Abstract
Rodent models have catalyzed major discoveries in the neocortex, a brain region unique to mammals. However, since the neocortex has expanded considerably in primates, employing rodent models has limitations. Human fetal brain tissue is a scarce resource with limitations for experimental manipulations. In order to create an experimentally tractable representation of human brain development, a number of labs have recently created in vitro models of the developing human brain. These models, generated using human embryonic stem cells or induced pluripotent stem cells, are called “organoids”. Organoids have successfully and rapidly uncovered new mechanisms of human brain development in health and disease. In the future, we envision that this strategy will enable faster and more efficient translation of basic neuroscience findings to therapeutic applications. In this review, we discuss the generation of the first human cerebral organoids, progress since their debut, and challenges to be overcome in the future.
Introduction
Targeted treatments for neurological and psychiatric disorders are unavailable due to difficulties in accessing primary human brain tissue and a failure of rodent model systems to faithfully recapitulate features of human neurophysiology and -pathology. The brain has expanded significantly in evolution, and the neocortex, a brain region only present in mammals, has expanded in the mammalian lineage (Molnar et al 2014, Nowakowski et al 2016). For example, the human neocortex is characterized by a more than 1,000-fold increase in surface area and number of neurons compared to the mouse (Herculano-Houzel et al 2006, Hofman 2014). Additionally, the human neocortex has a larger diversity of cell types than the rodent neocortex (Silbereis et al 2016). As an example, van Economo neurons are shown to be present only in mammals with large brain sizes (Allman et al 2011). Furthermore, proportions of cell types are altered in the human compared to the rodent neocortex (Hodge et al 2019, Lui et al 2011). For example, outer (or basal) radial glial cells, as well as astrocytes, are present in much larger numbers in human than in mouse neocortex (Beattie & Hippenmeyer 2017, Oberheim et al 2009, Wang et al 2011).
In order to better model features of the human neocortex absent in rodents, other mammals have been successfully used. For instance, the gyrencephalic ferret has a large number of outer radial glia cells, and has revealed important insights into the neuronal migration along these fibers (Fietz et al 2010, Gertz & Kriegstein 2015, Kalebic et al 2018, Reillo et al 2011). Moreover, sheep embryos may also be used to study outer radial glia cells (Reillo et al 2011) or to model neurodevelopmental disorders, as demonstrated for Arnold-Chiari syndrome, which involves hydrocephalus and polymicrogyria (Encinas et al 2011). However, it has also been found that there are differences in ferret and human neural progenitor populations (Fietz et al 2010) highlighting the importance of studying primate outer radial glia cells. A number of studies have worked with non-human primates (Mora-Bermudez et al 2016, Pollen et al 2015), but ethical and practical limitations make these animals impractical for large-scale studies.
Using primary human tissue to understand brain development
In order to overcome problems with modelling the human brain in other species, human fetal tissue may be obtained to study human neocortical development. Descriptive studies in such tissue have revealed the anatomical organization of the human neocortex at different developmental stages, different cell types and their transcriptional identities, as well as identifying spatiotemporal expression patterns of diverse genes (Fietz et al 2012, Lui et al 2011, Nowakowski et al 2017, Pletikos et al 2014, Reillo & Borrell 2012, Reillo et al 2017, Sousa et al 2017). The BrainSpan Atlas of the Developing Human Brain has an extensive collection of such data including transcriptomic and microarray atlases as well as an in situ hybridization resource (http://www.brainspan.org/). Single-cell transcriptomic resources are also available to query cell-type-specific gene expression in the developing human neocortex (e.g. https://cortexdev.cells.ucsc.edu/). In addition to descriptive studies, live cultures of either dissociated cells or organotypic slices can be performed with fetal tissue. These model systems have been used extensively in different labs- here we provide a few examples of their applications. For instance, the motile behavior of human progenitor cells has been described using live imaging of cortical cultures (Attardo et al 2008, Hansen et al 2010, Subramanian et al 2017). Organotypic slice cultures have also been employed to understand the cellular tropism of Zika virus (Onorati et al 2016, Retallack et al 2016). In our recent study (Mayer et al 2019), we dissociated cortical cells in order to perform calcium imaging coupled with single-cell transcriptomics, electrophysiology in acute slices, and pharmacological agents to study neurotransmitter signaling during cortical development. Despite their successes, primary human tissue during midgestation is a scarce resource and has limitations for experimental manipulations. For instance, slice cultures cannot be maintained for long and therefore extensive experimental manipulations are not feasible.
In vitro-derived models of human brain development
Stem cell-derived neuronal cultures represent another approach to study human brain development (Espuny-Camacho et al 2013, Shi et al 2012). However, 2-dimensional models lack the tissue architecture of the neocortex as well as cellular diversity. Thus, this approach cannot be used to investigate intercellular interactions, the microenvironment, and anatomical characteristics important for cell behavior. In order to address this gap, Sasai and colleagues developed a serum-free floating culture of embryoid body-like aggregates (SFEB) from mouse embryonic stem cells to study telencephalon development (Watanabe et al 2005) and further identified the importance of external signals for the formation of polarized cortical tissue (Eiraku et al 2008). Building on this idea, human induced pluripotent stem cells (hiPSCs) also can be guided towards a 3D structure (Mariani et al 2012). In the study by Vaccarino and colleagues, hiPSCs are allowed to form aggregates of cells, which are then induced toward forebrain fate by blocking Wingless-related integration site (Wnt), Bone morphogenic proteins (BMPs), and Transforming growth factor-beta (TGF-β)/activin/nodal signaling pathways (Mariani et al 2012). These structures are called multilayered structures (Mariani et al 2012). In 2013, two independent studies took advantage of Matrigel, secreted basement membrane extracts from Engelbreth-Holm-Swarm (EHS) mouse sarcoma cells, to induce neuronal differentiation (Kadoshima et al 2013, Lancaster et al 2013). These 3-dimensional structures formed using Matrigel has been called human cerebral organoids (Kadoshima et al 2013, Lancaster et al 2013).
Since the components of Matrigel are not well defined, in 2015, Pasca and colleagues applied a similar approach as Vaccarino lab (Mariani et al 2012) to induce neural fate in the absence of Matrigel (Pasca et al 2015). Pasca and co-workers named these 3-dimensional models cortical spheroids (Pasca et al 2015). At the same time, Mariani and colleagues use the Matrigel-based protocols to improve on their multilayered structure (Mariani et al 2015). The terms “organoids” and “spheroids” seem to represent the same 3-dimensional models of cortical development in vitro (Arlotta & Pasca 2019). In this review, we have referred to the models with the same terminology as the authors.
Due to their great promise, the field has quickly improved both the technical aspects of organoid culture as well as the number of brain regions which can be represented (Amin & Pasca 2018, Arlotta & Pasca 2019, Pasca 2018). After demonstrating feasibility, recent investigations have focused on optimizing reproducibility, recapitulating a diverse neuronal repertoire, and introducing non-neuronal cells. In this review, we will provide a brief overview of the rationale behind the on going development efforts of cerebral organoids and highlight critical differences to human cortical development with regards to anatomy, physiology, and vasculature.
