Abstract
Sporotrichosis generally shows no or a small number of fungal cells in tissue. Numerous fungal elements are usually associated with suppression of cellular immunity, either acquired or innate. The present case demonstrates that also topical immunosuppression can lead to increased fungal load at the affected site.
Keywords: Sporotrichosis, Sporothrix globosa, Fungal burden
1. Introduction
Members of the genus Sporothrix, agents of the implantation mycosis sporotrichosis, show two types of transmission. The large epidemics currently caused by Sporothrix brasiliensis in South America is almost exclusively transmitted by cats and dogs [1] , while outbreaks of Sporothix globosa are sapronotic [2]. Remarkably, Sporothrix schenckii shows both types of transmission [3]. In China, S. globosa is preponderant [4], except for the Nanchang area where S. schenckii occurs next to S. globosa [5].
In most sporotrichosis, histopathological detection of the etiologic agent is difficult due to a low fungal load, abundant cells being found in tissue only occasionally [6]. Sporotrichosis with numerous fungal elements is usually related to decreased immunity or underlying disorders, such as human immunodeficiency virus (HIV) infection, diabetes mellitus, alcoholism, or prolonged medication [[7], [8], [9]]. Corticosteroid therapy is one of the principal causes for this unusual histological feature. In the current paper we report a case of S. globosa infection with countless fungal cells in tissue, probably due to topical corticosteroid application.
2. Case presentation
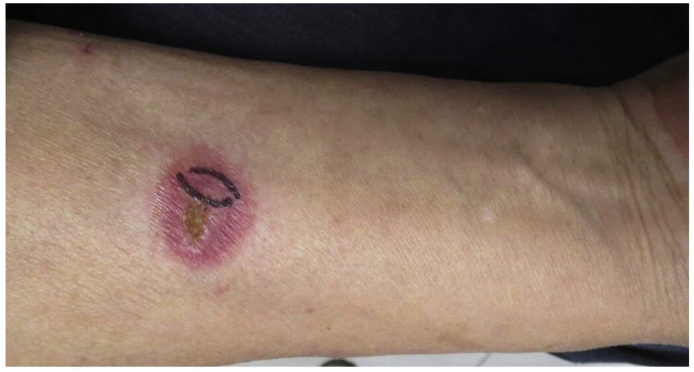
A male Greenhouse farmer in his 70s presented with an ulcerative plaque on his right forearm of 1 year's duration (day 0). According to the patient, the lesion was preceded by a wooden thorn on the vegetable rack (day-378) and subsequently a papule had developed slowly at the implantation site. The lesion was diagnosed as dermatitis at a local clinic (day -185) and topical 0.05% Fluoride Acetate Ointment was applied for 2 weeks. However the lesion expanded rapidly after that. He denied any other medication. On examination at our department (day 0), a solitary, well-defined, painless, red plaque measuring about 2 × 2 cm was located at his right forearm flexion. It had a central ulcer and yellow crusting at the center(Fig. 1). No similar lesions were found on any other part of the patient's body. Skin biopsy specimens were sent for histopathology and tissue culture. Routine complete blood cell count and complete metabolic panel were all within normal limits. An HIV test was negative.
Fig. 1.
A solitary, well-defined plaque located on the patient's right forearm with central yellow scab. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
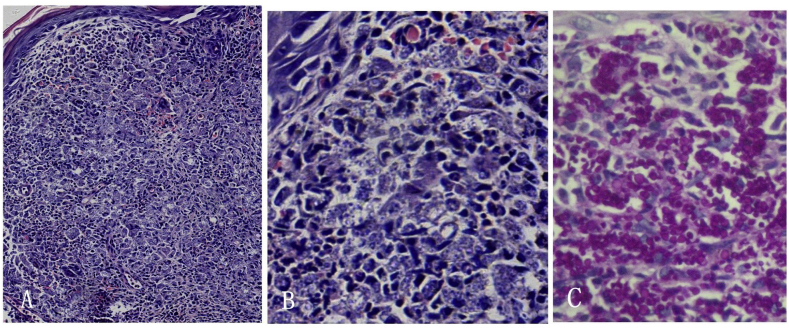
At day 5, a skin biopsy specimen revealed a dense histiocytic granulomatous infiltration surrounded by a few lymphocytes and eosinophils in the upper to middle dermis by hematoxylin and eosin staining(H&E). Few neutrophils and no abscess formation were noted. High magnification microscopy displayed disseminated, translucent, small spherical to ovoid bodies (Fig. 2A and B). Periodic-acid Schiff (PAS) staining confirmed presence of countless round fungal cells, about 2–8 μm in diam, within histiocytes and in the extracellular medium (Fig. 2C). No asteroid bodies were seen in the serial sections.
Fig. 2.
