Abstract
Metastatic lymphatic mapping in esophageal cancer is important to determine the optimal extent of the radiation field in case of neoadjuvant chemoradiotherapy and lymphadenectomy when esophagectomy is indicated. The objective of this review is to identify the distribution pattern of metastatic lymphatic spread in relation to histology, tumor location, and T-stage in patients with esophageal cancer. Embase and Medline databases were searched by two independent researchers. Studies were included if published before July 2019 and if a transthoracic esophagectomy with a complete 2- or 3-field lymphadenectomy was performed without neoadjuvant therapy. The prevalence of lymph node metastases was described per histologic subtype and primary tumor location. Fourteen studies were included in this review with a total of 8952 patients. We found that both squamous cell carcinoma and adenocarcinoma metastasize to cervical, thoracic, and abdominal lymph node stations, regardless of the primary tumor location. In patients with an upper, middle, and lower thoracic squamous cell carcinoma, the lymph nodes along the right recurrent nerve are often affected (34%, 24% and 10%, respectively). Few studies describe the metastatic pattern of adenocarcinoma. The current literature is heterogeneous in the classification and reporting of lymph node metastases. This complicates evidence-based strategies in neoadjuvant and surgical treatment.
Keywords: esophageal cancer, esophagectomy, lymphadenectomy, lymph node metastases, systematic review
1. Introduction
Esophageal cancer patients often present with an advanced disease stage, encompassing metastatic lymph nodes or distant metastases. The presence and number of lymph node metastases are among the most important prognostic factors in esophageal carcinoma and are independent predictors for long-term survival [1,2,3,4,5,6].
The location of metastatic lymph nodes depends on tumor histology, primary tumor location, T-stage and neo-adjuvant therapy [7]. The vessels in the dense lymphatic network surrounding the esophagus are complexly aligned and they contribute to a multidirectional spread of lymph node metastases in the abdomen, the mediastinum, and the neck [8,9]. Additionally, ‘skip metastasis’, skipping the first and directly metastasizing into the second or third lymph node echelons, are frequently seen in both esophageal adenocarcinoma and squamous cell carcinoma [10,11]. This contributes to the presence of lymph node metastases at unexpected distant sites, which makes standardization of the extent of the radiation field and lymphadenectomy in the treatment of esophageal cancer difficult. Not surprisingly, the optimal extent of the lymphadenectomy in esophagectomy has been subject of a global debate over the past decades [12,13]. In addition, several classification systems exist, contributing to the heterogeneity in the reporting of studies and clinical practice [14,15].
An extensive lymphadenectomy may result in more post-operative complications, while an insufficient lymphadenectomy carries the risk of understaging and undertreating patients, which may reduce long-term survival [16]. In Western countries, a two-field lymphadenectomy is preferred for distal esophageal adenocarcinomas. Especially in the upper mediastinum, the extent varies considerably among surgeons, centers, and countries [17]. In Asia, where predominantly squamous cell carcinoma is seen, an extensive three-field lymphadenectomy is common practice [12].
Current studies on metastatic lymphatic mapping in esophageal cancer suggest possible dissemination patterns but do not come forward with sufficient evidence to determine the optimal extent of the radiation field and lymphadenectomy. The lack of homogeneity concerning the classification of lymph node stations makes the interpretation of studies difficult and data hard to compare. In other types of cancer, such as pancreatic, breast, and in colon cancer, the standardization of lymphadenectomy has already been established and it has improved oncologic outcome in the long term [18,19,20].
The objective of this review is to identify the locoregional distribution of lymph node metastases in esophageal cancer patients in potentially curable patients, stratified for histology, tumor location, and T-stage. An outline on metastatic lymphatic distribution patterns may contribute to a uniform worldwide staging system and to the standardization of the extent of the radiation field and lymphadenectomy in esophageal carcinoma.
2. Methods
A review protocol was developed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (www.prisma-statement.org) and was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42018102804). PubMed and Embase were searched on 22 July 2019. The following terms were used (including synonyms and closely related words) as index terms or free-text words: ‘esophageal cancer (including junctional carcinomas)’ and ‘lymph node metastasis’ and ‘lymphadenectomy’. The full search strategies for PubMed and Embase.com can be found in Appendix A. Duplicate articles were excluded.
Articles were screened by two independent researchers (EH, SSG) in two stages: screening of titles and abstracts followed by the retrieval and screening of full-text articles. Inclusion criteria were as follows: studies describing a complete 2- or 3-field lymphadenectomy by transthoracic esophagectomy, the prevalence of patients with lymph node metastases per lymph node station is given or can be calculated, data is separately reported for adenocarcinoma and/or squamous cell carcinoma and tumor location. We excluded studies describing surgery following neo-adjuvant therapy (because the distribution may be different after neo-adjuvant chemo(radio)therapy), imaging studies, case reports, conference abstracts and reviews, and papers in another language than English or Dutch.
2.1. Data Extraction
The primary endpoint is the metastatic rate of lymph node metastases in esophageal carcinoma per lymph node station or region. When available, the following variables were extracted from the included studies: year of publication; country; study design; inclusion period; lymph node classification system used (JES, Japan Esophageal Society; AJCC, American Joint Committee on Cancer; other; none); description of how detailed lymph node regions or stations are described and reported; number of patients; patient characteristics (gender, age); tumor histology; tumor location (upper thoracic esophagus, middle thoracic esophagus, lower thoracic esophagus or gastroesophageal junction (GEJ)); c/pT-stage; c/pN-stage; type of surgical approach; lymphadenectomy (complete 2- or 3- field); use of immunohistochemistry staining; prevalence of lymph node metastases per lymph node station; number of patients with lymph node metastases; overall percentage of positive lymph nodes; number of patients per lymph node location with resected nodes and positive nodes in that station or region. Methodological quality was assessed using the Methodological Index for Non-Randomized Studies (MINORS) checklist [21].
2.2. Statistical Analysis
Descriptive statistics summarized the characteristics of included studies, patient characteristics, and the outcomes of each included study. Since not all studies used the same classification or definition for lymph node stations, and some studies only reported lymph node data for different regions instead of stations, we combined the two mostly used systems (JES and AJCC) and grouped lymph node stations into five regions: cervical, upper mediastinal, middle mediastinal, lower mediastinal, and abdominal (Table 1). Studies with a reported number of patients with metastatic lymph nodes per station were pooled separately from the studies only describing data per region. The prevalence of patients with lymph node metastases per station or region were calculated by summarizing all the patients with lymph node metastases per lymph node station or region and dividing them by the sum of all patients who had a lymph node dissection in this station or region. Results were stratified for tumor histology and primary tumor location. Finally, studies describing the lymphatic distribution pattern according to the pT-stage were pooled and described separately.
Table 1.
