Abstract
Cellular senescence was first described as a physiological tumor cell suppressor mechanism that leads to cell growth arrest with production of the senescence-associated secretory phenotype known as SASP. The main role of SASP in physiological conditions is to attract immune cells to clear senescent cells avoiding tumor development. However, senescence can be damage-associated and, depending on the nature of these stimuli, additional types of senescence have been described. In the context of cancer, damage-associated senescence has been described as a consequence of chemotherapy treatments that were initially thought of as a tumor suppressor mechanism. However, in certain contexts, senescence after chemotherapy can promote cancer progression, especially when immune cells become senescent and cannot clear senescent tumor cells. Moreover, aging itself leads to continuous inflammaging and immunosenescence which are responsible for rewiring immune cells to become defective in their functionality. Here, we define different types of senescence, pathways that activate them, and functions of SASP in these events. Additionally, we describe the role of senescence in cancer and its treatments, including how aging and chemotherapy contribute to senescence in tumor cells, before focusing on immune cell senescence and its role in cancer. Finally, we discuss potential therapeutic interventions to reverse cell senescence.
Keywords: senescence, SASP, senescence surveillance, inflammaging, immunotherapy
1. Defining Senescence
1.1. From Old to New and Different Concepts of Senescence
Cellular senescence is a cell fate that has the defining feature of stable growth arrest that is refractory to mitogenic stimulation. As such, senescent cells differ from those that are quiescent and able to reenter the cell cycle in favorable growth conditions. Senescence is distinct to apoptosis as well, and in fact, resistance to apoptosis is a feature of senescence [1]. Importantly, senescence does not correspond with a complete cellular shutdown, as evidenced by active metabolic reprogramming, changes to cell morphology and the large-scale production and secretion of proinflammatory molecules known as senescence-associated secretory phenotypes (SASP) [2].
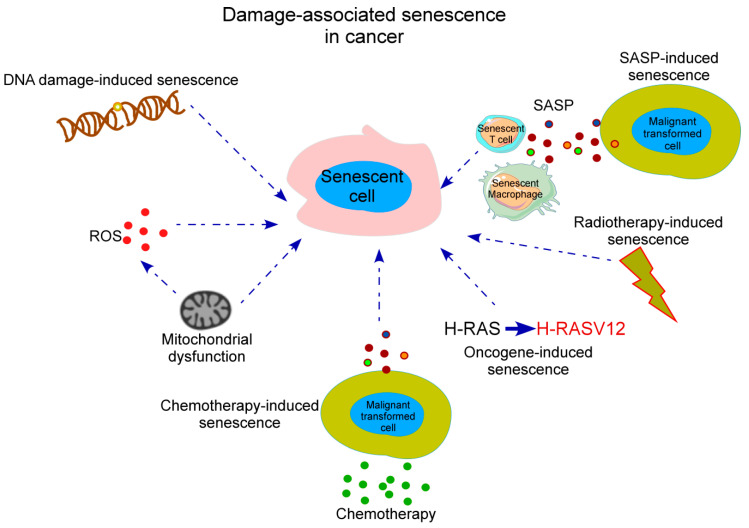
Historically, senescence was first identified in vitro by Hayflick and Moorhead in 1961 when they observed during serial passage of human diploid fibroblasts that these cultures had limited replicative potential, becoming irreversibly arrested [3]. This impaired proliferation seemed to be associated with a progressive shortage of telomeres, which induced a persistent DNA damage response (DDR) and activated signals including p53 and p16 [4]. These events were hypothesized to be a physiological mechanism to prevent cancer initiation by avoiding proliferation of damaged cells [5] as loss of telomerase activity appeared to show inhibition of tumor progression in various mice models [6]. Since these discoveries, a high number of studies have established that, with aging, progressive accumulation of DNA damage in the cell originate a cell senescent state initiated primarily to repair this DNA damage being termed “replicative senescence” or “DNA damage-associated senescence”. Replicative senescence occurs following excessive proliferation when a cell has reached its proliferative capacity and is no longer able to undergo cell division, a point known as the Hayflick limit. Initially, damage-associated senescence causes an arrest of the cell cycle progression to potentially allow the suppression of dysfunctional, transformed, or aged cells [2,7]. Thus, senescence can be perceived initially as a tumor-protective mechanism that prevents uncontrolled replication of those precancerous cells that contain some oncogene activation or the loss of tumor suppressor genes [8]. Furthermore, it has been found that the overexpression of p53 in malignant tumors has led to induction of cell senescence and tumor regression, supporting the idea of senescence as a neoplastic brake [9]. Moreover, cellular senescence can occur prematurely after exposure to a stress signal, being termed “premature senescence”, “stress-induced senescence”, or “oncogene-induced senescence” (OIS). The extracellular or internal triggers that cause premature senescence are wide-ranging and include a high-fat diet, cytokines, radiotherapy and chemotherapy drugs (known as “therapy-induced senescence”, (TIS), reactive oxygen species (ROS), mitochondria dysfunction, and oncogene activation [10]. These stress signals can cause other cell fates, primarily apoptosis, and therefore, the balance of many different signals is critical in the cellular decision to begin a program of senescence induction (Figure 1).
Figure 1.
Stress-mediated inducers of cellular senescence in cancer. Damage-associated senescence associated with cancer development can occur in cells of the immune system due to SASP released by tumor cells, after accumulation of DNA damage, after inflammatory insults such as ROS or mitochondrial dysfunction which also impacts ROS production, after radiotherapy damage and chemotherapy treatments in tumor cells and also as a result of oncogenic mutations. SASP, senescence-associated secretory phenotype; ROS, reactive oxygen species.
On the other hand, there is a physiological and non-damage-associated senescence termed “developmentally-programmed senescence” that occurs during mammalian embryonic development for efficient tissue remodeling. Developmentally-programmed senescence is strictly dependent on p21, followed by macrophage infiltration for clearance of senescent cells and, unlike age-related senescence, is independent of DNA damage. This mechanism has been proposed to be the origin of damage-associated senescence. Physiological cellular senescence occurring both during normal embryonic development and upon tissue damage follows a cycle of “senescence–clearance–regeneration” to trigger tissue remodeling and removal of senescent cells maintaining tissue homeostasis. This cycle is achieved with the help of the immune system that performs clearance of senescent cells. However, during aging, which is a relevant risk factor for cancer, a decline in the immune response termed “immunosenescence” causes these stages to not complete, compromising the clearance of senescent cells and exacerbating inflammation with detrimental effects [7,11].
1.2. Senescence Biomarkers and the SASP
Cellular senescence is complex and dynamic, with large variations in the observed phenotype depending on cell type, environment, and the time following senescence induction. Identification of senescent cells is dependent on multiple markers and functional properties, and no single marker has yet been identified to classify cells as being senescent. Other than the gold standard, detecting senescence-associated β-galactosidase (SA-β-gal) activity, some of the most popular senescence biomarkers are related to proliferation and the cell cycle, such as loss of Ki67 protein expression and increased levels of the cell cycle inhibitor p16. Lipofuscin, an aggregate of oxidized proteins, lipids, and metals that accumulates progressively mostly in aged postmitotic cells, colocalizes with SA-β-gal being also used as a senescence marker [12]. Other features of senescent cells include resistance to apoptosis by downregulation of caspase-3 and the proapoptotic BAX, and upregulation of antiapoptotic BCL-2, BCL-W and BCL-XL, and of cyclin-dependent kinase inhibitors p16 and p21 [13,14,15]. Genetic and epigenetic alterations can also serve as useful markers of senescence, in particular telomere length, trimethylation of histone H3 on lysine 9 (H3K9me3), phosphorylation of the histone H2AX (γH2AX), and loss of lamin B1 [16,17,18].
Secretion of SASP by senescent cells mediates many of their patho-physiological effects contributing to age-associated pathologies such as cancer [2,7,19]. The SASP contains a cocktail of factors that include proinflammatory cytokines, chemokines, growth factors, ROS, angiogenic factors, and proteases that are released by senescent cells [2,20] which can create a favorable cytokine microenvironment for tumorigenesis [21]. Although these proteins are secreted by many cell types in nonsenescent states, especially by tumor cells and immune cells, what makes the SASP unique is that it is induced by senescence. The exact components of the SASP are influenced by a variety of factors such as the senescence induction mechanism, cell type, and tissue of origin. Moreover, the SASP is dynamic and can change over time following the initiation of senescence [22]. The SASP is controlled by NF-κB, mammalian target of rapamycin (mTOR) [23], p38 mitogen-activated protein kinase (p38MAPK) signaling [24], and IL-1 signaling through the NLRP3 inflammasome [19]. In addition, damage-associated molecular patterns (DAMPs) can also activate the inflammasome triggering SASP activation [25]. SASP factors act in an autocrine way to create positive and negative feedback loops, and in a paracrine manner to modulate the activity of neighboring cells. Critically, the SASP can promote the propagation of senescence as it contains many prosenescent factors released in extracellular vesicles [26] which spread the SASP [19]. In the context of cancer, the SASP can have a major influence on tumor progression—it can either promote or hinder tumorigenesis depending on the exact components of the SASP [20]. In this regard, the SASP can be a double-edged sword in cancer treatment, as it is required by immune cells to mediate antitumor responses [27,28,29] promoting “senescence surveillance” and preventing tumor initiation [30,31], and at chronic levels and pathological conditions such as established tumors, SASP components, such as vascular endothelial growth factor (VEGF), CCL5, and IL-6, can induce cancer, drug resistance, cancer progression, and associated side effects such as cachexia [20,32,33,34,35,36,37,38,39,40,41]. Excellent reviews have addressed the different molecules and their functions present in the SASP [20]. Here, in Table 1 and Table 2 we have summarized some of the common molecules found in the SASP released by senescent and/or immune cells and their impact in cancer progression.
Table 1.
SASP factors released by immune cells.
| Senescent Immune Cell | SASP Factors | References |
|---|---|---|
| CD4+ T cells | IL-6, IL-10, TNFα, IFNγ, TGF-β1 | [102] |
| CD8+ T cells | IL-6, IL-8, TNFα, IL-18, IFNγ, TGF-β1, CCL16, ADAM28 | [102,111,117,118] |
| B cells | IL-6, IL-8, TNFα | [229] |
| NK cells | MMPs, cathepsins | [88] |
| Macrophages | IL-6, TNFα, PDGF-BB, TGFβ | Reviewed in [230] |
IL, interleukin; TNF, tumor necrosis factor; CCL, chemokine ligand; ADAM, a disintegrin and metalloproteinase; NK, natural killer; MMP, matrix metalloproteinase; PDGF, platelet-derived growth factor; TGF, transforming growth factor.
Table 2.
SASP factors that influence immune cell function in cancer.
| SASP Factor | Senescent Cells That Secrete Factor | Protumorigenic Mechanisms | Antitumorigenic Mechanisms | Reference |
|---|---|---|---|---|
| IL-6 | CD8+ T cells, B cells, macrophages | Recruit MDSCs, impair DC differentiation, inhibit antitumor T cell responses | Recruit macrophages and NKT cells | [231,232,233] |
| IL-8 | Tumor cells in solid tumors and hematological malignancies | Enhancement of angiogenesis, attraction of neutrophils and MDSCs | Reviewed in [234] | |
| IL-1α | Senescent fibroblasts. Breast cancer cells. Colon cancer cells | Regulation of IL-6 and IL-8 protumorigenic effects. Induction of production of tumor survival factors. Oncogene Ras-induced cell senescence, doxorubicin-induced cancer cell senescence and replicative senescence. |
Macrophage immune surveillance | [235,236,237,238] |
| IL-1β | Fibroblasts | Inflammaging, induction of ROS-mediated DDR | [58] | |
| IL-10 | Macrophages | Immunosuppression | [233] | |
| CXCL2/CXCR2 | Prostate cancer cells | T cell suppression through macrophage polarization to an anti-inflammatory phenotype. Recruit iMCs that hinder tumor cell senescence. |
[239,240] | |
| PGE2 | Hepatic stellate cells | Inhibit antitumor responses through PTGER4 receptor in hepatocellular carcinoma | [241] | |
| CCL2 | Senescent hepatocytes | Promotes accumulation of immunosuppressive iMCs promoting hepatocellular carcinoma through NK cell inhibition | Recruits myeloid cells that differentiate into macrophages to clear senescent precancerous cells | [39] |
| CCL3 (MIP-1 α) | Senescent hepatocytes | Recruit immune NK cells for clearance of senescent cells in hepatocellular carcinoma | [242] | |
| TGF-β1 | Fibroblasts | Inflammaging, induction of ROS-mediated DDR | [58] | |
| TGF-β3 | Senescent adipose-derived mesenchymal stem cells | Decreased angiogenic potential | [243] | |
| CCL5 | Melanoma cells | Recruit TILs to eliminate cancer cells | [244] | |
| TNFα | Induce T cell senescence | [109] | ||
| IFNγ | Bone marrow-derived macrophages | Induce M1 macrophage differentiation | [245] | |
| IFNα | Induce CD8+ T cell senescence, accelerates loss of CD27 and CD28 | [135] |
MDSC, myeloid-derived suppressor cell; NKT, natural killer T; TIL, tumor-infiltrating lymphocyte; iMC, Gr1+CD11b+ immature myeloid cell; DC, dendritic cell; IL, interleukin; PGE2, prostaglandin E2; TNF, tumor necrosis factor; IFN, interferon; ROS, reactive oxygen species; DDR, DNA damage response; CXCL, CXC-chemokine ligand; CXCR, CXC chemokine receptor; CCL, chemokine ligand; MIP; macrophage inflammatory protein; TGF, transforming growth factor; NK, natural killer
Other relevant features of senescent cells include decreased mitophagy, which results in increased mitochondria levels and defective mitochondrial networks that may contribute to the SASP and metabolic dysfunction with aging [42,43]. It is also important to highlight that senescence can be transmissible from senescent to nonsenescent cells through cytoplasmic bridges [2].
1.3. Epigenetics in Senescence
The progression of senescence is frequently associated with extensive epigenetic remodeling and large-scale chromatin reorganization [18]. Epigenetic alterations are vital for the senescence-associated DDR and p16 expression. However, epigenetic mechanisms that control senescence go much further [44]. For instance, the H3K9me3 histone modification is fundamental, and changes in the activity or levels of the enzymes associated with the deposition or removal of the methylation (methyltransferases or demethylases, respectively) can induce or reverse senescence [45,46,47]. Similarly, redistribution of histone variants is a senescence mechanism to control gene expression [44]. In particular, incorporation of histone variant H3.3 drives cell cycle arrest by downregulating proliferation-promoting genes [48], whereas, H2A.J and macroH2A1 promote the SASP [49,50]. Other important epigenetic regulators of senescence are the DNA-distorting proteins HMGB1 and HMGB2, which are diminished in the nuclei of senescent cells [51,52]. Nuclear HMGB1 inhibits SASP gene expression and HMGB2 loss is associated with the genomic reorganization that occurs early in senescence development [51,52]. Interestingly, secreted HMGB1 promotes the SASP in an autocrine/paracrine manner, in complete contrast to its role in the nucleus [52].
In addition, a common feature of OIS, but not replicative senescence, is the formation of nuclear structures known as senescence-associated heterochromatin foci (SAHFs) [16]. SAHFs occur due to reorganization of the nuclear architecture, in which areas of heterochromatin containing repressive marks, such as H3K9me3, are brought together into clusters. Formation of OIS SAHFs is dependent on the nuclear lamina component lamin B1 and DNMT1-mediated upregulation of HMGA2 [17,18]. The abundance of repressive marks and loss of modifications associated with gene expression meant that SAHFs were originally thought of as areas of gene silencing, although recent studies of OIS showing that heterochromatin decondenses as it forms SAHFs suggests that this may not be universally true [16,17]. In fact, the 3D realignment of chromatin that occurs in the formation of SAHFs actually enhances expression of senescence-associated genes in regions adjacent to SAHFs [17].
1.4. Signaling Pathways that Contribute to Senescence
Independent of the method of senescence induction, the DDR is often critical, but not the only cause, to translating the senescence trigger into cell cycle arrest; a key feature of senescence. In replicative senescence, telomere loss leads to the exposure of chromosome ends that the DDR detects as it would a double-strand break in DNA [53]. In OIS, hyperproliferation causes faults in DNA replication, resulting in a DDR [10]. Radiation and chemotherapeutic drugs can initiate a DDR by generating a number of different DNA aberrations, depending on the nature of the damaging agent.
Telomere attrition or irreparable/sustained DNA damage activate the kinases ataxia-telangiectasia mutated (ATM) and/or ATM and Rad3-related (ATR) to generate DDR foci (detected as γH2AX). When ATM/ATR activity at DDR foci exceeds a certain threshold, a signaling cascade is triggered in which CHK2 and CHK1, phosphorylated by ATM and ATR, respectively, translocate away from chromatin to phosphorylate a number of substrates involved in cell cycle control and protein expression [31]. As a result of this signaling, p53 is activated and stabilized, allowing p53 to drive the transcription of the cell cycle inhibitor p21, which ultimately causes cell cycle arrest at the G1/S checkpoint.