Histological examination showed dense histiocytic granulomatous infiltration and disseminated, translucent, small round or oval bodies in the dermis in H&E (A , × 10, B , × 40); Numerous yeast cells within histiocytes and in the extracellular medium are confirmed by PAS stain (C, × 40).
Culture on Sabouraud's glucose agar grew characteristic colonies of Sporothrix globosa after 5 days (day+5) of incubation at 25 °C. The initially cream-colored colonies turned brown to black after 7 days(day+12) (Fig. 3) and showed thermal dimorphism with yeast cells upon one-week incubation at 37 °C on blood glucose-cysteine agar(day+12). Microscopic structure showed branching, septate hyphae and acicular conidiogenous cells with apical conidia in a flower-like arrangement (day+15). Sequencing of the partial calmodulin gene confirmed the diagnosis as Sporothrix globosa(day+20).The patient was diagnosed as cutaneous fixed type sporotrichosis.
Fig. 3.
Culture on Sabouraud's glucose agar incubated at 25 °C, the initially cream-colored colonies turned brown to black at day 12. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Itraconazole therapy was initiated (day+15) at a dosage of 200 mg qd for 4 months while monitoring the liver and kidney function of patient every month. After a 1-year follow-up(day+12 months), the patient showed total recovery and no sign of recurrence.
3. Discussion
Sporotrichosis is a chronic, granulomatous, subcutaneous infection caused by dimorphic members of the genus Sporothrix [10]. Isolation of the fungus in culture, although sometimes difficult, is necessary to distinguish the different species which respond differently to antifungal therapy [11]. Histopathologically, the fungus is usually present in small numbers only. Observation of the agent in situ is frequently negative with H&E staining, and even when using specific techniques (e.g., PAS) [12]. A large histopathological study of 119 samples of confirmed cases of cutaneous sporotrichosis in Rio de Janeiro reported that no fungal structures were seen in 77 (64.7%) of the cases, whereas the fungus was detected only in a minority of cases (35.3%). Organisms, when detectable, were generally present in small numbers [6]. However, in contrast to these observations, the case reported here showed countless fungal elements in tissue. Sporothrix in human tissue can be seen in the form of yeast cells (spherical, ovoid or cigar-shaped), asteroid bodies (i.e. fungal structures surrounded by radial deposition of eosinophilic material), or sometimes hyphal fragments. Gori et al. [13] reported numerous branched hyphae in Giemsa-stained sputum smears obtained from an HIV-infected patient with pulmonary sporotrichosis. In our case, histopathology showed abundant spherical to ovoid yeast-like cells of variable size within histiocytes and in the extracellular medium, while asteroid bodies were not observed. As our patient had iatrogenic topical immunosuppression, discontinuation of immunosuppressive medication and prolonged treatment with sufficient-dose itraconazole were indicated. Given an exceptionally large number of organisms in the lesion, we decided for a dose of 200 mg once a day, prolonged for a 4-month course in our patient.
Although still rare, the number of sporotrichoses with abundant fungal elements upon histopathological examination seems to increase. At least 40 such cases have been reported in the English literature [6,8,14,15], and most of these concerned immunocompromised patients. Freitas et al., in 2012 reported 8 of 21 sporotrichoses with abundant fungal cells in HIV patients in Rio de Janeiro. Probably HIV-induced immunosuppression leads to uninhibited fungal reproduction. Our case showed no immunological deficiency in general, but patient underwent local application of corticosteroid ointments before biopsy. Similarly,Mohri et al. [14] revealed 14 cases of sporotrichosis with numerous fungal elements, 12 of which had topical corticosteroid application and one experienced corticosteroid administration per os. Although we are not aware of any reports addressing the effect of corticosteroids on sporotrichosis, it seems that impairment of both acquired and innate immunity enhances fungal expansion. In murine experiments of cutaneous sporotrichosis, it was shown that systemic cortisone acetate administration, after either subcutaneous or intrathoracic inoculation of S. schenckii, increased the extent and frequency of internal organ involvement and mortality rates [7]. In humans, corticosteroid therapy and other factors that interfere with the immune response are believed to play a role in the dissemination of systemic sporotrichosis [7,16]. We therefore conclude that the observed proliferation of the fungus in our patient was due to local immunosuppression induced by the topical steroids, probably via inhibition of neutrophil aggregation [14].
Declaration of competing interest
The authors have no conflicts of interest in this study.
Acknowledgements
This work was partly supported partly supported by Tianjin Municipal Science and Technology Planning Project (Grant No. 17ZXMFSY00030)and Tianjin Municipal Health Commission Scientific Research Project in Key Fields of Chinese Medicine (Grant No.2019002).
