Abstract
Brassinosteroids (BRs) are important plant growth hormones that regulate a wide range of plant growth and developmental processes. The BR signals are perceived by two cell surface-localized receptor kinases, Brassinosteroid-Insensitive1 (BRI1) and BRI1-Associated receptor Kinase (BAK1), and reach the nucleus through two master transcription factors, bri1-EMS suppressor1 (BES1) and Brassinazole-resistant1 (BZR1). The intracellular transmission of the BR signals from BRI1/BAK1 to BES1/BZR1 is inhibited by a constitutively active kinase Brassinosteroid-Insensitive2 (BIN2) that phosphorylates and negatively regulates BES1/BZR1. Since their initial discoveries, further studies have revealed a plethora of biochemical and cellular mechanisms that regulate their protein abundance, subcellular localizations, and signaling activities. In this review, we provide a critical analysis of the current literature concerning activation, inactivation, and other regulatory mechanisms of three key kinases of the BR signaling cascade, BRI1, BAK1, and BIN2, and discuss some unresolved controversies and outstanding questions that require further investigation.
Keywords: brassinosteroids, receptor-like kinases, GSK3-like kinases, somatic embryogenesis receptor-like kinases, protein phosphatases
1. Introduction
Brassinosteroids (BRs) are plant-specific steroid hormones and play essential roles in a broad range of plant growth and developmental processes, including cell elongation, cell division, and differentiation, seed germination, stomata formation, root development, vascular differentiation, plant architecture, flowering, male fertility, and senescence [1,2,3,4]. BRs are also involved in responding to various abiotic and biotic stresses, such as drought, flooding, salinity, extreme temperatures, microbial pathogens, and insect herbivores [5,6,7]. Plants with defects in BR biosynthesis or signaling show a characteristic set of developmental defects, including dwarfed statue, male sterility, delayed senescence and flowering, and photomorphogenesis in the dark [8,9].
Using various experimental and theoretical approaches, including genetics, biochemistry, cell biology, chemical biology, structural biology, proteomics, transcriptomics, genomics, mathematical modeling, and computational dynamics simulation, a series of important BR signaling components have been well established and intensively studied, revealing a protein phosphorylation-mediated BR signaling cascade [10,11,12] (Figure 1). BRs are perceived at the plasma membrane (PM) by the extracellular domains of BRI1 (Brassinosteroid-Insensitive1) receptor, a leucine-rich repeat receptor-like kinase (LRR-RLK) [9,13], and its co-receptor BAK1 (BRI1-Associated receptor Kinase1, also known as SERK3 for Somatic Embryogenesis Receptor Kinase3) [14,15,16], a versatile LRR-RLK involved in many signaling processes [17]. BR binding to the BR-binding pocket formed by the extracellular domains of BRI1 and BAK1 is thought to trigger conformational changes of their cytoplasmic domains [18,19]. BRI1 subsequently phosphorylates its inhibitor BKI1 (BRI1 Kinase Inhibitor1) and induces its dissociation from the PM [14,18,20,21], thus enabling heterodimerization, reciprocal phosphorylation, and full activation of the kinase activities of BRI1 and BAK1 [14,18,20,21,22,23]. The fully activated BRI1 triggers a series of phosphorylation or dephosphorylation events to transduce the extracellular BR signals into the cytosol. BRI1 phosphorylates BSK1 (BR-Signaling Kinase1), CDG1 (Constitutive Differential Growth1), and some of their homologs, leading to phosphorylation and subsequent activation of members of a unique family of protein phosphatases with Kelch-like domain (PPKLs) that include BSU1 (bri1 suppressor1) and BSU1-Like1-3 (BSL1-3) [24,25,26]. It is generally believed that the phosphorylated BSU1/BSLs inactivate BIN2 (Brassinosteroid-Insensitive2), which is a member of the plant GSK3 (Glycogen Synthase Kinase3)-like kinase family, via dephosphorylation of a phosphorylated tyrosine (Tyr) residue in the activation loop of BIN2 [27]. The dephosphorylated BIN2 also interacts with KIB1 (Kink suppressed in bzr1-1D1), an F-box E3 ubiquitin ligase, leading to BIN2 ubiquitination and proteasome-mediated degradation [28]. Upon BIN2 inactivation and degradation, two highly similar BIN2 substrates, BZR1 (Brassinazole-resistant1) and BES1 (bri1-EMS suppressor1) [29,30] are rapidly dephosphorylated by certain nuclear-localized members of the PP2A (protein phosphatase 2A) family [31], leading to their nuclear accumulation. The dephosphorylated BZR1 and BES1 bind to their target promoters containing BRRE (BR-response element) (CGTGC/TG) and/or E-box (CANNTG) motif to regulate expression of thousands of BR-responsive genes that are crucial for plant growth and development [32,33].
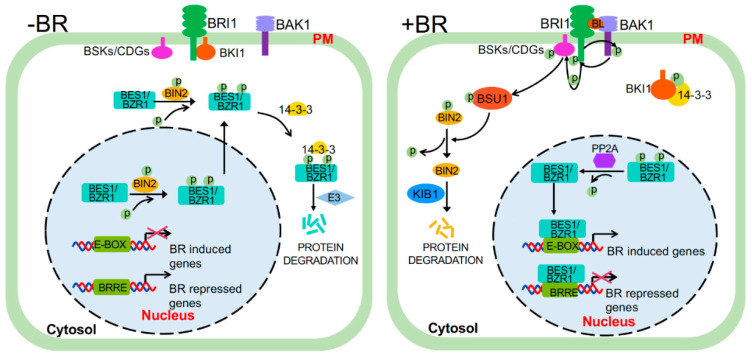
Figure 1.
A current model of BR signaling. When BRs (brassinosteroids) are absent (left), BRI1 (Brassinosteroid-Insensitive1) is kept inactive by its autoinhibitory C-terminus and BKI1 (BRI1 Kinase Inhibitor1) association. BIN2 (Brassinosteroid-Insensitive2) is constitutively active and phosphorylates BES1 (bri1-EMS suppressor1)/BZR1 (Brassinazole-resistant1) transcription factors to promote their degradation and 14-3-3-mediated cytosolic retention, and to directly inhibit their DNA-binding activities. When BRs are present (right) and bind to the extracellular domains of BRI1 and its co-receptor BAK1 (BRI1 Associated receptor Kinase1) to activate the two receptor kinases, leading to dissociation of BKI1 from BRI1, phosphorylation and activation of BSKs (BR-signaling kinases)/CDGs (Constitutive Differential Growth) and BSU1 (bri1 suppressor1). The activated BSU1 dephosphorylates and inactivates BIN2 while KIB1 (Kink suppressed in bzr1-1D1) promotes BIN2 degradation, causing nuclear accumulation of PP2A (protein phosphatase 2A)-dephosphorylated BES1/BZR1 that bind BRRE (BR response element)/E-box-containing promoters to regulate expression of thousands of BR-responsive genes important for plant growth and development.
In the past few years, several excellent reviews were published that summarized the significant progresses in understanding how the BR signal is perceived at the cell surface and how the extracellular BR signal is transduced into the nucleus to regulate a wide range of plant developmental and physiological processes [10,11,12,34]. In this review, we present a critical analysis of currently available data on three early BR signaling components, including the BR receptor BRI1, its coreceptor BAK1, and the crucial negative regulator BIN2, highlighting recent findings and discussing controversies and unanswered questions on the regulatory mechanisms that control their protein abundance, subcellular localizations, and signaling activities.
2. BRI1, the BR Receptor
BRI1 localizes to the PM and belongs to a large and plant-specific family of LRR-RLKs, which is composed of 223 members in Arabidopsis thaliana [35]. BRI1 consists of an extracellular LRR domain of 25 LRRs, a single-pass transmembrane segment, and a cytoplasmic kinase domain [9]. The 25 LRRs are interrupted by a 70-residue island domain (ID, from amino acid Lys586 to Met657), which constitutes the BR binding domain with the 22nd LRR [36]. Its cytoplasmic kinase domain can be subdivided into the JM (juxtamembrane) region, a canonical serine/threonine (Ser/Thr) kinase domain, and a short C-terminal tail (CT) of 36 amino acids (AAs). The Arabidopsis genome encodes three BRI1 homologs: BRL1 (BRI1-Like1), BRL2 (also known as VH1 for Vascular Highway1 [37]), and BRL3. BRL1 and BRL3 can also bind BRs and function as BR receptors [38,39], whereas BRL2/VH1 does not bind BR but functions in a BR-independent manner to regulate vascular development [37,40]. Gene expression analysis and genetic studies revealed that BRI1 is expressed in most plant tissues/organs, whereas BRLs are found only in the vascular tissues and stem cell niches [41]. BRLs have been shown to be involved in vascular development [42] and plant tolerance to abiotic stresses, such as hypoxia and drought [43,44].
2.1. Maintaining the Inactive State in the Absence of BR
In the absence of BR ligands, BRI1 is maintained at its inactive state via its inhibitory CT and its binding to BKI1 at the PM [20,23]. Removal of the BRI1 CT not only increased the in vitro kinase activity of a recombinant BRI1 kinase protein, but also led to a hyperactive BR receptor in vivo [23]. Further investigation will likely be required to fully understand the mechanism of this autoinhibitory activity of the BRI1 kinase activity given the missing CT in several available crystal structures of the BRI1 kinase domain [45,46]. The 36-AA CT could block the substrate binding site to interfere with the in vitro homodimerization for the auto(trans)phosphorylation activity or the in vivo binding of the kinase domains of BRI1 and BAK1. In addition to the “cis” autoinhibition, the binding of the PM-associated BKI1 to the BRI1 cytoplasmic domain demonstrates a “trans” inhibitory mechanism to prevent association and subsequent cross phosphorylation of the kinase domains of BRI1 and BAK1, which is likely triggered by BR-independent heterodimerization of their extracellular domains known to occur in plant cells [15,47,48]. A previous genetic demonstration of the absolute requirement of BAK1/SERKs for the BRI1’s activation [49] implies that BRI1 can be kept in its inactive state via competitive binding of BAK1 with certain BAK1-interacting proteins. Indeed, recent studies revealed that ligand-independent interaction of BAK1 with BIR3 (BAK1-Interacting Receptor-like kinase3) could inhibit BR-stimulated BAK1-BRI1 heterodimerization and BRI1 activation (see below for more discussion on the BAK1-BIR interaction) [50].
2.2. BRI1 Activation
It was well known that many kinases are activated via phosphorylation of crucial Ser/Thr residues in the activation segment catalyzed by upstream kinases [51]. In animal cells, many receptor kinases are activated by homodimerization and auto(trans)phosphorylation [52,53]. Despite several reports of BRI1 homodimerization in vitro or in vivo [15,23,54,55], BRI1 activation requires its heterodimerization and subsequent transphosphorylation with its coreceptor BAK1/SERKs. An earlier in vitro kinase assay with dephosphorylated recombinant kinases of BRI1 and BAK1 showed that neither BRI1 nor BAK1 was active when incubated alone with the ATP-containing kinase reaction buffer but became active when incubated together [45]. Similarly, the full-length BRI1 or BAK1 was inactive when expressed alone in yeast cells but became active kinases when they were coexpressed. Neither BRI1 nor BAK1 was active when coexpressed in yeast with a kinase-dead partner [18]. More important, a BRI1-FLAG fusion protein could not be activated by exogenously applied BR in a transgenic Arabidopsis line that lacks BAK1 and its two close homologs [49]. Furthermore, a recent study showed that ligand-independent heterodimerization of a chimeric LRR-RLK, which was composed of the extracellular domain of BIR3 and the intracellular domain of BRI1, and BAK1 led to constitutive activation of the BR signaling pathway [56]. All these biochemical and genetic/transgenic experiments argue against the widely accepted sequential-phosphorylation model. This model posits that BR binding-triggered conformational changes result in weak activation of BRI1 and its subsequent phosphorylation and activation of BAK1, which can then transphosphorylate BRI1 for its full activation [21]. Further studies, especially structural analysis of the ligand-bound full-length BRI1-BAK1 complexes and mass spectrometry (MS)-based quantitative phosphorylation analysis of BRI1 in bak1/serk mutants, are needed to fully understand the activation mechanism of BRI1 and the genetic requirement of BAK1/SERKs for its activation.
Structural analyses of the BRI1-BAK1 extracellular heterodimers and molecular dynamics simulations suggested that BR binding to the extracellular domain of BRI1 causes conformational changes in BRI1 [57,58]. Such subtle structural changes not only stabilize the ID to create a docking platform for the BRI1-BAK1 association, which is further stabilized by a bound active BR (functioning as “molecular glue” to interact with the N-terminal cap of BAK1), but also potentially create a secondary interface involving the N-terminal 12 LRRs of BRI1 to interact with BAK1 [16,19] (Moffett and Shukla, 2020 bioRxiv: http://doi.org/10.1101/630640). Despite lack of experimental evidence or structural information, it has been widely accepted that stable association of the extracellular domains of BRI1 and BAK1 brings their cytoplasmic domains into close proximity, thus permitting cross phosphorylation, especially at Ser1044 of BRI1 and Thr450 of BAK1 within their respective activation loop, and subsequent activation of both kinases [45,46,59]. A recent structural modeling study suggested that the cytoplasmic domains of BRI1 and BAK1 could weakly interact independently of BR binding to their extracellular domains [60]. However, it is important to point out that the structural models of the kinase domains of BRI1 and BAK1 used for the modeling study were derived from autophosphorylated and activated kinase domains with stabilized αC-helixes and activation segments.
Structural models of the activated BRI1 kinase domain [45,46] suggested that the phosphorylated Ser1044 is likely trapped in a positively-charged phosphate-binding pocket consisting of Arg922 (at the beginning of the αC-helix), Arg1008 [within the HRD (His-Arg-Asp) motif], and Arg1032 (at the beginning of the β9-strand), thus stabilizing the activation loop and establishing an active kinase conformation (Figure 2). Specifically, the interaction of the phosphorylated Ser1044 (pSer1044) and Arg922 of the αC-helix is likely critical for creating the so-called regulatory-spine (R-spine) consisting of 5 residues [the anchoring Asp1068 residue of the αF-helix, His1007 of the HRD motif, Phe1028 of the DFG (Asp-Phe-Gly) motif, Ile931 of the αC-helix, and Leu942 of the β4-strand] (Figure 2). The assembly of the R-spine has been considered as a crucial indicator of an active conformation of eukaryotic protein kinases [61,62]. As expected, a Ser1044-Ala mutation resulted in a strong loss of kinase and signaling activity of BRI1 in vitro and in vivo [22].
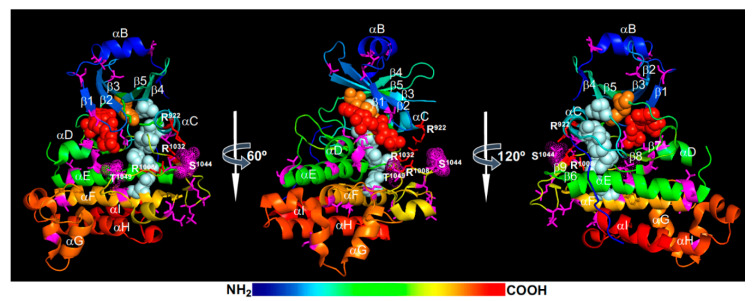
Figure 2.
A structural model of the activated BRI1 kinase domain. Shown here are three (0°, 60°, and 180°) rotational views of a rainbow-colored ribbon model of the crystal structure of the BRI1 kinase domain (Protein Data Bank No. 4oh4). Individual α-helices (αB-αI) and β-strands (β1-β5) were labelled. The magenta-colored sticks indicate phosphorylated Ser/Thr residues with the magenta dots surrounding Ser1044 and Thr1049 of the activation segment, the green sticks denote the Lys911 and Glu927 residues that form the salt bridge between the β3-strand and αC-helix, the red sticks mark the three positively charged residues of the phosphate-binding pocket, and the orange sticks represent the phosphorylated Tyr residue. The red spheres show adenylyl-imidodiphosphate (a non-hydrolysable ATP analog), the orange spheres indicate the gatekeeper Tyr956 residue that is also phosphorylated in in vitro assays, and the light-blue spheres denote the five regulatory-spine (R-spine) residues (from lower to upper: Asp1068, His1077, Phe1028, Ile931, and Leu942). The rainbow bar indicates the order of amino acids (AAs) from the N-terminus (blue) to the C-terminus (red).
In addition to the pSer1044 residue within the activation loop, previous studies revealed an essential role of a conserved Ser/Thr residue (Thr1049) in the “P+1 loop” [21,22], which is thought to be the docking site for the backbone of the substrate P-site and the side-chain of the P+1 residue [61]. Previous bioinformatic analysis indicated that this position is occupied by a Ser/Thr residue in >99% of the so-called “RD”-type RLKs containing Arg-Asp residues within the conserved HRD motif [21]. By contrast, only 30% of “non-RD”-type RLKs contain a Ser/Thr residue at this position. Mutating Thr1049 to Ala completely inhibited the in vitro phosphorylation activity of a recombinant BRI1 kinase domain and greatly reduced its signaling activity in transgenic Arabidopsis plants [23], demonstrating its crucial role in BRI1 kinase activity. Mutating the equivalent Ser/Thr residue in several other “RD”-type LRR-RLKs resulted in loss of in vitro phosphorylation activity [63]. Further studies will be needed to determine whether the phosphorylation of this residue is truly required for BRI1 activation as the phosphorylation-mimic Thr1049-Asp mutation also led to a strong loss of the in vitro phosphorylation activity of a recombinant BRI1 kinase [21]. Based on the structural model (Figure 2), the phosphorylation of this residue could potentially interfere with ATP binding and/or substrate binding.