Combination of JES and AJCC stations and five lymph node regions.
| Node Region | Station Number (JES) | Name of Station (JES) | Station Number (AJCC) | Name of Node Station (AJCC) |
|---|---|---|---|---|
| Cervical | 104R | Right supraclavicular lymph nodes | ||
| 104L | Left supraclavicular lymph nodes | |||
| 101R | Right cervical paraesophageal lymph nodes | 1R | Right lower cervical paratracheal lymph nodes | |
| 101L | Left cervical paraesophageal lymph nodes | 1L | Left lower cervical paratracheal lymph nodes | |
| 102 | Deep cervical lymph nodes | |||
| 103 | Peripharyngeal lymph nodes | |||
| Upper mediastinal | 105 | Upper thoracic paraesophageal lymph nodes | 8up | Posterior mediastinal lymph nodes |
| 106preR | Right pretracheal lymph nodes | |||
| 106preL | Left pretracheal lymph nodes | |||
| 106recR | Right recurrent nerve lymph nodes | 2R | Right and left upper paratracheal nodes (including lymph nodes along the recurrent laryngeal nerve and the cervical paratracheal lymph nodes) | |
| 106recL | Left recurrent nerve lymph nodes | 2L + 4L | Left upper paratracheal nodes + Left lower paratrachal lymph nodes | |
| 106tbR | Right tracheobronchial lymph nodes | Right lower paratrachal lymph nodes | ||
| 106tbL | Left tracheobronchial lymph nodes | 4L | Left lower paratrachal lymph nodes | |
| Middle mediastinal | 107 | Subcarinal lymph nodes | 7 | Subcarinal lymph nodes |
| 108 | Middle thoracic paraesophageal lymph nodes | 8M | Middle thoracic paraesophageal lymph nodes | |
| 109R | Right main bronchus lymph nodes | 7 | Subcarinal lymph nodes | |
| 109L | Left main bronchus lymph nodes | 7 | Subcarinal lymph nodes | |
| Lower mediastinal | 110 | Lower thoracic paraesophageal lymph nodes | 8Lo | Lower thoracic paraesophageal lymph nodes |
| 111 | Supradiaphragmatic lymph nodes | 15 | Diaphragmatic lymph nodes | |
| 112 | Posterior mediastinal lymph nodes | 9 | Pulmonary ligament lymph nodes | |
| Abdominal lymph node stations | 1 | Right paracardial lymph nodes | 16 | Paracardial lymph nodes |
| 2 | Left paracardial lymph nodes | 16 | Paracardial lymph nodes | |
| 3 | Lesser curvature lymph nodes | 17 | Lymph nodes along the left gastric artery | |
| 4 | Lymph nodes along the greater curvature | |||
| 7 | Lymph nodes along the left gastric artery | 17 | Lymph nodes along the left gastric artery | |
| 9 | Celiac lymph nodes | 20 | Celiac lymph nodes | |
| 8 | Lymph nodes along the common hepatic artery | 18 | Lymph nodes along the common hepatic artery | |
| 11 | Splenic artery lymph nodes | 19 | Splenic artery lymph nodes | |
| 19 | Infradiaphragmatic lymph nodes | 16 | Paracardial lymph nodes |
JES = Japan Esophageal Society; AJCC = American Joint Committee on Cancer; Lymph node stations 5, 6, 10, and 12 to 20 of the JES classification not described in this review.
3. Results
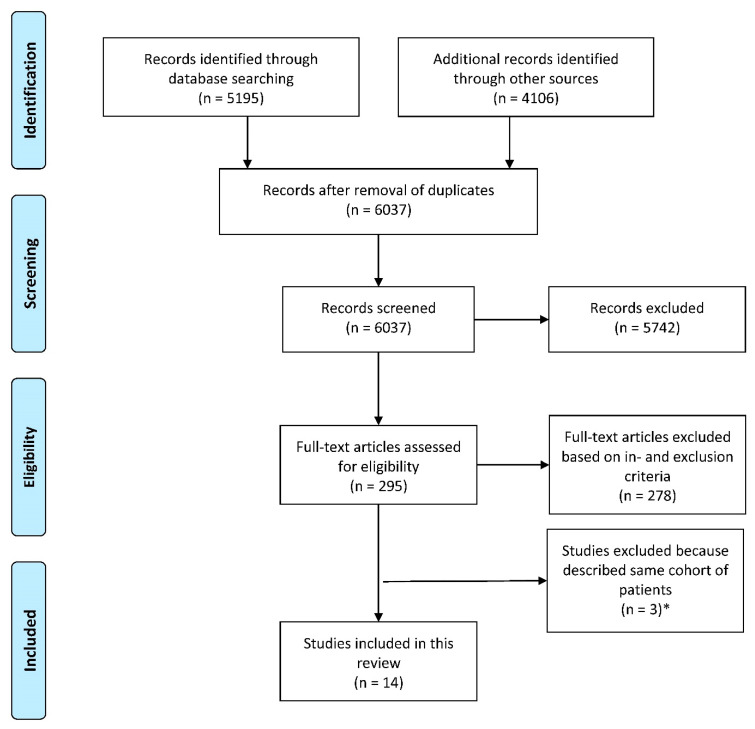
Details of the literature search and study selection are shown in Figure 1. Fourteen studies met the inclusion criteria and were included in this review [7,22,23,24,25,26,27,28,29,30,31,32,33,34]. Characteristics of the included studies are presented in Table 2. A total of 8952 patients were evaluated, including 409 (5%) with an adenocarcinoma and 8543 (95%) with a squamous cell carcinoma. Among all patients with a squamous cell carcinoma, 726 (9%) patients had a tumor located in the upper thoracic esophagus, 5130 (60%) patients had a tumor in the middle thoracic esophagus and 2687 (31%) had a tumor in the lower thoracic esophagus. None of these studies described patients with a cervical or GEJ squamous cell carcinoma. For adenocarcinoma, 32 (8%) tumors were located in the distal esophagus and 377 (92%) were located at the GEJ. The c/pT stage varied among studies, but most of the patients had a c/pT3 tumor. Details of the study populations can be found in Table 2 and Table 3.
Figure 1.
Flow-chart of study selection. * Three studies by Chen and colleagues and two by Li and colleagues described (partly) the same cohort of patients. Therefore, only one study of Chen et al. and one study of Li et al. were included. For both, this was the study where most of the lymph node stations and/or regions were described.
Table 2.
Characteristics of included studies.
| No | First Author | Year of Publication | Study Design | Country | Number of Patients | Inclusion Period | 2- or 3-Field Lymphadenectomy | Lymph Node Classification System Used | Use of Immunohistochemistry Staining | MINORS Score | How Detailed Are the Locations of Lymph Node Metastases Described? | How Are the Locations of Nodal Metastases Reported? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | S. Sharma [22] | 1994 | Retrospective study | Japan | 70 | 1985–1991 | 3-field | No standard classification used | NR | 10 | Description of several stations in cervical, thoracic, and abdominal regions. | Number of patients with resected and positive lymph nodes reported per station. |
| 2 | C. van de Ven [23] | 1999 | Prospective observational study | Belgium | 37 | 1994–1998 | 3-field | No standard classification used | NR | 11 | Description of several stations in cervical, thoracic, and abdominal regions. | Number of patients with resected and positive lymph nodes reported per region. |
| 3 | H. Igaki [24] | 2001 | Retrospective study | Japan | 96 | 1986–1998 | 3-field | No standard classification used | NR | 8 | No description of stations. Cervical, upper mediastinal, middle mediastinal, lower mediastinal, perigastric, and celiac regions described. | Number of patients with resected and positive lymph nodes reported per region. Numbers per station were not provided. |
| 4 | S.M. Dresner [25] | 2001 | Retrospective study | United Kingdom | 104 | 1996–1999 | 2-field | No standard classification used | NR | 10 | Description of stations in lower thoracic and abdominal regions. | Number of patients with resected and positive lymph nodes reported for the abdominal region. Numbers per station were not provided. |
| 5 | J. Chen [26] | 2009 | Retrospective study | China | 1850 | 1993–2006 | 3-field | JES | H&E | 12 | Description of stations according to JES in cervical, upper mediational, middle mediastinal, lower mediastinal and abdominal region. | Number of patients with resected and positive lymph nodes reported per region and station. |
| 6 | Y. Tachimori [27] | 2011 | Retrospective study | Japan | 356 | 2001–2005 | 3-field | No standard classification used | NR | 10 | No description of stations. Cervical, upper mediastinal, middle mediastinal, lower mediastinal, perigastric and celiac regions described. | Number of patients with resected and positive lymph nodes reported per region. Numbers per station were not provided. |
