excluding the potential tumorigenic effect of chemotherapy. In addition, this study concurrently explores the association of acquired T790M mutation with patient baseline demographics and clinical characteristics, treatment history, pattern of disease progression and investigation methods.
However, we do acknowledge that the study has several limitations. First, it was performed in a single centre, thus limiting the generalizability of the results. Second, this was a retrospective study with attendant limitations. Third, patients who experienced PD within six months of initial EGFR-TKI treatment or had interrupted EGFR-TKI treatment were excluded. Such patients were not uncommon in real-life practice. Fourth, patients with rare or complex EGFR mutation were not included because none of them had objective clinical benefit from initial EGFR-TKI treatment. Fifth, the acquired T790M mutation in patients who develop new symptomatic brain metastases on PD might have been underreported because brain biopsy was rarely performed. Sixth, the post-progression survival and overall survival of these patients were not assessed because of the heterogeneity or absence of subsequent lines of treatment.
Conclusions
This study concludes that acquired T790M mutation is the most common resistance mechanism leading to first- or second-generation EGFR-TKI treatment failure in Malaysian patients. The best tumor response of PR was an independent predictor of T790M mutation as a resistance mechanism. Patients with tumour harboring exon 19 deletion mutation as the original sensitizing mutation are more likely to acquire T790M mutation causing PD. These information are useful for clinicians to prognosticate and plan subsequent treatments for patients with advanced NSCLC harbouring EGFR mutations.
Acknowledgments
The abstract and parts of this article have been presented by the same authors above in the form of an abstract at the IASLC 2019 World Conference on Lung Cancer, Barcelona, Spain. The abstract presented at the conference was then published by the IASLC as:
Chong-Kin Liam, Chee-Shee Chai, Mau-Ern Poh et al Acquired T790M mutation in patients failing treatment with first or second-Generation EGFR-tyrosine kinase inhibitors. JTO 2019; 14(10):S1038.44
Funding Statement
This study is supported by an Educational Grant from AstraZeneca Sdn Bhd [PV003-2019].
Abbreviations
NSCLC, non-small cell lung cancer; EGFR, epidermal growth factor receptor; PI3K, phosphatidylinositol 3-kinase; MAPK, mitogen-activated protein kinase; TKIs, tyrosine kinase inhibitors; mPFS, median progression-free survival; PD, disease progression; UMMC, University Malaya Medical Center; CR, complete response; PR, partial response; SD, stable disease; CT, computed tomography; RECIST, Response Evaluation Criteria in Solid Tumours; TAP, thorax, abdomen and pelvis; FISH, fluorescent in-situ hybridisation; PCR, polymerase chain reaction; PNA-LNA PCR, peptic nucleic acid-locked nucleic acid PCR; ddPCR, droplet digital PCR; SD, standard deviation; SCLC, small cell lung cancer; EMT, epithelial–mesenchymal transition; ECOG, Eastern Cooperative Oncology Group; OR, odds ratio; 95% CI, 95% confidence interval.
Ethic Approval and Informed Consent
This study was performed in accordance with the Declaration of Helsinki. It was a retrospective study and all the data used for the statistical analysis were anonymous. Therefore, informed consent from the enrolled patients was waived. The Institutional Review Board and Medical Ethic Committee of UMMC approved this study, with ethic number of MECID. No 2,018,224-6046.
Author Contributions
All authors have contributed substantially to this study, including the conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; giving of their final approval of the version to be published; and agreement to be accountable for all aspects of the work.
