Abstract
Background
Acute kidney injury (AKI) is one of the serious complications of cardiac surgery. It is worsened when accompanied by low cardiac output syndrome.
Objectives
In this study, we compared kidney function based on the KDIGO criteria in isolated on-pump and off-pump coronary artery bypass graft (CABG) surgery.
Methods
In this cohort study, 52 patients with LCOS were enrolled after on-pump (28 patients) and off-pump (24 patients) CABG. In the first six hours after ICU entrance, blood samples were taken for serum creatinine based on routine. For determining AKI after surgery, we used the KDIGO criteria as a primary endpoint. Also, some clinical parameters were recorded before, during, and after surgery. The data were analyzed by SPSS software, version 24, using paired and independent t-test, ANOVA, and Pearson correlation test and non-parametric tests such as Mann-Whitney and Kruskal-Wallis tests at a significance level of P < 0.05.
Results
There was no significant difference in age (P = 0.3) and gender (P = 0.57) between the two groups. Among cardiac disease risk factors, only hypertension (P = 0.02) had a significant difference between the two groups, but AKI in patients with hypertension did not show a significant difference (P = 0.09). In paraclinical parameters, serum creatinine showed a significant difference before and after surgery in on-pump (P < 0.001) and off-pump (P = 0.007) groups. Also, this parameter had a significant difference at 6 h, 12 h, 24 h, and 48 h after surgery between the on-pump and on-pump groups. The AKI incidence showed a significant difference between the two groups (P < 0.001).
Conclusions
The incidence of AKI was more in on-pump patients than in off-pump patients. Also, a significant difference was observed between their clinical parameters. Thus, to improve the patients’ clinical outcomes and lower the health costs, we suggest that patients with a high risk of LCOS be followed up after CABG, especially on-pump CABG.
Keywords: Acute Kidney Injury, Coronary Artery Disease, Coronary Artery Bypass Graft Surgery
1. Background
Chronic diseases increased in the 21st century due to increased life expectancy. Among them, cardiovascular disease is the most common disease in the world with high morbidity and mortality (1). By increasing this disease, cardiac surgeries have become an essential strategy for patients that do not respond to medication and other therapeutic procedures (2). However, cardiac surgeries have some potential complications presenting in the postoperative period (3).
Acute kidney injury (AKI) is one of the serious complications of cardiac surgery characterized by a rapid loss of kidney function, leading to an acute increase in the serum creatinine concentration. Acute kidney injury occurs in up to 30% of patients after cardiac surgery and complicates 2.3% of the isolated coronary artery bypass grafting (CABG) patients (4-6).
Traditionally, CABG is done on cardiopulmonary bypass, and it is one of the most routinely performed procedures (7). Advancement in surgical techniques, including the use of intracoronary shunts and development of stabilization devices such as octopus, has allowed surgeons to regularly perform multivessel off-pump CABG surgery (8). As the protracted cardiopulmonary bypass (CPB) time is associated with postoperative AKI, some researchers have proposed off-pump coronary artery bypass (OPCAB) techniques for high-risk patients. However, randomized trials of OPCAB have not demonstrated benefits in a general population (9).
Low cardiac output syndrome in on-pump and off-pump CABG is probably an increasing risk factor for morbidity and mortality due to pulmonary complications, myocardial infarction, stroke, redo surgeries, and renal failure (10).
2. Objectives
Thus, this study aimed to determine the patients’ kidney function based on kidney disease improving global outcomes (KDIGO) criteria in isolated CABG surgery in on-pump and off-pump methods in patients with low cardiac output syndrome (LCOS) after surgery.
3. Methods
The present cohort study started in September 2017 and completed in July 2018. The sample of the study included all patients who were candidates for non-emergent isolated CABG surgery at the Imam Reza Cardiac Surgery Center, Mashhad, Iran. The participants were divided into two groups of on-pump CABG and off-pump CABG after considering the inclusion and exclusion criteria and obtaining the patients’ consent, as approved by the Ethics Committee of the University. The exclusion criteria included patients with (1) A history of supraventricular tachycardia; (2) a history of CABG; (3) contrast-induced nephropathy; (4) urologic impairment; (5) sustained respiratory disorder; (6) stroke and transient ischemic attack; (7) coagulopathies; (8) IABP before surgery, and (9) reduced ejection fraction and LCOS before surgery.
