Abstract
The protection of side-chain arginine in solid-phase peptide synthesis requires attention since current protecting groups have several drawbacks. Herein, the NO2 group, which is scarcely used, has been revisited. This work shows that it prevents the formation of δ-lactam, the most severe side-reaction during the incorporation of Arg. Moreover, it is stable in solution for long periods and can be removed in an easy-to-understand manner. Thus, this protecting group can be removed while the protected peptide is still anchored to the resin, with SnCl2 as reducing agent in mild acid conditions using 2-MeTHF as solvent at 55 °C. Furthermore, we demonstrate that sonochemistry can facilitate the removal of NO2 from multiple Arg-containing peptides.
Keywords: δ-lactam formation, microwave, NBP, orthogonal protection, protecting group, sonochemistry, side-reaction, solid-phase peptide synthesis, ultrasounds
1. Introduction
Arginine (Arg) is a key natural amino acid that is found in many biologically active peptides [1]. Its characteristic structural feature is the presence of a guanidino group in its side-chain. This group is strongly basic, with a pKa value of 12.5, which means the side-chain remains protonated under physiological conditions [2,3]. This guanidino moiety is key for the biological activity of Arg-containing peptides [4]. In this regard, several active pharmaceutical ingredients (APIs) contain one or even several Arg residues.
For instance, this intriguing amino acid is found in desmopressin, leuprolide, and the more recently approved abaloparatide, angiotensin II, bremelanotide and afamelanotide from the α-melanocyte-stimulating hormone (α-MSH), semaglutide and other drugs approved earlier from the glucagon-like peptide family (GLP) [5]. It is important to highlight the case of etelcalcetide, which was approved by the FDA in 2017 for the treatment of secondary hyperparathyroidism in adults with chronic kidney disease on hemodialysis. It is a simple octapeptide formed by a linear chain of seven D-amino acids (one D-Cys, one D-Ala, and five D-Arg) linked to an L-Cys through a disulphide bridge [6,7]. Multiple Arg residues are also very common in antimicrobial peptides (AMPs) and the so-called cell-penetrating peptides (CPPs) [8,9,10]. In fact, the unique characteristic of most CPPs is the presence of several Arg residues, as exemplified in the fragment 48–60 of the transactivator of transcription (TAT) of HIV and octa-Arg [11].
The majority of peptides used for research and industrial purposes are prepared by a chemical process, the fluorenylmethoxycarbonyl (Fmoc)/tert-butyl (tBu) Solid-Phase Peptide Synthesis (SPPS) approach being the most convenient [12]. Although the protonated guanidino group of Arg should show poor reactivity in front of acylation, which is the key reaction in peptide synthesis, it has to be protected to facilitate its solubility in the common solvents used in SPPS and, more importantly, to avoid the two possible side-reactions. On one hand, some Orn can be formed during the stepwise peptide chain elongation or during the cleavage [13,14], and on the other hand, the most important side-reaction, the δ-lactam formation, is a process driven by the six-member ring formed [15]. δ-Lactam formation takes place during the coupling step, when the carboxylic group of Arg is activated. In contrast to other side-reactions, this cyclic amide is not directly converted into an impurity in the target peptide. Instead, it causes an impractical consumption of the activated Arg, which in turn can be indirectly translated into incomplete incorporation of the Arg and therefore the presence of a deletion (des-Arg) peptide. To mitigate this, repetitive couplings of protected Arg are carried out, a protocol that increases the cost of the whole synthetic process. This drawback is exacerbated during the industrial preparation of Arg-containing peptides, where minimum excesses of reagents are used and repetitive couplings imply high solvent and reagent consumption and a longer process time, and therefore a greater overall production cost. Of note, in an industrial mode, the most currently used Arg derivative (Fmoc-Arg(Pbf)-OH, Pbf for 2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl, see below) is the most expensive of all protected proteinogenic amino acids. Thus, in a 100-g scale, the cost of Fmoc-Arg(Pbf)-OH is approximately 10 times more than Fmoc-Phe-OH (€360 vs. €36). Furthermore, it is important to consider atom economy, which is 0.27 for the former and 0.40 for the latter. These figures mean that the synthesis of a peptide containing Arg is approximately 15 times more expensive in comparison with those containing Phe or other amino acids. In this scenario, approaches to optimize Arg protection, coupling, and protecting group removal could have an important impact in the industrial sector.
