Abstract
Since originally isolated in 1899, the genus Bifidobacterium has been demonstrated to predominate in the gut microbiota of breastfed infants and to benefit the host by accelerating maturation of the immune response, balancing the immune system to suppress inflammation, improving intestinal barrier function, and increasing acetate production. In particular, Bifidobacterium longum subspecies infantis (B. infantis) is well adapted to the infant gut and has co-evolved with the mother-infant dyad and gut microbiome, in part due to its ability to consume complex carbohydrates found in human milk. B. infantis and its human host have a symbiotic relationship that protects the preterm or term neonate and nourishes a healthy gut microbiota prior to weaning. To provide benefits associated with B. infantis to all infants, a number of commercialized strains have been developed over the past decades. As new ingredients become available, safety and suitability must be assessed in preclinical and clinical studies. Consideration of the full clinical evidence for B. infantis use in pediatric nutrition is critical to better understand its potential impacts on infant health and development. Herein we summarize the recent clinical studies utilizing select strains of commercialized B. infantis.
Keywords: Bifidobacterium longum subspecies infantis, B. infantis, probiotics, pediatric nutrition, human milk oligosaccharides, inflammation, gut health, microbiome, short chain fatty acids, acetate
1. Introduction
The universal importance of bifidobacteria in human microbiota and gastrointestinal (GI) health from infancy to advanced years (as reviewed [1]), including the critical role of genus Bifidobacterium in the process of immune maturation early in life (as reviewed [2]), have been detailed in a wealth of literature. Bifidobacteria are Gram-positive, heterofermentative, anaerobic bacteria with a distinctive bifid (i.e., “Y”) shape [3]. Genus Bifidobacterium was originally isolated from the stool of breastfed infants in 1899, by Henri Tissier (as reviewed [2]). Bifidobacteria colonize the newborn gut within the first days and weeks after birth and represent the most abundant bacterial genus ranging from 40% to 80% of the total gut microbiota [4,5]. Vertical transmission of bifidobacteria from the mother (vagina, GI tract, or breast milk) has been demonstrated [6]. Vaginal delivery provides a higher abundance of Bifidobacterium spp. in infants compared to caesarean section (C-section) delivery [7,8]; however, the differences in the gut microbiomes of C-section and vaginally delivered neonates are not apparent until day five of life [9]. Further, differences in Bifidobacterium spp. colonization between vaginal and C-section delivery diminish by 30 days of age [10], highlighting the first month of life as a critical period to establish colonization. Reduced abundance of Bifidobacterium spp. in infants has been correlated to chronic diseases, including asthma and obesity [11], as well as to lower vaccine response [12]. Henrick and colleagues postulated that loss of Bifidobacterium spp. in the infant gut in populations of developed countries is linked to increased incidence of allergic and autoimmune diseases [13]. Bifidobacterium spp. abundance can be further influenced by nutrition, antibiotic use, and puberty [14,15,16]. Bifidobacterium longum subspecies infantis (B. infantis) in particular, dominates the gut microbiota of breastfed infants and benefits the host by accelerating maturation of the immune response, balancing the immune system to suppress inflammation, improving intestinal barrier function, and increasing acetate production [17]. This symbiotic relationship is an example of coevolution (humans and B. infantis) to protect the full term neonate and nourish a healthy gut microbiota prior to weaning (as reviewed [18]).
Here, we focus on the current status of knowledge for B. infantis, including the preclinical data and clinical evidence.
2. Current Status of Knowledge
2.1. Carbohydrate Metabolism and Short Chain Fatty Acids (SCFA) Production
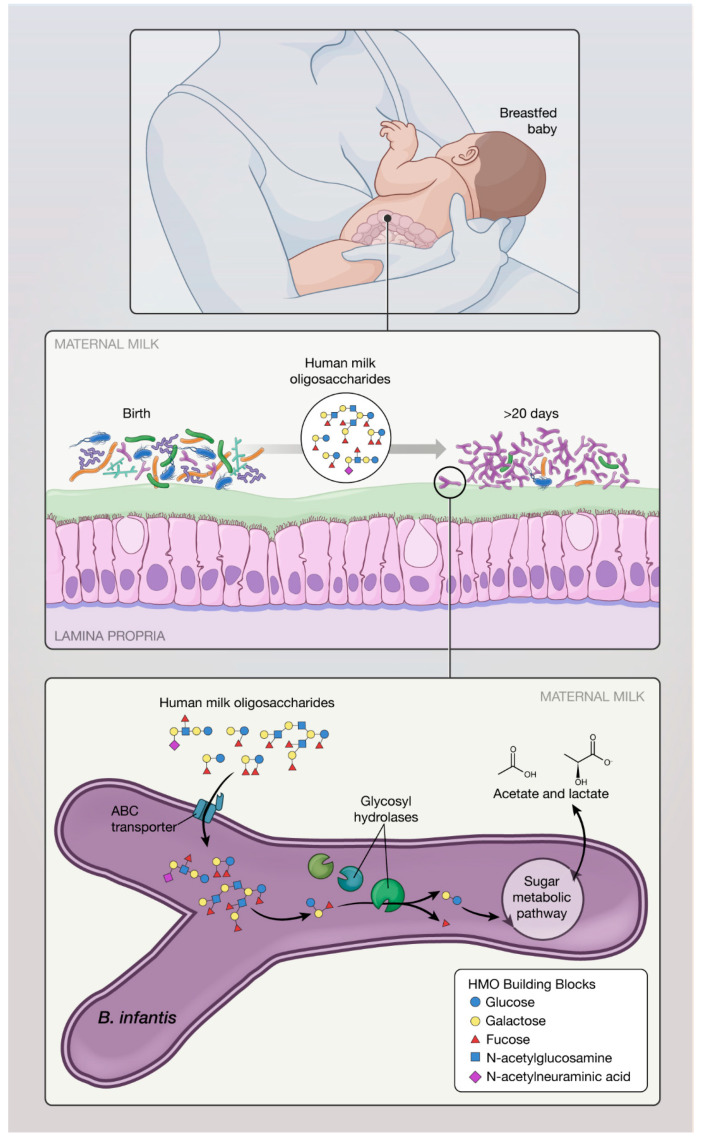
Human milk oligosaccharides (HMO) are the primary substrate for B. infantis metabolism (as reviewed [19]). HMO in human milk cannot be metabolized by the infant or by the majority of bacteria in the infant gut, which lack the required enzymes necessary to access and metabolize complex HMO. B. infantis demonstrates growth in vitro using HMO as the sole carbon source, reaching a cell density 3-fold higher than B. longum subsp. longum, B. breve, B. bifidum, and B. adolescentis [20,21]. Once consumed by the infant, intact HMO transit through the infant stomach and proximal small bowel, and are transported directly into the cytoplasm of B. infantis (Figure 1) to activate many genes involved in catabolism of HMO (“HMO cluster”) [22]. Compared to other Bifidobacterium species, the ability of B. infantis to sequester and facilitate complete digestion of HMO (via expression of up to 16 glycosyl hydrolases, such as α-fucosidases, β-galactosidases, β-hexosaminadases, and α-sialidases) [22,23,24] provides a competitive advantage over other GI microbiota, including pathogens (Figure 2A). Genes encoding carbohydrate transporters present in B. infantis also contribute to protection against Escherichia coli O157:H7 [25]. Of importance, HMO utilization genes are conserved across all B. infantis strains [26]. B. infantis preferentially consumes smaller HMO species (degree of polymerization <7) which are consistently produced over the course of lactation and represent the bulk of HMO present in pooled milk samples [27]. HMO metabolism by B. infantis produces short chain fatty acids (SCFA), such as acetate, which provides an important role in nutrition and intestinal and immune development, facilitates direct binding to intestinal cells, and stimulates anti-inflammatory/inhibits pro-inflammatory cytokine release by intestinal cells [3,28,29]. Acetate produced by bacteria acts in vivo to promote the defense functions of host epithelial cells [25]. Further acetate produced by B. infantis becomes a carbon source that stimulates growth and function of butyrate-producing microbes [30]. Thus, HMO stimulate the growth of bifidobacteria and by cross-feeding increase the production of butyrate, a preferred energy source for colonocytes [31]. In addition to their role in the gut, SCFA produced by B. infantis can enter circulation and directly affect the adipose tissue, lungs, brain, and liver, inducing overall beneficial metabolic effects [31]. For example, a small fraction of acetate crosses the blood–brain barrier, where it is taken up and activates hypothalamic neurons driving satiety [32], suggesting that acetate has a direct role in central appetite regulation. Lactate also was shown to cross blood–brain barrier and functions as an energy substrate of the brain [33].
