Abstract
Introduction
Understanding drug resistance is important in drug selection for Helicobacter pylori (H. pylori) eradication, and drug resistance data are lacking in Beijing.
Purpose
This cross-sectional study aimed to isolate H. pylori from patients with gastroduodenal diseases and to analyze drug resistance to clarithromycin (CLA) and levofloxacin (LEV), which are used frequently in China.
Patients and Methods
One hundred and seventy-six patients with gastroduodenal diseases undergoing gastroduodenoscopy were selected by convenient sampling. Gastric mucosa samples were cultured and sub-cultured using a new medium broth. Active H. pylori strains were confirmed by microscopy observation as Gram-negative curved bacilli with positive test results for urease, oxidase, and catalase, and H. pylori 16S rRNA amplification by polymerase chain reaction (PCR). CLA and LEV resistance was identified by minimum inhibitory concentration (MIC) tests and sequencing of 23S rRNA, gyrA, and gyrB genes.
Results
From the 176 clinical samples, 112 (112/176, 63.6%) were confirmed with H. pylori infection and 65 (65/176, 36.9%) active H. pylori strains were obtained and further confirmed by MIC assay. Overall, the rates of CLA-resistant and LEV-resistant mutations in the 112 samples were 50.9% and 33.0%, respectively. Mutation related to CLA resistance was A2143G in the 23S rRNA gene and mutations associated with LEV resistance were N87K, D91G, and D91Y in the gyrA gene. Of 112 samples, 22 (19.6%) presented dual resistance to CLA and LEV. Resistance of the H. pylori strains to CLA (r=0.846, P<0.001) and LEV (r=0.936, P<0.001) had a strong correlation in phenotypic and genotypic level.
Conclusion
The results indicated that resistance of CLA and LEV is severe among patients with gastroduodenitis. A good consistency could be found as to drug resistance between genotypic or phenotypic assay, suggested extending the detection of H. pylori drug resistance from the MIC method to a genotypic assay.
Keywords: Helicobacter pylori, clarithromycin, levofloxacin, antibiotic resistance
Introduction
Helicobacter pylori (H. pylori) is one of the most prevalent pathogens in the world, affecting 50% of the world’s population.1,2 It was first identified in samples of gastric biopsies by Warren and Marshall in Australia in 1983.3 H. pylori is a spiral-shaped Gram-negative facultative anaerobic bacterium, which is difficult to culture, and is reported to have huge genetic variation.4,5 Strains of H. pylori can be divided into two major subpopulations, based on their ability to produce a 120–145 kDa immunodominant protein called cytotoxin-associated gene A antigen (CagA), which is closely associated with severe atrophic gastritis and adenocarcinoma.6,7 This heterogeneity of H. pylori strains is thought to be associated with a strain’s ability to cause upper gastrointestinal diseases such as chronic gastritis, peptic ulcer, mucosa-associated lymphoid tissue (MALT) lymphoma, and gastric cancer.8,11 Recently, it has been suggested that H. pylori may be associated with extraintestinal diseases, including immune thrombocytopenic purpura, refractory iron deficiency anemia, and vitamin B12 deficiency.1,12
In China, the infection rate of H. pylori has reached to 50–60%13 Common antibiotics for the eradication of H. pylori include amoxicillin (AMX), clarithromycin (CLA), levofloxacin (LEV), metronidazole (MTZ), and tetracycline (TET).14,15 According to the Maastricht IV/Florence consensus report, it is recommended to use a proton-pump-inhibitor (PPI) in combination with CLA and AMX or MTZ for triple therapy and with the addition of bismuth for quadruple therapy as the treatment of choice, due to increasing antimicrobial resistance.1,16
Antibiotic resistance is the main factor accounting for the unsuccessful eradication of H. pylori. CLA has been extensively used because it was considered to be an efficient antibiotic during the past 10 years.17 With the increasing resistance of H. pylori to CLA, there is growing concern that the eradication of H. pylori infection may decline.18 Considering that CLA should be used with caution in countries with CLA resistance rates higher than 15–20%, regional data about H. pylori resistance to CLA is critical to clinical practice.19 Other treatment options including quinolones, such as LEV, have been evaluated as alternatives to standard antibiotics against H. pylori.20 Cross-resistance to LEV and other quinolones, which have been widely used clinically,15 has been reported. Although the LEV-containing regimen is not recommended for primary treatment,21 the prevalence of LEV usage and the high resistance rates to other quinolones will inevitably decrease the H. pylori eradication rate.
