Introduction
Nocardia are aerobic bacteria that exist ubiquitously in the environment (eg, soil) and typically cause disease in immunocompromised individuals (the occurrence rate may be as high as 2.65% in solid organ transplant recipients, with an associated overall mortality of approximately 17%).1 Nocardiosis typically presents as pneumonia in organ transplant recipients but may also cause primary cutaneous infections after traumatic inoculation and disseminate to multiple organs, including the brain, liver, kidney, bones, and pericardium.2,3 Microscopically, Nocardia have gram-positive, branched filaments that may fragment into coccal or bacillary elements.4 Both Actinomyces and Nocardia demonstrate similar gram-positive filaments but can be distinguished because Actinomyces stain negative on acid-fast staining, whereas Nocardia typically stain at least partially with the modified Kinyoun method.3 Rapidly growing Mycobacterium and Nocardia grow on the same media (eg, Löwenstein-Jensen) but may be distinguished by the fine filamentous morphology of Nocardia (rapidly growing Mycobacterium typically exhibit coccobacillary morphology).5,6 However, confirming the identity of Nocardia in culture according to the growth of aerial hyphae typically requires at least 3 to 5 days and can be obfuscated by more rapidly growing bacteria.3,5,7
Case report
A 72-year-old man with a complex medical history (factor V Leiden deficiency, ischemic cardiomyopathy, treated pulmonary cryptococcosis, thin basement membrane disease, and renal transplantation after nephrectomy for clear cell renal cell carcinoma) sustained a laceration to the right forearm after a fall onto gravel. A tetanus vaccine booster was administered, the wound was irrigated, and bacitracin was applied daily. He received maintenance mycophenolate, tacrolimus, and prednisone for immunosuppression without trimethoprim-sulfamethoxazole prophylaxis.
Three weeks after sustaining the laceration, he reported onset of a painful, pustular rash on the extensor surface of the right arm that did not respond to outpatient clindamycin or ceftriaxone (Fig 1). On admission, computed tomographic imaging of the arm showed no organized fluid, gas, or acute osseous abnormality. He began receiving cefepime, metronidazole, vancomycin, and acyclovir empirically, and skin biopsies for hematoxylin-eosin staining and tissue culture were obtained. Acyclovir was discontinued after