Figure 1.
During breastfeeding, infant ingests human milk oligosaccharides (HMO) which allow selective growth of B. infantis in the lower gut. B. infantis thrives and dominates the gut of breastfed infant, providing numerous benefits important in healthy development. B. infantis is evolutionary unique in its ability to metabolize HMO and produce short chain fatty acids (SCFA).
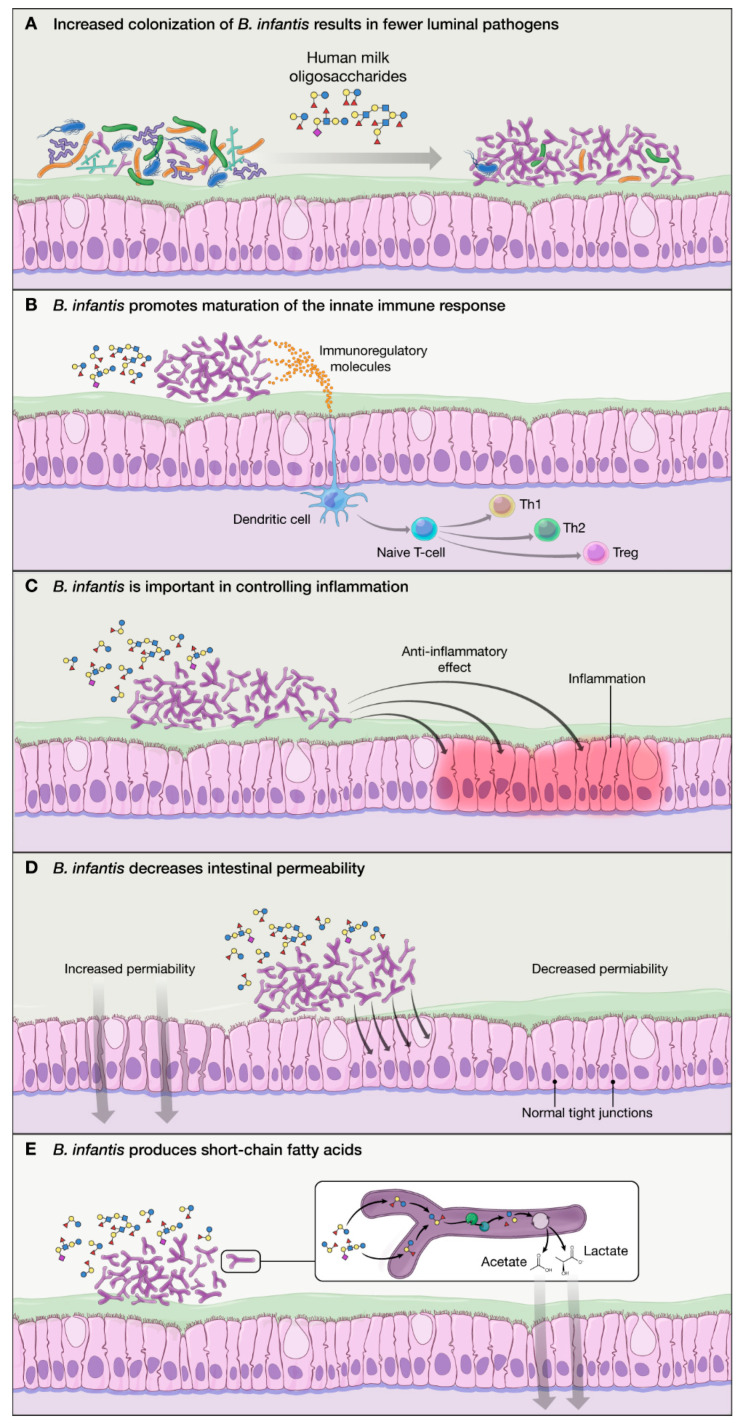
Figure 2.
Mechanisms of action of B. infantis: (A) B. infantis is equipped in “enzymatic machinery” to metabolize HMO, allowing increased colonization and fewer luminal pathogens via competitive advantage; (B) B. infantis produces exogenous substances that promote maturation of the immature innate immune response in breastfed infants; (C) B. infantis has anti-inflammatory properties benefiting the epithelial layer in the gut; (D) B. infantis reduces intestinal permeability and helps with “leaky gut”; and (E) B. infantis produces SCFA (particularly acetate) which have a beneficial effect on the host.
