Abstract
Amyloid beta (Aβ) accumulation in the brain is one of the major pathological features of Alzheimer’s disease. The active form of vitamin D (1,25(OH)2D3), which acts via its nuclear hormone receptor, vitamin D receptor (VDR), has been implicated in the treatment of Aβ pathology, and is thus considered as a neuroprotective agent. However, its underlying molecular mechanisms of action are not yet fully understood. Here, we aim to investigate whether the molecular mechanisms of 1,25(OH)2D3 in ameliorating Aβ toxicity involve an interplay of glial cell line-derived neurotrophic factor (GDNF)-signaling in SH-SY5Y cells. Cells were treated with Aβ(25-35) as the source of toxicity, followed by the addition of 1,25(OH)2D3 with or without the GDNF inhibitor, heparinase III. The results show that 1,25(OH)2D3 modulated Aβ-induced reactive oxygen species, apoptosis, and tau protein hyperphosphorylation in SH-SY5Y cells. Additionally, 1,25(OH)2D3 restored the decreasing GDNF and the inhibited phosphorylation of the phosphatidylinositol 3 kinase (PI3K)/protein kinase B (Akt)/glycogen synthase kinase-3β (GSK-3β) protein expressions. In the presence of heparinase III, these damaging effects evoked by Aβ were not abolished by 1,25(OH)2D3. It appears 1,25(OH)2D3 is beneficial for the alleviation of Aβ neurotoxicity, and it might elicit its neuroprotection against Aβ neurotoxicity through an interplay with GDNF-signaling.
Keywords: Amyloid beta, Alzheimer’s disease, vitamin D, vitamin D receptor, GDNF, tau protein
1. Introduction
Alzheimer’s disease (AD) is one of the most commonly occurring neurodegenerative diseases, and it is characterized by two pathologic feature: aberrant deposition of amyloid beta (Aβ) in extracellular plaques and intracellular accumulation of phosphorylated tau proteins in the brain [1,2,3]. This disease clinically presents slow progressive memory loss and cognitive deficits. Whether aberrant Aβ and tau proteins are key mechanisms in response to the AD-associated neuronal loss and death is still poorly understood. Aberrant Aβ has been gaining increasing attention due to the possibility of AD pathogenesis being initiated by this event and as a probable mediator of tau-pathology [4], although there is controversy concerning whether aberrant Aβ is a prerequisite for the hyperphosphorylation of tau protein [5]. The neurotoxicity of Aβ(1-42), which is the predominant Aβ species, has been addressed in both in vivo and in vitro models, and putative underlying mechanisms of its actions include reactive oxygen species (ROS) production and cell apoptosis [6,7]. A direct link between Aβ neurotoxicity and ROS production has been increasingly shown with methionine located at residue 35 (methionine 35) of Aβ(1-42) [8]. It has been proposed that methionine 35 of Aβ has the greatest vulnerability to oxidation, and is prone to being attacked by various radicals [7,8]. In in vitro and in vivo experiments, when methionine 35 residue of Aβ(25-35) and Aβ (1-42) was substituted with norleucine, oxidative stress and neurotoxicity were prevented [7,9]. It is noted that attention has been specifically directed to neurotoxicity and apoptotic cell death induced by Aβ(25-35), the short fragment of full length Aβ (1-42) with a retained methionine 35 residue and greater toxicity [10]. In this context, methionine 35 residue of Aβ(25-35) has been proposed to be an important contributor to apoptotic cell death involved in Aβ(25-35)-mediated neurotoxic properties [11]. Collectively, Aβ itself, under pathological conditions, has high potential to promote ROS production, which in turn may exacerbate the damage of oxidative stress (including lipid peroxidation). Notably, enhanced oxidative stress has been implicated in the pathogenesis of AD [12]. It is probable that excessive production of ROS in response to aberrant Aβ also activates apoptosis, suggesting an interplay among the production of ROS, the induction of apoptosis, and the toxicity of Aβ at the cellular level. A putative cellular signaling pathway that regulates this interplay is assumed to be the PI3K/Akt/GSK-3β pathway [13,14,15]. Thus, it is possible that antioxidants capable of mediating this pathway may be potential therapeutic targets for modulating Aβ-induced neurotoxicity.
Intriguingly, vitamin D, particularly its active form, 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3), has emerged recently as a new, attractive agent for combating AD [16,17]. Epidemiological studies strongly associated higher vitamin D intake with a lower risk of AD [18]. A significant decrease in the vitamin D repository in the body was found in patients with mild cognitive impairment status [19]. In addition, vitamin D deficiency occurring in older adults is closely linked to neurological dysfunction and cognitive decline [20]. Collectively, it is apparent that the active form of vitamin D (1,25(OH)2D3) may be of therapeutic value in AD. It is well-documented that 1,25(OH)2D3 exerts its actions via its nuclear hormone receptor, the vitamin D receptor (VDR), known as a transcription factor [21]. The interaction of VDR with its ligand 1,25(OH)2D3 is responsible for regulation of the target gene transcription in relation to a wide array of physiological functions, such as calcium homeostasis, cell proliferation, and cell differentiation [21,22]. VDRs are widely distributed in the brain, suggesting the importance of vitamin D in maintaining neurophysiological functions of the brain including neurodevelopment, neuronal proliferation, and neuronal survival [16,22]. The neuroprotective potential of 1,25(OH)2D3 against AD may be mediated by multiple mechanisms, such as anti-inflammatory effects [23], antioxidant properties [24], and enhancement of Aβ clearance [25]. However, the molecular mechanisms by which1,25(OH)2D3 exerts its neuroprotective effects have not yet been completely elucidated. One possible mode of action may be related to the regulation of the neurotrophic factors such as the brain glial cell line-derived neurotrophic factor (GDNF) at the cellular level [22].
Evidence exists that the 1,25(OH)2D3-VDR pathway plays a key role in the stimulation of the synthesis of GDNF, which promotes neuronal functions including neuron survival and differentiation, and neurite branching in the brain [26,27,28,29,30]. Interestingly, it was shown that a deficiency of vitamin D in the brain causes a decrease in GDNF expression [31]. Likewise, decreased GDNF levels in the blood and increased GDNF levels in cerebrospinal fluid samples of AD patients have been detected, suggesting that GDNF could be a putative target protein for AD-related pathology [32]. Therefore, the common protein, GDNF, involving two distinct cellular events, the 1,25(OH)2D3-VDR pathway and AD-pathological targets, is conducive for positing a mechanistic hypothesis that the protective mechanisms of 1,25(OH)2D3 against Aβ neurotoxicity could be driven by VDR-ligand 1,25(OH)2D3/GDNF interplay that targets the PI3K/AKT/GSK-3β pathway. To test this hypothesis, the human neuroblastoma cell line, SH-SY5Y, was pre-incubated with Aβ(25-35) followed by treatment with the addition of 1,25(OH)2D3. In the present study, cell viability, intracellular ROS, apoptosis, and phosphorylated tau protein were determined to reflect the putative neuroprotective effects of 1,25(OH)2D3 on modulating Aβ-related pathology. Moreover, Western blot analysis was employed to examine the Aβ-induced alterations in cellular mediators, including VDR and GDNF, and molecules of the PI3K/AKT/GSK-3β signaling pathway in SH-SY5Y cells.
