Figure 1.
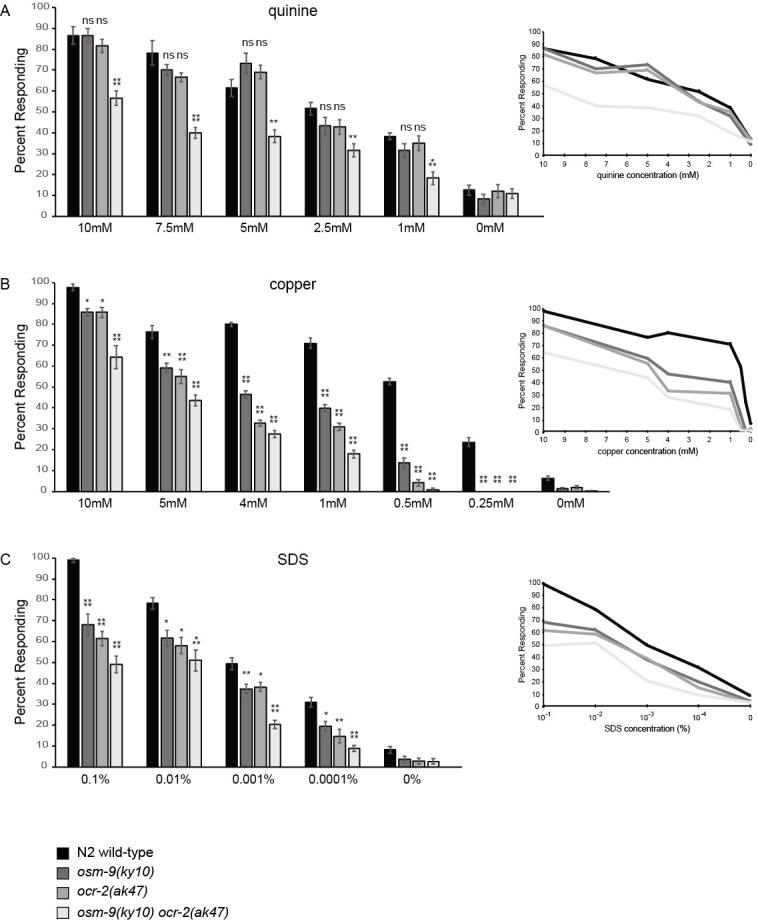
Behavioral response of well-fed young adults to aversive chemical stimuli was assessed using the drop assay 20 minutes after transfer to NGM plates lacking bacteria (“off food”). Bar graphs show the percentage of animals responding to the indicated concentrations of (A) the bitter tastant quinine, (B) the heavy metal copper (CuCl2), and (C) the detergent SDS. Inserts represent the corresponding dose-response curves for each genotype across the range of concentrations tested, for readability showing only the means from the bar graphs in panels A-C. n >/= 60 animals for each genotype were assayed at each concentration. Assays were performed on at least three separate days. All tastants were dissolved in M13 buffer, pH 7.4 (Wood 1988). Error bars represent the standard error of the mean (SEM). The one-way ANOVA with Tukey’s Honestly Significant Difference (HSD) was used for statistical analysis. * denotes p < 0.05, ** denotes p < 0.01, *** denotes p < 0.001, and **** denotes p < 0.0001 compared to wild-type animals. ns denotes p >/= 0.05. F values for 10mM – 1mM quinine, respectively: 16.7, 20.4, 16.1, 5.6, 9.2. F values for 10mM – 0.25mM copper, respectively: 19.9, 22.6, 245.7, 133.8, 212.5, 112.7. F values for 0.1% – 0.0001% SDS, respectively: 29.9, 7.6, 24.0, 11.5.
Description
Transient receptor potential (TRP) channels are a family of cation channels that are important for response to diverse external stimuli across eukaryotes (Venkatachalam and Montell 2007; Samanta et al. 2018). In C. elegans, only members of the TRPV family, which includes osm-9, ocr-1, ocr-2, ocr-3 and ocr-4 (Colbert and Bargmann 1995; Colbert et al. 1997; Tobin et al. 2002), have been shown to play a role in chemosensory behavior (Bargmann 2006). OSM-9 and OCR-2 are co-expressed in six pairs of sensory neurons: AWA, ASH, ADL, ADF, PHA and PHB (Colbert et al. 1997; Tobin et al. 2002). In these cells, they are thought to come together to function as a single channel complex to mediate sensory transduction since their localization to the cilia is mutually dependent upon each other (Tobin et al. 2002).