Previous studies have found that CLA resistance is caused by point mutations (adenine–guanine transition) at positions 2142 (11.7%) and 2143 (69.8%) of the 23S rRNA gene, preventing macrolides from binding to the 50S nucleosomes.22 The mechanism of LEV resistance is related to mutations in the quinolone resistance-determining regions (QRDRs) of the gyrA gene.23 Although mutations in the gyrB gene have been reported in LEV-resistant strains in Japan, these mutations usually occur in conjunction with gyrA mutation.24
Despite the growing drug resistance in H. pylori, it is not a priority in clinical practice to perform H. pylori culture and drug susceptibility tests, given that the culture of H. pylori as a microaerobic bacteria is a tedious job with prolonged turn-around-time and low isolation rate. Most physicians choose the eradication program based on clinical practice, which makes the situation even worse by producing new drug-resistant strains. Periodical and regional data on H. pylori antibiotic resistance would facilitate the rational use of antibiotics; however, such data are seldom reported in Beijing.
In this study, H. pylori was isolated from the biopsy gastric mucosa of patients with chronic gastroduodenitis by an improved and rapid H. pylori culture method, with the purpose to find the situation of H. pylori drug resistance to CLA and LEV, at phenotypic and genotypic levels, and to evaluate the correlation between the two methods.
Patients and Methods
Patient Recruitment and Ethics Statement
One hundred and seventy-six patients were selected for H. pylori culture from 6779 cases by convenient sampling. All the patients came to pursue medical consultation at the Gastroenterology Department of Beijing Tsinghua Changgung Hospital during April 2018 to April 2019. Only those patients who accepted a gastroscopy check on Tuesday and Wednesday mornings, who presented positive results of 13C-Urea Breath Test (13C-UBT) within one month, and who had never been treated with antibiotics or PPI or had discontinued previous treatment for more than a month were selected for H. pylori culture. Samples of gastric mucosa in the antrum or fundus were taken when they were undergoing gastroduodenoscopy.
In accordance with the Declaration of Helsinki, this study was approved by the Institutional Review Board of Beijing Tsinghua Changgung Hospital (No. 18023-0-02). All the patients signed informed consent prior to their gastroduodenoscopy, including consent access to pathological tissue samples.
H. pylori Culture and Active Strain Isolation
The gastric mucosa samples were transferred into a one-step commercial isolation broth with rich broth medium and indicator (Yimin Bioengineering, Guangdong, China) and immediately placed in a 37°C incubator. According to the manufacturer’s instruction, the sample was considered to be H. pylori-positive when the culture medium turns red and some floating granules could be seen. The more granules, the larger the bacterial quantity. After 5 days of culture, no visible change indicated H. pylori-negative. The growth of active H. pylori was accelerated and the culture was purified by transferring 100–200 μL of the positive culture medium into another tube containing fresh isolation broth. Strains of H. pylori were identified by microscopy observation as Gram‑negative curved bacilli and positive test results for urease, oxidase, catalase, and polymerase chain reaction (PCR) using a specific primer to amplify H. pylori 16S rRNA.25 All active strains were stored at −80°C in special frozen broth (Yimin Bioengineering, Guangdong, China) for subculture, and both drug susceptibility test and PCR for sequencing.
MIC Determination
MIC was determined by broth microdilution at the lowest concentration, according to the recommendations of the Clinical and Laboratory Standards Institute (CLSI)26 and the manufacturer’s instructions. Based on the recommendations of the European Committee on Antimicrobial Susceptibility Testing (EUCAST), H. pylori 26695 was used as the quality control strain. The resistance breakpoints of CLA and LEV were defined as >0.5 μg/mL and >1 μg/mL, as previously suggested.27
PCR Amplification and Sequencing
The H. pylori genome was extracted by TIANamp Bacteria DNA Kit (Tiangen Biotech, Beijing, China) and using the 112 primary culture-positive samples. Briefly, 200 μL culture broth from each sample was centrifugated to precipitate the bacteria, and the precipitate was used for DNA extraction and PCR. Antibiotic resistance of H. pylori to CLA includes A2142C, A2142G, and A2143G in 23S rRNA28 and resistance mutations to LEV include gyrA, gyrB,29 which were amplified using previously reported primers (Table 1). The PCR products were obtained by agarose gel electrophoresis. Positive PCR products were sent to BGI Technology Company (Shengzhen, China) for sequencing using the PCR primers. The sequences were then compared with the published sequence of the H. pylori 23S rRNA, gyrA and gyrB genes (GenBank accession number NC_000915.1).
Table 1.
Primer Sequences for the Amplification of the Drug Resistance Gene of H. pylori
Statistical Analysis
The result of MIC drug sensitivity and the genotype mutations of the H. pylori is compared by Spearman correlation analysis, with 0.05 as the statistical significance level. SPSS 22.0 was used for all statistical analyses (SPSS Inc., Chicago, IL, USA).
Results
The Culture and Isolation of Active H. pylori Strains
The 176 patients aged from 17 to 82, with a mean age of 48.4. Of the 176 patients, 93 (52.8%) were male, 83 (47.2%) were female, and the ratio of male to female was 1.1.