The objectives of the study were explained to the patients, and their written informed consent was obtained according to the Declaration of Helsinki. Furthermore, it was explained that the patients could withdraw from the study at any time. This study was approved by the Ethics Committee of Mashhad University of Medical Sciences (no.: 960096) and complied with the Declaration of Helsinki. Informed consent was obtained from the subjects.
The demographic characteristics included age, gender, weight, height, body mass index, history of smoking, history of diabetes mellitus (insulin-dependent and non-insulin dependent), metabolic syndrome (according to the American Heart Association criteria (11)), history of hypertension, the status of peripheral arterial disease (after primary evaluation for approving the disorder by ankle-brachial index measurement and determination of peripheral arterial disease in patients based on the Rutherford classification system (12)), estimated serum creatinine based on the modification of diet in renal disease (MDRD), the cardiac disease classification based on New York Heart Association (NYHA) class and Canadian Cardiovascular Society (CCS), the type of occluded coronary artery, the number of involved coronary arteries, and the number of grafts. The left ventricular ejection fraction (EF%) was measured 24 h before surgery via trans-thoracic echocardiography. The laboratory indices before surgery included serum levels of sodium, potassium, calcium, magnesium, cholesterol, fasting blood sugar, neutrophil, lymphocyte, hemoglobin, hematocrit (according to the last measured laboratory parameters before surgery), and EuroSCORE II.
In the on-pump method, after ascending aorta cross-clamping, we used median sternotomy, elegant dissection of the pericardium and peripheral tissues, and standard cannulation, respectively. Before aortic cannulation, we used the epi-aortic scan to determine the lack of mural thrombus or calcification. In these patients, before cannulation, heparin sodium was prescribed, and cardiopulmonary bypass (CPB) was not initiated until active coagulation time (ACT) was over 480 seconds. The cardioplegic arrest was done by antegrade cardioplegic catheter insertion in coronary Ostia and retrograde cardioplegic catheter in the coronary sinus. Cardioplegic solution prescription was done in the intermittent route and repeated in every 20 min. The oxygenator in all of the patients was Fusion® (Medtronic Corporation, Minneapolis MN). Non-pulsatile perfusion with roller pump recommended that perfusion was maintained between 2 and 2.8 Lit/min/m2. During CPB, for the evaluation of arterial blood gas, the alpha-stat strategy was administrated. Thus, PaCO2 and PaO2 were maintained 35 - 45 mmHg and 150 - 250 mmHg, respectively. The prime solution was crystalloid for all the patients in the on-pump group. The cardioplegia solution (St. Thomas Hospital solution) was the same after general anesthesia induction in all the patients. Mild hypothermia with the thermal range of 32°C to 34°C was used during surgery. During hemodilution, the hematocrit level was maintained in the optimal range (greater than 25%). During CPB, the patients did not receive furosemide or mannitol as diuretics.
In the off-pump procedure, after median sternotomy and free of dehiscence by pericardial deep tractions, the heart was getting in the suitable position. Then, the anastomosis site was immobile by a standard octopus stabilizer. In this group, the patients were heparinized with 1 mg/kg dose, and the surgery was not initiated until the ACT was 300 seconds. During grafting, we used an intra-coronary shunt (Medtronic® Corporation, Minneapolis, MN).
The variables recorded during surgery included the time of CPB, blood product during surgery (on-pump and off-pump), the last arterial blood gas sample parameters before weaning from CPB (on-pump group) and before the removal of octopus (off-pump group). All patients were monitored with cerebral oximetry during surgery. Thermal monitoring was done with the nasopharyngeal probe. Due to the reverse anticoagulation effect of heparin, we used protamine sulfate at 1 - 1.3 mg/kg/100 units of heparin ratio. During cardiac surgery, transfused iso-group and iso-Rh red blood cells were used to maintain the hemoglobin level above 10 g/dL if the hemoglobin level was lower than 10 g/dL during surgery. The mean arterial pressure (MAP) was 60 - 80 mmHg during surgery.