Whereas other bacteria consume HMO (e.g., Bacteroidaceae), only Bifidobacteriaceae converts HMO to acidic end products that affect stool pH [13]. Therefore, acidic fermentation of selected HMO by B. infantis may result in fecal pH changes and reduction of dysbiotic taxa in the gut. Preclinical models, including the necrotizing enterocolitis (NEC) model, have demonstrated that B. infantis could potentially decrease intestinal permeability and increase stabilization of tight junction proteins [28,34]. For example, indole-3-lactic acid (ILA) is an anti-inflammatory molecule which significantly attenuates lipopolysaccharide-induced activation of NF-κB in macrophages; it also attenuates an increase in the pro-inflammatory cytokine IL-8. B. infantis contributes to maintaining the intestinal barrier integrity through ILA, a metabolite of tryptophan [35], and may protect the epithelium via activation of the aryl hydrogen receptor, which can further promote normal intestinal immune function. B. infantis could also potentially protect against excessive intestinal inflammation which often affects the premature infant and may be a contributing factor in NEC.
2.2. Proposed Mechanisms of Action of B. infantis Based on In Vitro Studies:
B. infantis is highly specialized for the consumption of HMO and has a competitive advantage against other bacteria, allowing increased colonization and resulting in fewer luminal pathogens (Figure 2A) [36].
B. infantis produces exogenous substances that promote maturation of the immature innate immune response (Figure 2B) [28].
HMO “turn on” the repertoire of genes in B. infantis that are important in controlling inflammation within the infant gut (Figure 2C) [22].
B. infantis becomes dominant in the gut and reduces pH by its unique ability to metabolize all HMO into acidic end products, lactate, and acetate (Figure 2E) [36].
B. infantis improves the intestinal barrier integrity through the production of tryptophan metabolite, indole-3-lactic acid [35].
2.3. Clinical Evidence: Safety and Efficacy of Select Commercialized Strains of B. infantis Used in Pediatric Populations
In 2007, the European Food Safety Authority (EFSA) assigned qualified presumption of safety (QPS) status to the bacterial species B. longum, which includes subspecies infantis, indicating that this taxonomic group does not raise any safety concerns [37]. The QPS status, which applies to all strains of B. infantis listed in this review, indicates that none of those strains have been associated with human clinical disease. In addition, based on our knowledge, B. infantis has not been associated with any reports on antibiotic resistance. Here, we summarize clinical evidence for safety and efficacy of select commercialized B. infantis strains used in pediatric populations (Table 1).
Table 1.
Clinical trials in pediatric populations using select strains of B. infantis.
|
B. infantis Strain (Manufacturer) |
Trial ID | Site | Enrollment | Feeding Period | Study Design/ Study Groups |
Study Outcomes | Conclusions | Year |
|---|---|---|---|---|---|---|---|---|
| M63 (Morinaga) |
n/a | France | 66 infants | 1 month | Term infants identified with colic a at enrollment (3 w to 3 months) multicenter double-blind randomized controlled trial (DBRCT)
|
|
|
2010 [38] |
| ClinicalTrials.gov NCT00920166 |
France | 97 infants | 6 months | Term infants (<postnatal day [PND] 3 at enrollment. Multicenter DBRCT
Stool samples collected at 1 and 6 months. |
Primary
|
|
2011 [39] | |
| n/a | Italy | 55 children (4–12 years old [yo]) with functional constipation. | 8 weeks | Prospective, placebo-controlled, randomized trial
|
|
|
2017 [40] | |
| ClinicalTrials.gov NCT02807064 |
Italy | 40 children (9 yo) with allergic rhinitis and asthma. | 4 weeks | Prospective, placebo-controlled, randomized trial
|
Primary
|
|
2017 [41] | |
| ClinicalTrials.gov NCT02566876 |
Italy | 73 children (8–16 yo) with abdominal pain (AP)-associated functional GI disorders (FGID). | 6 weeks | Prospective, placebo-controlled, randomized trial
|
Primary
|
|
2017 [42] | |
| not known | Japan | 44 infants (low-birthweight). | 6 weeks |
|
|
|
2013 [43] | |
| ATCC 15697 | ClinicalTrials.gov NCT00810160 |
US | 12 premature infants. | 5 weeks |
|
|
|
2013 [44] |
| ClinicalTrials.gov NCT01316510 |
US | 24 infants with gastroschisis. | 6 weeks (or hospital discharge) |
|
Primary
|
|
2016 [45] | |
| UCD272 (Culture Systems Inc) | ClinicalTrials.gov NCT02086110 (listed as SC268) |
US | 11 children (2–11 yo) with ASD | 12 weeks | DBRC
|
Primary
|
|
2019 [46] |
| EVC001 (Evolve Biosystems) |
ClinicalTrials.gov NCT02457338 |
US | 80 mother/ infant dyads |
21 days | Randomized, parallel assignment
stool samples collected through PND 60. |
|
|
2017 [47] |
|
In infants who received EVC001:
|
2017 [36] | ||||||
|
|
2018 [48] | ||||||
|
|
2018 [49] |
||||||
|
|
2019 [50] | ||||||
| CECT 7210 (IM-1®, Ordesa S.L.) |
Clinicaltrials.gov NCT02096302 |
Spain | 151 term infants | 12 weeks | Multicenter DBRCT
|
Primary
|
In CECT 7210 group:
|
2018 [51] |
| BB-02 (Chr. Hansen) | Australia and New Zealand Clinical Trials Register, ACTRN012607000144415 |
Australia/New Zealand | 1099 preterm infants | Through hospital discharge or term corrected age |
Multicenter DBRCT
|
Primary
|
Incidence of NEC was significantly reduced in infants receiving the probiotic combination but not definite late-onset sepsis or mortality. | 2013 [52] |
|
Higher levels of Bifidobacterium spp. found in infants who received the probiotics; Enterococcus reduced in infants receiving the probiotic mix during the supplementation period | 2018 [53] | ||||||
| R0033 (Lallemand) | Clinicaltrials.gov NCT02215304 | Spain | 221 infants | 8 weeks | Multicenter DBRCT
|
Primary
|
Use of R0033 was safe and well tolerated. No impact on growth (weight, height, and head circumference), adverse events, or serious adverse events. Increased ratio of IL-10/IL-12 and significant reduction in Collinsella, Enterococcus, and Klebsiella genera in infants receiving R0033. |
2017 [54] 2018 [55] |
| 135 children (3–7 yo) |
3 months | Multicenter DBRCT
|
Percentage of children free of any episodes of ear, nose and throat, respiratory tract, or gastrointestinal illness |
Synbiotic preparation decreased the risk of occurrence of common infectious diseases. No side effects were detected in either group. | 2010 [56] | |||
| BT1 | germanctr.de (DRKS00003660) |
Germany | 106 infants | 12 months | Double-blind, randomized, placebo-controlled study
|
Composition of the fecal microbiota (16s rRNA sequencing) and fecal metabolome (HPLC). | Probiotic formula modulated the infant stool microbiome (e.g., Bacteroides) and metabolome (e.g., lipids) at very early stages of life, with no detectable long-term consequences. | 2017 [57] |
a colic defined using Wessel criteria [58]; b from postnatal day (PND) 7.