The protection of Arg in the Fmoc strategy has its roots in tert-butoxycarbonyl (Boc) chemistry, where Arg is protected with the tosyl (Tos) group, which is removed by anhydrous HF, trifluoromethanesulfonic (TFMSA) or related acids [16]. Thus, Yajima’s group developed mesityl-2-sulfonyl (Mts) [17], and later Masahiko et al., after the in-depth study of several candidates, developed the more labile 4-methoxy-2,3,6-trimethylphenylsulfonyl (Mtr) [18], which is removed with almost neat trifluoroacetic acid (TFA) in the presence of scavengers over a prolonged period. An important breakthrough in this field was the development of the 2,2,5,7,8-pentamethylchroman-6-sulfonyl group (Pmc) by Ramage and co-workers [19,20]. The structure of this group recasts the methoxy in position 4 on Mtr in a cyclic ether and keeps three methyl groups on the aryl moiety. Pmc is more labile than Mtr. Carpino and co-workers went a step further when they discovered that the five-member ring present in the Pbf makes this group more labile than the six-member ring of the Pmc [21]. Verdini et al. [22] developed the bis-Boc protection, blocking both Nω and Nω’ for the guanidino group of Arg. Both Boc groups are removed by TFA-H2O (95:5) at room temperature (rt) in 1 h. Although our group has reported that 1,2-dimethylindole-3-sulfonyl (MIS) is much more labile than the Pbf group [23], the latter continues to be the most widely used protecting group for the side-chain of Arg. However, in addition to its high price, which is mainly due to an extremely difficult production process, Pbf is not exempt from the side-reactions outlined earlier, such as δ-lactam formation as has been recently reported by our group [24] (see Figure 1 for the structures of these protecting groups).
Figure 1.
Most used Arg side-chain protecting groups.
In the context of SPPS, little attention has been paid to the use of the NO2 group, which was introduced by Bergmann et al. [25] at the beginning of the era of protecting groups and has been applied mostly in solution chemistry. The strong electron-withdrawing character of the NO2 group reduces the basic nature of the guanidino group, thereby modulating its reactivity. Catalytic hydrogenation using a wide range of catalysts such Pd black, [26] SnCl2, [27] and TiCl3 [28] has been proposed for the removal of the NO2 group. Catalytic hydrogenation is only friendly used in organic chemistry laboratories, but not in those devoted to other biosciences. Furthermore, this reaction works relatively without difficulty for shorter peptides containing a single Arg(NO2) residue. However, with multiple Arg(NO2) residues, catalytic hydrogenation can lead to difficulties, which include a long reaction time with the concomitant degradation of the target peptide [29]. Conversion of a Arg(NO2) residue into an aminoguanidino-derivative [30] and reduction of the aromatic ring of a Trp and even Phe residue [31] have been observed in some cases.
Here we revisit the use of the NO2 group for Arg protection in SPPS. In this regard, we first compared the stability of the NO2 derivative of Arg with the Pbf and (Boc)2 derivatives in N,N-dimethylformamide (DMF) and the green solvent N-butylpyrrolidone (NBP), as well as their tendency to render δ-lactam formation in both solvents [32]. We then developed a method for removing the NO2 group without the concourse of catalytic hydrogenation.
2. Results and Discussion
2.1. Stability of Fmoc-Arg(x)-OH in Solution
The stability of three Fmoc-Arg(X)-OH analogues [X = (Boc)2, NO2, and Pbf] was studied in solution over a period of time. Solutions (0.2 M) of each analogue in both DMF and NBP were prepared in closed high-performance liquid chromatography (HPLC) vials following the procedure described previously by our group [24]. The reaction was monitored by reverse phase HPLC (Figures S1–S6).
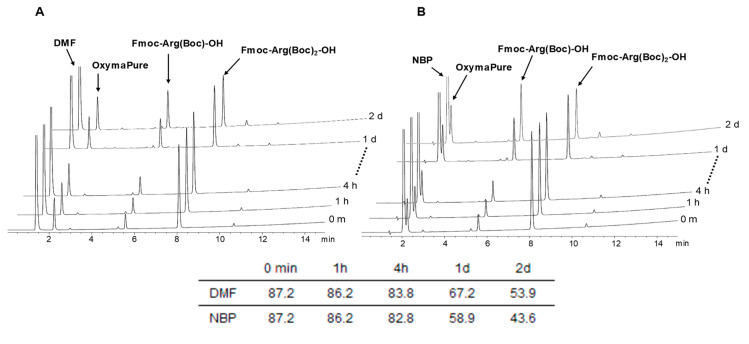
Fmoc-Arg(Boc)2-OH slowly degraded in both solvents over time, rendering mainly the mono protected Fmoc-Arg(Boc)2-OH, which was already present in the commercial sample (Table 1). Nevertheless, solutions of Fmoc-Arg(Boc)2-OH in the two solvents could be kept up to one week, thereby making it compatible with peptide synthesizers and also for industrial manufacturing where the solution is prepared at the time of coupling. For longer periods, the degradation was slightly higher in NBP. In contrast, the NO2 and Pbf analogues were totally stable.
Table 1.