After cardiac surgery, the patients were monitored in the Postanesthesia Care Unit (PACU) and admitted to the intensive cardiac care unit after complete recovery. In the first hour after the ICU entrance, the patients were enrolled in the study if they had the symptoms of LCOS. The criteria for determining LCOS included a decreased Cardiac index (CI) to lower than 2.0 L/min/m2, the need for inotropic agent infusion, and the use of intra-aortic balloon pump (IABP) to maintain systolic blood pressure greater than 90 mmHg. After the correction of electrolyte disturbances and arterial blood gas parameters, other clinical symptoms included a systolic blood pressure of lower than 90 mmHg along with tissue hypoperfusion symptoms such as clammy skin, lethargy, oliguria or anuria, and elevated serum lactate.
In the first six hours after ICU entrance, venous blood samples were taken for serum creatinine level as a routine method. Other serum creatinine evaluations were done 12, 24, and 48 h after ICU entrance. Also, the urinary outputs of the patients were recorded in the first six, 12, and 24 h after surgery. The volume of urinary output of all participants was maintained at about 0.5 mL/kg/h without the use of furosemide or other diuretic agents. The left ventricular function (EF%) was measured 24 h after surgery in the ICU.
To define the acid-base disturbance incidence, we used the Henderson-Hasselbalch equation. To determine AKI in the patients, we used the KDIGO criteria. In this study, based on the KDIGO criteria, AKI was defined as the increase of serum creatinine by 0.3 mg/dL or more within 48 h. The severity of AKI was determined according to the KDIGO staging system as follows:
Stage 1: The level of serum creatinine of 1.5 - 1.9 times the baseline value or an increase of more than 0.3 mg/dL; stage 2: the level of serum creatinine of 2 - 2.9 times the baseline value; stage 3: the level of serum creatinine of three times the baseline value or an increase by greater than 4 mg/dL or the initiation of renal replacement therapy.
The statistical analysis was done by the Statistical Package for the Social Sciences (SPSS) version 24 software. The data were analyzed by descriptive statistics, variance analysis, Fisher’s exact test, Kruskal-Wallis test, paired t-test, and independent t-test. The level of significance was set at 0.05.
4. Results
In the present study, 485 patients underwent CABG, 249 of whom were in the on-pump group, and others in the off-pump group. After applying the exclusion criteria, 121 and 115 patients remained in the on-pump and off-pump groups, respectively. Finally, 28 patients in the on-pump group and 24 patients in the off-pump group took part in the study. The main finding of this study showed a significant difference in the stages of AKI between the groups of surgery (P < 0.001) so that the incidence of AKI was significantly lower in off-pump CABG patients than in on-pump CABG patients.
In our study, 52 patients were enrolled of whom, 30 (57.6%) were male, and 22 (42.3%) were female. The mean age was 57.96 ± 9.58 years in the on-pump group and 60.1 ± 57.74 years in the off-pump group. There were no significant differences in age (P = 0.57) and gender (P = 0.3) between the on-pump and off-pump groups. Among cardiovascular risk factors, only hypertension showed a significant difference (P = 0.02) between the two groups. Nevertheless, there were no significant differences between the two groups of study in other risk factors such as smoking (P = 0.26), diabetes mellitus (P = 0.16), and metabolic syndrome (P = 0.32). Fisher’s exact test showed no significant relationship between the staging of AKI (mild, moderate, and severe) and hypertension (P = 0.09). Also, no significant relationship was found between NYHA and CCS class (P = 0.09), the type and the number of occluded vessels (P = 0.32), the number of grafts (P = 0.08), and EuroSCORE II (P = 0.08). There was no significant difference in the left ventricular ejection fraction (EF%) before and after the surgery between the on-pump and off-pump groups (P = 0.08).