2.3.1. B. infantis M63
Term infants identified with colic were enrolled (n = 66) in a multicenter double-blind randomized controlled trial (DBRCT); participants received a standard infant formula (IF) or an IF with added probiotics (10 million CFU B. infantis M63 per 100 mL formula (Morinaga, Japan)) and 10 million CFU Lactobacillus rhamnosus LCS742 per 100 mL formula [38]) over a one month feeding period. Feeding-related GI adverse events were significantly lower in infants receiving M63. The same probiotic mix was used in another DBRCT trial, where term infants (n = 97; <postnatal day (PND) three of age) received M63 and LCS742 (140 million CFU per 100 mL formula, each) for six months [39]. After one month of feeding, infants receiving IF with added probiotics exhibited less crying or agitation and more quiet behavior (p = 0.03) and at six months, atopic dermatitis was less frequently observed (p < 0.05). Overall, the results from these two studies suggest the potential beneficial effect in a population of children with colic as being improved tolerance and a protective effect against the development of atopic dermatitis.
A probiotic mix of M63 (1 billion CFU/day), B. breve M16 (1 billion CFU/day), and B. longum BB536 (3 billion CFU/day) has been studied in several clinical trials. For example, children (n = 55, 4–12 years of age) with functional constipation received polyethylene glycol (PEG, a laxative) or PEG and the probiotic mix over an eight week period [40]. The probiotic mix was demonstrated as safe; however, no difference in efficacy was demonstrated between groups. In a placebo-controlled study, children (n = 40; 9 ± 2.2 years of age) with allergic rhinitis and asthma received the same probiotic mix over a four week period [41] and showed significant improvement of symptoms. In a DBRCT crossover trial, children (8–16 years of age) with irritable bowel syndrome (IBS) (n = 48) and functional dyspepsia (n = 25) received the same probiotic mix over a six week period [42]. Administration of probiotics improved abdominal pain in children with IBS but not with functional dyspepsia. The proportion of IBS children who reported an improvement in QoL (quality of life) was significantly higher after probiotics than after placebo (48% vs. 17%). In a trial conducted in Japan (n = 44, six weeks old), low-birthweight infants assigned to a modified probiotic mix of M63, M16, and BB536 (500 million CFU/day of each strain) over a six week feeding period demonstrated higher bifidobacteria GI colonization compared to those receiving M16 alone [43]. The above studies used a probiotic mix including B. infantis M63. Although the benefits cannot be directly attributed exclusively to B. infantis, the results are in agreement with several mechanisms of B. infantis described above.
2.3.2. B. infantis ATCC 15697
A study by Underwood et al. [44] consisted of two phases; in phase one, premature infants (n = 12, five weeks old) received formula feedings and were randomized to receive B. infantis ATCC 15697 (4 billion CFU twice daily) or B. animalis subsp. lactis with doses increased over a five week feeding period. In phase two, nine premature infants receiving their mother’s milk received each of those two bifidobacteria for two weeks separated by a one-week washout period. Fecal bifidobacteria count was significantly higher and, in case of phase two, Proteobacteria were significantly lower in the ATCC 15697 group, with authors concluding that ATCC 15697 was more effective at colonizing the premature infants compared to B. animalis sbsp. lactis [44]. The study also demonstrated that the combination of human milk and B. infantis was most effective at “normalizing” the fecal microbiota confirming the specialized ability of B. infantis to metabolize HMO.
Infants with confirmed gastroschisis were enrolled (n = 24, gestational age at birth > 34 weeks) in a placebo-controlled pilot study. Participants were randomized to receive either ATCC 15697 (1 billion CFU) or a placebo twice daily for six weeks or until hospital discharge [45]. ATCC 15697 was well tolerated, even during the period of gastric suctioning. Infants fed B. infantis had higher fecal Bifidobacteriaceae, lower Clostridiaceae, and trends toward lower Enterobacteriaceae, Enterococcaceae, Staphylococcaceae, and Streptococcaceae. Clinical outcomes, including length of hospital stay did not differ between groups.
2.3.3. B. infantis UCD272
Children with autism spectrum disorders (ASD; n = 11, 2 to 11 years of age) and a history of chronic constipation, diarrhea, or IBS were enrolled in a crossover study; participants received a combination of B. infantis UCD272 (20 billion CFU/day; Culture Systems, Inc., Mishawaka, IN) and a prebiotic (bovine colostrum product) over a five-week period, followed by a two-week washout, and five weeks of prebiotic alone [46]. UCD272 was well tolerated; however, a trend toward greater reduction in GI symptoms, aberrant behavior and immune imbalance was observed during use of the prebiotic alone. Conclusions were limited due to the small number of participants enrolled.
2.3.4. B. infantis EVC001
Clinical outcomes related to B. infantis EVC001 in breastfed infants were described in several recent publications. Mother/infant dyads (n = 80) were randomized by parallel assignment to either lactation support (LS; Control) or LS + EVC001 (Evolve Biosystems, Davis, CA) over a 21-day feeding period [47]. EVC001 was packaged in single-use sachets (156 mg; 18 billion CFU) and delivered diluted with lactose starting at postnatal day (PND) seven [47]. Primary outcomes included health and safety reporting (body temperature, GI symptom ratings, number of illnesses and sick doctor visits, use of antibiotics or gas-relieving medications, presence of colic, jaundice, flatulence, or bloody stool) and stool Bifidobacterium spp. counts from PND 6 to 28. Stool samples were collected through PND 60. In each group, 34 participants were included in the analysis. Stool Bifidobacterium spp. count was significantly higher and stool frequency significantly lower in infants receiving EV0001 from PND 6 to 28. No group differences in health and safety outcomes were detected. One month after discontinuing feeding EVC001, stool count of EVC001 persisted and was significantly higher compared to the control group [36]. The dominance of EVC001 influenced beta diversity (diversity between samples); however, there were no differences in terms of microbial species richness (alpha diversity) as the Shannon diversity index was similar between Control and EVC001 groups. Lack of differences in alpha diversity between Control and EVC001 is consistent with previous reports on breast-fed infants. Recently, lower alpha diversity was reported in infants receiving human milk compared to infant formula [59]. In addition, stool HMO from PND 6 to 29 were significantly lower (suggesting increased bifidobacteria metabolism) in the EVC001 group compared to the control group; acetate and lactate were significantly higher in the EVC001 vs. the control group. Increased production of lactate and acetate alters the intestinal environment to prohibit the growth of pH-sensitive pathogenic populations (e.g., Enterobacteriaceae and Clostridia). Specifically, average fecal pH was 5.15 in infants colonized by EVC001, compared to 5.97 fecal pH and 10-fold higher fecal HMO in infants lacking EVC001 colonization [36]. Stool endotoxins were 4-fold lower in the EVC001 group, consistent with observed lower levels of Gram-negative Proteobacteria and Bacteroidetes. The relative abundance of virulent pathogens such as E. coli, Klebsiella, Clostridium and Staphylocossus were decreased by over 93% [48]. Shotgun metagenomics was used to examine the effect of feeding EVC001 upon antibiotic resistance genes (ARG, i.e., the resistome) in infants (Control, n = 31; EVC001, n = 29) from PND 7 to 21 [48]. The resistome is the collection of all the antibiotic resistance genes, including those associated with pathogenic bacteria, non-pathogenic antibiotic producing bacteria, and all other resistance genes [60]. ARG are associated with resistance to a wide range of drugs including β-lactamase, fluoroquinolone, and tetracycline. In the EVC001 group 87.5% fewer ARG were detected in the microbiome; 38 ARG were significantly reduced, and relative and absolute abundance of Escherichia (which predominantly harbored ARG) were also significantly reduced [48]. It is possible that infants with fewer ARG could be less likely to exhibit resistance to a wider spectrum of drug classes, however clinical studies are needed to confirm this theory. Further analyses indicated much less evidence of mucous layer erosion and inflammation in infants in the EVC001 compared to the control group. Specifically, using nano-HPLC chip/time-of-flight mass spectrometry, EVC001 was demonstrated to help maintain barrier function by diminishing colonic glycan degradation [49]. In continuation of analyses conducted on the same cohort, significantly more stool N-glycans (specific complex carbohydrates released from milk glycoproteins and available for select use by B. infantis) were measured in stool samples from the EVC001 group. The release of selectively fermentable N-glycans may increase the prebiotic activity and increase the growth of EVC001 in vivo. Finally, in stool samples from the same cohort, significantly lower calprotectin and proinflammatory cytokines were reported in infants receiving EVC001 [50] suggesting lower enteric inflammation.