Neural Induction
During the third week of human development in vivo, the nervous system forms from the ectoderm in a process called neural induction. Initially, inhibition of BMPs signalling leads to the establishment of a neural fate (Munoz-Sanjuan & Brivanlou 2002). The neuroectoderm then invaginates to form the neural tube, which will later contain cerebrospinal fluid (Figure 1A). Following neural induction, the neural tube is subdivided through signalling factors such as Wnt, fibroblast growth factor (FGF), BMPs, and retinoic acid (RA), which induce areal fate progressively (Sadler 2005, Wen et al 2009). The walls of the neural tube consist of neuroepithelial cells, which give rise to radial glia cells (Kriegstein & Alvarez-Buylla 2009), the progenitor cells that produce transit-amplifying progenitor cells or postmitotic neurons (Malatesta et al 2008, Paridaen & Huttner 2014).
Figure 1: Cerebral organoids mimic human brain development.
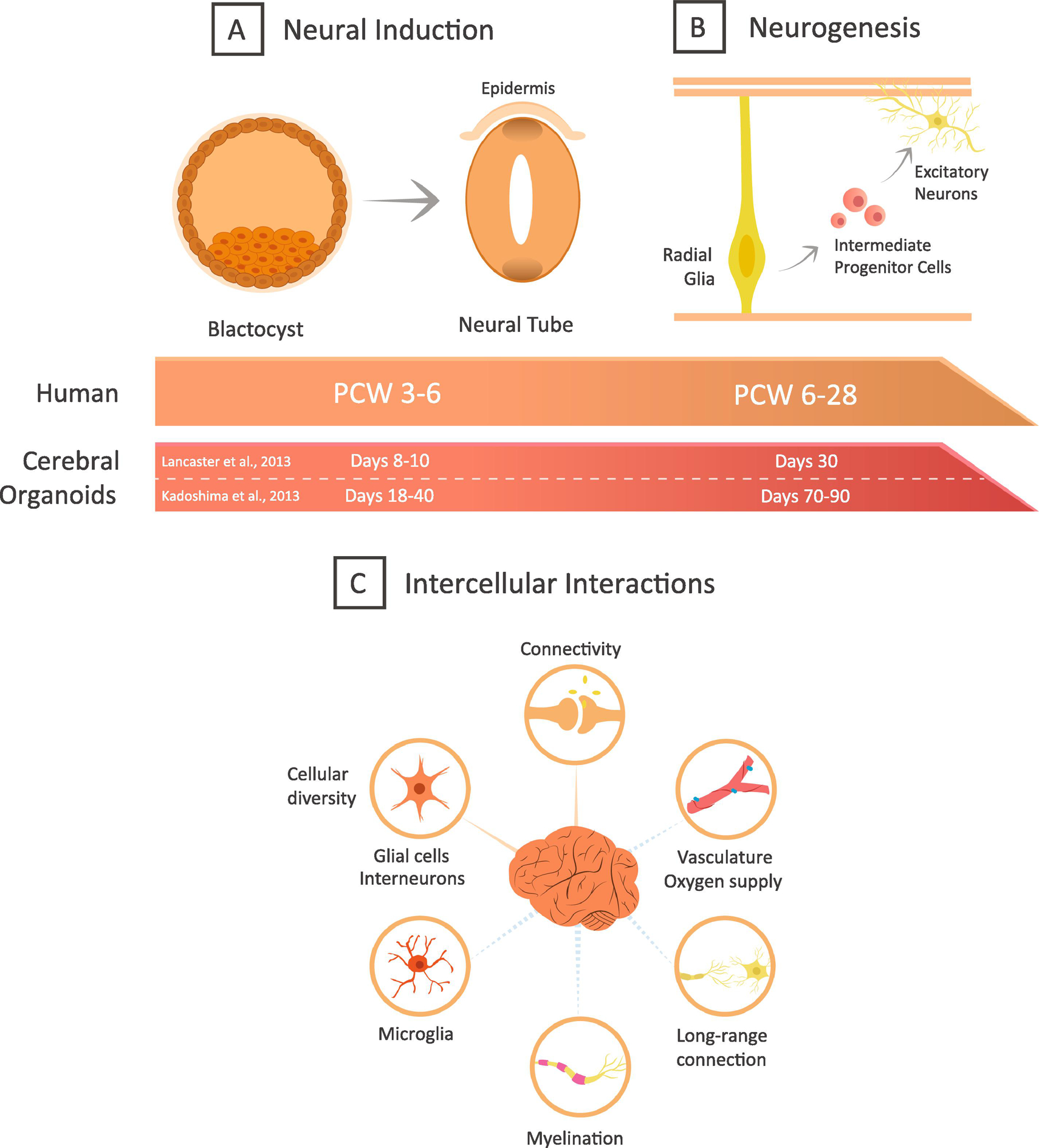
Graphic representation of neocortical development and timing of equivalent events in organoids protocols (A-B). Novel improvements to cerebral organoids (C). The dotted line indicates that the results are still at the preliminary stage.
Brain organoids aim to mimic neural induction by exogenously applying the appropriate growth factors and signaling pathway inhibitors at specific time points. Pluripotent stem cells derived from human embryonic stem cells or iPSC can be used for organoid generation. In the first step of organoid protocols, these cells are cultured in a floating manner to allow the formation of aggregates of pluripotent stem cells, so-called embryoid bodies (Kadoshima et al 2013, Lancaster et al 2013). This step is achieved by seeding the cells in low-cell-adhesion-wells with a low dose of basic fibroblast growth factor (bFGF) to maintain proliferation and a high dose of rho-associated protein kinase (ROCK) inhibitor to prevent apoptosis (Kadoshima et al 2013, Lancaster et al 2013). Embryoid bodies are then differentiated into neuroectodermal tissue (Xia & Zhang 2009), which is further developed in the presence or absence of the droplet of Matrigel (Lancaster & Knoblich 2014, Lancaster et al 2013, Mariani et al 2012).
In all protocols to date, neuroepithelial cells then give rise to radial glial cells mimicking human neocortical development, but the timing can be modified by exogenous factors (Kadoshima et al 2013, Lancaster et al 2013). In the so-called “unguided” protocol, pluripotent stem cells spontaneously differentiate into neural progenitor cells after only 8–10 days without the addition of exogenous factors that would guide regional specification (Lancaster et al 2013). In contrast, the “guided” protocol exposes serum-free embryoid body aggregates to inhibitors of TGF-β, Wnt, and BMPs pathways to promote forebrain specification (Kadoshima et al 2013, Pasca et al 2015). Addition of other factors can promote the development of different brain regions, such as midbrain or hippocampus (Jo et al 2016, Sakaguchi et al 2015).