| 7 | C. Castoro [7] | 2011 | Retrospective study | Italy | 248 | 1992–2007 | 2-field and 3-field | No standard classification used | H&E and PAS | 11 | Description of stations in cervical, thoracic and abdominal regions. | Number of patients with resected and positive lymph nodes reported per station.* |
| 8 | H. Li [28] | 2012 | Retrospective study | China | 200 | 2000–2010 | 3-field | No standard classification used | H&E | 11 | No description of stations. Cervical, mediastinal, recurrent laryngeal nerve and abdominal regions described. | Number of patients with resected and positive lymph nodes reported per region and the recurrent laryngeal nerve station. |
| 9 | S. Kosugi [29] | 2013 | Retrospective study | Japan | 86 | 1992–2011 | 3-field | JES | NR | 11 | Description of stations according to JES in cervical, upper mediational, middle mediastinal, lower mediastinal, perigastric, and suprapancreatic regions. | Number of patients with resected and positive lymph nodes reported per station. |
| 10 | J. Cheng [30] | 2013 | Retrospective study | China | 1893 | 2003–2011 | 2-field and 3-field | JES | H&E | 9 | Description of stations according to JES in cervical, upper mediational, middle mediastinal, lower mediastinal, and abdominal region. | Number of patients with resected and positive lymph nodes reported per region and per a selected number of stations. |
| 11 | Z. Lin [31] | 2016 | Prospective observational study | China | 260 | 2009–2013 | 3-field | AJCC | H&E | 13 | Description of stations according to AJCC in the thoracic and abdominal region. | Number of patients with resected and positive lymph nodes reported per station. |
| 12 | Y. Dong [32] | 2015 | Retrospective study | China | 3587 | 2000–2014 | 2-field and 3-field | JES | NR | 10 | Description of stations according to JES in the cervical, upper mediastinal, middle mediastinal, lower mediastinal, and abdominal regions. | Number of patients with resected and positive lymph nodes reported per region. Numbers per station were not provided.* |
| 13 | X. Duan [33] | 2017 | Retrospective study | China | 136 | 2014–2016 | 2-field and 3-field | No standard classification used | NR | 11 | Description of stations in thoracic and abdominal regions. | Number of patients with resected and positive lymph nodes reported per station. |
| 14 | S. Park [34] | 2018 | Prospective observational study | Korea | 29 | 2014–2018 | 3-field | JES | H&E | 10 | Description of stations according to JES in the cervical, upper mediastinal, middle mediastinal, lower mediastinal, and abdominal regions. | Number of patients with resected and positive lymph nodes reported per station. |
Studies are shown in chronological order; JES = Japan Esophageal Society; AJCC = American Joint Committee on Cancer; * Partly combined locations of the tumor in displayed data; H&E = hematoxylin & eosin; PAS = periodic acid-Schiff; NR = not reported; MINORS score ranges from 0 to 16 for non-comparative studies with 16 being the ideal score.
Table 3.
Study population characteristics.
| No | First Author | Sex, Male n (%) | Age, in years | Histology | T-Stage *, n (%) | N-Stage *, n (%) | Location of the Tumor, n (%) | Surgical Approach | Number of Dissected Lymph Nodes per Patient | Percentage of Patients with Lymph Node Metastases | Overall Percentage of Positive Lymph Nodes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | S. Sharma [22] | 62 (89) | mean 58.5 | SCC | pT1 11 (16) pT2 12 (17) pT3 46 (65) pT5 1 (2) |
pN0 20 (29) pN1 50 (71) |
UTE 10 (14) MTE 37 (53) LTE 23 (33) |
Open procedures | mean 82 | 71% (50/70) |
4% (208/5720) |
| 2 | C. van de Ven [23] | NR | NR | AC | cT3 | NR | LTE 17 (46) GEJ 20 (54) |
Open procedures | mean 60, SD 17 |
NR | 14% (323/2240) |
| 3 | H. Igaki [24] | 85 (97) | mean 62 [range 42–86] |
SCC | pT1 27 (28) pT2 16 (16) pT3 53 (56) |
pN0 36 (38) pN1 60 (62) |
LTE | Open procedures | NR | 66% (63/96) |
NR |
| 4 | S.M. Dresner [25] | 91 (88) | mean 63 [range 30–78] |
AC | NR | NR | GEJ | Open procedures | median 22 [range 11–57] |
70% (73/104) | 21% (508/2476) |
| 5 | J. Chen [26] | 1351 (73) | median 55 [range 27–54] |
SCC | cT1 109 (6) cT2 348 (19) cT3 1215 (65) cT4 178 (10) |
NR | UTE 289 (16) MTE 1381 (74) LTE 180 (10) |
Open procedures | mean 26 [range 15–71] |
58% (1081/1850) |
9% (4350/47470) |
| 6 | Y. Tachimori [27] | 314 (88) | mean 63 [range 41–80] |
SCC | pT1 127 (36) pT2 40 (11) pT3 183 (51) pT4 6 (2) |
pN0 110 (31) pN1 116 (33) pN2 81 (23) pN3 49 (13) |
UTE 55 (15) MTE 173 (49) LTE 128 (36) |
Open procedures | NR | NR | NR |
| 6 | C. Castoro [7] | 327 (81) | median 63 [IQR 56–70] | SCC 116 (47) AC 132 (53) |
cT1 5 (2) cT2 42 (17) cT3 201 (81) |
cN0 107 (43) cN1 141 (57) |
UTE (all SCC) 25 (10) MTE (all SCC) 50 (20) LTE (AC 15, SCC 41) 56 (23) GEJ (all AC) 117 (47) |
Open procedures | AC median 19.5 [IQR 15–27] SCC median 16 [IQR 12–21] |
AC 54% (63/116) SCC 67% (88/132) |
NR |
| 8 | H. Li [28] | 163 (82) | mean 57, SD 9 |
SCC | pT1 18 (9) pT2 45 (23) pT3 114 (56) pT4 23 (12) |
NR | UTE 31 (15) MTE 137 (69) LTE 32 (16) |
Open procedures | NR | NR | NR |
| 9 | S. Kosugi [29] | 78 (91) | mean 60, SD 7 |
SCC | pT1a 7 (8) pT1b 75 (87) pT2 4 (5) |
pN0 48 (56) pN1 31 (36) pN2 6 (7) pN3 1 (1) |
UTE 17 (20) MTE 59 (69) LTE 10 (11) |
Open procedures | NR | 47% (40/86) |
NR |
| 10 | J. Cheng [30] | 1474 (78) | < 40: 1% 41–59: 48% ≥60: 51% |
SCC | cTis 10 (1) cT1 103 (5) cT2 345 (18) cT4 1173 (62) cT4 262 (14) |
NR | UTE 82 (4) MTE 1266 (67) LTE 545 (29) |
Open procedures | mean 13 | 46% (865/1893) |
NR |
| 11 | Z. Lin [31] | 59 (23) | median 61 [IQR 52–67] |
SCC | pT1 30 (11) pT2 44 (17) pT3 164 (63) pT4 22 (9) |
pN0 119 (46) pN1 67 (25) pN2 54 (21) pN3 20 (8) |
UTE 28 (11) MTE 173 (67) LTE 59 (22) |
Open procedures | median 35 [IQR 25–46] |
54% (141/260) |
15% (316/2097) |
| 12 | Y. Dong [32] | 2536 (72) | median 61 | SCC | pT1 435 (14) pT2 935 (25) pT3 1992 (55) pT4 225 (6) |
pN0 2223 (62) pN1 1233 (34) pN2 98 (3) pN3 33 (1) |
UTE 189 (5) MTE 1837 (51) LTE 1561 (44) |
Hybrid procedures | mean 20 [range 16–50] |
38% (1.364/3587) |
4% (2870/71740) |
| 13 | X. Duan [33] | 128 (95) | mean 63, SD 9 |
AC | pT1-2 17 (13) pT3-4 119 (87) |
pN0 44 (32) pN1 64 (47) pN2 21 (15) pN3 7 (6) |
GEJ | Open procedures | mean 15 | 68% (92/136) |
21% (431/2083) |
| 14 | S. Park [34] | 26 (90) | mean 63, SD 7 |
SCC | cT1 | cN0 25 (86) cN1 4 (14) |
MTE 17 (59) LTE 12 (41) |
Robot-assisted procedures | mean 55, SD 17 |
86% (25/29) |
NR |
Studies are shown in chronological order; SCC = squamous cell carcinoma; AC = adenocarcinoma; GEJ = gastroesophageal junction; CE = Cervical esophagus; UTE = Upper thoracic esophagus; MTE = middle thoracic esophagus; LTE = lower thoracic esophagus; NR = not reported; SD = standard deviation; IQR = interquartile range; * either pathological or clinical T and N-stage, based on what is reported in the paper.