2.3.5. B. infantis CECT 7210
Term infants (<3 months of age) enrolled in a multicenter DBRCT were randomized to receive a standard IF (Control, n = 97) or IF with added B. infantis CECT 7210 at 10 million CFU per 100 mL formula (Laboratorio Ordesa SL, Spain) (n = 93) for a 12-week feeding period [51]. Parent-reported incidence of diarrhea was the primary outcome. Secondary outcomes included incidence of infection, salivary IgA levels, stool microbiota, infant growth, and tolerance measures. A total of 151 infants completed the study (Control, n = 78; CECT 7210, n = 73). Overall diarrhea events per infant (median; Control: 0.29 ± 1.07, CECT 7210: 0.05 ± 0.28) were not significantly different between groups. Stool frequency was significantly lower in the control group after four weeks of study feeding. By study end, total stool Bifidobacterium spp. was similar between groups; however, B. infantis was significantly higher in the CECT7210 group. No differences in growth, formula intake, or other secondary outcomes were observed. Overall, the authors concluded that formula with added CECT was safe, effective, and well tolerated in healthy term infants.
2.3.6. B. infantis BB02
Jacobs and colleagues studied a mix of three probiotics, including the strain B. infantis BB02 in preterm infants (<32 completed weeks of gestation, 1500 g or less) enrolled in a multicenter DBRCT. Infants were randomized to receive daily administration of a probiotic combination (BB02, Streptococcus thermophilus TH4, and B. lactis BB12; 1 billion CFU/day; Solgar, NJ, USA; n = 548) or a placebo (maltodextrin; n = 551) [52]. The primary outcome was at least one episode of definite late-onset sepsis. Incidence of NEC (Bell stage 2 or higher) was significantly reduced in infants receiving the probiotic combination; no group difference in definite late-onset sepsis or all-cause mortality was detected. Stool microbiome analysis of the study cohort demonstrated significantly increased Bifidobacterium spp. count and reduced genus Enterococcus count in infants who received probiotics [53]. Considering that this probiotic combination included other probiotic strains in addition to B. infantis, the observed benefits cannot be attributed definitively to BB02. However, the observed increase in Bifidobacterium spp. and reduction in NEC are consistent with the mechanisms we described above, e.g., increase of beneficial microbiota and improvement of gut barrier integrity.
2.3.7. B. infantis R0033
Healthy term infants were enrolled (n = 208; 3 to 12 months of age) and received B. infantis R0033 (3 billion CFU/d; Lallemand Health Solutions, Montreal, Canada) over an eight week feeding period [54]. Other study groups included L. helveticus and B. bifidum. R0033 was safe, well tolerated, and had no impact on growth (weight, height, and head circumference). A significant decrease in fecal Blautia, Collinsella, Enterococcus and Klebsiella genera and increase in the IL-10/IL-12 ratio were demonstrated in infants receiving R0033, suggesting anti-pathogenic and anti-inflammatory activity [55]. The anti-inflammatory effect of R0033 can be potentially attributed to production of ILA (see “Carbohydrate Metabolisms and SCFA Production” above), although ILA was not measured in this study.
In another DBRCT, children three to seven years of age (n = 135) received a daily synbiotic preparation (R0033, L. helveticus R0052, B. bifidum R0071 (3 billion CFU total), and fructo-oligosaccharide (FOS)) over a three month feeding period [56]. Otherwise healthy, participants suffered from at least three episodes of ear, nose and throat, respiratory tract, or GI illness during the previous winter. Overall, supplementation with a probiotic mix, which included R0033, significantly decreased the risk of occurrence of common infectious diseases in children. No side effects were detected in either group. As in several other studies listed in our review, the associated benefits cannot be exclusively attributed to R003 alone. However, the decrease in infectious diseases, including GI illnesses is supported by well-described mechanisms of B. infantis, which include prohibition of pH-sensitive pathogenic organisms.
2.3.8. B. infantis BT1
Term infants were enrolled (n = 106, newborn) in a DBRCT; participants received a control IF or IF with an added probiotic mix of B. infantis BT1, B. bifidum BF3, B. breve BR3, and B. longum BG7 (total 10 million CFU/g) through 12 months of age to measure fecal microbiota diversity and composition [57]. Although Bacteroides and Blautia spp. counts were lower after one month of feeding in infants receiving probiotics, no group differences in microbiome or metabolite profile were detected after 12 months of age. No significant differences were observed between infant feeding groups regarding growth, antibiotic uptake, or other health variables.
3. Conclusions
Studies reviewed in this manuscript suggest that colonization with B. infantis might have potential beneficial effects in infants and children. B. infantis is well adapted to the infant gut, in part due to its ability to consume complex carbohydrates found in human milk (HMO). As evidenced in clinical studies, the administration of B. infantis leads to successful colonization in the gut, where the highly selective and acidic fermentation of HMO results in increased production of lactate and acetate, and subsequent reduction of gut dysbiosis and lower pH. This potentially includes an important role in development and maturation of the immune system. Modern practices, including C-section and perinatal antibiotics have disrupted the mother-to-infant transfer of B. infantis at birth, leading to a loss of this key member of the infant gut microbiota, which has resulted in an increase of stool pH in infants [13]. Continuing to gather evidence of the protective effects of B. infantis strains within the infant gut will help us better understand the critical role of the infant gut microbiome in establishing long-term health.