2. Results
2.1. Effects of 1,25(OH)2D3 on Cell Morphology, Cell Viability, and Protein Expression of VDR and GDNF After Aβ(25-35) Treatment
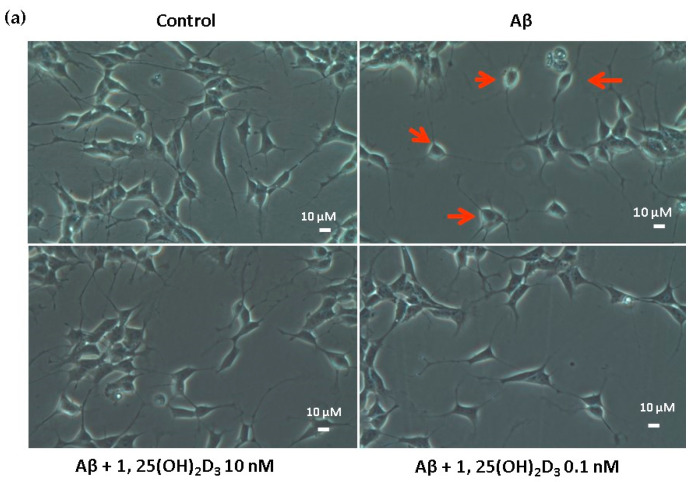
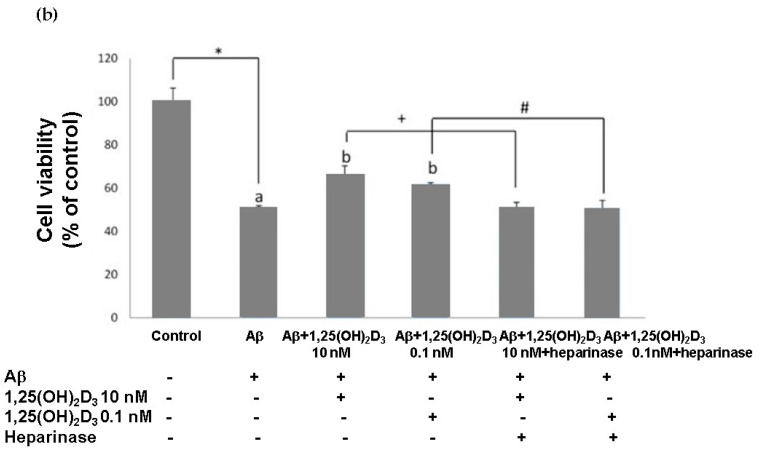
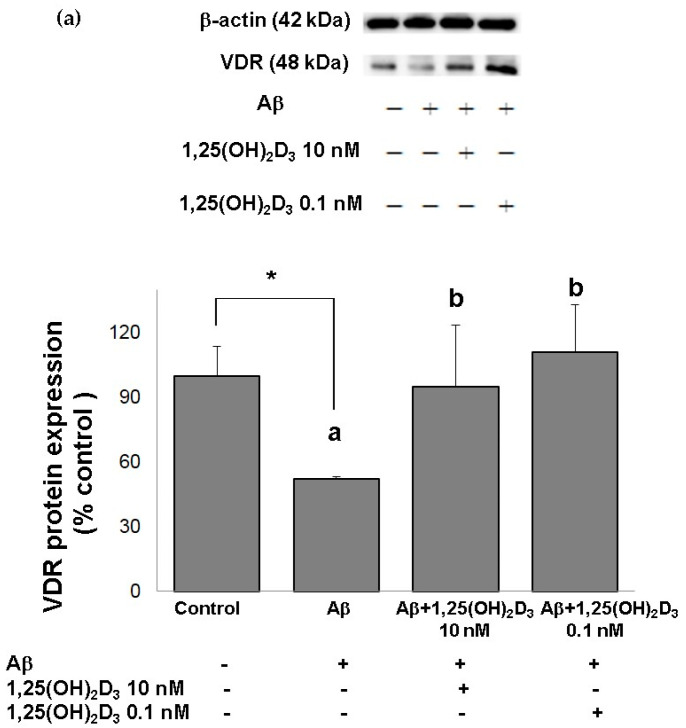
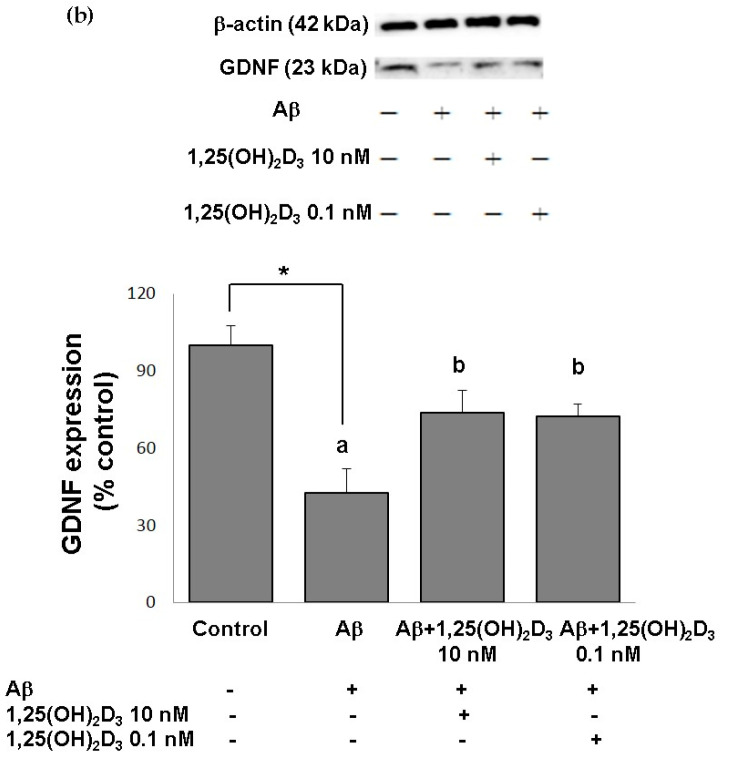
In this study, microscopic observation revealed overt abnormality of SH-SY5Y cell morphology after Aβ(25-35) stimulation, in which round-shaped cells without the extension of neurites were observed (shown by red arrows in Figure 1a), implying the deteriorating effect induced by Aβ(25-35) on neurite extension. However, as shown in Figure 1a, an angular shape and longer neurites of SH-SY5Y cells after 1,25(OH)2D3 treatment for 24 h were seen, suggesting that neurite extension was promoted by 1,25(OH)2D3. Moreover, the results from the MTT assay showed that Aβ(25-35) exposure significantly reduced cell viability when compared to the control group, whilst post-treatment with 1,25(OH)2D3 blocked this effect (Figure 1b). Further, to understand the toxic effects of Aβ(25-35) on protein expressions of VDR and GDNF, Western blotting analysis was employed. It was observed that Aβ(25-35) significantly decreased VDR (Figure 2a) and GDNF (Figure 2b) protein expressions compared to the control group (p < 0.05). Different dosages of 1,25(OH)2D3, 0.1 and 10 nM, were added after the Aβ(25-35) treatment, and both dosages significantly increased VDR and GDNF protein expressions compared to the Aβ(25-35) group (p < 0.05) (Figure 2). These results suggest that Aβ(25-35) was cytotoxic to the SH-SY5Y cells, leading to downregulations of VDR and GDNF, but these effects were able to be attenuated by 1,25(OH)2D3. Next, to verify the role of GDNF in mediating the neuroprotection of 1,25(OH)2D3 against Aβ(25-35) cytotoxicity, cells were then pretreated with Aβ(25-35) for 24 h prior to the addition of 1,25(OH)2D3 with or without the GDNF inhibitor, heparinase III, for 24 h. It was found that the presence of heparinase III significantly suppressed the cell viability induced by 1,25(OH)2D3 (Figure 1b). Altogether, these results support our hypothesis that the action of GDNF might be required for 1,25(OH)2D3-induced attenuation of cell survival evoked by Aβ(25-35).
Figure 1.
Effects of 1,25(OH)2D3 on Aβ-induced changes in SH-SY5Y cell morphology and cell viability. (a) SH-SY5Y cell morphology. Bar, 10 μM. Images were analyzed with SPOT 4.7 Advanced software. The arrows indicate the shorter neurite outgrowth of SH-SY5Y cells after the Aβ(25-35) challenge. (b) SH-SY5Y cell viability was analyzed by an MTT assay. SH-SY5Y cells were incubated with 1 μM Aβ(25-35) prior to the addition of 0.1 and 10 nM 1,25(OH)2D3 with or without heparinase III for 24 h. Data are presented as the mean ± SD of three experiments, and each experiment included triplicate repeats. *,+,# Significantly differs between the two groups (statistical analysis was performed using Student’s t test). Bars of Aβ, Aβ + 0.1 nM 1,25(OH)2D3, and Aβ + 10 nM 1,25(OH)2D3 with different letters significantly differ (p < 0.05) (statistical analysis was performed using one-way analysis of variance (ANOVA) with Duncan’s post-hoc analysis).
Figure 2.
Effects of 1,25(OH)2D3 on Aβ-induced changes in vitamin D receptor (VDR) (a) and glial cell line-derived neurotrophic factor (GDNF) (b) protein expressions. SH-SY5Y cells were incubated with 1 μM Aβ(25-35) prior to the addition of 0.1 and 10 nM 1,25(OH)2D3 for 24 h.* Significantly differs from the control group (statistical analysis was performed using Student’s t test). Bars of Aβ, Aβ + 0.1 nM 1,25(OH)2D3, and Aβ + 10 nM 1,25(OH)2D3 with different letters significantly differ from each other (p < 0.05) (statistical analysis was performed using one-way analysis of variance (ANOVA) with Duncan’s post-hoc analysis). All data are expressed as mean ± SD of three experiments, and each experiment included triplicate repeats.