The activated BRI1 is thought to autophosphorylate additional Ser, Thr, and Tyr residues in both N-lobe and C-lobe (Figure 2). The phosphorylated N-lobe residues include Ser838, Thr842, Thr846, Thr851, and Ser858 of the JM (not shown in Figure 2), Thr872 and Thr880 on the αB-helix that caps the N-lobe, Ser887 and Ser891 on both ends of the β1-strand, Ser906 in the β2-β3 loop, Ser917 in the β3-αC loop (missing in the BRI1 crystal structure), and Thr930 on the αC-helix. The phosphorylated C-lobe residues include Ser963 at the start of the αD-helix, Ser981, Thr982, and Ser990 of the αE-helix, Ser1012 and Ser1013 of a half helical twist before the β7-strand, Ser1026 located between the β8-strand and the DFG motif, Thr1039, Ser1042, and Thr1045 of the activation segment, Ser1071 and Thr1081 of the αF-helix, Ser1109 located at the start of the twisted helical αG-αH linker, Thr1147 at the start of the αI-helix, and at least 5 residues in the CT (Ser1166, Ser1168, Thr1169, Ser1179, and Ser1187) [21,22,63,64,65,66] (Figure 2). Among those phosphorylated residues, at least 11 were identified from immunoprecipitated BRI1 fusion protein from transgenic Arabidopsis plants, including 6 uniquely identified (Ser838, Ser858, Thr872, Thr880, Thr982, and Ser1168), 2 additional Thr sites (most likely Thr842 and Thr846) and 3 ambiguous sites within the activation fragment [22]. In addition, at least 3 Tyr residues were reported to be autophosphorylated, including Tyr831 (in the JM), Tyr956 (the gatekeeper residue on the β5-strand), and Tyr1072 (on the αF-helix) [67,68] (Figure 2). It should be noted that the published MS data from several in vitro and in vivo phosphorylation site-mapping experiments of BRI1 did not identify any phosphorylated Tyr residue [21,22,63,64,65,66]. The 3 phosphorylated Tyr residues (Tyr831, Tyr956, and Tyr1072) that were discussed in the current literature were deduced from immunoblot assays with generic and site-specific anti-phosphorylated Tyr (anti-pTyr) antibodies [67,68]. It is generally believed that these phosphorylated Ser/Thr/Tyr sites could regulate the abundance and/or signaling activity of BRI1 and create docking sites for binding downstream signaling components or regulators to build a BRI1 signaling complex, which likely include scaffolding proteins such as TTLs (Tetratricopeptide Thioredoxin-Like proteins) and BSK3 [69,70]. The activated BRI1 can then transphosphorylate these regulators and downstream targets to transduce the extracellular BR signal into the cytosol, ultimately reaching the nucleus to alter gene expression.
One question that remains to be answered is how binding of BR to BRI1 triggers phosphorylation of BKI1. As discussed above, the activation of BRI1’s kinase activity requires its heterodimerization and transphosphorylation with BAK1, a process that is prevented by the PM-localized BRI1-binding BKI1, while BKI1 dissociation from BRI1 and the PM absolutely requires phosphorylation by activated BRI1 [71,72]. Previous studies showed that BRI1-catalyzed Tyr phosphorylation at the conserved Tyr211, which likely requires BRI1-catalyzed phosphorylation at Ser270 and Ser274 [72], is necessary and sufficient for its dissociation from the PM [71]. It might be possible that BR binding to the BRI1’s extracellular domain triggers yet undefined conformational changes in its cytoplasmic domain. Such structural changes could weaken the BRI1-BKI1 binding and permit weak BRI1-BAK1 association to allow the BAK1-catalyzed transphosphorylation of Thr1044 in the activation loop of BRI1, leading to further conformational changes and full activation of BRI1. Activated BRI1 can subsequently phosphorylate BKI1 at Ser270/274 and Tyr211, resulting in BKI1 dissociation from BRI1 and the PM and a stronger BRI1-BAK1 binding. A better understanding of the BRI1-BKI1 and BRI1-BAK1 interaction requires further structural analyses of the protein complexes of full-length proteins in the absence or presence of active BRs.
2.3. Attenuation and Deactivation
The magnitude and duration of the BR signaling is dynamically regulated by activation and deactivation of the BR receptor. One simple mechanism of receptor inactivation or attenuation is mediated by autophosphorylation. Previous studies suggested that autophosphorylation at certain residues inhibited the in vitro kinase activity of an E. coli expressed BRI1 kinase domain and reduced the physiological activity of BRI1 in transgenic Arabidopsis plants [21,23,67,73]. For example, mutating Thr872 to Ala, which is located at the start of the αB-helix that caps the N-lobe of 5 antiparallel β-strands (Figure 2), could significantly enhance the in vitro autophosphorylation activity or transphosphorylation activity towards an artificial peptide substrate [22]. It is interesting to note that Thr872 and Thr880, known to be phosphorylated and located at the start and end of the αB-helix (Figure 2), are highly conserved among 213 Arabidopsis LRR-RLK [22], suggesting that phosphorylation of these residues might be a conserved attenuation mechanism for many plant LRR-RLKs. Similarly, Ser891, located in the β1-β2 ATP-binding loop (better known as “glycine-rich loop” or “G-loop”) containing the GXGXXG-motif (X indicating any AA), was previously shown to deactivate BRI1 [73], likely by interfering with ATP binding, as the “G-loop” is known for positioning the three phosphate groups and the adenine ring of ATP [61]. An earlier study also suggested that phosphorylation of two tyrosine residues, Tyr831 (located in the JM) and Tyr956 (a critical gatekeeper of the ATP binding pocket), might also inhibit the BRI1 kinase and signaling activity [67]. However, the lack of appropriate phosphorylation-mimic AA for a pTyr residue makes it complicated to interpret the published experimental results on the regulatory roles of BRI1’s Tyr phosphorylation.
In addition to the “cis” attenuation by autophosphorylation, the signaling activity of BRI1 could be “trans” attenuated by dephosphorylation of certain phosphorylated Ser/Thr residues through PP2A [66,74]. A PP2A holoenzyme is composed of three subunits: a catalytic C subunit, a regulatory B subunit, and a scaffolding A subunit. A B subunit from one of the 3 subfamilies in Arabidopsis: B, B’, and B’’, determines the substrate specificity, while an A subunit brings the B and C subunits together to form an active protein phosphatase complex [75]. An earlier study implicated the Arabidopsis SBI1 (suppressor of bri1), a leucine carboxylmethyltransferase, in methylating the C subunits of PP2A to enhance the PM-association of certain PP2A holoenzymes [74]. As a result, PP2A could bind and dephosphorylate activated BRI1, leading to increased BRI1 degradation and attenuated BR signaling. Further support for the involvement of PP2A in attenuating BR signaling came from a recent study showing that at least four cytoplasm-localized PP2A B’ subunits, including B’γ, B’η, B’θ, and B’ζ [75], interact with BRI1 and mediate its dephosphorylation and inactivation [66]. Overexpression of any of these PP2A-B’ genes in a weak bri1-5 mutant significantly enhanced its dwarf phenotype and further reduced its BR signaling activity. By contrast, simultaneously eliminating these four PP2A-B’ genes could enhance BR signaling and partially suppressed the dwarf phenotype of a weak BR-deficient mutant det2 (de-etiolation2) [66]. Quantitative MS assays of autophosphorylated BRI1 kinase domain incubated with immunoprecipitated PP2A complexes identified several autophosphorylated Ser/Thr residues as the potential dephosphorylation sites of PP2A, including a phosphorylated residue (Thr872, Thr880, or Ser887 on the αB-helix and the αB-β1 loop), Ser917 (in the β3-αC loop), Ser981 (on the αD helix), and four phosphorylated Ser/Thr residues in the CT (Ser1166, Ser1168, Thr1169, or Ser1172, and S1179/Thr1180) [66]. An earlier mutagenesis experiment indicated that individual mutations of these Ser/Thr residues (except Thr872) had little impact on the in vitro autophosphorylation activity of the BRI1 kinase but did reduce its transphosphorylation activity towards a peptide substrate [22]. In addition, the dephosphorylated CT was previously shown to exert inhibitory effects on BRI1 signaling activity [23]. Further MS analyses of the endogenous BRI1 in PP2A B’-overexpressing transgenic Arabidopsis lines and the Arabidopsis quadruple mutant lacking B’γ, B’η, B’θ, and B’ζ are needed to pinpoint the exact Ser/Thr residues that are dephosphorylated by PP2A. Given the importance of Tyr phosphorylation in BR signaling [67,68,71] and a previous report of Tyr dephosphorylation of an LRR-RLK immunity receptor EFR (Elongation Factor-Tu Receptor) by a bacterial tyrosine phosphatase [76], it will be interesting to investigate if the BRI1 signaling activity could also be regulated by a member of the Arabidopsis PTP/DSPP (protein tyrosine phosphatase/dual specificity protein phosphatase) family [77].
2.4. Regulating the Abundance of BRI1 on the PM
2.4.1. Trafficking from the ER to the PM
The BR signaling activity at the PM is also controlled by the amount of the PM-localized BRI1 receptor, which starts its secretory journey in the endoplasmic reticulum (ER) (Figure 3). It was well known that the ER houses several stringent quality control (QC) systems that permit export of only correctly folded proteins into the Golgi apparatus but retain incompletely or incorrectly folded proteins in the ER for chaperone-assisted folding repair or degradation via ER-associated degradation mechanism (ERAD) [78,79]. Two structurally defect but biochemically competent mutant variants of BRI1, bri1-5 with Cys69-Tyr mutation and bri1-9 carrying a Ser662-Phe mutation, are retained in the ER and degraded by ERAD [80,81,82] (Figure 3). Loss-of-function mutations in the ER quality control (ERQC) or ERAD system resulted in increased amounts of mutant bri1 receptors on the PM, thus partially suppressing the dwarfism phenotype of these bri1 mutants as the corresponding mutant BRI1 proteins still retain partial signaling activity after being correctly targeted to the PM [83,84,85,86]. A recent study also suggested the presence of a yet to be defined QC system at the PM to remove misfolded/damaged PM-localized proteins, such as bri1-301 (with a Gly989-Ile mutation) that escapes from ERQC/ERAD [87,88]. TWD1 (Twisted Dwarf1), a well-studied cochaperone protein that was previously shown to be localized in the ER and at the PM, where it regulates auxin transport [89], also interacts with both BRI1 and BAK1 to enhance BR signaling [90,91]. Given its demonstrated interaction with HSP90 (heat shock protein 90) [92,93], it is tempting to speculate that TWD1 could promote optimal folding of BRI1 and its coreceptor, thus maximizing their BR signaling activities at the PM.
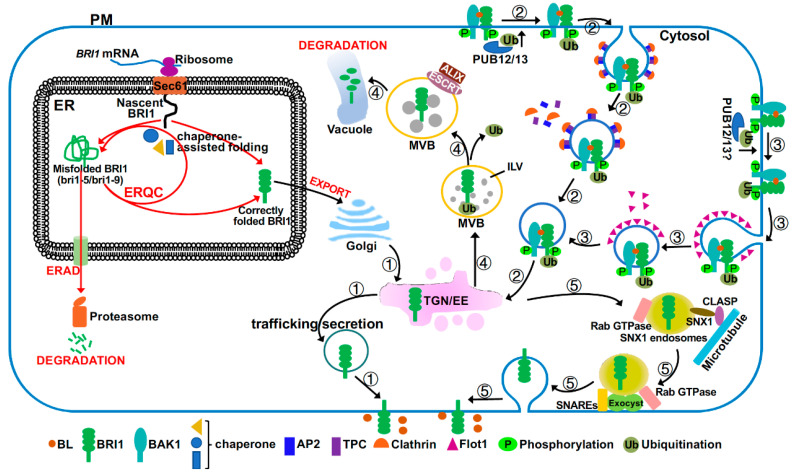
Figure 3.
A current model of regulating the BRI1 abundance on the plasma membrane (PM). Newly synthesized BRI1 is translocated through the Sec61 translocon from the ER (endoplasmic reticulum)-associated ribosomes into the ER where it undergoes chaperone-assisted protein folding. Correctly folded BRI1 is exported into the Golgi to continue its secretory journey through the TGN (trans-Golgi network)/EE (early endosome) to be targeted on the PM (1), whereas the incorrectly/misfolded BRI1, such as its mutant variants bri1-5 and bri1-9, are retained in the ER by ERQC (ER quality control) for refolding and degradation via ERAD (ER-associated degradation) that involves retrotranslocation and cytosolic proteasome. The PM-localized BRI1 or BRI1/BAK1 heterodimer is presumably ubiquitinated by the PUB12/13 (plant U-box protein12/13) E3 ligases and undergoes constitutive CME (clathrin-mediated endocytosis)-mediated internalization (2) or ligand-induced CIE (clathrin-independent endocytosis)-mediated internalization (3). The endocytosed BRI1 at the TGN/EE could be packaged into ILVs (intraluminal vesicles) and delivered to the vacuole for degradation via ESCRT (endosomal sorting complex required for transport)-mediated biogenesis of MVBs (multivesicular bodies) and eventual MVB-vacuole fusion (4). Alternatively, the TGN/EE-localized BRI1 can be recycled back to the PM via retromer-mediated cargo selection, microtubule-assisted vesicle trafficking, and exocyst/SNARE (soluble N-ethylmaleimide-sensitive factor-attachment protein receptor)-involved exocytosis (5). The circled numbers indicate different secretion/trafficking routes. SNX1 (sorting nexin1) is one of the core retromer subunits, CLASP (cytoplasmic linker-associated protein) is a microtubule-associated protein, and ALIX (the Arabidopsis homolog of apoptosis-linked gene 2-interacting protein X) is a cytosolic ESCRT-associated protein.
2.4.2. Targeting to Unique PM Nano/Microdomains
Recent fluorescence microscopy studies coupled with live-cell imaging techniques have revealed that plant LRR-RLKs, including BRI1 and BAK1, are not uniformly distributed on the PM but are rather localized to specific PM subcompartments known as lipid rafts, nanodomains, or microdomains with unique lipid and protein compositions [48,94,95]. It was thought that members of the two protein families: Flotillins (Flot) and plant-specific remorins, might function as organization centers to form protein nanoclusters that are important for receptor signaling [55,95]. Interestingly, AtFlot1, one of the two Arabidopsis Flot homologs, was shown to coexist with BRI1 in a PM microdomain to influence BRI1 endocytosis [55] while OsREM4.1 (Oryza sativa remorin4.1), a member of the rice remorin family, interacted with OsSERK1, a rice homolog of BAK1, to regulate the heterodimerization of OsSERK1 with a rice homolog of BRI1, OsBRI1 [96]. It remains to be determined how AtFlot1 and OsREM4.1 recruit BRI1/OsBRI1 to unique nano/microdomains on the PM given the presence of >600 RLKs, but only 2 Flots and 16 remorins in Arabidopsis [97,98].
2.4.3. Endocytosis
Like many PM-localized proteins, the wild-type BRI1 is also known to dynamically undergo clathrin-mediated endocytosis (CME) or clathrin-independent endocytosis (CIE) from the PM to the trans-Golgi network or early endosomes (TGN/EE) [15,55,99], where BRI1 can then be recycled back to the PM or sorted into the vacuole for degradation via multiple multimeric ESCRT (endosomal sorting complex required for transport) complexes [100,101,102,103,104]. Thus, BRI1 endocytosis serves to attenuate BR signaling despite an early report that suggested endosomal initiation of BR signaling [99,105]. CME is a highly conserved cellular process that requires coordination of several groups of proteins, including clathrin triskelia consisting of clathrin heavy chains and light chains, small GTPases and their regulators such as ADP-ribosylation factor-guanine nucleotide exchange factors (ARF-GEFs), and adapter complexes, including the canonical heterotetrameric adapter protein 2 (AP2)-complex and the heterooctomeric TPLATE complex (TPC) [106]. Consistent with this, inhibition of BRI1 endocytosis by tyrphostin A23, which is a widely-used chemical that specifically blocks the cargo recruitment step of CME [107], could inhibit BRI1 endocytosis and enhance BR signaling [55,104]. Similarly, genetic mutations or transgenic interferences (dominant-negative and/or gene silencing) of the CME components, including clathrins, Rab GTPases, two ARF-GEFs (GNOM and GNOM-LIKE1), the AP2-complex, and the TPC complex, impaired BRI1 endocytosis, leading to increased BRI1 abundance at the PM and enhanced BR signaling [104,108,109]. It was generally thought that the CME-mediated BRI1 internalization is a constitutive process; however, a recent study suggested that BRI1 internalization could be stimulated by BR treatment, which is mediated by a CIE pathway involving a membrane microdomain-associated protein AtFlot1 (Arabidopsis thaliana Flot1 [110]. AtFlot1 directly associates with BRI1 and interference with AtFlot1 protein affects BRI1 endocytosis, leading to increased BRI1 on the PM and enhanced BR signaling [55]. It remains to be determined to what degree coordination of the CME and CIE pathways regulate BRI1 endocytosis and downstream BR signaling.
2.4.4. The Endocytic Pathway to the Vacuole for Degradation
Endocytosed BRI1 proteins accumulate in the TGN/EE compartments where they are sorted into the vacuole for degradation, an endocytic pathway that is mediated through sorting/packaging of BRI1 into ILVs (intraluminal vesicles) of the LE/MVBs (late endosomes/multivesicular bodies) and eventual MVB-vacuole fusion [111]. Previous studies suggested that BRI1 endocytosis and its subsequent sorting at TGN/EE into MVBs is likely regulated by the E3 ligases PUB12/13 (plant U-box protein12/13)-catalyzed ubiquitination of BRI1 [112] while its packaging into MVBs is regulated by ESCRT protein complexes and their associated proteins [113]. Interestingly, BR treatment stimulated the BRI1-PUB13 interaction and PUB13 phosphorylation and simultaneous elimination of PUB12 and PUB13 inhibited BRI1 internalization, leading to increased BRI1 abundance and enhanced BR sensitivity [112]. It was known that K63-linked polyubiquitination is involved in endocytosis and subsequent ESCRT-mediated vacuolar delivery, whereas K48-linked polyubiquitination is the signal for proteasome-mediated degradation [114,115]. A previous study indicated that PUB12/13-catalyzed ubiquitination of FLS2 (Flagellin Sensing2), another LRR-RLK plant immunity receptor that senses bacterial flagellin [116], is involved in proteasome-mediated FLS2 degradation [117], implying that PUB12/13 likely catalyzes the K48-linked polyubiquitination of FLS2. Thus, it will be interesting to determine the exact type of the ubiquitin-linkage and the exact site(s) of the PUB12/13-catalyzed BRI1 ubiquitination given an earlier report indicating that the K63-linked polyubiquitination at Lys866 of an immunoprecipitated BRI1 is the likely signal to drive the BRI1 endocytosis [115]. Experiments are also needed to fully understand the discrepancy between the BR-dependent BRI1-PUB12/13 interaction [112] and the kinase-dependent but ligand-independent BRI1 ubiquitination [115].