Although p21 is important for senescence initiation, it is thought that sustained cell G1 phase arrest requires a second cell cycle inhibitory protein, p16 [54]. The function of p16 is critical because it removes the inhibition of retinoblastoma protein, allowing it to repress E2F-mediated expression of DNA replication genes. Expression of p16 from the INK4A/ARF locus, which also encodes the tumor suppressor proteins p14 and p15, is tightly controlled by chromatin modifiers, cofactor proteins and RNA molecules [55]. Although many details of this regulation are still unknown, it is well-established that polycomb repressive complexes restrain p16 transcription by adding chromatin-compacting modifications to the INK4A/ARF locus, especially H3K27 trimethylation (H3K27me3). Stress signals can contribute to senescence by suppressing the polycomb repressive complexes or by activating demethylases such as JMJD3 that removes the H3K27me3 mark, both of which abolish gene silencing at the INK4A/ARF locus and facilitate the transcription of p16 [56,57].
A number of signaling pathways cooperate to induce the development of the SASP. The DDR and signal transduction pathways mediated by oncogene activation, p38 MAPK, cGAS/STING, and JAK/STAT ultimately converge to control the activity of NF-κB and/or C/EBPβ transcription factors. In turn, NF-κB and C/EBPβ promote the expression of SASP factors, such as IL-6, IL-8, and IL-1β, which act in an autocrine and paracrine manner to generate a positive feedback loop and increase SASP production. Moreover, SASP-derived IL-1β and TGFβ promote senescence in surrounding cells by promoting a ROS-dependent DDR [58]. mTOR signaling is key to the regulation of the SASP as well. mTOR controls the translation of key proteins involved in the SASP, such as IL-1α and MAPKAPK2 [59]. There are signaling pathways that control the flavor of the SASP as well, such as those downstream of NOTCH1 which inhibit a C/EBPβ-mediated proinflammatory SASP in favor of a TGFβ-rich secretome [60].
Cellular senescence is also elicited independently of the DDR. Thus, metabolic rewiring is another important contributor to the senescent phenotype, particularly in cell cycle arrest and SASP production. Senescent cells often exhibit a glycolytic state, albeit with a reduced energy profile and dysfunction in other metabolic pathways, such as the malate–aspartate shuttle [61,62,63]. Reduced malate–aspartate shuttle activity causes a decrease in the cytosolic NAD+/NADH ratio, which is critical for replicative senescence and mitochondrial dysfunction-associated senescence (MiDAS) [61,64]. The associated increase in ADP/ATP and AMP/ATP ratios trigger AMP-activated protein kinase (AMPK) activation, which promotes p53-mediated cell cycle arrest [65]. In turn, p53 causes decreased expression of the ME1 and ME2 enzymes, which convert malate into pyruvate, to further increase p53 expression and enhance senescence [66].
The metabolite pyruvate is another important molecule for senescence induction, although the fate of pyruvate can differ depending on the senescence trigger. In replicative senescence and MiDAS, the increase in lactate dehydrogenase activity/expression causes more pyruvate to be converted into lactate, and thus taken away from potential use in the TCA cycle [61,62]. However, in models of OIS and TIS, both glycolytic flux and TCA cycle activity are heightened [63]. Increased activity of the enzyme pyruvate dehydrogenase directs pyruvate into the TCA cycle, and as such, mitochondrial energy production is increased [67,68]. Another major driver of heightened mitochondrial metabolism in OIS is the oxidation of fatty acids [69], which are generated more in OIS cells through the action of fatty acid synthase [70]. Interestingly, OIS is sensitive to perturbation of nucleotide metabolism as well—oncogenic Ras-driven repression of a critical dNTP synthesis enzyme results in a lack of dNTP production, stalled replication forks, and, as a result, DDR [71].
The mechanisms of many other aspects of senescence, including inhibition of autophagy, morphological changes, and resistance to apoptosis, have been studied to a varied degree, but the precise cellular signal transduction pathways that control them are as yet unclear or remain controversial.
1.5. Contribution of Inflammaging to Senescence Surveillance
The clearance of senescent cells by immune cells known as “senescence surveillance” is a critical step for resolution of senescence. In a physiological context, the SASP promotes senescence surveillance, activating immune cells to drive the clearance of senescent cells and thus preventing tumor initiation [30,31]. With aging, the senescence of immune cells themselves, termed “immunosenescence”, avoids the elimination of senescent cells. Moreover, during aging, the SASP is responsible for the development of two different processes that lead to a chronic, sterile, highly self-reactive, systemic inflammatory condition termed “inflammaging” which enhances immunosenescence. The first process is thymic involution related to aging that leads to a decline in immune function or “immunosenescence”, causing insufficient production of naïve T cells and amplified oligo-clonal expansion of memory T cells with reduced immune repertoire diversity [72]. This immunosenescence compromises the clearance of senescent cells and exacerbates inflammation through the SASP, causing inflammaging [73]. Second, thymic involution leads to a reduced capacity of regulatory T cells (Tregs) for autoimmune suppression and to an amplified release of autoreactive T cells leading to tissue damage with chronic inflammation that exacerbates inflammaging [73,74].
2. Senescence in Cancer
One important question that still remains in the oncology field is the extent to which senescence can be placed as one of the drivers of neoplastic malignancy, as opposed to a consequence of the pathology itself. While some studies support senescence as an aftereffect willing to stop tumor growth as a proliferation-suppressive mechanism [75], others support the idea of senescence as a mechanism to create a favorable microenvironment through SASP for the tumor cells to be protected from immunoclearance [4]. In addition, it is important to highlight the dual role of immune cells in the context of cancer, as multiple lines of evidence indicate that immune inflammatory cells attracted by chemokines present in the SASP can be actively tumor-promoting, capable of fostering angiogenesis, cancer cell proliferation, and invasiveness through the secretion of proinflammatory components [76]. Thus, there is a very-heterogeneous landscape of opinions about the role of senescence in cancer. Moreover, the positive and negative aspects of cellular senescence sometimes just depend on the time senescent cells remain in the organism and whether they can be cleared by the immune system. Context-dependent outcomes and possible different scenarios regarding the relationship between senescence and cancer are discussed here.
2.1. Before Cancer Treatment: Age-Related Senescence and Cancer
Before cancer treatment the existence of senescence can have two different effects. On one hand, senescence can be beneficial when it is followed by immune clearance and tissue remodeling [7]. Moreover, senescence can prevent tumorigenesis by avoiding replication of precancerous cells [77]. In this context, the capacity of senescent cells entering cell cycle arrest was attributed to be a tumor-suppressive mechanism [4] and the loss of telomerase activity appeared to inhibit tumor progression in various mice models [6]. Senescence seemed to prevent uncontrolled replication of those precancerous cells that contain some oncogene activation or loss of tumor suppressor genes [8]. These observations suggested that cellular senescence does not simply arise from the accumulation of cell divisions but can also be prompted by stress inducers, such as oncogenic activation [10].
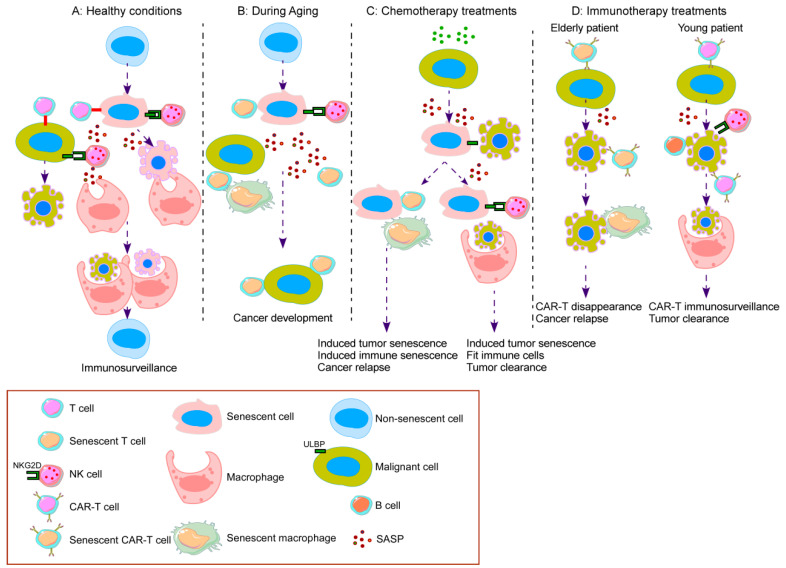
On the other hand, immunosenescence is a hallmark of aging which concerns both the innate and adaptive immune system. This deterioration of the immune system contributes to the increased susceptibility to neoplastic transformation with age. Senescence can present a detrimental outcome during aging when emerging senescent cells are not cleared by the immune system leading to their accumulation. This accumulation promotes SASP and a proinflammatory state within the tissue that can cause or facilitate the appearance of neoplastic pathologies, especially in the elderly population [20]. The linkage between age, cancer, and cellular senescence remains one of the examples why senescence could end in detrimental outcomes for patients [78]. The elderly population faces different degenerative and neoplastic pathologies derived from the loss of proper cellular function. Age-related diseases, such as cancer, occur on a background of dysfunctional tissue and moreover, the appearance and accumulation of senescent cells with age could worsen the tissue status. Although the emergence of ‘primary’ senescent cells is a physiologic process of tissue maintenance for regeneration, when these cells are not efficiently cleared by the immune system due to impaired or aged immune cells, the senescent cells accumulate within the tissue [7]. Those are known as ‘secondary’ senescent cells and are generated at sites of pathology after disease initiation and are thought to be responsible for disease amplification [79] (Figure 2).
Figure 2.
The complex and contrasting roles of senescence in the immune response to cancer. (A) Healthy conditions: proliferative cells react to stresses, such as DNA damage or oncogene activation, by inducing senescence to avoid malignancy. Immune cells, recruited by immunosupportive elements of the SASP, prevent the harmful effects of senescent cell accumulation by mediating their clearance in a process known as immunosurveillance. As a further protective mechanism, malignant cells are detected and eliminated by effector T cells and NK cells. (B) During aging: immunosenescence leads to loss of immune cell function and thus a diminished ability to detect and clear senescent cells. The SASP released from persistent senescent cells has multiple roles in cancer progression, including promoting tumor cell expansion and the development of an immunosuppressive tumor microenvironment. Cancer cells reinforce T cell senescence through metabolite competition and other mechanisms. (C) Chemotherapy treatments: senescence-inducing chemotherapeutic agents force malignant cells into senescence. An undesirable effect of these chemotherapies is the induction of immune cell senescence, resulting in lack of senescent cell clearance and increased risk of cancer relapse. Nonetheless, when immune cell senescence is avoided, immune cells effectively remove senescent cancer cells and tumor progression is prevented. (D) Immunotherapy treatments: senescence in CAR-T cells causes cell cycle arrest and loss of function, and is therefore associated with decreased in vivo persistence and cancer relapse. This problem is likely to be more pronounced in elderly cancer patients due to immunosenescence of many immune cell types. Nondysfunctional chimeric antigen receptor modified T (CAR-T) cells expand in patients to become an effective antitumor therapy. Functional NK cells, macrophages and B cells also have important roles to play in tumor clearance, making these cell types exciting future immunotherapeutic options.
2.2. After Cancer Treatment with Chemotherapy
Therapy-induced senescence (TIS) appears as a result of pharmacological intervention. TIS can end either in an advantageous outcome or an unwanted side effect [79]. The most dangerous side effect derived from TIS is cancer relapse. Cancer relapse comes from dormant cancer cells that survive therapy because they become apoptosis-resistant. Anticancer agents possess the ability to induce senescent-like phenotypes in some subset of tumor cells, creating a chemo-resistant niche. Although senescent cells remain in a durable and prolonged growth arrest, it may not be permanent. In fact, studies show that while replicative senescence maintains the irreversibility of senescence [80], those caused by premature stress such as TIS or OIS can reactivate the cell cycle and bring on cancer daughter cells more transformed than the original population, suggesting that the arrested state is not indefinite. TIS cells could be contemplated as the minimal residual disease and be the origin of relapse [81]. The main hypothesis is that some senescent cancer cells generated by therapeutic intervention can eventually escape from the arrest and acquire self-renewing properties. Saleh et al. [82] showed high expression of senescence markers in cancer cell lines after they escaped TIS induced by etoposide and doxorubicin. Some detrimental features observed in those cells include aggressiveness and stemness, making them a dangerous source of therapeutic failure and cancer relapse [83] (Figure 2).
2.3. After Cancer Treatment with Immunotherapy
Senescence occurrence after TIS is an explored pathway after several decades of using chemotherapeutic agents for cancer treatment. Nevertheless, more and more approaches are appearing to address anticancer treatment, such as immunotherapy [84]. Thus, the appearance of senescence after immunotherapy has not been explored as deeply as TIS. Recently, it has been identified that IFNγ and TNFα, two cytokines highly secreted after cell immunotherapy [85], induce senescence in different murine and human neoplastic diseases [86]. Moreover, we observed that natural killer (NK) cells, which are used as a source for immunotherapy [87], secrete a high variety of proinflammatory molecules, including matrix metalloproteases, heat shock proteins [88] and others present in the SASP.
3. Senescence in the Immune Response to Fight Cancer
The removal of senescent tumor and harmful nontumor cells by immune cells requires the inter-collaboration of different immune cell populations [9,30,89,90]. Therefore, restricting the proliferation of immune cells is likely to promote tumor growth and progression. This is particularly true for T and B lymphocytes whose function declines with aging as immunosenescence and further diminishes the natural anticancer response in older cancer patients [72]. The central hallmarks of immunosenescence include a poor immune response to novel antigens due to a decrease in naïve cells, an increase in memory cells, and also the chronic, low level of inflammation or ‘inflammaging’ with subsequent development of age-related diseases, such as cancer [73,91,92]. The cellular senescence that occurs in individual cells of the immune system is detailed below.
3.1. T Cells
T cell dysfunction occurs through the development of exhaustion, anergy, and/or senescence [93]. T cell senescence occurs naturally with aging as a result of multiple rounds of proliferation, loss of activity of the telomere-extending enzyme complex telomerase, and shortening of telomeres. As a result, this form of T cell senescence is common in older individuals but can also be found in younger people whose T cells have undergone excessive proliferation, such as in X-linked lymphoproliferative syndrome patients [94]. With aging, a thymic atrophy results in a decrease of functional naïve CD4+ and CD8 T cells [95]. This decline is more pronounced in males than in females [96] and is more noticeable for CD8 T cells with a peripheral oligo-clonal expansion of memory T cells, which in general provides a contracted T cell antigen receptor (TCR)-repertoire diversity inducing immunosenescence. In addition, aging causes CD4+ T cells to become longer-lived but functionally impaired with reduced proliferation and IL-2 production. These cells express reduced levels of the proapoptotic BCL-2 interacting mediator of cell death (Bim), which is associated with development of age-associated dysfunctions [97,98]. This T cell dysfunctionality is a common phenomenon occurring in cancer patients that leads to deficient antitumor immune responses.
Moreover, this replicative senescence is exacerbated by certain diseases, including cancer and chronic viral infections (especially cytomegalovirus (CMV)), whereby long-term exposure of antigen causes repeated T cell stimulation [99]. An alternative method of senescence induction in T cells occurs that is independent of telomere length. This premature senescence is likely to be triggered by mechanisms that provoke the DNA damage response, such as excessive ROS [100,101]. Correspondingly, metabolic competition by Tregs can promote DNA damage in effector T cells and consequently induce senescence [102,103,104]. It appears that both types of T cell senescence are immunosuppressive in the tumor microenvironment [105,106].
Senescent T cells are found within CD4+ and CD8+ T cell compartments that have lost expression of CD27 and CD28. Absence of the costimulatory molecules CD27 and CD28 in T cells corresponds with short telomeres and an upregulation of CD57 and KLRG1 [100,107,108,109,110]. Further classifying CD27-CD28- T cells with CD45RA expression identifies a CD45RA+ or TEMRA population that has multiple characteristics of senescence, including decreased proliferation, an inability to upregulate telomerase activity, and higher levels of γH2AX [100,109,111]. Interestingly, these EMRA T cells do not have shorter telomeres than CD27-CD45RA- cells and the senescence phenotype is reversible, which suggests that their senescence is not purely telomere-dependent [109,112,113]. Furthermore, CD27-CD28- T cells are not resistant to apoptosis, in contrast to senescent fibroblasts, and in fact, they are more prone to activation-induced apoptosis [112,114]. Long term T cell cultures are, however, resistant to apoptosis, possibly because they are in a later stage of senescence than freshly isolated senescent T cells, or because they have been selected during the in vitro T cell expansion [115,116]. Senescent T cells also persist in vivo due to the survival signals they receive, and senescent T cells from rheumatoid arthritis patients also appear to be apoptosis-resistant, which could have implications for overall T cell responses in elderly people with cancer.