Concerning the median levels of laboratory findings before surgery, only the calcium level (P = 0.03) and hematocrit (P = 0.002) showed significant differences between the two groups. However, after the surgery, the creatinine level (P = 0.006), potassium (P = 0.008), hemoglobin (P = 0.001), and hematocrit (P < 0.001) had significant differences between the two groups. After dividing the two groups of surgery and comparing the laboratory findings before and after the surgery separately for each group, the findings revealed that all of the laboratory indices had significant differences before and after the surgery in the on-pump group. However, this comparison in the off-pump group showed that only the creatinine level (P = 0.007), sodium (P = 0.011), blood sugar (P < 0.001), hemoglobin (P < 0.001), and hematocrit (P < 0.001) had significant differences before and after the surgery. There were significant differences in serum creatinine at six hours (P = 0.02), 12 h (P = 0.02), 24 h (P = 0.001), and 48 h (P = 0.000) between the two groups.
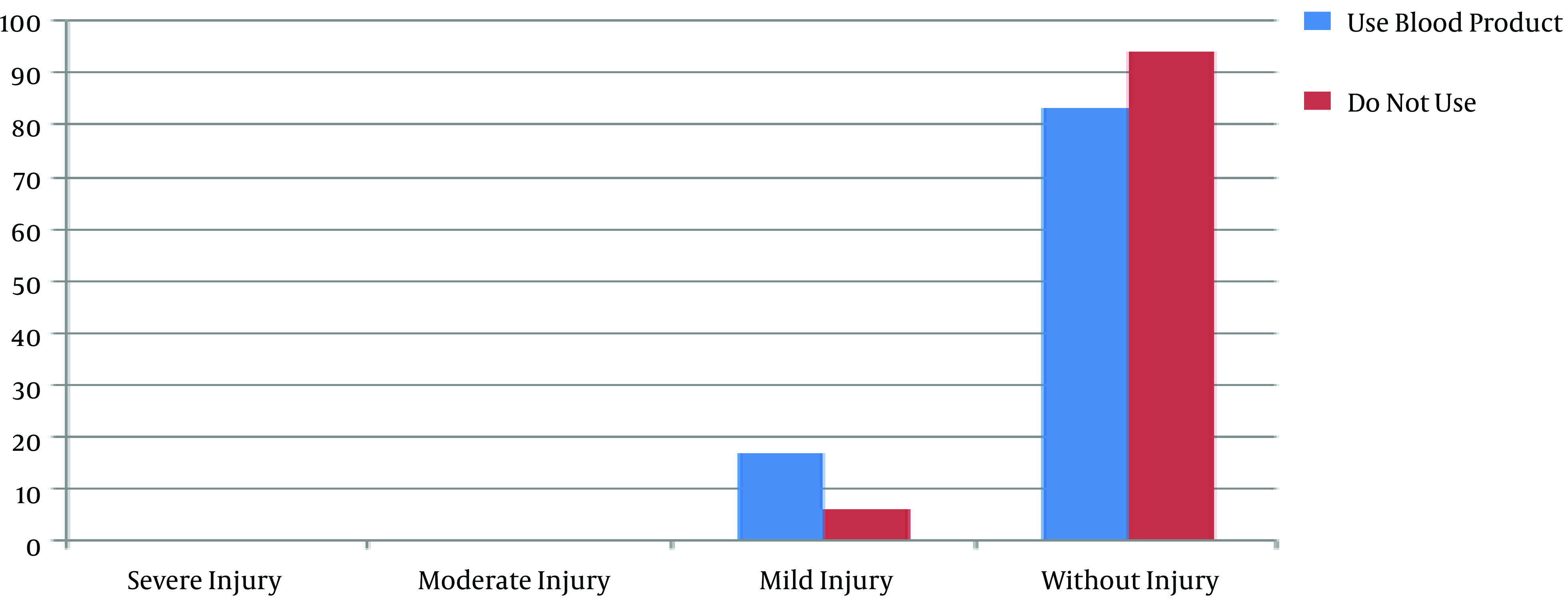
There were no significant differences between the groups in body mass index (P = 0.9) and body surface area (P = 0.89). There was also no significant difference between the groups in peripheral arterial disease based on the ankle-brachial index and Rutherford classification (P = 0.23). Also, there was no significant relationship between the staging of AKI and peripheral arterial disease in the on-pump (P = 0.67) and off-pump (P = 0.1) groups. Moreover, 11 out of 26 (42.3%) patients in the on-pump group and 15 out of 23 (57.7%) patients in the off-pump group used blood products during the surgery, and there was no difference in the blood product use between the groups. Also, Fisher’s exact test showed no significant relationship between the different stages of AKI and blood product use in the on-pump (P = 0.78) and off-pump (P = 0.46) groups (Figures 1 and 2).