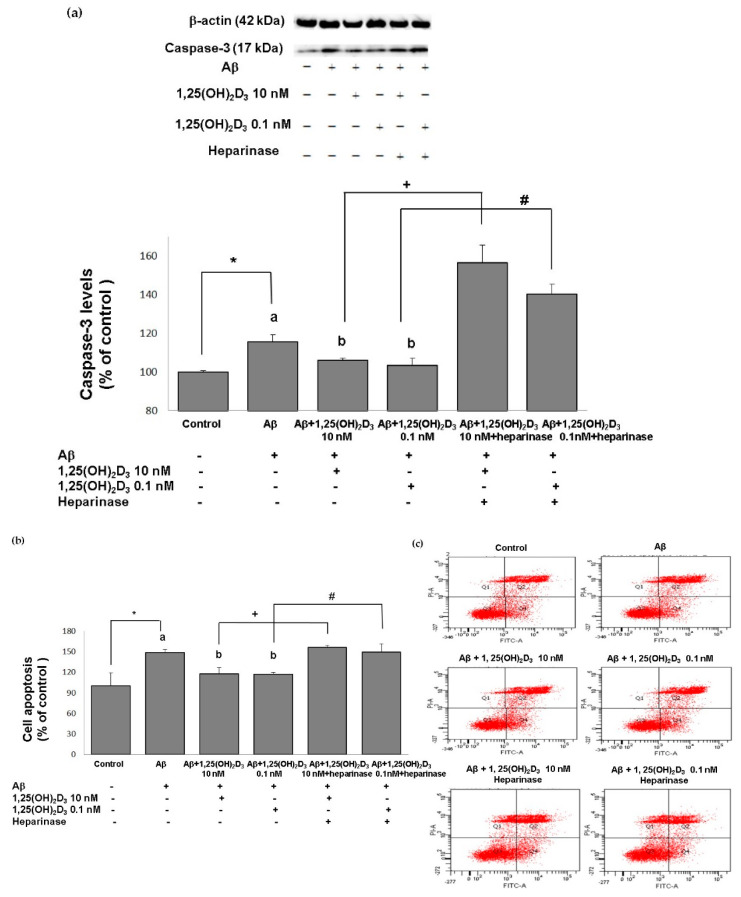
2.2. Effects of 1,25(OH)2D3 on Activating Caspase-3 and Cell Apoptosis after Aβ(25-35) Treatment
The neuroprotective role of 1,25(OH)2D3 was also validated by the apoptosis-related approaches, and similar results were revealed. The group treated with Aβ(25-35) exhibited significantly increased expression of activated caspase-3, a marker of cell death (Figure 3a), compared to the control group (p < 0.05), along with significant promotion of cell apoptosis (p < 0.05, Figure 3b,c). When 1,25(OH)2D3 was added after Aβ(25-35) treatment, it significantly decreased activated caspase-3 expression and cell apoptosis, compared to those of the Aβ(25-35) group (p < 0.05, Figure 3). These findings demonstrate that the Aβ(25-35) exposure resulted in apoptotic cell death, and this effect was attenuated by the 1,25(OH)2D3 treatment. In addition, Aβ(25-35)-induced apoptotic cell death and caspase-3 activation was unaffected by the 1,25(OH)2D3 treatment in the presence of heparinase III (Figure 3). Altogether, these observations support that the neuroprotective effects of 1,25(OH)2D3 on Aβ(25-35)-induced apoptosis might be elicited through the action of GDNF.
Figure 3.
Effects of 1,25(OH)2D3 on Aβ-induced changes in cell apoptosis. (a) Western blot analysis of caspase-3 protein expression in SH-SY5Y cells. (b) Percentages of apoptotic cells in each group quantified from (c). (c) Representative profiles of cell apoptosis detected by flow cytometry with Annexin V/propidium iodide double-staining. SH-SY5Y cells were incubated with 1 μM Aβ(25-35) prior to the addition of 0.1 and 10 nM 1,25(OH)2D3 with or without heparinase III for 24 h. Data are presented as the mean ± SD of three experiments and each experiment included triplicate repeats. *,+,# Significantly differs between the two groups (statistical analysis was performed using Student’s t test). Bars of Aβ, Aβ + 0.1 nM 1,25(OH)2D3, and Aβ + 10 nM 1,25(OH)2D3 with different letters significantly differ (p < 0.05) (statistical analysis was performed using one-way analysis of variance (ANOVA) with Duncan’s post-hoc analysis).
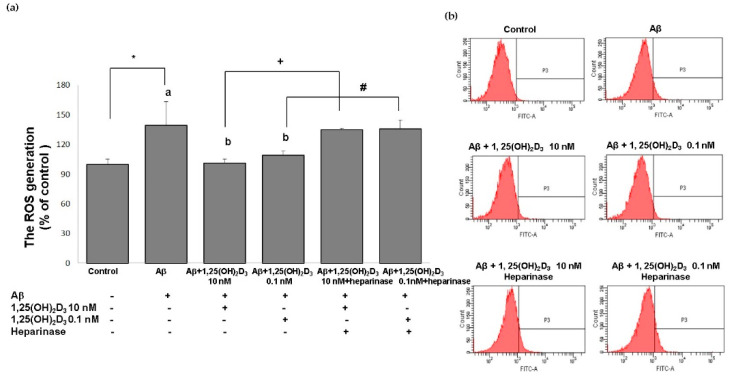
2.3. Effects of 1,25(OH)2D3 on Intracellular ROS after Aβ(25-35) Treatment
One promising mechanism underlying Aβ-induced apoptotic cell death is attributed to excessive production of ROS [33]. In order to better understand the neuroprotective mechanisms of 1,25(OH)2D3, in this study, the intracellular ROS levels were determined in SH-SY5Y cells using the DCF-DA assay. Figure 4 shows that the group treated with Aβ(25-35) exhibited significantly increased intracellular ROS production compared to the control group (p < 0.05). When 1,25(OH)2D3 at 0.1 and 10 nM was added after Aβ treatment, it caused a significant decrease in intracellular ROS compared to the Aβ group (p < 0.05). Heparinase III counteracted the effect of 1,25(OH)2D3 on intracellular ROS generation (p < 0.05, Figure 4). From these results, 1,25(OH)2D3 could significantly scavenge intracellular ROS triggered by Aβ(25-35), suggesting an antioxidant potential of 1,25(OH)2D3. Furthermore, these results support our central hypothesis that the role of GDNF was closely associated with the antioxidative effect of 1,25(OH)2D3 against Aβ(25-35)-induced intracellular ROS generation.
Figure 4.
Effects of 1,25(OH)2D3 on Aβ-induced intracellular reactive oxygen species (ROS) production. (a) Quantitative results of ROS levels in each group according to (b). (b) Representative profiles of the intracellular ROS levels detected by flow cytometry using the 2’,7’-dichlorofluoroescin diacetate (DCFH-DA) assay. SH-SY5Y cells were incubated with 1 μM Aβ(25-35) prior to the addition of 0.1 and 10 nM 1,25(OH)2D3 with or without heparinase III for 24 h. Data are presented as the mean ± SD of three experiments, and each experiment included triplicate repeats. *,+,# Significantly differs between the two groups (statistical analysis was performed using Student’s t test). Bars of Aβ, Aβ + 0.1 nM 1,25(OH)2D3, and Aβ + 10 nM 1,25(OH)2D3 with different letters significantly differ (p < 0.05) (statistical analysis was performed using one-way analysis of variance (ANOVA) with Duncan’s post-hoc analysis).
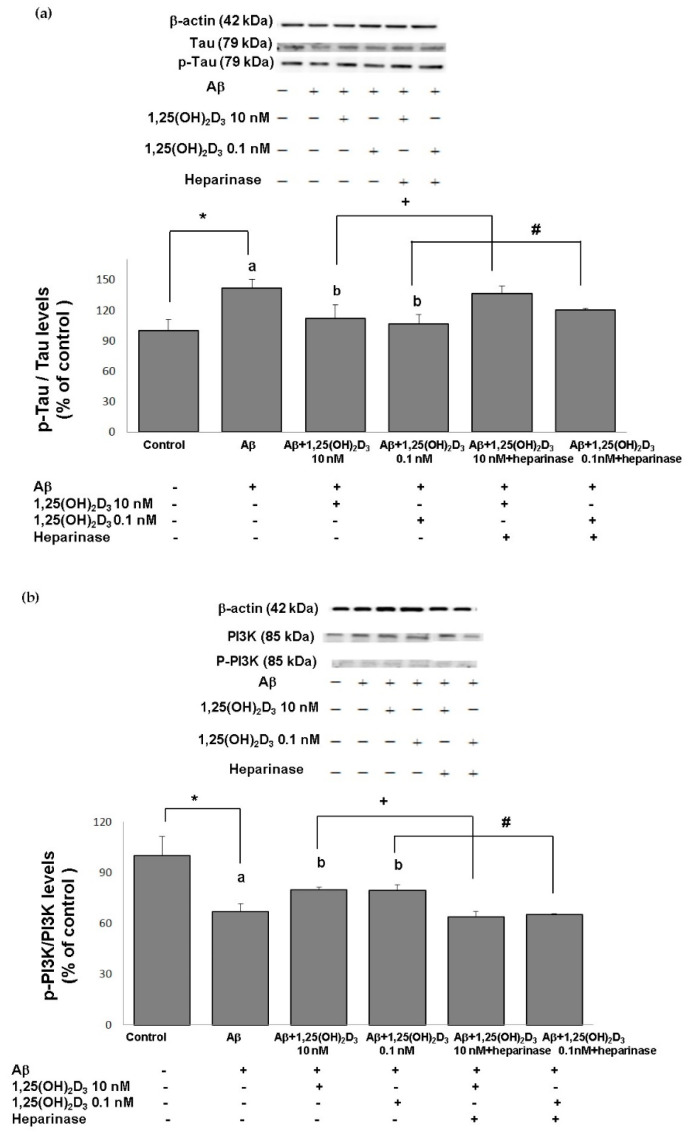
2.4. Effects of 1,25(OH)2D3 on the p-Tau/Tau Ratio after Aβ(25-35) Treatment
Excess generation of ROS has been shown to play a crucial role in the mechanisms associated with Aβ-induced neurotoxicity as well as tau pathology [34,35]. Herein, we first examine the level of tau phosphorylation in cells exposed to Aβ(25-35) prior to 1,25(OH)2D3 treatment. The group treated with Aβ exhibited a significant increase in the p-tau/tau ratio compared to the control group (Figure 5a). When 1,25(OH)2D3 at 0.1 and 10 nM was added after the Aβ(25-35) treatment, it caused a significant decrease in the p-tau/tau ratio compared to the Aβ group (p < 0.05, Figure 5a). The presence of heparinase III significantly increased the p-tau/tau ratio (p < 0.05, Figure 5a). These results show that 1,25(OH)2D3 was able to inhibit Aβ(25-35)-stimulated tau phosphorylation, and this action was linked to GDNF action.