Stability of Fmoc-Arg(X)-OH in DMF (N,N-dimethylformamide) and NBP (N-butylpyrrolidone) at room temperature.
| 0 h | 1 h | 24 h | 48 h | 10 d | 15 d | 20 d | 30 d | |
|---|---|---|---|---|---|---|---|---|
| Fmoc-Arg(Boc)2-OH, DMF | 88.8 | 88.6 | 86.9 | 85.0 | 77.6 | 65.1 | 58.5 | 51.2 |
| Fmoc-Arg(Boc)2-OH, NBP | 88.8 | 88.4 | 85.8 | 83.5 | 71.8 | 62.0 | 52.2 | 37.7 |
|
Fmoc-Arg(NO2)-OH
(DMF or NBP) 1 |
100 | 100 | 100 | 100 | 100 | |||
|
Fmoc-Arg(Pbf)-OH
(DMF or NBP) 1 |
100 | 100 | 100 | 100 | 100 |
1 The study was stopped after 10 days, no changes were observed during this period.
Stability was examined again at 45 °C in DMF and NBP, in the presence of OxymaPure, which are conditions commonly used in SPPS. Again, Pbf and NO2 analogues showed total stability (Figures S7–S10), while the bis Boc derivative degraded slightly faster than in the previous experiment, but could still be considered compatible with the standard reaction times for coupling (up to 4 h). As in the previous case, the degree of degradation was higher in NBP than DMF over a longer period (Figure 2).
Figure 2.
Stability of the mixture of Fmoc-Arg(Boc)2-OH/OxymaPure 1:1 in DMF (A) and NBP (B) at 45 °C. Elution: 30-95% of B into A in 15 min.
2.2. δ-Lactam Formation
The formation of δ-lactam was studied following Scheme 1. The carboxylic groups of the three protected Arg derivatives were activated using N,N’-diisopropylcarbodiimide (DIC) and OxymaPure as additive in an equimolar mixture (1:1:1) in DMF or NBP at 45 °C. The mixture (1.5 equiv.) was then added onto the peptide H-Gly-Phe-Leu-NH-Rink-amide-polystyrene-resin. Aliquots of the supernatant were taken at different times and analyzed by HPLC. After 120 min of coupling, the resin was filtered, washed and Fmoc removed before cleavage and total deprotection (except in case of the NO2 group) using high TFA-triisopropylsilane (TIS)-H2O (95:2.5:2.5). The crude peptides were then analyzed by HPLC [24].
Scheme 1.
Activation of protected Arg derivatives and incorporation on a tripeptidyl resin.
The analysis of the supernatants of each derivative revealed that DMF and NBP showed a similar behavior in the three protecting groups. More importantly, the same analysis showed that Fmoc-Arg(NO2)-OH had the least tendency to form δ-lactam. There was a good fertile consumption (the decrease in protected Arg was not translated into the formation of δ-lactam), (In the context of this manuscript, “fertile consumption” refers to the decrease in the presence of the protected Arg derivative when it remains as an active ester after activation or acylates the peptide resin) because at 30 min the 75% decrease in Fmoc-Arg(NO2)-OH (It is also important to consider that part of the protected Arg shown after 30 min could derive from the hydrolysis of the corresponding active ester and therefore would be part of the “fertile consumption”.) was translated into only 3% of δ-lactam, having approximately 15% of active ester (Figure 3A). This finding implies that the rest of the monomer was incorporated into the peptidyl resin, which was corroborated by high coupling (>99% yield). For Fmoc-Arg(Pbf)-OH, the kinetics was slower, except for δ-lactam formation (Figure 3B). Thus, at 30 min, the formation of δ-lactam was greater (12%, four times more) than for the NO2 derivative. Furthermore, although the fertile consumption of the initial Fmoc-Arg(Pbf)-OH (40% decrease of the initial protected Arg2 and 8% formation of the active ester) was less than that achieved by the NO2 analogue, the result was that after 120 min it yielded same coupling efficiency (>99%). Finally, Fmoc-Arg(Boc)2-OH showed the fastest kinetics of δ-lactam formation (60%) (Figure 3C), which translated into low coupling efficiency (28%) as a result of the side-reaction. Overall, the lower tendency of the NO2 analogue vs. the (Boc)2 and Pbf analogues to render δ-lactam is reflected by the observation that the important increment of the side-product occurred after 60 min in NO2, when presumably the coupling had been completed and therefore the active ester could no longer be fertile. Even at 120 min, a ratio of almost 1:1 of δ-lactam and active ester was present. In the case of Pbf analogue at 120 min, almost all the active ester had been converted to the side-product. Finally, in the case of the (Boc)2 analogue, the active ester was not present at any time, only the δ-lactam was found.
Figure 3.
High-performance liquid chromatography (HPLC) chromatograms of the supernatants after the protected Arg derivatives [A, NO2; B, Pbf; C, (Boc)2] were activated with DIC (N,N’-diisopropylcarbodiimide) and OxymaPure and then added to the peptidyl resin. Elution: 30–95% of B into A in 15 min.