Interestingly, a partial loss-of-function mutation in Arabidopsis ALIX (apoptosis-linked gene 2-interacting protein X), which is required for localizing an Arabidopsis deubiquitinating enzyme in LE/MVBs and associates with an ESCRT complex to mediate packaging of cargos into ILVs [118], was found to be defective in the vacuolar delivery of BRI1 [113]. It remains unknown whether the failure to package endocytosed BRI1 into ILVs is functionally related to the mislocalization of the deubiquitinating enzyme in the alix mutant. A recent study also implicated BIL4 (Brassinazole-Insensitive-Long hypocotyl4), a 7-transmembrane protein localized in the TGN/EE, LE/MVB, and vacuolar membrane, in regulating the endocytic trafficking of BRI1 to the vacuole [119]. RNAi-mediated BIL4 silencing resulted in increased BRI1 localization in the vacuole, whereas BIL4 overexpression reduced the BRI1 trafficking from the TGN/EE to the vacuole. It remains to be investigated to fully understand the biochemical function of BIL4 in the plant endocytic pathway.
2.4.5. Endocytic Recycling
Most of the endocytosed BRI1 are thought to be recycled back to the PM to replenish the PM pool of the BR receptor, thus enhancing BR signaling [99,101,102,104]. It was thought that the TGN/EE-PM recycling involves retromer complex-mediated cargo retrieval, microtubule-assisted trafficking of recycling endosomes, and SNARE (soluble N-ethylmaleimide-sensitive factor attachment proteins receptor)-mediated exocytosis [111]. A hypomorphic allele of DET3 that encodes the cytosolic C subunit of the Arabidopsis vacuolar ATPase (V-ATPase) reduces the ability of V-ATPase to acidify the TGN/EE but not the Golgi or the vacuole, leading to compromised secretion and recycling of BRI1 and reduced BR sensitivity [120,121]. An Arabidopsis microtubule-associated protein CLASP (cytoplasmic linker protein-associated protein) was previously known to interact with SNX1 (sorting nexin1), a component of the Arabidopsis retromer complex, to mediate the endosome-microtubule association [122]. Interestingly, the expression of CLASP is regulated by BR in a BRI1-dependent manner and CLASP also mediates the BR-induced microtubule reorganization [123]. Importantly, a loss-of-function mutation in CLASP compromised the TGN/EE-PM trafficking of the constitutive cycle of BRI1 endocytosis-exocytosis, leading to reduced BRI1 abundance on the PM and dampened BR sensitivity [123]. The exocytosis is known to be coordinated by Rab GTPase, the vesicle tethering complex known as exocyst, and SNAREs [124]. Mutations in EXO70A1 (exocyst subunit 70A1, a component of the Arabidopsis exocyst complex) or components of the SNARE complex reduced BRI1 recycling back to the PM [125,126]. A recent study also implicated BIG3 (brefeldin A-inhibited guanine nucleotide-exchange protein3) and BIG5, two members of the BIG subfamily of the Arabidopsis ARF-GEFs, in BRI1 recycling. Simultaneous elimination of BIG3 and BIG5 resulted in dwarfed plants with reduced BR sensitivity [127], but their exact cellular functions remain to be defined. Given the widely accepted model of constitutive endocytosis of BRI1, it will be interesting to investigate how plant cells integrate developmental cues and environmental signals to balance the endocytic vacuolar degradation and the endocytic recycling process to control the abundance of signaling competent BRI1 on the PM.
3. BAK1, the Coreceptor
BAK1/SERK3 and three other members of the SERK family, SERK1, SERK4, and SERK5 (nonfunction in the Col-0 ecotype of Arabidopsis thaliana but remains function in the Ler-0 ecotype [128]), are required to function as the BRI1 coreceptor to initiate the BR signaling at the PM [14,18,129,130]. The 5 members of the SERK family share the same structural organization with an extracellular domain of 5 LRRs plus the N-terminal cap, a single transmembrane helix, a cytoplasmic kinase domain, and a CT with a conserved Ser-Gly-Pro-Arg motif at their C-terminal end known to be important for their kinase activity [131]. Structural analysis of the BRI1-BAK1 heterodimer of their extracellular domains identified key residues involved in forming and stabilizing the BAK1-BRI1 dimer [19]. In addition to functioning as the coreceptors for BRI1 to activate the ligand-bound BRI1, BAK1/SERKs were found to be versatile coreceptors that heterodimerize with many ligand-bound RLKs for their activation [17] and intracellular signal transduction.
3.1. Phosphorylation of BAK1
As discussed above, BAK1 activation requires its heterodimerization with BRI1 when assayed with E. coli expressed kinase domains of BRI1 and BAK1 [45] or with yeast expressed full-length proteins [18]. An earlier transgenic experiment, which expressed a BAK1-GFP fusion protein in a strong bri1-1 mutant background, showed that exogenous BR application resulted in no detectable change in BAK1 phosphorylation [21], suggesting that BAK1/SERKs activation in vivo also requires its heterodimerization with BRI1.
MS analyses of E. coli-expressed recombinant BAK1 kinase, in vitro phosphorylated forms, and immunoprecipitated BAK1 fusion proteins from transgenic Arabidopsis plants identified a total of 23 phosphorylation sites in both N-lobe and C-lobe (Figure 4). The phosphorylated residues in the N-lobe include Ser286 of the αB-helix, Ser290 of the αB-β1 loop, Thr312 of the β2-β3 loop, Ser324 within the β3-αC loop (that is missing in the BAK1 kinase structure), Thr333 and Ser339 of the αC-helix, and Thr355 and Thr357 of the β4-β5 loop. The phosphorylated residues of the C-lobe include Ser370 and Ser373 on the αD-helix, Ser381 of the αD-αE loop, Tyr443, Thr446, Thr449, Thr450, and Thr455 on the activation segment, Ser465 and Thr466 located on a short α-helix that links the P+1 loop with the αF helix, Ser557 of the αI-helix, and 4 residues (Ser602, Thr603, Ser604, and Ser612) in the CT [21,59,65,130,132,133] (Figure 4). Among these phosphorylation sites, a total of 9 residues, including Ser290, Thr312, Thr446, Thr449, Thr455, and the 4 Ser/Thr residues in the CT, were discovered from immunoprecipitated BAK1 fusion proteins of transgenic Arabidopsis plants [21,134]. Whereas no single pTyr residue of BRI1 was detected by MS, a pTyr residue, Tyr443 located at the tip of the activation loop (Figure 4), was identified from MS analysis of an E. coli expressed recombinant BAK1 kinase [65]. Two additional Tyr residues, Tyr463 (located at a short α-helix that links the P+1 loop with the αF-helix) (Figure 4) and Tyr610 (on the CT that is not shown on the BAK1 kinase crystal structure), were previously shown to be phosphorylated based on immunoblotting analysis with anti-pTyr antibodies and loss-of-phosphorylation (Tyr-Phe) mutagenesis [73,135].
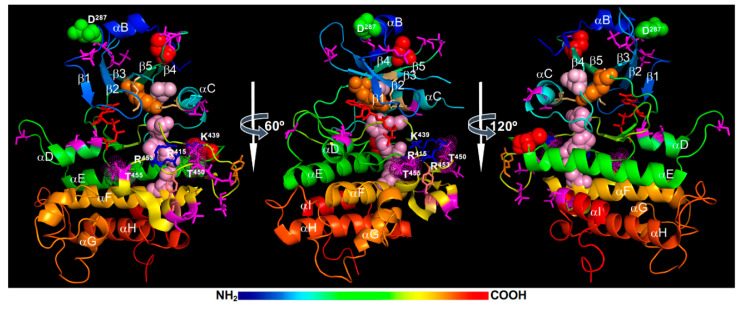
Figure 4.
A crystal structure of the BAK1 kinase domain. Shown here are three rotation (0°, 60°, and 180°) views of a rainbow-colored ribbon model of the crystal structure of the BAK1 kinase domain (Protein Data Bank No: 3uim). Individual α-helices (αB-αI) and β-strands (β1-β5) were labelled. The purple sticks indicate phosphorylated Ser/Thr residues with the Thr450 and Thr455 residues surrounded with purple dots. The red sticks show the bound adenylyl-imidodiphosphate (a non-hydrolysable ATP analog), the light-orange sticks denote the two residues that form the conserved salt bridge between the β3-strand and the αC-helix, the blue sticks represent the three positively charged residues that make up the phosphate-binding pocket, and the dark-orange sticks indicate phosphorylated Tyr residues. The pink spheres mark the R-spine residues, the red spheres show the two Cys residues that were S-glutathionylated in vitro, the orange spheres denote the gatekeeper Tyr residue, and the green spheres designate the Asp287 residue important for Ca2+-dependent BAK1 cleavage. The rainbow bar indicates the order of AAs from the N-terminus (blue) to the C-terminus (red).
Structural analysis and molecular dynamics simulations suggested that the Thr450 phosphorylation is likely the most critical for BAK1 activation as its attached phosphate group likely binds to a positively-charged phosphate-binding pocket, which consists of Arg415 (within the HRD motif), Lys439 (on the β9-strand), and Arg453 (on the activation segment), to stabilize the activation segment (Figure 4). It is interesting to note that the third Arg residue of the BAK1’s phosphate binding pocket is from the activation segment itself instead the αC-helix that contributes the third Arg residue for the phosphate-binding pocket of the BRI1 kinase (Figure 2). The interaction between Arg922 with the phosphorylated Ser1044 is important to stabilize the N-lobe-C-lobe interaction in BRI1 to assemble the R-spine. It remains to be determined whether this is the likely cause for the apparent failure to incorporate a hydrophobic residue (likely Ile338) from the αC-helix to the R-spine of BAK1 and an apparent gap between the Lys317 (from the β3-strand) and Glu334 (of the αC-helix) that should form the conserved salt bridge important for the kinase activity. Consistent with the structural analysis, Thr450 was found to be essential for its autophosphorylation and transphosphorylation activities when assayed in vitro [59,132,136]. However, these results were contradictory to an earlier study demonstrating that the Thr450-Ala mutation only marginally affected the in vitro phosphorylation and in vivo signaling activities of BAK1 [21]. Further studies are needed to fully establish if the transphosphorylation of BAK1 by BRI1 at the Thr450 residue is absolutely required to activate the BAK1 kinase activity in vivo. It is also interesting to investigate if the gatekeeper residue Tyr363 might contribute to the assembly of the R-spine crucial for BAK1 activation (Figure 4).
Similar to what was discovered with the BRI1 kinase domain, the Thr residue in the P+1 loop, Thr455, is essential for the BAK1 activity. Mutating Thr455 to Ala or Asp/Glu resulted in a strong loss of BAK1’s phosphorylation activity in vitro and its BR-signaling function in transgenic Arabidopsis plants [21,59,132], suggesting that autophosphorylation of this highly conserved Ser/Thr residue might serve to inhibit rather activate BAK1 activity. Similar to what was discussed for the BRI1’s Thr1049 residue, the phosphorylation of Thr455 could interfere with ATP binding or substrate binding due to its strategic position at the P+1 loop. However, it remains a possibility that the phosphorylation of Thr455 is required for BAK1 activation but the Thr-Asp/Glu mutation might not be able to mimic the pThr455 residue at this strategic position. Site-directed mutagenesis coupled with in vitro phosphorylation assays and Arabidopsis transgenic experiments revealed another Ser/Thr residue whose phosphorylation serves to attenuate the BAK1 activity. Mutating Ser286 to Ala, which is located at the αB-helix capping the N-lobe, had little impact on the BAK1 activity; however, mutating Ser286 to Asp resulted in almost complete loss of in vitro phosphorylation activity of BAK1 and caused a dominant negative effect in transgenic Arabidopsis plants [21]. The impact of pSer286 on BAK1 activity is very similar to that of pThr872 on BRI1, which is also localized on the αB-helix that caps the BRI1’s N-lobe [22]. Further studies are needed to fully appreciate the negative impact of phosphorylating the Ser/Thr residues of the αB-helix. It is equally important to determine whether these Ser/Thr residues are intramolecularly autophosphorylated or intermolecularly transphosphorylated in order to fully understand the activation or autoinhibitory mechanisms of BAK1/SERKs and other LRR-RLKs.
In addition to the “cis” attenuation mechanism via auto/trans-phosphorylation of Ser286/Thr455, the BAK1 signaling activity could also be attenuated by a “trans” regulatory mechanism involving a phosphatase. A recent study suggested a role of a PP2A (consisting of subunits A1, C4, and B’η/ζ) in regulating the phosphorylation status of BAK1 in plant immunity response [137]. Given the demonstrated constitutive BAK1-PP2A association [137] and implication of PP2A in regulating BRI1 phosphorylation status [66], it is quite possible that this PP2A or its close homolog(s) could negatively influence BR signaling by controlling the phosphorylation level of the BRI1-associated BAK1/SERKs.
3.2. Regulating BAK1 Availability for BRI1 Interaction
Given the importance of BAK1/SERKs in activating the BR-bound BRI1 [49], it is no surprise that plant cells could regulate the availability of BAK1/SERKs to control BR signaling. The ligand-independent/dependent heterodimerization of BAK1 with BRI1 was previously known to induce endocytosis of both LRR-RLKs [15]. An Arabidopsis protein, MSBP1 (membrane steroid binding protein1) was previously found to inhibit BR signaling by stimulating BAK1 endocytosis to limit the amount of BAK1 on the PM [138]. Recently, several members of a small subfamily of LRR-RLKs known as BIRs were found to constitutively interact with BAK1/SERKs, thus interfering the signal initiation processes of many BAK1/SERKs-required LRR-RLKs [50,60,139,140,141,142,143]. However, only the BIR3-BAK1 interaction affects the BR-induced BRI1-BAK1/SERK heterodimerization and inhibits BR signaling [50]. A previously known gain-of-function mutant of BAK1, elg1-D (elongated1-Dominant, carrying an Asp122-Asn mutation) that was previously identified as a suppressor of an Arabidopsis gibberellin-deficient dwarf mutant [144,145], exhibits a much weaker binding affinity to the extracellular domain of BIR3 [143], thus increasing availability of BAK1 for BRI1 interaction to enhance BR signaling. However, it remains to be studied why overexpression of BIR3 led to a strong bri1-like dwarf phenotype with complete BR insensitivity but its loss-of-function bir3 mutation enhanced the dwarf phenotype of a weak bri1 mutant and exhibited no morphological similarity to the elg1-D mutant or weak phenotype of BRI1-overexpression. Further studies are also needed to fully understand why BIR3, but not its two other homologs, BIR1 and BIR2, can inhibit BR signaling. One possible explanation is its ability to interact with BRI1 and form ligand-independent BRI1-BAK1/SERKs-BIR3 receptor nanoclusters [60,95]. In addition to BIRs, members of another small subfamily of LRR-RLK [LRR-RLK IX subfamily, named BAK1-Associated Receptor-like Kinase1 (BARK1) and BARK1-Like Kinase 1-3 (BLK1-3)], could also bind BAK1/SERKs to influence BR signaling [146]. However, overexpression of BARK1 resulted in a hypersensitive phenotype, suggesting a potentially positive role of BARK1 in BR signaling. Unlike BIR3 that likely carries a cytoplasmic pseudokinase domain [50,147], the kinase domain of BARK1 is predicted to be an active kinase that could potentially help to phosphorylate and activate BAK1/SERKs or downstream signaling components to enhance BR signaling. A recent rice study also implicated OsREM4.1, a member of the remorin family thought to be associated with micro/nanodomains on the PM [97], as an interactor of OsSERK1 to interfere with the OsSERK1-OsBRI1 heterodimerization [96], suggesting that competitive BAK1/SERK-binding is likely a conserved mechanism to control BR signal initiation on the cell surface.
3.3. BAK1 Regulation by Other Mechanisms
Increasing evidence suggested an important role of S-glutathionylation, a post-translational modification of a Cys residue via its disulfide linkage with the Cys residue of glutathione (a γ-Glu-Cys-Gly tripeptide) [148], in regulating protein stability and activity in response to cellular oxidative stress. A recent in vitro study showed that BAK1 could be S-glutathionylated at Cys353 and Cys408 (shown in red spheres in Figure 4) by AtGRXC2 (Arabidopsis thaliana glutaredoxin C2) via a thiol-dependent reaction with glutathione disulfide, leading to a reduction of the in vitro BAK1’s kinase activity [149]. Molecular dynamics simulations suggested that S-glutathionylation of Cys408 promotes an inactive kinase conformation state [150]. The S-glutathionylation of Cys408 might directly affect the positioning of the αC-helix by steric hinderance or interfere with the interaction of the αC-β4 loop with the αE-helix, which is a crucial interaction that stabilizes the αC-helix to maintain an active kinase conformation [61]. It is interesting to note that Cys408 was mutated to Tyr in bak1-5, which compromises some plant innate immunity responses but has little effect on BR signaling [151]. It remains to be tested if BAK1/SERKs are S-glutathionylated in vivo and whether S-glutathionylation interferes their heterodimerization and transphosphorylation with BRI1 or other BAK1/SERKs-required LRR-RLKs and whether such a modification exhibits different impacts on BR signaling, plant immunity, and other BAK1/SERK-mediated processes.
A recent study suggested potential involvement of a Ca2+-dependent BAK1 proteolytic cleavage process in BR-mediated plant development and growth [152]. Biochemical studies suggested that the Asp287 residue (shown in green spheres in Figure 4), which is conserved among SERK family members and located right after the important regulatory Ser286 residue [17], is critical for its proteolytic cleavage. However, functional verification of the cleavage process in BR signaling was complicated by retention of a mutated BAK1 (carrying Asp287-Ala mutation) in the ER, most likely caused by ERQC-associated processes. This study suggested that considerable caution is needed in interpreting results with mutated transgenic constructs because some introduced mutations could potentially cause structural defects that could be detected by stringent quality control systems in plant cells, thus altering their protein abundance and/or subcellular locations.
One question that remains to be answered is whether or not BAK1/SERKs interact with downstream BR signaling components to directly influence the intracellular transmission of the extracellular BR signals in addition to their essential role of activating BRI1. Previous studies indicated that a C-terminally-tagged BAK1 or a bak1 allele (known as sobir7-1 for suppressor of bir1 7-1, carrying a non-sense mutation at Trp597 and lacking the CT) affected plant defense signaling but had little impact on BR signaling [131,153], leading to a conclusion that BAK1/SERKs require their CTs to interact with downstream signaling components to influence the plant immunity. It is interesting to note that the Arabidopsis BIK1 (Botrytis-Induced Kinase1), a receptor-like cytoplasmic kinase that is rapidly phosphorylated by FLS2-associated BAK1 and transduces the plant immunity signal to downstream targets [154], inhibits BR signaling by interacting with BRI1 but not the BRI1-interacting BAK1 [26]. Given the versatile roles of BAK1/SERKs in plant growth/development and plant defense [17], understanding the molecular mechanism(s) that determine the biochemical functions of BAK1/SERKs in BR signaling and FLS2/EFR-mediated plant defense responses will have a huge impact on plant biology.