Figure 5.
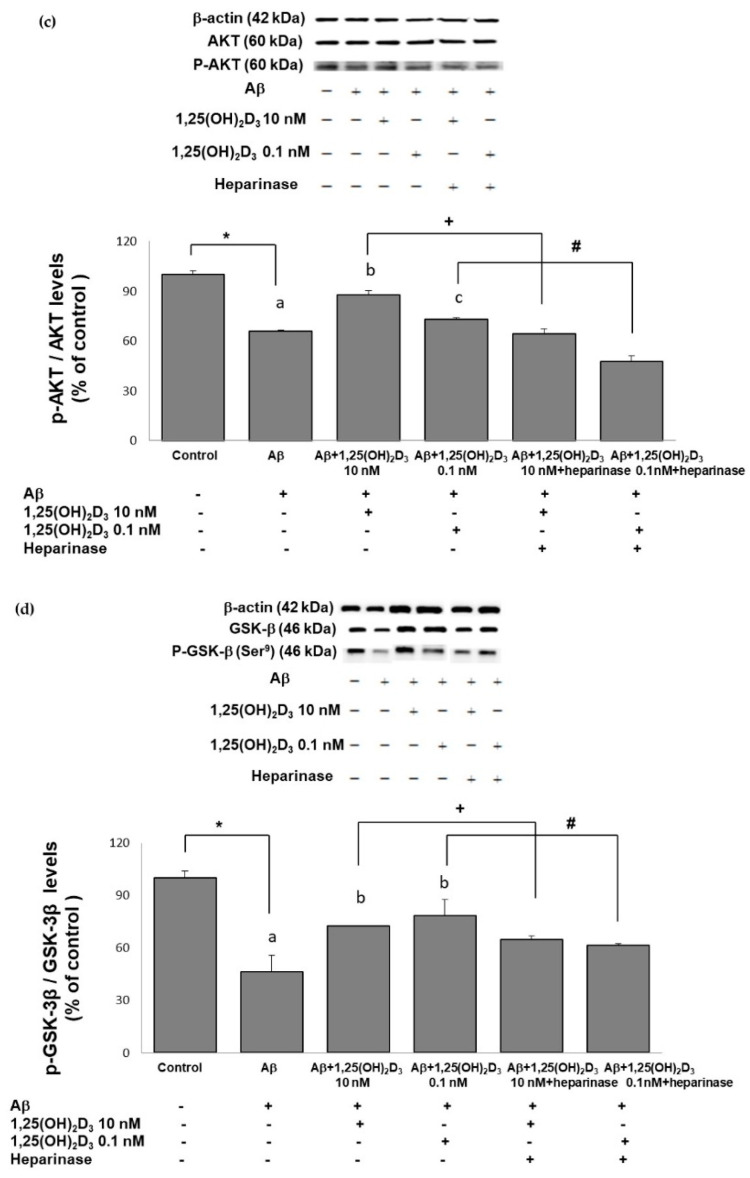
Effects of 1,25(OH)2D3 on Aβ-induced changes in the phosphorylated (p)-tau/tau ratio (a), phosphorylated (p)-phosphatidylinositol 3K (PI3K)/PI3K ratio (b), the phosphorylated (p)-Akt/Akt ratio (c), and the phosphorylated (p)-glycogen synthase kinase (GSK)-3β (Ser9)/GSK-3β ratio (d) of protein expressions. SH-SY5Y cells were incubated with 1 μM Aβ(25-35) prior to the addition of 0.1 and 10 nM 1,25(OH)2D3 with or without heparinase III for 24 h. Data are presented as the mean ± SD of three experiments, and each experiment included triplicate repeats. *,+,# Significantly differs between the two groups (statistical analysis was performed using Student’s t test). Bars of Aβ, Aβ + 0.1 nM 1,25(OH)2D3, and Aβ + 10 nM 1,25(OH)2D3 with different letters significantly differ (p < 0.05) (statistical analysis was performed using one-way analysis of variance (ANOVA) with Duncan’s post-hoc analysis).
2.5. Effects of 1,25(OH)2D3 on the p-PI3K/PI3K, p-Akt/Akt, and p-GSK-3β (Ser9)/GSK-3β Ratios after Aβ(25-35) Treatment
One of the putative cellular mechanisms by which Aβ(25-35) induces ROS and apoptotic cell death involves the aforementioned dysregulation of the PI3K/Akt/GSK-3β signaling pathway. Therefore, we next examined the neuroprotective effects of 1,25(OH)2D3 on several proteins of this pathway after Aβ(25-35) exposure by the use of Western blotting analysis. The group treated with Aβ(25-35) exhibited significant decreases in the p-PI3K/PI3K (Figure 5b), p-Akt/Akt (Figure 5c), and p-GSK-3β (Ser9)/GSK-3β (Figure 5d) ratios compared to the control group (p < 0.05). Treatment with 1,25(OH)2D3 at 0.1 and 10 nM after Aβ(25-35) treatment significantly increased the phosphorylation of these proteins compared to the Aβ(25-35) group (p < 0.05, Figure 5). However, with the presence of heparinase III, a GDNF-signaling inhibitor, the effect of 1,25(OH)2D3 on phosphorylation was reduced (p < 0.05, Figure 5). These results revealed that 1,25(OH)2D3 was able to stimulate Aβ(25-35)-inhibited phosphorylation of PI3K, Akt, and GSK-3β, and such stimulation appeared to be related to GDNF action.
3. Discussion
It is recognized that aberrant Aβ exhibits neurotoxicity that contributes to neuronal death, and this event is thought to be the primary factor that initiates the pathogenesis of AD [36,37]. Several neurotoxic effects of Aβ shown in the present study are consistent with previous studies [38,39]. For instance, we observed changes in cell morphology and tau phosphorylation, and increase in the number of apoptotic cells in parallel with the excess generation of ROS after Aβ treatment. These findings support that Aβ-associated oxidative stress was involved in the observed neuronal damage, and thus played an important role in Aβ neurotoxicity [34]. Furthermore, the morphology of neuronal cells is stabilized by the tau protein [40]. Once the tau protein is hyperphosphorylated, as observed after Aβ treatment in this study, it failed to maintain the cell structure [41] and caused cell apoptosis [2,42,43]. It is worth mentioning that neurons are capable of protecting against oxidative damage through secreting neurotrophic factors; as neurotrophic factors decrease, neurons are unable to eliminate the accumulated ROS [15,44]. In this study, we observed that both ROS production and cellular apoptosis increased as GDNF expression decreased after Aβ treatment. Hence, we speculate that Aβ might exert its toxic effects by inhibiting the action of GDNF and augmenting oxidative stress and apoptosis. In this regard, given the strong implication of excessive production of ROS and the reduction in GDNF levels in the mechanisms of Aβ neurotoxicity, it is plausible that antioxidants could be effective in the treatment of Aβ-related pathological processes [45].
In recent years, 1,25(OH)2D3 has received great attention due to its therapeutic potential as a potent antioxidant and neuroprotectant [24,46]. In the brain, 1,25(OH)2D3 regulates the neurotrophic factors via VDR, thereby controlling neuronal survival, development, and function [47]. There is evidence that protein and gene expressions of GDNF can be elevated by the binding of 1,25(OH)2D3 to the VDR [48,49]. As 1,25(OH)2D3 binds to the VDR, the protein and gene expressions of the VDR also increase [50,51]. In contrast, it was found that Aβ suppresses the protein and gene expressions of the VDR [52]. In the present study, we first confirmed that VDR and GDNF expressions were both suppressed by Aβ treatment in our model. This suppression of VDR and GDNF expression was reversed after the addition of 1,25(OH)2D3, indicating that the upregulation of GDNF may be a consequence of the formation of the 1,25(OH)2D3/VDR complex. These data suggest that VDR activity may be linked to GDNF production [53]. Taken together, we hypothesized that for 1,25(OH)2D3 to elicit its anti-Aβ cytotoxicity, GDNF-signaling may be required as a cooperating event. Our hypothesis is supported by a previous study reporting that a GDNF mechanism potentially participates in anti-neurotoxicity of 1,25(OH)2D3, regardless of the type of toxic substances administered [54]. Our observations described below corroborate such statements. Suppression of ROS production and apoptotic cell death after the administration of 1,25(OH)2D3 supports the theory that 1,25(OH)2D3 may act as an antioxidant as well as a neuroprotectant to ameliorate Aβ-induced oxidative damage [22]. To determine whether this protection involves the upregulation of GDNF in response to 1,25(OH)2D3, we utilized heparinase III to block GDNF signaling and found that the generation of ROS was indeed not affected. A recent study has established that GDNF-signaling in dopaminergic neurons is regulated by 1,25(OH)2D3 [55], which supports our discovery of an interplay between 1,25(OH)2D3-VDR and GDNF signaling. We therefore postulate that the ability of 1,25(OH)2D3 to decrease ROS produced by Aβ may occur, at least in part, through direct interactions of 1,25(OH)2D3 with GDNF at the cellular levels.