3.1. Reporting Standard
Standard reported lymphadenectomy differed among studies. In 1 study, patients underwent a 2-field lymphadenectomy, in 9 studies, patients underwent a 3-field lymphadenectomy, and in 4 studies, both procedures were included, resulting in a different nodal yield per study. In addition, the definition of anatomical locations of lymph node stations differed amongst studies; 1 study used AJCC, 5 used JES, and 8 did not use a standard classification system. Moreover, 6 studies described the prevalence of lymph node metastases per lymph node station, 5 only described the prevalence of lymph node metastases per region, and 3 reported a combination of both. The reported regions and stations also varied among studies (Table 2). Some studies described for some stations both sides together, and other studies separated left and right. One study combined tumor locations when reporting the number of patients per lymph node station and could therefore not be pooled with the others studies [7].
3.2. Distribution Pattern for Esophageal Squamous Cell Carcinoma
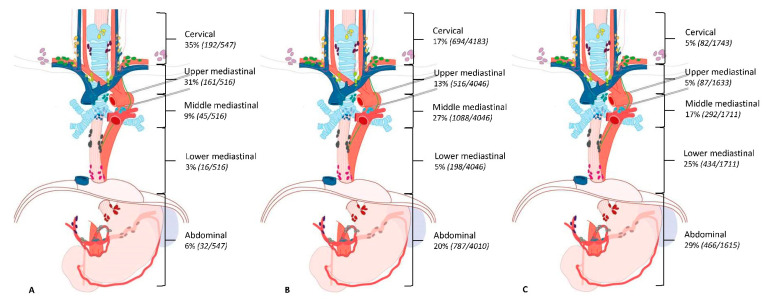
Eleven studies [7,22,24,26,27,28,29,30,31,32,34] described the location of lymph node metastases in patients with a squamous cell carcinoma (n = 8543). Table 4 shows the prevalence of lymph node metastases per lymph node station among the seven studies [22,26,28,29,30,31,34] that reported data per lymph node station per tumor location. For patients with an upper thoracic tumor, lymph node metastases are most frequently seen along the right recurrent nerve (60%) and cervical paraesophageal lymph nodes (right 34% and left 22%). For patients with a middle thoracic tumor, the prevalence of lymph node metastases was highest along the right recurrent nerve (23%), right cervical paraesophageal lymph nodes (24%), and middle thoracic paraesophageal lymph nodes (23%). The lymph nodes along the left gastric artery (28%) and lower thoracic esophagus (23%) had the highest prevalence of lymph node metastases in patients with a tumor in the lower thoracic esophagus. Six studies [24,26,27,28,30,32] described the location of the lymph node metastases per region. The results of these studies are shown in Figure 2.
Table 4.
Prevalence of lymph node metastases per lymph node station.
| Lymph Node Station | Squamous Cell Carcinoma | Adenocarcinoma | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Upper Thoracic Esophagus | Middle Thoracic Esophagus | Lower Thoracic Esophagus | Gastroesophageal Junction | |||||||||||||
| Cervical region | ||||||||||||||||
| Right supraclavicular lymph nodes | 9% | 34 | / | 381 | 10% | 266 | / | 2684 | 13% | 100 | / | 748 | NR | |||
| Left supraclavicular lymph nodes | 5% | 14 | / | 299 | 5% | 67 | / | 1418 | 3% | 6 | / | 203 | NR | |||
| Right cervical paraesophageal lymph nodes | 34% | 103 | / | 299 | 24% | 345 | / | 1418 | 10% | 20 | / | 203 | NR | |||
| Left cervical paraesophageal lymph nodes | 22% | 65 | / | 299 | 11% | 152 | / | 1418 | 4% | 8 | / | 203 | NR | |||
| Right deep cervical lymph nodes | 2% | 5 | / | 289 | <1% | 4 | / | 1381 | 0% | 0 | / | 180 | NR | |||
| Left deep cervical lymph nodes | 2% | 5 | / | 289 | 1% | 8 | / | 1381 | 0% | 0 | / | 180 | NR | |||
| Peripharyngeal lymph nodes | 1% | 2 | / | 289 | <1% | 1 | / | 1381 | 0% | 0 | / | 180 | NR | |||
| Upper mediastinal region | ||||||||||||||||
| Upper thoracic paraesophageal lymph nodes | 10% | 40 | / | 388 | 6% | 163 | / | 2811 | 3% | 7 | / | 214 | NR | |||
| Right pretracheal lymph nodes | 12% | 43 | / | 371 | 6% | 81 | / | 1381 | 2% | 3 | / | 180 | NR | |||
| Left pretracheal lymph nodes | 9% | 28 | / | 299 | 7% | 101 | / | 1418 | 2% | 4 | / | 203 | NR | |||
| Right recurrent nerve lymph nodes | 60% | 6 | / | 10 | 23% | 15 | / | 66 | 15% | 8 | / | 52 | NR | |||
| Left recurrent nerve lymph nodes | 11% | 32 | / | 289 | 7% | 102 | / | 1410 | 3% | 7 | / | 209 | NR | |||
| Tracheobronchial lymph nodes | 12% | 10 | / | 82 | 12% | 17 | / | 145 | 6% | 3 | / | 49 | NR | |||
| Middle mediastinal region | ||||||||||||||||
| Subcarinal lymph nodes | 8% | 32 | / | 398 | 18% | 517 | / | 2913 | 14% | 121 | / | 836 | 25% | 1 | / | 4 |
| Middle thoracic paraesophageal lymph nodes | 5% | 20 | / | 388 | 23% | 595 | / | 2542 | 21% | 170 | / | 804 | 2% | 1 | / | 45 |
| Right main bronchus lymph nodes | <1% | 1 | / | 289 | 2% | 24 | / | 1410 | 2% | 4 | / | 209 | 0% | 0 | / | 10 |
| Left main bronchus lymph nodes | 1% | 2 | / | 289 | 3% | 37 | / | 1410 | 2% | 5 | / | 209 | 6% | 2 | / | 31 |
| Lower mediastinal region | ||||||||||||||||
| Lower thoracic paraesophageal lymph nodes | 3% | 12 | / | 388 | 8% | 221 | / | 2851 | 23% | 184 | / | 809 | 10% | 10 | / | 96 |
| Supradiaphragmatic lymph nodes | 0% | 0 | / | 306 | <1% | 3 | / | 1504 | 5% | 39 | / | 778 | 0% | 0 | / | 5 |
| Posterior mediastinal lymph nodes | 3% | 10 | / | 316 | 7% | 102 | / | 1545 | 5% | 14 | / | 259 | 0% | 0 | / | 17 |
| Abdominal region | ||||||||||||||||
| Right paracardial lymph nodes | 1% | 2 | / | 199 | 3% | 48 | / | 1447 | 12% | 27 | / | 232 | 26% | 33 | / | 128 |
| Left paracardial lymph nodes | 3% | 10 | / | 299 | 7% | 201 | / | 2684 | 13% | 97 | / | 748 | 37% | 48 | / | 131 |
| Lesser curvature lymph nodes | 3% | 9 | / | 299 | 10% | 273 | / | 2713 | 11% | 89 | / | 777 | 29% | 37 | / | 127 |
| Lymph nodes along the greater curvature | 0% | 0 | / | 289 | <1% | 1 | / | 1381 | 0% | 0 | / | 180 | 12% | 5 | / | 41 |
| Lymph nodes along the left gastric artery | 4% | 11 | / | 299 | 16% | 238 | / | 1528 | 28% | 76 | / | 269 | 48% | 29 | / | 60 |
| Celiac lymph nodes | NR | 2% | 1 | / | 56 | 3% | 1 | / | 33 | 14% | 4 | / | 29 | |||
| Lymph nodes along the common hepatic artery | <1% | 1 | / | 289 | 3% | 40 | / | 1483 | 5% | 37 | / | 774 | 14% | 5 | / | 37 |
| Splenic artery lymph nodes | NR | 2% | 1 | / | 59 | 0% | 0 | / | 31 | 26% | 11 | / | 43 | |||
| Infradiaphragmatic lymph nodes | NR | NR | NR | NR | ||||||||||||
| Subaortic lymph nodes | NR | 10% | 2 | / | 21 | 0% | 0 | / | 6 | NR | ||||||
| Para-aortic lymph nodes | NR | 10% | 1 | / | 10 | 0% | 0 | / | 3 | NR | ||||||
NR = not reported. Data presented as percentage of patients with lymph node metastases with number of patients with (number of patients with metastatic lymph nodes/number of patients with lymph node dissection of this station). Only studies that presented data per lymph node station were included in this table.