Senescent T cells lack proliferative ability and display features of cell cycle arrest at the G1/S phase transition [117]. In terms of effector function, CD27-CD45RA+ senescent T cells are very much active, retaining the ability to release inflammatory cytokines (including IFNγ and TNFα) and produce cytotoxic molecules (granzyme B and perforin) [100,110,111,114]. Interestingly, in CD4+ T cells, IL-2 production is high in CD27-CD45RA+ cells, but for CD8+ T cells, the opposite is true [113,114]. Senescent CD8+ T cells also release a cocktail of molecules that shows similarities with the SASP generated by other senescent cell types [118]. It is also important to remember that senescent T cells remain metabolically active, although senescence has a profound impact on cellular metabolism and vice versa. The ability to uptake nutrients and mitochondrial mass directly impacts on T cell senescence, as evidenced when comparing the more senescent/less proliferative CD8+ TEMRA cells with less senescent/more proliferative CD8+ TEM cell or CD4+ TEMRA cells [100,119].
In many types of cancer, the presence of senescent-like T cells has been associated with malignancy and poor prognosis [120,121,122]. Indeed, tumor-infiltrating lymphocytes (TILs) have short telomeres and lack telomerase activity [123]. Although not well-researched, it has been shown that tumor cells themselves can induce a senescent-like phenotype in human T cells [120,124,125]. In a nonsuppressive environment, CD8+ TILs react to tumor antigens by proliferating, differentiating, and producing effector molecules. However, senescence causes downregulation of costimulatory molecules and, at least in some cases, effector molecules [126]. Moreover, senescent T cells repress the activity of other immune cells in the tumor microenvironment, making it even more immunosuppressive [102,103].
Recently, it has been shown that senescent T cells are increased in different hematologic malignancies, including leukemias, lymphomas, and multiple myeloma (MM) [122,127,128]. In chronic lymphocytic leukemia (CLL), patients with a CD4+:CD8+ T cell ratio below 1 had more senescent-like CD8+ T cells and a poorer prognosis [122]. A comprehensive analysis of T cells from acute myeloblastic leukemia (AML) patients showed an increase in senescent CD8+ T cells compared to healthy controls and a correlation between the expression of senescent markers and the patient response to chemotherapy [129]. Similarly, T cell senescence is associated with therapy response in MM. Specifically, when treated with autologous stem cell transplantation (ASCT), relapsed patients had drastically higher percentages of CD28-CD57+ cells in both the CD8+ and CD4+ T cell compartments compared to patients showing a complete response [130].
For solid tumors, one of the major mechanisms used by tumor cells to cause T cell dysfunction is to outcompete them for essential nutrients, especially glucose, in the tumor microenvironment [131]. Glucose deprivation induces senescence in T cells by provoking the DDR, which in turn, initiates AMPK/p38 MAPK activation [104,106,125]. In addition, tumor cells produce metabolites that induce senescence in T cells. For example, tumor cells initiate inhibitory signaling pathways in T cells by secreting adenosine, which binds to the T cell adenosine receptor, or by transferring cyclic AMP (cAMP) through gap junctions [125,132]. Another interesting senescent-promoting process that tumor cells may utilize is the release of protein- and RNA-containing exosomes, which when taken up by T cells, cause loss of CD27/CD28 expression, proliferation and IFNγ production [133]. Finally, suppressive immune cells in the tumor microenvironment promote effector T cell senescence through a number of mechanisms, such as the production of proinflammatory cytokines and nutrient competition. For instance, CD4+CD25hi Tregs and tumor-derived γδ Tregs cause DNA damage-associated senescence in effector T cells through nutrient competition, resulting in the activation of a senescence signaling network involving ATM, MAPKs (ERK1/2 and p38 MAPK), and STAT1/3 protein [103,104]. However, there are still many unknowns regarding the interplay between T cell senescence and cancer, making this area an exciting prospective area of study.
Mechanistically, T cell senescence is regulated by signaling networks downstream of the TCR, costimulatory molecules, cytokine receptors, and inhibitory receptors. p38 MAPK is an important promoter of the senescent phenotype [100,109,134], and there are at least three pathways through which p38 MAPK can be regulated: canonical, alternative, and AMPK-mediated [134]. The canonical pathway in T cells, activated by the TCR, CD28 engagement and cytokines such as TNFα and IFNα, initiates the classical MAPK cascade [100,135,136]. The alternative pathway occurs downstream of TCR ligation whereby ZAP70 induces p38 MAPK autophosphorylation [136,137]. However, senescent T cells, which lack CD28 and TCR signalosome components, do not engage the canonical or alternative signaling cascades to regulate p38 MAPK activity [138,139]. Instead, they take advantage of a third p38 MAPK pathway in which low glucose or DNA damage, sensed by sestrins, activate AMPK, leading to TAB1-dependent autophosphorylation of p38 MAPK [138,140]. Inhibition of p38 MAPK or disruption of this pathway in senescent CD4+ or CD8+ T cells increases hTERT expression, telomerase activity, telomere length, proliferation, and TNFα secretion [100,109,138,140]. Furthermore, in senescent CD8+ T cells, p38 MAPK controls the SASP, autophagy, and mitochondrial mass [100,118], which is critical for the metabolic contribution to T cell senescence [119].
As well as p38 MAPK, the MAPKs ERK1/2 and Jnk also enhance T cell senescence using a similar mechanism of activation by AMPK, but without a dependence on TAB1 [140]. In addition, a recent study has discovered that CD8+ senescent-like T cells have deficient TCR activity, but adopt innate signaling mechanisms normally found in NK cells [139]. In detail, stress-sensing sestrins control this switch from TCR to NK cell signaling by regulating the expression of the NK receptor NKG2D, which then forms a complex with the adaptor molecule DAP12 to control effector molecule production and killing ability [139].
3.2. B Cells
B cells known as tumor-infiltrating B cells (TIBs) commonly permeate solid tumor microenvironments, where they can function to either inhibit or enhance tumor progression [141]. The antibodies produced by TIBs act to kill tumor cells by forming conjugates with antigens and activating complement-mediated lysis, by flagging tumor cells for destruction by other immune cells, or by stimulating the uptake of tumor cells by dendritic cells, thus activating T cells through presentation of tumor antigens [141]. In addition, TIBs act as tumor antigen-presenting cells themselves, and produce immunostimulatory cytokines and chemokines. On the other hand, B cell-derived molecules can directly enhance tumorigenesis or dampen the antitumor responses of other immune cells. As such, lymphotoxin secreted by TIBs stimulates growth in prostate cancer cells [142], and antibodies produced by B cells can form circulating immune complexes that induce tumor-resident myeloid cells to promote angiogenesis and carcinogenesis [143]. Furthermore, a subset of TIBs known as regulatory B cells suppress cytolytic immune cells through production of IL-10 and promote the formation of Tregs by secreting TGFβ [144]. Although the effect of aging on B cell tumor responses has not been studied in detail, it is tempting to hypothesize that B cell immunosenescence and cellular senescence mechanisms could dampen B cell function in cancer, particularly through the reduction of antibody-driven responses and cytokine production.
3.3. NK Cells
NK cells are important antitumor cells of our innate immune system whose activity is mediated by a complex array of activating and inhibitory receptors and their ligands on tumor cells [87]. However, NK cells are involved in many other biological processes, such as immune regulation, antimicrobial immune responses [87], and clearance of senescent cells. Therefore, a decrease in NK cell function that accompanies aging and/or cancer will have wide implications. It is important to consider the short lifespan of NK cells when talking about NK cell senescence. Estimates of the in vivo half-life of NK cells are possibly less than 10 days in humans [145], though advances in cell identification have shown a persistence of months for specific NK clones in rhesus macaque [146]. Currently, whereas for T cells there are defined phenotypes to define dysfunctional T cells differentiating exhaustion, anergy, and senescence, these phenotypes have not been clearly defined for NK cells [147]. However, given the universality of the phenomenon of senescence, NK senescence should be studied to define its existence in NK cells.
The possibility of NK cell senescence during aging has been observed with an increase in the total NK cell number with aging being the majority the CD56dim NK subset. Importantly, these NK cells show decreased cell proliferation ability [148]. Usually, NK effector cytotoxic function is defined by CD107a degranulation, IFNγ, and granzyme B production. Regarding functionality and phenotype with aging different studies have defined some changes. Specifically, from newborns to adults there is a transition from a KIR-NKG2A+ to a KIR+NKG2A- NK cell repertoire and an increase in the frequency of the senescence marker CD57 in NK cells in the elderly, which associates with human CMV infection [149]. Another study has also observed an increase in the cytotoxic NK cell subset CD16+CD56dim with greater expression of CD57, and a decrease in the immunoregulatory NK cell subset CD16-CD56bright [150].
In the field of cancer, there are not conclusive studies to define NK cell senescence in cancer patients. However, healthy allogeneic NK cells, such as cord-blood-derived NK cells or haploidentical NK cells are currently being used as a source of immunotherapy for the treatment of cancer patients [87]. Most groups use in vitro approaches to expand NK cells to obtain a high number of NK cells to treat patients. It could be expected that this approach, by generating high numbers of NK cells, could give rise to NK cell senescence as a result of continued in vitro proliferation. In vitro techniques to expand NK cells are able to prolong NK cell lifespan, a phenomenon observed using K562-based artificial antigen presenting cells (aAPC) with membrane-bound IL-15 or IL-21. The use of K562-based aAPC with membrane-bound IL-21 has provided superior results and interestingly, this method causes increased telomere length in NK cells [151]. However, these in vitro-expanded NK cells do not persist long in patients [152], suggesting that the enhancement of NK cell in vitro persistence and proliferation might be inducing NK cell senescence due to continuous proliferation. The phenotype of the different NK cell clones after expansion with K562-based aAPC with membrane-bound IL-21 has been investigated with no conclusive results. After 2–8 weeks of culture, NKG2A was increased and CD57 was lost. No difference in IFNγ production, NK proliferation, or NK cell cytotoxicity between CD57 positive and negative subsets was observed [153]. However, other studies, using K562 aAPC, have shown a difference evaluating the CD57 subsets and observed that CD57+ NK cells had greater IFNγ production, greater cytotoxicity, and lower proliferation after exposure to target cells than the CD57- subset [154]. Regarding SASP secretion, our group recently described that cord-blood-derived NK cells expanded with K562-based aAPC secrete a high variety of proinflammatory molecules including proteases, such as cathepsins and matrix metalloproteases [88] which are described to be part of the SASP. Further studies should be performed to find out whether these in vitro techniques to expand NK cells are actually increasing senescence in NK cells.
3.4. Macrophages
Aging seems to hold an undesirable effect on macrophages as well, affecting their normal functionality. It has been observed that macrophages from old mice fail to lyse tumor cells and produce nitric oxide (NO). Moreover, aged macrophages from mice are less responsive to IFNγ, which can participate in the dysregulation of immune function in general terms [155]. Macrophages do not exhibit a stable terminally differentiated state and they represent a highly plastic population of immune cells that readjust to changing environmental circumstances. Proinflammatory cytokines such as IL-1β, IL-6, and TNFα are increased and observed in the serum of elderly individuals, suggesting the existence of inflammaging [156]. It is believed that a proinflammatory M1 macrophage phenotype prevails at tumor sites of chronic inflammation, promoting tumor survival. Once the tumor has been established, the immunosuppressor microenvironment drives macrophages towards an M2 phenotype with immunosuppressive features, which indicates a worse prognosis. In elderly mice, M2 macrophages have been found to be increased in bone marrow and spleen when compared to young mice [157]. Regarding senescent cell clearance, at all stages macrophages are a key innate immune phagocytic cell and are generally recruited by SASP molecules [158,159]. Immature myeloid cells (iMCs) characterized by CD11b+Gr1+ can differentiate into dendritic cells (DCs), neutrophils, or macrophages. However, in the presence of tumor-derived factors, they lose their capability to differentiate, supporting an immunotolerant environment [160]. Eggert et al. [39] proved that the production of CCL2 by senescent cells is able to recruit iMCs CD11b+Gr1+ cells that subsequently mature into macrophages for senescence clearance. In contrast, the absence of macrophage differentiation results in the accumulation of senescent cells [160].
3.5. Other Immune Cells
The deleterious effects of aging on immunosenescence in other immune cell types, such as DCs, invariant natural killer T (NKT) cells, and neutrophils, has been investigated [89], but very little information is available regarding the mechanism of cellular senescence that occurs in these immune cells. Interestingly, one study has reported that DCs cultured with tumor-derived γδ Tregs exhibit senescent-like features, including SA-β-gal induction and loss of function, but not the development of a SASP [103]. It is possible that mature terminally differentiated immune cells are subject to cellular senescence as well. Although unable to undergo replicative senescence as they are non-proliferative, it has been shown that terminally differentiated cells, such as neurons and hepatocytes, can exhibit signs of stress-induced senescence [161]. Intriguingly, lack of the oxidative-burst-induced DDR in neutrophils, which normally leads to apoptosis downstream of ATM signaling and ROS production, allows for the overproduction of proinflammatory cytokines, including typical SASP component IL-8, p38 MAPK activation, and repressed apoptosis—hallmarks of cellular senescence [162].
4. Clinical Relevance of Senescence in Immunotherapy: A Balance between Induction and Reversal of Senescence
4.1. Induction or Reversal of Senescence
The development of cancer treatments targeting senescence should consider both sides of senescence. Initially, the ability for a cell to induce senescence as an alternative to aberrant proliferation is a major tumor-suppressive mechanism. However, the long-term effects of senescent cancer cells are often unfavorable as SASP secretion can contribute to an enhancement of local and systemic inflammation exacerbating the side effects of cancer treatment and promoting tumor relapse and metastasis. Additionally, the senescence of noncancerous cells can aid in the generation of a procancer microenvironment and promote tumor progression. Moreover, senescent tumor cells might represent one avenue whereby they evade the cytotoxic impact of therapy, allowing for prolonged survival in a dormant state, with the potential to recover self-renewal capacity and contribute to relapse. In the case of immune cells, cellular senescence in T cells can diminish their anticancer functions and moreover, a higher number of senescent T cells prior to chemotherapy is associated with increased risk of chemotherapy-induced fatigue [82]. On the other hand, senescence may promote infiltration of immune cells to the tumor through chemokine and proinflammatory molecules secreted within the SASP [163,164]. Importantly, prosenescent therapies achieve tumor growth suspension but do not cause regression or elimination on their own, as it involves the need for a two-step therapy [165] that includes an optimal immune system capable of clearing senescent cells and eliminating residual cancerous cells [166]. As such, adoptive immune cell transfer may prove to be a useful adjuvant therapy to promote the clearance of senescent cells.
Taken all together, combination and optimization of immune-modulatory approaches with senescence-enhancing or senolytic agents [167,168,169,170] appear as an unexplored area of research for the treatment of neoplastic diseases depending on the stage of the disease. As such, an increasing number of senolytic agents that induce apoptosis in senescent cancer cells have been studied, including dasatinib and quercetin [168] which improve clinical symptoms in pulmonary fibrosis, in aging, and after radiotherapy [171,172,173]. Others include BCL-2 family inhibitors, such as Navitoclax (ABT-263) [174]; the flavonoid fisetin [167], FOXO4-p53 interfering peptides [169], HSP90 inhibitors [170], FOXO4 D-retro reverse peptide, the mTOR inhibitor rapamycin, and antibodies targeting SASP factors such as anakinra (anti-IL-1β), tocilizumab (anti-IL6R), and infliximab (TNF-α antibody) [20].
4.2. Clearance of Senescent Cancer Cells by NK Cells
NK cells, which can be used in the clinic as a source of immunotherapy [87], play an important role in the removal of tumor senescent cells as demonstrated by different studies. In this regard, perforin-1 is crucial in this clearance; mice deficient in perforin-1 lack functional T, NK, or NKT lymphocytes and accumulate more senescent cells in multiple organs with signs of inflammation and overexpression of SASP. In addition, these mice exhibit premature aging in different organs and a reduced median lifespan [175].
NK cell receptors are involved in the NK-mediated clearance of senescent cells. NKG2D is the most studied receptor involved in these mechanisms playing a relevant role in the clearance of p53-induced senescent cells in hepatocellular cancer [176]. However, opposing roles have been demonstrated depending on the type of tumor, such as lung adenocarcinoma, where NK cells limited the clearance of senescent tumor cells and instead, infiltration of monocytes, neutrophils, and interstitial macrophages was induced [177]. Importantly, NKG2D ligands, such as MICA, MICB, and ULBP proteins, are expressed in a variety of tumor cells, which enable their targeting by NK cells [87]. Certain chemotherapies which induce senescence [2] cause over-expression of these NKG2D ligands in different types of tumors making their NK cell-mediated removal possible [178,179].