In an attempt to further understand the interaction between 1,25(OH)2D3-VDR and GDNF-signaling against Aβ neurotoxicity, the PI3K/AKT/GSK-3β pathway was examined due to its involvement in the promotion of cell survival and the pathogenesis of AD [56,57]. The GDNF-stimulated PI3K/Akt pathway regulates phosphorylation of GSK-3β (Ser9) and cell survival [58,59,60,61]. It was indicated that Aβ also decreases GDNF secretion and increases activation of GSK-3β, promoting tau protein hyperphosphorylation and neuronal apoptosis in the brain [62,63,64,65,66,67]. Inactivation of Akt in the brain causes the amyloid protein precursor (APP) to accumulate [64]. In the present study, we observed that Aβ treatment downregulated the activated form of PI3K/Akt and that 1,25(OH)2D3 reversed this dysregulation. Akt is the main regulator of GSK-3β [9]. Activation of GSK-3β causes greater Aβ accumulation and promotion of cell apoptosis through caspase-3 activation [62,68]. Activation of GSK-3β decreases as Akt is phosphorylated (i.e., activated), which results in greater cell survival [62]. Importantly, the major cause of the decrease in activation of Akt is downregulation of neurotrophic factors [62]. In the present study, we found that Aβ treatment enhanced the activated forms of GSK-3β and caspase-3, and tau protein hyperphosphorylation, but 1,25(OH)2D3 administration normalized these hyperactivations caused by Aβ. Altogether, in the present study, 1,25(OH)2D3 potentiated PI3K/Akt activation and subsequently led to the inactivation of downstream GSK-3β upon Aβ challenge. These findings demonstrate a putative neuroprotective role of 1,25(OH)2D3 against Aβ neurotoxicity by acting on the PI3K/AKT/GSK-3β pathway. In addition, blockage of the GDNF upregulation by heparinase III was likely to prevent the aforementioned beneficial effects of 1,25(OH)2D3. Our data suggest that the protective PI3K/AKT/GSK-3β pathway involving GSK-3β inactivation may be partially mediated through GDNF [65]. Therefore, we propose that GDNF signaling might be an important driving mechanism underlying the 1,25(OH)2D3-mediated modulating effects on Aβ-induced neurotoxicity.
As mentioned previously, PI3K/AKT pathway activation mediated by GDNF contributes to neuronal survival, making cells resistant to apoptosis [58]. In this study, PI3K/Akt downregulation may have resulted in activation of GSK-3β and caspase-3, thus inducing cell apoptosis. Aβ accumulation to cause toxicity may exacerbate neuronal damage, leading to cell apoptosis [69]. In our study, treatment with 1,25(OH)2D3 appeared to attenuate Aβ-induced apoptosis, supporting that 1,25(OH)2D3 may be anti-apoptotic. In addition, combinational treatment with1,25(OH)2D3 and the GDNF inhibitor showed inhibition of the counteracting of apoptosis in the presence of Aβ. As a possible consequence to these findings, upregulation of GDNF seemed to be a key mechanism through which 1,25(OH)2D3 neutralized Aβ-induced excessive ROS production and apoptotic death in SH-SY5Y cells. Collectively, 1,25(OH)2D3 might elicit its neuroprotection via actions of GDNF-signaling, with the signals subsequently and indirectly leading to the resistance of SH-SY5Y cells to Aβ neurotoxicity.
4. Materials and Methods
4.1. Aβ(25-35) and 1,25(OH)2D3 Preparations
A short fragment of full length Aβ(1-42), Aβ(25-35) (A4559, Sigma, St. Louis, MO, USA), was employed in the present study due to the fact that it has been demonstrated to exist in AD brains [70], it exhibits the same neurotoxicity as Aβ(1-42), and has exhibited rapidly developed toxic effects in in vitro studies [10]. In brief, Aβ(25-35) was dissolved in sterile distilled water at a concentration of 1 mM as a stock solution before being diluted to desired concentrations. It was then incubated in capped vials at 37 °C for 5 days to form aggregates and develop full toxicity. It was stored frozen at −20 °C until use [71,72]. Next, the stock solution of Aβ(25-35) was diluted and added to cultures in a final concentration of 1 μM for 24 h prior to the treatment of 1,25(OH)2D3. The dose of Aβ(25-35) at 1 μM was chosen based on the literature [73] and the results of our preliminary experiments, in which cell viability, ROS, and apoptosis assays were conducted to test maximal toxic effects (data not shown).
For the preparation of 1,25(OH)2D3, the biological concentration of vitamin D in the peripheral circulation in healthy people is around 20 ng/mL (50 nM) [74] and 10 nM was indicated to induce GDNF expression [48,49]. Considering the conversion rate from 25-hydroxyvitamin D3 to 1,25(OH)2D3 and much lower concentrations in the brain, we treated cells with 0.1 and 10 nM of 1,25(OH)2D3. For this, 1,25(OH)2D3 (D1530, Sigma, St. Louis, MO, USA) was dissolved in 99.5% ethanol to a concentration of 10 mM as a stock solution before being diluted in 99.5% ethanol to desired concentrations.
4.2. Cell Culture Preparation
The SH-SY5Y human neuroblastoma cell line is a well-established cell model for studying neurodegenerative diseases [75]. The cell line was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in Dulbecco’s modified Eagle medium (DMEM) mixed with F12 (Gibco, Paisley, UK), 10% fetal bovine serum, and sodium bicarbonate at 37 °C in a 5% CO2 incubator. The medium was replaced every 2~3 days. Each aliquot (vial) of cells was grown for no more than 10 passages. Experiments were performed at 80% cell confluence. Then, cells were incubated with 1 μM of Aβ(25-35) for 24 h, followed by washing and incubation with two different concentrations of 1,25(OH)2D3 (0.1 or 10nM) for 24 h. Heparinase III (H8891, Sigma, St. Louis, MO, USA), an inhibitor of the GDNF-signaling, was used with 1,25(OH)2D3 treatment in some of the experiments to elucidate the role of GDNF on 1,25(OH)2D3-stimulated effects.
4.3. Cell Morphology
Cell morphology was observed under a microscope (Nikon, Tokyo, Japan) at 40× and 400× magnifications, and photos were processed with SPOT 4.7 Advanced software (SPOT Imaging Solutions, Sterling Heights, MI, USA).
4.4. Cell Viability Analysis
A 3-[4,5-cimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) assay was performed to determine the viability of SH-SY5Y cells that were pre-treated with Aβ(25-35) for 24 h and then further treated with two different concentrations of 1,25(OH)2D3 for 24 h. Briefly, MTT was added to each well of a 24-well plate and incubated at 37 °C for 1 h. Purple-colored precipitates of the living cell metabolite, formazan, were then dissolved in 500 µL of dimethyl sulfoxide (DMSO) and were analyzed in a 96-well plate. The color absorbance was recorded at 590 nm. Cell viability was calculated by the absorbance ratio of the treated group over the control.
4.5. Intracellular ROS Analysis
The production of intracellular ROS was determined by the 2’,7’-dichlorofluoroescin diacetate (DCFH-DA) probe, which is converted to the fluorescent dichlorofluorescein (DCF) in the presence of peroxides. Cells were seeded in 6-well dishes at 5 × 105 cells per well before the allotted experimental treatments were performed. After being treated, cells were trypsinized and washed with phosphate-buffered saline (PBS) once by centrifugation at 200× g for 3 min at 25 °C. After removing the supernatant, DCFH-DA dissolved in PBS was added to each sample. Samples were then incubated in the complete absence of light for 60 min. Each sample was moved to a Falcon tube prior to analysis by flow cytometry (Flowcytometer-3, FACSCantoII, BD Biosciences, Franklin Lake, NJ, USA).
4.6. Protein Extraction and Quantification
After being treated, cells were harvested, washed three times with PBS, and lysed using cold RIPA buffer supplemented with a protease inhibitor and an EDTA solution at a ratio of 100:1:1, respectively, then centrifuged at 13,000× g at 4 °C for 30 min. The supernatant was collected, and the protein concentration was estimated with a BCA Protein Assay Kit (Milpitas, CA, USA) using bovine serum albumin as the standard.
4.7. Western Blot Analysis
A Western blot analysis was performed to examine expression levels of certain proteins. Equal quantities (30 µg) of proteins were separated by 10% sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis and then transferred onto nitrocellulose membranes. After being transferred, membranes were blocked with Tris-buffered saline (TBS) containing 0.1% Tween-20 (TBST) and 5% non-fat milk for 1 h. Membranes were subsequently incubated with specific primary antibodies: β-actin (A3854, Sigma, St. Louis, MO, USA), GDNF (MAB212, R&D System, Minneapolis, MN, USA), vitamin D receptor (VDR; ab8756, Abcam, Cambridge, MA, USA), phosphorylated (p)-phosphatidylinositol 3K (PI3K; 4228, Cell Signaling Technology, Danvers, MA, USA), PI3K (4292, Cell Signaling Technology), p-Akt (9271, Cell Signaling Technology), Akt (9272, Cell Signaling Technology), p-glycogen synthase kinase (GSK)-3β (Ser9) (9336, Cell Signaling Technology), GSK-3β (9315, Cell Signaling Technology), activated caspase-3 (ab13847, Abcam), p-tau (ab109390, Abcam), and tau (ab22261, Abcam) overnight at 4 °C. After washing three times with TBST for 30 min, the membranes were incubated with an anti-mouse (A9024, Sigma) or an anti-rabbit (R5506, Sigma) immunoglobulin G (IgG) secondary antibody for 1 h, and then washed with TBST three times for 30 min. Immunoreactive proteins were detected and quantified using an enhanced chemiluminescence (ECL; Bionovas, Toronto, Canada) Western blot detection system and Image-Pro Plus Software (Cybernetics, Rockville, MD, USA), respectively.