4. BIN2, the Negative Regulator
The GSK3-like kinase BIN2 was originally discovered in a forward genetic screen for mutants similar to loss-of-function bri1 mutants [30] and was subsequently shown to phosphorylate and negatively regulate BES1 and BZR1 [29,155,156] to block the intracellular transduction of the extracellular BR signals. The Arabidopsis genome encodes a total 10 GSK3-like kinases that are divided into four different subgroups [157], but genetic screens for BR-related dwarf mutants (bin2 or dwarf12) [30,158] or leaf development mutants (ucu for ultracurvata) [159] discovered gain-of-function mutations only in BIN2 but not in any of the remaining 9 GSK3-like kinases. Interestingly, at least 7 of the 8 known bin2/dwarf12/ucu1 mutants carry single AA changes in a 4-AA “TREE” (Thr261Arg262Glu263Glu264) motif that is a part of a surface-exposed α-helix (Figure 5) on the long connection fragment that links the αG and αH helices. However, subsequent reverse genetic studies showed that BIN2 functions redundantly with at least 6 other members of the Arabidopsis GSK3-like kinase family in regulating BR signaling [27,160,161,162,163].
Figure 5.
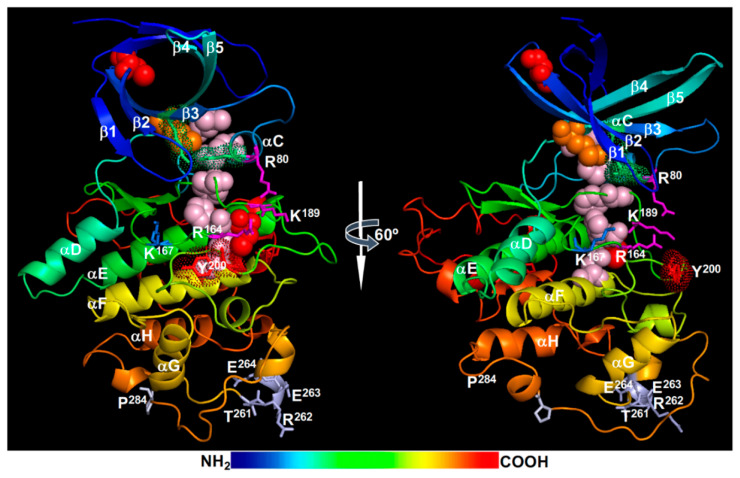
A structural model of BIN2. Shown here are two rotational (0° and 60°) views of a modeled BIN2 structure obtained at SWISS-MODEL (https://swissmodel.expasy.org/). Individual β-strands (β1-β5) and α-helices (αC-αH) were labeled. The two extra antiparallel-β-strands that cap the N-lobe are not labelled. The TREE motif and the Pro284 residue mutated in known bin2/dwarf12/ucu1 alleles are indicated with grey-colored sticks. The magenta sticks show the three positively charged residues [Arg80, Arg164, and Lys189 (also acetylated)] of the conserved phosphate-binding pocket, the red sticks with red dots denote the Tyr200 residue, the green sticks with green dots mark the Lys69-Glu81 salt bridge between the β3-strand and the αC-helix, and the blue sticks represent the Lys167 residue that corresponds to the acetylated Lys183 residue of the human GSK3β. The red spheres show the Cys residues that were shown to be S-nitrosylated or S-glutathionylated in vitro, the orange spheres indicate the gatekeeper Met115 residue, and the pink spheres mark the 5 R-spine residues (from lower to top: Asp223, His163, Phe185, Met85, and Leu96). Important residues are also labelled with single letter codes with positions. The rainbow bar indicates the order of AAs from the N-terminus (blue) to the C-terminus (red).
4.1. BIN2 Regulation by Dephosphorylation
The signaling activity of BIN2 is likely regulated by post-translational modifications that include phosphorylation, acetylation, S-glutathionylation, S-nitrosylation, and ubiquitination. Like its animal homologs, BIN2 is a constitutively active kinase but is inactivated in response to BRI1/BAK1 activation. Molecular modeling revealed that BIN2 likely adopts an active kinase conformation with a well assembled R-spine consisting of Asp223 (the anchorage residue on the αF-helix), His163 (of the HRD motif), Phe185 (of the DFG motif), Met85 (of the αC-helix), and Leu96 (of the β4-strand), the Lys69-Glu81 salt bridge between the β3-strand and the αC-helix, and an opened activation segment (Figure 5). In animal cells, GSK3 phosphorylates its substrates via two different mechanisms: one requiring a priming phosphorylation of the substrate by a different kinase and the other requiring an adapter protein that binds both GSK3 and its substrate [164]. Accordingly, an animal GSK3 kinase can be inactivated by two general mechanisms: phosphorylation of a key Ser/Thr residue in its autoinhibitory N-terminal fragment and interaction of a GSK3-binding protein [164]. The phosphorylated N-terminal fragment competes with a “primed” substrate for the phosphate-binding pocket consisting of three positively-charged residues, Arg96, Arg180, and Lys205 in the human GSK3β (corresponding to Arg80, Arg164, and Lys189 in BIN2, respectively) (Figure 5), while a GSK3-binding protein competitively prevents the GSK3-substrate binding necessary to phosphorylate a non-primed GSK3 substrate.
There has been no evidence so far for a similar phosphorylation-mediated autoinhibitory mechanism in plant cells to inactivate BIN2 in response to BR. An earlier biochemical study showed that the BIN2-catalyzed BES1/BZR1 phosphorylation does not need a priming phosphorylation but instead requires a direct binding between BIN2 and BES1/BZR1 that carry a 12-AA docking motif [165]. However, phosphorylation is involved in BIN2 regulation, which is likely mediated by several protein Ser/Thr phosphatases (PSPs) instead of protein kinases [27,166]. It is widely believed that BIN2 is inhibited through dephosphorylating a phosphorylated Tyr residue (pTyr200) in the activation segment by BSU1/BSLs [27]. The phosphorylation of the corresponding Tyr residue in the human GSK3 kinases was known to occur intramolecularly during HSP90-facilitated folding process [167,168], and peptides of BIN2 or its closest homologs containing the pTyr200 residue were identified previously by phosphoproteomic studies [169,170]. However, the requirement of the pTyr residue (pTyr216 in human GSK3β) for the GSK3 kinase activity remains controversial for animal GSK3 kinases [171]. An early study reported that the mammalian GSK3 kinases required the pTyr residue for their maximum enzymatic activities [172], but later studies showed that non-phosphorylated GSK3β adopted an active conformation [173] and mutating the Tyr residue to Phe had a marginal impact on the GSK3β kinase activity [174,175]. As expected from published GSK3 structures, structure modeling of BIN2 suggests that Tyr200 adopts an anti-conformation (away from the substrate binding site) to avoid steric clash with a substrate (Figure 5). However, mutating Tyr200 to Phe was shown to completely inactivate BIN2 in transgenic Arabidopsis plants [27], but the inhibitory impact of the Tyr-Phe mutation could be caused by lacking of a hydroxyl group or a phosphate group on the aromatic ring as the Tyr residue is absolutely conserved among GSK3 and GSK3-like kinases [158]. Thus, further investigation is needed to confirm whether dephosphorylation of the pTyr200 residue is capable of complete inactivating BIN2.
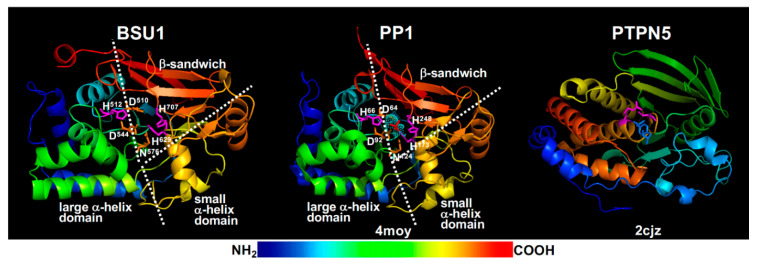
The current BR signaling model suggests that the phosphate group of pTyr200 is removed by BSU1/BSLs [27], members of a plant-specific PPKL family [176]. BSU1, which was originally discovered as an activation-tagged bri1 suppressor [177] but later found to be exclusively present in the Brassicaceae family and exclusively expressed in pollen with yet unknown physiological function [178], was predicted and shown to be a functional PSP [176,177]. Consistently, molecular modeling revealed that BSU1 has the conserved PSP structure of an α/β fold with a β-sandwich surrounded by a large and a small α-helical domains (Figure 6) and contains three catalytic signature motifs, GDXHG (GlyAsp510IleHis511Gly in BSU1), GDXVDRG (GlyAsp544TyrValAspArgGly in BSU1), and GNHE (GlyAsn576HisGlu in BSU1), plus two conserved His residues (His629 and His707 in BSU1). It was well established that these conserved residues coordinate two metal ions that bind and activate a water molecule for its nucleophilic attack on the phosphate ester linkage with a Ser/Thr residue [179]. These signature motifs are quite different from the HCX5R motif (Figure 6), the catalytic signature of PTP/DSPP with the Cys residue being the enzymatic nucleophile and Arg directly binding the phosphate group of pTyr/pSer/pThr [180]. Furthermore, a previous genetic study coupled with phylogeny/evolution analysis of BSU1/BSLs questioned a major BR signaling role of the pollen-expressed BSU1 and its three homologs whose loss-of-function mutations had a marginal impact on the in vivo BIN2 activity [178]. It is important to note that a role of BSU1/BSLs in BR signaling was supported by a recent study in rice that investigated genetic and biochemical interactions between a rice homolog of BSL1, qGL3 (quantitative trait locus regulating grain length3, also known as OsPPKL1), and OsGSK3 (a rice homolog of BIN2) [181]. However, the rice study did not test whether qGL3 was capable of dephosphorylating the conserved Tyr residue of OsGSK3. Given the crucial role of BIN2 and its homologs in regulating the intracellular transduction of the extracellular BR signals into the nucleus, further genetic, biochemistry, and structural studies are needed to fully understand the biochemical roles of pTyr200 and BSU1/BSLs in BR signaling and other relevant physiological processes. The results of these future studies will not only increase our knowledge of BR signaling, but will also significantly enhance our understating of GSK3 regulation in plants and catalytic mechanism of the plant specific PPKLs.
Figure 6.
Comparison between a structure model of BSU1’s phosphatase domain with crystal structures of PSP (protein Ser/Thr phosphatase) and PTP (protein tyrosine phosphatase). Shown here are a predicted structure of the BSU1’s C-terminal phosphatase domain [obtained at SWISS-MODEL at https://swissmodel.expasy.org/ using the protein sequence of BSU1 (accession No: NP_171844)], a crystal structure of a human PSP (HsPP1; Protein Data Bank No. 4moy), and a crystal structure of a human PTP (HsPTPN5 for the human PTP non-receptor type 5; Protein Data Bank No. 2cjz). The conserved metal-coordinating amino acid residues are indicated by colored sticks: orange colored Asp(D)510, Asp(D)544, and Asn(N)576, and three magenta-colored His(H) residues. The three dotted white lines separate the β-sandwich of two β-sheets from the two flanking α-helix domains in the structure models of BSU1 and PP1. The dotted spheres and the red sticks in PP1 represent two metal ions and the phosphate, respectively. The two conserved residues (Cys and Arg of the HCX5R catalytic signature motif) are colored with magenta in the human PTPN5 structure and a phosphorylated tyrosine is shown with blue sticks. The rainbow bar indicates the order of AAs from the N-terminus (blue) to the C-terminus (red) of each peptide.
BIN2 could also be regulated through dephosphorylation by ABI1 (ABA-Insensitive1) and ABI2 [166], two protein phosphatase 2C-type PSPs known to play inhibitory roles in transducing the signal of the plant stress hormone abscisic acid (ABA) [182]. While this discovery seems to support earlier findings that the ABA-BR antagonism is mediated by a biochemical event located between BRI1 activation and BIN2 inhibition of the BR signaling cascade [183], it remains to be determined which phosphorylated residue(s) are dephosphorylated by ABA1/2. Quantitative analysis of the in vivo BIN2 phosphorylation dynamics in response to ABA and/or BR treatment coupled with structural analysis of BIN2 will not only lead to a better understanding of the well-known ABA-BR antagonism but will also provide biochemical insights into the regulatory mechanism(s) of plant GSK3-like kinases.
4.2. BIN2 Regulation by Other Post-Translational Modifications
In additional to phosphorylation/dephosphorylation, BIN2 can be regulated by other post-translational modifications. A recent study implicated a role of an Arabidopsis histone deacetylase HDA6 (histone deacetylase6) in BIN2 inactivation [184]. HDA6 interacts with BIN2 and deacetylates BIN2 at Lys189 in vitro. Because Lys189 is one of the positively charged residues that make up the highly conserved phosphate-binding pocket (Figure 5), its acetylation should neutralize its positive charge and likely reduce the BIN2 activity whereas deacetylation of the acetylated Lys189 is expected to increase the BIN2 activity via enhanced BIN2 binding with BES1/BZR1 that has multiple conserved (Ser/Thr)X3(Ser/Thr) GSK3 phosphorylation repeats. Consistent with the published GSK3 structures and the role of the conserved phosphate binding pocket [173,185], acetylation of Lys205 in the human GSK3β (corresponding to Lys189 of BIN2) was found to reduce its phosphorylation activity while a histone deacetylase sirtuin1 (Sirt1) and the Lys205-Arg mutation increased the GSK3β activity [186]. By contrast, the Lys189-Arg mutation, which should eliminate its presumed acetylation, actually reduced the in vitro kinase activity and in vivo signaling function of BIN2 [184], suggesting that acetylation of Lys189 stimulates BIN2 activity via a yet to be defined biochemical mechanism in plants. However, the mutagenesis results should be interpreted with caution as previous sequence analyses showed that Lys189 is absolutely conserved between BIN2 and its homologs from other plants or other eukaryotic organisms [30,158]. By contrast, both Arg and Lys were found at the corresponding position in plant LRR-RLKs [9,187]. It is noteworthy that acetylation at another conserved Lys residue, Lys183 (corresponding to Lys167 in BIN2) known to be important for ATP binding was recently shown to inhibit the mammalian GSK3β activity, whereas its deacetylation by another histone deacetylase Sirt7 served to promote ATP binding and to stimulate GSK3 activity [188]. Further studies, such as mapping the in vivo BIN2 acetylation sites and identification of BIN2 acetylation enzyme(s), are needed to fully understand the role of acetylation and deacetylation in BIN2 regulation.
Nitric oxide (NO), an important signaling molecular, was shown to inhibit the kinase activity of BIN2 by S-nitrosylation at the conserved Cys162 site in an in vitro assay [189]. It is important to note that Cys162 sits right before the conserved HRD motif (Figure 5) and its S-nitrosylation could thus interfere with the assembly of the R-spine (involving the His163 residue) or the interaction between a phosphate group of BES1/BZR1 with the Arg164 residue, one of the three positively charged residues of the BIN2’s phosphate binding pocket (Figure 5). S-nitrosylation was recently shown to regulate the human GSK3β activity in a very interesting way that inhibits its phosphorylation of cytosolic substrates containing the (Ser/Thr)X3(Ser/Thr) phosphorylation motif but promotes its nuclear localization to phosphorylate its nuclear substrates containing the (Ser/Thr)-Pro motif [190]. In addition to the NO-triggered S-nitrosylation of Cys residues, a recent study suggested that certain cysteine residues of BIN2, such as Cys59 (sits at the end of the β2-strand) and Cys162, could also be S-glutathionylated in vitro and are likely involved in the reactive oxygen species (ROS)-induced activation of BIN2 in vivo [191]. Further studies are needed to confirm these S-nitrosylation and S-glutathionylation sites in vivo and to investigate their biochemical/cellular impacts on the catalytic activity and subcellular localization of BIN2 and other plant GSK3-like kinases. It will also be interesting to determine if additional post-translational modifications, such as ribosylation, SUMOylation, and methylation that were implicated in regulating the mammalian GSK3 kinases [171], are also involved in regulating the in vivo BIN2 activity.
4.3. BIN2 Regulation by Protein–Protein Interactions
Given the earlier discovery of a direct BIN2-BES1/BZR1 binding [165] that was recently confirmed by a single-molecular analysis [191], it is no surprise that BIN2 is also regulated by its competitive binding with other cellular proteins in plant cells. Recent studies showed that BIN2 interacted in vivo with several well-studied light signaling components, including CRY1 (Cryptochrome1, a blue-light photoreceptor) [192], the COP1 (Constitutive Photomorphogenesis1)/SPA (Suppressor of phyA-105) complex (a light regulated E3 ubiquitin ligase) [193], and HY5 (long hypocotyl5, a bZIP-type transcriptional factor) [194]. CRY1 exhibits a blue light-dependent binding with both BIN2 and BZR1 to enhance the BIN2-catalyzed BZR1 phosphorylation [192], thus inhibiting its nuclear translocation (likely by increased interaction with 14-3-3 proteins [195]) and its DNA binding activity through CRY1’s competitive binding to the BZR1’s DNA binding domain. Thus, the blue light-activated CRY1 functions as an adapter to further promote the recruitment of BZR1, which carries a BIN2-binding motif near its C-terminus [165], to the BIN2 kinase. While the blue light-dependent CRY1-BIN2 interaction stimulates the BIN2-catalyzed BZR1 phosphorylation, COP1/SPA was recently found to inhibit the BIN2-catalyzed phosphorylation of PIF3 (phytochrome interacting factor3) [193]. COP1/SPA1 is a well-studied crucial photomorphogenesis repressor complex [196] that was previously shown to interact with CRY1 [197,198], while PIF3 functions together with its homologs to repress photomorphogenesis in the dark [199]. Despite being an E3 ubiquitin ligase [196], COP1/SPA did not promote BIN2 degradation to inhibit its PIF3 phosphorylation activity. Instead, the inhibitory effect of the COP1/SPA complex on the BIN2-catalyzed PIF3 phosphorylation is mediated by preventing the BIN2-PIF3 binding via competitive bindings of COP1/SPA with the kinase and the substrate [193]. Further experiments are needed to see whether these two studies actually revealed two general mechanisms to regulate the phosphorylation activities of BIN2 and other plant GSK3-like kinases: one using an adapter protein that facilitates the BIN2-substrate phosphorylation while the other relying on a disrupter that interferes the BIN2-substrate binding.