Figure 2.
Prevalence of lymph node metastases per tumor location in patients with squamous cell carcinoma. (A) = Upper thoracic esophageal tumor, (B) = Middle thoracic esophageal tumor, (C) = Distal thoracic esophageal tumor. Data presented as percentage of patients with lymph node metastases in this region.
3.3. Distribution Pattern for Esophageal Adenocarcinoma
Four studies [7,23,25,33] described the location of lymph node metastases in patients with an adenocarcinoma (n = 409). One study [33] described the prevalence of metastatic lymph nodes per lymph node station; this was for GEJ tumors (Table 4). Lymph node stations with the highest prevalence of patients with metastatic lymph nodes were lymph nodes along the left gastric artery (48%), lesser curvature (29%), splenic artery (26%), right paracardial lymph nodes (26%), and subcarinal lymph nodes (25%).
Two studies [23,25] described the location of lymph node metastases in regions. Pooled numbers show that for patients with a GEJ tumor, 20% (4 out of 10) had lymph node metastases in the cervical region and 25% (31 out of 124) had metastases in the abdominal lymph node stations (other regions were not reported). For patients with an adenocarcinoma of the lower thoracic esophagus, 35% (6 out of 17) had lymph node metastases in the cervical region, 71% (12 out of 17) had lymph node metastases in the lower mediastinal region, and 71% (12 out of 17) had lymph node metastases in the abdominal region.
One study [7] combined patients with a tumor of the distal esophagus and GEJ. In this study, a prevalence of 30% in the periesophageal lymph nodes, 37% in the paracardial lymph nodes, 35% in the perigastric lymph nodes, and 14% in the celiac axis was reported.
3.4. Distribution of LN Metastases in Relation to pT-Stage
Three studies [24,27,30] stratified the prevalence of nodal metastases per pT-stage. All three studies described patients with squamous cell carcinoma. Table 5 shows the rate of patients with lymph node metastases per region, divided into four groups: patients with pT1-2 and pT3-4 and patients with pT1 and pT2-4. Among patients with higher T-stages, a higher prevalence of lymph node metastases is seen per region, while the distribution remained similar.
Table 5.
Lymph node metastases per pathological T-stage of patients with esophageal carcinoma.
| Lymph Node Region | MTE | LTE | UTE | MTE | LTE | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| pT1-2 | pT2-3 | pT1-2 | pT2-3 | pT1 | pT2-4 | pT1 | pT2-4 | pT1 | pT2-4 | |
| n = 315 | n = 915 | n = 171 | n = 470 | n = 22 | n = 33 | n = 67 | n = 106 | n = 38 | n = 90 | |
| Cervical region | 3% | 5% | 2% | 3% | 14% | 21% | 12% | 25% | 0% | 6% |
| Upper mediastinal region | 3% | 6% | 2% | 5% | 55% | 85% | 22% | 61% | 13% | 27% |
| Middle mediastinal region | 18% | 38% | 9% | 19% | 5% | 9% | 6% | 49% | 5% | 23% |
| Lower mediastinal region | 2% | 3% | 27% | 39% | 0% | 9% | 9% | 25% | 5% | 27% |
| Abdominal region | 11% | 16% | 25% | 33% | NR | NR | NR | NR | NR | NR |
| Perigastric region | NR | NR | NR | NR | 0% | 6% | 24% | 54% | 39% | 66% |
| Celiac region | NR | NR | NR | NR | 0% | 9% | 3% | 5% | 0% | 9% |
Data presented as percentage of patients with lymph node metastases in this region. UTE = Upper thoracic esophagus; MTE = Middle thoracic esophagus; LTE = Lower thoracic esophagus; NR = not reported.
4. Discussion
This study describes the sites of lymph node metastases in esophageal cancer patients according to the histology and primary tumor location based on literature published before July 2019. This is the first study systematically combining available evidence on lymph node metastases pattern, contributing toward revealing the lymphatic metastatic pattern of esophageal carcinoma. This study showed that both squamous cell carcinoma and adenocarcinoma metastasize to cervical, thoracic, and abdominal lymph node stations, regardless of the location of the primary tumor.
4.1. Lymphatic Distribution Pattern for Squamous Cell Carcinoma
Multiple studies have attempted to define the lymph node metastases pattern in esophageal cancer [7,22,23,25,26,27,30,31,32,33]. Most of these studies describe squamous cell carcinoma only. The available evidence of lymph node metastases pattern in adenocarcinoma of the esophagus is scarce, and the available literature for both tumor types is very heterogeneous. Whereas some studies report data per lymph node station, others report per region. To make this even more complex, not all studies adhere to the same boundaries of lymph node regions and not all studies use the same anatomical definition of lymph node stations. To define anatomical sites on lymph node stations, some use standardized classification systems such as the AJCC or JES, while others do not use any standardized classification system. Moreover, not all studies report the exact extent of lymphadenectomy. In addition, some studies excluded from this review combined patients with adenocarcinoma and squamous cell carcinoma and/or different tumor locations, which makes data hard to interpret, since these factors could influence the distribution pattern [35,36,37] All together, these factors make comparing available evidence on the lymph node metastases distribution in esophageal cancer difficult.
For upper, middle, as well as lower thoracic esophageal squamous cell carcinoma, the stations around the esophagus are among those with the highest prevalence of lymph node metastases. However, not only lymph node stations in the same region as the tumor have a high prevalence of nodal metastases; stations in different regions are affected as well. For example, 13% of the patients with a lower esophageal tumor have right cervical lymph node metastases.
4.2. Lymphatic Distribution Pattern for Adenocarcinoma
For adenocarcinoma, although data are more limited, similar results are seen. One-quarter (25%) of patients with a GEJ adenocarcinoma had middle thoracic paraesopahgeal lymph node metastases, and when looking at zones, 20% of the patients with a GEJ adenocarcinoma had lymph node metastases in the cervical zone. An explanation could be the presence of an extensive lymphatic network in the submucosa and even in the lamina propria of the esophagus, with both intramural and longitudinal lymphatic drainage. The longitudinal nature of this network explains the variation in anatomic sites of lymph node metastases [38,39,40]. Another result of the complexity of the lymphatic vessel system is the phenomenon of skip metastases [37]. Skip metastases are distant lymph nodes with metastatic involvement, without tumor infiltration in the regional lymph nodes, and they are more often seen in early tumors [10]. It is unclear what the exact clinical value is since the literature is conflicting on the prognostic relevance [41,42,43].