Kadoshima and colleagues suggest that their self-organizing guided method resembles the timing of corticogenesis in the first trimester of human gestation (18–40 days in cerebral organoids and 21–42 days post-conception in human) (Kadoshima et al 2013). Similarly, the Matrigel-free guided protocol from Mariani shows a multilayered structure reminiscent of the human cerebral cortex at 32–40 days post-conception (Mariani et al 2012). In 2015, Pasca and colleagues introduced a cortical spheroid guided system with the use of TGF-β and BMP inhibitor treatment to induce neural induction within six days of culture (Pasca et al 2015). In summary, these protocols differ in both the timing of neural induction and the signals which orchestra this change. It will be valuable in the future to determine how these modifications, impact the potential and output of radial glial cells.
Neurogenesis
Neurogenesis in the human neocortex starts around postconceptional week six and finishes around postconceptional week 28 in humans (Silbereis et al 2016). The second trimester of gestation is the peak of neurogenesis, and various anatomical zones can be distinguished in the cerebral neuroepithelium. In the human neocortex, ventricular radial glia cells are located close to the ventricle in the ventricular zone, while intermediate progenitor cells and a large population of outer radial glia cells (also called basal radial glial cells) are located in the subventricular zone (Lui et al 2011). The progenitor cells divide asymmetrically to give rise to excitatory neurons, which migrate radially towards the pial surface through the intermediate zone and the subplate. Eventually, they form the cortical plate, which over time forms into the six-layered neocortex (Lui et al 2011, Nowakowski et al 2017). Importantly, the outer subventricular zone is enlarged in humans compared to other species such as the mouse due to a large number of outer radial glia cells (Molnar et al 2019) (Figure 1B).
Remarkably, the published organoid protocols all generate an anatomical structure resembling the developing human neocortex, with regions corresponding to the ventricular zone, subventricular zone, intermediate zone, cortical plate, and marginal zone (Kadoshima et al 2013, Lancaster et al 2013, Mariani et al 2012, Pasca et al 2015). On a cellular level, cerebral organoids show the presence of radial glial cells immunopositive for the transcription factors Pax6 and Sox2 (Kadoshima et al 2013), which are separated from the Tuj1-immunopositive neuronal layer, and a layer representing the layer I of the cortical plate, which is immunopositive for Reelin (Kadoshima et al 2013, Lancaster et al 2013). Cerebral organoids also display the presence of the Tbr2-immunopositive intermediate progenitor cells. Interestingly, the early-born, deep layer Ctip2-immunopositive neurons and late-born upper layer Satb2-positive neurons are located in distinct locations in cerebral organoids as in human cortical development (Camp et al 2015, Kadoshima et al 2013, Lancaster et al 2013, Mariani et al 2012, Pasca et al 2015, Pollen et al 2019, Watanabe et al 2017). Furthermore, 40 to 60 days old cerebral organoids possess similar epigenetic markers, to the genome of a human mid-fetal cortex (Luo et al 2016). Based on the expression of the layer II and III markers CUX1 and BRN2, neurons of these layers may be produced in organoids in a miniaturized spinning bioreactor (Qian et al 2018, Qian et al 2016). Remarkably, the same organoids also display a separation of the progenitor zones reminiscent of the inner subventricular zone, where predominantly Tbr2-immunopositive intermediate progenitor cells are located, and the outer subventricular zone, where predominantly Hopx immunopositive outer radial glial cells are located at day 84 (Qian et al 2018, Qian et al 2016). These bioreactors may enable the generation of these distinct anatomical layers by improving oxygen and nutrient delivery to the center of the sphere (Qian et al 2016). Additionally, bioreactors in conjunction with other factors have enabled the production of midbrain and hypothalamus organoids (Qian et al 2016).
Importantly, the timing in which the anatomical hallmarks are achieved varies widely between the different protocols. The multilayered structure produced by Mariani and colleagues (Mariani et al 2012) showed forebrain-like identity by day 50, and synapses could be observed between the neurons in these spheroids by day 70 (Mariani et al 2012). Brain organoids produced using the Lancaster protocol display evidence of neurogenesis within 30 days of culture (Lancaster et al 2013). In contrast, the Kadoshima protocol initiates neurogenesis within 70–90 days of culture (Kadoshima et al 2013). The Pasca protocol uses neurotrophic factors to induce differentiation of neural progenitors to neurons, and demonstrates zones reminiscent of the ventricular zone and subventricular zone within 52 days and the presence of layers resembling deep and superficial layers in the cortical plate within 137 days (Pasca et al 2015). Bearing in mind the difference in the timing of neurogenesis in the organoid protocols, one needs to be careful when comparing results between different protocols and in relation to in vivo brain development. For example, cerebral organoids from human and chimpanzee have been used to study species differences between primates by growing organoids (Kanton et al 2019, Mora-Bermudez et al 2016, Pollen et al 2019). In these studies, according to the protocol used, different times points are chosen to compare cell-type-specific differences in gene expression (Kanton et al 2019, Mora-Bermudez et al 2016, Pollen et al 2019).
Since organoids faithfully represent many stages of neurogenesis, this system can easily be adapted to study neurodevelopmental diseases (Table 1). Organoids have been utilized to study cellular mechanisms underlying genetic mutations that induce microcephaly and lissencephaly (Bershteyn et al 2017, Lancaster et al 2013). Cerebral organoids produced from Miller Dieker Syndrome patients, a classic type of lissencephaly, show increased apoptosis and an alteration in the radial glial spindle orientation (Bershteyn et al 2017). A change in spindle orientation is also observed in an organoid model of genetic microcephaly caused by a mutation in cyclin-dependent kinase 5 regulatory subunit-associated protein 2, CDK5RAP2 (Lancaster et al 2013). This disorganization causes a reduction in the number of progenitor cells and an increase in the Tuj1-immunopositive neuronal layer compared to control cerebral organoids on day 22 (Lancaster et al 2013). Tuberous sclerosis organoids models are created by introducing homozygous mutations in either TSC1 or TSC2 genes (Blair et al 2018). These mutations lead to more activity of the mTORC1 signaling pathway (Blair et al 2018). mTORC1 signaling increases the phosphorylation of STAT3 and tips the neurogenic/gliogenic balance towards the production of glia (Blair et al 2018). According to the organoid model of periventricular heterotopia, mutations in two genes of cadherin receptor ligands are responsible for defective neuronal migration and morphological disturbance in neural progenitor cells (Klaus et al 2019). Another study with forebrain organoids produced from Autism spectrum disorder patients shows a thicker cortical plate-like area with early born neurons that have longer neurites in comparison to control (Mariani et al 2015, Schafer et al 2019).