2.3. On-Resin Removal of NO2 from the Arg Side-Chain
Once the suitability of the NO2 derivative of the Arg had been demonstrated in terms of stability and the low tendency of δ-lactam formation, our attention turned to the development of a new removal method with broad applicability and easy handling. Inspired by our previous work on the removal of p-nitrobenzyloxycarbonyl (pNZ) as protecting group of amines in SPPS [33], the capacity of SnCl2 in a slightly acid medium to remove the NO2 group was investigated. A similar method has been extensively used in the early times of combinatorial chemistry to reduce polymer-supported nitro aromatic compounds [34,35]. Taking the tripeptide H-Leu-Arg(NO2)-Phe-NH-Rink-amide-resin as a model, an initial fine-tune of the removal strategy was carried out. The conditions were then further adjusted with the peptidyl resins shown in Figure 4. The list included the lineal precursor of Cilengitide and bradykinin, two peptides of biological interest, and also two peptides containing the Arg-Trp sequence repeated twice and three times. These molecules thus allowed us to study the effect of an increasing number of NO2 groups to be removed, as well as the effect of the removal conditions on a fragile residue like Trp. As references, the same peptides were synthesized using Fmoc-Arg(Pbf)-OH. All peptides synthesized with NO2- or Pbf-protecting groups were cleaved from the resin using TFA-TIS-H2O (95:2.5:2.5) for 1 h at r.t.
Figure 4.
Peptidyl resins used in this study.
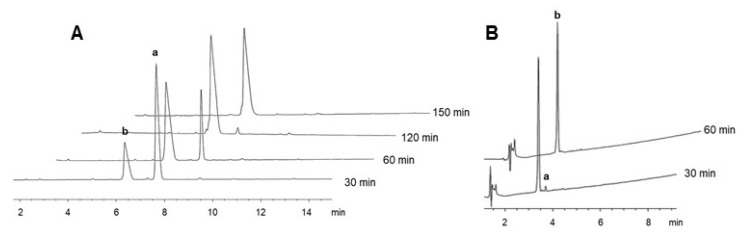
As a starting point, using the H-Leu-Arg(NO2)-Phe-NH-Rink-amide-resin as substrate and the conditions developed by our group to remove pNZ (solutions of 6–8 M SnCl2, 0.4 M phenol, either 0.064 M HCl-dioxane or 0.0016 M AcOH in DMF at 55 °C), the following parameters were studied: concentration of SnCl2 (taking into consideration that 8 M SnCl2 is a supersaturate solution of difficult handling), the need of phenol, the best acid rectifier and its concentration, the temperature, and the solvent (SI, Table 1). The results can be summarized as follows: (i) after assaying DMF, DCM/DMF, and the green solvents MeOH, EtOH, NBP, cyclopentylmethyl ether (CPME), and 2-methyltetrahydrofurane (2Me-THF), the best results were found with the latter. While DMF gave acceptable results, NBP was, on this occasion, less suitable. The use of EtOH gave slower kinetics and MeOH and CPME yielded the poorest results, probably due to its poor capacity to swell the resin; (ii) 2 M SnCl2 rendered similar (or even better) performance than 6-8 M solutions and therefore is the concentration preferred; (iii) the absence of phenol gave slightly poorer results; and (iv) the use of 0.2 M aq HCl, which is easier to use, proved to be good substitute for HCl-dioxane. This final concentration of acid is compatible with the presence of acid-labile protecting groups and it does not cause cleavage from the resin since it represents only 0.64% of the solution. Additionally, in some of these experiments, it was observed that the kinetics during the first hour were very slow, and that the reaction was faster after addition of fresh solution. Therefore, a previous washing with a solution of 0.2 M aq HCl in 2-MeTHF was carried out before the removal treatment. This approach greatly enhanced the removal. This acidic washing can neutralize traces of the piperidine used to remove the Fmoc group at the end of the peptide chain elongation. As a result of this preliminary set of experiments, washings with 0.2 M aq HCl in 2-MeTHF (3 × 1 min) followed by 2 M SnCl2-0.04 M phenol-0.2 M aq HCl in 2-MeTHF (2–3 × 1 h) at 55 °C were considered optimal conditions for the LRF model sequence (Figure 5A). These conditions were then applied successfully to the RGD peptides (Figure 5B). Interestingly, the removal kinetics for the RGD pentapeptide were much faster than for the LRF tripeptide. After a single treatment of 30 min, the RGD peptide showed more than 97% deprotection whilst only 30% was observed in case of LRF. This finding prompted us to do a new assay for this peptide reducing the amount of SnCl2 to a concentration of 1M, in which the total deprotection was achieved after 3 × 30 min treatment (Figure S11). This result suggested that the removal of the NO2 group could be sequence-dependent, thus potentially facilitating the use of even milder conditions than those generally proposed in some cases.
Figure 5.
HPLC of the removal of the NO2 group from H-Leu-Arg(NO2)-Phe-NH-Rinkamide-resin (A) and H-Asp(OtBu)-Phe-Gly-Arg(NO2)-Gly-NH-Rink-amide-resin (B) using 2 M SnCl2-0.04 M phenol-0.2 M aq HCl in 2-MeTHF at 55 °C. a: protected peptide; b: unprotected peptide. Elution: 10-25% of B into A for (A) and 5-95% of B into A for (B) in 15 min.