Disclosure
Prof. Dr. Gwo-Fuang Ho reports grants from Merck Sharp & Dohme, Regeneron, Astellas, Eli Lily, Tessa Therapeutics, and AB science, personal fees from Roche, Boehringer Ingelheim, and Eli Lily (M) Sdn Bhd, grants and personal fees from Pfizer, and non-financial support from AstraZeneca and Eisai, outside the submitted work. The authors report no other possible conflicts of interest in this work.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–98. doi: 10.1056/NEJMoa011954 [DOI] [PubMed] [Google Scholar]
- 3.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(21):3543–3551. doi: 10.1200/JCO.2007.15.0375 [DOI] [PubMed] [Google Scholar]
- 4.Perez-Soler R. HER1/EGFR targeting: refining the strategy. Oncologist. 2004;9(1):58–67. doi: 10.1634/theoncologist.9-1-58 [DOI] [PubMed] [Google Scholar]
- 5.Morgillo F, Della Corte CM, Fasano M, Ciardiello F. Mechanisms of resistance to EGFR-targeted drugs: lung cancer. ESMO Open. 2016;1(3):e000060. doi: 10.1136/esmoopen-2016-000060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–2139. doi: 10.1056/NEJMoa040938 [DOI] [PubMed] [Google Scholar]
- 7.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–1500. doi: 10.1126/science.1099314 [DOI] [PubMed] [Google Scholar]
- 8.Jackman D, Pao W, Riely GJ, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non–small-cell lung cancer. J Clin Onco. 2009;28(2):357–360. doi: 10.1200/JCO.2009.24.7049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 10.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4 [DOI] [PubMed] [Google Scholar]
- 11.Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27(34):4702–4711. doi: 10.1038/onc.2008.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solca F, Dahl G, Zoephel A, et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther. 2012;343(2):342–350. doi: 10.1124/jpet.112.197756 [DOI] [PubMed] [Google Scholar]
- 13.Cappuzzo F, Janne PA, Skokan M, et al. MET increased gene copy number and primary resistance to gefitinib therapy in non-small-cell lung cancer patients. Ann Oncol. 2009;20(2):298–304. doi: 10.1093/annonc/mdn635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26–75ra26. doi: 10.1126/scitranslmed.3002003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oxnard GR, Arcila ME, Sima CS, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res. 2011;17(6):1616–1622. doi: 10.1158/1078-0432.CCR-10-2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240–2247. doi: 10.1158/1078-0432.CCR-12-2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun JM, Ahn MJ, Choi YL, Ahn JS, Park K. Clinical implications of T790M mutation in patients with acquired resistance to EGFR tyrosine kinase inhibitors. Lung Cancer. 2013;82(2):294–298. doi: 10.1016/j.lungcan.2013.08.023 [DOI] [PubMed] [Google Scholar]
- 18.Kuiper JL, Heideman DA, Thunnissen E, et al. Incidence of T790M mutation in (sequential) rebiopsies in EGFR-mutated NSCLC-patients. Lung Cancer. 2014;85(1):19–24. doi: 10.1016/j.lungcan.2014.03.016 [DOI] [PubMed] [Google Scholar]
- 19.Matsuo N, Azuma K, Sakai K, et al. Association of EGFR exon 19 deletion and EGFR-TKI treatment duration with frequency of T790M mutation in EGFR-mutant lung cancer patients. Sci Rep. 2016;6:36458. doi: 10.1038/srep36458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ke EE, Zhou Q, Zhang Q-Y, et al. A higher proportion of the EGFR T790M mutation may contribute to the better survival of patients with exon 19 deletions compared with those with L858R. J Thorac Oncol. 2017;12(9):1368–1375. doi: 10.1016/j.jtho.2017.05.018 [DOI] [PubMed] [Google Scholar]
- 21.Kogure Y, Shigematsu F, Oki M, Saka H. T790M correlates with longer progression-free survival in non-small cell lung carcinomas harboring EGFR mutations. In vivo. 2018;32(5):1199–1204. doi: 10.21873/invivo.11364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaut D, Sim MS, Yue Y, et al. Clinical implications of the T790M mutation in disease characteristics and treatment response in patients with epidermal growth factor receptor (EGFR)-mutated non-small-cell lung cancer (NSCLC). Clin Lung Cancer. 2018;19(1):e19–e28. doi: 10.1016/j.cllc.2017.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hata A, Katakami N, Yoshioka H, et al. Rebiopsy of non-small cell lung cancer patients with acquired resistance to epidermal growth factor receptor-tyrosine kinase inhibitor: comparison between T790M mutation-positive and mutation-negative populations. Cancer. 2013;119(24):4325–4332. doi: 10.1002/cncr.28364 [DOI] [PubMed] [Google Scholar]