Three studies [24,27,30] described lymph node metastases per lymph node station in relation to pT-stage in patients with squamous cell carcinoma. An increased prevalence of lymph node metastases is seen per region in patients with a higher pT-stage, while the distribution remained similar. These are small numbers; nevertheless, the literature points out that a higher T-stage is associated with more lymph node metastases [44].
It should be pointed out that this study defines the lymph node metastases pattern based on patients without neoadjuvant treatment because lymph node involvement may differ after neoadjuvant chemo(radio)therapy [45]. Currently, neoadjuvant chemoradiation or perioperative or neoadjuvant chemotherapy is the standard of care in most countries. This makes our results less applicable to current surgical patients, and one of the main questions for the future is whether the lymphadenectomy strategy should be based on the pattern of lymph node metastases before neoadjuvant treatment or after neoadjuvant treatment. However, the location of lymph node metastases in untreated esophageal cancer patients tells us more about the behavior of the disease, and this is fundamental, as this allows for accurately defining neoadjuvant treatment strategies by targeting high-risk regions for lymph node metastases in patients with specific characteristics. A recent study showed that after neoadjuvant chemoradiotherapy, almost half of the patients in that cohort had lymph node metastases outside the radiation field, indicating that the current radiation fields are not sufficient [46]. Although, it should be noted that radiotherapy to an elective nodal area (both metastatic and non-metastatic) does not guarantee a better outcome [47].
There are some limitations of the present study. Firstly, as previously mentioned, studies were very heterogeneous in lymph node dissection and the reporting of anatomical sites of nodal metastases. This heterogeneity might have made our pooled results less reliable. Moreover, not all studies could be pooled per station (since they only described lymph node regions) and vice versa. The inclusion of different studies in Table 2 and Table 4 makes displayed percentages slightly different. In addition, few of the studies subdivided patients for T-stage and location of the primary tumor, whilst it has been proven that these factors influence lymph node metastases [26,48,49].
If we want to determine the exact distribution pattern of esophageal cancer, large well-designed prospective studies are needed. One initiative of such a study is the multinational prospective TIGER study (ClinicalTrials.gov Identifier: NCT03222895) [50].
5. Conclusions
Both esophageal squamous cell carcinoma and adenocarcinoma are aggressive diseases that can metastasize to cervical, thoracic, as well as abdominal lymph node stations, regardless of the location of the primary tumor. The prevalence of patients with metastatic lymph nodes per station and region could be determined for squamous cell carcinoma. However, few studies described the distribution of lymph node metastases for esophageal adenocarcinoma, and the data for both tumor types was very heterogeneous. This complicates evidence-based treatment strategies in both neoadjuvant (radiation field) and surgical (lymphadenectomy) treatment. Well-designed prospective studies are needed to determine the exact lymphatic distribution pattern of esophageal cancer.
Appendix A
Table A1.
Search strategy for PubMed (22 July 2019).
| Search | Query | Items Found |
|---|---|---|
| #1 | (((((((((((“Esophageal Neoplasms”[Mesh] OR “Esophagectomy”[Mesh] OR ((esophagus[tiab] OR esophageal[tiab] OR esophagogastric[tiab] OR oesophagus[tiab] OR oesophageal[tiab] OR oesophagogastric[tiab] OR gastroesophag*[tiab] OR gastrooesophag*[tiab]) AND (neoplas*[tiab] OR cancer*[tiab] OR carcino*[tiab] OR adenocarcino*[tiab] OR tumor[tiab] OR tumors[tiab] OR tumour[tiab] OR tumours[tiab] OR malig*[tiab])) OR esophagectom*[tiab])) AND (“Lymph Nodes”[Mesh] OR “Lymphatic Metastasis”[Mesh] OR ((lymph[tiab] OR lymphatic[tiab]) AND (node*[tiab] OR nodal[tiab] OR metastas*[tiab])))))) AND ((“Neoplasm Staging”[Mesh] OR staging[tiab] OR TNM[tiab] OR number[tiab] OR extent[tiab] OR extended[tiab] OR scoring[tiab] OR score[tiab] OR classif*[tiab] OR categor*[tiab] OR criteria[tiab] OR 2-field*[tiab] OR two-field*[tiab] OR 3-field*[tiab] OR three-field*[tiab] OR node status[tiab] OR nodal status[tiab] OR D1[tiab] OR D2[tiab] OR N0[tiab] OR N1[tiab] OR N2[tiab] OR N3[tiab] OR pattern*[tiab] OR drainage[tiab] OR spread[tiab] OR pathway*[tiab] OR depth[tiab])))) NOT ((express*[ti] OR overexpress*[ti] OR gene[ti] OR genes[ti] OR protein*[ti] OR p53[ti] OR serum[ti] OR (case[ti] AND report[ti]))) NOT (Animals[Mesh] NOT Humans[Mesh])) AND (english[la] OR dutch[la])))) | 4106 |
[Mesh] = Medical subject headings; [tiab] = words in title OR abstract; [ti] = words in title; [la] = language.
Table A2.
Search strategy for Embase.com (22 July 2019).
| # | Searches | Results |
|---|---|---|
| 1 | exp esophagus tumor/or esophagus resection/or esophagectom*.ti,ab,kw. or ((esophagus or esophageal or esophagogastric or oesophagus or oesophageal or oesophagogastric or gastroesophag* or gastrooesophag*) and (neoplas* or cancer* or carcino* or adenocarcino* or tumor or tumors or tumour or tumours or malig*)).ti,ab,kw. | 127708 |
| 2 | (exp lymph node/or exp lymph node metastasis/or ((lymph or lymphatic) and (node* or nodal or metastas*)).ti,ab,kw.) and (cancer staging/or (staging or TNM or number or extent or extended or scoring or score or classif* or categor* or criteria or 2-field* or two-field* or 3-field* or three-field* or node status or nodal status or D1 or D2 or N0 or N1 or N2 or N3 or pattern* or drainage or spread or pathway* or depth).ti,ab,kw.) | 208651 |
| 3 | 1 and 2 | 10765 |
| 4 | (express* or overexpress* or gene or genes or protein* or p53 or serum or (case and report)).ti. | 2592018 |
| 5 | animal/ not human/ | 1423187 |
| 6 | conference abstract.pt. or conference paper/ or letter/ | 4893909 |
| 7 | 3 not 4 not 5 not 6 | 6444 |
| 8 | limit 7 to (dutch or english) | 5195 |
exp = EMtree keyword with explosion; de = EMtree keyword without explosion; .ab,ti,kw = words in title OR abstract OR keyword; .ti = words in title; .pt = publication type.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Mariette C., Piessen G., Briez N., Triboulet J.P. The Number of Metastatic Lymph Nodes and the Ratio Between Metastatic and Examined Lymph Nodes Are Independent Prognostic Factors in Esophageal Cancer Regardless of Neoadjuvant Chemoradiation or Lymphadenectomy Extent. Ann. Surg. 2008;247:365–371. doi: 10.1097/SLA.0b013e31815aaadf. [DOI] [PubMed] [Google Scholar]