Figure 1. The distribution of acute kidney injury stages in the on-pump group according to the use of blood product.
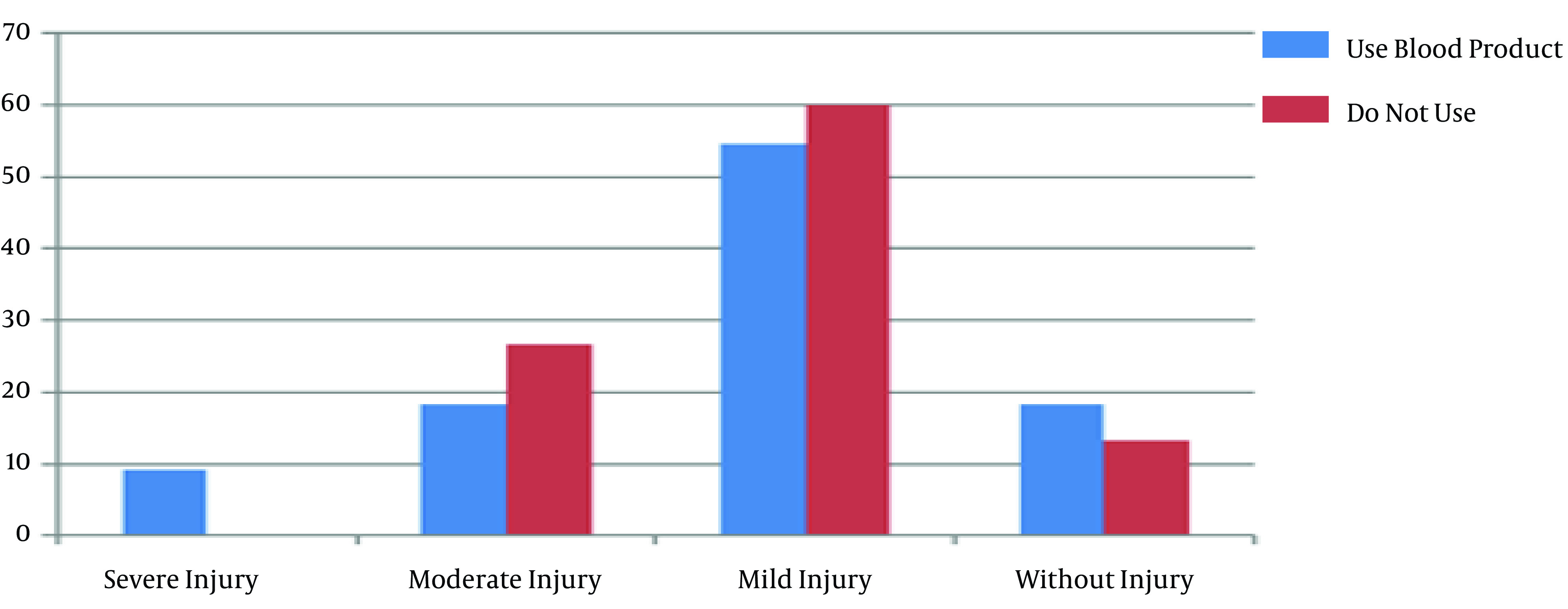
Figure 2. The distribution of acute kidney injury stages in the off-pump group according to the use of blood product.