- 24.Tanaka K, Nosaki K, Otsubo K, et al. Acquisition of the T790M resistance mutation during afatinib treatment in EGFR tyrosine kinase inhibitor-naive patients with non-small cell lung cancer harboring EGFR mutations. Oncotarget. 2017;8(40):68123–68130. doi: 10.18632/oncotarget.19243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nosaki K, Satouchi M, Kurata T, et al. Re-biopsy status among non-small cell lung cancer patients in Japan: a retrospective study. Lung Cancer. 2016;101:1–8. doi: 10.1016/j.lungcan.2016.07.007 [DOI] [PubMed] [Google Scholar]
- 26.Del Re M, Petrini I, Mazzoni F, et al. Incidence of T790M in patients with NSCLC progressed to gefitinib, erlotinib, and afatinib: a study on circulating cell-free DNA. Clin Lung Cancer. 2019. [DOI] [PubMed] [Google Scholar]
- 27.Li W, Ren S, Li J, et al. T790M mutation is associated with better efficacy of treatment beyond progression with EGFR-TKI in advanced NSCLC patients. Lung Cancer. 2014;84(3):295–300. doi: 10.1016/j.lungcan.2014.03.011 [DOI] [PubMed] [Google Scholar]
- 28.Tseng JS, Su KY, Yang TY, et al. The emergence of T790M mutation in EGFR-mutant lung adenocarcinoma patients having a history of acquired resistance to EGFR-TKI: focus on rebiopsy timing and long-term existence of T790M. Oncotarget. 2016;7(30):48059–48069. doi: 10.18632/oncotarget.10351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352(8):786–792. doi: 10.1056/NEJMoa044238 [DOI] [PubMed] [Google Scholar]
- 30.Yun CH, Mengwasser KE, Toms AV, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A. 2008;105(6):2070–2075. doi: 10.1073/pnas.0709662105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cortot AB, Janne PA. Molecular mechanisms of resistance in epidermal growth factor receptor-mutant lung adenocarcinomas. Eur Respir Rev. 2014;23(133):356–366. doi: 10.1183/09059180.00004614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hata AN, Niederst MJ, Archibald HL, et al. Tumor cells can follow distinct evolutionary paths to become resistant to epidermal growth factor receptor inhibition. Nat Med. 2016;22(3):262–269. doi: 10.1038/nm.4040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suda K, Mizuuchi H, Maehara Y, Mitsudomi T. Acquired resistance mechanisms to tyrosine kinase inhibitors in lung cancer with activating epidermal growth factor receptor mutation–diversity, ductility, and destiny. Cancer Metastasis Rev. 2012;31(3–4):807–814. doi: 10.1007/s10555-012-9391-7 [DOI] [PubMed] [Google Scholar]
- 34.Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol. 2014;11(8):473–481. doi: 10.1038/nrclinonc.2014.104 [DOI] [PubMed] [Google Scholar]
- 35.Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359(4):366–377. doi: 10.1056/NEJMoa0800668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosell R, Molina MA, Costa C, et al. Pretreatment EGFR T790M Mutation and BRCA1 mRNA Expression in Erlotinib-Treated Advanced Non–Small-Cell Lung Cancer Patients with EGFR Mutations. Clin Cancer Res. 2011;17(5):1160–1168. doi: 10.1158/1078-0432.CCR-10-2158 [DOI] [PubMed] [Google Scholar]
- 37.Su KY, Chen HY, Li KC, et al. Pretreatment epidermal growth factor receptor (EGFR) T790M mutation predicts shorter EGFR tyrosine kinase inhibitor response duration in patients with non-small-cell lung cancer. J Clin Oncol. 2012;30(4):433–440. doi: 10.1200/JCO.2011.38.3224 [DOI] [PubMed] [Google Scholar]
- 38.Sharma SV, Lee DY, Li B, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141(1):69–80. doi: 10.1016/j.cell.2010.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee HJ, Zhuang G, Cao Y, Du P, Kim HJ, Settleman J. Drug resistance via feedback activation of Stat3 in oncogene-addicted cancer cells. Cancer Cell. 2014;26(2):207–221. doi: 10.1016/j.ccr.2014.05.019 [DOI] [PubMed] [Google Scholar]
- 40.Wilson TR, Fridlyand J, Yan Y, et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature. 2012;487(7408):505–509. doi: 10.1038/nature11249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheng M, Wang F, Zhao Y, et al. Comparison of clinical outcomes of patients with non-small-cell lung cancer harbouring epidermal growth factor receptor exon 19 or exon 21 mutations after tyrosine kinase inhibitors treatment: a meta-analysis. Eur J Clin Pharmacol. 2016;72(1):1–11. doi: 10.1007/s00228-015-1966-0 [DOI] [PubMed] [Google Scholar]
- 42.Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97(5):339–346. doi: 10.1093/jnci/dji055 [DOI] [PubMed] [Google Scholar]
- 43.Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361(10):958–967. doi: 10.1056/NEJMoa0904554 [DOI] [PubMed] [Google Scholar]
- 44.Liam CK, Chai CS, Poh ME, et al. Acquired T790M mutation in patients failing treatment with first or second-generation EGFR-tyrosine kinase inhibitors. JTO. 2019;14(10):S1038. doi: 10.1016/j.jtho.2019.08.2503 [DOI] [PMC free article] [PubMed] [Google Scholar]