A more interesting discovery on the impact of protein–protein interaction on the BIN2 phosphorylation activity came from a recent study [194] that investigated the genetic and biochemical interactions between BIN2 and HY5, a bZIP-type transcription factor with versatile roles in plant growth and development [200] and a known substrate of the COP1/SPA E3 ligase [201]. Biochemical experiments coupled with molecular modeling and computational simulations suggested that the HY5-BIN2 binding stimulated the BIN2 catalytic activity assayed by a HY5-stimulated increase in pTyr200 signal [194], suggesting that HY5 might function similarly as the mammalian HSP90 known to facilitate GSK3β folding during which its Tyr206 (the equivalent of BIN2’s Tyr200 residue) was intramolecularly autophosphorylated [167]. Further experimentation is needed to confirm its hypothetic “chaperone-like” function to assist BIN2 folding into an active kinase in vivo.
4.4. BIN2 Regulation by Subcellular Localization
Given that many of the known BIN2 substrates are transcriptional factors [157], it is no surprise that differential subcellular localization is an important regulatory mechanism to control the BIN2 phosphorylation activity in vivo. An earlier study with the GFP-tagged BIN2 or the mutant bin2-1 [carrying the Glu264-Lys mutation in the TREE motif (Figure 5)] revealed that the bin2-1 protein mainly accumulated in the nucleus while the wild-type BIN2 seemed to exhibit more or less equal distributions at the PM, in the cytosol and the nucleus [163]. More importantly, adding a nuclear localization signal (NLS) to the wild-type BIN2 resulted in a stronger BIN2 activity to block BR signaling, whereas fusing a nuclear export signal (NES) greatly reduced its BR signaling-inhibition activity. Consistent with these findings, Arabidopsis OPS (OCTOPUS), a PM-associated protein crucial for phloem differentiation in Arabidopsis roots [202], binds and recruits BIN2 to the PM, thus reducing the BIN2 phosphorylation activity towards BES1/BZR1 and enhancing BR signaling to promote phloem differentiation [203]. Similarly, a stomata lineage cell-expressed POLAR (polar localization during asymmetric division and redistribution) protein [204], interacts with BIN2 and several other GSK3-like kinases to prevent their nuclear localization and transiently polarizes their subcellular distributions, thus driving asymmetric cell division essential for stomata development [205]. It is noteworthy that the rice PM-associated protein GW5 (grain width and weight5) interacts with OsGSK2, a rice homolog of BIN2, to inhibit the OsGSK2’s phosphorylation activity, presumably by recruiting OsGSK2 to the PM, and to enhance BR signaling that regulates grain width and weight in rice [206]. The regulation of BIN2/GSKs via differential subcellular localization was also discovered in Sorghum bicolor, which uses DW1 (Dwarfing1), a PM/cytosol-localized protein, to interact with SbBIN2 (a Sorghum bicolor BIN2 homolog), thus preventing the nuclear localization of SbBIN2 and increasing BR signaling [207]. It will be interesting to know if their Arabidopsis homologs can also bind BIN2/GSK3s to enhance BR signaling. More importantly, further studies are needed to understand how these four distinct proteins with little sequence homology interact with BIN2/GSK3s to regulate their subcellular distributions. Careful biochemical analyses of their association with BIN2/GSK3s could define the minimum binding motifs while structural biology studies might reveal similar docking mechanisms to allow their BIN2/GSK3-binding.
In contrast to these four proteins that enhance BR signaling by preventing nuclear localization of BIN2/GSK3s, at least two members of the Arabidopsis HSP90 family whose mammalian homologs were known to assist the folding and activation of GSK3β [167], were shown to keep BIN2 inside the nucleus in an ATP-dependent manner to inhibit BR signaling [208]. Treatment with geldanamycin, a widely used inhibitor of HSP90-dependent ATPase, or active BR, resulted in nuclear export of the HSP90-BIN2 complexes [208]. Further studies are needed to determine 1) whether or not the Arabidopsis HSP90s are needed for the cytosolic folding and autoactivation of BIN2/GSK3s, 2) if the HSP90-bound BIN2 is still capable of phosphorylating its many substrates, and 3) how the extracellular BR signal is rapidly sensed by the nuclear-localized HSP90-BIN2 complexes that translocate into the cytosol [208].
4.5. BIN2 Inhibition by Degradation
Proteasome-mediated BIN2 degradation is another important mechanism to keep this negative regulator of BR signaling at low levels. An earlier study suggested that the bin2-1 (Glu264-Lys) mutation greatly stabilized the mutant kinase, which was partially responsible for its increased BES1/BZR1 phosphorylation activity [209]. A recent study demonstrated that the Arabidopsis KIB1, a Kelch-repeat-containing F-box E3 ubiquitin ligase that was identified as a genetic suppressor of a constitutively active form of BZR1, bzr1-1D, ubiquitinated BIN2 in vitro and stimulated the proteasome-mediated BIN2 degradation in vivo [28]. In addition to its role in BIN2 degradation, the Kelch repeat-mediated KIB1-BIN2 interaction blocked the BIN2-BZR1 binding, thus further reducing the BIN2 phosphorylation activity [28]. It remains to be determined whether proteasome-mediated degradation is an evolutionally conserved mechanism in other plant species to regulate the phosphorylation activity of BIN2 homologs given a recent discovery of restricted distribution of KIB1 and its homologs in the Brassicaceae family and their extremely low expression in vegetative tissues [210]. It is interesting to note that the proteasome-mediated GSK3 degradation was previously shown to be induced by glucocorticoid in mammalian cells although the identity of a GSK3-interacting E3 ubiquitin ligase remains unknown [211].
5. Conclusions and Remarks
The basic scheme of the BR signaling pathway is quite simple, involving a PM-localized BR receptor complex, a cytosolic/nuclear-localized inhibitor BIN2, and two key transcription factors, BES1 and BZR1. In the absence of BR, BIN2 is constitutively active to phosphorylate and inhibit BES1/BZR1. BR binding to BRI1 triggers its conformational changes to allow stable heterodimerization, transphosphorylation, and activation of BRI1 and BAK1, leading to inhibition of BIN2 and nuclear accumulation of non/de-phosphorylated BES1/BZR1. These BES1/BZR1 proteins bind to their target promoters to control expression of thousands of genes important for plant growth, development, and stress tolerance. Studies in the last twenty years have provided molecular understanding of key BR signaling events and discovered a wide range of biochemical and cellular mechanisms that regulate the abundance, subcellular locations, and biochemical activities of these key signaling components, which have dramatically enhanced our understanding of BR signaling processes. Despite these great achievements, there are still outstanding questions and unresolved controversies (discussed throughout this review) that require further investigation. Emerging technologies and novel experimental approaches will not only help to answer questions and resolve controversies, but will also lead to new discoveries that will provide a high-resolution atomic portrait of the BR signaling events to further our understanding of the BR signaling pathway and its interactions with other plant signaling processes.
Acknowledgments
We thank Erik Nielsen (University of Michigan) for careful editing of the revised manuscript.
Abbreviations
| AA | amino acids |
| ABA | abscisic acid |
| ABI1/2 | ABA-Insensitive1/2 |
| ALIX | apoptosis-linked gene 2-interacting protein X |
| AP2 | adapter protein 2 |
| ARF-GEF | ADP-ribosylation factor-guanine nucleotide exchange factor |
| AtFlot1 | Arabidopsis thaliana Flot1 |
| BAK1 | BRI1-Associated receptor Kinase1 |
| BARK1 | BAK1-Associated Receptor-like Kinase1 |
| BES1 | bri1-EMS suppressor1 |
| BIG3/5 | brefeldin A-inhibited guanine nucleotide-exchange protein3/5 |
| BIK1 | Botrytis-Induced Kinase1 |
| BIL4 | Brassinazole-Insensitive-Long hypocotyl4 |
| BIN2 | Brassinosteroid-Insensitive2 |
| BIR1-3 | BAK1-Interacting Receptor-like kinase1-3 |
| BKI1 | BRI1 Kinase Inhibitor1 |
| BLK1-3 | BARK1-Like Kinase1-3 |
| BR | Brassinosteroid |
| BRI1 | Brassinosteroid-Insensitive1 |
| BRL1-3 | BRI1-Like1-3 |
| BRRE | BR-response element |
| BSK1/3 | BR-Signaling Kinase1/3 |
| BSL1-3 | BSU1-Like1-3 |
| BSU1 | bri1 suppressor1 |
| BZR1 | Brassinazole-Resistant1 |
| CDG1 | Constitutive Differential Growth1 |
| CIE | clathrin-independent endocytosis |
| CLASP | cytoplasmic linker-associated protein |
| CME | clathrin-mediated endocytosis |
| COP1 | Constitutive Photomorphogenesis1 |
| CRY1 | Cryptochrome1 |
| CT | C-terminal tail |
| det2/DET3 | de-etiolated2/3 |
| DW1 | Dwarfing1 |
| EFR | Elongation Factor-Tu Receptor |
| elg1-D | elongated 1-Dominant |
| ER | endoplasmic reticulum |
| ERAD | ER-associated degradation |
| ERQC | ER-quality control |
| ESCRT | endosomal sorting complex required for transport |
| EXO70A1 | exocyst subunit 70A1 |
| FLS2 | Flagellin Sensing2 |
| G-loop | glycine-rich loop |
| AtGRXC2 | Arabidopsis thaliana glutaredoxin C2 |
| GSK3 | Glycogen Synthase Kinase3 |
| GW5 | grain width and weight5 |
| HDA6 | histone deacetylase6 |
| HSP90 | heat shock protein 90 |
| HsPTPN5 | human PTP non-receptor type 5 |
| HY5 | long hypocotyl5 |
| ID | island domain |
| ILVs | intraluminal vesicles |
| JM | juxtamembrane |
| KIB1 | Kink suppressed in bzr1-1D1 |
| LE/MVBs | late endosomes/multivesicular bodies |
| LRR-RLK | leucine-rich repeat receptor-like kinase |
| MS | mass spectrometry |
| MSBP1 | membrane steroid binding protein1 |
| NES | nuclear export signal |
| NLS | nuclear localization signal |
| NO | nitric oxide |
| OPS | OCTOPUS |
| OsGSK3 | Oryza sativa GSK3-like kinase3 |
| OsREM4.1 | Oryza sativa Remorin 4.1 |
| PIF3 | Phytochrome-Interacting Factor3 |
| PM | plasma membrane |
| POLAR | polar localization during asymmetric division and redistribution |
| PP2A | protein phosphatase 2A |
| PPKL | protein phosphatase with Kelch-like domains |
| PSP | protein Ser/Thr phosphatase |
| PTP/DSPP | protein tyrosine phosphatase/dual specificity protein phosphatase |
| pTyr | phosphorylated tyrosine residue |
| PUB12/13 | plant U-box protein12/13 |
| QC | quality control |
| qGL3 | quantitative trait locus regulating grain length3 |
| ROS | reactive oxygen species |
| R-spine | regulatory-spine |
| SbBIN2 | Sorghum bicolor BIN2 homolog |
| SBI1 | suppressor of bri1 1 |
| SERK | Somatic Embryogenesis Receptor Kinase |
| Sirt1/7 | Sirtuin1/7 |
| SNARE | soluble N-ethylmaleimide-sensitive factor attachment proteins receptor |
| SNX1 | sorting nexin1 |
| sobir7-1 | suppressor of bir1 7-1 |
| SPA | Suppressor of phyA-105 |
| TGN/EE | trans-Golgi network/early endosome |
| TPC | TPLATE complex |
| TTL | Tetratricopeptide Thioredoxin-Like |
| TWD1 | Twisted Dwarf1 |
| ucu | ultracurvata |
| V-ATPase | Vacuolar ATPase |
| VH1 | Vascular Highway1 |
Author Contributions
The authors confirm their contributions to this work: J.M. in conceptualization, original draft preparation, and writing; and J.L. in writing, review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by grants from the National Natural Science Foundation of China (No. 31900176 to Juan Mao and No. 31870253 to Jianming Li).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Clouse S.D., Sasse J.M. Brassinosteroids: Essential regulators of plant growth and development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998;49:427–451. doi: 10.1146/annurev.arplant.49.1.427. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z.Y., Bai M.Y., Oh E., Zhu J.Y. Brassinosteroid signaling network and regulation of photomorphogenesis. Annu. Rev. Genet. 2012;46:701–724. doi: 10.1146/annurev-genet-102209-163450. [DOI] [PubMed] [Google Scholar]
- 3.Guo H., Li L., Aluru M., Aluru S., Yin Y. Mechanisms and networks for brassinosteroid regulated gene expression. Curr. Opin. Plant Biol. 2013;16:545–553. doi: 10.1016/j.pbi.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Yang C.J., Zhang C., Lu Y.N., Jin J.Q., Wang X.L. The mechanisms of brassinosteroids’ action: From signal transduction to plant development. Mol. Plant. 2011;4:588–600. doi: 10.1093/mp/ssr020. [DOI] [PubMed] [Google Scholar]
- 5.Nawaz F., Naeem M., Zulfiqar B., Akram A., Ashraf M.Y., Raheel M., Shabbir R.N., Hussain R.A., Anwar I., Aurangzaib M. Understanding brassinosteroid-regulated mechanisms to improve stress tolerance in plants: A critical review. Environ. Sci. Pollut. Res. Int. 2017;24:15959–15975. doi: 10.1007/s11356-017-9163-6. [DOI] [PubMed] [Google Scholar]
- 6.Saini S., Sharma I., Pati P.K. Versatile roles of brassinosteroid in plants in the context of its homoeostasis, signaling and crosstalks. Front Plant Sci. 2015;6:950. doi: 10.3389/fpls.2015.00950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Q.-F., Lu J., Yu J.-W., Zhang C.-Q., He J.-X., Liu Q.-Q. The brassinosteroid-regulated transcription factors BZR1/BES1 function as a coordinator in multisignal-regulated plant growth. Biochim. Biophys. Acta Gene Regul. Mech. 2018;1861:561–571. doi: 10.1016/j.bbagrm.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Li J., Nagpal P., Vitart V., McMorris T.C., Chory J. A role for brassinosteroids in light-dependent development of Arabidopsis. Science. 1996;272:398–401. doi: 10.1126/science.272.5260.398. [DOI] [PubMed] [Google Scholar]
- 9.Li J., Chory J. A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell. 1997;90:929–938. doi: 10.1016/S0092-8674(00)80357-8. [DOI] [PubMed] [Google Scholar]
- 10.Nolan T.M., Vukasinovic N., Liu D., Russinova E., Yin Y. Brassinosteroids: Multidimensional regulators of plant growth, development, and stress responses. Plant Cell. 2020;32:295–318. doi: 10.1105/tpc.19.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Planas-Riverola A., Gupta A., Betegon-Putze I., Bosch N., Ibanes M., Cano-Delgado A.I. Brassinosteroid signaling in plant development and adaptation to stress. Development. 2019;146:dev151894. doi: 10.1242/dev.151894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belkhadir Y., Jaillais Y. The molecular circuitry of brassinosteroid signaling. New Phytol. 2015;206:522–540. doi: 10.1111/nph.13269. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z.Y., Seto H., Fujioka S., Yoshida S., Chory J. BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature. 2001;410:380–383. doi: 10.1038/35066597. [DOI] [PubMed] [Google Scholar]
- 14.Li J., Wen J., Lease K.A., Doke J.T., Tax F.E., Walker J.C. BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell. 2002;110:213–222. doi: 10.1016/S0092-8674(02)00812-7. [DOI] [PubMed] [Google Scholar]
- 15.Russinova E., Borst J.-W., Kwaaitaal M., Caño-Delgado A., Yin Y., Chory J., de Vries S.C. Heterodimerization and endocytosis of Arabidopsis brassinosteroid receptors BRI1 and AtSERK3 (BAK1) Plant Cell. 2004;16:3216–3229. doi: 10.1105/tpc.104.025387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santiago J., Henzler C., Hothorn M. Molecular mechanism for plant steroid receptor activation by somatic embryogenesis co-receptor kinases. Science. 2013;341:889–892. doi: 10.1126/science.1242468. [DOI] [PubMed] [Google Scholar]
- 17.Ma X., Xu G., He P., Shan L. SERKing coreceptors for receptors. Trends. Plant Sci. 2016;21:1017–1033. doi: 10.1016/j.tplants.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Nam K.H., Li J. BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell. 2002;110:203–212. doi: 10.1016/S0092-8674(02)00814-0. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y., Han Z., Tang J., Hu Z., Chai C., Zhou B., Chai J. Structure reveals that BAK1 as a co-receptor recognizes the BRI1-bound brassinolide. Cell Res. 2013;23:1326–1329. doi: 10.1038/cr.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X., Chory J. Brassinosteroids regulate dissociation of BKI1, a negative regulator of BRI1 signaling, from the plasma membrane. Science. 2006;313:1118–1122. doi: 10.1126/science.1127593. [DOI] [PubMed] [Google Scholar]