herpes simplex virus and varicella zoster virus polymerase chain reaction swab results were negative. One day later, he had proximal extension of tender erythema. Empirical therapy was switched to amphotericin B for fungal coverage, ciprofloxacin for atypical water/environmental bacteria, and meropenem and linezolid for empirical Nocardia coverage. Typical processing methods were used for gram, modified Kinyoun, Fite, fungal, and auramine-rhodamine staining. Microbiology laboratory analysis showed no organisms on gram or calcofluor stain. Auramine rhodamine acid-fast bacilli screening showed greater than 2 acid-fast bacilli; thus, empirical therapy was modified to azithromycin, imipenem, tigecycline, ciprofloxacin, and linezolid to cover rapidly growing Mycobacterium (eg, M abscessus, M chelonae, M fortuitum). Results for 2 series of blood cultures were negative. An initial surface bacterial swab grew only coagulase-negative staphylococcus.
Fig 1.
Clustered pustules on a diffuse erythematous, edematous plaque of the elbow.
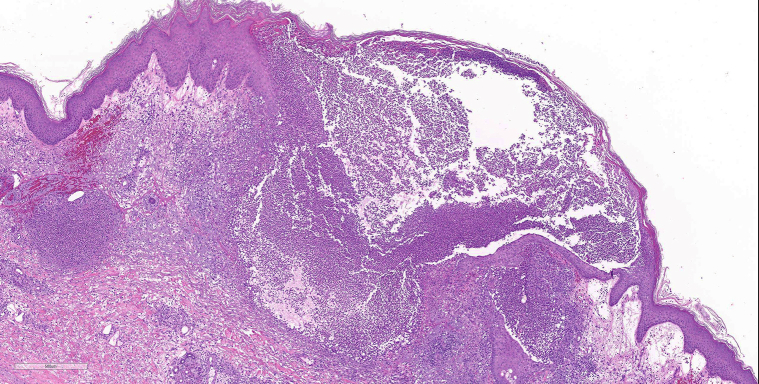
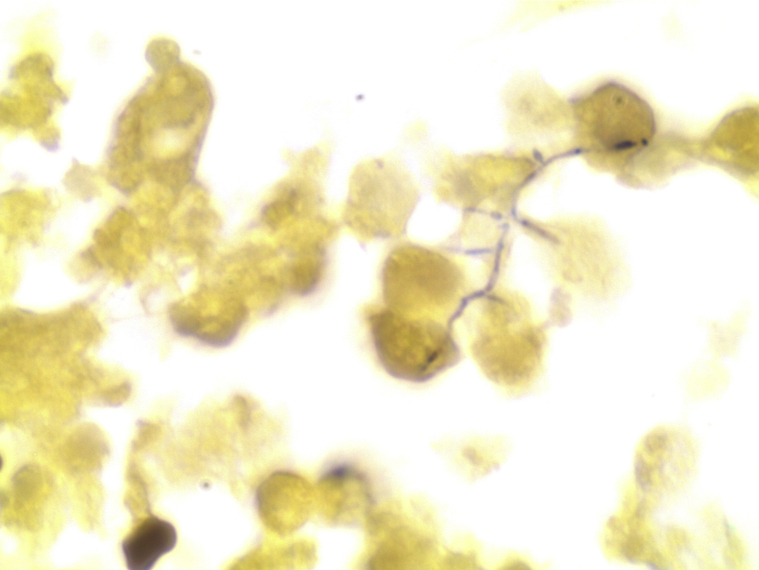
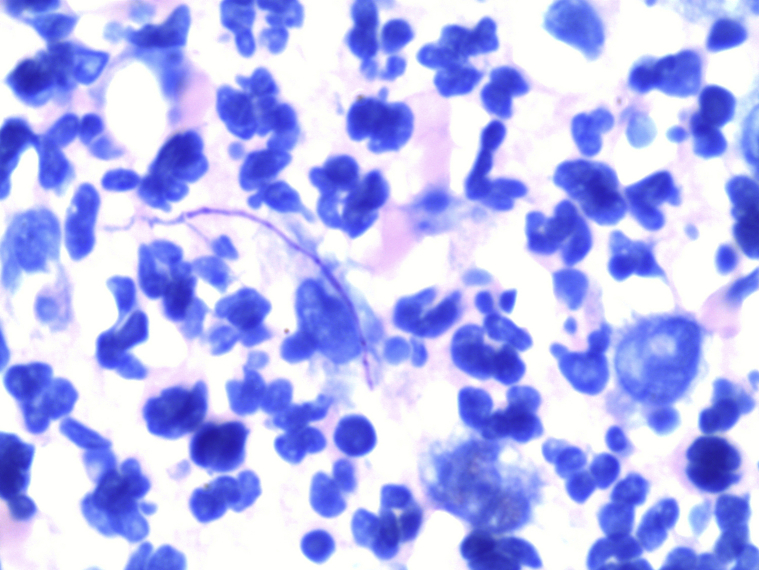
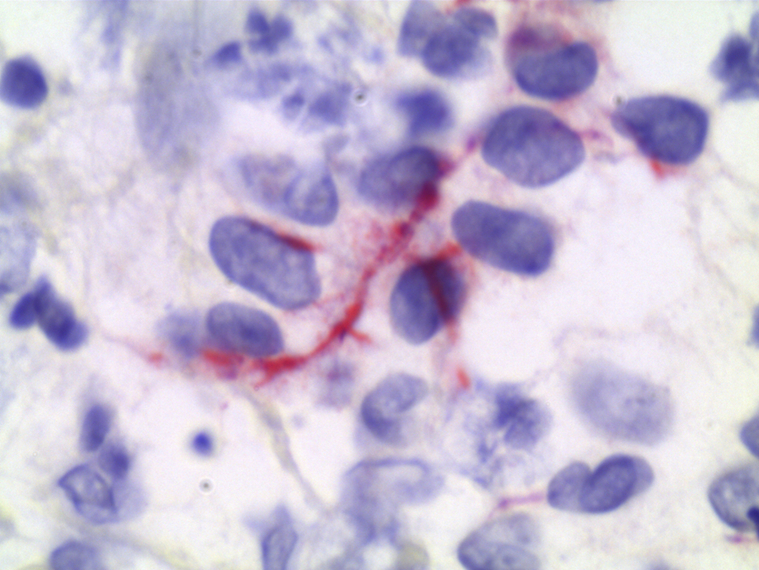
On microscopic examination, hematoxylin-eosin staining demonstrated extensive neutrophilic dermal infiltration concerning for infection, but no organisms were observed (Fig 2). Fine, filamentous bacteria weakly stained with tissue gram stain (Fig 3) and acid-fast bacilli stain (Fig 4) but were rare and difficult to visualize. Because of the initial difficulty in finding these structures on tissue gram and acid-fast bacilli stains, an immunohistochemistry stain for M tuberculosis was used. The staining clearly highlighted the filamentous bacteria and showed long, branching forms (Fig 5) characteristic of Nocardia infection. The immunohistochemistry staining consisted of polyclonal rabbit IgG to M tuberculosis and was used according to manufacturer specifications (902-140-062713, Biocare Medical, Concord, CA). Furthermore, the filamentous morphology of the Nocardia organisms was better appreciated in the immunohistochemistry M tuberculosis staining in tissue sections than in the previous auramine-rhodamine–stained smear. Based on the morphologic findings in the histologic slides, the antibiotic therapy was modified to cover nocardiosis with linezolid, trimethoprim-sulfamethoxazole, and renally dosed amoxicillin/clavulanate.