4.8. Apoptotic Cell Analysis
Apoptosis cell analyses were performed using flow cytometry by double-staining with propidium iodide (PI) and annexin-V dye. Cells were seeded in 6-well dishes at 5 × 105 cells per well before the allotted experimental treatments were performed. After being treated, cells were trypsinized and washed with PBS at least twice by centrifugation at 200× g for 3 min at 4 °C. The supernatant was removed, and the pellet was re-suspended in 1 mL of cold PBS and centrifuged for 3 min at 200× g and 4 °C. After removing the supernatant, 100 µL of binding buffer, 2 µL of PI dye, and 2 µL of annexin-V dye were added to each sample. Samples were then incubated at room temperature in the complete absence of light for 15 min. Each sample was resuspended in 600 µL of cold PBS and moved to a Falcon tube prior to analysis by flow cytometry (Flowcytometer-3, FACSCantoII, BD Biosciences, San Jose, CA, USA).
4.9. Statistical Analysis
Statistical comparisons were performed with a t-test (control vs. Aβ; Aβ+1,25(OH)2D3 vs. Aβ+1,25(OH)2D3+heparinase) and one-way analysis of variance (ANOVA) with Duncan’s post-hoc analysis (Aβ vs. different concentrations of 1,25(OH)2D3). The level of significance was set at p < 0.05. Data are presented as the mean value and standard deviation (SD).
5. Conclusions
In conclusion, this study demonstrates the neuroprotective effects of 1,25(OH)2D3 against Aβ neurotoxicity and concomitant changes in phosphorylated tau protein in SH-SY5Y cells. The underlying mechanisms of the action of 1,25(OH)2D3 were attributed to its ability to counteract excessive production of ROS and apoptotic cell death. However, the optimal responses of 1,25(OH)2D3 to Aβ neurotoxicity in SH-SY5Y cells required an interplay with GDNF-signaling by targeting inactivation of the PI3K/Akt/GSK-3β pathway at the cellular level. However, more studies are warranted to explore the additional pathways through which GDNF works to mediate neuroprotection of 1,25(OH)2D3 and to better understand the interactions with the PI3K/Akt/GSK-3β signaling in relation to Aβ neurotoxicity. Nonetheless, we have demonstrated the pivotal role of GDNF in 1,25(OH)2D3-elicited neuroprotection following an Aβ challenge in SH-SY5Y cells, which could add value as the basis of 1,25(OH)2D3 treatment for limiting Aβ-related pathology.
Abbreviations
| Aβ | Amyloid beta |
| AD | Alzheimer’s disease |
| 1,25(OH)2D3 | 1α,25-dihydroxyvitamin D3 |
| VDR | Vitamin D receptor |
| ROS | Reactive oxygen species |
| GDNF | Glial cell line-derived neurotrophic factor |
| PI3K | Phosphoinositide 3-kinase |
| Akt | Protein kinase B |
| GSK-3β | Glycogen synthase kinase-3β |
| APP | Amyloid protein precursor |
| DCFH-DA | 2′,7′-dichlorodihydrofluorescein diacetate |
| DMEM | Dulbecco’s modified Eagle medium |
| DCF | Dichlorofluorescein |
| MTT | 3-[4,5-cimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide |
| PBS | Phosphate-buffered saline |
| DMSO | Dimethyl sulfoxide |
| SDS | Sodium dodecyl sulfate |
| TBS | Tris-buffered saline |
| TBST | Tris-buffered saline containing 0.1% Tween-20 |
| ECL | Enhanced chemiluminescence |
| IgG | Immunoglobulin G |
| PI | Propidium iodide |
| ANOVA | Analysis of variance |
| SD | Standard deviation |
Author Contributions
Conceptualization, C.-IL., Y.-C.C., and S.-H.L.; methodology, Y.-C.C. and S.-H.L.; software, Y.-C.C.; validation, C.-IL., N.-J.K., and S.-H.L.; formal analysis, Y.-C.C.; investigation, C.-IL., Y.-C.C., and S.-H.L.; data curation, C.-IL. and Y.-C.C.; writing—original draft preparation, C.-IL., Y.-C.C., and T.-W.C.; writing—review and editing, C.-IL. and S.-H.L.; visualization, N.-J.K. and W.-J.L.; supervision, C.-IL. and S.-H.L.; project administration, S.-H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Misonou H., Morishima-Kawashima M., Ihara Y. Oxidative stress induces intracellular accumulation of amyloid beta-protein (Abeta) in human neuroblastoma cells. Biochemistry. 2000;39:6951–6959. doi: 10.1021/bi000169p. [DOI] [PubMed] [Google Scholar]
- 2.Querfurth H.W., LaFerla F.M. Alzheimer’s disease. N. Engl. J. Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 3.Sebastian-Serrano A., de Diego-Garcia L., Diaz-Hernandez M. The Neurotoxic Role of Extracellular Tau Protein. Int. J. Mol. Sci. 2018;19:998. doi: 10.3390/ijms19040998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang H.C., Jiang Z.F. Accumulated amyloid-beta peptide and hyperphosphorylated tau protein: Relationship and links in Alzheimer’s disease. J. Alzheimer’s Dis. JAD. 2009;16:15–27. doi: 10.3233/JAD-2009-0960. [DOI] [PubMed] [Google Scholar]
- 5.Van der Kant R., Goldstein L.S.B., Ossenkoppele R. Amyloid-β-independent regulators of tau pathology in Alzheimer disease. Nat. Rev. Neurosci. 2020;21:21–35. doi: 10.1038/s41583-019-0240-3. [DOI] [PubMed] [Google Scholar]
- 6.Reddy P.H. Amyloid precursor protein-mediated free radicals and oxidative damage: Implications for the development and progression of Alzheimer’s disease. J. Neurochem. 2006;96:1–13. doi: 10.1111/j.1471-4159.2005.03530.x. [DOI] [PubMed] [Google Scholar]
- 7.Varadarajan S., Yatin S., Aksenova M., Butterfield D.A. Review: Alzheimer’s amyloid beta-peptide-associated free radical oxidative stress and neurotoxicity. J. Struct. Boil. 2000;130:184–208. doi: 10.1006/jsbi.2000.4274. [DOI] [PubMed] [Google Scholar]
- 8.Butterfield D.A., Boyd-Kimball D. The critical role of methionine 35 in Alzheimer’s amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity. Biochim. Biophys. Acta. 2005;1703:149–156. doi: 10.1016/j.bbapap.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Varadarajan S., Yatin S., Kanski J., Jahanshahi F., Butterfield D.A. Methionine residue 35 is important in amyloid beta-peptide-associated free radical oxidative stress. Brain Res. Bull. 1999;50:133–141. doi: 10.1016/S0361-9230(99)00093-3. [DOI] [PubMed] [Google Scholar]
- 10.Millucci L., Ghezzi L., Bernardini G., Santucci A. Conformations and biological activities of amyloid beta peptide 25-35. Curr. Protein Pept. sci. 2010;11:54–67. doi: 10.2174/138920310790274626. [DOI] [PubMed] [Google Scholar]
- 11.Clementi M.E., Marini S., Coletta M., Orsini F., Giardina B., Misiti F. Abeta(31-35) and Abeta(25-35) fragments of amyloid beta-protein induce cellular death through apoptotic signals: Role of the redox state of methionine-35. FEBS Lett. 2005;579:2913–2918. doi: 10.1016/j.febslet.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 12.Butterfield D.A., Boyd-Kimball D. Oxidative Stress, Amyloid-β Peptide, and Altered Key Molecular Pathways in the Pathogenesis and Progression of Alzheimer’s Disease. J. Alzheimer’s Dis. JAD. 2018;62:1345–1367. doi: 10.3233/JAD-170543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma R., Xiong N., Huang C., Tang Q., Hu B., Xiang J., Li G. Erythropoietin protects PC12 cells from beta-amyloid(25-35)-induced apoptosis via PI3K/Akt signaling pathway. Neuropharmacology. 2009;56:1027–1034. doi: 10.1016/j.neuropharm.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Matsuda S., Nakagawa Y., Tsuji A., Kitagishi Y., Nakanishi A., Murai T. Implications of PI3K/AKT/PTEN Signaling on Superoxide Dismutases Expression and in the Pathogenesis of Alzheimer’s Disease. Diseases. 2018;6:28. doi: 10.3390/diseases6020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hetman M., Cavanaugh J.E., Kimelman D., Xia Z. Role of glycogen synthase kinase-3beta in neuronal apoptosis induced by trophic withdrawal. J. Neurosci. Off. J. Soc. Neurosci. 2000;20:2567–2574. doi: 10.1523/JNEUROSCI.20-07-02567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anjum I., Jaffery S.S., Fayyaz M., Samoo Z., Anjum S. The Role of Vitamin D in Brain Health: A Mini Literature Review. Cureus. 2018;10:e2960. doi: 10.7759/cureus.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel P., Shah J. Role of Vitamin D in Amyloid clearance via LRP-1 upregulation in Alzheimer’s disease: A potential therapeutic target? J. Chem. Neuroanat. 2017;85:36–42. doi: 10.1016/j.jchemneu.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Annweiler C., Rolland Y., Schott A.M., Blain H., Vellas B., Herrmann F.R., Beauchet O. Higher vitamin D dietary intake is associated with lower risk of alzheimer’s disease: A 7-year follow-up. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012;67:1205–1211. doi: 10.1093/gerona/gls107. [DOI] [PubMed] [Google Scholar]