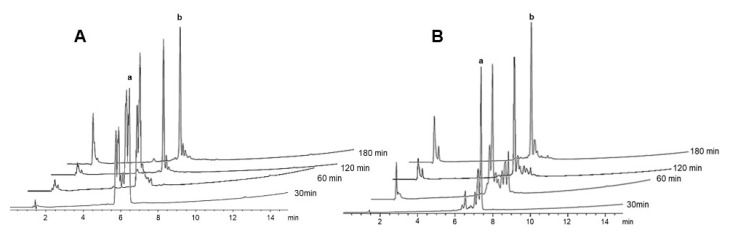
Next, these conditions were applied to remove the two NO2 groups of bradykinin. On this occasion, the kinetics was slower than in the preceding cases. In the search for alternatives to accelerate the reaction, two new assays were then run, one in an ultrasonic bath (Figure S12) and the other under microwave (MW) (Figure 6). Both rendered the expected results, namely a faster reaction, with ultrasound leading to total deprotection in 3 × 1 h treatments (as in the case of the LRF peptide) and MW in 3 × 30 min treatments. Although MW was faster, the ultrasonic bath may be a more accessible device.
Figure 6.
HPLC of the removal of the NO2 groups from protected bradykinin-NH-Rink-amide-resin using 2 M SnCl2-0.04 M phenol-0.2 M aq HCl in 2-MeTHF at 55 °C (MW). a: protected peptide; b: unprotected peptide. Elution: 5–95% of B into A in 15 min.
Finally, to continue studying the removal of multiple NO2 groups, (RW)nP-NH2 (n = 2 or 3) were synthesized. The conditions applied were the general ones used previously under sonication. For both peptides, the results obtained after 3 × 1 h treatments were highly satisfactory and more than acceptable (Figure 7).
Figure 7.
HPLC of the removal of the NO2 groups from H-[Trp(Boc)-Arg(NO2)]2-Pro-NH-Rink-amide resin (A) and H-[Trp(Boc)-Arg(NO2)]3-Pro-NH-Rink-amide resin (B) using 2 M SnCl2-0.04 M phenol-0.2 M aq HCl in 2-MeTHF at 55 °C. a: protected peptide; b: unprotected peptide. elution: 5-95% of B into A in 15 min.
3. Conclusions
Arg is a key amino acid for the construction of peptides with relevant biological activity. Its guanidino side-chain requires protection to avoid side-reactions and to facilitate its solubility. Pbf, which is the most widely used protecting group, shows some drawbacks, mainly δ-lactam formation, stability to the TFA-based global deprotection conditions, and a high price, the latter due mainly to the preparation of the 2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl chloride intermediate and its posterior incorporation into the guanidino group.
The NO2 group, which was first described by Bergmann, has been scarcely used in SPPS mainly because it has to be removed by catalytic hydrogenation in a post-cleavage step and this does not always provide satisfactory results. Furthermore, there has been some discrepancy in the literature regarding the potential of the NO2 group to suppress δ-lactam. Herein, we demonstrated that the electron-withdrawing effect of the NO2 group minimizes its nucleophilicity, which is translated into a reduction of the side-reaction when compared with Pbf protection. In contrast, bis-Boc protection is highly prone to δ-lactam formation. Furthermore, this group showed limited stability in DMF and in OxymaPure containing DMF, while the NO2 and the Pbf were stable.
Importantly, here we report on the development of a new and straightforward method for removing the NO2 group from Arg side chain while the protected peptide is still on the resin. This removal strategy is based on the use of SnCl2 as reducing agent in mild acid conditions using aq HCl as acid rectifier in a solution of 2-MeTHF, which is a green solvent, at 55 °C. As commonly found in peptide chemistry, we observed that the removal reaction could be sequence-dependent. However, in the cases in which the removal is not efficient, the concourse of sonochemistry significantly accelerates the removal reactions. The use of microwave heating also gave positive results. However, accessibility to this type of heating is limited in bioscience laboratories and therefore ultrasound baths emerge as the method of choice. In some demanding sequences involving the unstable Trp residue, the appearance of small satellite peaks could be attributable to side-reactions, which should be further studied for each case. Although currently the price of the Fmoc-Arg(NO2)-OH is, in a 25-g scale, slightly more expensive than Fmoc-Arg(Pbf)-OH, in large-scale production the former should be more economical due to it using less expensive raw materials.
Overall, in terms of effectiveness and handling, Fmoc-Arg(NO2)-OH emerges as an interesting alternative to the existing Arg derivatives.
4. Materials and Methods
4.1. Materials
All reagents and solvents were from commercial suppliers and were used without further purification. Fmoc amino acids and Fmoc-Rink-amide AM PS resin (loading 0.74 mmol/g) were purchased from Iris Biotech GMBH (Marktredwitz, Germany). DIC and OxymaPure were a gift from Luxembourg Biotech. (Ness Ziona, Israel) and N,N-diidsopropylethylamine (DIEA), SnCl2, and piperidine were supplied by Sigma-Aldrich (St. Louis, MO, USA). Organic solvents (DMF, CH2Cl2 (DCM)) and HPLC quality acetonitrile (CH3CN) were purchased from Merck (Kenilworth, NJ, USA). Milli-Q water was used for RP-HPLC analyses. Microwave treatments were carried using a Discover SP (CEM, Matthews, NC, USA). Scientech Ultrasonic Cleaner (Labotec, Durban, South Africa) was used for the ultrasound treatment. Analytical HPLC was performed on an Agilent 1100 system using a Phenomenex AerisTMC18 (3.6 μm, 4.6 × 150 mm) column, with flow rate of 1.0 mL/min and UV detection at 220 nm. Chemstation software was used for data processing. Buffer A: 0.1% TFA in H2O; buffer B: 0.1% TFA in CH3CN. LC-MS was performed on Ultimate™ 3000, AerisTM 3.6 µm Wide pore column, Phenomenex C18 (150 × 4.6 mm) column.