In the literature, OSM-9 and OCR-2 are often referred to as being required for all ASH-mediated avoidance behaviors. Indeed, osm-9 and ocr-2 mutant animals are defective for response to nose touch and high osmolarity, although ocr-2 mutants did retain some ability to respond to the highest osmotic strength tested (4M) (Colbert et al. 1997; Tobin et al. 2002). osm-9 and ocr-2 mutants are also severely defective in avoidance of 2-octanone (Tobin et al. 2002) and 1-octanol (Ezak et al. 2010). Both mutants are defective in avoidance of high pH (Sassa et al. 2013; Wang et al. 2016), and osm-9 mutants are defective in CuSO4 avoidance when the assay was performed in a plate chemotaxis format (Wang et al. 2015). However, osm-9 and ocr-2 single mutants, as well as osm-9 ocr-2 double mutant animals, retain partial response to bitter tastants, including quinine, when tested at 10mM (Hilliard et al. 2004; Ezak et al. 2010). Animals lacking either or both channels also retain an intermediate level of response to the heavy metal copper (10mM CuCl2) and the detergent SDS (0.1%) (Ezak et al. 2010).
We sought to determine the extent to which OSM-9 and OCR-2 contribute to the ASH-mediated behavioral avoidance of quinine (Hilliard et al. 2004), copper (Sambongi et al. 1999) and SDS (Hilliard et al. 2002). We used the drop assay as previously described (Hilliard et al. 2002; Fukuto et al. 2004; Hilliard et al. 2004; Ezak et al. 2010; Krzyzanowski et al. 2013) to assess the percentage of wild-type, osm-9(ky10), ocr-2(ak47), and osm-9(ky10) ocr-2(ak47) animals responding to each soluble chemical stimulus across a range of concentrations (Figure 1).
Although in one study osm-9 mutant animals were reported to have a modest but statistically significant defect in 10mM quinine avoidance (Hilliard et al. 2004), we found that individual loss of neither OSM-9 nor OCR-2 function affected response to 10mM quinine (Figure 1A), similar to our previous report (Ezak et al. 2010). Further, osm-9 and ocr-2 single mutants responded similarly to wild-type animals across the range of quinine concentrations tested (10mM – 1mM, Figure 1A). Only in the osm-9 ocr-2 double mutant animals was a partial defect in quinine avoidance seen, at each concentration (Figure 1A).
In response to copper (CuCl2), at each concentration the osm-9 and ocr-2 single mutants, as well as the osm-9 ocr-2 double mutant, were partially defective in the avoidance response (Figure 1B). Only at the lowest concentrations tested (0.5mM and 0.25mM) were either the single or double mutant animals essentially lacking response (Figure 1B). Similarly, in response to SDS only a partial avoidance defect was seen for osm-9 and ocr-2 single mutants at 0.1% – 0.001%, with the double mutant showing a somewhat greater defect at 0.001% (Figure 1C).
Taken together, assaying animals at intermediate concentrations of quinine, copper and SDS demonstrated that even when lacking the function of both OSM-9 and OCR-2, a substantial percentage of animals retained behavioral response to these aversive stimuli. Thus, although OSM-9 and OCR-2 are key components in ASH-mediated chemosensory avoidance, their loss leads to diminished, but not absent, avoidance responses at most concentrations tested. This suggests that an additional channel(s) may contribute to chemical avoidance in the absence of these TRPV channels.
Reagents
The strains N2 Bristol wild-type, CX10 osm-9(ky10) and CX4544 ocr-2(ak47) were obtained from the Caenorhabditis Genetics Center, which is funded in part by the National Institutes of Health – Office of Research Infrastructure Programs. LX748 osm-9(ky10) ocr-2(ak47) was a gift from Michael Koelle and has not been sent to the CGC.
Acknowledgments
Acknowledgments
We thank Michael Koelle for the LX748 strain and Aditi Chaubey for help with statistical analysis.