Acknowledgments
We would like to recognize the professional illustration services of Haderer & Müller–Biomedical Art, LLC (haderermuller.com).
Author Contributions
All authors (M.C., N.S., J.L.W., S.S.W. and J.A.V.) contributed to the drafting, editing, and revision of this review. All authors have approved the submitted version and agree to be personally accountable for its accuracy and integrity. All authors have read and agreed to the published version of the manuscript.
Funding
This review received no external funding.
Conflicts of Interest
M.C., N.S., J.L.W. and S.S.W. are employed by the study sponsor, Mead Johnson Nutrition. Mead Johnson Nutrition’s parent company, Reckitt Benckiser, entered into a partnership with Evolve Biosystems in December 2019; however, this had no influence on the outcome of the review. J.A.V. has been a consultant for Mead Johnson Nutrition.
References
- 1.Arboleya S., Watkins C., Stanton C., Ross R.P. Gut bifidobacteria populations in human health and aging. Front. Microbiol. 2016;7:1204. doi: 10.3389/fmicb.2016.01204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Neill I., Schofield Z., Hall L.J. Exploring the role of the microbiota member Bifidobacterium in modulating immune-linked diseases. Emerg. Top. Life Sci. 2017;1:333–349. doi: 10.1042/etls20170058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pokusaeva K., Fitzgerald G.F., van Sinderen D. Carbohydrate metabolism in Bifidobacteria. Genes Nutr. 2011;6:285–306. doi: 10.1007/s12263-010-0206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ling X., Linglong P., Weixia D., Hong W. Protective effects of bifidobacterium on intestinal barrier function in LPS-induced enterocyte barrier injury of caco-2 monolayers and in a rat NEC model. PLoS ONE. 2016;11:e0161635. doi: 10.1371/journal.pone.0161635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makino H., Martin R., Ishikawa E., Gawad A., Kubota H., Sakai T., Oishi K., Tanaka R., Ben-Amor K., Knol J., et al. Multilocus sequence typing of bifidobacterial strains from infant’s faeces and human milk: Are bifidobacteria being sustainably shared during breastfeeding? Benef. Microbes. 2015;6:563–572. doi: 10.3920/BM2014.0082. [DOI] [PubMed] [Google Scholar]
- 6.Duranti S., Lugli G.A., Mancabelli L., Armanini F., Turroni F., James K., Ferretti P., Gorfer V., Ferrario C., Milani C., et al. Maternal inheritance of bifidobacterial communities and bifidophages in infants through vertical transmission. Microbiome. 2017;5:66. doi: 10.1186/s40168-017-0282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milani C., Mancabelli L., Lugli G.A., Duranti S., Turroni F., Ferrario C., Mangifesta M., Viappiani A., Ferretti P., Gorfer V., et al. Exploring vertical transmission of bifidobacteria from mother to child. Appl. Environ. Microbiol. 2015;81:7078–7087. doi: 10.1128/AEM.02037-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J., Cai W., Feng Y. Development of intestinal bifidobacteria and lactobacilli in breast-fed neonates. Clin. Nutr. 2007;26:559–566. doi: 10.1016/j.clnu.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Wampach L., Heintz-Buschart A., Hogan A., Muller E.E.L., Narayanasamy S., Laczny C.C., Hugerth L.W., Bindl L., Bottu J., Andersson A.F., et al. Colonization and succession within the human gut microbiome by archaea, bacteria, and microeukaryotes during the first year of life. Front. Microbiol. 2017;8:738. doi: 10.3389/fmicb.2017.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grönlund M.M., Lehtonen O.P., Eerola E., Kero P. Fecal microflora in healthy infants born by different methods of delivery: Permanent changes in intestinal flora after cesarean delivery. J. Pediatr. Gastroenterol. Nutr. 1999;28:19–25. doi: 10.1097/00005176-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Ly N.P., Litonjua A., Gold D.R., Celedón J.C. Gut microbiota, probiotics, and vitamin D: Interrelated exposures influencing allergy, asthma, and obesity? J. Allergy Clin. Immunol. 2011;127:1087–1094. doi: 10.1016/j.jaci.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huda M.N., Lewis Z., Kalanetra K.M., Rashid M., Ahmad S.M., Raqib R., Qadri F., Underwood M.A., Mills D.A., Stephensen C.B. Stool microbiota and vaccine responses of infants. Pediatrics. 2014;134:e362–e372. doi: 10.1542/peds.2013-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henrick B.M., Hutton A.A., Palumbo M.C., Casaburi G., Mitchell R.D., Underwood M.A., Smilowitz J.T., Frese S.A. Elevated fecal ph indicates a profound change in the breastfed infant gut microbiome due to reduction of Bifidobacterium over the past century. mSphere. 2018;3 doi: 10.1128/mSphere.00041-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voreades N., Kozil A., Weir T.L. Diet and the development of the human intestinal microbiome. Front. Microbiol. 2014;5 doi: 10.3389/fmicb.2014.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yatsunenko T., Rey F.E., Manary M.J., Trehan I., Dominguez-Bello M.G., Contreras M., Magris M., Hidalgo G., Baldassano R.N., Anokhin A.P., et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koenig J.E., Spor A., Scalfone N., Fricker A.D., Stombaugh J., Knight R., Angenent L.T., Ley R.E. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. USA. 2011;108:4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Underwood M.A., German J.B., Lebrilla C.B., Mills D.A. Bifidobacterium longum subspecies infantis: Champion colonizer of the infant gut. Pediatr. Res. 2015;77:229–235. doi: 10.1038/pr.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sela D.A., Mills D.A. Nursing our microbiota: Molecular linkages between bifidobacteria and milk oligosaccharides. Trends Microbiol. 2010;18:298–307. doi: 10.1016/j.tim.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chichlowski M., German J.B., Lebrilla C.B., Mills D.A. The influence of milk oligosaccharides on microbiota of infants: Opportunities for formulas. Ann. Rev. Food Sci. Technol. 2011;2:331–351. doi: 10.1146/annurev-food-022510-133743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ward R.E., Ninonuevo M., Mills D.A., Lebrilla C.B., German J.B. In vitro fermentability of human milk oligosaccharides by several strains of bifidobacteria. Mol. Nutr. Food Res. 2007;51:1398–1405. doi: 10.1002/mnfr.200700150. [DOI] [PubMed] [Google Scholar]