- 19.Briones T.L., Darwish H. Vitamin D mitigates age-related cognitive decline through the modulation of pro-inflammatory state and decrease in amyloid burden. J. Neuroinflamm. 2012;9:244. doi: 10.1186/1742-2094-9-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Annweiler C. Vitamin D in dementia prevention. Ann. N. Y. Acad. Sci. 2016;1367:57–63. doi: 10.1111/nyas.13058. [DOI] [PubMed] [Google Scholar]
- 21.Haussler M.R., Whitfield G.K., Haussler C.A., Hsieh J.C., Thompson P.D., Selznick S.H., Dominguez C.E., Jurutka P.W. The nuclear vitamin D receptor: Biological and molecular regulatory properties revealed. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 1998;13:325–349. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- 22.Buell J.S., Dawson-Hughes B. Vitamin D and neurocognitive dysfunction: Preventing “D”ecline? Mol. Asp. Med. 2008;29:415–422. doi: 10.1016/j.mam.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nissou M.F., Guttin A., Zenga C., Berger F., Issartel J.P., Wion D. Additional clues for a protective role of vitamin D in neurodegenerative diseases: 1,25-dihydroxyvitamin D3 triggers an anti-inflammatory response in brain pericytes. J. Alzheimer’s Dis. JAD. 2014;42:789–799. doi: 10.3233/JAD-140411. [DOI] [PubMed] [Google Scholar]
- 24.Ibi M., Sawada H., Nakanishi M., Kume T., Katsuki H., Kaneko S., Shimohama S., Akaike A. Protective effects of 1 alpha,25-(OH)(2)D(3) against the neurotoxicity of glutamate and reactive oxygen species in mesencephalic culture. Neuropharmacology. 2001;40:761–771. doi: 10.1016/S0028-3908(01)00009-0. [DOI] [PubMed] [Google Scholar]
- 25.Masoumi A., Goldenson B., Ghirmai S., Avagyan H., Zaghi J., Abel K., Zheng X., Espinosa-Jeffrey A., Mahanian M., Liu P.T., et al. 1alpha,25-dihydroxyvitamin D3 interacts with curcuminoids to stimulate amyloid-beta clearance by macrophages of Alzheimer’s disease patients. J. Alzheimer’s Dis. JAD. 2009;17:703–717. doi: 10.3233/JAD-2009-1080. [DOI] [PubMed] [Google Scholar]
- 26.Sun C., Ou X., Farley J.M., Stockmeier C., Bigler S., Brinton R.D., Wang J.M. Allopregnanolone increases the number of dopaminergic neurons in substantia nigra of a triple transgenic mouse model of Alzheimer’s disease. Curr. Alzheimer Res. 2012;9:473–480. doi: 10.2174/156720512800492567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Airaksinen M.S., Titievsky A., Saarma M. GDNF family neurotrophic factor signaling: Four masters, one servant? Mol. Cell. Neurosci. 1999;13:313–325. doi: 10.1006/mcne.1999.0754. [DOI] [PubMed] [Google Scholar]
- 28.Chen P.S., Peng G.S., Li G., Yang S., Wu X., Wang C.C., Wilson B., Lu R.B., Gean P.W., Chuang D.M., et al. Valproate protects dopaminergic neurons in midbrain neuron/glia cultures by stimulating the release of neurotrophic factors from astrocytes. Mol. Psychiatr. 2006;11:1116–1125. doi: 10.1038/sj.mp.4001893. [DOI] [PubMed] [Google Scholar]
- 29.Gordon T. The role of neurotrophic factors in nerve regeneration. Neurosurg. Focus. 2009;26:E3. doi: 10.3171/FOC.2009.26.2.E3. [DOI] [PubMed] [Google Scholar]
- 30.Duarte E.P., Curcio M., Canzoniero L.M., Duarte C.B. Neuroprotection by GDNF in the ischemic brain. Growth Factors. 2012;30:242–257. doi: 10.3109/08977194.2012.691478. [DOI] [PubMed] [Google Scholar]
- 31.Eyles D., Brown J., Mackay-Sim A., McGrath J., Feron F. Vitamin D 3 and brain development. Neuroscience. 2003;118:641–653. doi: 10.1016/S0306-4522(03)00040-X. [DOI] [PubMed] [Google Scholar]
- 32.Straten G., Eschweiler G.W., Maetzler W., Laske C., Leyhe T. Glial cell-line derived neurotrophic factor (GDNF) concentrations in cerebrospinal fluid and serum of patients with early Alzheimer’s disease and normal controls. J. Alzheimer’s Dis. JAD. 2009;18:331–337. doi: 10.3233/JAD-2009-1146. [DOI] [PubMed] [Google Scholar]
- 33.Jiang T., Sun Q., Chen S. Oxidative stress: A major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog. Neurobiol. 2016;147:1–19. doi: 10.1016/j.pneurobio.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Padurariu M., Ciobica A., Lefter R., Serban I.L., Stefanescu C., Chirita R. The oxidative stress hypothesis in Alzheimer’s disease. Psychiatr. Danub. 2013;25:401–409. [PubMed] [Google Scholar]
- 35.Giraldo E., Lloret A., Fuchsberger T., Vina J. Abeta and tau toxicities in Alzheimer’s are linked via oxidative stress-induced p38 activation: Protective role of vitamin E. Redox Boil. 2014;2:873–877. doi: 10.1016/j.redox.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh A.K., Bissoyi A., Kashyap M.P., Patra P.K., Rizvi S.I. Autophagy Activation Alleviates Amyloid-beta-Induced Oxidative Stress, Apoptosis and Neurotoxicity in Human Neuroblastoma SH-SY5Y Cells. Neurotox. Res. 2017;32:351–361. doi: 10.1007/s12640-017-9746-5. [DOI] [PubMed] [Google Scholar]
- 37.Leong Y.Q., Ng K.Y., Chye S.M., Ling A.P.K., Koh R.Y. Mechanisms of action of amyloid-beta and its precursor protein in neuronal cell death. Metab. Brain Dis. 2020;35:11–30. doi: 10.1007/s11011-019-00516-y. [DOI] [PubMed] [Google Scholar]
- 38.Gao L., Zhou F., Wang K.X., Zhou Y.Z., Du G.H., Qin X.M. Baicalein protects PC12 cells from Abeta25-35-induced cytotoxicity via inhibition of apoptosis and metabolic disorders. Life Sci. 2020;248:117471. doi: 10.1016/j.lfs.2020.117471. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L., Guo Y., Wang H., Zhao L., Ma Z., Li T., Liu J., Sun M., Jian Y., Yao L., et al. Edaravone reduces Abeta-induced oxidative damage in SH-SY5Y cells by activating the Nrf2/ARE signaling pathway. Life Sci. 2019;221:259–266. doi: 10.1016/j.lfs.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 40.Mohamed N.V., Herrou T., Plouffe V., Piperno N., Leclerc N. Spreading of tau pathology in Alzheimer’s disease by cell-to-cell transmission. Eur. J. Neurosci. 2013;37:1939–1948. doi: 10.1111/ejn.12229. [DOI] [PubMed] [Google Scholar]
- 41.Bloom G.S. Amyloid-beta and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71:505–508. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- 42.Lee C.K., Weindruch R., Prolla T.A. Gene-expression profile of the ageing brain in mice. Nat. Genet. 2000;25:294–297. doi: 10.1038/77046. [DOI] [PubMed] [Google Scholar]
- 43.Ghribi O., Herman M.M., Pramoonjago P., Spaulding N.K., Savory J. GDNF regulates the A beta-induced endoplasmic reticulum stress response in rabbit hippocampus by inhibiting the activation of gadd 153 and the JNK and ERK kinases. Neurobiol. Dis. 2004;16:417–427. doi: 10.1016/j.nbd.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Alberghina L., Colangelo A. The modular systems biology approach to investigate the control of apoptosis in Alzheimer’s disease neurodegeneration. BMC Neurosci. 2006;7:1–26. doi: 10.1186/1471-2202-7-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng Y., Wang X. Antioxidant therapies for Alzheimer’s disease. Oxidative Med. Cell. Longev. 2012;2012:472932. doi: 10.1155/2012/472932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Molinari C., Morsanuto V., Ghirlanda S., Ruga S., Notte F., Gaetano L., Uberti F. Role of Combined Lipoic Acid and Vitamin D3 on Astrocytes as a Way to Prevent Brain Ageing by Induced Oxidative Stress and Iron Accumulation. Oxidative Med. Cell. Longev. 2019;2019:2843121. doi: 10.1155/2019/2843121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Banerjee A., Khemka V.K., Ganguly A., Roy D., Ganguly U., Chakrabarti S. Vitamin D and Alzheimer’s Disease: Neurocognition to Therapeutics. Int. J. Alzheimer’s Dis. 2015;2015:192747. doi: 10.1155/2015/192747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Orme R.P., Bhangal M.S., Fricker R.A. Calcitriol Imparts Neuroprotection In Vitro to Midbrain Dopaminergic Neurons by Upregulating GDNF Expression. PLoS ONE. 2013;8:e62040. doi: 10.1371/journal.pone.0062040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naveilhan P., Neveu I., Wion D., Brachet P. 1,25-Dihydroxyvitamin D3, an inducer of glial cell line-derived neurotrophic factor. Neuroreport. 1996;7:2171–2175. doi: 10.1097/00001756-199609020-00023. [DOI] [PubMed] [Google Scholar]