Table 1:
Disease models in human brain organoids.
| Disease | Paper | Guided/Unguided system | Region | Main findings | Protocol used |
|---|---|---|---|---|---|
| Microcephaly | Lancaster et al 2013 (Nature) | Unguided | Whole-brain organoids | Increase in immature neuronal differentiation | Lancaster et al 2013 |
| Autism spectrum disorder | Mariani et al 2015 (Cell) | Guided | Telencephalon organoids | Disruption of excitatory/inhibitor balance | Mariani et al 2015 |
| ZIKA virus exposure | Qian et al 2016 (Cell) | Guided | Forebrain organoids | Increase in apoptosis and suppressing neuronal progenitor proliferation | Qian et al 2016 |
| Alzheimer disease | Raja et al 2016 (PLOS PNE) | Guided | Forebrain organoids | Amyloid and tau pathology is mimicked and their responsiveness to drug treatment | Adopted from Kadoshima et al 2013 protocol |
| Lissencephaly (Miller Dieker Syndrome) | Bershteyn et al 2017 (Cell Stem Cell) | Guided | Cerebral organoids | A decrease in radial glial cells population and defect in neuronal migration | Kadoshima et al 2013 |
| Zika virus | Watanabe et al 2017 (Cell Report) | Guided | Cerebral organoids | Increase apoptosis and hence smaller organoids size. Further efficiency of different drug treatment | Adopted from Kadoshima et al 2013 protocol |
| Schizophrenia | Ye et al 2017 (Neuron) | Guided | Forebrain organoids | Reduced proliferation of neural stem cells in the ventricular zone | Qian et al 2016 |
| Microcephaly | Li et al 2017 (Protein & Cell) | Guided | Telencephalon organoids | A decrease in neuronal progenitors and mature neurons. | Adopted from Lancaster et al 2013 (Li et al 2017) |
| Frontotemporal dementia | Seo et al 2017 (The Journal of neuroscience) | Guided | Forebrain organoids | The pathway involved in Tau phosphorylation | Adopted from Kadoshima et al 2013 protocol |
| Brain tumor formation | Bian et al 2018 (Nature method) | Unguided | Cerebral organoids | Targeted drug testing on different brain tumors | Lancaster et al 2013 |
| Periventricular heterotopia | Blair et al 2018 (Nature medicine) | Unguided | Cerebral organoids | Defective neuronal migration, and morphological disturbance in neural progenitor cells | Lancaster et al 2013 |
| Autism spectrum disorder | Schafer et al 2019 (Nature neuroscience) | Guided and Unguided organoids | Cerebral and forebrain organoids | Increase in neurite length of the early born neurons | Lancaster et al 2013; Qian et al 2016 |
| Tuberous sclerosis | Klaus et al 2019 (Nature medicine) | Guided | Cortical spheroids | Disruption of mTORCI signaling pathway during development | Pasca et al 2015 |
| Hypoxic brain injury of prematurity | Pasca et al 2019 (Nature medicine) | Guided | Cortical spheroids | Premature differentiation of intermediate progenitor cells | Pasca et al 2019 |
| Prenatal Hypoxic Injury | Daviaud et al 2019 (frontiers in Cellular Neuroscience) | Unguided | Cerebral organoids | Varying resilience from different neural populations. | Adopted from Lancaster et al 2013 |
In addition to studying genetic mutations that lead to neurodevelopmental disorders, organoids have also been used to interrogate environmental impacts on brain development. Zika infection of organoids profoundly affects the neural progenitor cells, including outer radial glial cells, at all ages (Retallack et al 2016). Furthermore, the relative simplicity of organoid cultures offers advantages to identify specific receptors and test candidate drugs, which could combat Zika microcephaly (Retallack et al 2016, Watanabe et al 2017). To understand the neurodevelopmental deficits in formerly preterm infants, investigators varied the oxygen level of organoids or spheroids at time points which correspond to extreme prematurity. These manipulations demonstrate a reduction in intermediate progenitors and offering insights into a pathology that has been notoriously difficult to study in rodent models (Daviaud et al 2019, Pasca et al 2019).
Intercellular interactions
During the second trimester of gestation, newborn neurons and progenitor cells start to form the first network interactions through calcium waves as well as the first chemical synapses (Luhmann et al 2016, Moore et al 2014). We have recently shown a high degree of cell-type specificity in responses to neurotransmitter stimulation in the developing human neocortex during the second trimester of gestation, highlighting the importance of intercellular signalling at this stage (Mayer et al 2019). Therefore, it is crucial to test whether cerebral organoids recapitulate these physiological responses, using calcium imaging and electrophysiological recording (Lancaster et al 2013, Mansour et al 2018, Pasca et al 2015, Quadrato et al 2017, Sloan et al 2017). Excitatory postsynaptic currents dependent on α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) and N-methyl-D-aspartate (NMDA) receptors were shown in neurons from the cortical spheroids, (Pasca et al 2015). Further, cerebral organoids placed on multi-electrode arrays display electrophysiological network activity similar to spontaneous and synchronized network activity to the developing human neocortex (Trujillo et al 2019).
Interestingly, organoids generated in spinning bioreactors displayed a neuronal maturation similar to the in vivo human cortex as evidenced by the expression of Na-K-Cl cotransporter (NKCC1), and K-Cl co-transporter (KCC2) at different time points during development (Qian et al 2016). In addition, telencephalon organoids have been designed to study the activity of glutamatergic and GABAergic neurons in Autism spectrum disorder (Mariani et al 2015). In this setting, the excitatory/ inhibitory balance is disrupted because of an increase in GABAergic neurons compared to glutamatergic neurons (Mariani et al 2015). Furthermore, calcium activity in microcephaly modeled in telencephalon organoid shows less functional synchronization between cells indicating fewer mature cells (Li et al 2017).
Other essential players in intercellular interaction include long-distance neuronal projections from distinct brain regions (Giandomenico et al 2019, Paul et al 2017) and other cell types such as inhibitory neurons, oligodendrocytes, astrocytes, and microglial cells. Several studies have addressed these absences by introducing astrocytes, microglia, oligodendrocytes, myelinated neurons, and migratory interneurons as explained below (Abud et al 2017, Bagley et al 2017, Madhavan et al 2018, Mansour et al 2018, Ormel et al 2018, Sloan et al 2017) (Figure 1C).
In vivo, astrocytes execute functions vital to neurodevelopment, including synaptogenesis as well as blood-brain barrier formation and maintenance (Molofsky & Deneen 2015). Pasca and colleagues cultivate mature astrocytes in their cortical spheroids (Pasca et al 2015, Sloan et al 2017). Oligodendrocytes were introduced to the organoids by the addition of platelet-derived growth factor AA (PDGF-AA), and insulin-like growth factor 1 (IGF-1) (Madhavan et al 2018). In organoids, these oligodendrocyte-like cells myelinated axons for the first time after 20–30 weeks in the cerebral organoid (Madhavan et al 2018).
To incorporate microglia, human cerebral organoids are transplanted into immunocompromised mouse brains allowed endogenous murine microglia to colonize the cerebral organoid (Daviaud et al 2018, Mansour et al 2018). Also, microglial-like cells can be induced from iPSCs and added to the human brain organoids (Abud et al 2017) or using the unguided brain organoids model (Lancaster et al 2013) to allow the presence of mesodermal cells inducing microglial-like cells (Ormel et al 2018).