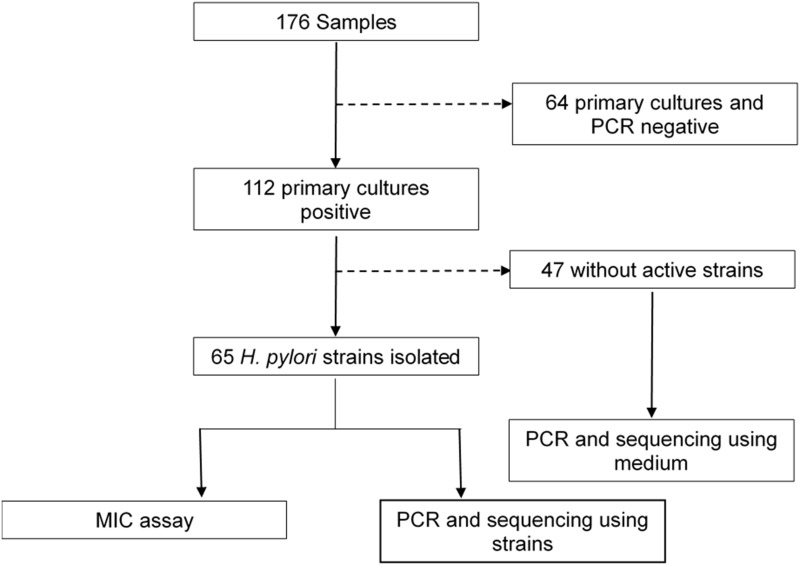
Of the 176 samples, 112 were confirmed to be H. pylori-positive by primary culture and were further confirmed by 16S rRNA PCR; the overall detection rate was 112/176 (63.6%). By subculturing the 112 samples, we finally obtained 65 active H. pylori strains. No active strain was isolated from the other 47 primary culture-positive samples, which could be a result of either contamination or H. pylori dying during the subculture process. The isolation rate of active strains was 36.9% (65/176) and the 65 strains were used for phenotype study by the MIC method. Figure 1 presents the flowchart of this study.
Figure 1.
Flowchart of this study.
Antibiotic Susceptibility by MIC
The MICs of CLA and LEV were determined in 65 active strains of H. pylori. Based on the EUCAST breakpoints, the prevalence of drug resistance to CLA (MIC>0.5 μg/mL) and LEV (MIC>1 μg/mL) was 52.3% (34/65) and 38.5% (25/65), respectively. Of the 65 H. pylori strains, 35.4% (23/65) were sensitive to both CLA and LEV, while 26.2% (17/65) were dual resistant to CLA and LEV (Table 2).
Table 2.
Spearman Correlation Between Phenotype and Genotype of 65 H. pylori Strains
| Susceptibility Test Results | MIC Results | Molecular Results | r | P-value |
|---|---|---|---|---|
| Total sensitive | 23 (35.4%) | 27 (41.5%) | 0.813 | <0.001 |
| Total resistance | ||||
| CLA | 34 (52.3%) | 33 (50.8%) | 0.846 | <0.001 |
| LEV | 25 (38.5%) | 23 (35.4%) | 0.936 | <0.001 |
| Dual resistance | 17 (26.2%) | 18 (27.7%) | 0.962 | <0.001 |
Abbreviations: CLA, clarithromycin; LEV, levofloxacin; MIC, minimum inhibitory concentration.
Characteristics of the Drug-Resistant Gene to CLA and LEV
Of the 112 samples with positive results in the primary culture used for molecular tests, the frequency of resistant genes to CLA and LEV was 50.9% (57/112) and 33.0% (37/112), respectively. The CLA-resistant mutation was all mapped to A2143G (Figure 2A). For gyrA resistance, 13 of the strains had mutations corresponding to Asp-91 (C271T, A272G) and 24 had mutations corresponding to Asn-87 (C261A, C261G) (Figure 2B). We did not find any mutation in the gyrB gene. There were 19.6% (22/112) of the strains showed dual resistance to both CLA and LEV (Table 3).
Figure 2.
Comparison of 23S rRNA allele and gyrA allele sequences of H. pylori. (A) 23S rRNA allele sequences of H. pylori. Sequence alignment showed that 23S rRNA allele was in the V region. Part of the RNA sequence of 23S rRNA V region shows the allele sequence found in different H. pylori clinical isolates. Compared with ATCC 26695, the mutant was 2143 adenine-guanine mutation. The point mutation has been marked black. (B) gyrA allele sequences of H. pylori. Sequence alignment showed that the amino acid sequence of gyrA was in QRDRs. Compared with ATCC 26695, mutant N87K was 87 asparagine-lysine mutations, mutant D91G was 91 aspartic acid-glycine mutations, and mutant D91Y was 91 aspartic acid-tyrosine mutations. The point mutation has been marked black.