- 2.O’Riordan J.M., Rowley S., Murphy J.O., Ravi N., Byrne P.J., Reynolds J.V. Impact of solitary involved lymph node on outcome in localized cancer of the esophagus and esophagogastric junction. J. Gastrointest. Surg. 2007;11:493–499. doi: 10.1007/s11605-006-0027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okholm C., Svendsen L.B., Achiam M.P. Status and prognosis of lymph node metastasis in patients with cardia cancer—A systematic review. Surg. Oncol. 2014;23:140–146. doi: 10.1016/j.suronc.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Koenig A.M., Prenzel K.L., Bogoevski D., Yekebas E.F., Bubenheim M., Faithova L., Vashist Y.K., Gawad K.A., Baldus S.E., Schneider P.M., et al. Strong impact of micrometastatic tumor cell load in patients with esophageal carcinoma. Ann. Surg. Oncol. 2009;16:454–462. doi: 10.1245/s10434-008-0169-7. [DOI] [PubMed] [Google Scholar]
- 5.Smit J.K., Pultrum B.B., van Dullemen H.M., Van Dam G.M., Groen H., Plukker J.T.M. Prognostic factors and patterns of recurrence in esophageal cancer assert arguments for extended two-field transthoracic esophagectomy. Am. J. Surg. 2010;200:446–453. doi: 10.1016/j.amjsurg.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Phillips A.W., Lagarde S.M., Navidi M., Disep B., Griffin S.M. Impact of extent of lymphadenectomy on survival, post neoadjuvant chemotherapy and trans-thoracic esophagectomy. Ann Surg. 2016;265:750–756. doi: 10.1097/SLA.0000000000001737. [DOI] [PubMed] [Google Scholar]
- 7.Castoro C., Scarpa M., Cagol M., Ruol A., Cavallin F., Alfieri R., Zanchettin G., Rugge M., Ancona E. Nodal Metastasis From Locally Advanced Esophageal Cancer: How Neoadjuvant Therapy Modifies Their Frequency and Distribution. Ann. Surg. Oncol. 2011;18:3743–3754. doi: 10.1245/s10434-011-1753-9. [DOI] [PubMed] [Google Scholar]
- 8.Sharma D., Thakur A., Toppo S., Chandrakar S.K. Lymph node counts in indians in relation to lymphadenectomy for carcinoma of the oesophagus and stomach. Asian J. Surg. 2005;28:116–120. doi: 10.1016/S1015-9584(09)60274-8. [DOI] [PubMed] [Google Scholar]
- 9.Mizutani M., Murakami G., Nawata S.I., Hitrai I., Kimura W. Anatomy of right recurrent nerve node: Why does early metastasis of esophageal cancer occur in it? Surg. Radiol. Anat. 2006;28:333–338. doi: 10.1007/s00276-006-0115-y. [DOI] [PubMed] [Google Scholar]
- 10.Prenzel K.L., Bollschweiler E., Schröder W., Mönig S.P., Drebber U., Vallboehmer D., Hölscher A.H. Prognostic relevance of skip metastases in esophageal cancer. Ann. Thorac. Surg. 2010;90:1662–1667. doi: 10.1016/j.athoracsur.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Liu J., Liu Q., Wang Y., Xia Z., Zhao G. Nodal skip metastasis is associated with a relatively poor prognosis in thoracic esophageal squamous cell carcinoma. Eur. J. Surg. Oncol. 2016;42:1202–1205. doi: 10.1016/j.ejso.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 12.Akutsu Y., Matsubara H. Lymph node dissection for esophageal cancer. Gen. Thorac. Cardiovasc. Surg. 2013;61:397–401. doi: 10.1007/s11748-013-0237-1. [DOI] [PubMed] [Google Scholar]
- 13.Mariette C., Piessen G. Oesophageal cancer: How radical should surgery be? Eur. J. Surg. Oncol. 2012;38:210–213. doi: 10.1016/j.ejso.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 14.Japanese Esophageal Sociey Japanese Classification of Esophageal Cancer, 11th Edition: Part I. Esophagus. 2017;14:1–36. doi: 10.1007/s10388-016-0551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rice T.W., Patil D.T., Blackstone E.H. AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: Application to clinical practice. Ann. Cardiothorac. Surg. 2017;6:119–130. doi: 10.21037/acs.2017.03.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye T., Sun Y., Zhang Y., Zhang Y., Chen H. Three-field or two-field resection for thoracic esophageal cancer: A meta-analysis. Ann. Thorac. Surg. 2013;96:1933–1941. doi: 10.1016/j.athoracsur.2013.06.050. [DOI] [PubMed] [Google Scholar]
- 17.van Rijswijk A.S., Hagens E.R.C., van der Peet D.L., van Berge Henegouwen M.I., Gisbertz S.S. Differences in Esophageal Cancer Surgery in Terms of Surgical Approach and Extent of Lymphadenectomy: Findings of an International Survey. Ann. Surg. Oncol. 2019;26:2063–2072. doi: 10.1245/s10434-019-07316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willaert W., Mareel M., Van De Putte D., Van Nieuwenhove Y., Pattyn P., Ceelen W. Lymphatic spread, nodal count and the extent of lymphadenectomy in cancer of the colon. Cancer Treat. Rev. 2014;40:405–413. doi: 10.1016/j.ctrv.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Salhab M., Patani N., Mokbel K. Sentinel lymph node micrometastasis in human breast cancer: An update. Surg. Oncol. 2011;20:e195–e206. doi: 10.1016/j.suronc.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Nappo G., Perinel J., El Bechwaty M., Adham M. The standardization of pancreatoduodenectomy where are we? Pancreas. 2016;45:493–502. doi: 10.1097/MPA.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 21.Slim K., Nini E., Forestier D., Kwiatkowski F., Panis Y., Chipponi J. Methodological index for non-randomized studies (Minors): Development and validation of a new instrument. ANZ J. Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 22.Sharma S., Fujita H., Yamana H., Kakegawa T. Patterns of lymph node metastasis in 3-field dissection for carcinoma in the thoracic esophagus. Surg. Today. 1994;24:410–414. doi: 10.1007/BF01427033. [DOI] [PubMed] [Google Scholar]
- 23.Van De Ven C., De Leyn P., Coosemans W., Van Raemdonck D., Lerut T. Three-field lymphadenectomy and pattern of lymph node spread in T3 adenocarcinoma of the distal esophagus and the gastro-esophageal junction. Eur. J. Cardio Thorac. Surg. 1999;15:769–773. doi: 10.1016/S1010-7940(99)00122-0. [DOI] [PubMed] [Google Scholar]
- 24.Igaki H., Kato H., Tachimori Y., Sato H. Prognostic evaluation for squamous cell carcinomas of the lower thoracic esophagus treated with three-field lymph node dissection. Eur. J. Cardiothorac. Surg. 2001;19:887–893. doi: 10.1016/S1010-7940(01)00701-1. [DOI] [PubMed] [Google Scholar]
- 25.Dresner S.M., Lamb P.J., Bennett M.K., Hayes N., Griffin S.M. The pattern of metastatic lymph node dissemination from adenocarcinoma of the esophagogastric junction. Surgery. 2001;129:103–109. doi: 10.1067/msy.2001.110024. [DOI] [PubMed] [Google Scholar]
- 26.Chen J., Liu S., Pan J., Zheng X., Zhu K., Zhu J., Xiao J., Ying M. The pattern and prevalence of lymphatic spread in thoracic oesophageal squamous cell carcinoma. Eur. J. Cardiothorac. Surg. 2009;36:480–486. doi: 10.1016/j.ejcts.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 27.Tachimori Y., Nagai Y., Kanamori N., Hokamura N., Igaki H. Pattern of lymph node metastases of esophageal squamous cell carcinoma based on the anatomical lymphatic drainage system. Dis. Esophagus. 2011;24:33–38. doi: 10.1111/j.1442-2050.2010.01086.x. [DOI] [PubMed] [Google Scholar]
- 28.Li H., Yang S., Zhang Y., Xiang J., Chen H. Thoracic recurrent laryngeal lymph node metastases predict cervical node metastases and benefit from three-field dissection in selected patients with thoracic esophageal squamous cell carcinoma. J. Surg. Oncol. 2012;105:548–552. doi: 10.1002/jso.22148. [DOI] [PubMed] [Google Scholar]