However, the role of chemotherapy in the induction of oxidative stress, SASP and senescence and in the clearance of senescent cells is controversial and can lead to opposite roles also for NK cells [4,180]. In this regard, DNA-damage chemotherapy induces secretion of SASP in different types of resistant senescent tumor cells leading to inhibition of clearance of senescent cells by NK cells. This mechanism is performed through proteases present in the SASP which cleave and release soluble NKG2D ligands [181,182,183]. These proteases include the families of matrix metalloproteinases and a disintegrin and metalloproteases (ADAMs). Among them, ADAM10 cleaves MICA, MICB, and ULBP-2 in different types of cancer cells and its increased expression correlates with cancer progression in MM [184], malignant pleural mesothelioma [185], oral squamous cell carcinoma [186], and gastric cancer [187]. Of note, inhibition of these proteases recovers immune recognition of tumor cells by NK cells [188]. On the other hand, studies demonstrated the beneficial effects of SASP components in the clearance of senescent cells, such as CCL2 secretion by senescent tumor cells in liver carcinoma which induces NKG2D ligand expression on tumor cells to activate NK-mediated clearance through NKG2D [176]. It is important to highlight that SASP drives inflammation and inflammation is recognized as one of the drivers of cancer. This association has been observed for NKG2D where in a context of exacerbated inflammation in hepatocellular carcinoma, NKG2D accelerated tumor growth [189]. Moreover, in previous stages to cancer such as hepatic fibrosis, NKG2D binding to their ligands in senescent cells induces clearance of senescent cells by NK cells, facilitating the resolution of fibrosis [190]. However, in certain cases a downregulation of NKG2D can limit these effects leading to liver fibrosis [191].
In advanced non-small-cell lung cancer, the chemotherapy drug pemetrexed induces senescence and increases in vitro sensitivity to NK-cell-mediated clearance of tumor cells through upregulation of ULBP proteins [192].
The inhibitory NK cell receptor NKG2A limits the clearance of senescent cells by NK cells. This mechanism is mediated through the SASP which induces HLA-E overexpression in senescent cells of human skin and melanocytic nevi during aging leading to NK cell inhibition [193].
Additional mechanisms involved in the NK-mediated clearance of senescent cells include other receptors and production of cytokines that promote macrophage activation. To give an example, in liver fibrosis, senescent cells are preferentially killed through NK cell granule exocytosis with IFNγ production protecting against liver fibrosis. In this context, granule exocytosis appears to be the preferential cytolytic mechanism instead of death receptor–death ligand interaction. The DCR2 receptor in senescent cells acts as a competitive inhibitor of death receptor signaling by death ligands, such as Fas or TRAIL, inhibiting the NK cell granule exocytosis pathway [89]. IL-15 is also involved in elimination of senescent cells after chemotherapy-associated senescence. Chemotherapy induces SASP containing IL15RA and IL-15 in MM and increased expression of IL-15/IL15RA on the membrane of senescent myeloma cells. These events allow the functional trans-presentation of IL-15 to neighboring NK cells leading to NK cell recognition and cytotoxicity [194]. Moreover, additional NK mechanisms such as antibody-dependent cellular cytotoxicity (ADCC) are activated after chemotherapy to eliminate senescent breast cancer cells, where doxorubicin enhances senescence through SASP secretion leading to a perforin-dependent increase in ADCC by NK cells [195].
The role of oxidative stress in the NK cell-mediated clearance of senescent tumor cells has been studied in breast cancer where elevated granzyme F levels and the NK cell marker NKTR correlated with recruitment of NK cells into tumors and their clearance. These activities are impaired by upregulation of the protein acyltransferase DHHC3 which supports tumor growth and metastasis. DHHC3 expression correlates with diminished patient survival in breast cancer and six other human cancer types, an effect which is mediated through a decrease in senescence and oxidative stress in tumor cells. DHHC3 ablation in mammary tumor cell xenografts increases oxidative stress promoting cellular senescence with SASP secretion that leads to recruitment of macrophages and NK cells with reduced sizes of metastasis [196].
The role of senolytics might be controversial for NK cells. For instance, mTOR and TNFα which are targets of senolytic drugs [20] are both required for NK cell activity. It has been shown that mTOR inhibition through peroxisome proliferator-activated receptor causes lipid accumulation in NK cells, blunting their antitumor response [197]. mTOR is also critical for IL-15 signaling in NK cells, their development, and peripheral activation [198]. Therefore, senolytic drugs targeting these pathways would potentially negatively impact NK cells and should be studied in the future.
4.3. Reversal of T Cell Senescence
In the last few years, a high number of cancer immunotherapies have been developed that target CD8+ effector T cells. Currently, the most widely used immunotherapies for cancer patients are checkpoint inhibitors, specifically monoclonal antibodies against PD-1/PD-L1 and CTLA-4, that work by blocking one of the pathways that contribute to CD8+ T cell exhaustion [199]. Despite the incredible ability of these therapies to treat even late-stage cancer, 40–85% of patients treated with checkpoint inhibitors do not exhibit a sustained clinical response and many present with adverse side effects caused by autoimmune responses [200]. A novel immunotherapeutic strategy could be to reverse T cell senescence, because as explained in the previous sections, senescent T cells have an impaired ability to clear tumors and can even promote tumor progression. Indeed, senescent T cells accumulate in a multitude of different cancer types, and the increase in immune cell senescence in the elderly could be linked with their increased likelihood to develop cancer [120]. Moreover, there is evidence that treatment with checkpoint inhibitors can increase the presence of senescent T cells [201].
Among the most obvious targets to reverse T cell senescence is p38 MAPK due to its position at the heart of senescence signaling in CD4+ and CD8+ T cells. Beyond T cell senescence, impeding p38 MAPK activity is appealing for cancer treatment as a mechanism to prevent tumor cell SASP development [20]. Inhibitors that target the p38α or both the p38α and p38β MAPK isoenzymes (SCIO-469 and ralimetinib, respectively) have been used in clinical trials for myelodysplastic syndrome, MM, breast cancer, ovarian cancer, glioblastoma, and a range of advanced/metastatic cancers, albeit with mixed results [202,203,204]. Overall, the results from these trials suggest that p38α/β inhibitors are safe, and that when used in combination with chemotherapies, are promising cancer treatments.
In T cell senescence signaling, p38 MAPK lies downstream of sestrins and AMPK [138,140], which could be other targets for senescence-reversing therapy. However, it is unclear how useful AMPK inhibitors would be in the context of an in vivo tumor as some studies found that AMPK was tumorigenic, but in other cases it acts as a tumor suppressor [205,206]. In any case, there are no specific AMPK inhibitors that are currently available [205,206]. Inhibition of sestrins, which activate AMPK, could also reduce effector T cell senescence, but could equally be detrimental because sestrins promote senescent-like CD8+ T cells to adopt an NK cell-like phenotype, which could be beneficial in tumor clearance [139].
Sestrins and AMPK regulate the ERK and Jnk MAPKs as well as p38 MAPK in senescent T cells [140]. Inhibition of MEK1/2, the MAPKKs which activate ERK1/2, prevented Treg- and tumor cell-induced responder T cell senescence in an in vivo model [102,104,125]. Three MEK1/2 inhibitors, trametinib, cobimetinib, and binimetinib, are currently approved to treat advanced metastatic melanoma where the tumor has a B-Raf V600E/K mutation, with the aim of inhibiting the constitutively active Raf/Ras/MEK/ERK pathway. As well as dampening oncogenic signaling in tumor cells, MEK1/2 inhibitors increase the number of CD4+ and CD8+ TILs without restraining their effector function [207,208,209]. In the clinic, MEK inhibitors are often used in combination with a B-Raf inhibitor, although multiple studies in different cancers have shown that they augment checkpoint inhibitor treatment as well [208,209,210,211,212], further highlighting their therapeutic potential. The extent to which MEK inhibitors control T cell senescence reversal in cancer patients, and how much that contributes to their therapeutic benefit, remains to be elucidated.
Another way to prevent T cell senescence in the tumor microenvironment is to hinder cancer cell nutrient uptake. Tumor cells are highly glycolytic and depend on a constant supply of glucose to feed their hyperactive metabolism, which in the tumor microenvironment, deprives effector T cells of glucose [131], thus promoting T cell senescence. As such, the use of drugs that obstruct glycolysis, such as glucose transporter blockers or hexokinase inhibitors, would harm tumor cells, but could also increase T cell senescence. Therefore, an interesting therapeutic approach could be to combine the use of reversible glycolysis inhibitors, which would generate a glucose-rich tumor microenvironment, with subsequent autologous T cell therapy, which would likely be more active in killing the tumor and resisting senescence.
An alternate strategy could be to activate TLR8 signaling in tumor cells and Tregs. TLR8 agonists decrease production of cAMP in tumor cells and inhibit glycolysis in tumor-derived Tregs, but do not interfere with effector T cell metabolism, thus preventing senescence of tumor-killing TILs without limiting their function [106,125]. In a murine melanoma model, TLR8 ligands diminished the inhibitory effect of tumor cells and Tregs on effector T cells, resulting in reduced tumor size [106,125,213,214]. Clinical trials testing combination therapies containing the TLR8-specific agonist motolimod (VTX-2337) for the treatment of various tumor types have been published [215,216,217,218,219,220], and more are currently ongoing or planned (clinicaltrials.gov; NCT03906526, NCT02431559 and NCT04272333).
Finally, chimeric antigen receptor modified T cells (CAR-T cells) have appeared in recent years as a successful immunotherapy for certain hematological malignancies [84]. However, some patients treated with CAR-T cells relapse, which is partially caused by a lack of functional persistence following treatment administration [221]. As the effectiveness of CAR-T cell therapy is dependent on the fitness of the patients’ T cells from which they are derived [222], the lack of sustained functional capacity of CAR-T cells could be due to the inflated levels of T cell senescence observed in cancer patients [107]. Furthermore, many cancer patients, including those with hematological malignancies, are elderly, meaning that they are likely to exhibit immunosenescence. In the case of anti-BCMA CAR-T cell clinical trials in MM, individuals receive CAR-T cell therapy after relapse, but at this stage of disease progression, the patient T cells are highly senescent and have a phenotype associated with a diminished response to anti-BCMA CAR-T cell treatment [130,222,223]. Therefore, therapeutic interventions that reverse CAR-T cell senescence, either in the process of their development or in vivo, would be of great benefit.
4.4. Potential Use of B Cells in Immunotherapy and Reversal of B Cell Senescence
B cells clearly play an important role in the progression of many cancer types [141], and should therefore not be overlooked when discussing novel immunotherapies. Although some studies have shown that B cells are able to promote tumor progression and abrogate immunogenic chemotherapies [141,144], there is a clear correlation between increased TIBs and better patient outcome [141]. In fact, recent studies have shown a link between better response to immunotherapy and higher numbers of TIBs, and moreover, that this positive relationship is caused by the formation of tertiary lymphoid structures which promote T cell activation [224,225,226]. Interestingly, Helmink et al. found that there was a trend for fewer late memory CD27- IgD- B cells, which correspond to the cells that display senescent-like features, in the tumors of responders compared to nonresponders of immune checkpoint blockers [224], suggesting that fewer senescent B cells could promote immunotherapy response.
A possible strategy to reverse B cell senescence could be to enhance autophagy. Reduced autophagy levels in old B lymphocytes corresponds with compromised B cell responses, but treatment with spermidine restores autophagy in old B cells so that they regain function [227].
4.5. Retuning the Tumor Microenvironment to Promote Macrophage-Mediated Cancer Cell Clearance
In the case of macrophages, the repolarization from M2 to M1 seems to be more important than senescence reversal, as M1 appears to be the phenotype capable of proinflammatory actions to initiate clearance of senescent cells [228]. The reversal of immune incompetence could be prevented by the restoration of the tumor microenvironment into one with a less immunosuppressive profile. There are studies that suggest a different role of molecules present in the SASP depending on the tumor stage [39]. Thus, in models of hepatocellular carcinoma, early stages of senescent precancerous hepatocytes secrete CCL2 via their SASP which acts as a tumor suppressive mechanism promoting the recruitment of macrophages to remove senescent cells. However, in developed tumors, senescent peritumoral tissue induces NK cell inhibition via CCL2-CCR2, promoting the growth of hepatocellular carcinoma. Moreover, CCL2 inhibits maturation of monocytes to macrophages causing more accumulation of senescent cells and contributing to tumor immune escape. This study demonstrates a dual context-dependent function of SASP components depending on the stage of the disease [39].
5. Conclusions
To summarize, in recent years, senescence is attracting increased attention in the field of cancer treatment as new studies are revealing its complex roles in cancer prevention, development, and progression. Whereas originally, in physiological conditions, senescence was described as a tumor suppressor mechanism, additional studies showed that damage-associated senescence after chemotherapy treatment can both hinder and promote cancer progression. The SASP has a crucial role in these seemingly contradictory outcomes and in the promotion of senescence in cells of the immune system that are crucial for the removal of tumor cells. Immunotherapy requires fit immune cells in order to succeed, and the development of novel cancer therapies should consider treatments that do not negatively affect immune cells, and moreover, that the achievement of senescence reversal in immune cells is likely to provide effective immunotherapy treatments.