Table 3.
Gene Mutations Related to CLA and LEV Resistance of the 112 H. pylori Positive Samples
| Antibiotics | Sample Type | n/N (%) | Mutations | Percentage of Mutation |
|---|---|---|---|---|
| CLA | AS | 33/65 (50.8%) | A2143G | 33/33 (100%) |
| NS | 24/47 (51.1%) | A2143G | 24/24 (100%) | |
| LEV | AS | 23/65 (35.4%) | N87K | 15/23 (65.2%) |
| D91Gly | 7/23 (30.5%) | |||
| D91Y | 1/23 (4.3%) | |||
| NS | 14/47 (29.8%) | N87K | 9/14 (64.2%) | |
| D91Gly | 4/14 (28.6%) | |||
| D91Y | 1/14 (7.0%) | |||
| CLA + LEV | AS | 18/65 (27.7%) | A2143G + N87K | 11/18 (61.1%) |
| A2143G + D91Gly | 6/18 (33.3%) | |||
| A2143G + D91Y | 1/18 (5.6%) | |||
| NS | 4/47 (8.5%) | A2143G + N87K | 3/4 (75%) | |
| A2143G + D91Gly | 1/4 (25%) |
Abbreviations: A, adenine; AS, active strain; CLA, clarithromycin; D, aspartic acid; G, guanine; Gly, glycine; K, lysine; LEV, levofloxacin; N, asparagine; Y, tyrosine; NS, no strain (ie, no active H. pylori strain isolated from the sample).
Comparison of the Genotype and Phenotype of CLA and LEV Resistance
Genotype and phenotype resistance to the two drugs were compared in the 65 H. pylori strains between MIC results and the presenting of drug-resistant mutation. Briefly, no drug-resistant mutation was detected from 27 strains, among which 23 (23/27) strains were sensitive to CLA and LEV by MIC (r=0.813, P<0.001). As shown in Table 2, the results of the Spearman correlation test indicated that resistance of the H. pylori strains to CLA (r=0.846, P<0.001) and LEV (r=0.936, P<0.001) had a strong correlation to the presenting of reported drug-resistant mutations. There were 18 strains of H. pylori presenting resistance genes to CLA and LEV in 23S rRNA and gyrA gene altogether (Table 4). However, 7 strains showed inconsistencies in the phenotype and genotype resistance to CLA and LEV, with 5 of them exhibiting inconsistencies to CLA, 3 of them indicating drug resistance by MIC but no mutation, 2 of them with mutation but no MIC resistance; the other 2 strains showed inconsistencies in LEV resistance in that drug resistance was detected while no related mutation was found (Table 5).
Table 4.
18 H. pylori Strains with MIC Resistance to Both CLA and LEV and the Corresponding Mutations
| Strains Number | MIC (μg/mL) | Mutations of 23S rRNA | Mutations of gyrA | |
|---|---|---|---|---|
| CLA | LEV | |||
| 3 | 8 | 16 | A2143G | N87K |
| 5 | 32 | 8 | A2143G | D91Gly |
| 7 | 16 | 4 | A2143G | N87K |
| 10 | 16 | 8 | A2143G | D91Gly |
| 14 | 64 | 8 | A2143G | N87K |
| 19 | 4 | 4 | A2143G | N87K |
| 28 | 32 | 16 | A2143G | D91Gly |
| 38 | ≥256 | 4 | A2143G | D91Gly |
| 39 | 64 | 4 | A2143G | N87K |
| 40 | 64 | 8 | A2143G | N87K |
| 43 | 64 | 8 | A2143G | D91Gly |
| 44 | 16 | 4 | A2143G | D91Gly |
| 48 | 8 | 8 | A2143G | N87K |
| 52 | 8 | 4 | A2143G | N87K |
| 54 | 16 | 4 | A2143G | N87K |
| 58 | 16 | 4 | A2143G | D91Y |
| 65 | ≥256 | 128 | A2143G | N87K |
| 37 | <0.0156 | 16 | A2143G | N87K |
Note: CLA > 0.5 μg/mL is sensitive, LEV > 1 μg/mL is sensitive.
Abbreviations: A, adenine; CLA, clarithromycin; D, aspartic acid; G, guanine; Gly, glycine; K, lysine; LEV, levofloxacin; MIC, minimal inhibitory concentration; N, asparagine; Y, tyrosine.
Table 5.