To add interneurons, Bagley and colleagues fused dorsal and ventral forebrain organoids to introduce migratory interneurons derived from the ventral forebrain in vivo into cerebral organoids forming assembloids (Bagley et al 2017). Similar fusion experiments also show the migration of interneurons in cortical spheroids (Birey et al 2017, Xiang et al 2017). Further, a fusion of thalamic organoids with cortical organoids/spheroids mimic projections between thalamus and cortex similar to the human neocortex (Xiang et al 2019). By incorporating different cell types and organoids of different brain regions individually, these approaches will allow to isolate the contributions of specific intercellular interactions to brain development. Ultimately, the integration of the full diversity of brain cell types will more faithfully recapitulate the maturing intercellular interactions and microenvironment.
Vascularization
Organoids develop a prominent hypoxic or glycolytic group of cells over time (Amiri et al 2018, Pollen et al 2019, Xiang et al 2019) which likely reflects the lack of oxygen and nutrient delivery to the center of the organoid without blood vessels. Very little is known about human brain vascular development. In the mouse brain, the vascular system develops simultaneously with the nervous system (Paredes et al 2018). At the onset of murine neurogenesis (embryonic day 9.5, or E9.5), pial vessels surround the brain. Blood vessels then grow into the parenchyma, starting with the ventral telencephalon. They extend to the dorsal ventricular wall by E11.5 when neurogenesis is highly functional (Arnold et al 2014). In the mouse ganglionic eminences, the endfeet of radial glial fibers contact periventricular vessels and severing this contact changes the cellular progeny of radial glia (Tan et al 2016).
Interestingly, neurogenic niches in vivo are hypoxic, and this state is required for neural stem cell proliferation (Mohyeldin et al 2010). As the vascular component of the germinal zones grows, the reversal of hypoxia by angiogenesis promotes neural stem cell differentiation (Lange et al 2016). In support of this hypothesis, experimentally blocking vascular growth increases neural stem cell expansion at the expense of differentiation (Lange et al 2016). It is tempting, therefore, to speculate that including vascular cells in organoids may improve the diversity and reproducibility of cell types, which are generated from radial glia. In line with this idea, human progenitor cells may be specifically dependent on vascular interactions, as they have been shown to contact blood vessels during the late stages of neurogenesis (Nowakowski et al 2016). While the importance of the vasculature in embryonic neurogenesis is evident, less is known about the specific molecular players governing neurovascular interactions during development. In other neural stem cell systems such as the adult ventricular-subventricular zone, endothelial and mural cells produce growth factors that have a significant impact on neural stem cells (Crouch et al 2015, Paredes et al 2018). Therefore, the signalling and mechanical contributions from blood vessel cells are absent in the current state of organoids. To address this issue, different studies in 2018 focused on vascularizing human cerebral organoids using an immunocompromised host mouse brain (Daviaud et al 2018, Mansour et al 2018, Pham et al 2018), which then received functional vasculature as well as other host cells. Importantly, the engraftment of host blood vessels was critical for organoid survival. New strategies are incorporating vascular cells into organoids in vitro with either human umbilical vein endothelial cells (huvec) or endothelial cells driven from iPSc with ectopic expression of ETV-2 (Cakir et al 2019). Similar to their function in vivo, endothelial cells in organoids acquire blood-brain-barrier characteristics and promote neuronal maturation. As the vasculature is increasingly recognized to contribute to healthy and diseased brain function (Silva-Vargas et al 2013, Sweeney et al 2018), engineering systems to simultaneously develop the vasculature and neural lineages in vitro will catalyse both a better understanding of brain vasculature and the ability to manipulate it therapeutically.
Future perspectives
Due to the considerable promise of organoids as a model for human brain development, physiology, and pathology, there has been a great effort to optimize the technical aspects of culture. The Arlotta lab has recently improved the reproducibility of the Kadoshima protocol by omitting the 40% O2 and introducing four separate cortical differentiation media with or without Matrigel at different time points to better direct cellular differentiation during cerebral organoids development (Velasco et al 2019). In sum, integrating multiple organoid strategies may build the most comprehensive strategy yet to interrogate human cerebral development. With the basis of a highly reproducible protocol, this approach could build on the intrinsic brain-building properties of neural stem cells to include critical structural and cellular elements and finally fuse different regional modules to create a full brain organoid.
The success of organoids to study for neurodevelopment and neurodevelopmental disease has brought about both extensions to adult diseases as well as appropriate ethical concerns. Due to their longevity, organoids have shown early promise to study neurodegeneration (Amin & Pasca 2018, Gonzalez et al 2018). For example, forebrain organoids modeling schizophrenia have been used and these demonstrate a reduction in proliferation of the neural stem cells in the ventricular zone (Ye et al 2017) indicating possible neurodevelopmental contributions to the disease. In recent years, forebrain organoids modeling Alzheimer’s disease have mimicked amyloid and tau pathology and responded to drug treatment to eliminate the abnormal proteins (Raja et al 2016). Moreover, Frontotemporal Dementia-like organoids develop an increase in tau phosphorylation (Seo et al 2017), which is reduced by inhibition of association of the P25, a truncated form of a regulatory activator called P35 with cyclin-dependent kinase 5 (Seo et al 2017). The 3D environment of organoids also represents an advance in our ability to study brain cancers, as has recently been demonstrated with glioblastoma (Bian et al 2018, Linkous et al 2019). Knoblich and colleagues modeled glioblastoma in cerebral organoids by showing astrocytic neoplasm (Bian et al 2018). These glioblastoma models are suitable for targeted drug tests such as afatinib (Bian et al 2018). As evidence of the significant potential of organoids to model sophisticated processes such as cognition, the scientific community has recently discussed ethical regulations around their experimental manipulation that will be important to consider in the future (Farahany et al 2018).
Due to their scalability and species specificity, brain organoids may represent a powerful complement or even replacement for animal studies for studying the effects of teratogenic substances (Herrmann 2019). When applied to growing organoids, nicotine, alcohol, and cocaine cause premature neuronal differentiation and an increase in apoptosis (Lee et al 2017, Wang et al 2018, Zhu et al 2017). In addition, proteomic study of Dimethyltryiptamine treated organoids hints on differential regulation of proteins from various singling pathways (Dakic et al 2017).
In the future, one could imagine that when a patient presents to their clinical provider with a neurological condition, a skin biopsy is taken to produce induced pluripotent stem cells. The cells are then grown into organoids with a specific milieu of differentiated cell types relevant to the patient’s condition. The organoids are then examined for any defects at the cellular level, and potential therapies tested relatively quickly. These laboratory results are then returned to the clinician with a specific list of therapeutic options. While not possible with current technologies, the invention of organoids enables this type of vision for the future of personalized medicine for genetic diseases and acquired ones such as cancer or infection.
Highlights.