Author Contributions
A.M.B., M.B. and B.M.-A. wrote and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Instituto de Salud Carlos III, grant number PI17/01043, and the “la Caixa” Foundation, grant number P-CP042702.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Wang E. Senescent Human Fibroblasts Resist Programmed Cell Death, and Failure to Suppress bell Is Involved. Cancer Res. 1995;55:2284–2292. [PubMed] [Google Scholar]
- 2.Gorgoulis V., Adams P.D., Alimonti A., Bennett D.C., Bischof O., Bishop C., Campisi J., Collado M., Evangelou K., Ferbeyre G., et al. Cellular Senescence: Defining a Path Forward. Cell. 2019;179:813–827. doi: 10.1016/j.cell.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Hayflick L., Moorhead P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Mancera P.A., Young A.R., Narita M. Inside and out: The activities of senescence in cancer. Nat. Rev. Cancer. 2014;14:547–558. doi: 10.1038/nrc3773. [DOI] [PubMed] [Google Scholar]
- 5.Collado M., Blasco M.A., Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Suarez E., Samper E., Flores J.M., Blasco M.A. Telomerase-deficient mice with short telomeres are resistant to skin tumorigenesis. Nat. Genet. 2000;26:114–117. doi: 10.1038/79089. [DOI] [PubMed] [Google Scholar]
- 7.Munoz-Espin D., Serrano M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014;15:482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 8.Sieben C.J., Sturmlechner I., van de Sluis B., van Deursen J.M. Two-Step Senescence-Focused Cancer Therapies. Trends Cell Biol. 2018;28:723–737. doi: 10.1016/j.tcb.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xue W., Zender L., Miething C., Dickins R.A., Hernando E., Krizhanovsky V., Cordon-Cardo C., Lowe S.W. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Micco R., Fumagalli M., Cicalese A., Piccinin S., Gasparini P., Luise C., Schurra C., Garre M., Nuciforo P.G., Bensimon A., et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- 11.McHugh D., Gil J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018;217:65–77. doi: 10.1083/jcb.201708092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georgakopoulou E.A., Tsimaratou K., Evangelou K., Fernandez Marcos P.J., Zoumpourlis V., Trougakos I.P., Kletsas D., Bartek J., Serrano M., Gorgoulis V.G. Specific lipofuscin staining as a novel biomarker to detect replicative and stress-induced senescence. A method applicable in cryo-preserved and archival tissues. Aging (Albany N. Y.) 2013;5:37–50. doi: 10.18632/aging.100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yosef R., Pilpel N., Tokarsky-Amiel R., Biran A., Ovadya Y., Cohen S., Vadai E., Dassa L., Shahar E., Condiotti R., et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat. Commun. 2016;7:11190. doi: 10.1038/ncomms11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcotte R., Lacelle C., Wang E. Senescent fibroblasts resist apoptosis by downregulating caspase-3. Mech. Ageing Dev. 2004;125:777–783. doi: 10.1016/j.mad.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Sanders Y.Y., Liu H., Zhang X., Hecker L., Bernard K., Desai L., Liu G., Thannickal V.J. Histone modifications in senescence-associated resistance to apoptosis by oxidative stress. Redox Biol. 2013;1:8–16. doi: 10.1016/j.redox.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandra T., Ewels P.A., Schoenfelder S., Furlan-Magaril M., Wingett S.W., Kirschner K., Thuret J.Y., Andrews S., Fraser P., Reik W. Global reorganization of the nuclear landscape in senescent cells. Cell Rep. 2015;10:471–483. doi: 10.1016/j.celrep.2014.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sati S., Bonev B., Szabo Q., Jost D., Bensadoun P., Serra F., Loubiere V., Papadopoulos G.L., Rivera-Mulia J.-C., Fritsch L., et al. 4D Genome Rewiring during Oncogene-Induced and Replicative Senescence. Mol. Cell. 2020;78:1–17. doi: 10.1016/j.molcel.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah P.P., Donahue G., Otte G.L., Capell B.C., Nelson D.M., Cao K., Aggarwala V., Cruickshanks H.A., Rai T.S., McBryan T., et al. Lamin B1 depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape. Genes Dev. 2013;27:1787–1799. doi: 10.1101/gad.223834.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Acosta J.C., Banito A., Wuestefeld T., Georgilis A., Janich P., Morton J.P., Athineos D., Kang T.W., Lasitschka F., Andrulis M., et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013;15:978–990. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faget D.V., Ren Q., Stewart S.A. Unmasking senescence: Context-dependent effects of SASP in cancer. Nat. Rev. Cancer. 2019;19:439–453. doi: 10.1038/s41568-019-0156-2. [DOI] [PubMed] [Google Scholar]
- 21.Bottazzi B., Riboli E., Mantovani A. Aging, inflammation and cancer. Semin. Immunol. 2018;40:74–82. doi: 10.1016/j.smim.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez-Segura A., de Jong T.V., Melov S., Guryev V., Campisi J., Demaria M. Unmasking Transcriptional Heterogeneity in Senescent Cells. Curr. Biol. 2017;27:2652–2660.e4. doi: 10.1016/j.cub.2017.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saxton R.A., Sabatini D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salminen A., Kauppinen A., Kaarniranta K. Emerging role of NF-kappaB signaling in the induction of senescence-associated secretory phenotype (SASP) Cell Signal. 2012;24:835–845. doi: 10.1016/j.cellsig.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Feldman N., Rotter-Maskowitz A., Okun E. DAMPs as mediators of sterile inflammation in aging-related pathologies. Ageing Res. Rev. 2015;24:29–39. doi: 10.1016/j.arr.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Takasugi M., Okada R., Takahashi A., Virya Chen D., Watanabe S., Hara E. Small extracellular vesicles secreted from senescent cells promote cancer cell proliferation through EphA2. Nat. Commun. 2017;8:15729. doi: 10.1038/ncomms15728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong W., Hoffmann J.M., Stock S., Wang L., Liu Y., Schubert M.L., Neuber B., Huckelhoven-Krauss A., Gern U., Schmitt A., et al. Comparison of IL-2 vs IL-7/IL-15 for the generation of NY-ESO-1-specific T cells. Cancer Immunol. Immunother. 2019;68:1195–1209. doi: 10.1007/s00262-019-02354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okoye I.S., Xu L., Walker J., Elahi S. The glucocorticoids prednisone and dexamethasone differentially modulate T cell function in response to anti-PD-1 and anti-CTLA-4 immune checkpoint blockade. Cancer Immunol. Immunother. 2020 doi: 10.1007/s00262-020-02555-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yi F., Frazzette N., Cruz A.C., Klebanoff C.A., Siegel R.M. Beyond Cell Death: New Functions for TNF Family Cytokines in Autoimmunity and Tumor Immunotherapy. Trends Mol. Med. 2018;24:642–653. doi: 10.1016/j.molmed.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang T.W., Yevsa T., Woller N., Hoenicke L., Wuestefeld T., Dauch D., Hohmeyer A., Gereke M., Rudalska R., Potapova A., et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–551. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 31.d’Adda di Fagagna F. Living on a break: Cellular senescence as a DNA-damage response. Nat. Rev. Cancer. 2008;8:512–522. doi: 10.1038/nrc2440. [DOI] [PubMed] [Google Scholar]
- 32.Kumari N., Dwarakanath B.S., Das A., Bhatt A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016;37:11553–11572. doi: 10.1007/s13277-016-5098-7. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y.X., Xu B.W., Chen Y.J., Fu X.Q., Zhu P.L., Bai J.X., Chou J.Y., Yin C.L., Li J.K., Wang Y.P., et al. Inhibiting the Src/STAT3 signaling pathway contributes to the anti-melanoma mechanisms of dioscin. Oncol. Lett. 2020;19:2508–2514. doi: 10.3892/ol.2020.11315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang L., So J.Y., Skrypek N., Yang H.H., Merchant A.S., Nelson G.W., Chen W.D., Ishii H., Chen J.M., Hu G., et al. Induction of DNMT3B by PGE2 and IL6 at distant metastatic sites promotes epigenetic modification and breast cancer colonization. Cancer Res. 2020 doi: 10.1158/0008-5472.CAN-19-3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel H.J., Patel B.M. TNF-alpha and cancer cachexia: Molecular insights and clinical implications. Life Sci. 2017;170:56–63. doi: 10.1016/j.lfs.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 36.Itzkowitz S.H., Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: The role of inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G7–G17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 37.Maynard J.P., Ertunc O., Kulac I., Baena-Del Valle J.A., De Marzo A.M., Sfanos K.S. IL8 Expression Is Associated with Prostate Cancer Aggressiveness and Androgen Receptor Loss in Primary and Metastatic Prostate Cancer. Mol. Cancer Res. 2020;18:153–165. doi: 10.1158/1541-7786.MCR-19-0595. [DOI] [PubMed] [Google Scholar]
- 38.Orosz P., Kruger A., Hubbe M., Ruschoff J., Von Hoegen P., Mannel D.N. Promotion of experimental liver metastasis by tumor necrosis factor. Int. J. Cancer. 1995;60:867–871. doi: 10.1002/ijc.2910600624. [DOI] [PubMed] [Google Scholar]
- 39.Eggert T., Wolter K., Ji J., Ma C., Yevsa T., Klotz S., Medina-Echeverz J., Longerich T., Forgues M., Reisinger F., et al. Distinct Functions of Senescence-Associated Immune Responses in Liver Tumor Surveillance and Tumor Progression. Cancer Cell. 2016;30:533–547. doi: 10.1016/j.ccell.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eyman D., Damodarasamy M., Plymate S.R., Reed M.J. CCL5 secreted by senescent aged fibroblasts induces proliferation of prostate epithelial cells and expression of genes that modulate angiogenesis. J. Cell Physiol. 2009;220:376–381. doi: 10.1002/jcp.21776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown J.A., Yonekubo Y., Hanson N., Sastre-Perona A., Basin A., Rytlewski J.A., Dolgalev I., Meehan S., Tsirigos A., Beronja S., et al. TGF-beta-Induced Quiescence Mediates Chemoresistance of Tumor-Propagating Cells in Squamous Cell Carcinoma. Cell Stem Cell. 2017;21:650–664.e8. doi: 10.1016/j.stem.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun N., Youle R.J., Finkel T. The Mitochondrial Basis of Aging. Mol. Cell. 2016;61:654–666. doi: 10.1016/j.molcel.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Correia-Melo C., Marques F.D., Anderson R., Hewitt G., Hewitt R., Cole J., Carroll B.M., Miwa S., Birch J., Merz A., et al. Mitochondria are required for pro-ageing features of the senescent phenotype. EMBO J. 2016;35:724–742. doi: 10.15252/embj.201592862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paluvai H., Di Giorgio E., Brancolini C. The Histone Code of Senescence. Cells. 2020;9:466. doi: 10.3390/cells9020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Narita M., Nũnez S., Heard E., Narita M., Lin A.W., Hearn S.A., Spector D.L., Hannon G.J., Lowe S.W. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–716. doi: 10.1016/S0092-8674(03)00401-X. [DOI] [PubMed] [Google Scholar]
- 46.Peters A.H., O’Carroll D., Scherthan H., Mechtler K., Sauer S., Schofer C., Weipoltshammer K., Pagani M., Lachner M., Kohlmaier A., et al. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell. 2001;107:323–337. doi: 10.1016/S0092-8674(01)00542-6. [DOI] [PubMed] [Google Scholar]
- 47.Yu Y., Schleich K., Yue B., Ji S., Lohneis P., Kemper K., Silvis M.R., Qutob N., van Rooijen E., Werner-Klein M., et al. Targeting the Senescence-Overriding Cooperative Activity of Structurally Unrelated H3K9 Demethylases in Melanoma. Cancer Cell. 2018;33:322–336.e8. doi: 10.1016/j.ccell.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duarte L.F., Young A.R.J., Wang Z., Wu H.A., Panda T., Kou Y., Kapoor A., Hasson D., Mills N.R., Ma’ayan A., et al. Histone H3.3 and its proteolytically processed form drive a cellular senescence programme. Nat. Commun. 2014;5:5210. doi: 10.1038/ncomms6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Contrepois K., Coudereau C., Benayoun B.A., Schuler N., Roux P.F., Bischof O., Courbeyrette R., Carvalho C., Thuret J.Y., Ma Z., et al. Histone variant H2A.J accumulates in senescent cells and promotes inflammatory gene expression. Nat. Commun. 2017;8:14995. doi: 10.1038/ncomms14995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen H., Ruiz P.D., McKimpson W.M., Novikov L., Kitsis R.N., Gamble M.J. MacroH2A1 and ATM Play Opposing Roles in Paracrine Senescence and the Senescence-Associated Secretory Phenotype. Mol. Cell. 2015;59:719–731. doi: 10.1016/j.molcel.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zirkel A., Nikolic M., Sofiadis K., Mallm J.P., Brackley C.A., Gothe H., Drechsel O., Becker C., Altmuller J., Josipovic N., et al. HMGB2 Loss upon Senescence Entry Disrupts Genomic Organization and Induces CTCF Clustering across Cell Types. Mol. Cell. 2018;70:730–744.e6. doi: 10.1016/j.molcel.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 52.Davalos A.R., Kawahara M., Malhotra G.K., Schaum N., Huang J., Ved U., Beausejour C.M., Coppe J.P., Rodier F., Campisi J. p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. J. Cell Biol. 2013;201:613–629. doi: 10.1083/jcb.201206006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.D’Adda Di Fagagna F., Reaper P.M., Clay-Farrace L., Fiegler H., Carr P., Von Zglinicki T., Saretzki G., Carter N.P., Jackson S.P. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 54.Beausejour C.M., Krtolica A., Galimi F., Narita M., Lowe S.W., Yaswen P., Campisi J. Reversal of human cellular senescence: Roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–4222. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gil J., Peters G. Regulation of the INK4b-ARF-INK4a tumour suppressor locus: All for one or one for all. Nat. Rev. Mol. Cell Biol. 2006;7:667–677. doi: 10.1038/nrm1987. [DOI] [PubMed] [Google Scholar]
- 56.Bracken A.P., Kleine-Kohlbrecher D., Dietrich N., Pasini D., Gargiulo G., Beekman C., Theilgaard-Monch K., Minucci S., Porse B.T., Marine J.C., et al. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev. 2007;21:525–530. doi: 10.1101/gad.415507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Agger K., Cloos P.A., Rudkjaer L., Williams K., Andersen G., Christensen J., Helin K. The H3K27me3 demethylase JMJD3 contributes to the activation of the INK4A-ARF locus in response to oncogene- and stress-induced senescence. Genes Dev. 2009;23:1171–1176. doi: 10.1101/gad.510809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hubackova S., Krejcikova K., Bartek J., Hodny Z. IL1-and TGFβ-Nox4 signaling, oxidative stress and DNA damage response are shared features of replicative, oncogene-induced, and drug-induced paracrine ‘Bystander senescence’. Aging. 2012;4:932–951. doi: 10.18632/aging.100520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herranz N., Gallage S., Mellone M., Wuestefeld T., Klotz S., Hanley C.J., Raguz S., Acosta J.C., Innes A.J., Banito A., et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat. Cell Biol. 2015;17:1205–1217. doi: 10.1038/ncb3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoare M., Ito Y., Kang T.W., Weekes M.P., Matheson N.J., Patten D.A., Shetty S., Parry A.J., Menon S., Salama R., et al. NOTCH1 mediates a switch between two distinct secretomes during senescence. Nat. Cell Biol. 2016;18:979–992. doi: 10.1038/ncb3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zwerschke W., Mazurek S., Stöckl P., Hütter E., Eigenbrodt E., Jansen-Dürr P. Metabolic analysis of senescent human fibroblasts reveals a role for AMP in cellular senescence. Biochem. J. 2003;376:403–411. doi: 10.1042/bj20030816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.James E.L., Michalek R.D., Pitiyage G.N., De Castro A.M., Vignola K.S., Jones J., Mohney R.P., Karoly E.D., Prime S.S., Parkinson E.K. Senescent human fibroblasts show increased glycolysis and redox homeostasis with extracellular metabolomes that overlap with those of irreparable DNA damage, aging, and disease. J. Proteome Res. 2015;14:1854–1871. doi: 10.1021/pr501221g. [DOI] [PubMed] [Google Scholar]
- 63.Dörr J.R., Yu Y., Milanovic M., Beuster G., Zasada C., Däbritz J.H.M., Lisec J., Lenze D., Gerhardt A., Schleicher K., et al. Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature. 2013;501:421–425. doi: 10.1038/nature12437. [DOI] [PubMed] [Google Scholar]
- 64.Lee S.M., Dho S.H., Ju S.K., Maeng J.S., Kim J.Y., Kwon K.S. Cytosolic malate dehydrogenase regulates senescence in human fibroblasts. Biogerontology. 2012;13:525–536. doi: 10.1007/s10522-012-9397-0. [DOI] [PubMed] [Google Scholar]
- 65.Moiseeva O., Bourdeau V., Roux A., Deschenes-Simard X., Ferbeyre G. Mitochondrial Dysfunction Contributes to Oncogene-Induced Senescence. Mol. Cell. Biol. 2009;29:4495–4507. doi: 10.1128/MCB.01868-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang P., Du W., Mancuso A., Wellen K.E., Yang X. Reciprocal regulation of p53 and malic enzymes modulates metabolism and senescence. Nature. 2013;493:689–693. doi: 10.1038/nature11776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaplon J., Zheng L., Meissl K., Chaneton B., Selivanov V.A., MacKay G., Van Der Burg S.H., Verdegaal E.M.E., Cascante M., Shlomi T., et al. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature. 2013;498:109–112. doi: 10.1038/nature12154. [DOI] [PubMed] [Google Scholar]