References
- 1.Montenegro H., Rodrigues A.M., Dias M.A., da S.E.A., Bernardi F., de Camargo Z.P. Feline sporotrichosis due to Sporothrix brasiliensis: an emerging animal infection in São Paulo, Brazil. BMC Vet. Res. 2014;10:269. doi: 10.1186/s12917-014-0269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taa M., Nms K., Al Z.H.S. Origin and distribution of Sporothrix globosa causing sapronoses in Asia. J. Med. Microbiol. 2017;66(5):560–569. doi: 10.1099/jmm.0.000451. [DOI] [PubMed] [Google Scholar]
- 3.de Lima Barros M.B., Schubach A.O., de Vasconcellos Carvalhaes de Oliveira R., Martins E.B., Teixeira J.L., Wanke B. Treatment of cutaneous sporotrichosis with itraconazole--study of 645 patients. Clin. Infect. Dis. 2011;52(12):e200–206. doi: 10.1093/cid/cir245. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y., Hagen F., Wan Z. Two cases of sporotrichosis of the right upper extremity in right-handed patients with diabetes mellitus. Rev. Iberoam. De. Micol. 2016;33(1):38–42. doi: 10.1016/j.riam.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Li J., Zhan P., Jiang Q. Prevalence and antifungal susceptibility of Sporothrix species in jiangxi, central China, med. Mycol. 2019;57(8):954–961. doi: 10.1093/mmy/myy163. [DOI] [PubMed] [Google Scholar]
- 6.Quintella L.P., Passos S.R., do V.A.C. Histopathology of cutaneous sporotrichosis in Rio de Janeiro: a series of 119 consecutive cases. J. Cutan. Pathol. 2011;38(1):25–32. doi: 10.1111/j.1600-0560.2010.01626.x. [DOI] [PubMed] [Google Scholar]
- 7.Bickley L.K., Berman I.J., Hood A.F. Fixed cutaneous sporotrichosis: unusual histopathology following intralesional corticosteroid administration. J. Am. Acad. Dermatol. 1985;12(6):1007–1012. doi: 10.1016/s0190-9622(85)70129-6. [DOI] [PubMed] [Google Scholar]
- 8.Freitas D.F., de Siqueira Hoagland B., do V.A.C. Sporotrichosis in HIV-infected patients: report of 21 cases of endemic sporotrichosis in Rio de Janeiro, Brazil, Med. Mycol. 2012;50(2):170–178. doi: 10.3109/13693786.2011.596288. [DOI] [PubMed] [Google Scholar]
- 9.Schechtman R.C., Crignis G.S., Pockstaller M.P., Azulay-Abulafia L., Quintella L.P., Belo M. Molluscum-like lesions in a patient with sporotrichosis. An. Bras. Dermatol. 2011;86(6):1217–1219. doi: 10.1590/s0365-05962011000600028. [DOI] [PubMed] [Google Scholar]
- 10.Barros M.B., de Almeida Paes R., Schubach A.O. Sporothrix schenckii and sporotrichosis. Clin. Microbiol. Rev. 2011;24(4):633–654. doi: 10.1128/CMR.00007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliveira D.C., Lopes P.G., Spader T.B. Antifungal susceptibilities of Sporothrix albicans, S. brasiliensis, and S. luriei of the S. schenckii complex identified in Brazil. J. Clin. Microbiol. 2011;49(8):3047–3049. doi: 10.1128/JCM.00255-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerhard R., de Moscoso P.C., Gabbi T.V., Valente N.Y. Fine-needle aspiration biopsy of disseminated sporotrichosis: a case report. Diagn. Cytopathol. 2008;36(3):174–177. doi: 10.1002/dc.20777. [DOI] [PubMed] [Google Scholar]
- 13.Gori S., Lupetti A., Moscato G., Parenti M., Lofaro A. Pulmonary sporotrichosis with hyphae in a human immunodeficiency virus-infected patient. A case report. Acta Cytol. 1997;41(2):519–521. doi: 10.1159/000332549. [DOI] [PubMed] [Google Scholar]
- 14.Mohri S., Nakajima H., Kurosawa T., Takanashi Y., Takahashi Y., Nagai R. Three cases of sporotrichosis with numerous fungal elements. J. Dermatol. 1987;14(4):382–387. doi: 10.1111/j.1346-8138.1987.tb03597.x. [DOI] [PubMed] [Google Scholar]
- 15.Fujii H., Tanioka M., Yonezawa M. A case of atypical sporotrichosis with multifocal cutaneous ulcers. Clin. Exp. Dermatol. 2008;33(2):135–138. doi: 10.1111/j.1365-2230.2007.02572.x. [DOI] [PubMed] [Google Scholar]
- 16.Coondoo A., Phiske M., Verma S., Lahiri K. Side-effects of topical steroids: a long overdue revisit. Indian Dermatol. Online J. 2014;5(4):416–425. doi: 10.4103/2229-5178.142483. [DOI] [PMC free article] [PubMed] [Google Scholar]