- 21.Wang X., Kota U., He K., Blackburn K., Li J., Goshe M.B., Huber S.C., Clouse S.D. Sequential transphosphorylation of the BRI1/BAK1 receptor kinase complex impacts early events in brassinosteroid signaling. Dev. Cell. 2008;15:220–235. doi: 10.1016/j.devcel.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Wang X., Goshe M.B., Soderblom E.J., Phinney B.S., Kuchar J.A., Li J., Asami T., Yoshida S., Huber S.C., Clouse S.D. Identification and functional analysis of in vivo phosphorylation sites of the Arabidopsis Brassinosteroid-Insensitive1 receptor kinase. Plant Cell. 2005;17:1685–1703. doi: 10.1105/tpc.105.031393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X., Li X., Meisenhelder J., Hunter T., Yoshida S., Asami T., Chory J. Autoregulation and homodimerization are involved in the activation of the plant steroid receptor BRI1. Dev. Cell. 2005;8:855–865. doi: 10.1016/j.devcel.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Tang W., Kim T.W., Oses-Prieto J.A., Sun Y., Deng Z., Zhu S., Wang R., Burlingame A.L., Wang Z.Y. BSKs mediate signal transduction from the receptor kinase BRI1 in Arabidopsis. Science. 2008;321:557–560. doi: 10.1126/science.1156973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim T.W., Guan S., Burlingame A.L., Wang Z.Y. The CDG1 kinase mediates brassinosteroid signal transduction from BRI1 receptor kinase to BSU1 phosphatase and GSK3-like kinase BIN2. Mol. Cell. 2011;43:561–571. doi: 10.1016/j.molcel.2011.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin W., Lu D., Gao X., Jiang S., Ma X., Wang Z., Mengiste T., He P., Shan L. Inverse modulation of plant immune and brassinosteroid signaling pathways by the receptor-like cytoplasmic kinase BIK1. Proc. Natl. Acad. Sci. USA. 2013;110:12114–12119. doi: 10.1073/pnas.1302154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim T.W., Guan S., Sun Y., Deng Z., Tang W., Shang J.X., Sun Y., Burlingame A.L., Wang Z.Y. Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors. Nat. Cell Biol. 2009;11:1254–1260. doi: 10.1038/ncb1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu J.Y., Li Y., Cao D.M., Yang H., Oh E., Bi Y., Zhu S., Wang Z.Y. The F-box protein KIB1 mediates brassinosteroid-induced inactivation and degradation of GSK3-like kinases in Arabidopsis. Mol. Cell. 2017;66:648–657. doi: 10.1016/j.molcel.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin Y., Wang Z.Y., Mora-Garcia S., Li J., Yoshida S., Asami T., Chory J. BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell. 2002;109:181–191. doi: 10.1016/S0092-8674(02)00721-3. [DOI] [PubMed] [Google Scholar]
- 30.Li J., Nam K.H. Regulation of brassinosteroid signaling by a GSK3/SHAGGY-like kinase. Science. 2002;295:1299–1301. doi: 10.1126/science.1065769. [DOI] [PubMed] [Google Scholar]
- 31.Tang W., Yuan M., Wang R., Yang Y., Wang C., Oses-Prieto J.A., Kim T.W., Zhou H.W., Deng Z., Gampala S.S., et al. PP2A activates brassinosteroid-responsive gene expression and plant growth by dephosphorylating BZR1. Nat. Cell Biol. 2011;13:124–131. doi: 10.1038/ncb2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He J.X., Gendron J.M., Sun Y., Gampala S.S., Gendron N., Sun C.Q., Wang Z.Y. BZR1 is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses. Science. 2005;307:1634–1638. doi: 10.1126/science.1107580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yin Y., Vafeados D., Tao Y., Yoshida S., Asami T., Chory J. A new class of transcription factors mediates brassinosteroid-regulated gene expression in Arabidopsis. Cell. 2005;120:249–259. doi: 10.1016/j.cell.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 34.Peres A., Soares J.S., Tavares R.G., Righetto G., Zullo M.A.T., Mandava N.B., Menossi M. Brassinosteroids, the sixth class of phytohormones: A molecular view from the discovery to hormonal interactions in plant development and stress adaptation. Int. J. Mol. Sci. 2019;20:331. doi: 10.3390/ijms20020331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiu S.H., Bleecker A.B. Plant receptor-like kinase gene family: Diversity, function, and signaling. Sci. STKE. 2001;113:re22. doi: 10.1126/stke.2001.113.re22. [DOI] [PubMed] [Google Scholar]
- 36.Kinoshita T., Cano-Delgado A., Seto H., Hiranuma S., Fujioka S., Yoshida S., Chory J. Binding of brassinosteroids to the extracellular domain of plant receptor kinase BRI1. Nature. 2005;433:167–171. doi: 10.1038/nature03227. [DOI] [PubMed] [Google Scholar]
- 37.Clay N.K., Nelson T. VH1, a provascular cell-specific receptor kinase that influences leaf cell patterns in Arabidopsis. Plant Cell. 2002;14:2707–2722. doi: 10.1105/tpc.005884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cano-Delgado A., Yin Y., Yu C., Vafeados D., Mora-Garcia S., Cheng J.C., Nam K.H., Li J., Chory J. BRL1 and BRL3 are novel brassinosteroid receptors that function in vascular differentiation in Arabidopsis. Development. 2004;131:5341–5351. doi: 10.1242/dev.01403. [DOI] [PubMed] [Google Scholar]
- 39.Zhou A., Wang H., Walker J.C., Li J. BRL1, a leucine-rich repeat receptor-like protein kinase, is functionally redundant with BRI1 in regulating Arabidopsis brassinosteroid signaling. Plant J. 2004;40:399–409. doi: 10.1111/j.1365-313X.2004.02214.x. [DOI] [PubMed] [Google Scholar]
- 40.Ceserani T., Trofka A., Gandotra N., Nelson T. VH1/BRL2 receptor-like kinase interacts with vascular-specific adaptor proteins VIT and VIK to influence leaf venation. Plant J. 2009;57:1000–1014. doi: 10.1111/j.1365-313X.2008.03742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lozano-Elena F., Caño-Delgado A.I. Emerging roles of vascular brassinosteroid receptors of the BRI1-like family. Curr. Opin. Plant Biol. 2019;51:105–113. doi: 10.1016/j.pbi.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Fàbregas N., Lozano-Elena F., Blasco-Escámez D., Tohge T., Martínez-Andújar C., Albacete A., Osorio S., Bustamante M., Riechmann J.L., Nomura T., et al. Overexpression of the vascular brassinosteroid receptor BRL3 confers drought resistance without penalizing plant growth. Nat. Commun. 2018;9:4680. doi: 10.1038/s41467-018-06861-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klok E.J., Wilson I.W., Wilson D., Chapman S.C., Ewing R.M., Somerville S.C., Peacock W.J., Dolferus R., Dennis E.S. Expression profile analysis of the low-oxygen response in Arabidopsis root cultures. Plant Cell. 2002;14:2481–2494. doi: 10.1105/tpc.004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohashi-Ito K., Kubo M., Demura T., Fukuda H. Class III homeodomain leucine-zipper proteins regulate xylem cell differentiation. Plant Cell Physiol. 2005;46:1646–1656. doi: 10.1093/pcp/pci180. [DOI] [PubMed] [Google Scholar]
- 45.Wang J., Jiang J., Wang J., Chen L., Fan S.L., Wu J.W., Wang X., Wang Z.X. Structural insights into the negative regulation of BRI1 signaling by BRI1-interacting protein BKI1. Cell Res. 2014;24:1328–1341. doi: 10.1038/cr.2014.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bojar D., Martinez J., Santiago J., Rybin V., Bayliss R., Hothorn M. Crystal structures of the phosphorylated BRI1 kinase domain and implications for brassinosteroid signal initiation. Plant J. 2014;78:31–43. doi: 10.1111/tpj.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bucherl C.A., van Esse G.W., Kruis A., Luchtenberg J., Westphal A.H., Aker J., van Hoek A., Albrecht C., Borst J.W., de Vries S.C. Visualization of BRI1 and BAK1(SERK3) membrane receptor heterooligomers during brassinosteroid signaling. Plant Physiol. 2013;162:1911–1925. doi: 10.1104/pp.113.220152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hutten S.J., Hamers D.S., den Toorn M.A., van Esse W., Nolles A., Bucherl C.A., de Vries S.C., Hohlbein J., Borst J.W. Visualization of BRI1 and SERK3/BAK1 nanoclusters in arabidopsis roots. PLoS ONE. 2017;12:e0169905. doi: 10.1371/journal.pone.0169905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gou X., Yin H., He K., Du J., Yi J., Xu S., Lin H., Clouse S.D., Li J. Genetic evidence for an indispensable role of somatic embryogenesis receptor kinases in brassinosteroid signaling. PLoS Genet. 2012;8:e1002452. doi: 10.1371/journal.pgen.1002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Imkampe J., Halter T., Huang S., Schulze S., Mazzotta S., Schmidt N., Manstretta R., Postel S., Wierzba M., Yang Y., et al. The Arabidopsis leucine-rich repeat receptor kinase BIR3 negatively regulates BAK1 receptor complex formation and stabilizes BAK1. Plant Cell. 2017;29:2285–2303. doi: 10.1105/tpc.17.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson L.N., Noble M.E., Owen D.J. Active and inactive protein kinases: Structural basis for regulation. Cell. 1996;85:149–158. doi: 10.1016/S0092-8674(00)81092-2. [DOI] [PubMed] [Google Scholar]
- 52.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/S0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 53.Heldin C.H., Moustakas A. Signaling receptors for TGF-beta family members. Cold Spring Harb. Perspect. Biol. 2016 doi: 10.1101/cshperspect.a022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hink M.A., Shah K., Russinova E., de Vries S.C., Visser A.J. Fluorescence fluctuation analysis of Arabidopsis thaliana somatic embryogenesis receptor-like kinase and BRASSINOSTEROID INSENSITIVE 1 receptor oligomerization. Biophys. J. 2008;94:1052–1062. doi: 10.1529/biophysj.107.112003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang L., Li H., Lv X., Chen T., Li R., Xue Y., Jiang J., Jin B., Baluska F., Samaj J., et al. Spatiotemporal dynamics of the BRI1 receptor and its regulation by membrane microdomains in living arabidopsis cells. Mol. Plant. 2015;8:1334–1349. doi: 10.1016/j.molp.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 56.Hohmann U., Ramakrishna P., Wang K., Lorenzo-Orts L., Nicolet J., Henschen A., Barberon M., Bayer M., Hothorn M. Constitutive activation of leucine-rich repeat receptor kinase signaling pathways by BAK1-interacting receptor-like kinase 3 chimera. bioRxiv. 2020 doi: 10.1101/2020.02.18.954479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.She J., Han Z., Kim T.W., Wang J., Cheng W., Chang J., Shi S., Wang J., Yang M., Wang Z.Y., et al. Structural insight into brassinosteroid perception by BRI1. Nature. 2011;474:472–476. doi: 10.1038/nature10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hothorn M., Belkhadir Y., Dreux M., Dabi T., Noel J.P., Wilson I.A., Chory J. Structural basis of steroid hormone perception by the receptor kinase BRI1. Nature. 2011;474:467–471. doi: 10.1038/nature10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan L., Ma Y., Liu D., Wei X., Sun Y., Chen X., Zhao H., Zhou J., Wang Z., Shui W., et al. Structural basis for the impact of phosphorylation on the activation of plant receptor-like kinase BAK1. Cell Res. 2012;22:1304–1308. doi: 10.1038/cr.2012.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grosseholz R., Feldman-Salit A., Wanke F., Schulze S., Glockner N., Kemmerling B., Harter K., Kummer U. Specifying the role of BAK1-interacting receptor-like kinase 3 in brassinosteroid signaling. J. Integr. Plant Biol. 2020;62:456–469. doi: 10.1111/jipb.12803. [DOI] [PubMed] [Google Scholar]
- 61.Kornev A.P., Taylor S.S. Dynamics-driven allostery in protein kinases. Trends Biochem. Sci. 2015;40:628–647. doi: 10.1016/j.tibs.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kornev A.P., Haste N.M., Taylor S.S., Eyck L.F. Surface comparison of active and inactive protein kinases identifies a conserved activation mechanism. Proc. Natl. Acad. Sci. USA. 2006;103:17783–17788. doi: 10.1073/pnas.0607656103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mitra S.K., Chen R., Dhandaydham M., Wang X., Blackburn R.K., Kota U., Goshe M.B., Schwartz D., Huber S.C., Clouse S.D. An autophosphorylation site database for leucine-rich repeat receptor-like kinases in Arabidopsis thaliana. Plant J. 2015;82:1042–1060. doi: 10.1111/tpj.12863. [DOI] [PubMed] [Google Scholar]
- 64.Oh M.H., Ray W.K., Huber S.C., Asara J.M., Gage D.A., Clouse S.D. Recombinant BRASSINOSTEROID INSENSITIVE 1 receptor-like kinase autophosphorylates on serine and threonine residues and phosphorylates a conserved peptide motif in vitro. Plant Physiol. 2000;124:751–766. doi: 10.1104/pp.124.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu X., Oh M.H., Kim H.S., Schwartz D., Imai B.S., Yau P.M., Clouse S.D., Huber S.C. Transphosphorylation of E. coli proteins during production of recombinant protein kinases provides a robust system to characterize kinase specificity. Front. Plant Sci. 2012;3:262. doi: 10.3389/fpls.2012.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang R., Liu M., Yuan M., Oses-Prieto J.A., Cai X., Sun Y., Burlingame A.L., Wang Z.Y., Tang W. The brassinosteroid-activated BRI1 receptor kinase is switched off by dephosphorylation mediated by cytoplasm-localized PP2A B’ subunits. Mol. Plant. 2016;9:148–157. doi: 10.1016/j.molp.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 67.Oh M.H., Wang X., Kota U., Goshe M.B., Clouse S.D., Huber S.C. Tyrosine phosphorylation of the BRI1 receptor kinase emerges as a component of brassinosteroid signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2009;106:658–663. doi: 10.1073/pnas.0810249106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oh M.H., Clouse S.D., Huber S.C. Tyrosine phosphorylation of the BRI1 receptor kinase occurs via a post-translational modification and is activated by the juxtamembrane domain. Front. Plant Sci. 2012;3:175. doi: 10.3389/fpls.2012.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Amorim-Silva V., Garcia-Moreno A., Castillo A.G., Lakhssassi N., Del Valle A.E., Perez-Sancho J., Li Y., Pose D., Perez-Rodriguez J., Lin J., et al. TTL proteins scaffold brassinosteroid signaling components at the plasma membrane to optimize signal transduction in arabidopsis. Plant Cell. 2019;31:1807–1828. doi: 10.1105/tpc.19.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ren H., Willige B.C., Jaillais Y., Geng S., Park M.Y., Gray W.M., Chory J. Brassinosteroid-signaling kinase 3, a plasma membrane-associated scaffold protein involved in early brassinosteroid signaling. PLoS Genet. 2019;15:e1007904. doi: 10.1371/journal.pgen.1007904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jaillais Y., Hothorn M., Belkhadir Y., Dabi T., Nimchuk Z.L., Meyerowitz E.M., Chory J. Tyrosine phosphorylation controls brassinosteroid receptor activation by triggering membrane release of its kinase inhibitor. Genes Dev. 2011;25:232–237. doi: 10.1101/gad.2001911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang H., Yang C., Zhang C., Wang N., Lu D., Wang J., Zhang S., Wang Z.X., Ma H., Wang X. Dual role of BKI1 and 14-3-3 s in brassinosteroid signaling to link receptor with transcription factors. Dev. Cell. 2011;21:825–834. doi: 10.1016/j.devcel.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 73.Oh M.H., Wang X., Clouse S.D., Huber S.C. Deactivation of the Arabidopsis BRASSINOSTEROID INSENSITIVE 1 (BRI1) receptor kinase by autophosphorylation within the glycine-rich loop. Proc. Natl. Acad. Sci. USA. 2012;109:327–332. doi: 10.1073/pnas.1108321109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu G., Wang X., Li X., Kamiya Y., Otegui M.S., Chory J. Methylation of a phosphatase specifies dephosphorylation and degradation of activated brassinosteroid receptors. Sci. Signal. 2011;4:ra29. doi: 10.1126/scisignal.2001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Janssens V., Goris J. Protein phosphatase 2A: A highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J. 2001;353:417–439. doi: 10.1042/bj3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Macho A.P., Schwessinger B., Ntoukakis V., Brutus A., Segonzac C., Roy S., Kadota Y., Oh M.H., Sklenar J., Derbyshire P., et al. A bacterial tyrosine phosphatase inhibits plant pattern recognition receptor activation. Science. 2014;343:1509–1512. doi: 10.1126/science.1248849. [DOI] [PubMed] [Google Scholar]
- 77.Kerk D., Templeton G., Moorhead G.B. Evolutionary radiation pattern of novel protein phosphatases revealed by analysis of protein data from the completely sequenced genomes of humans, green algae, and higher plants. Plant Physiol. 2008;146:351–367. doi: 10.1104/pp.107.111393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Araki K., Nagata K. Protein folding and quality control in the ER. Cold Spring Harb. Perspect. Biol. 2011;3:a007526. doi: 10.1101/cshperspect.a007526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Strasser R. Protein quality control in the endoplasmic reticulum of plants. Annu. Rev. Plant Biol. 2018;69:147–172. doi: 10.1146/annurev-arplant-042817-040331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jin H., Hong Z., Su W., Li J. A plant-specific calreticulin is a key retention factor for a defective brassinosteroid receptor in the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA. 2009;106:13612–13617. doi: 10.1073/pnas.0906144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jin H., Yan Z., Nam K.H., Li J. Allele-specific suppression of a defective brassinosteroid receptor reveals a physiological role of UGGT in ER quality control. Mol. Cell. 2007;26:821–830. doi: 10.1016/j.molcel.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hong Z., Jin H., Tzfira T., Li J. Multiple mechanism-mediated retention of a defective brassinosteroid receptor in the endoplasmic reticulum of Arabidopsis. Plant Cell. 2008;20:3418–3429. doi: 10.1105/tpc.108.061879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Su W., Liu Y., Xia Y., Hong Z., Li J. Conserved endoplasmic reticulum-associated degradation system to eliminate mutated receptor-like kinases in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2011;108:870–875. doi: 10.1073/pnas.1013251108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Su W., Liu Y., Xia Y., Hong Z., Li J. The Arabidopsis homolog of the mammalian OS-9 protein plays a key role in the endoplasmic reticulum-associated degradation of misfolded receptor-like kinases. Mol. Plant. 2012;5:929–940. doi: 10.1093/mp/sss042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin L., Zhang C., Chen Y., Wang Y., Wang D., Liu X., Wang M., Mao J., Zhang J., Xing W., et al. PAWH1 and PAWH2 are plant-specific components of an Arabidopsis endoplasmic reticulum-associated degradation complex. Nat. Commun. 2019;10:3492. doi: 10.1038/s41467-019-11480-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu Y., Zhang C., Wang D., Su W., Liu L., Wang M., Li J. EBS7 is a plant-specific component of a highly conserved endoplasmic reticulum-associated degradation system in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2015;112:12205–12210. doi: 10.1073/pnas.1511724112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang X., Zhou L., Qin Y., Chen Y., Liu X., Wang M., Mao J., Zhang J., He Z., Liu L., et al. A temperature-sensitive misfolded bri1-301 receptor requires its kinase activity to promote growth. Plant Physiol. 