Fig 2.
Extensive papillary dermal edema with sheets of neutrophils in the upper and mid reticular dermis, accompanying hemorrhage, and rare multinucleated giant cells (well-formed granulomas were not found). The deeper dermis and subcutaneous fat show edema with patent blood vessels and few inflammatory cells. The epidermis shows acanthosis, spongiosis, focal parakeratosis, and foci of neutrophils. No bacteria were noted. No herpetic viral cytopathic changes were noted. (Hematoxylin-eosin stain; original magnification: ×5.)
Fig 3.
Weakly stained filamentous structures suggestive of Nocardia infection. (Gram stain; original magnification: ×100.)
Fig 4.
Weakly stained filamentous structures suggestive of Nocardia infection. (Acid-fast bacilli stain; original magnification: ×100.)
Fig 5.
Staining using M tuberculosis antibody, showing rare filamentous structures suggestive of Nocardia infection. (Immunohistochemistry stain; original magnification: ×100.)
Three days after biopsy, white chalky colonies typical of Nocardia spp were observed on chocolate and colistin nalidixic acid agars of the routine culture of the biopsy. The same organism also grew on the fungal culture, but the acid-fast bacilli culture remained without growth. The organism was identified as N brasiliensis via matrix-assisted laser desorption ionization–time of flight mass spectrometry using the VITEK MS (bioMérieux, Durham, NC) with knowledge base v3.2 according to the manufacturer's recommended protocol for Nocardia and mycobacteria. Antibody results for blastomycosis, aspergillus, histoplasmosis, and coccidioidomycosis were negative. On postbiopsy day 4, trimethoprim-sulfamethoxazole was discontinued because of acute renal dysfunction, and the patient continued receiving linezolid and amoxicillin/clavulanate for nocardiosis (sensitivity testing confirmed organism susceptibility). The patient declined recommended brain magnetic resonance imaging and dedicated chest computed tomography to assess for occult dissemination. After 14 days of therapy, his right-arm wounds were markedly improved. However, 25 days after skin biopsy, he died because of cardiac failure.
Discussion
In the current case, misidentification of Nocardia as rapidly growing Mycobacterium led to a delay in pathogen-directed anti-infective therapy by 1 day. Because Nocardia can take days to weeks to grow in culture, it is important to use reliable staining techniques to promptly obtain a more narrow presumptive diagnosis and to avoid prematurely discarding cultures with falsely negative results.7
Although auramine-rhodamine staining allows greater sensitivity and ease of slide review, there is decreased specificity and the potential for false-positive Mycobacterium results, as in our case.8 Nocardia may also be misidentified as mycobacteria when acid-fast staining is used, particularly in older cultures in which partially acid-fast branched long filaments fragment into bacilli and cocci that may be misinterpreted as mycobacteria.4,5 In contrast, younger cultures demonstrate the branched filaments characteristic of Nocardia.5 Although the use of 1% acid in the modified Kinyoun protocol allows distinction between partially acid-fast Nocardia and nonstaining Actinomyces, there is a greater potential for misdiagnosis because of the retention of the stain by a wider range of organisms than just Mycobacterium.3,7 In this case, although auramine-rhodamine confirmed the presence of acid-fast organisms, rapidly growing Mycobacterium rather than Nocardia was initially suspected.
Because of equivocal acid-fast bacilli staining showing filamentous structures and difficulty identifying organisms on tissue gram stain, we used an immunohistochemistry M tuberculosis stain to confirm our suspicion of nocardiosis before verification with culture and matrix-assisted laser desorption ionization–time of flight analysis. This method allowed pathogen-directed narrowing of antimicrobial therapy 24 hours before culture-based identification. The immunohistochemistry M tuberculosis stain we used has previously demonstrated cross-reactivity with Nocardia species, a feature that is likely due to shared cell wall mycolic acids and polysaccharides (arabinomannan and arabinogalactan) among the Corynebacteriaceae suborder (eg, Nocardia spp and Mycobacterium spp).2,9 Although the immunohistochemistry stain for M tuberculosis antibody we used reacts with other atypical organisms (eg, M avium, M. phlei, and M parafortuitum), atypical mycobacteria tend to appear shorter, thicker, more clustered, and sometimes beaded, and have less branching than Nocardia spp. Thus, on reexamination of the skin biopsy with immunohistochemistry stain for M tuberculosis, the branching filamentous appearance of the bacteria in concert with weak staining on tissue gram staining and acid-fast bacilli staining facilitated the diagnosis of nocardiosis. Nocardiosis was subsequently confirmed by culture and matrix-assisted laser desorption ionization–time of flight results.