Several different protocols to generate human cerebral organoids are compared
Organoid protocols recapitulate key stages of human cortical development
Time in culture to reach milestones varies considerably between organoid protocols
Modeling all cell types including glia and the vasculature is a major challenge
Organoids are a promising approach to model diseases and develop new treatments
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abud EM, Ramirez RN, Martinez ES, Healy LM, Nguyen CHH, et al. 2017. iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases. Neuron 94: 278–93 e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman JM, Tetreault NA, Hakeem AY, Manaye KF, Semendeferi K, et al. 2011. The von Economo neurons in the frontoinsular and anterior cingulate cortex. Ann N Y Acad Sci 1225: 59–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin ND, Pasca SP. 2018. Building Models of Brain Disorders with Three-Dimensional Organoids. Neuron 100: 389–405 [DOI] [PubMed] [Google Scholar]
- Amiri A, Coppola G, Scuderi S, Wu F, Roychowdhury T, et al. 2018. Transcriptome and epigenome landscape of human cortical development modeled in organoids. Science 362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlotta P, Pasca SP. 2019. Cell diversity in the human cerebral cortex: from the embryo to brain organoids. Curr Opin Neurobiol 56: 194–98 [DOI] [PubMed] [Google Scholar]
- Arnold TD, Niaudet C, Pang MF, Siegenthaler J, Gaengel K, et al. 2014. Excessive vascular sprouting underlies cerebral hemorrhage in mice lacking alphaVbeta8-TGFbeta signaling in the brain. Development 141: 4489–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attardo A, Calegari F, Haubensak W, Wilsch-Brauninger M, Huttner WB. 2008. Live imaging at the onset of cortical neurogenesis reveals differential appearance of the neuronal phenotype in apical versus basal progenitor progeny. PLoS One 3: e2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley JA, Reumann D, Bian S, Levi-Strauss J, Knoblich JA. 2017. Fused cerebral organoids model interactions between brain regions. Nat Methods 14: 743–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie R, Hippenmeyer S. 2017. Mechanisms of radial glia progenitor cell lineage progression. FEBS Letters 591: 3993–4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershteyn M, Nowakowski TJ, Pollen AA, Di Lullo E, Nene A, et al. 2017. Human iPSC-Derived Cerebral Organoids Model Cellular Features of Lissencephaly and Reveal Prolonged Mitosis of Outer Radial Glia. Cell Stem Cell 20: 435–49 e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian S, Repic M, Guo Z, Kavirayani A, Burkard T, et al. 2018. Genetically engineered cerebral organoids model brain tumor formation. Nat Methods 15: 631–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birey F, Andersen J, Makinson CD, Islam S, Wei W, et al. 2017. Assembly of functionally integrated human forebrain spheroids. Nature 545: 54–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair JD, Hockemeyer D, Bateup HS. 2018. Genetically engineered human cortical spheroid models of tuberous sclerosis. Nat Med 24: 1568–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakir B, Xiang Y, Tanaka Y, Kural MH, Parent M, et al. 2019. Engineering of human brain organoids with a functional vascular-like system. Nat Methods 16: 1169–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp JG, Badsha F, Florio M, Kanton S, Gerber T, et al. 2015. Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc Natl Acad Sci U S A 112: 15672–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch EE, Liu C, Silva-Vargas V, Doetsch F. 2015. Regional and stage-specific effects of prospectively purified vascular cells on the adult V-SVZ neural stem cell lineage. J Neurosci 35: 4528–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakic V, Minardi Nascimento J, Costa Sartore R, Maciel RM, de Araujo DB, et al. 2017. Short term changes in the proteome of human cerebral organoids induced by 5-MeO-DMT. Sci Rep 7: 12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviaud N, Chevalier C, Friedel RH, Zou H. 2019. Distinct Vulnerability and Resilience of Human Neuroprogenitor Subtypes in Cerebral Organoid Model of Prenatal Hypoxic Injury. Front Cell Neurosci 13: 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviaud N, Friedel RH, Zou H. 2018. Vascularization and Engraftment of Transplanted Human Cerebral Organoids in Mouse Cortex. eNeuro 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, et al. 2008. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3: 519–32 [DOI] [PubMed] [Google Scholar]
- Encinas JL, Garcia-Cabezas MA, Barkovich J, Fontecha CG, Peiro JL, et al. 2011. Maldevelopment of the cerebral cortex in the surgically induced model of myelomeningocele: implications for fetal neurosurgery. J Pediatr Surg 46: 713–22 [DOI] [PubMed] [Google Scholar]
- Espuny-Camacho I, Michelsen KA, Gall D, Linaro D, Hasche A, et al. 2013. Pyramidal neurons derived from human pluripotent stem cells integrate efficiently into mouse brain circuits in vivo. Neuron 77: 440–56 [DOI] [PubMed] [Google Scholar]
- Farahany NA, Greely HT, Hyman S, Koch C, Grady C, et al. 2018. The ethics of experimenting with human brain tissue. Nature 556: 429–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fietz SA, Kelava I, Vogt J, Wilsch-Brauninger M, Stenzel D, et al. 2010. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat Neurosci 13: 690–9 [DOI] [PubMed] [Google Scholar]
- Fietz SA, Lachmann R, Brandl H, Kircher M, Samusik N, et al. 2012. Transcriptomes of germinal zones of human and mouse fetal neocortex suggest a role of extracellular matrix in progenitor self-renewal. Proc Natl Acad Sci U S A 109: 11836–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertz CC, Kriegstein AR. 2015. Neuronal Migration Dynamics in the Developing Ferret Cortex. J Neurosci 35: 14307–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giandomenico SL, Mierau SB, Gibbons GM, Wenger LMD, Masullo L, et al. 2019. Cerebral organoids at the air-liquid interface generate diverse nerve tracts with functional output. Nat Neurosci 22: 669–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C, Armijo E, Bravo-Alegria J, Becerra-Calixto A, Mays CE, Soto C. 2018. Modeling amyloid beta and tau pathology in human cerebral organoids. Molecular Psychiatry 23: 2363–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen DV, Lui JH, Parker PR, Kriegstein AR. 2010. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 464: 554–61 [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel S, Mota B, Lent R. 2006. Cellular scaling rules for rodent brains. Proc Natl Acad Sci USA 103: 12138–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann K 2019. Beyond the 3Rs: Expanding the use of human-relevant replacement methods in biomedical research. Altex: 343–52 [DOI] [PubMed] [Google Scholar]