The comparison of the groups for neutrophil, lymphocyte, and the ratio of neutrophil to lymphocyte showed that only neutrophils had a significant difference between the on-pump and off-pump groups (P = 0.001). The assessment of arterial blood gas in the on-pump and off-pump groups showed a significant difference in lactate (P = 0.003) and bicarbonate (P = 0.01) levels. The independent t-test revealed significant differences between the two groups in the mean ICU stay (P < 0.001) and the weaning time of mechanical ventilation (P < 0.001).
The mean time of cardiopulmonary bypass (P = 0.08) and cross-clamp of the aorta time (P = 0.34) had no significant relationship with different stages of AKI (Table 1). On the other hand, our results showed a significant relationship (P = 0.026) between the mean duration of surgery and different stages of AKI in the off-pump group.
Table 1. The Comparison of the Mean Time of CPB Use and Cross-Clamp Time Among Patients in the On-Pump Groupa.
| Variable | KDIGO | Frequency | Mean ± ST | Test | P Value |
|---|---|---|---|---|---|
| CPB use time | 0 | 4 (15.4) | 64.15 ± 25.08 | Kruskal-Wallis test | P = 0.08 |
| 1 | 15 (57.7) | 59.15 ± 6.35 | |||
| 2 | 6 (23.1) | 17 ± 78.81 | |||
| 3 | 1 (3.8) | 0 ± 78 | |||
| Cross-clamp time | 0 | 4 (15.4) | 17 ± 51.14 | Kruskal-Wallis test | P = 0.34 |
| 1 | 15 (57.7) | 17 ± 40.76 | |||
| 2 | 6 (23.1) | 57.11 ± 67.25 | |||
| 3 | 1 (3.8) | 0 ± 61 |
aKDIGO 0, without injury; KDIGO 1, mild injury; KDIGO 2, moderate injury; KDIGO 3, severe injury.
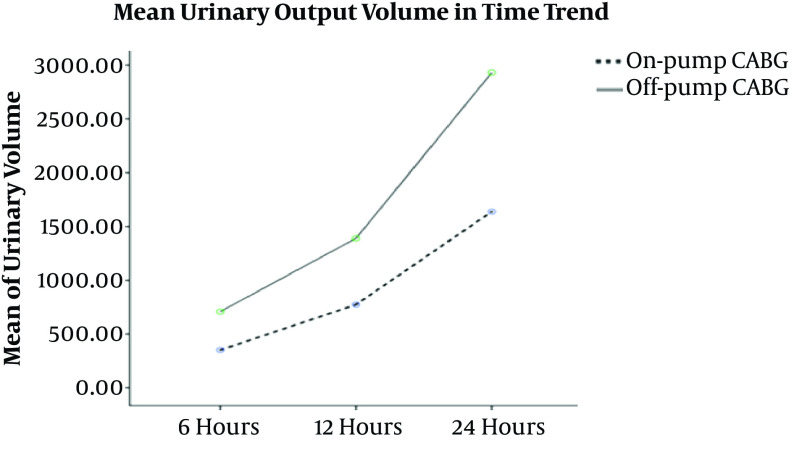
The results of our study showed that a powerful statistical difference between the urinary output volume and the first six hours (P < 0.001), 12 hours (P = 0.001), and 24 hours (P < 0.001). Figure 3 shows the mean urinary output volume over time.
Figure 3. Mean urinary output volume over time at six, 12, and 24 h after surgery.
5. Discussion
The aim of this study was to determine the effect of two different methods of coronary artery bypass graft surgery on kidney function based on the KDIGO criteria in patients with low cardiac output syndrome. The most important finding of this study was a statistical difference in different stages of AKI between the groups of surgery. This finding revealed that off-pump CABG privileged on-pump CABG in the AKI incidence in low cardiac output syndrome patients. This finding is in line with those of other studies (5, 13-16). On the other hand, some studies (3, 17, 18) reported different findings. This contradiction may be due to the patients’ status after cardiac surgery (low cardiac output syndrome) and different inclusion and exclusion criteria. This is approved by Ding et al. (10) that showed patients with LCOS after isolated CABG had different degrees of multi-organ dysfunction, especially kidney function.