- 29.Kosugi S.I., Kawaguchi Y., Kanda T., Ishikawa T., Sakamoto K., Akaike H., Fujii H., Wakai T. Cervical lymph node dissection for clinically submucosal carcinoma of the thoracic esophagus. Ann. Surg. Oncol. 2013;20:4016–4021. doi: 10.1245/s10434-013-3141-0. [DOI] [PubMed] [Google Scholar]
- 30.Cheng J., Kong L., Huang W., Li B., Li H., Wang Z., Zhang J., Zhou T., Sun H. Explore the radiotherapeutic clinical target volume delineation for thoracic esophageal squamous cell carcinoma from the pattern of lymphatic metastases. J. Thorac. Oncol. 2013;8:359–365. doi: 10.1097/JTO.0b013e31827e1f6d. [DOI] [PubMed] [Google Scholar]
- 31.Lin Z., Chen W., Chen Y., Peng X., Zhu K., Lin Y., Lin Q., Hu Z. A new classification of lymph node metastases according to the lymph node stations for predicting prognosis in surgical patients with esophageal squamous cell carcinoma. Oncotarget. 2016;7:76261–76273. doi: 10.18632/oncotarget.12842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong Y., Guan H., Huang W., Zhang Z., Zhao D., Liu Y., Zhou T., Li B. Precise delineation of clinical target volume for crossing-segments thoracic esophageal squamous cell carcinoma based on the pattern of lymph node metastases. J. Thorac. Dis. 2015;7:2313–2320. doi: 10.3978/j.issn.2072-1439.2015.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duan X.-F., Tang P., Shang X.-B., Jiang H.-J., Yu Z.-T. The prevalence of lymph node metastasis for pathological T1 esophageal cancer: A retrospective study of 143 cases. Surg. Oncol. 2018;27:1–6. doi: 10.1016/j.suronc.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Park S.Y., Suh J.W., Kim D.J., Park J.C., Kim E.H., Lee C.Y., Lee J.G., Paik H.C., Chung K.Y. Near-Infrared Lymphatic Mapping of the Recurrent Laryngeal Nerve Nodes in T1 Esophageal Cancer. Ann. Thorac. Surg. 2018;105:1613–1620. doi: 10.1016/j.athoracsur.2018.01.083. [DOI] [PubMed] [Google Scholar]
- 35.Pedrazzani C., de Manzoni G., Marrelli D., Giacopuzzi S., Corso G., Minicozzi A.M., Rampone B., Roviello F. Lymph node involvement in advanced gastroesophageal junction adenocarcinoma. J. Thorac. Cardiovasc. Surg. 2007;134:378–385. doi: 10.1016/j.jtcvs.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 36.Gertler R., Stein H.J., Schuster T., Rondak I.-C., Hofler H., Feith M. Prevalence and topography of lymph node metastases in early esophageal and gastric cancer. Ann. Surg. 2014;259:96–101. doi: 10.1097/SLA.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 37.Künzli H.T., van Berge Henegouwen M.I., Gisbertz S.S., van Esser S., Meijer S.L., Bennink R.J., Wiezer M.J., Seldenrijk C.A., Bergman J.J.G.H.M., Weusten B.L.A.M., et al. Pilot-study on the feasibility of sentinel node navigation surgery in combination with thoracolaparoscopic lymphadenectomy without esophagectomy in early esophageal adenocarcinoma patients. Dis. Esophagus. 2017;30:1–8. doi: 10.1093/dote/dox097. [DOI] [PubMed] [Google Scholar]
- 38.Management C. Anatomy of lymphatic drainage of the esophagus and lymph node metastasis of thoracic esophageal cancer. Cancer Manag. Res. 2018;10:6295–6303. doi: 10.2147/CMAR.S182436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murakami G., Sato I., Shimada K., Dong C., Kato Y., Imazeki T. Direct lymphatic drainage from the esophagus into the thoracic duct. Surg. Radiol. Anat. 1994;16:399–407. doi: 10.1007/BF01627660. [DOI] [PubMed] [Google Scholar]
- 40.Akiyama H., Tsurumaru M., Udagawa H., Kajiyama Y. Radical lymph node dissection for cancer of the thoracic esophagus. Ann. Surg. 1994;220:363–364. doi: 10.1097/00000658-199409000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang F., Zheng Y., Wang Z., Zheng Q., Huang Q., Liu S. Nodal Skip Metastasis in Esophageal Squamous Cell Carcinoma Patients Undergoing Three-Field Lymphadenectomy. Ann. Thorac. Surg. 2017;104:1187–1193. doi: 10.1016/j.athoracsur.2017.03.081. [DOI] [PubMed] [Google Scholar]
- 42.He S.-L., Yang Y.-S., Wang W.-P., Zhang H.-L., Wang Y.-C., Chen L.-Q. Prognostic Evaluation of Nodal Skip Metastasis for Thoracic Esophageal Squamous Cell Carcinoma. Ann. Thorac. Surg. 2019;108:1717–1723. doi: 10.1016/j.athoracsur.2019.03.081. [DOI] [PubMed] [Google Scholar]
- 43.Kumakura Y., Yokobori T., Yoshida T., Hara K., Sakai M., Sohda M., Miyazaki T., Yokoo H., Handa T., Yorifuji H., et al. Elucidation of the Anatomical Mechanism of Nodal Skip Metastasis in Superficial Thoracic Esophageal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2018;25:1221–1228. doi: 10.1245/s10434-018-6390-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shin S., Kim H.K., Choi Y.S., Kim K., Shim Y.M. Clinical stage T1-T2N0M0 oesophageal cancer: Accuracy of clinical staging and predictive factors for lymph node metastasis. Eur. J. Cardiothorac. Surg. 2014;46:274–279. doi: 10.1093/ejcts/ezt607. [DOI] [PubMed] [Google Scholar]
- 45.Talsma A.K., Shapiro J., Looman C.W., van Hagen P., Steyerberg E.W., van der Gaast A., van Berge Henegouwen M.I., Wijnhoven B.P.L., van Lanschot J.J.B., CROSS Study Group Lymph node retrieval during esophagectomy with and without neoadjuvant chemoradiotherapy: Prognostic and therapeutic impact on survival. Ann. Surg. 2014;260:783–786. doi: 10.1097/SLA.0000000000000965. [DOI] [PubMed] [Google Scholar]
- 46.Hagens E.R.C., Künzli H.T., van Rijswijk A.S., Meijer S.L., Mijnals R.C.D., Weusten B.L., Geijsen E.D., van Laarhoven H.W.M., van Berge Henegouwen M.I., Gisbertz S.S. Distribution of lymph node metastases in esophageal adenocarcinoma after neoadjuvant chemoradiation therapy: A prospective study. Surg. Endosc. 2019 doi: 10.1007/s00464-019-07205-y. [DOI] [PubMed] [Google Scholar]
- 47.Ma J.B., Song Y.P., Yu J.M., Zhou W., Cheng E.C., Zhang X.Q., Kong L. Feasibility of involved-field conformal radiotherapy for cervical and upper-thoracic esophageal cancer. Oncol. Res. Treat. 2011;34:599–604. doi: 10.1159/000334194. [DOI] [PubMed] [Google Scholar]
- 48.Huang W., Li B., Gong H., Yu J., Sun H., Zhou T., Zhang Z., Liu X. Pattern of lymph node metastases and its implication in radiotherapeutic clinical target volume in patients with thoracic esophageal squamous cell carcinoma: A report of 1077 cases. Radiother. Oncol. 2010;95:229–233. doi: 10.1016/j.radonc.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J., Liu Y., Che F., Luo Y., Huang W., Heng X., Li B. Pattern of lymph node metastasis in thoracic esophageal squamous cell carcinoma with poor differentiation. Mol. Clin. Oncol. 2018;8:760–766. doi: 10.3892/mco.2018.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hagens E.R.C., van Berge Henegouwen M.I., Van Sandick J.W., Cuesta M.A., Van Der Peet D.L., Heisterkamp J., Nieuwenhuijzen G.A.P., Rosman C., Scheepers J.J.G., Van Hillegersberg R., et al. Distribution of lymph node metastases in esophageal carcinoma [TIGER study]: Study protocol of a multinational observational study. BMC Cancer. 2019;19:662. doi: 10.1186/s12885-019-5761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]