Funding
This work was supported by the National Institutes of Health (grant R01DC015758 to DMF).
References
- Bargmann CI. Chemosensation in C. elegans. WormBook. 2006 Oct 25;:1–29. doi: 10.1895/wormbook.1.123.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert HA, Bargmann CI. Odorant-specific adaptation pathways generate olfactory plasticity in C. elegans. Neuron. 1995 Apr 01;14(4):803–812. doi: 10.1016/0896-6273(95)90224-4. [DOI] [PubMed] [Google Scholar]
- Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 1997 Nov 01;17(21):8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezak MJ, Hong E, Chaparro-Garcia A, Ferkey DM. Caenorhabditis elegans TRPV channels function in a modality-specific pathway to regulate response to aberrant sensory signaling. Genetics. 2010 Feb 22;185(1):233–244. doi: 10.1534/genetics.110.115188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuto HS, Ferkey DM, Apicella AJ, Lans H, Sharmeen T, Chen W, Lefkowitz RJ, Jansen G, Schafer WR, Hart AC. G protein-coupled receptor kinase function is essential for chemosensation in C. elegans. Neuron. 2004 May 27;42(4):581–593. doi: 10.1016/s0896-6273(04)00252-1. [DOI] [PubMed] [Google Scholar]
- Hilliard MA, Bargmann CI, Bazzicalupo P. C. elegans responds to chemical repellents by integrating sensory inputs from the head and the tail. Curr Biol. 2002 Apr 30;12(9):730–734. doi: 10.1016/s0960-9822(02)00813-8. [DOI] [PubMed] [Google Scholar]
- Hilliard MA, Bergamasco C, Arbucci S, Plasterk RH, Bazzicalupo P. Worms taste bitter: ASH neurons, QUI-1, GPA-3 and ODR-3 mediate quinine avoidance in Caenorhabditis elegans. EMBO J. 2004 Feb 26;23(5):1101–1111. doi: 10.1038/sj.emboj.7600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzanowski MC, Brueggemann C, Ezak MJ, Wood JF, Michaels KL, Jackson CA, Juang BT, Collins KD, Yu MC, L'etoile ND, Ferkey DM. The C. elegans cGMP-dependent protein kinase EGL-4 regulates nociceptive behavioral sensitivity. PLoS Genet. 2013 Jul 11;9(7):e1003619–e1003619. doi: 10.1371/journal.pgen.1003619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta A, Hughes TET, Moiseenkova-Bell VY. Transient Receptor Potential (TRP) Channels. Subcell Biochem. 2018;87:141–165. doi: 10.1007/978-981-10-7757-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambongi Y, Nagae T, Liu Y, Yoshimizu T, Takeda K, Wada Y, Futai M. Sensing of cadmium and copper ions by externally exposed ADL, ASE, and ASH neurons elicits avoidance response in Caenorhabditis elegans. Neuroreport. 1999 Mar 17;10(4):753–757. doi: 10.1097/00001756-199903170-00017. [DOI] [PubMed] [Google Scholar]
- Sassa T, Murayama T, Maruyama IN. Strongly alkaline pH avoidance mediated by ASH sensory neurons in C. elegans. Neurosci Lett. 2013 Jun 12;555:248–252. doi: 10.1016/j.neulet.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Tobin DM, Madsen DM, Kahn-Kirby A, Peckol EL, Moulder G, Barstead R, Maricq AV, Bargmann CI. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron. 2002 Jul 18;35(2):307–318. doi: 10.1016/s0896-6273(02)00757-2. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Xu ZJ, Wu YQ, Qin LW, Li ZY, Wu ZX. Off-response in ASH neurons evoked by CuSO4 requires the TRP channel OSM-9 in Caenorhabditis elegans. Biochem Biophys Res Commun. 2015 Apr 12;461(3):463–468. doi: 10.1016/j.bbrc.2015.04.017. [DOI] [PubMed] [Google Scholar]
- Wang X, Li G, Liu J, Liu J, Xu XZ. TMC-1 Mediates Alkaline Sensation in C. elegans through Nociceptive Neurons. Neuron. 2016 Jun 16;91(1):146–154. doi: 10.1016/j.neuron.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, W.B. (1988) <i>The Nematode Caenorhabditis elegans</i>. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York.