- 68.Takebayashi S.I., Tanaka H., Hino S., Nakatsu Y., Igata T., Sakamoto A., Narita M., Nakao M. Retinoblastoma protein promotes oxidative phosphorylation through upregulation of glycolytic genes in oncogene-induced senescent cells. Aging Cell. 2015;14:689–697. doi: 10.1111/acel.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Quijano C., Cao L., Fergusson M.M., Romero H., Liu J., Gutkind S., Rovira, Mohney R.P., Karoly E.D., Finkel T. Oncogene-induced senescence results in marked metabolic and bioenergetic alterations. Cell Cycle. 2012;11:1383–1392. doi: 10.4161/cc.19800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fafián-Labora J., Carpintero-Fernández P., Jordan S.J.D., Shikh-Bahaei T., Abdullah S.M., Mahenthiran M., Rodríguez-Navarro J.A., Niklison-Chirou M.V., O’Loghlen A. FASN activity is important for the initial stages of the induction of senescence. Cell Death Dis. 2019;10:318. doi: 10.1038/s41419-019-1550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aird K.M., Zhang G., Li H., Tu Z., Bitler B.G., Garipov A., Wu H., Wei Z., Wagner S.N., Herlyn M., et al. Suppression of Nucleotide Metabolism Underlies the Establishment and Maintenance of Oncogene-Induced Senescence. Cell Rep. 2013;3:1252–1265. doi: 10.1016/j.celrep.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Drabkin M.J., Meyer J.I., Kanth N., Lobel S., Fogel J., Grossman J., Krumenacker J.H. Age-stratified Patterns of Thymic Involution on Multidetector CT. J. Thorac. Imaging. 2018;33:409–416. doi: 10.1097/RTI.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 73.Thomas R., Wang W., Su D.M. Contributions of Age-Related Thymic Involution to Immunosenescence and Inflammaging. Immun. Ageing. 2020;17:2. doi: 10.1186/s12979-020-0173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coder B.D., Wang H., Ruan L., Su D.M. Thymic involution perturbs negative selection leading to autoreactive T cells that induce chronic inflammation. J. Immunol. 2015;194:5825–5837. doi: 10.4049/jimmunol.1500082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lowe S.W., Cepero E., Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–315. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- 76.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 77.Serrano M., Lin A.W., McCurrach M.E., Beach D., Lowe S.W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/S0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 78.Campisi J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Childs B.G., Durik M., Baker D.J., van Deursen J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015;21:1424–1435. doi: 10.1038/nm.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 2001;11:S27–S31. doi: 10.1016/S0962-8924(01)02151-1. [DOI] [PubMed] [Google Scholar]
- 81.Pluquet O., Abbadie C., Coqueret O. Connecting cancer relapse with senescence. Cancer Lett. 2019;463:50–58. doi: 10.1016/j.canlet.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 82.Saleh T., Tyutyunyk-Massey L., Gewirtz D.A. Tumor Cell Escape from Therapy-Induced Senescence as a Model of Disease Recurrence after Dormancy. Cancer Res. 2019;79:1044–1046. doi: 10.1158/0008-5472.CAN-18-3437. [DOI] [PubMed] [Google Scholar]
- 83.Milanovic M., Fan D.N.Y., Belenki D., Dabritz J.H.M., Zhao Z., Yu Y., Dorr J.R., Dimitrova L., Lenze D., Monteiro Barbosa I.A., et al. Senescence-associated reprogramming promotes cancer stemness. Nature. 2018;553:96–100. doi: 10.1038/nature25167. [DOI] [PubMed] [Google Scholar]
- 84.Perez-Amill L., Marzal B., Urbano-Ispizua A., Juan M., Martin-Antonio B. CAR-T Cell Therapy: A Door Is Open to Find Innumerable Possibilities of Treatments for Cancer Patients. Turk. J. Haematol. 2018;35:217–228. doi: 10.4274/tjh.2018.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shimabukuro-Vornhagen A., Godel P., Subklewe M., Stemmler H.J., Schlosser H.A., Schlaak M., Kochanek M., Boll B., von Bergwelt-Baildon M.S. Cytokine release syndrome. J. Immunother. Cancer. 2018;6:56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Braumuller H., Wieder T., Brenner E., Assmann S., Hahn M., Alkhaled M., Schilbach K., Essmann F., Kneilling M., Griessinger C., et al. T-helper-1-cell cytokines drive cancer into senescence. Nature. 2013;494:361–365. doi: 10.1038/nature11824. [DOI] [PubMed] [Google Scholar]
- 87.Martin-Antonio B., Sune G., Perez-Amill L., Castella M., Urbano-Ispizua A. Natural Killer Cells: Angels and Devils for Immunotherapy. Int. J. Mol. Sci. 2017;18:1868. doi: 10.3390/ijms18091868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martin-Antonio B., Sune G., Najjar A., Perez-Amill L., Antonana-Vildosola A., Castella M., Leon S., Velasco-de Andres M., Lozano F., Lozano E., et al. Extracellular NK histones promote immune cell antitumor activity by inducing cell clusters through binding to CD138 receptor. J. Immunother. Cancer. 2019;7:259. doi: 10.1186/s40425-019-0739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sagiv A., Biran A., Yon M., Simon J., Lowe S.W., Krizhanovsky V. Granule exocytosis mediates immune surveillance of senescent cells. Oncogene. 2013;32:1971–1977. doi: 10.1038/onc.2012.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Egashira M., Hirota Y., Shimizu-Hirota R., Saito-Fujita T., Haraguchi H., Matsumoto L., Matsuo M., Hiraoka T., Tanaka T., Akaeda S., et al. F4/80+ Macrophages Contribute to Clearance of Senescent Cells in the Mouse Postpartum Uterus. Endocrinology. 2017;158:2344–2353. doi: 10.1210/en.2016-1886. [DOI] [PubMed] [Google Scholar]
- 91.Aiello A., Farzaneh F., Candore G., Caruso C., Davinelli S., Gambino C.M., Ligotti M.E., Zareian N., Accardi G. Immunosenescence and its hallmarks: How to oppose aging strategically? A review of potential options for therapeutic intervention. Front. Immunol. 2019;10:1–19. doi: 10.3389/fimmu.2019.02247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coppe J.P., Patil C.K., Rodier F., Sun Y., Munoz D.P., Goldstein J., Nelson P.S., Desprez P.Y., Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Crespo J., Sun H., Welling T.H., Tian Z., Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr. Opin. Immunol. 2013;25:214–221. doi: 10.1016/j.coi.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Plunkett F.J., Franzese O., Belaramani L.L., Fletcher J.M., Gilmour K.C., Sharifi R., Khan N., Hislop A.D., Cara A., Salmon M., et al. The impact of telomere erosion on memory CD8+ T cells in patients with X-linked lymphoproliferative syndrome. Mech. Ageing Dev. 2005;126:855–865. doi: 10.1016/j.mad.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 95.Hale J.S., Boursalian T.E., Turk G.L., Fink P.J. Thymic output in aged mice. Proc. Natl. Acad. Sci. USA. 2006;103:8447–8452. doi: 10.1073/pnas.0601040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hun M.L., Wong K., Gunawan J.R., Alsharif A., Quinn K., Chidgey A.P. Gender Disparity Impacts on Thymus Aging and LHRH Receptor Antagonist-Induced Thymic Reconstitution Following Chemotherapeutic Damage. Front. Immunol. 2020;11:302. doi: 10.3389/fimmu.2020.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tsukamoto H., Huston G.E., Dibble J., Duso D.K., Swain S.L. Bim dictates naive CD4 T cell lifespan and the development of age-associated functional defects. J. Immunol. 2010;185:4535–4544. doi: 10.4049/jimmunol.1001668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Goronzy J.J., Lee W.W., Weyand C.M. Aging and T-cell diversity. Exp. Gerontol. 2007;42:400–406. doi: 10.1016/j.exger.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van de Berg P.J.E.J., Griffiths S.J., Yong S.-L., Macaulay R., Bemelman F.J., Jackson S., Henson S.M., ten Berge I.J.M., Akbar A.N., van Lier R.A.W. Cytomegalovirus Infection Reduces Telomere Length of the Circulating T Cell Pool. J. Immunol. 2010;184:3417–3423. doi: 10.4049/jimmunol.0903442. [DOI] [PubMed] [Google Scholar]
- 100.Henson S.M., Lanna A., Riddel N.E., Franzese O., Macaulay R., Griffiths S.J., Puleston D.J., Watson A.S., Simon A.K., Tooze S.A., et al. P38 signaling inhibits mTORC1-independent autophagy in senescent human CD8+ T cells. J. Clin. Investig. 2014;124:4004–4016. doi: 10.1172/JCI75051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Passos J.F., Nelson G., Wang C., Richter T., Simillion C., Proctor C.J., Miwa S., Olijslagers S., Hallinan J., Wipat A., et al. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol. Syst. Biol. 2010;6:347. doi: 10.1038/msb.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ye J., Huang X., Hsueh E.C., Zhang Q., Ma C., Zhang Y., Varvares M.A., Hoft D.F., Peng G. Human regulatory T cells induce T-lymphocyte senescence. Blood. 2012;120:2021–2031. doi: 10.1182/blood-2012-03-416040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ye J., Ma C., Hsueh E.C., Eickhoff C.S., Zhang Y., Varvares M.A., Hoft D.F., Peng G. Tumor-Derived γδ Regulatory T Cells Suppress Innate and Adaptive Immunity through the Induction of Immunosenescence. J. Immunol. 2013;190:2403–2414. doi: 10.4049/jimmunol.1202369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu X., Mo W., Ye J., Li L., Zhang Y., Hsueh E.C., Hoft D.F., Peng G. Regulatory T cells trigger effector T cell DNA damage and senescence caused by metabolic competition. Nat. Commun. 2018;9:249. doi: 10.1038/s41467-017-02689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huff W.X., Kwon J.H., Henriquez M., Fetcko K., Dey M. The Evolving Role of CD8+CD28- Immunosenescent T Cells in Cancer Immunology. Int. J. Mol. Sci. 2019;20:2810. doi: 10.3390/ijms20112810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li L., Liu X., Sanders K.L., Edwards J.L., Ye J., Si F., Gao A., Huang L., Hsueh E.C., Ford D.A., et al. TLR8-Mediated Metabolic Control of Human Treg Function: A Mechanistic Target for Cancer Immunotherapy. Cell Metab. 2019;29:103–123.e5. doi: 10.1016/j.cmet.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kasakovski D., Xu L., Li Y. T cell senescence and CAR-T cell exhaustion in hematological malignancies. J. Hematol. Oncol. 2018;11:91. doi: 10.1186/s13045-018-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Monteiro J., Batliwalla F., Ostrer H., Gregersen P.K. Shortened telomeres in clonally expanded CD28-CD8+ T cells imply a replicative history that is distinct from their CD28+CD8+ counterparts. J. Immunol. 1996;156:3587–3590. [PubMed] [Google Scholar]
- 109.Di Mitri D., Azevedo R.I., Henson S.M., Libri V., Riddell N.E., Macaulay R., Kipling D., Soares M.V.D., Battistini L., Akbar A.N. Reversible Senescence in Human CD4 + CD45RA + CD27 − Memory T Cells. J. Immunol. 2011;187:2093–2100. doi: 10.4049/jimmunol.1100978. [DOI] [PubMed] [Google Scholar]
- 110.Romero P., Zippelius A., Kurth I., Pittet M.J., Touvrey C., Iancu E.M., Corthesy P., Devevre E., Speiser D.E., Rufer N. Four Functionally Distinct Populations of Human Effector-Memory CD8 + T Lymphocytes. J. Immunol. 2007;178:4112–4119. doi: 10.4049/jimmunol.178.7.4112. [DOI] [PubMed] [Google Scholar]
- 111.Henson S.M., Macaulay R., Riddell N.E., Nunn C.J., Akbar A.N. Blockade of PD-1 or p38 MAP kinase signaling enhances senescent human CD8+ T-cell proliferation by distinct pathways. Eur. J. Immunol. 2015;45:1441–1451. doi: 10.1002/eji.201445312. [DOI] [PubMed] [Google Scholar]
- 112.Plunkett F.J., Franzese O., Finney H.M., Fletcher J.M., Belaramani L.L., Salmon M., Dokal I., Webster D., Lawson A.D.G., Akbar A.N. The Loss of Telomerase Activity in Highly Differentiated CD8 + CD28 − CD27 − T Cells Is Associated with Decreased Akt (Ser 473) Phosphorylation. J. Immunol. 2007;178:7710–7719. doi: 10.4049/jimmunol.178.12.7710. [DOI] [PubMed] [Google Scholar]
- 113.Riddell N.E., Griffiths S.J., Rivino L., King D.C.B., Teo G.H., Henson S.M., Cantisan S., Solana R., Kemeny D.M., Macary P.A., et al. Multifunctional cytomegalovirus (CMV)-specific CD8+ T cells are not restricted by telomere-related senescence in young or old adults. Immunology. 2015;144:549–560. doi: 10.1111/imm.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Libri V., Azevedo R.I., Jackson S.E., Di Mitri D., Lachmann R., Fuhrmann S., Vukmanovic-Stejic M., Yong K., Battistini L., Kern F., et al. Cytomegalovirus infection induces the accumulation of short-lived, multifunctional CD4+CD45RA+CD27- T cells: The potential involvement of interleukin-7 in this process. Immunology. 2011;132:326–339. doi: 10.1111/j.1365-2567.2010.03386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Spaulding C., Guo W., Effros R.B. Resistance to apoptosis in human CD8+ T cells that reach replicative senescence after multiple rounds of antigen-specific proliferation. Exp. Gerontol. 1999;34:633–644. doi: 10.1016/S0531-5565(99)00033-9. [DOI] [PubMed] [Google Scholar]
- 116.Akbar A.N., Henson S.M. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat. Rev. Immunol. 2011;11:289–295. doi: 10.1038/nri2959. [DOI] [PubMed] [Google Scholar]
- 117.Mondal A.M., Horikawa I., Pine S.R., Fujita K., Morgan K.M., Vera E., Mazur S.J., Appella E., Vojtesek B., Blasco M.a., et al. p53 isoforms regulate aging- and tumor-associated replicative senescence in T lymphocytes. J. Clin. Investig. 2013;123:5247–5257. doi: 10.1172/JCI70355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Callender L.A., Carroll E.C., Beal R.W.J., Chambers E.S., Nourshargh S., Akbar A.N., Henson S.M. Human CD8(+) EMRA T cells display a senescence-associated secretory phenotype regulated by p38 MAPK. Aging Cell. 2018;17:e12675. doi: 10.1111/acel.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Callender L.A., Carroll E.C., Bober E.A., Akbar A.N., Solito E., Henson S.M. Mitochondrial mass governs the extent of human T cell senescence. Aging Cell. 2020;19:e13067. doi: 10.1111/acel.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu X., Hoft D.F., Peng G. Senescent T cells within suppressive tumor microenvironments: Emerging target for tumor immunotherapy. J. Clin. Investig. 2020;130:1073–1083. doi: 10.1172/JCI133679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kubuki Y., Suzuki M., Sasaki H., Toyama T., Yamashita K., Maeda K., Ido A., Matsuoka H., Okayama A., Nakanishi T., et al. Telomerase activity and telomere length as prognostic factors of adult T-cell leukemia. Leuk. Lymphoma. 2005;46:393–399. doi: 10.1080/10428190400018349. [DOI] [PubMed] [Google Scholar]
- 122.Nunes C., Wong R., Mason M., Fegan C., Man S., Pepper C. Expansion of a CD8 +PD-1 + replicative senescence phenotype in early stage CLL patients is associated with inverted CD4:CD8 ratios and disease progression. Clin. Cancer Res. 2012;18:678–687. doi: 10.1158/1078-0432.CCR-11-2630. [DOI] [PubMed] [Google Scholar]
- 123.Shen X., Zhou J., Hathcock K.S., Robbins P., Powell D.J., Rosenberg S.A., Hodes R.J. Persistence of tumor infiltrating lymphocytes in adoptive immunotherapy correlates with telomere length. J. Immunother. 2007;30:123–129. doi: 10.1097/01.cji.0000211321.07654.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Montes C.L., Chapoval A.I., Nelson J., Orhue V., Zhang X., Schulze D.H., Strome S.E., Gastman B.R. Tumor-induced senescent T cells with suppressor function: A potential form of tumor immune evasion. Cancer Res. 2008;68:870–879. doi: 10.1158/0008-5472.CAN-07-2282. [DOI] [PubMed] [Google Scholar]
- 125.Ye J., Ma C., Hsueh E.C., Dou J., Mo W., Liu S., Han B., Huang Y., Zhang Y., Varvares M.A., et al. TLR 8 signaling enhances tumor immunity by preventing tumor-induced T-cell senescence. EMBO Mol. Med. 2014;6:1294–1311. doi: 10.15252/emmm.201403918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ye S.W., Wang Y., Valmori D., Ayyoub M., Han Y., Xu X.L., Zhao A.L., Qu L., Gnjatic S., Ritter G., et al. Ex-vivo analysis of CD8+ T cells infiltrating colorectal tumors identifies a major effector-memory subset with low perforin content. J. Clin. Immunol. 2006;26:447–456. doi: 10.1007/s10875-006-9040-4. [DOI] [PubMed] [Google Scholar]
- 127.Urbaniak-Kujda D., Kapelko-Słowik K., Wołowiec D., Dybko J., Hałoń A., Jaźwiec B., Maj J., Jankowska-Konsur A., Kuliczkowski K. Increased percentage of CD8+CD28- suppressor lymphocytes in peripheral blood and skin infiltrates correlates with advanced disease in patients with cutaneous T-cell lymphomas. Postepy Hig. Med. Dosw. 2009;63:355–359. [PubMed] [Google Scholar]