- Hodge RD, Bakken TE, Miller JA, Smith KA, Barkan ER, et al. 2019. Conserved cell types with divergent features in human versus mouse cortex. Nature 573: 61–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman MA. 2014. Evolution of the human brain: when bigger is better. Front Neuroanat 8: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo J, Xiao Y, Sun AX, Cukuroglu E, Tran HD, et al. 2016. Midbrain-like Organoids from Human Pluripotent Stem Cells Contain Functional Dopaminergic and Neuromelanin-Producing Neurons. Cell Stem Cell 19: 248–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoshima T, Sakaguchi H, Nakano T, Soen M, Ando S, et al. 2013. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc Natl Acad Sci U S A 110: 20284–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalebic N, Gilardi C, Albert M, Namba T, Long KR, et al. 2018. Human-specific ARHGAP11B induces hallmarks of neocortical expansion in developing ferret neocortex. Elife 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanton S, Boyle MJ, He Z, Santel M, Weigert A, et al. 2019. Organoid single-cell genomic atlas uncovers human-specific features of brain development. Nature 574: 418–22 [DOI] [PubMed] [Google Scholar]
- Klaus J, Kanton S, Kyrousi C, Ayo-Martin AC, Di Giaimo R, et al. 2019. Altered neuronal migratory trajectories in human cerebral organoids derived from individuals with neuronal heterotopia. Nat Med 25: 561–68 [DOI] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. 2009. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 32: 149–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Knoblich JA. 2014. Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc 9: 2329–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, et al. 2013. Cerebral organoids model human brain development and microcephaly. Nature 501: 373–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C, Turrero Garcia M, Decimo I, Bifari F, Eelen G, et al. 2016. Relief of hypoxia by angiogenesis promotes neural stem cell differentiation by targeting glycolysis. EMBO J 35: 924–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CT, Chen J, Kindberg AA, Bendriem RM, Spivak CE, et al. 2017. CYP3A5 Mediates Effects of Cocaine on Human Neocorticogenesis: Studies using an In Vitro 3D Self-Organized hPSC Model with a Single Cortex-Like Unit. Neuropsychopharmacology 42: 774–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Sun L, Fang A, Li P, Wu Q, Wang X. 2017. Recapitulating cortical development with organoid culture in vitro and modeling abnormal spindle-like (ASPM related primary) microcephaly disease. Protein Cell 8: 823–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkous A, Balamatsias D, Snuderl M, Edwards L, Miyaguchi K, et al. 2019. Modeling Patient-Derived Glioblastoma with Cerebral Organoids. Cell Rep 26: 3203–11 e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhmann HJ, Sinning A, Yang JW, Reyes-Puerta V, Stuttgen MC, et al. 2016. Spontaneous Neuronal Activity in Developing Neocortical Networks: From Single Cells to Large-Scale Interactions. Front Neural Circuits 10: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui JH, Hansen DV, Kriegstein AR. 2011. Development and evolution of the human neocortex. Cell 146: 18–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Lancaster MA, Castanon R, Nery JR, Knoblich JA, Ecker JR. 2016. Cerebral Organoids Recapitulate Epigenomic Signatures of the Human Fetal Brain. Cell Rep 17: 3369–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan M, Nevin ZS, Shick HE, Garrison E, Clarkson-Paredes C, et al. 2018. Induction of myelinating oligodendrocytes in human cortical spheroids. Nat Methods 15: 700–06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malatesta P, Appolloni I, Calzolari F. 2008. Radial glia and neural stem cells. Cell Tissue Res 331: 165–78 [DOI] [PubMed] [Google Scholar]
- Mansour AA, Goncalves JT, Bloyd CW, Li H, Fernandes S, et al. 2018. An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol 36: 432–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani J, Coppola G, Zhang P, Abyzov A, Provini L, et al. 2015. FOXG1-Dependent Dysregulation of GABA/Glutamate Neuron Differentiation in Autism Spectrum Disorders. Cell 162: 375–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani J, Simonini MV, Palejev D, Tomasini L, Coppola G, et al. 2012. Modeling human cortical development in vitro using induced pluripotent stem cells. Proc Natl Acad Sci U S A 109: 12770–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer S, Chen J, Velmeshev D, Mayer A, Eze UC, et al. 2019. Multimodal Single-Cell Analysis Reveals Physiological Maturation in the Developing Human Neocortex. Neuron 102: 143–58 e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. 2010. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell 7: 150–61 [DOI] [PubMed] [Google Scholar]
- Molnar Z, Clowry GJ, Sestan N, Alzu’bi A, Bakken T, et al. 2019. New insights into the development of the human cerebral cortex. J Anat [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar Z, Kaas JH, de Carlos JA, Hevner RF, Lein E, Nemec P. 2014. Evolution and development of the mammalian cerebral cortex. Brain Behav Evol 83: 126–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Deneen B. 2015. Astrocyte development: A Guide for the Perplexed. Glia 63: 1320–9 [DOI] [PubMed] [Google Scholar]
- Moore AR, Zhou WL, Sirois CL, Belinsky GS, Zecevic N, Antic SD. 2014. Connexin hemichannels contribute to spontaneous electrical activity in the human fetal cortex. Proc Natl Acad Sci U S A 111: E3919–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora-Bermudez F, Badsha F, Kanton S, Camp JG, Vernot B, et al. 2016. Differences and similarities between human and chimpanzee neural progenitors during cerebral cortex development. Elife 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Sanjuan I, Brivanlou AH. 2002. Neural induction, the default model and embryonic stem cells. Nat Rev Neurosci 3: 271–80 [DOI] [PubMed] [Google Scholar]
- Nowakowski TJ, Bhaduri A, Pollen AA, Alvarado B, Mostajo-Radji MA, et al. 2017. Spatiotemporal gene expression trajectories reveal developmental hierarchies of the human cortex. Science 358: 1318–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowakowski TJ, Pollen AA, Sandoval-Espinosa C, Kriegstein AR. 2016. Transformation of the Radial Glia Scaffold Demarcates Two Stages of Human Cerebral Cortex Development. Neuron 91: 1219–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim NA, Takano T, Han X, He W, Lin JHC, et al. 2009. Uniquely Hominid Features of Adult Human Astrocytes. Journal of Neuroscience 29: 3276–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onorati M, Li Z, Liu F, Sousa AMM, Nakagawa N, et al. 2016. Zika Virus Disrupts Phospho-TBK1 Localization and Mitosis in Human Neuroepithelial Stem Cells and Radial Glia. Cell Rep 16: 2576–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormel PR, Vieira de Sa R, van Bodegraven EJ, Karst H, Harschnitz O, et al. 2018. Microglia innately develop within cerebral organoids. Nat Commun 9: 4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes I, Himmels P, Ruiz de Almodovar C. 2018. Neurovascular Communication during CNS Development. Dev Cell 45: 10–32 [DOI] [PubMed] [Google Scholar]
- Paridaen JT, Huttner WB. 2014. Neurogenesis during development of the vertebrate central nervous system. EMBO Rep 15: 351–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasca AM, Park JY, Shin HW, Qi Q, Revah O, et al. 2019. Human 3D cellular model of hypoxic brain injury of prematurity. Nat Med 25: 784–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasca AM, Sloan SA, Clarke LE, Tian Y, Makinson CD, et al. 2015. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods 12: 671–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasca SP. 2018. The rise of three-dimensional human brain cultures. Nature 553: 437–45 [DOI] [PubMed] [Google Scholar]