4.2. Peptide Synthesis
The peptides used in this study were synthesized manually using a syringe fitted with a porous polyethylene disc and attached to a vacuum trap for easy filtration. Synthesis were carried out 0.1 or 0.2 mmol scale Rink-amide-PS resin (loading = 0.74 mmol/g) using Fmoc/tBu protocols. For Fmoc removal, 20% of piperidine in DMF was used. Couplings were performed by 1.5 eq. of an equimolar mixture of Fmoc-amino acid derivative, DIC and Oxyma in DMF or NBP, which was allowed to react for 1 h. Global deprotection and cleavage was performed by adding TFA-TIS-H2O (95:2.5:2.5) to the peptidyl-resin for 1 h at rt. Then, chilled ether was added to precipitate the peptides and after centrifugation and decantation, the peptides were taken up in water. The crudes obtained were analyzed by HPLC and finally were lyophilized. The peptide identities were confirmed by LC-MS.
4.3. Stability Study Procedure
Solutions (0.2 M) of each protected Arg analogue in the absence or presence of OxymaPure (0.2 M) were prepared and kept in sealed vials. Aliquots (10 µL) at different times of each one were taken and diluted with CH3CN (490 µL), and 1µL of this mixture was injected in the HPLC.
4.4. δ-Lactam Formation
An equimolar solution of Fmoc-Arg(X)-OH/DIC/OxymaPure (1.5 equiv.) in DMF or NBP was added to the tripeptidyl resin (H-GFL-resin) at 45 °C and kept reacting for 2 h. A 10-μL aliquot of the supernatant was taken from the mixture at 0, 30, 60, and 120 min and diluted to 0.5 mL with CH3CN. From this solution, 1 μL was injected and analyzed by RP-HPLC (30–95% B into A in 15 min). After 120 min, Fmoc was removed using 20% piperidine in DMF, and peptides were cleaved from the resin as described before. Amino acid coupling was quantified by integration of the peaks obtained in their HPLC (10–25% B into A in 15 min).
4.5. Removal of the NO2 Group
The removal solution was prepared by taking SnCl2 (1.8 g), phenol (4 mg) in 2-MeTHF (2 mL), then aqHCl (98 µL) was added and the mixture was sonicated until total solubility of SnCl2. The final volume was then made up to 5 mL using 2-MeTHF, which gave a 0.2 N concentration of HCl.
Peptide resin (50 mg) was washed with a solution of 2-MeTHF-HClaq (0.1%) (1.5 mL, 2 × 1 min). After filtration, the SnCl2 solution was added (1.5 mL) and the syringe was sealed. The reaction was then left for 30 min at 55 °C with sporadic stirring. The solution was filtered off and the resin was washed with 2-MeTHF. An aliquot was then taken for mini cleavage using TFA-TIS-H2O (95:2.5:2.5) (60 µL) as before. Fresh removal solution was added to the peptide resin and the process was repeated.
Supplementary Materials
The supplementary information is available online at https://www.mdpi.com/1422-0067/21/12/4464/s1.
Author Contributions
The work was designed by J.L., F.A, and B.G.d.l.T.; the experimental part was carried out by M.A. and A.K. under the supervision of F.A., and B.G.d.l.T. All authors contributed in the results and discussion. The first draft of the manuscript was prepared by M.A. and A.K. and all authors contributed to the final version. All authors have read and agreed to the published version of the manuscript.