- 21.LoCascio R.G., Niñonuevo M.R., Kronewitter S.R., Freeman S.L., German J.B., Lebrilla C.B., Mills D.A. A versatile and scalable strategy for glycoprofiling bifidobacterial consumption of human milk oligosaccharides. Microb. Biotechnol. 2009;2:333–342. doi: 10.1111/j.1751-7915.2008.00072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sela D.A., Chapman J., Adeuya A., Kim J.H., Chen F., Whitehead T.R., Lapidus A., Rokhsar D.S., Lebrilla C.B., German J.B., et al. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc. Natl. Acad. Sci. USA. 2008;105:18964–18969. doi: 10.1073/pnas.0809584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sela D.A., Garrido D., Lerno L., Wu S., Tan K., Eom H.J., Joachimiak A., Lebrilla C.B., Mills D.A. Bifidobacterium longum subsp. infantis ATCC 15697 α-fucosidases are active on fucosylated human milk oligosaccharides. Appl. Environ. Microbiol. 2012;78:795–803. doi: 10.1128/AEM.06762-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sela D.A., Li Y., Lerno L., Wu S., Marcobal A.M., German J.B., Chen X., Lebrilla C.B., Mills D.A. An infant-associated bacterial commensal utilizes breast milk sialyloligosaccharides. J. Biol. Chem. 2011;286:11909–11918. doi: 10.1074/jbc.M110.193359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukuda S., Toh H., Hase K., Oshima K., Nakanishi Y., Yoshimura K., Tobe T., Clarke J.M., Topping D.L., Suzuki T., et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 26.LoCascio R.G., Desai P., Sela D.A., Weimer B., Mills D.A. Broad conservation of milk utilization genes in Bifidobacterium longum subsp. infantis as revealed by comparative genomic hybridization. Appl. Environ. Microbiol. 2010;76:7373–7381. doi: 10.1128/AEM.00675-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ninonuevo M.R., Perkins P.D., Francis J., Lamotte L.M., LoCascio R.G., Freeman S.L., Mills D.A., German J.B., Grimm R., Lebrilla C.B. Daily variations in oligosaccharides of human milk determined by microfluidic chips and mass spectrometry. J. Agric. Food Chem. 2008;56:618–626. doi: 10.1021/jf071972u. [DOI] [PubMed] [Google Scholar]
- 28.Chichlowski M., De Lartigue G., German J.B., Raybould H.E., Mills D.A. Bifidobacteria isolated from infants and cultured on human milk oligosaccharides affect intestinal epithelial function. J. Pediatr. Gastroenterol. Nutr. 2012;55:321–327. doi: 10.1097/MPG.0b013e31824fb899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Underwood M.A., Arriola J., Gerber C.W., Kaveti A., Kalanetra K.M., Kananurak A., Bevins C.L., Mills D.A., Dvorak B. Bifidobacterium longum subsp. infantis in experimental necrotizing enterocolitis: Alterations in inflammation, innate immune response, and the microbiota. Pediatr. Res. 2014;76:326. doi: 10.1038/pr.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duncan S.H., Holtrop G., Lobley G.E., Calder A.G., Stewart C.S., Flint H.J. Contribution of acetate to butyrate formation by human faecal bacteria. Br. J. Nutr. 2007;91:915–923. doi: 10.1079/BJN20041150. [DOI] [PubMed] [Google Scholar]
- 31.Koh A., De Vadder F., Kovatcheva-Datchary P., Backhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 32.Frost G., Sleeth M.L., Sahuri-Arisoylu M., Lizarbe B., Cerdan S., Brody L., Anastasovska J., Ghourab S., Hankir M., Zhang S., et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nature Commun. 2014;5:3611. doi: 10.1038/ncomms4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bozzo L., Puyal J., Chatton J.Y. Lactate modulates the activity of primary cortical neurons through a receptor-mediated pathway. PLoS ONE. 2013;8:e71721. doi: 10.1371/journal.pone.0071721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergmann K.R., Liu S.X.L., Tian R., Kushnir A., Turner J.R., Li H.L., Chou P.M., Weber C.R., De Plaen I.G. Bifidobacteria Stabilize Claudins at Tight Junctions and Prevent Intestinal Barrier Dysfunction in Mouse Necrotizing Enterocolitis. Am. J. Pathol. 2013;182:1595–1606. doi: 10.1016/j.ajpath.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meng D., Sommella E., Salviati E., Campiglia P., Ganguli K., Djebali K., Zhu W., Walker W.A. Indole-3-lactic acid, a metabolite of tryptophan, secreted by Bifidobacterium longum subspecies infantis is anti-inflammatory in the immature intestine. Pediatr. Res. 2020 doi: 10.1038/s41390-019-0740-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frese S.A., Hutton A.A., Contreras L.N., Shaw C.A., Palumbo M.C., Casaburi G., Xu G., Davis J.C.C., Lebrilla C.B., Henrick B.M., et al. Persistence of supplemented Bifidobacterium longum subsp. infantis EVC001 in breastfed infants. mSphere. 2017;2 doi: 10.1128/mSphere.00501-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.EFSA Introduction of a Qualified Presumption of Safety (QPS) approach for assessment of selected microorganisms referred to EFSA—Opinion of the Scientific Committee. EFSA J. 2007;5:587. doi: 10.2903/j.efsa.2007.587. [DOI] [Google Scholar]
- 38.Dupont C., Rivero M., Grillon C., Belaroussi N., Kalindjian A., Marin V. α-Lactalbumin-enriched and probiotic-supplemented infant formula in infants with colic: Growth and gastrointestinal tolerance. Eur. J. Clin. Nutr. 2010;64:765. doi: 10.1038/ejcn.2010.81. [DOI] [PubMed] [Google Scholar]
- 39.Rozé J.C., Barbarot S., Butel M.J., Kapel N., Waligora-Dupriet A.J., De Montgolfier I., Leblanc M., Godon N., Soulaines P., Darmaun D., et al. An α-lactalbumin-enriched and symbiotic-supplemented v. a standard infant formula: A multicentre, double-blind, randomised trial. Br. J. Nutr. 2011;107:1616–1622. doi: 10.1017/S000711451100479X. [DOI] [PubMed] [Google Scholar]