Despite the delay in pathogen-directed therapy, our patient improved clinically with linezolid and amoxicillin/clavulanate. Given a high rate of susceptibility, trimethoprim-sulfamethoxazole and linezolid are considered the treatment of choice, with a treatment duration of at least 3 months up to 1 year.4,10 Regardless, prompt initiation of appropriate antimicrobials is crucial to mitigating morbidity and mortality, especially in immunosuppressed patients.
In summary, careful evaluation of bacteria morphology in skin biopsy sections with gram, acid-fast, and immunohistochemistry M tuberculosis stains may allow prompt distinction between nocardiosis and other types of acid-fast bacterial infections before organism confirmation by culture analysis.
Acknowledgments
We would like to honor the memory of our wonderful patient and thank his gracious spouse for encouraging us to report his case, which we hope will benefit the medical community and other patients. We would also like to thank Dr Kevin Alby for his expertise and guidance on the use of matrix-assisted laser desorption ionization–time of flight and cultures for this case.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Majeed A., Beatty N., Iftikhar A. A 20-year experience with nocardiosis in solid organ transplant (SOT) recipients in the Southwestern United States: a single-center study. Transpl Infect Dis. 2018;20(4):e12904. doi: 10.1111/tid.12904. [DOI] [PubMed] [Google Scholar]
- 2.Stevens D.A. Clinical and clinical laboratory aspects of nocardial infection. J Hyg (Lond) 1983;91(3):377–384. doi: 10.1017/s0022172400060423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McHugh K.E., Sturgis C.D., Procop G.W., Rhoads D.D. The cytopathology of Actinomyces, Nocardia, and their mimickers. Diagn Cytopathol. 2017;45(12):1105–1115. doi: 10.1002/dc.23816. [DOI] [PubMed] [Google Scholar]
- 4.Queipo-Zaragoza J.A., Broseta-Rico E., Alapont-Alacreu J.M., Santos-Durantez M., Sanchez-Plumed J., Jimenez-Cruz J.F. Nocardial infection in immunosuppressed kidney transplant recipients. Scand J Urol Nephrol. 2004;38(2):168–173. doi: 10.1080/00365590410025353. [DOI] [PubMed] [Google Scholar]
- 5.Muricy E.C., Lemes R.A., Bombarda S., Ferrazoli L., Chimara E. Differentiation between Nocardia spp. and Mycobacterium spp.: critical aspects for bacteriological diagnosis. Rev Inst Med Trop Sao Paulo. 2014;56(5):397–401. doi: 10.1590/S0036-46652014000500005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kothavade R.J., Dhurat R.S., Mishra S.N., Kothavade U.R. Clinical and laboratory aspects of the diagnosis and management of cutaneous and subcutaneous infections caused by rapidly growing mycobacteria. Eur J Clin Microbiol Infect Dis. 2013;32(2):161–188. doi: 10.1007/s10096-012-1766-8. [DOI] [PubMed] [Google Scholar]
- 7.Olson E.S., Simpson A.J., Norton A.J., Das S.S. Not everything acid fast is Mycobacterium tuberculosis–a case report. J Clin Pathol. 1998;51(7):535–536. doi: 10.1136/jcp.51.7.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kommareddi S., Abramowsky C.R., Swinehart G.L., Hrabak L. Nontuberculous mycobacterial infections: comparison of the fluorescent auramine-O and Ziehl-Neelsen techniques in tissue diagnosis. Hum Pathol. 1984;15(11):1085–1089. doi: 10.1016/s0046-8177(84)80253-1. [DOI] [PubMed] [Google Scholar]
- 9.Solomon I.H., Johncilla M.E., Hornick J.L., Milner D.A., Jr. The utility of immunohistochemistry in mycobacterial infection: a proposal for multimodality testing. Am J Surg Pathol. 2017;41(10):1364–1370. doi: 10.1097/PAS.0000000000000925. [DOI] [PubMed] [Google Scholar]
- 10.Wang H.L., Seo Y.H., LaSala P.R., Tarrand J.J., Han X.Y. Nocardiosis in 132 patients with cancer: microbiological and clinical analyses. Am J Clin Pathol. 2014;142(4):513–523. doi: 10.1309/AJCPW84AFTUWMHYU. [DOI] [PubMed] [Google Scholar]