Funding
The work was funded in part by the following: the National Research Foundation (NRF) (# 105892 and Blue Sky’s Research Programme # 120386) and the University of KwaZulu-Natal (South Africa); the Spanish Ministry of Science, Innovation, and Universities (RTI2018-093831-B-100), and the Generalitat de Catalunya (2017 SGR 1439) (Spain). We thank to Dr. Peter White and Millipore-Sigma-Aldrich for supporting part of this research. We thank Dr. Jonathan A. Collins (CEM) for facilitating the use of the microwave instrument.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Futaki S. Membrane-permeable arginine-rich peptides and the translocation mechanisms. Adv. Drug Deliv. Rev. 2005;57:547–558. doi: 10.1016/j.addr.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Glasel J.A. Chapter 1—Basic Physical Properties of Proteins and Nucleic Acids. In: Glasel J.A., Deutscher M.P., editors. Introduction to Biophysical Methods for Protein and Nucleic Acid Research. Academic Press; San Diego, CA, USA: 1995. pp. 36–37. [Google Scholar]
- 3.Fitch C.A., Platzer G., Okon M., Garcia-Moreno B.E., McIntosh L.P. Arginine: Its pKa value revisited. Protein Sci. 2015;24:752–761. doi: 10.1002/pro.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hannon C.L., Anslyn E.V. The Guanidinium Group: Its Biological Role and Synthetic Analogs. In: Dugas H., Schmidtchen F.P., editors. Bioorganic Chemistry Frontiers. Volume 3 Springer; Berlin, Germany: 1993. [Google Scholar]
- 5.Al Shaer D., Al Musaimi O., Albericio F., de la Torre B.G. 2019 FDA TIDES (Peptides and Oligonucleotides) Harvest. Pharmaceuticals. 2020;13:40. doi: 10.3390/ph13030040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al Musaimi O., Alshaer D., de la Torre B.G., Albericio F. 2017 FDA Peptide Harvest. Pharmaceuticals. 2018;11:42. doi: 10.3390/ph11020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henninot A., Collins J.C., Nuss J.M. The Current State of Peptide Drug Discovery: Back to the Future? J. Med. Chem. 2018;61:1382–1414. doi: 10.1021/acs.jmedchem.7b00318. [DOI] [PubMed] [Google Scholar]
- 8.Seo M.-D., Won H.-S., Kim J.-H., Mishig-Ochir T., Lee B.-J. Antimicrobial Peptides for Therapeutic Applications: A Review. Molecules. 2012;17:12276–12286. doi: 10.3390/molecules171012276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramesh S., Govender T., Kruger H.G., de la Torre B.G., Albericio F. Short AntiMicrobial Peptides (SAMPs) as a class of extraordinary promising therapeutic agents. J. Pept. Sci. 2016;22:438–451. doi: 10.1002/psc.2894. [DOI] [PubMed] [Google Scholar]
- 10.Takechi-Haraya Y., Saito H., Science P. Current understanding of physicochemical mechanisms for cell membrane penetration of arginine-rich cell penetrating peptides: Role of glycosaminoglycan interactions. Curr. Protein Pept. Sci. 2018;19:623–630. doi: 10.2174/1389203719666180112100747. [DOI] [PubMed] [Google Scholar]
- 11.Moku G., Layek B., Trautman L., Putnam S., Panyam J., Prabha S. Improving Payload Capacity and Anti-Tumor Efficacy of Mesenchymal Stem Cells Using TAT Peptide Functionalized Polymeric Nanoparticles. Cancers. 2019;11:491. doi: 10.3390/cancers11040491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behrendt R., White P., Offer J. Advances in Fmoc solid-phase peptide synthesis. J. Pept. Sci. 2016;22:4–27. doi: 10.1002/psc.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y. Side Reactions in Peptide Synthesis. Academic Press; Oxford, UK: 2016. pp. 20–21. [Google Scholar]
- 14.Rink H., Sieber P., Raschdorf F. Conversion of NGurethane protected arginine to ornithine in peptide solid phase synthesis. Tetrahedron Lett. 1984;25:621–624. doi: 10.1016/S0040-4039(00)99954-4. [DOI] [Google Scholar]
- 15.Cezari M., Juliano L. Studies on lactam formation during coupling procedures of N alpha-N omega-protected arginine derivatives. Pept. Res. 1996;9:88–91. [PubMed] [Google Scholar]
- 16.Isidro Llobet A., Alvarez M., Albericio F. Amino Acid-Protecting Groups. Chem. Rev. 2009;109:2455–2504. doi: 10.1021/cr800323s. [DOI] [PubMed] [Google Scholar]
- 17.Yajima H., Takeyama M., Kanaki J., Mitani K. The mesitylene-2-sulphonyl group, an acidolytically removable N-protecting group for arginine. J. Chem. Soc. Chem. Commun. 1978:482–483. doi: 10.1039/c39780000482. [DOI] [Google Scholar]
- 18.Fujino M., Wakimasu M., Kitada C. Further Studies on the Use of Multi-substituted Benzenesulfonyl Groups for Protection of the Guanidino Function of Arginine. Chem. Pharm. Bull. 1981;29:2825–2831. doi: 10.1248/cpb.29.2825. [DOI] [Google Scholar]