- 40.Russo M., Giugliano F.P., Quitadamo P., Mancusi V., Miele E., Staiano A. Efficacy of a mixture of probiotic agents as complementary therapy for chronic functional constipation in childhood. Ital. J. Pediatr. 2017;43:24. doi: 10.1186/s13052-017-0334-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miraglia Del Giudice M., Indolfi C., Capasso M., Maiello N., Decimo F., Ciprandi G. Bifidobacterium mixture (B longum BB536, B infantis M-63, B breve M-16V) treatment in children with seasonal allergic rhinitis and intermittent asthma. Ital. J. Pediatr. 2017;43:25. doi: 10.1186/s13052-017-0340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giannetti E., Maglione M., Alessandrella A., Strisciuglio C., De Giovanni D., Campanozzi A., Miele E., Staiano A. A Mixture of 3 Bifidobacteria Decreases Abdominal Pain and Improves the Quality of Life in Children With Irritable Bowel Syndrome: A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Crossover Trial. J. Clin. Gastroenterol. 2017;51:e5–e10. doi: 10.1097/MCG.0000000000000528. [DOI] [PubMed] [Google Scholar]
- 43.Ishizeki S., Sugita M., Takata M., Yaeshima T. Effect of administration of bifidobacteria on intestinal microbiota in low-birth-weight infants and transition of administered bifidobacteria: A comparison between one-species and three-species administration. Anaerobe. 2013;23:38–44. doi: 10.1016/j.anaerobe.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Underwood M.A., Kalanetra K.M., Bokulich N.A., Lewis Z.T., Mirmiran M., Tancredi D.J., Mills D.A. A comparison of two probiotic strains of bifidobacteria in premature infants. J. Pediatr. 2013;163:1585–1591. doi: 10.1016/j.jpeds.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Powell W.T., Borghese R.A., Kalanetra K.M., Mirmiran M., Mills D.A., Underwood M.A. Probiotic administration in infants with gastroschisis: A pilot randomized placebo-controlled trial. J. Pediatr. Gastroenterol. Nutr. 2016;62:852–857. doi: 10.1097/MPG.0000000000001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanctuary M.R., Kain J.N., Chen S.Y., Kalanetra K., Lemay D.G., Rose D.R., Yang H.T., Tancredi D.J., German J.B., Slupsky C.M., et al. Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms. PLoS ONE. 2019;14:e0210064. doi: 10.1371/journal.pone.0210064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smilowitz J.T., Moya J., Breck M.A., Cook C., Fineberg A., Angkustsiri K., Underwood M.A. Safety and tolerability of Bifidobacterium longum subspecies infantis EVC001 supplementation in healthy term breastfed infants: A phase I clinical trial. BMC Pediatr. 2017;17:133. doi: 10.1186/s12887-017-0886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Casaburi G., Vance D., Duar R., Frese S., Smilowitz J., Underwood M. Targeted probiotic supplementation reduces antibiotic resistance gene carriage in breastfed infants. J. Pediatr. Gastroenterol. Nutr. 2018;66:874. [Google Scholar]
- 49.Karav S., Casaburi G., Frese S.A. Reduced colonic mucin degradation in breastfed infants colonized by Bifidobacterium longum subsp. infantis EVC001. FEBS Open Bio. 2018;8:1649–1657. doi: 10.1002/2211-5463.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henrick B.M., Chew S., Casaburi G., Brown H.K., Frese S.A., Zhou Y., Underwood M.A., Smilowitz J.T. Colonization by B. infantis EVC001 modulates enteric inflammation in exclusively breastfed infants. Pediatr. Res. 2019;86:749–757. doi: 10.1038/s41390-019-0533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Escribano J., Ferré N., Gispert-Llaurado M., Luque V., Rubio-Torrents C., Zaragoza-Jordana M., Polanco I., Codoñer F.M., Chenoll E., Morera M., et al. Bifidobacterium longum subsp infantis CECT7210-supplemented formula reduces diarrhea in healthy infants: A randomized controlled trial. Pediatr. Res. 2018;83:1120. doi: 10.1038/pr.2018.34. [DOI] [PubMed] [Google Scholar]
- 52.Jacobs S.E., Tobin J.M., Opie G.F., Donath S., Tabrizi S.N., Pirotta M., Morley C.J., Garland S.M. Probiotic effects on late-onset sepsis in very preterm infants: A randomized controlled trial. Pediatrics. 2013;132:1055–1062. doi: 10.1542/peds.2013-1339. [DOI] [PubMed] [Google Scholar]
- 53.Plummer E.L., Bulach D.M., Murray G.L., Jacobs S.E., Tabrizi S.N., Garland S.M., ProPrems Study G. Gut microbiota of preterm infants supplemented with probiotics: Sub-study of the ProPrems trial. BMC Microbiol. 2018;18:184. doi: 10.1186/s12866-018-1326-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manzano S., Andrés J.D., Castro I., Rodríguez J.M., Jiménez E., Espinosa-Martos I. Safety and tolerance of three probiotic strains in healthy infants: A multi-centre randomized, double-blind, placebo-controlled trial. Benef. Microbes. 2017;8:569–578. doi: 10.3920/BM2017.0009. [DOI] [PubMed] [Google Scholar]
- 55.Andrés J.D., Manzano S., García C., Rodríguez J.M., Espinosa-Martos I., Jiménez E. Modulatory effect of three probiotic strains on infants’ gut microbial composition and immunological parameters on a placebo-controlled, double-blind, randomised study. Benef. Microbes. 2018;9:573–584. doi: 10.3920/BM2017.0132. [DOI] [PubMed] [Google Scholar]
- 56.Cazzola M., Pham-Thi N., Kerihuel J.C., Durand H., Bohbot S. Efficacy of a synbiotic supplementation in the prevention of common winter diseases in children: A randomized, double-blind, placebo-controlled pilot study. Ther. Adv. Respir. Dis. 2010;4:271–278. doi: 10.1177/1753465810379010. [DOI] [PubMed] [Google Scholar]
- 57.Bazanella M., Maier T.V., Clavel T., Lagkouvardos I., Lucio M., Maldonado-Gòmez M.X., Autran C., Walter J., Bode L., Schmitt-Kopplin P., et al. Randomized controlled trial on the impact of early-life intervention with bifidobacteria on the healthy infant fecal microbiota and metabolome. Am. J. Clin. Nutr. 2017;106:1274–1286. doi: 10.3945/ajcn.117.157529. [DOI] [PubMed] [Google Scholar]
- 58.Wessel M.A., Cobb J.C., Jackson E.B., Harris G.S., Detwiler A.C. Paroxysmal fussing in infancy, sometimes called “colic”. Pediatrics. 1954;14:421–435. [PubMed] [Google Scholar]
- 59.Brink L.R., Mercer K.E., Piccolo B.D., Chintapalli S.V., Elolimy A., Bowlin A.K., Matazel K.S., Pack L., Adams S.H., Shankar K., et al. Neonatal diet alters fecal microbiota and metabolome profiles at different ages in infants fed breast milk or formula. Am. J. Clin. Nutr. 2020 doi: 10.1093/ajcn/nqaa076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wright G.D. The antibiotic resistome: The nexus of chemical and genetic diversity. Nature Rev. Microbiol. 2007;5:175–186. doi: 10.1038/nrmicro1614. [DOI] [PubMed] [Google Scholar]