The results of the comparison of demographic variables showed a lack of significant differences between them and the method of surgery. This finding is consistent with Ghanei et al. (14) and Houlind et al. (6) studies. One of the reasons for the similarities of the results can be the echogenic distribution of patients in the on-pump and off-pump groups. Also, most participants in our study and similar studies were aged over 50 years, and most of them were males. Although men were more than women in our study and other studies (6, 14), this difference was not significant. In another study performed by Machado et al. (19), a significant difference was found between the age and gender of patients. In their study, the age distribution was in the excess range. Also, the sample size was greater in that study than in our study that can be a probable reason for the significant difference between the two groups of the study.
In the comparison of on-pump and off-pump CABG groups for cardiovascular risk factors, only significant differences were observed in hypertension and dyslipidemia; the same results were seen in other studies (6, 14). However, in another study (5), patients had no significant difference in cardiovascular risk factors, especially hypertension (P = 0.44) and diabetes mellitus (P = 0.45). In our study, the between-group comparison revealed insignificant differences in metabolic syndrome. Although in the diagnostic criteria of metabolic syndrome, other cardiovascular risk factors such as hypertension and dyslipidemia were seen and also these factors in our study had significant differences, separately, it is probable that by increasing the sample size of the study and precise evaluation of the effect of these factors in AKI incidence in patients undergoing CABG in the two different methods, different results would be obtained.
In this study, there were no significant differences in the NYHA class between the groups of study. This result is contrary to the findings by Jyrala et al. (20) that showed a significant difference between the on-pump and off-pump CABG groups in the NYHA class (P < 0.001). One of the most important reasons for these different results can be different criteria for the inclusion of patients to the study. That study used only patients with mild renal dysfunction before surgery. The presence of kidney dysfunction in all stages can be effective in cardiac function, and the cardiac function is diminished by the progression of renal dysfunction (21). The results of a similar study showed an insignificant difference (P = 0.080) in the NYHA class between the groups of CABG surgery (6). The sample size and distribution of patients in that study were similar to the inclusion criteria used in our study.
The findings of our study showed that left ventricular ejection fraction (LVEF) had no significant difference between the on-pump and off-pump CABG groups. This result is parallel with the findings of other studies (3, 6, 18). The similarity of our result and those studies can be due to the evaluation of all patients with reduced ejection fraction before surgery. However, in another study (20), a significant difference was seen between the groups of surgery in LVEF (P = 0.001). This contradiction may be due to the evaluation of LVEF patients before all types of cardiac surgery, including CABG, heart valve surgery, etc. via CPB while in our study, only patients undergoing CABG were enrolled in two different methods (on-pump and off-pump).
The results of our study showed the lack of a significant difference in the type of occluded coronary artery, the number of involved vessels, and the number of grafts between the on-pump versus off-pump groups. Zakkar et al. study (22) also showed parallel findings with our study. Likewise, Houlind et al. (6) evaluated patients for one-vessel, two-vessel, and three-vessel diseases of coronary arteries in on-pump and off-pump CABG, and showed no differences between them.
Nowadays, an increasing interest has been created in the prediction of mortality and adverse complications after cardiac surgery. Therefore, many models have been developed to predict these events (23). One of these applied models is the EuroSCORE II questionnaire that has a predictive value to determine early mortality after cardiac surgery (24, 25). In the comparison of the two groups for EuroSCORE II, we observed no significant difference, which is compatible with Jamaati et al. (23) and Atashi et al. (25) studies. On the other hand, there was a significant difference between the on-pump and off-pump groups in EuroSCORE (22). These contrary results may be due to the fragile ability of this measurement in Iranian patients. Thus, it is crucial to indigenize and calibrate this scale for use in Iranian people.