- 128.Zelle-Rieser C., Thangavadivel S., Biedermann R., Brunner A., Stoitzner P., Willenbacher E., Greil R., Johrer K. T cells in multiple myeloma display features of exhaustion and senescence at the tumor site. J. Hematol. Oncol. 2016;9:116. doi: 10.1186/s13045-016-0345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Knaus H.A., Berglund S., Hackl H., Blackford A.L., Zeidner J.F., Montiel-Esparza R., Mukhopadhyay R., Vanura K., Blazar B.R., Karp J.E., et al. Signatures of CD8+ T cell dysfunction in AML patients and their reversibility with response to chemotherapy. JCI Insight. 2018;3:1–20. doi: 10.1172/jci.insight.120974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chung D.J., Pronschinske K.B., Shyer J.A., Sharma S., Leung S., Curran S.A., Lesokhin A.M., Devlin S.M., Giralt S.A., Young J.W. T-cell exhaustion in Multiple myeloma relapse after autotransplant: Optimal timing of immunotherapy. Cancer Immunol. Res. 2016;4:61–71. doi: 10.1158/2326-6066.CIR-15-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chang C.H., Qiu J., O’Sullivan D., Buck M.D., Noguchi T., Curtis J.D., Chen Q., Gindin M., Gubin M.M., van der Windt G.J., et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Parish S.T., Kim S., Sekhon R.K., Wu J.E., Kawakatsu Y., Effros R.B. Adenosine deaminase modulation of telomerase activity and replicative senescence in human CD8 T lymphocytes. J. Immunol. 2010;184:2847–2854. doi: 10.4049/jimmunol.0903647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Maybruck B.T., Pfannenstiel L.W., Diaz-Montero M., Gastman B.R. Tumor-derived exosomes induce CD8+ T cell suppressors. J. Immunother. Cancer. 2017;5:1–15. doi: 10.1186/s40425-017-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Akbar A.N., Henson S.M., Lanna A. Senescence of T Lymphocytes: Implications for Enhancing Human Immunity. Trends Immunol. 2016;37:866–876. doi: 10.1016/j.it.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 135.Lanna A., Coutavas E., Levati L., Seidel J., Rustin M.H.A., Henson S.M., Akbar A.N., Franzese O. IFN-α Inhibits Telomerase in Human CD8 + T Cells by Both hTERT Downregulation and Induction of p38 MAPK Signaling. J. Immunol. 2013;191:3744–3752. doi: 10.4049/jimmunol.1301409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dodeller F., Schulze-Koops H. The p38 mitogen-activated protein kinase signaling cascade in CD4 T cells. Arthritis Res. Ther. 2006;8:205. doi: 10.1186/ar1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Salvador J.M., Mittelstadt P.R., Guszczynski T., Copeland T.D., Yamaguchi H., Appella E., Fornace A.J., Jr., Ashwell J.D. Alternative p38 activation pathway mediated by T cell receptor-proximal tyrosine kinases. Nat. Immunol. 2005;6:390–395. doi: 10.1038/ni1177. [DOI] [PubMed] [Google Scholar]
- 138.Lanna A., Henson S.M., Escors D., Akbar A.N. The kinase p38 activated by the metabolic regulator AMPK and scaffold TAB1 drives the senescence of human T cells. Nat. Immunol. 2014;15:965–972. doi: 10.1038/ni.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pereira B.I., De Maeyer R.P.H., Covre L.P., Nehar-Belaid D., Lanna A., Ward S., Marches R., Chambers E.S., Gomes D.C.O., Riddell N.E., et al. Sestrins induce natural killer function in senescent-like CD8+ T cells. Nat. Immunol. 2020;21:684–694. doi: 10.1038/s41590-020-0643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lanna A., Gomes D.C.O., Muller-Durovic B., McDonnell T., Escors D., Gilroy D.W., Lee J.H., Karin M., Akbar A.N. A sestrin-dependent Erk-Jnk-p38 MAPK activation complex inhibits immunity during aging. Nat. Immunol. 2017;18:354–363. doi: 10.1038/ni.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yuen G.J., Demissie E., Pillai S. B lymphocytes and cancer: A love-hate relationship. Trends Cancer. 2016;2:747–757. doi: 10.1016/j.trecan.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ammirante M., Luo J.L., Grivennikov S., Nedospasov S., Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464:302–305. doi: 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Andreu P., Johansson M., Affara N.I., Pucci F., Tan T., Junankar S., Korets L., Lam J., Tawfik D., DeNardo D.G., et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17:121–134. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shalapour S., Font-Burgada J., Di Caro G., Zhong Z., Sanchez-Lopez E., Dhar D., Willimsky G., Ammirante M., Strasner A., Hansel D.E., et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature. 2015;521:94–98. doi: 10.1038/nature14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nayar S., Dasgupta P., Galustian C. Extending the lifespan and efficacies of immune cells used in adoptive transfer for cancer immunotherapies—A review. Oncoimmunology. 2015;4:e1002720. doi: 10.1080/2162402X.2014.1002720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wu C., Espinoza D.A., Koelle S.J., Yang D., Truitt L., Schlums H., Lafont B.A., Davidson-Moncada J.K., Lu R., Kaur A., et al. Clonal expansion and compartmentalized maintenance of rhesus macaque NK cell subsets. Sci. Immunol. 2018;3:eaat9781. doi: 10.1126/sciimmunol.aat9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Judge S.J., Murphy W.J., Canter R.J. Characterizing the Dysfunctional NK Cell: Assessing the Clinical Relevance of Exhaustion, Anergy, and Senescence. Front. Cell Infect. Microbiol. 2020;10:49. doi: 10.3389/fcimb.2020.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gounder S.S., Abdullah B.J.J., Radzuanb N., Zain F., Sait N.B.M., Chua C., Subramani B. Effect of Aging on NK Cell Population and Their Proliferation at Ex Vivo Culture Condition. Anal. Cell Pathol. (Amst.) 2018;2018:7871814. doi: 10.1155/2018/7871814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Manser A.R., Uhrberg M. Age-related changes in natural killer cell repertoires: Impact on NK cell function and immune surveillance. Cancer Immunol. Immunother. 2016;65:417–426. doi: 10.1007/s00262-015-1750-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Przemska-Kosicka A., Childs C.E., Maidens C., Dong H., Todd S., Gosney M.A., Tuohy K.M., Yaqoob P. Age-Related Changes in the Natural Killer Cell Response to Seasonal Influenza Vaccination Are Not Influenced by a Synbiotic: A Randomised Controlled Trial. Front. Immunol. 2018;9:591. doi: 10.3389/fimmu.2018.00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Denman C.J., Senyukov V.V., Somanchi S.S., Phatarpekar P.V., Kopp L.M., Johnson J.L., Singh H., Hurton L., Maiti S.N., Huls M.H., et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS ONE. 2012;7:e30264. doi: 10.1371/journal.pone.0030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shah N., Li L., McCarty J., Kaur I., Yvon E., Shaim H., Muftuoglu M., Liu E., Orlowski R.Z., Cooper L., et al. Phase I study of cord blood-derived natural killer cells combined with autologous stem cell transplantation in multiple myeloma. Br. J. Haematol. 2017;177:457–466. doi: 10.1111/bjh.14570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Streltsova M.A., Erokhina S.A., Kanevskiy L.M., Lee D.A., Telford W.G., Sapozhnikov A.M., Kovalenko E.I. Analysis of NK cell clones obtained using interleukin-2 and gene-modified K562 cells revealed the ability of “senescent” NK cells to lose CD57 expression and start expressing NKG2A. PLoS ONE. 2018;13:e0208469. doi: 10.1371/journal.pone.0208469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lopez-Verges S., Milush J.M., Pandey S., York V.A., Arakawa-Hoyt J., Pircher H., Norris P.J., Nixon D.F., Lanier L.L. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. 2010;116:3865–3874. doi: 10.1182/blood-2010-04-282301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dace D.S., Apte R.S. Effect of senescence on macrophage polarization and angiogenesis. Rejuvenation Res. 2008;11:177–185. doi: 10.1089/rej.2007.0614. [DOI] [PubMed] [Google Scholar]
- 156.Linehan E., Fitzgerald D.C. Ageing and the immune system: Focus on macrophages. Eur. J. Microbiol. Immunol. (Bp) 2015;5:14–24. doi: 10.1556/EuJMI-D-14-00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jackaman C., Tomay F., Duong L., Abdol Razak N.B., Pixley F.J., Metharom P., Nelson D.J. Aging and cancer: The role of macrophages and neutrophils. Ageing Res. Rev. 2017;36:105–116. doi: 10.1016/j.arr.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 158.Sagiv A., Krizhanovsky V. Immunosurveillance of senescent cells: The bright side of the senescence program. Biogerontology. 2013;14:617–628. doi: 10.1007/s10522-013-9473-0. [DOI] [PubMed] [Google Scholar]
- 159.Reimann M., Lee S., Loddenkemper C., Dorr J.R., Tabor V., Aichele P., Stein H., Dorken B., Jenuwein T., Schmitt C.A. Tumor stroma-derived TGF-beta limits myc-driven lymphomagenesis via Suv39h1-dependent senescence. Cancer Cell. 2010;17:262–272. doi: 10.1016/j.ccr.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 160.Llanos S., Serrano M. Senescence and Cancer: In the Name of Immunosuppression. Cancer Cell. 2016;30:507–508. doi: 10.1016/j.ccell.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 161.Herranz N., Gil J. Mechanisms and functions of cellular senescence. J. Clin. Investig. 2018;128:1238–1246. doi: 10.1172/JCI95148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Harbort C.J., Soeiro-Pereira P.V., von Bernuth H., Kaindl A.M., Costa-Carvalho B.T., Condino-Neto A., Reichenbach J., Roesler J., Zychlinsky A., Amulic B. Neutrophil oxidative burst activates ATM to regulate cytokine production and apoptosis. Blood. 2015;126:2842–2851. doi: 10.1182/blood-2015-05-645424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Vilgelm A.E., Johnson C.A., Prasad N., Yang J., Chen S.C., Ayers G.D., Pawlikowski J.S., Raman D., Sosman J.A., Kelley M., et al. Connecting the Dots: Therapy-Induced Senescence and a Tumor-Suppressive Immune Microenvironment. J. Natl. Cancer Inst. 2016;108:djv406. doi: 10.1093/jnci/djv406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ewald J.A., Desotelle J.A., Wilding G., Jarrard D.F. Therapy-induced senescence in cancer. J. Natl. Cancer Inst. 2010;102:1536–1546. doi: 10.1093/jnci/djq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.von Kobbe C. Targeting senescent cells: Approaches, opportunities, challenges. Aging (Albany N. Y.) 2019;11:12844–12861. doi: 10.18632/aging.102557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nardella C., Clohessy J.G., Alimonti A., Pandolfi P.P. Pro-senescence therapy for cancer treatment. Nat. Rev. Cancer. 2011;11:503–511. doi: 10.1038/nrc3057. [DOI] [PubMed] [Google Scholar]
- 167.Yousefzadeh M.J., Zhu Y., McGowan S.J., Angelini L., Fuhrmann-Stroissnigg H., Xu M., Ling Y.Y., Melos K.I., Pirtskhalava T., Inman C.L., et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. 2018;36:18–28. doi: 10.1016/j.ebiom.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhu Y., Tchkonia T., Pirtskhalava T., Gower A.C., Ding H., Giorgadze N., Palmer A.K., Ikeno Y., Hubbard G.B., Lenburg M., et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Baar M.P., Brandt R.M.C., Putavet D.A., Klein J.D.D., Derks K.W.J., Bourgeois B.R.M., Stryeck S., Rijksen Y., van Willigenburg H., Feijtel D.A., et al. Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell. 2017;169:132–147.e16. doi: 10.1016/j.cell.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fuhrmann-Stroissnigg H., Ling Y.Y., Zhao J., McGowan S.J., Zhu Y., Brooks R.W., Grassi D., Gregg S.Q., Stripay J.L., Dorronsoro A., et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat. Commun. 2017;8:422. doi: 10.1038/s41467-017-00314-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Justice J.N., Nambiar A.M., Tchkonia T., LeBrasseur N.K., Pascual R., Hashmi S.K., Prata L., Masternak M.M., Kritchevsky S.B., Musi N., et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–563. doi: 10.1016/j.ebiom.2018.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Xu M., Pirtskhalava T., Farr J.N., Weigand B.M., Palmer A.K., Weivoda M.M., Inman C.L., Ogrodnik M.B., Hachfeld C.M., Fraser D.G., et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018;24:1246–1256. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang H., Wang Z., Huang Y., Zhou Y., Sheng X., Jiang Q., Wang Y., Luo P., Luo M., Shi C. Senolytics (DQ) Mitigates Radiation Ulcers by Removing Senescent Cells. Front. Oncol. 2019;9:1576. doi: 10.3389/fonc.2019.01576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhu Y., Tchkonia T., Fuhrmann-Stroissnigg H., Dai H.M., Ling Y.Y., Stout M.B., Pirtskhalava T., Giorgadze N., Johnson K.O., Giles C.B., et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell. 2016;15:428–435. doi: 10.1111/acel.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ovadya Y., Landsberger T., Leins H., Vadai E., Gal H., Biran A., Yosef R., Sagiv A., Agrawal A., Shapira A., et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat. Commun. 2018;9:5435. doi: 10.1038/s41467-018-07825-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Iannello A., Thompson T.W., Ardolino M., Lowe S.W., Raulet D.H. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J. Exp. Med. 2013;210:2057–2069. doi: 10.1084/jem.20130783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Stokes K.L., Cortez-Retamozo V., Acosta J., Lauderback B., Robles-Oteiza C., Cicchini M., Pittet M.J., Feldser D.M. Natural killer cells limit the clearance of senescent lung adenocarcinoma cells. Oncogenesis. 2019;8:24. doi: 10.1038/s41389-019-0133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Soriani A., Zingoni A., Cerboni C., Iannitto M.L., Ricciardi M.R., Di Gialleonardo V., Cippitelli M., Fionda C., Petrucci M.T., Guarini A., et al. ATM-ATR-dependent upregulation of DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK-cell susceptibility and is associated with a senescent phenotype. Blood. 2009;113:3503–3511. doi: 10.1182/blood-2008-08-173914. [DOI] [PubMed] [Google Scholar]
- 179.Ciaglia E., Laezza C., Abate M., Pisanti S., Ranieri R., D’Alessandro A., Picardi P., Gazzerro P., Bifulco M. Recognition by natural killer cells of N6-isopentenyladenosine-treated human glioma cell lines. Int. J. Cancer. 2018;142:176–190. doi: 10.1002/ijc.31036. [DOI] [PubMed] [Google Scholar]
- 180.Gorrini C., Harris I.S., Mak T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013;12:931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 181.Waldhauer I., Goehlsdorf D., Gieseke F., Weinschenk T., Wittenbrink M., Ludwig A., Stevanovic S., Rammensee H.G., Steinle A. Tumor-associated MICA is shed by ADAM proteases. Cancer Res. 2008;68:6368–6376. doi: 10.1158/0008-5472.CAN-07-6768. [DOI] [PubMed] [Google Scholar]
- 182.Liu G., Atteridge C.L., Wang X., Lundgren A.D., Wu J.D. The membrane type matrix metalloproteinase MMP14 mediates constitutive shedding of MHC class I chain-related molecule A independent of A disintegrin and metalloproteinases. J. Immunol. 2010;184:3346–3350. doi: 10.4049/jimmunol.0903789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Munoz D.P., Yannone S.M., Daemen A., Sun Y., Vakar-Lopez F., Kawahara M., Freund A.M., Rodier F., Wu J.D., Desprez P.Y., et al. Targetable mechanisms driving immunoevasion of persistent senescent cells link chemotherapy-resistant cancer to aging. JCI Insight. 2019;5:e124716. doi: 10.1172/jci.insight.124716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zingoni A., Cecere F., Vulpis E., Fionda C., Molfetta R., Soriani A., Petrucci M.T., Ricciardi M.R., Fuerst D., Amendola M.G., et al. Genotoxic Stress Induces Senescence-Associated ADAM10-Dependent Release of NKG2D MIC Ligands in Multiple Myeloma Cells. J. Immunol. 2015;195:736–748. doi: 10.4049/jimmunol.1402643. [DOI] [PubMed] [Google Scholar]
- 185.Sepult C., Bellefroid M., Rocks N., Donati K., Gerard C., Gilles C., Ludwig A., Duysinx B., Noel A., Cataldo D. ADAM10 mediates malignant pleural mesothelioma invasiveness. Oncogene. 2019;38:3521–3534. doi: 10.1038/s41388-018-0669-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ko S.Y., Lin S.C., Wong Y.K., Liu C.J., Chang K.W., Liu T.Y. Increase of disintergin metalloprotease 10 (ADAM10) expression in oral squamous cell carcinoma. Cancer Lett. 2007;245:33–43. doi: 10.1016/j.canlet.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 187.Carl-McGrath S., Lendeckel U., Ebert M., Roessner A., Rocken C. The disintegrin-metalloproteinases ADAM9, ADAM12, and ADAM15 are upregulated in gastric cancer. Int. J. Oncol. 2005;26:17–24. doi: 10.3892/ijo.26.1.17. [DOI] [PubMed] [Google Scholar]