Seven Strains with Discordant Phenotype to Genotype Resistance to CLA and LEV
| Strain Number | MIC (μg/mL) | Mutations of 23S rRNA | Mutations of gyrA | |
|---|---|---|---|---|
| CLA | LEV | |||
| 1 | 4 | 0.25 | – | – |
| 18 | 0.03 | 2 | – | – |
| 21 | 0.06 | 4 | – | – |
| 23 | 2 | 0.06 | – | – |
| 47 | 8 | 0.25 | – | – |
| 22 | 0.06 | 0.25 | A2143G | – |
| 37 | <0.0156 | 16 | A2143G | N87K |
Notes: CLA > 0.5 μg/mL is sensitive, LEV > 1 μg/mL is sensitive.
Abbreviations: A, adenine; CLA, clarithromycin; G, guanine; LEV, levofloxacin; MIC, minimal inhibitory concentration; -, no mutation.
Discussion
The present study was a cross-sectional study based in a tertiary hospital in Beijing, with the purpose of getting updated regional data about H. pylori resistance to two widely used antibiotics (CLA and LEV) in clinical practice in Beijing, and comparing the phenotype resistance (results of the MIC tests) to the genotype resistance (gene mutations associated with drug resistance). MIC is still the golden standard for antibiotic resistance, providing that live bacteria strains are available. One hundred and seventy-six non-repetitive gastric mucosa samples were accepted for H. pylori culture following a convenient rule as described, but the researcher had no bias on the sampling of the culture. From those 176 patients, 112 (63.6%) were confirmed with H. pylori infection by H. pylori primary culture and 16S rRNA PCR, and 65 (36.9%) active H. pylori strains were obtained after subculture. The culture of H. pylori has seldom been used for clinical diagnosis due to low efficiency, and some laboratories used it only for research purposes.30 In this study, we used the commercial improved broth of one-step culture as the separation medium, with the gastric mucosa samples not needing to be ground and could be directly added to the broth. The one-step medium also includes antibiotics to prevent the growth of other bacteria and growth indicator of 2, 3, 5-triphenyte-trazoliumchloride (TTC) to inhibit some bacteria and make it easier for researchers to judge the positive results of the culture. This method can reduce the experimental steps and increase the chance of obtaining a pure culture. We finally isolated 65 live H. pylori strains that could be analyzed by the MIC tests. The positive rate was 36.9%, which was similar to other studies.31 To overcome the lower efficiency in obtaining positive culture, current guidelines recommend culture and antibiotic susceptibility tests after two rounds of failed H. pylori eradication attempts.1 Where testing is unavailable, epidemiological data on H. pylori resistance are essential to help the rational use of antibiotics.
It was realized that molecular assay might increase the sensitivity of H. pylori identification significantly compared to bacterial culture. Some molecular assays were adapted to use gastric tissues or stool samples to detect H. pylori and antibiotic resistance and to identify the recurrence or reinfection after eradication, in addition to providing new means for understanding clinical and epidemiological drug resistance of H. pylori.31
Base on the Maastricht V/Florence consensus report, if the local CLA resistance rate exceeds 15%, it is recommended to avoid using CLA-based triple therapy.21 Among the 112 clinical samples, 57 (50.1%) were detected as resistant to CLA. The rate was much greater than the 15% limitation, suggesting that the CLA-based triple regime was not suitable for the treatment of H. pylori infection in this region. Resistance to CLA is usually associated with point mutations in the 23S rRNA gene, especially at loci of 2142 and 214322 In our study, all mutations were A2143G, the most predominant mutation reported previously.22
Of the 112 samples, genotypic assay indicated that 37 (33.0%) were resistant to LEV. Among these LEV-resistance samples, we did not find any mutation in the gyrB gene; all mutations were found in the gyrA gene. N87K and D91G were more frequent than others. Resistance to LEV was due to substitution at positions 87 (K) and 91 (G, Y) of gyrA QRDRs. Our results were consistent with other reports indicating that D91G and N87K appeared to be the major mutations described worldwide.33
It has been indicated that CLA resistance increased annually in a linear manner and was associated with increasing age.34 In neighboring regions of Japan and South Korea, the CLA resistance rate increased from 1.8% in 1996 to 27.1% in 200835 and 11% in 2005 to 60% in 2009,36 respectively. In the United States, the CLA resistance rate increased from 9.1% in 2009–2010 to 24.2% in 2011–2013.37 In a US study focused on children, the rate of CLA resistance was as high as 50%.37 Some guidelines suggest that LEV should be part of rescue treatment based on antibiotic susceptibility testing.1,38 The resistance rate of LEV in Italy and Portugal is also 22–24%.39,40 Other studies indicated that LEV resistance decreased annually, with resistance for a certain cohort at 3.5%.34 In a multicenter retrospective study of children in China, the rate of LEV resistance was low, accounting for 6.7% of the study population,41 because quinolones are not indicated in children younger than 18 years old. In a multicenter study among patients who had gastroduodenoscopy in Morocco, the rate of LEV resistance was 11%, and these patients had never received treatment.42 In Nepal, the primary LEV resistance rate was as high as 42.9%, which might lead to cross-resistance of other quinolones.43 Our results showed that the rate of CLA resistance was 50.9% and the rate of LEV resistance was 33.0%, which indicated a severe situation of H. pylori resistance to both CLA and LEV. In addition, we found that 22 (19.6%) of the 112 samples had dual resistance genes to CLA (23S rRNA) and LEV (gyrA). This is the first report showing H. pylori dual resistance to CLA and LEV in the geographic region of Beijing, suggesting that H. pylori eradication rate would be significantly reduced with a CLA plus LEV regimen.