2018;178:1704–1719. doi: 10.1104/pp.18.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lv M., Li M., Chen W., Wang Y., Sun C., Yin H., He K., Li J. Thermal-enhanced bri1-301 instability reveals a plasma membrane protein quality control system in plants. Front. Plant Sci. 2018;9:1620. doi: 10.3389/fpls.2018.01620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu G., Otegui M.S., Spalding E.P. The ER-localized TWD1 immunophilin is necessary for localization of multidrug resistance-like proteins required for polar auxin transport in Arabidopsis roots. Plant Cell. 2010;22:3295–3304. doi: 10.1105/tpc.110.078360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao B., Lv M., Feng Z., Campbell T., Liscum E., Li J. TWISTED DWARF 1 associates with BRASSINOSTEROID-INSENSITIVE 1 to regulate early events of the brassinosteroid signaling pathway. Mol. Plant. 2016;9:582–592. doi: 10.1016/j.molp.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 91.Chaiwanon J., Garcia V.J., Cartwright H., Sun Y., Wang Z.Y. Immunophilin-like FKBP42/TWISTED DWARF1 interacts with the receptor kinase BRI1 to regulate brassinosteroid signaling in Arabidopsis. Mol. Plant. 2016;9:593–600. doi: 10.1016/j.molp.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Geisler M., Kolukisaoglu H.U., Bouchard R., Billion K., Berger J., Saal B., Frangne N., Koncz-Kalman Z., Koncz C., Dudler R., et al. TWISTED DWARF1, a unique plasma membrane-anchored immunophilin-like protein, interacts with Arabidopsis multidrug resistance-like transporters AtPGP1 and AtPGP19. Mol. Biol. Cell. 2003;14:4238–4249. doi: 10.1091/mbc.e02-10-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhu J., Bailly A., Zwiewka M., Sovero V., Di Donato M., Ge P., Oehri J., Aryal B., Hao P., Linnert M., et al. TWISTED DWARF1 mediates the action of auxin transport inhibitors on actin cytoskeleton dynamics. Plant Cell. 2016;28:930–948. doi: 10.1105/tpc.15.00726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ott T. Membrane nanodomains and microdomains in plant-microbe interactions. Curr. Opin. Plant Biol. 2017;40:82–88. doi: 10.1016/j.pbi.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 95.Bucherl C.A., Jarsch I.K., Schudoma C., Segonzac C., Mbengue M., Robatzek S., MacLean D., Ott T., Zipfel C. Plant immune and growth receptors share common signalling components but localise to distinct plasma membrane nanodomains. Elife. 2017;6:e25114. doi: 10.7554/eLife.25114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gui J., Zheng S., Liu C., Shen J., Li J., Li L. OsREM4.1 interacts with OsSERK1 to coordinate the interlinking between abscisic acid and brassinosteroid signaling in rice. Dev. Cell. 2016;38:201–213. doi: 10.1016/j.devcel.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 97.Jaillais Y., Ott T. The nanoscale organization of the plasma membrane and its importance in signaling: A proteolipid perspective. Plant Physiol. 2020;182:1682–1696. doi: 10.1104/pp.19.01349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shiu S.H., Karlowski W.M., Pan R., Tzeng Y.H., Mayer K.F., Li W.H. Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell. 2004;16:1220–1234. doi: 10.1105/tpc.020834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Geldner N., Hyman D.L., Wang X., Schumacher K., Chory J. Endosomal signaling of plant steroid receptor kinase BRI1. Genes Dev. 2007;21:1598–1602. doi: 10.1101/gad.1561307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Claus L.A.N., Savatin D.V., Russinova E. The crossroads of receptor-mediated signaling and endocytosis in plants. J. Integr. Plant Biol. 2018;60:827–840. doi: 10.1111/jipb.12672. [DOI] [PubMed] [Google Scholar]
- 101.Dettmer J., Hong-Hermesdorf A., Stierhof Y.D., Schumacher K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell. 2006;18:715–730. doi: 10.1105/tpc.105.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Viotti C., Bubeck J., Stierhof Y.D., Krebs M., Langhans M., van den Berg W., van Dongen W., Richter S., Geldner N., Takano J., et al. Endocytic and secretory traffic in Arabidopsis merge in the trans-Golgi network/early endosome, an independent and highly dynamic organelle. Plant Cell. 2010;22:1344–1357. doi: 10.1105/tpc.109.072637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jaillais Y., Fobis-Loisy I., Miege C., Gaude T. Evidence for a sorting endosome in Arabidopsis root cells. Plant J. 2008;53:237–247. doi: 10.1111/j.1365-313X.2007.03338.x. [DOI] [PubMed] [Google Scholar]
- 104.Irani N.G., Di Rubbo S., Mylle E., van den Begin J., Schneider-Pizon J., Hnilikova J., Sisa M., Buyst D., Vilarrasa-Blasi J., Szatmari A.M., et al. Fluorescent castasterone reveals BRI1 signaling from the plasma membrane. Nat. Chem. Biol. 2012;8:583–589. doi: 10.1038/nchembio.958. [DOI] [PubMed] [Google Scholar]
- 105.Geldner N., Robatzek S. Plant receptors go endosomal: A moving view on signal transduction. Plant Physiol. 2008;147:1565–1574. doi: 10.1104/pp.108.120287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reynolds G.D., Wang C., Pan J., Bednarek S.Y. Inroads into internalization: Five years of endocytic exploration. Plant Physiol. 2018;176:208–218. doi: 10.1104/pp.17.01117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Banbury D.N., Oakley J.D., Sessions R.B., Banting G. Tyrphostin A23 inhibits internalization of the transferrin receptor by perturbing the interaction between tyrosine motifs and the medium chain subunit of the AP-2 adaptor complex. J. Biol. Chem. 2003;278:12022–12028. doi: 10.1074/jbc.M211966200. [DOI] [PubMed] [Google Scholar]
- 108.Gadeyne A., Sánchez-Rodríguez C., Vanneste S., Di Rubbo S., Zauber H., Vanneste K., van Leene J., de Winne N., Eeckhout D., Persiau G., et al. The TPLATE adaptor complex drives clathrin-mediated endocytosis in plants. Cell. 2014;156:691–704. doi: 10.1016/j.cell.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 109.Di Rubbo S., Irani N.G., Kim S.Y., Xu Z.Y., Gadeyne A., Dejonghe W., Vanhoutte I., Persiau G., Eeckhout D., Simon S., et al. The clathrin adaptor complex AP-2 mediates endocytosis of brassinosteroid insensitive1 in Arabidopsis. Plant Cell. 2013;25:2986–2997. doi: 10.1105/tpc.113.114058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li R., Liu P., Wan Y., Chen T., Wang Q., Mettbach U., Baluska F., Samaj J., Fang X., Lucas W.J., et al. A membrane microdomain-associated protein, Arabidopsis Flot1, is involved in a clathrin-independent endocytic pathway and is required for seedling development. Plant Cell. 2012;24:2105–2122. doi: 10.1105/tpc.112.095695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rodriguez-Furlan C., Minina E.A., Hicks G.R. Remove, recycle, degrade: Regulating plasma membrane protein accumulation. Plant Cell. 2019;31:2833–2854. doi: 10.1105/tpc.19.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhou J., Liu D., Wang P., Ma X., Lin W., Chen S., Mishev K., Lu D., Kumar R., Vanhoutte I., et al. Regulation of Arabidopsis brassinosteroid receptor BRI1 endocytosis and degradation by plant U-box PUB12/PUB13-mediated ubiquitination. Proc. Natl. Acad. Sci. USA. 2018;115:E1906–E1915. doi: 10.1073/pnas.1712251115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cardona-Lopez X., Cuyas L., Marin E., Rajulu C., Irigoyen M.L., Gil E., Puga M.I., Bligny R., Nussaume L., Geldner N., et al. ESCRT-III-associated protein ALIX mediates high-affinity phosphate transporter trafficking to maintain phosphate homeostasis in arabidopsis. Plant Cell. 2015;27:2560–2581. doi: 10.1105/tpc.15.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mattern M., Sutherland J., Kadimisetty K., Barrio R., Rodriguez M.S. Using ubiquitin binders to decipher the ubiquitin code. Trends. Biochem. Sci. 2019;44:599–615. doi: 10.1016/j.tibs.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 115.Martins S., Dohmann E.M., Cayrel A., Johnson A., Fischer W., Pojer F., Satiat-Jeunemaitre B., Jaillais Y., Chory J., Geldner N., et al. Internalization and vacuolar targeting of the brassinosteroid hormone receptor BRI1 are regulated by ubiquitination. Nat. Commun. 2015;6:6151. doi: 10.1038/ncomms7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gomez-Gomez L., Boller T. FLS2: An LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol. Cell. 2000;5:1003–1011. doi: 10.1016/S1097-2765(00)80265-8. [DOI] [PubMed] [Google Scholar]
- 117.Lu D., Lin W., Gao X., Wu S., Cheng C., Avila J., Heese A., Devarenne T.P., He P., Shan L. Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innate immunity. Science. 2011;332:1439–1442. doi: 10.1126/science.1204903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kalinowska K., Nagel M.K., Goodman K., Cuyas L., Anzenberger F., Alkofer A., Paz-Ares J., Braun P., Rubio V., Otegui M.S., et al. Arabidopsis ALIX is required for the endosomal localization of the deubiquitinating enzyme AMSH3. Proc. Natl. Acad. Sci. USA. 2015;112:E5543–E5551. doi: 10.1073/pnas.1510516112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yamagami A., Saito C., Nakazawa M., Fujioka S., Uemura T., Matsui M., Sakuta M., Shinozaki K., Osada H., Nakano A., et al. Evolutionarily conserved BIL4 suppresses the degradation of brassinosteroid receptor BRI1 and regulates cell elongation. Sci. Rep. 2017;7:5739. doi: 10.1038/s41598-017-06016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Luo Y., Scholl S., Doering A., Zhang Y., Irani N.G., Rubbo S.D., Neumetzler L., Krishnamoorthy P., Van Houtte I., Mylle E., et al. V-ATPase activity in the TGN/EE is required for exocytosis and recycling in Arabidopsis. Nat. Plants. 2015;1:15094. doi: 10.1038/nplants.2015.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schumacher K., Vafeados D., McCarthy M., Sze H., Wilkins T., Chory J. The Arabidopsis det3 mutant reveals a central role for the vacuolar H(+)-ATPase in plant growth and development. Genes Dev. 1999;13:3259–3270. doi: 10.1101/gad.13.24.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ambrose C., Ruan Y., Gardiner J., Tamblyn L.M., Catching A., Kirik V., Marc J., Overall R., Wasteneys G.O. CLASP interacts with sorting nexin 1 to link microtubules and auxin transport via PIN2 recycling in Arabidopsis thaliana. Dev. Cell. 2013;24:649–659. doi: 10.1016/j.devcel.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 123.Ruan Y., Halat L.S., Khan D., Jancowski S., Ambrose C., Belmonte M.F., Wasteneys G.O. The microtubule-associated protein CLASP sustains cell proliferation through a brassinosteroid signaling negative feedback loop. Curr. Biol. 2018;28:2718–2729. doi: 10.1016/j.cub.2018.06.048. [DOI] [PubMed] [Google Scholar]
- 124.Zhang L., Xing J., Lin J. At the intersection of exocytosis and endocytosis in plants. New Phytol. 2019;224:1479–1489. doi: 10.1111/nph.16018. [DOI] [PubMed] [Google Scholar]
- 125.Zhang L., Liu Y., Zhu X.F., Jung J.H., Sun Q., Li T.Y., Chen L.J., Duan Y.X., Xuan Y.H. SYP22 and VAMP727 regulate BRI1 plasma membrane targeting to control plant growth in Arabidopsis. New Phytol. 2019;223:1059–1065. doi: 10.1111/nph.15759. [DOI] [PubMed] [Google Scholar]
- 126.Drdova E.J., Synek L., Pecenkova T., Hala M., Kulich I., Fowler J.E., Murphy A.S., Zarsky V. The exocyst complex contributes to PIN auxin efflux carrier recycling and polar auxin transport in Arabidopsis. Plant J. 2013;73:709–719. doi: 10.1111/tpj.12074. [DOI] [PubMed] [Google Scholar]
- 127.Xue S., Zou J., Liu Y., Wang M., Zhang C., Le J. Involvement of BIG5 and BIG3 in BRI1 trafficking reveals diverse functions of BIG-subfamily ARF-GEFs in plant growth and gravitropism. Int. J. Mol. Sci. 2019;20:2339. doi: 10.3390/ijms20092339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wu W., Wu Y., Gao Y., Li M., Yin H., Lv M., Zhao J., Li J., He K. Somatic embryogenesis receptor-like kinase 5 in the ecotype Landsberg erecta of Arabidopsis is a functional RD LRR-RLK in regulating brassinosteroid signaling and cell death control. Front Plant Sci. 2015;6:852. doi: 10.3389/fpls.2015.00852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chinchilla D., Zipfel C., Robatzek S., Kemmerling B., Nürnberger T., Jones J.D.G., Felix G., Boller T. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature. 2007;448:497–500. doi: 10.1038/nature05999. [DOI] [PubMed] [Google Scholar]
- 130.Karlova R., Boeren S., Russinova E., Aker J., Vervoort J., de Vries S. The arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE1 protein complex includes BRASSINOSTEROID-INSENSITIVE1. Plant Cell. 2006;18:626–638. doi: 10.1105/tpc.105.039412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wu D., Liu Y., Xu F., Zhang Y. Differential requirement of BAK1 C-terminal tail in development and immunity. J. Integr. Plant Biol. 2018;60:270–275. doi: 10.1111/jipb.12623. [DOI] [PubMed] [Google Scholar]
- 132.Wang Y., Li Z., Liu D., Xu J., Wei X., Yan L., Yang C., Lou Z., Shui W. Assessment of BAK1 activity in different plant receptor-like kinase complexes by quantitative profiling of phosphorylation patterns. J. Proteomics. 2014;108:484–493. doi: 10.1016/j.jprot.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 133.Shah K., Vervoort J., de Vries S.C. Role of threonines in the Arabidopsis thaliana somatic embryogenesis receptor kinase 1 activation loop in phosphorylation. J. Biol. Chem. 2001;276:41263–41269. doi: 10.1074/jbc.M102381200. [DOI] [PubMed] [Google Scholar]
- 134.Perraki A., DeFalco T.A., Derbyshire P., Avila J., Sere D., Sklenar J., Qi X., Stransfeld L., Schwessinger B., Kadota Y., et al. Phosphocode-dependent functional dichotomy of a common co-receptor in plant signalling. Nature. 2018;561:248–252. doi: 10.1038/s41586-018-0471-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Singh V., Perraki A., Kim S.Y., Shrivastava S., Lee J.H., Zhao Y., Schwessinger B., Oh M.H., Marshall-Colon A., Zipfel C., et al. Tyrosine-610 in the receptor kinase BAK1 does not play a major role in brassinosteroid signaling or innate immunity. Front Plant Sci. 2017;8:1273. doi: 10.3389/fpls.2017.01273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lin W., Li B., Lu D., Chen S., Zhu N., He P., Shan L. Tyrosine phosphorylation of protein kinase complex BAK1/BIK1 mediates Arabidopsis innate immunity. Proc. Natl. Acad. Sci. USA. 2014;111:3632–3637. doi: 10.1073/pnas.1318817111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Segonzac C., Macho A.P., Sanmartin M., Ntoukakis V., Sanchez-Serrano J.J., Zipfel C. Negative control of BAK1 by protein phosphatase 2A during plant innate immunity. EMBO J. 2014;33:2069–2079. doi: 10.15252/embj.201488698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Song L., Shi Q.M., Yang X.H., Xu Z.H., Xue H.W. Membrane steroid-binding protein 1 (MSBP1) negatively regulates brassinosteroid signaling by enhancing the endocytosis of BAK1. Cell Res. 2009;19:864–876. doi: 10.1038/cr.2009.66. [DOI] [PubMed] [Google Scholar]
- 139.Gao M., Wang X., Wang D., Xu F., Ding X., Zhang Z., Bi D., Cheng Y.T., Chen S., Li X., et al. Regulation of cell death and innate immunity by two receptor-like kinases in Arabidopsis. Cell Host Microbe. 2009;6:34–44. doi: 10.1016/j.chom.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 140.Halter T., Imkampe J., Mazzotta S., Wierzba M., Postel S., Bucherl C., Kiefer C., Stahl M., Chinchilla D., Wang X., et al. The leucine-rich repeat receptor kinase BIR2 is a negative regulator of BAK1 in plant immunity. Curr. Biol. 2014;24:134–143. doi: 10.1016/j.cub.2013.11.047. [DOI] [PubMed] [Google Scholar]
- 141.Liu Y., Huang X., Li M., He P., Zhang Y. Loss-of-function of Arabidopsis receptor-like kinase BIR1 activates cell death and defense responses mediated by BAK1 and SOBIR1. New Phytol. 2016;212:637–645. doi: 10.1111/nph.14072. [DOI] [PubMed] [Google Scholar]
- 142.Halter T., Imkampe J., Blaum B.S., Stehle T., Kemmerling B. BIR2 affects complex formation of BAK1 with ligand binding receptors in plant defense. Plant Signal. Behav. 2014;9:e28944. doi: 10.4161/psb.28944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hohmann U., Nicolet J., Moretti A., Hothorn L.A., Hothorn M. The SERK3 elongated allele defines a role for BIR ectodomains in brassinosteroid signalling. Nat. Plants. 2018;4:345–351. doi: 10.1038/s41477-018-0150-9. [DOI] [PubMed] [Google Scholar]
- 144.Chung Y., Choe V., Fujioka S., Takatsuto S., Han M., Jeon J.S., Park Y.I., Lee K.O., Choe S. Constitutive activation of brassinosteroid signaling in the Arabidopsis elongated-D/bak1 mutant. Plant Mol. Biol. 2012;80:489–501. doi: 10.1007/s11103-012-9963-5. [DOI] [PubMed] [Google Scholar]
- 145.Halliday K., Devlin P.F., Whitelam G.C., Hanhart C., Koornneef M. The ELONGATED gene of Arabidopsis acts independently of light and gibberellins in the control of elongation growth. Plant J. 1996;9:305–312. doi: 10.1046/j.1365-313X.1996.09030305.x. [DOI] [PubMed] [Google Scholar]
- 146.Kim M.H., Kim Y., Kim J.W., Lee H.S., Lee W.S., Kim S.K., Wang Z.Y., Kim S.H. Identification of Arabidopsis BAK1-associating receptor-like kinase 1 (BARK1) and characterization of its gene expression and brassinosteroid-regulated root phenotypes. Plant Cell Physiol. 2013;54:1620–1634. doi: 10.1093/pcp/pct106. [DOI] [PubMed] [Google Scholar]