- 188.Wolpert F., Tritschler I., Steinle A., Weller M., Eisele G. A disintegrin and metalloproteinases 10 and 17 modulate the immunogenicity of glioblastoma-initiating cells. Neuro Oncol. 2014;16:382–391. doi: 10.1093/neuonc/not232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sheppard S., Guedes J., Mroz A., Zavitsanou A.M., Kudo H., Rothery S.M., Angelopoulos P., Goldin R., Guerra N. The immunoreceptor NKG2D promotes tumour growth in a model of hepatocellular carcinoma. Nat. Commun. 2017;8:13930. doi: 10.1038/ncomms13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Krizhanovsky V., Yon M., Dickins R.A., Hearn S., Simon J., Miething C., Yee H., Zender L., Lowe S.W. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sagiv A., Burton D.G., Moshayev Z., Vadai E., Wensveen F., Ben-Dor S., Golani O., Polic B., Krizhanovsky V. NKG2D ligands mediate immunosurveillance of senescent cells. Aging (Albany N. Y.) 2016;8:328–344. doi: 10.18632/aging.100897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Okimoto T., Kotani H., Iida Y., Koyanagi A., Tanino R., Tsubata Y., Isobe T., Harada M. Pemetrexed sensitizes human lung cancer cells to cytotoxic immune cells. Cancer Sci. 2020;111:1910–1920. doi: 10.1111/cas.14401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pereira B.I., Devine O.P., Vukmanovic-Stejic M., Chambers E.S., Subramanian P., Patel N., Virasami A., Sebire N.J., Kinsler V., Valdovinos A., et al. Senescent cells evade immune clearance via HLA-E-mediated NK and CD8(+) T cell inhibition. Nat. Commun. 2019;10:2387. doi: 10.1038/s41467-019-10335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Borrelli C., Ricci B., Vulpis E., Fionda C., Ricciardi M.R., Petrucci M.T., Masuelli L., Peri A., Cippitelli M., Zingoni A., et al. Drug-Induced Senescent Multiple Myeloma Cells Elicit NK Cell Proliferation by Direct or Exosome-Mediated IL15 Trans-Presentation. Cancer Immunol. Res. 2018;6:860–869. doi: 10.1158/2326-6066.CIR-17-0604. [DOI] [PubMed] [Google Scholar]
- 195.Inao T., Kotani H., Iida Y., Kartika I.D., Okimoto T., Tanino R., Shiba E., Harada M. Different sensitivities of senescent breast cancer cells to immune cell-mediated cytotoxicity. Cancer Sci. 2019;110:2690–2699. doi: 10.1111/cas.14116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sharma C., Wang H.X., Li Q., Knoblich K., Reisenbichler E.S., Richardson A.L., Hemler M.E. Protein Acyltransferase DHHC3 Regulates Breast Tumor Growth, Oxidative Stress, and Senescence. Cancer Res. 2017;77:6880–6890. doi: 10.1158/0008-5472.CAN-17-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Michelet X., Dyck L., Hogan A., Loftus R.M., Duquette D., Wei K., Beyaz S., Tavakkoli A., Foley C., Donnelly R., et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat. Immunol. 2018;19:1330–1340. doi: 10.1038/s41590-018-0251-7. [DOI] [PubMed] [Google Scholar]
- 198.Marcais A., Cherfils-Vicini J., Viant C., Degouve S., Viel S., Fenis A., Rabilloud J., Mayol K., Tavares A., Bienvenu J., et al. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat. Immunol. 2014;15:749–757. doi: 10.1038/ni.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Esfahani K., Roudaia L., Buhlaiga N., Del Rincon S.V., Papneja N., Miller W.H., Jr. A review of cancer immunotherapy: From the past, to the present, to the future. Curr. Oncol. (Toronto Ont.) 2020;27(Suppl. 2):S87–S97. doi: 10.3747/co.27.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Das S., Johnson D.B. Immune-related adverse events and antitumor efficacy of immune checkpoint inhibitors. J. Immunother. Cancer. 2019;7:306. doi: 10.1186/s40425-019-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Arasanz H., Zuazo M., Bocanegra A., Gato M., Martínez-Aguillo M., Morilla I., Fernández G., Hernández B., López P., Alberdi N., et al. Early detection of hyperprogressive disease in non-small cell lung cancer by monitoring of systemic T cell dynamics. Cancers. 2020;12:344. doi: 10.3390/cancers12020344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sokol L., Cripe L., Kantarjian H., Sekeres M.A., Parmar S., Greenberg P., Goldberg S.L., Bhushan V., Shammo J., Hohl R., et al. Randomized, dose-escalation study of the p38α MAPK inhibitor SCIO-469 in patients with myelodysplastic syndrome. Leukemia. 2013;27:977–980. doi: 10.1038/leu.2012.264. [DOI] [PubMed] [Google Scholar]
- 203.Patnaik A., Haluska P., Tolcher A.W., Erlichman C., Papadopoulos K.P., Lensing J.L., Beeram M., Molina J.R., Rasco D.W., Arcos R.R., et al. A first-in-human phase i study of the oral p38 MAPK inhibitor, ralimetinib (LY2228820 Dimesylate), in patients with advanced cancer. Clin. Cancer Res. 2016;22:1095–1102. doi: 10.1158/1078-0432.CCR-15-1718. [DOI] [PubMed] [Google Scholar]
- 204.Vergote I., Heitz F., Buderath P., Powell M., Sehouli J., Lee C.M., Hamilton A., Fiorica J., Moore K.N., Teneriello M., et al. A randomized, double-blind, placebo-controlled phase 1b/2 study of ralimetinib, a p38 MAPK inhibitor, plus gemcitabine and carboplatin versus gemcitabine and carboplatin for women with recurrent platinum-sensitive ovarian cancer. Gynecol. Oncol. 2020;156:23–31. doi: 10.1016/j.ygyno.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 205.Vara-Ciruelos D., Dandapani M., Russell F.M., Grzes K.M., Atrih A., Foretz M., Viollet B., Lamont D.J., Cantrell D.A., Hardie D.G. Phenformin, But Not Metformin, Delays Development of T Cell Acute Lymphoblastic Leukemia/Lymphoma via Cell-Autonomous AMPK Activation. Cell Rep. 2019;27:690–698.e4. doi: 10.1016/j.celrep.2019.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Vara-Ciruelos D., Russell F.M., Hardie D.G. The strange case of AMPK and cancer: Dr Jekyll or Mr Hyde? Open Biol. 2019;9:190099. doi: 10.1098/rsob.190099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Frederick D.T., Piris A., Cogdill A.P., Cooper Z.A., Lezcano C., Ferrone C.R., Mitra D., Boni A., Newton L.P., Liu C., et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin. Cancer Res. 2013;19:1225–1231. doi: 10.1158/1078-0432.CCR-12-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Liu L., Mayes P.A., Eastman S., Shi H., Yadavilli S., Zhang T., Yang J., Seestaller-Wehr L., Zhang S.Y., Hopson C., et al. The BRAF and MEK inhibitors dabrafenib and trametinib: Effects on immune function and in combination with immunomodulatory antibodies targeting PD-1, PD-L1, and CTLA-4. Clin. Cancer Res. 2015;21:1639–1651. doi: 10.1158/1078-0432.CCR-14-2339. [DOI] [PubMed] [Google Scholar]
- 209.Ebert P.J.R., Cheung J., Yang Y., McNamara E., Hong R., Moskalenko M., Gould S.E., Maecker H., Irving B.A., Kim J.M., et al. MAP Kinase Inhibition Promotes T Cell and Antitumor Activity in Combination with PD-L1 Checkpoint Blockade. Immunity. 2016;44:609–621. doi: 10.1016/j.immuni.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 210.Moshofsky K.B., Cho H.J., Wu G., Romine K.A., Newman M.T., Kosaka Y., McWeeney S.K., Lind E.F. Acute myeloid leukemia–induced T-cell suppression can be reversed by inhibition of the MAPK pathway. Blood Adv. 2019;3:3038–3051. doi: 10.1182/bloodadvances.2019000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Loi S., Dushyanthen S., Beavis P.A., Salgado R., Denkert C., Savas P., Combs S., Rimm D.L., Giltnane J.M., Estrada M.V., et al. RAS/MAPK Activation Is Associated with Reduced Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer: Therapeutic Cooperation Between MEK and PD-1/PD-L1 Immune Checkpoint Inhibitors. Clin. Cancer Res. 2016;22:1499–1509. doi: 10.1158/1078-0432.CCR-15-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kang S.H., Keam B., Ahn Y.O., Park H.R., Kim M., Kim T.M., Kim D.W., Heo D.S. Inhibition of MEK with trametinib enhances the efficacy of anti-PD-L1 inhibitor by regulating antitumor immunity in head and neck squamous cell carcinoma. Oncoimmunology. 2019;8:e1515057. doi: 10.1080/2162402X.2018.1515057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Peng G., Guo Z., Kiniwa Y., Voo K.S., Peng W., Fu T., Wang D.Y., Li Y., Wang H.Y., Wang R.F. Immunology: Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–1384. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 214.Peng G., Wang H.Y., Peng W., Kiniwa Y., Seo K.H., Wang R.F. Tumor-Infiltrating γδ T Cells Suppress T and Dendritic Cell Function via Mechanisms Controlled by a Unique Toll-like Receptor Signaling Pathway. Immunity. 2007;27:334–348. doi: 10.1016/j.immuni.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 215.Ferris R.L., Saba N.F., Gitlitz B.J., Haddad R., Sukari A., Neupane P., Morris J.C., Misiukiewicz K., Bauman J.E., Fenton M., et al. Effect of Adding Motolimod to Standard Combination Chemotherapy and Cetuximab Treatment of Patients With Squamous Cell Carcinoma of the Head and Neck. JAMA Oncol. 2018;4:1583. doi: 10.1001/jamaoncol.2018.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Dietsch G.N., Randall T.D., Gottardo R., Northfelt D.W., Ramanathan R.K., Cohen P.A., Manjarrez K.L., Newkirk M., Bryan J.K., Hershberg R.M. Late-Stage Cancer Patients Remain Highly Responsive to Immune Activation by the Selective TLR8 Agonist Motolimod (VTX-2337) Clin. Cancer Res. 2015;21:5445–5452. doi: 10.1158/1078-0432.CCR-15-0578. [DOI] [PubMed] [Google Scholar]
- 217.Monk B.J., Brady M.F., Aghajanian C., Lankes H.A., Rizack T., Leach J., Fowler J.M., Higgins R., Hanjani P., Morgan M., et al. A phase 2, randomized, double-blind, placebo- controlled study of chemo-immunotherapy combination using motolimod with pegylated liposomal doxorubicin in recurrent or persistent ovarian cancer: A Gynecologic Oncology Group partners study. Ann. Oncol. 2017;28:996–1004. doi: 10.1093/annonc/mdx049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Monk B.J., Facciabene A., Brady W.E., Aghajanian C.A., Fracasso P.M., Walker J.L., Lankes H.A., Manjarrez K.L., Danet-Desnoyers G.H., Bell-McGuinn K.M., et al. Integrative Development of a TLR8 Agonist for Ovarian Cancer Chemoimmunotherapy. Clin. Cancer Res. 2017;23:1955–1966. doi: 10.1158/1078-0432.CCR-16-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Chow L.Q.M., Morishima C., Eaton K.D., Baik C.S., Goulart B.H., Anderson L.N., Manjarrez K.L., Dietsch G.N., Bryan J.K., Hershberg R.M., et al. Phase Ib Trial of the Toll-like Receptor 8 Agonist, Motolimod (VTX-2337), Combined with Cetuximab in Patients with Recurrent or Metastatic SCCHN. Clin. Cancer Res. 2017;23:2442–2450. doi: 10.1158/1078-0432.CCR-16-1934. [DOI] [PubMed] [Google Scholar]
- 220.Shayan G., Kansy B.A., Gibson S.P., Srivastava R.M., Bryan J.K., Bauman J.E., Ohr J., Kim S., Duvvuri U., Clump D.A., et al. Phase Ib Study of Immune Biomarker Modulation with Neoadjuvant Cetuximab and TLR8 Stimulation in Head and Neck Cancer to Overcome Suppressive Myeloid Signals. Clin. Cancer Res. 2018;24:62–72. doi: 10.1158/1078-0432.CCR-17-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Roex G., Feys T., Beguin Y., Kerre T., Poire X., Lewalle P., Vandenberghe P., Bron D., Anguille S. Chimeric Antigen Receptor-T-Cell Therapy for B-Cell Hematological Malignancies: An Update of the Pivotal Clinical Trial Data. Pharmaceutics. 2020;12:194. doi: 10.3390/pharmaceutics12020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Garfall A.L., Dancy E.K., Cohen A.D., Hwang W.T., Fraietta J.A., Davis M.M., Levine B.L., Siegel D.L., Stadtmauer E.A., Vogl D.T., et al. T-cell phenotypes associated with effective CAR T-cell therapy in postinduction vs relapsed multiple myeloma. Blood Adv. 2019;3:2812–2815. doi: 10.1182/bloodadvances.2019000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cohen A.D., Garfall A.L., Stadtmauer E.A., Melenhorst J.J., Lacey S.F., Lancaster E., Vogl D.T., Weiss B.M., Dengel K., Nelson A., et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J. Clin. Investig. 2019;129:2210–2221. doi: 10.1172/JCI126397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Helmink B.A., Reddy S.M., Gao J., Zhang S., Basar R., Thakur R., Yizhak K., Sade-Feldman M., Blando J., Han G., et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577:549–555. doi: 10.1038/s41586-019-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Petitprez F., de Reynies A., Keung E.Z., Chen T.W., Sun C.M., Calderaro J., Jeng Y.M., Hsiao L.P., Lacroix L., Bougouin A., et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020;577:556–560. doi: 10.1038/s41586-019-1906-8. [DOI] [PubMed] [Google Scholar]
- 226.Cabrita R., Lauss M., Sanna A., Donia M., Skaarup Larsen M., Mitra S., Johansson I., Phung B., Harbst K., Vallon-Christersson J., et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577:561–565. doi: 10.1038/s41586-019-1914-8. [DOI] [PubMed] [Google Scholar]
- 227.Zhang H., Alsaleh G., Feltham J., Sun Y., Napolitano G., Riffelmacher T., Charles P., Frau L., Hublitz P., Yu Z., et al. Polyamines Control eIF5A Hypusination, TFEB Translation, and Autophagy to Reverse B Cell Senescence. Mol. Cell. 2019;76:110–125.e9. doi: 10.1016/j.molcel.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lujambio A., Akkari L., Simon J., Grace D., Tschaharganeh D.F., Bolden J.E., Zhao Z., Thapar V., Joyce J.A., Krizhanovsky V., et al. Non-cell-autonomous tumor suppression by p53. Cell. 2013;153:449–460. doi: 10.1016/j.cell.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Frasca D., Diaz A., Romero M., Blomberg B.B. Ageing and obesity similarly impair antibody responses. Clin. Exp. Immunol. 2017;187:64–70. doi: 10.1111/cei.12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Prieto L.I., Baker D.J. Cellular Senescence and the Immune System in Cancer. Gerontology. 2019;65:505–512. doi: 10.1159/000500683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kansara M., Leong H.S., Lin D.M., Popkiss S., Pang P., Garsed D.W., Walkley C.R., Cullinane C., Ellul J., Haynes N.M., et al. Immune response to RB1-regulated senescence limits radiation-induced osteosarcoma formation. J. Clin. Investig. 2013;123:5351–5360. doi: 10.1172/JCI70559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ruhland M.K., Loza A.J., Capietto A.H., Luo X., Knolhoff B.L., Flanagan K.C., Belt B.A., Alspach E., Leahy K., Luo J., et al. Stromal senescence establishes an immunosuppressive microenvironment that drives tumorigenesis. Nat. Commun. 2016;7:11762. doi: 10.1038/ncomms11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wculek S.K., Cueto F.J., Mujal A.M., Melero I., Krummel M.F., Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020;20:7–24. doi: 10.1038/s41577-019-0210-z. [DOI] [PubMed] [Google Scholar]
- 234.David J.M., Dominguez C., Hamilton D.H., Palena C. The IL-8/IL-8R Axis: A Double Agent in Tumor Immune Resistance. Vaccines (Basel) 2016;4:22. doi: 10.3390/vaccines4030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Orjalo A.V., Bhaumik D., Gengler B.K., Scott G.K., Campisi J. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc. Natl. Acad. Sci. USA. 2009;106:17031–17036. doi: 10.1073/pnas.0905299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kuan E.L., Ziegler S.F. A tumor-myeloid cell axis, mediated via the cytokines IL-1alpha and TSLP, promotes the progression of breast cancer. Nat. Immunol. 2018;19:366–374. doi: 10.1038/s41590-018-0066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Su Y., Xu C., Sun Z., Liang Y., Li G., Tong T., Chen J. S100A13 promotes senescence-associated secretory phenotype and cellular senescence via modulation of non-classical secretion of IL-1alpha. Aging (Albany N. Y.) 2019;11:549–572. doi: 10.18632/aging.101760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wiggins K.A., Parry A.J., Cassidy L.D., Humphry M., Webster S.J., Goodall J.C., Narita M., Clarke M.C.H. IL-1alpha cleavage by inflammatory caspases of the noncanonical inflammasome controls the senescence-associated secretory phenotype. Aging Cell. 2019;18:e12946. doi: 10.1111/acel.12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Di Mitri D., Mirenda M., Vasilevska J., Calcinotto A., Delaleu N., Revandkar A., Gil V., Boysen G., Losa M., Mosole S., et al. Re-education of Tumor-Associated Macrophages by CXCR2 Blockade Drives Senescence and Tumor Inhibition in Advanced Prostate Cancer. Cell Rep. 2019;28:2156–2168.e5. doi: 10.1016/j.celrep.2019.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Di Mitri D., Toso A., Chen J.J., Sarti M., Pinton S., Jost T.R., D’Antuono R., Montani E., Garcia-Escudero R., Guccini I., et al. Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature. 2014;515:134–137. doi: 10.1038/nature13638. [DOI] [PubMed] [Google Scholar]
- 241.Loo T.M., Kamachi F., Watanabe Y., Yoshimoto S., Kanda H., Arai Y., Nakajima-Takagi Y., Iwama A., Koga T., Sugimoto Y., et al. Gut Microbiota Promotes Obesity-Associated Liver Cancer through PGE2-Mediated Suppression of Antitumor Immunity. Cancer Discov. 2017;7:522–538. doi: 10.1158/2159-8290.CD-16-0932. [DOI] [PubMed] [Google Scholar]
- 242.Iannello A., Raulet D.H. Immunosurveillance of senescent cancer cells by natural killer cells. Oncoimmunology. 2014;3:e27616. doi: 10.4161/onci.27616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ratushnyy A., Ezdakova M., Buravkova L. Secretome of Senescent Adipose-Derived Mesenchymal Stem Cells Negatively Regulates Angiogenesis. Int. J. Mol. Sci. 2020;21:1802. doi: 10.3390/ijms21051802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Vilgelm A.E., Pawlikowski J.S., Liu Y., Hawkins O.E., Davis T.A., Smith J., Weller K.P., Horton L.W., McClain C.M., Ayers G.D., et al. Mdm2 and aurora kinase a inhibitors synergize to block melanoma growth by driving apoptosis and immune clearance of tumor cells. Cancer Res. 2015;75:181–193. doi: 10.1158/0008-5472.CAN-14-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Huang X., Li Y., Fu M., Xin H.B. Polarizing Macrophages In Vitro. Methods Mol. Biol. 2018;1784:119–126. doi: 10.1007/978-1-4939-7837-3_12. [DOI] [PMC free article] [PubMed] [Google Scholar]