By comparing genotypic and phenotypic characteristics of CLA and LEV resistance of the 65 strains, it is obvious that most of the genotypic results were consistent with the phenotypic results. The findings supported the rationale of using the molecular method to predict antibiotic-resistant of H. pylori instead of MIC assay. However, 7 strains had different results between the genotypic and phenotypic tests. Two strains had CLA-resistant A2143G, but it was sensitive to CLA in the MIC test. The other 5 strains showed no mutations in drug resistance genes, but 2 were resistant to CLA, and 3 were resistant to LEV. In this study, we focused on previously reported mutations. However, there could be other mutations that have not yet been identified and the samples should be subjected to follow-up studies (Table 5). The results also suggested that there might be inconsistencies between the results of molecular detection and phenotypic analysis, and this possibility should be taken into consideration in clinical practice.
Conclusion
Our study indicated that the resistance of H. pylori to CLA and LEV in Beijing is in a serious situation, with dual resistance to the two drugs reaching 19.6%. It would be necessary to select a therapeutic regime based on information on H. pylori resistance. Information on the drug-resistant gene was well correlated to MIC assay and could serve as an alternative to support drug selection in a more convenient way than MIC assay. However, there were some limitations to this study. First, it was a cross-sectional study and previous treatments were not taken into account; therefore, previous antibiotic treatments might have affected the H. pylori isolation and presentation of the resistance. Secondly, the selection of the 176 patients for H. pylori isolation followed a convenient sampling manner but not random design, though the researcher had no interference on sampling, but the typical of this study is discounted. Third, the phenotypic results were considered to be the standard method for antibiotic resistance estimation, but the MICs used to define drug resistance were inconsistent in different studies, leading to bias when comparing our drug resistance to others.
Acknowledgment
Many thanks to our colleagues in the microbiology lab for assistance with the experiments. We also appreciate Gaohui Nong at the Zhu Hai Health School for providing technical instructions when we performed the H. pylori culture. This study was supported by Beijing Tsinghua Changgung Hospital Fund (Grant No. 12017C1019).
Disclosure
The authors declare no potential conflicts of interest.
References
- 1.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of helicobacter pylori infection–the maastricht iv/florence consensus report. GUT. 2012;61(5):646–664. doi: 10.1136/gutjnl-2012-302084 [DOI] [PubMed] [Google Scholar]
- 2.Williams MP, Pounder RE. Helicobacter pylori: from the benign to the malignant. Am J Gastroenterol. 1999;94(11 Suppl):S11–6. doi: 10.1016/S0002-9270(99)00657-7 [DOI] [PubMed] [Google Scholar]
- 3.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. LANCET. 1984;1(8390):1311–1315. doi: 10.1016/S0140-6736(84)91816-6 [DOI] [PubMed] [Google Scholar]
- 4.Atherton JC, Peek RJ, Tham KT, Cover TL, Blaser MJ. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. GASTROENTEROLOGY. 1997;112(1):92–99. doi: 10.1016/S0016-5085(97)70223-3 [DOI] [PubMed] [Google Scholar]
- 5.Logan RP, Berg DE. Genetic diversity of Helicobacter pylori. LANCET. 1996;348(9040):1462–1463. [DOI] [PubMed] [Google Scholar]
- 6.Covacci A, Censini S, Bugnoli M, et al. Molecular characterization of the 128-kDa immunodominant antigen of helicobacter pylori associated with cytotoxicity and duodenal ulcer. P NATL ACAD SCI USA. 1993;90(12):5791–5795. doi: 10.1073/pnas.90.12.5791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatakeyama M. Helicobacter pylori CagA—a bacterial intruder conspiring gastric carcinogenesis. Int J Cancer. 2006;119(6):1217–1223. doi: 10.1002/ijc.21831 [DOI] [PubMed] [Google Scholar]