- 147.Blaum B.S., Mazzotta S., Noldeke E.R., Halter T., Madlung J., Kemmerling B., Stehle T. Structure of the pseudokinase domain of BIR2, a regulator of BAK1-mediated immune signaling in Arabidopsis. J. Struct. Biol. 2014;186:112–121. doi: 10.1016/j.jsb.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 148.Grek C.L., Zhang J., Manevich Y., Townsend D.M., Tew K.D. Causes and consequences of cysteine S-glutathionylation. J. Biol. Chem. 2013;288:26497–26504. doi: 10.1074/jbc.R113.461368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bender K.W., Wang X., Cheng G.B., Kim H.S., Zielinski R.E., Huber S.C. Glutaredoxin AtGRXC2 catalyses inhibitory glutathionylation of Arabidopsis BRI1-associated receptor-like kinase 1 (BAK1) in vitro. Biochem. J. 2015;467:399–413. doi: 10.1042/BJ20141403. [DOI] [PubMed] [Google Scholar]
- 150.Moffett A.S., Bender K.W., Huber S.C., Shukla D. Allosteric control of a plant receptor kinase through s-glutathionylation. Biophys. J. 2017;113:2354–2363. doi: 10.1016/j.bpj.2017.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schwessinger B., Roux M., Kadota Y., Ntoukakis V., Sklenar J., Jones A., Zipfel C. Phosphorylation-dependent differential regulation of plant growth, cell death, and innate immunity by the regulatory receptor-like kinase BAK1. PLoS Genet. 2011;7:e1002046. doi: 10.1371/journal.pgen.1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhou J., Wang P., Claus L.A.N., Savatin D.V., Xu G., Wu S., Meng X., Russinova E., He P., Shan L. Proteolytic processing of SERK3/BAK1 regulates plant immunity, development, and cell death. Plant Physiol. 2019;180:543–558. doi: 10.1104/pp.18.01503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ntoukakis V., Schwessinger B., Segonzac C., Zipfel C. Cautionary notes on the use of C-terminal BAK1 fusion proteins for functional studies. Plant Cell. 2011;23:3871–3878. doi: 10.1105/tpc.111.090779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lu D., Wu S., Gao X., Zhang Y., Shan L., He P. A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proc. Natl. Acad. Sci. USA. 2010;107:496–501. doi: 10.1073/pnas.0909705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhao J., Peng P., Schmitz R.J., Decker A.D., Tax F.E., Li J. Two putative BIN2 substrates are nuclear components of brassinosteroid signaling. Plant Physiol. 2002;130:1221–1229. doi: 10.1104/pp.102.010918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang Z.Y., Nakano T., Gendron J., He J., Chen M., Vafeados D., Yang Y., Fujioka S., Yoshida S., Asami T., et al. Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev. Cell. 2002;2:505–513. doi: 10.1016/S1534-5807(02)00153-3. [DOI] [PubMed] [Google Scholar]
- 157.Youn J.H., Kim T.W. Functional insights of plant GSK3-like kinases: Multi-taskers in diverse cellular signal transduction pathways. Mol. Plant. 2015;8:552–565. doi: 10.1016/j.molp.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 158.Choe S., Schmitz R.J., Fujioka S., Takatsuto S., Lee M.O., Yoshida S., Feldmann K.A., Tax F.E. Arabidopsis brassinosteroid-insensitive dwarf12 mutants are semidominant and defective in a glycogen synthase kinase 3beta-like kinase. Plant Physiol. 2002;130:1506–1515. doi: 10.1104/pp.010496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Perez-Perez J.M., Ponce M.R., Micol J.L. The UCU1 Arabidopsis gene encodes a SHAGGY/GSK3-like kinase required for cell expansion along the proximodistal axis. Dev. Biol. 2002;242:161–173. doi: 10.1006/dbio.2001.0543. [DOI] [PubMed] [Google Scholar]
- 160.Yan Z., Zhao J., Peng P., Chihara R.K., Li J. BIN2 functions redundantly with other Arabidopsis GSK3-like kinases to regulate brassinosteroid signaling. Plant Physiol. 2009;150:710–721. doi: 10.1104/pp.109.138099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Youn J.H., Kim T.W., Kim E.J., Bu S., Kim S.K., Wang Z.Y., Kim T.W. Structural and functional characterization of Arabidopsis GSK3-like kinase AtSK12. Mol. Cells. 2013;36:564–570. doi: 10.1007/s10059-013-0266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.De Rybel B., Audenaert D., Vert G., Rozhon W., Mayerhofer J., Peelman F., Coutuer S., Denayer T., Jansen L., Nguyen L., et al. Chemical inhibition of a subset of Arabidopsis thaliana GSK3-like kinases activates brassinosteroid signaling. Chem. Biol. 2009;16:594–604. doi: 10.1016/j.chembiol.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Vert G., Chory J. Downstream nuclear events in brassinosteroid signalling. Nature. 2006;441:96–100. doi: 10.1038/nature04681. [DOI] [PubMed] [Google Scholar]
- 164.Beurel E., Grieco S.F., Jope R.S. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol. Ther. 2015;148:114–131. doi: 10.1016/j.pharmthera.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Peng P., Zhao J., Zhu Y., Asami T., Li J. A direct docking mechanism for a plant GSK3-like kinase to phosphorylate its substrates. J. Biol. Chem. 2010;285:24646–24653. doi: 10.1074/jbc.M110.142547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang H., Tang J., Liu J., Hu J., Liu J., Chen Y., Cai Z., Wang X. Abscisic acid signaling inhibits brassinosteroid signaling through dampening the dephosphorylation of BIN2 by ABI1 and ABI2. Mol. Plant. 2018;11:315–325. doi: 10.1016/j.molp.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 167.Lochhead P.A., Kinstrie R., Sibbet G., Rawjee T., Morrice N., Cleghon V. A chaperone-dependent GSK3beta transitional intermediate mediates activation-loop autophosphorylation. Mol. Cell. 2006;24:627–633. doi: 10.1016/j.molcel.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 168.Cole A., Frame S., Cohen P. Further evidence that the tyrosine phosphorylation of glycogen synthase kinase-3 (GSK3) in mammalian cells is an autophosphorylation event. Biochem. J. 2004;377:249–255. doi: 10.1042/bj20031259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sugiyama N., Nakagami H., Mochida K., Daudi A., Tomita M., Shirasu K., Ishihama Y. Large-scale phosphorylation mapping reveals the extent of tyrosine phosphorylation in Arabidopsis. Mol. Syst. Biol. 2008;4:193. doi: 10.1038/msb.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lin L.L., Hsu C.L., Hu C.W., Ko S.Y., Hsieh H.L., Huang H.C., Juan H.F. Integrating phosphoproteomics and bioinformatics to study brassinosteroid-regulated phosphorylation dynamics in arabidopsis. BMC Genomics. 2015;16:533. doi: 10.1186/s12864-015-1753-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hoffmeister L., Diekmann M., Brand K., Huber R. GSK3: A kinase balancing promotion and resolution of inflammation. Cells. 2020;9:820. doi: 10.3390/cells9040820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hughes K., Nikolakaki E., Plyte S.E., Totty N.F., Woodgett J.R. Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. EMBO J. 1993;12:803–808. doi: 10.1002/j.1460-2075.1993.tb05715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dajani R., Fraser E., Roe S.M., Young N., Good V., Dale T.C., Pearl L.H. Crystal structure of glycogen synthase kinase 3 beta: Structural basis for phosphate-primed substrate specificity and autoinhibition. Cell. 2001;105:721–732. doi: 10.1016/S0092-8674(01)00374-9. [DOI] [PubMed] [Google Scholar]
- 174.Buescher J.L., Phiel C.J. A noncatalytic domain of glycogen synthase kinase-3 (GSK-3) is essential for activity. J. Biol. Chem. 2010;285:7957–7963. doi: 10.1074/jbc.M109.091603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Itoh K., Tang T.L., Neel B.G., Sokol S.Y. Specific modulation of ectodermal cell fates in Xenopus embryos by glycogen synthase kinase. Development. 1995;121:3979–3988. doi: 10.1242/dev.121.12.3979. [DOI] [PubMed] [Google Scholar]
- 176.Moorhead G.B., de Wever V., Templeton G., Kerk D. Evolution of protein phosphatases in plants and animals. Biochem. J. 2009;417:401–409. doi: 10.1042/BJ20081986. [DOI] [PubMed] [Google Scholar]
- 177.Mora-Garcia S., Vert G., Yin Y., Cano-Delgado A., Cheong H., Chory J. Nuclear protein phosphatases with Kelch-repeat domains modulate the response to brassinosteroids in Arabidopsis. Genes Dev. 2004;18:448–460. doi: 10.1101/gad.1174204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Maselli G.A., Slamovits C.H., Bianchi J.I., Vilarrasa-Blasi J., Cano-Delgado A.I., Mora-Garcia S. Revisiting the evolutionary history and roles of protein phosphatases with Kelch-like domains in plants. Plant Physiol. 2014;164:1527–1541. doi: 10.1104/pp.113.233627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shi Y. Serine/threonine phosphatases: Mechanism through structure. Cell. 2009;139:468–484. doi: 10.1016/j.cell.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 180.Tonks N.K. Protein tyrosine phosphatases: From genes, to function, to disease. Nat. Rev. Mol. Cell Biol. 2006;7:833–846. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- 181.Gao X., Zhang J.Q., Zhang X., Zhou J., Jiang Z., Huang P., Tang Z., Bao Y., Cheng J., Tang H., et al. Rice qGL3/OsPPKL1 functions with the GSK3/SHAGGY-like kinase OsGSK3 to modulate brassinosteroid signaling. Plant Cell. 2019;31:1077–1093. doi: 10.1105/tpc.18.00836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Merlot S., Gosti F., Guerrier D., Vavasseur A., Giraudat J. The ABI1 and ABI2 protein phosphatases 2C act in a negative feedback regulatory loop of the abscisic acid signalling pathway. Plant J. 2001;25:295–303. doi: 10.1046/j.1365-313x.2001.00965.x. [DOI] [PubMed] [Google Scholar]
- 183.Zhang S., Cai Z., Wang X. The primary signaling outputs of brassinosteroids are regulated by abscisic acid signaling. Proc. Natl. Acad. Sci. USA. 2009;106:4543–4548. doi: 10.1073/pnas.0900349106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hao Y., Wang H., Qiao S., Leng L., Wang X. Histone deacetylase HDA6 enhances brassinosteroid signaling by inhibiting the BIN2 kinase. Proc. Natl. Acad. Sci. USA. 2016;113:10418–10423. doi: 10.1073/pnas.1521363113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.ter Haar E., Coll J.T., Austen D.A., Hsiao H.M., Swenson L., Jain J. Structure of GSK3beta reveals a primed phosphorylation mechanism. Nat. Struct. Biol. 2001;8:593–596. doi: 10.1038/89624. [DOI] [PubMed] [Google Scholar]
- 186.Monteserin-Garcia J., Al-Massadi O., Seoane L.M., Alvarez C.V., Shan B., Stalla J., Paez-Pereda M., Casanueva F.F., Stalla G.K., Theodoropoulou M. Sirt1 inhibits the transcription factor CREB to regulate pituitary growth hormone synthesis. FASEB J. 2013;27:1561–1571. doi: 10.1096/fj.12-220129. [DOI] [PubMed] [Google Scholar]
- 187.Taylor I., Wang Y., Seitz K., Baer J., Bennewitz S., Mooney B.P., Walker J.C. Analysis of phosphorylation of the receptor-like protein kinase HAESA during arabidopsis floral abscission. PLoS ONE. 2016;11:e0147203. doi: 10.1371/journal.pone.0147203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sarikhani M., Mishra S., Maity S., Kotyada C., Wolfgeher D., Gupta M.P., Singh M., Sundaresan N.R. SIRT2 deacetylase regulates the activity of GSK3 isoforms independent of inhibitory phosphorylation. Elife. 2018;7:e32925. doi: 10.7554/eLife.32952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang P., Du Y., Hou Y.J., Zhao Y., Hsu C.C., Yuan F., Zhu X., Tao W.A., Song C.P., Zhu J.K. Nitric oxide negatively regulates abscisic acid signaling in guard cells by S-nitrosylation of OST1. Proc. Natl. Acad. Sci. USA. 2015;112:613–618. doi: 10.1073/pnas.1423481112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang S.B., Venkatraman V., Crowgey E.L., Liu T., Fu Z., Holewinski R., Ranek M., Kass D.A., O’Rourke B., van Eyk J.E. Protein s-nitrosylation controls glycogen synthase kinase 3beta function independent of its phosphorylation state. Circ. Res. 2018;122:1517–1531. doi: 10.1161/CIRCRESAHA.118.312789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Song S., Wang H., Sun M., Tang J., Zheng B., Wang X., Tan Y.W. Reactive oxygen species-mediated BIN2 activity revealed by single-molecule analysis. New Phytol. 2019;223:692–704. doi: 10.1111/nph.15669. [DOI] [PubMed] [Google Scholar]
- 192.He G., Liu J., Dong H., Sun J. The blue-light receptor CRY1 interacts with BZR1 and BIN2 to modulate the phosphorylation and nuclear function of BZR1 in repressing BR signaling in Arabidopsis. Mol. Plant. 2019;12:689–703. doi: 10.1016/j.molp.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 193.Ling J.J., Li J., Zhu D., Deng X.W. Noncanonical role of Arabidopsis COP1/SPA complex in repressing BIN2-mediated PIF3 phosphorylation and degradation in darkness. Proc. Natl. Acad. Sci. USA. 2017;114:3539–3544. doi: 10.1073/pnas.1700850114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Li J., Terzaghi W., Gong Y., Li C., Ling J.J., Fan Y., Qin N., Gong X., Zhu D., Deng X.W. Modulation of BIN2 kinase activity by HY5 controls hypocotyl elongation in the light. Nat. Commun. 2020;11:1592. doi: 10.1038/s41467-020-15394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gampala S.S., Kim T.W., He J.X., Tang W., Deng Z., Bai M.Y., Guan S., Lalonde S., Sun Y., Gendron J.M., et al. An essential role for 14-3-3 proteins in brassinosteroid signal transduction in Arabidopsis. Dev. Cell. 2007;13:177–189. doi: 10.1016/j.devcel.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hoecker U. The activities of the E3 ubiquitin ligase COP1/SPA, a key repressor in light signaling. Curr. Opin. Plant Biol. 2017;37:63–69. doi: 10.1016/j.pbi.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 197.Liu B., Zuo Z., Liu H., Liu X., Lin C. Arabidopsis cryptochrome 1 interacts with SPA1 to suppress COP1 activity in response to blue light. Genes Dev. 2011;25:1029–1034. doi: 10.1101/gad.2025011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lian H.L., He S.B., Zhang Y.C., Zhu D.M., Zhang J.Y., Jia K.P., Sun S.X., Li L., Yang H.Q. Blue-light-dependent interaction of cryptochrome 1 with SPA1 defines a dynamic signaling mechanism. Genes Dev. 2011;25:1023–1028. doi: 10.1101/gad.2025111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pham V.N., Kathare P.K., Huq E. Phytochromes and phytochrome interacting factors. Plant Physiol. 2018;176:1025–1038. doi: 10.1104/pp.17.01384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gangappa S.N., Botto J.F. The multifaceted roles of HY5 in plant growth and development. Mol. Plant. 2016;9:1353–1365. doi: 10.1016/j.molp.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 201.Osterlund M.T., Hardtke C.S., Wei N., Deng X.W. Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature. 2000;405:462–466. doi: 10.1038/35013076. [DOI] [PubMed] [Google Scholar]
- 202.Truernit E., Bauby H., Belcram K., Barthelemy J., Palauqui J.C. OCTOPUS, a polarly localised membrane-associated protein, regulates phloem differentiation entry in Arabidopsis thaliana. Development. 2012;139:1306–1315. doi: 10.1242/dev.072629. [DOI] [PubMed] [Google Scholar]
- 203.Anne P., Azzopardi M., Gissot L., Beaubiat S., Hematy K., Palauqui J.C. OCTOPUS negatively regulates BIN2 to control phloem differentiation in Arabidopsis thaliana. Curr. Biol. 2015;25:2584–2590. doi: 10.1016/j.cub.2015.08.033. [DOI] [PubMed] [Google Scholar]
- 204.Pillitteri L.J., Peterson K.M., Horst R.J., Torii K.U. Molecular profiling of stomatal meristemoids reveals new component of asymmetric cell division and commonalities among stem cell populations in Arabidopsis. Plant Cell. 2011;23:3260–3275. doi: 10.1105/tpc.111.088583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Houbaert A., Zhang C., Tiwari M., Wang K., de Marcos Serrano A., Savatin D.V., Urs M.J., Zhiponova M.K., Gudesblat G.E., Vanhoutte I., et al. POLAR-guided signalling complex assembly and localization drive asymmetric cell division. Nature. 2018;563:574–578. doi: 10.1038/s41586-018-0714-x. [DOI] [PubMed] [Google Scholar]
- 206.Liu J., Chen J., Zheng X., Wu F., Lin Q., Heng Y., Tian P., Cheng Z., Yu X., Zhou K., et al. GW5 acts in the brassinosteroid signalling pathway to regulate grain width and weight in rice. Nat. Plants. 2017;3:17043. doi: 10.1038/nplants.2017.43. [DOI] [PubMed] [Google Scholar]
- 207.Hirano K., Kawamura M., Araki-Nakamura S., Fujimoto H., Ohmae-Shinohara K., Yamaguchi M., Fujii A., Sasaki H., Kasuga S., Sazuka T. Sorghum DW1 positively regulates brassinosteroid signaling by inhibiting the nuclear localization of BRASSINOSTEROID INSENSITIVE 2. Sci. Rep. 2017;7:126. doi: 10.1038/s41598-017-00096-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Samakovli D., Margaritopoulou T., Prassinos C., Milioni D., Hatzopoulos P. Brassinosteroid nuclear signaling recruits HSP90 activity. New Phytol. 2014;203:743–757. doi: 10.1111/nph.12843. [DOI] [PubMed] [Google Scholar]
- 209.Peng P., Yan Z., Zhu Y., Li J. Regulation of the Arabidopsis GSK3-like kinase BRASSINOSTEROID-INSENSITIVE 2 through proteasome-mediated protein degradation. Mol. Plant. 2008;1:338–346. doi: 10.1093/mp/ssn001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lama S., Broda M., Abbas Z., Vaneechoutte D., Belt K., Sall T., Vandepoele K., van Aken O. Neofunctionalization of mitochondrial proteins and incorporation into signaling networks in plants. Mol. Biol. Evol. 2019;36:974–989. doi: 10.1093/molbev/msz031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Failor K.L., Desyatnikov Y., Finger L.A., Firestone G.L. Glucocorticoid-induced degradation of glycogen synthase kinase-3 protein is triggered by serum- and glucocorticoid-induced protein kinase and Akt signaling and controls beta-catenin dynamics and tight junction formation in mammary epithelial tumor cells. Mol. Endocrinol. 2007;21:2403–2415. doi: 10.1210/me.2007-0143. [DOI] [PubMed] [Google Scholar]