- 19.Ramage R., Green J. NG-2,2,5,7,8-pentamethylchroman-6-sulphonyl-L-arginine: A new acid labile derivative for peptide synthesis. Tetrahedron Lett. 1987;28:2287–2290. doi: 10.1016/S0040-4039(00)96103-3. [DOI] [Google Scholar]
- 20.Ramage R., Green J., Blake A.J. An acid labile arginine derivative for peptide synthesis: NG-2,2,5,7,8-pentamethylchroman-6-sulphonyl-L-arginine. Tetrahedron. 1991;47:6353–6370. doi: 10.1016/S0040-4020(01)86564-9. [DOI] [Google Scholar]
- 21.Carpino L.A., Shroff H., Triolo S.A., Mansour E.-S.M.E., Wenschuh H., Albericio F. The 2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl group (Pbf) as arginine side chain protectant. Tetrahedron Lett. 1993;34:7829–7832. doi: 10.1016/S0040-4039(00)61487-9. [DOI] [Google Scholar]
- 22.Verdini A.S., Lucietto P., Fossati G., Giordani C. A facile preparation of Fmoc-Argω,ω′(Boc)2-OH and Z-Argω,ω′(Boc)2-OH, new arginine derivatives for peptide synthesis. Tetrahedron Lett. 1992;33:6541–6542. doi: 10.1016/S0040-4039(00)79037-X. [DOI] [Google Scholar]
- 23.Isidro-Llobet A., Latassa D., Giraud M., Álvarez M., Albericio F. 1, 2-Dimethylindole-3-sulfonyl (MIS) as protecting group for the side chain of arginine. Org. Biomol. Chem. 2009;7:2565–2569. doi: 10.1039/b904836g. [DOI] [PubMed] [Google Scholar]
- 24.de la Torre B.G., Kumar A., Alhassan M., Bucher C., Albericio F., Lopez J. Successful development of a method for the incorporation of Fmoc-Arg(Pbf)-OH in solid-phase peptide synthesis using N-butylpyrrolidinone (NBP) as solvent. Green Chem. 2020;22:3162–3169. doi: 10.1039/C9GC03784E. [DOI] [Google Scholar]
- 25.Bergmann M., Zervas L., Rinke H. Neues Verfahren zur Synthese von Peptiden des Arginins. Hoppe Seyler´s Z. Für Physiol. Chem. 1934;224:40–44. doi: 10.1515/bchm2.1934.224.1-2.40. [DOI] [Google Scholar]
- 26.Moroder L., Borin G., Marchiori F., Scoffone E. Studies on cytochrome c. Part I. Synthesis of the protected hexadecapeptide (sequence 1–16) of Baker’s Yeast iso-1-cytochrome c. Biopolymers. 1973;12:477–492. doi: 10.1002/bip.1973.360120303. [DOI] [PubMed] [Google Scholar]
- 27.Tadao H., Yukihiko F., Junzo N. A New Method of Reducing Nitroarginine-peptide into Arginine-peptide, with Reference to the Synthesis of Poly-L-arginine Hydrochloride. Bull. Chem. Soc. Jpn. 1967;40:1205–1208. doi: 10.1246/bcsj.40.1205. [DOI] [PubMed] [Google Scholar]
- 28.Freidinger R.M., Hirschmann R., Veber D.F. Titanium (III) as a selective reducing agent for nitroarginyl peptides: Synthesis of arginine vasotocin. J. Org. Chem. 1978;43:4800–4803. doi: 10.1021/jo00419a019. [DOI] [Google Scholar]
- 29.Schafer D.J., Young G.T., Elliott D.F., Wade R. Amino-acids and peptides. Part XXXII. A simplified synthesis of bradykinin by use of the picolyl ester method. J. Chem. Soc. C. 1971:46–49. doi: 10.1039/j39710000046. [DOI] [Google Scholar]
- 30.Gros C., de Garilhe M.P., Costopanagiotis A., Schwyzer R. Isolement à partir de l’hypophyse postérieure du tripeptide leucyl-arginyl-leucine et sa synthèse par une route nouvelle quant à l’incorporation de l’arginine. Helv. Chim. Acta. 1961;44:2042–2048. doi: 10.1002/hlca.19610440730. [DOI] [Google Scholar]
- 31.Bodanszky M., Martinez J. Side reactions in peptide synthesis. Synthesis. 1981;1981:333–356. doi: 10.1055/s-1981-29442. [DOI] [Google Scholar]
- 32.Lopez J., Pletscher S., Aemissegger A., Bucher C., Gallou F. N-Butylpyrrolidinone as Alternative Solvent for Solid-Phase Peptide Synthesis. Org. Process Res. Dev. 2018;22:494–503. doi: 10.1021/acs.oprd.7b00389. [DOI] [Google Scholar]
- 33.Isidro-Llobet A., Guasch-Camell J., Álvarez M., Albericio F. p-Nitrobenzyloxycarbonyl (pNZ) as a Temporary Nα-Protecting Group in Orthogonal Solid-Phase Peptide Synthesis—Avoiding Diketopiperazine and Aspartimide Formation. Eur. J. Org. Chem. 2005;2005:3031–3039. doi: 10.1002/ejoc.200500167. [DOI] [Google Scholar]
- 34.Kiselyov A.S., Smith II L., Armstrong R.W. Solid Support Synthesis of Polysubstituted Tetrahydroquinolines via Three-Component Condensation Catalyzed by Yb(OTf)3. Tetrahedron. 1998;54:5089–5096. doi: 10.1016/S0040-4020(98)00248-8. [DOI] [Google Scholar]
- 35.Schwarz M.K., Tumelty D., Gallop M.A. Solid-Phase Synthesis of 3,5-Disubstituted 2,3-Dihydro-1,5-benzothiazepin-4(5H)-ones. J. Org. Chem. 1999;64:2219–2231. doi: 10.1021/jo981567p. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.