- 8.Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J Clin Invest. 2004;113(3):321–333. doi: 10.1172/JCI20925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinis-Ribeiro M, Areia M, de Vries AC, et al. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European society of gastrointestinal endoscopy (ESGE), European helicobacter study group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). ENDOSCOPY. 2012;44(1):74–94. doi: 10.1055/s-0031-1291491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vakil N, Megraud F. Eradication therapy for Helicobacter pylori. GASTROENTEROLOGY. 2007;133(3):985–1001. doi: 10.1053/j.gastro.2007.07.008 [DOI] [PubMed] [Google Scholar]
- 11.Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. LANCET. 2008;372(9636):392–397. doi: 10.1016/S0140-6736(08)61159-9 [DOI] [PubMed] [Google Scholar]
- 12.Banic M, Franceschi F, Babic Z, Gasbarrini A. Extragastric manifestations of Helicobacter pylori infection. HELICOBACTER. 2012;17(Suppl 1):49–55. doi: 10.1111/j.1523-5378.2012.00983.x [DOI] [PubMed] [Google Scholar]
- 13.Hooi J, Lai WY, Ng WK, et al. Global prevalence of helicobacter pylori infection: systematic review and meta-analysis. GASTROENTEROLOGY. 2017;153(2):420–429. doi: 10.1053/j.gastro.2017.04.022 [DOI] [PubMed] [Google Scholar]
- 14.Li M, Gao F. Progress in research on therapeutic regimen for helicobacter pylori eradication. Chin J Gastroenter. 2016;10:623–625. [Google Scholar]
- 15.Fifth Chinese LW. National consensus report on the management of helicobacter pylori infection. Chin J Gastroenter. 2017;6:321–324. [Google Scholar]
- 16.Graham DY, Lee SY. How to effectively use bismuth quadruple therapy: the good, the bad, and the ugly. GASTROENTEROL CLIN N. 2015;44(3):537–563. doi: 10.1016/j.gtc.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fallone CA, Chiba N, van Zanten SV, et al. The toronto consensus for the treatment of helicobacter pylori infection in adults. GASTROENTEROLOGY. 2016;151(1):51–69. doi: 10.1053/j.gastro.2016.04.006 [DOI] [PubMed] [Google Scholar]
- 18.Gumurdulu Y, Serin E, Ozer B, et al. Low eradication rate of Helicobacter pylori with triple 7–14 days and quadruple therapy in Turkey. World J Gastroenterol. 2004;10(5):668–671. doi: 10.3748/wjg.v10.i5.668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mascellino MT, Porowska B, De Angelis M, Oliva A. Antibiotic susceptibility, heteroresistance, and updated treatment strategies in Helicobacter pylori infection. Drug design devther. 2017;11(2209–2220). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Group CHPR, Gastroenterology CSO, Hong C, Fulian HU, Yong X, Pinjin HU. Prevalence of helicobacter pylori resistance to antibiotics and its influence on the treatment outcome in china: a multicenter clinical study. Chin J Gastroenter. 2007;9:525–530. [Google Scholar]
- 21.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of helicobacter pylori infection—the maastricht v/florence consensus report. GUT. 2016;66(1):6–30. [DOI] [PubMed] [Google Scholar]
- 22.Nishizawa T, Suzuki H. Mechanisms of Helicobacter pylori antibiotic resistance and molecular testing. Fron Mol Biosci. 2014;1. doi: 10.3389/fmolb.2014.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cattoir V, Nectoux J, Lascols C, et al. Update on fluoroquinolone resistance in Helicobacter pylori: new mutations leading to resistance and first description of a gyrA polymorphism associated with hypersusceptibility. Int J Antimicrob Agents. 2007;29(4):389–396. doi: 10.1016/j.ijantimicag.2006.11.007 [DOI] [PubMed] [Google Scholar]
- 24.Miyachi H, Miki I, Aoyama N, et al. Primary levofloxacin resistance and gyrA/B mutations among Helicobacter pylori in Japan. HELICOBACTER. 2006;11(4):243–249. doi: 10.1111/j.1523-5378.2006.00415.x [DOI] [PubMed] [Google Scholar]
- 25.Singh V, Mishra S, Rao GRK, et al. Evaluation of nested PCR in detection of helicobacter pylori targeting a highly conserved gene: HSP60. HELICOBACTER. 2008;13(1):30–34. doi: 10.1111/j.1523-5378.2008.00573.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CLSI. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria. In: Approved Standard–Third Edition. CLSI Document M45-A2. vol. Wayne, PA: Clinical and Laboratory Standards Institute; 2015. [Google Scholar]