- 50.Celli A., Treves C., Stio M. Vitamin D receptor in SH-SY5Y human neuroblastoma cells and effect of 1,25-dihydroxyvitamin D3 on cellular proliferation. Neurochem. Int. 1999;34:117–124. doi: 10.1016/S0197-0186(98)00075-8. [DOI] [PubMed] [Google Scholar]
- 51.Garcion E., Wion-Barbot N., Montero-Menei C.N., Berger F., Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol. Metab. TEM. 2002;13:100–105. doi: 10.1016/S1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- 52.Dursun E., Gezen-Ak D., Yilmazer S. A novel perspective for Alzheimer’s disease: Vitamin D receptor suppression by amyloid-beta and preventing the amyloid-beta induced alterations by vitamin D in cortical neurons. J. Alzheimer’s Dis. JAD. 2011;23:207–219. doi: 10.3233/JAD-2010-101377. [DOI] [PubMed] [Google Scholar]
- 53.Neveu I., Naveilhan P., Baudet C., Wion D., De Luca H.F., Brachet P. 1, 25-dihydroxyvitamin D 3 regulates the synthesis of nerve growth factor in primary cultures of glial cells. Mol. Brain Res. 1994;24:70–76. doi: 10.1016/0169-328X(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 54.Wang J.-Y., Wu J.-N., Cherng T.-L., Hoffer B.J., Chen H.-H., Borlongan C.V., Wang Y. Vitamin D 3 attenuates 6-hydroxydopamine-induced neurotoxicity in rats. Brain Res. 2001;904:67–75. doi: 10.1016/S0006-8993(01)02450-7. [DOI] [PubMed] [Google Scholar]
- 55.Pertile R.A.N., Cui X., Hammond L., Eyles D.W. Vitamin D regulation of GDNF/Ret signaling in dopaminergic neurons. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018;32:819–828. doi: 10.1096/fj.201700713R. [DOI] [PubMed] [Google Scholar]
- 56.Kitagishi Y., Kobayashi M., Kikuta K., Matsuda S. Roles of PI3K/AKT/GSK3/mTOR pathway in cell signaling of mental illnesses. Depress. Res. Treat. 2012;2012:752563. doi: 10.1155/2012/752563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jolivalt C., Lee C., Beiswenger K., Smith J., Orlov M., Torrance M., Masliah E. Defective insulin signaling pathway and increased GSK-3 activity in the brain of diabetic mice: Parallels with Alzheimer’s disease and correction by insulin. J. Neurosci. Res. 2008;86:3265. doi: 10.1002/jnr.21787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Villegas S.N., Njaine B., Linden R., Carri N.G. Glial-derived neurotrophic factor (GDNF) prevents ethanol (EtOH) induced B92 glial cell death by both PI3K/AKT and MEK/ERK signaling pathways. Brain Res. Bull. 2006;71:116–126. doi: 10.1016/j.brainresbull.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 59.Srinivasan S., Anitha M., Mwangi S., Heuckeroth R.O. Enteric neuroblasts require the phosphatidylinositol 3-kinase/Akt/Forkhead pathway for GDNF-stimulated survival. Mol. Cell. Neurosci. 2005;29:107–119. doi: 10.1016/j.mcn.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 60.Mograbi B., Bocciardi R., Bourget I., Busca R., Rochet N., Farahi-Far D., Juhel T., Rossi B. Glial cell line-derived neurotrophic factor-stimulated phosphatidylinositol 3-kinase and Akt activities exert opposing effects on the ERK pathway: Importance for the rescue of neuroectodermic cells. J. Boil. Chem. 2001;276:45307–45319. doi: 10.1074/jbc.M101220200. [DOI] [PubMed] [Google Scholar]
- 61.Besset V., Scott R.P., Ibanez C.F. Signaling complexes and protein-protein interactions involved in the activation of the Ras and phosphatidylinositol 3-kinase pathways by the c-Ret receptor tyrosine kinase. J. Boil. Chem. 2000;275:39159–39166. doi: 10.1074/jbc.M006908200. [DOI] [PubMed] [Google Scholar]
- 62.Grimes C.A., Jope R.S. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog. Neurobiol. 2001;65:391–426. doi: 10.1016/S0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 63.Mwangi S., Anitha M., Fu H., Sitaraman S.V., Srinivasan S. Glial cell line-derived neurotrophic factor-mediated enteric neuronal survival involves glycogen synthase kinase-3beta phosphorylation and coupling with 14-3-3. Neuroscience. 2006;143:241–251. doi: 10.1016/j.neuroscience.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 64.Abbott J.J., Howlett D.R., Francis P.T., Williams R.J. Abeta (1-42) modulation of Akt phosphorylation via alpha7 nAChR and NMDA receptors. Neurobiol. Aging. 2008;29:992–1001. doi: 10.1016/j.neurobiolaging.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 65.Schindowski K., Belarbi K., Buee L. Neurotrophic factors in Alzheimer’s disease: Role of axonal transport. Genes Brain Behav. 2008;7(Suppl. 1):43–56. doi: 10.1111/j.1601-183X.2007.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cora O.N. PI3-kinase/Akt/mTOR signaling: Impaired on/off switches in aging, cognitive decline and Alzheimer’s disease. Exp. Gerontol. 2013;48:647–653. doi: 10.1016/j.exger.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 67.Nayak G., Cooper G.M. p53 is a major component of the transcriptional and apoptotic program regulated by PI 3-kinase/Akt/GSK3 signaling. Cell Death Dis. 2012;3:e400. doi: 10.1038/cddis.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu H.-C., Leu S.-J., Chuang D.-M. Roles of Glycogen Synthase Kinase-3 in Alzheimer’s Disease: From Pathology to Treatment Target. J. Exp. Clin. Med. 2012;4:135–139. doi: 10.1016/j.jecm.2012.04.001. [DOI] [Google Scholar]
- 69.Mohsenzadegan M., Mirshafiey A. The immunopathogenic role of reactive oxygen species in Alzheimer disease. Iran. J. Allerg. Asthma Immunol. 2012;11:203–216. [PubMed] [Google Scholar]
- 70.Kubo T., Nishimura S., Kumagae Y., Kaneko I. In vivo conversion of racemized beta-amyloid ([D-Ser 26]A beta 1-40) to truncated and toxic fragments ([D-Ser 26]A beta 25-35/40) and fragment presence in the brains of Alzheimer’s patients. J. Neurosci. Res. 2002;70:474–483. doi: 10.1002/jnr.10391. [DOI] [PubMed] [Google Scholar]
- 71.Yang R., Chen L., Wang H., Xu B., Tomimoto H., Chen L. Anti-amnesic effect of neurosteroid PREGS in Abeta25-35-injected mice through sigma1 receptor- and alpha7nAChR-mediated neuroprotection. Neuropharmacology. 2012;63:1042–1050. doi: 10.1016/j.neuropharm.2012.07.035. [DOI] [PubMed] [Google Scholar]
- 72.Liu J.Y., Guan Y.L., Zou L.B., Gong Y.X., Hua H.M., Xu Y.N., Zhang H., Yu Z.G., Fan W.H. Saponins with neuroprotective effects from the roots of Pulsatilla cernua. Molecules. 2012;17:5520–5531. doi: 10.3390/molecules17055520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Awale S., Tohda C., Tezuka Y., Miyazaki M., Kadota S. Protective Effects of Rosa damascena and Its Active Constituent on Aβ (25-35)-Induced Neuritic Atrophy. Evid.-Based Complement. Altern. Med. eCAM. 2011;2011:131042. doi: 10.1093/ecam/nep149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Przybelski R.J., Binkley N.C. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch. Biochem. Biophys. 2007;460:202–205. doi: 10.1016/j.abb.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 75.Uhrig M., Ittrich C., Wiedmann V., Knyazev Y., Weninger A., Riemenschneider M., Hartmann T. New Alzheimer amyloid beta responsive genes identified in human neuroblastoma cells by hierarchical clustering. PLoS ONE. 2009;4:e6779. doi: 10.1371/journal.pone.0006779. [DOI] [PMC free article] [PubMed] [Google Scholar]