- Paul A, Chaker Z, Doetsch F. 2017. Hypothalamic regulation of regionally distinct adult neural stem cells and neurogenesis. Science 356: 1383–86 [DOI] [PubMed] [Google Scholar]
- Pham MT, Pollock KM, Rose MD, Cary WA, Stewart HR, et al. 2018. Generation of human vascularized brain organoids. Neuroreport 29: 588–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletikos M, Sousa AM, Sedmak G, Meyer KA, Zhu Y, et al. 2014. Temporal specification and bilaterality of human neocortical topographic gene expression. Neuron 81: 321–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollen AA, Bhaduri A, Andrews MG, Nowakowski TJ, Meyerson OS, et al. 2019. Establishing Cerebral Organoids as Models of Human-Specific Brain Evolution. Cell 176: 743–56 e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollen AA, Nowakowski TJ, Chen J, Retallack H, Sandoval-Espinosa C, et al. 2015. Molecular identity of human outer radial glia during cortical development. Cell 163: 55–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Jacob F, Song MM, Nguyen HN, Song H, Ming GL. 2018. Generation of human brain region-specific organoids using a miniaturized spinning bioreactor. Nat Protoc 13: 565–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, et al. 2016. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell 165: 1238–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadrato G, Nguyen T, Macosko EZ, Sherwood JL, Min Yang S, et al. 2017. Cell diversity and network dynamics in photosensitive human brain organoids. Nature 545: 48–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja WK, Mungenast AE, Lin YT, Ko T, Abdurrob F, et al. 2016. Self-Organizing 3D Human Neural Tissue Derived from Induced Pluripotent Stem Cells Recapitulate Alzheimer’s Disease Phenotypes. PLoS One 11: e0161969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reillo I, Borrell V. 2012. Germinal zones in the developing cerebral cortex of ferret: ontogeny, cell cycle kinetics, and diversity of progenitors. Cereb Cortex 22: 2039–54 [DOI] [PubMed] [Google Scholar]
- Reillo I, de Juan Romero C, Cardenas A, Clasca F, Martinez-Martinez MA, Borrell V. 2017. A Complex Code of Extrinsic Influences on Cortical Progenitor Cells of Higher Mammals. Cereb Cortex 27: 4586–606 [DOI] [PubMed] [Google Scholar]
- Reillo I, de Juan Romero C, Garcia-Cabezas MA, Borrell V. 2011. A role for intermediate radial glia in the tangential expansion of the mammalian cerebral cortex. Cereb Cortex 21: 1674–94 [DOI] [PubMed] [Google Scholar]
- Retallack H, Di Lullo E, Arias C, Knopp KA, Laurie MT, et al. 2016. Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc Natl Acad Sci U S A 113: 14408–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler TW. 2005. Embryology of neural tube development. Am J Med Genet C Semin Med Genet 135C: 2–8 [DOI] [PubMed] [Google Scholar]
- Sakaguchi H, Kadoshima T, Soen M, Narii N, Ishida Y, et al. 2015. Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat Commun 6: 8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer ST, Paquola ACM, Stern S, Gosselin D, Ku M, et al. 2019. Pathological priming causes developmental gene network heterochronicity in autistic subject-derived neurons. Nat Neurosci 22: 243–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J, Kritskiy O, Watson LA, Barker SJ, Dey D, et al. 2017. Inhibition of p25/Cdk5 Attenuates Tauopathy in Mouse and iPSC Models of Frontotemporal Dementia. J Neurosci 37: 9917–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Kirwan P, Livesey FJ. 2012. Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat Protoc 7: 1836–46 [DOI] [PubMed] [Google Scholar]
- Silbereis JC, Pochareddy S, Zhu Y, Li M, Sestan N. 2016. The Cellular and Molecular Landscapes of the Developing Human Central Nervous System. Neuron 89: 248–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Vargas V, Crouch EE, Doetsch F. 2013. Adult neural stem cells and their niche: a dynamic duo during homeostasis, regeneration, and aging. Curr Opin Neurobiol 23: 935–42 [DOI] [PubMed] [Google Scholar]
- Sloan SA, Darmanis S, Huber N, Khan TA, Birey F, et al. 2017. Human Astrocyte Maturation Captured in 3D Cerebral Cortical Spheroids Derived from Pluripotent Stem Cells. Neuron 95: 779–90 e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa AMM, Zhu Y, Raghanti MA, Kitchen RR, Onorati M, et al. 2017. Molecular and cellular reorganization of neural circuits in the human lineage. Science 358: 1027–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian L, Bershteyn M, Paredes MF, Kriegstein AR. 2017. Dynamic behaviour of human neuroepithelial cells in the developing forebrain. Nat Commun 8: 14167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MD, Kisler K, Montagne A, Toga AW, Zlokovic BV. 2018. The role of brain vasculature in neurodegenerative disorders. Nat Neurosci 21: 1318–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Liu WA, Zhang XJ, Shi W, Ren SQ, et al. 2016. Vascular Influence on Ventral Telencephalic Progenitors and Neocortical Interneuron Production. Dev Cell 36: 624–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo CA, Gao R, Negraes PD, Gu J, Buchanan J, et al. 2019. Complex Oscillatory Waves Emerging from Cortical Organoids Model Early Human Brain Network Development. Cell Stem Cell 25: 558–69 e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco S, Kedaigle AJ, Simmons SK, Nash A, Rocha M, et al. 2019. Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Tsai JW, LaMonica B, Kriegstein AR. 2011. A new subtype of progenitor cell in the mouse embryonic neocortex. Nat Neurosci 14: 555–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang L, Zhu Y, Qin J. 2018. Human brain organoid-on-a-chip to model prenatal nicotine exposure. Lab Chip 18: 851–60 [DOI] [PubMed] [Google Scholar]
- Watanabe K, Kamiya D, Nishiyama A, Katayama T, Nozaki S, et al. 2005. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci 8: 288–96 [DOI] [PubMed] [Google Scholar]
- Watanabe M, Buth JE, Vishlaghi N, de la Torre-Ubieta L, Taxidis J, et al. 2017. Self-Organized Cerebral Organoids with Human-Specific Features Predict Effective Drugs to Combat Zika Virus Infection. Cell Rep 21: 517–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen S, Li H, Liu J. 2009. Dynamic signaling for neural stem cell fate determination. Cell Adhesion & Migration 3:1: 107–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Zhang SC. 2009. Differentiation of neuroepithelia from human embryonic stem cells. Methods Mol Biol 549: 51–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Tanaka Y, Cakir B, Patterson B, Kim KY, et al. 2019. hESC-Derived Thalamic Organoids Form Reciprocal Projections When Fused with Cortical Organoids. Cell Stem Cell 24: 487–97 e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Tanaka Y, Patterson B, Kang YJ, Govindaiah G, et al. 2017. Fusion of Regionally Specified hPSC-Derived Organoids Models Human Brain Development and Interneuron Migration. Cell Stem Cell 21: 383–98 e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F, Kang E, Yu C, Qian X, Jacob F, et al. 2017. DISC1 Regulates Neurogenesis via Modulating Kinetochore Attachment of Ndel1/Nde1 during Mitosis. Neuron 96: 1041–54 e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Wang L, Yin F, Yu Y, Wang Y, et al. 2017. Probing impaired neurogenesis in human brain organoids exposed to alcohol. Integr Biol (Camb) 9: 968–78 [DOI] [PubMed] [Google Scholar]