- 27.Shetty V, Lamichhane B, Tay CY, et al. High primary resistance to metronidazole and levofloxacin, and a moderate resistance to clarithromycin in Helicobacter pylori isolated from Karnataka patients. GUT PATHOG. 2019;11(1):1. doi: 10.1186/s13099-019-0305-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.HO S, EL TAN, CK SAM, GOH K. Clarithromycin resistance and point mutations in the 23S rRNA gene in Helicobacter pylori isolates from Malaysia. J DIGEST DIS. 2010;11(2):101–105. doi: 10.1111/j.1751-2980.2010.00423.x [DOI] [PubMed] [Google Scholar]
- 29.Wang L. Distribution of gyrA mutations in fluoroquinolone-resistant Helicobacter pylori strains. WORLD J GASTROENTERO. 2010;16(18):2272. doi: 10.3748/wjg.v16.i18.2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez-Perez GI. Accurate diagnosis of Helicobacter pylori. Culture, including transport. Gastroenterol Clin North Am. 2000;29(4):879–884. doi: 10.1016/S0889-8553(05)70155-2 [DOI] [PubMed] [Google Scholar]
- 31.BJ HU, ZHAO FJ, ZL CHAI, et al. Prevalence and antibiotic resistance profi le of Helicobacter pylori in Shanghai. Chin J Infect Chemother. 2016;16(3):346–352. [Google Scholar]
- 32.Wang YH, Li Z, Wang L, et al. A systematic review and meta‐analysis of genotypic methods for detecting antibiotic resistance in Helicobacter pylori. Helicobacter. 2018;23(2):e12467. doi: 10.1111/hel.12467 [DOI] [PubMed] [Google Scholar]
- 33.Garcia M, Raymond J, Garnier M, Cremniter J, Burucoa C. Distribution of Spontaneous gyrA Mutations in 97 Fluoroquinolone-Resistant Helicobacter pylori Isolates Collected in France. Antimicrobial Agents and Chemotherapy. 2012;56(1):550–551. doi: 10.1128/AAC.05243-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boltin D, Ben-Zvi H, Perets TT, et al. Trends in secondary antibiotic resistance of Helicobacter pylori from 2007 to 2014: has the tide turned? J Clin Microbiol. 2015;53(2):522–527. doi: 10.1128/JCM.03001-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horiki N, Omata F, Uemura M, et al. Annual change of primary resistance to clarithromycin among Helicobacter pylori isolates from 1996 through 2008 in Japan. HELICOBACTER. 2009;14(5):86–90. doi: 10.1111/j.1523-5378.2009.00714.x [DOI] [PubMed] [Google Scholar]
- 36.Kim JJ, Reddy R, Lee M, et al. Analysis of metronidazole, clarithromycin and tetracycline resistance of Helicobacter pylori isolates from Korea. J Antimicrob Chemother. 2001;47(4):459–461. doi: 10.1093/jac/47.4.459 [DOI] [PubMed] [Google Scholar]
- 37.Shiota S, Reddy R, Alsarraj A, El-Serag HB, Graham DY. Antibiotic resistance of helicobacter pylori among male united states veterans. Clin Gastroenterol Hepatol. 2015;13(9):1616–1624. doi: 10.1016/j.cgh.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitui M, Patel A, Leos NK, Doern CD, Park JY. Novel Helicobacter pylori sequencing test identifies high rate of clarithromycin resistance. J Pediatr Gastroenterol Nutr. 2014;59(1):6–9. doi: 10.1097/MPG.0000000000000380 [DOI] [PubMed] [Google Scholar]
- 39.Saracino IM, Zullo A, Holton J, et al. High prevalence of primary antibiotic resistance in Helicobacter pylori isolates in Italy. J Gastrointestin Liver Dis. 2012;21(4):363–365. [PubMed] [Google Scholar]
- 40.Cabrita J, Oleastro M, Matos R, et al. Features and trends in Helicobacter pylori antibiotic resistance in Lisbon area, Portugal (1990–1999). J Antimicrob Chemother. 2000;46(6):1029–1031. doi: 10.1093/jac/46.6.1029 [DOI] [PubMed] [Google Scholar]
- 41.Li L, Ke Y, Yu C, et al. Antibiotic resistance of Helicobacter pylori in Chinese children: A multicenter retrospective study over 7 years. HELICOBACTER. 2017;22(3):e12373. doi: 10.1111/hel.12373 [DOI] [PubMed] [Google Scholar]
- 42.Bouihat N, Burucoa C, Benkirane A, et al. Helicobacter pylori primary antibiotic resistance in 2015 in morocco: a phenotypic and genotypic prospective and multicenter study. Microb Drug Resist. 2017;23(6):727–732. doi: 10.1089/mdr.2016.0264 [DOI] [PubMed] [Google Scholar]
- 43.Miftahussurur M, Shrestha PK, Subsomwong P, Sharma RP, Yamaoka Y. Emerging Helicobacter pylori levofloxacin resistance and novel genetic mutation in Nepal. BMC Microbiol. 2016;16(1):1. doi: 10.1186/s12866-016-0873-6 [DOI] [PMC free article] [PubMed] [Google Scholar]