One of the most important results of our study was the significant difference between the two groups in the hematocrit level, as the hematocrit level between two groups before and after the study. Also, in the examination of the relationship between hematocrit level and AKI incidence, we observed a significant relationship between different stages of AKI and the hematocrit level before and after the study (P = 0.02). This correlation shows that patients in the two study groups experienced a higher severity of AKI by a decrease in the hematocrit level. This principle finding is monitoring in the patients during CPB, while the minimum level of hematocrit during bypass considered overt than 25% (26). Since now, no study has evaluated the relationship between hematocrit before and after on-pump and off-pump CABG. Also, there is no report on the relationship between hematocrit before and after CABG and the AKI incidence and its modality, and thus this is one of the rare results of our study.
In our study, there was no relationship between the body mass index and body surface area. Other studies (5, 14) showed the same results, as well. However, in some other studies (8, 16, 19, 27). The reason for this discrepancy may be the examination of patients with the same race in our study. Sajja et al. study (16) showed that European and African races had a higher body surface area and a higher probability of AKI incidence. It is suggested that future studies evaluate the effect of BSA and BMI on the incidence of AKI in all types of cardiac surgeries in Iranian people.
Most of the patients that participated in our study were affected by peripheral arterial disease (PAD), which had no significant difference between the on-pump and off-pump groups. This finding is part of the unique findings of this study. Although our findings did not show a significant difference between the groups, we observed that according to the KDIGO criteria, patients with a high degree of PAD based on the Rutherford classification system had a high incidence of AKI.
The results of our investigation in terms of the use or non-use of blood products during surgery showed no significant difference between the groups. In another study performed on 1,175 patients undergoing cardiac surgery, the findings showed that AKI in different stages occurred following the use of red blood and other blood products (28). Nevertheless, Amouzeshi et al. (18) showed no significant difference in the AKI incidence between the on-pump or off-pump CABG methods, which is consistent with our results. One of the reasons for the lack of a statistical relationship can be the maintenance of the hematocrit level during surgery with red blood cell administration. The decreased level of hematocrit after cardiac surgery can lead to AKI incidence. On the other hand, Kindzelski et al. (28) showed that the administration of red blood cells during surgery over three units could subsequently lead to the progressive AKI incidence. Thus, it is suggested that the hematocrit level of patients be managed during surgery with the least use of blood products to prevent the risk of AKI.
The measurement of the neutrophil-to-lymphocyte ratio as an inflammatory factor and the predictor of organ injury incidence is suggested in patients with coronary artery disease (29). In this study, there was no relationship between AKI and the neutrophil-to-lymphocyte ratio in the on-pump and off-pump groups. However, other studies showed a significant relationship between the neutrophil-to-lymphocyte ratio and the predictive role in organ failure (30-33).
In our study, we investigated the difference in arterial blood gas (ABG) parameters between the two groups of on-pump and off-pump. However, bicarbonate and lactate were the only parameters that had significant differences between the two groups. Since now, no study has considered the difference in ABG during surgery between on-pump and off-pump CABG. Similar studies (7, 34-36) only examined ABG after surgery. This is while we can better manage patients during surgery with the clinical evidence of ABG to avoid post-surgery complications such as AKI.
In the evaluation of ICU stay, there was a significant difference between the on-pump and off-pump CABG groups. These findings showed a higher hospitalization rate of patients undergoing on-pump CABG than that of the other group. Similar studies (6, 7) showed that the ICU stay was significantly lower after off-pump CABG than on-pump CABG. These findings revealed the superiority of the off-pump method in CABG over the conventional method. In another study, the use of off-pump CABG decreased the ICU stay and reduced the health care costs. Moreover, the rate of ICU stays increases with the increase of AKI incidence in CABG patients (37). Therefore, we should use proper treatment and the best surgical method to reduce the severity of AKI in CABG and subsequently reduce the ICU stay.
In our investigation, the time of weaning from mechanical ventilation had a significant difference between on-pump and off-pump CABG. This finding is similar to other studies (30, 31) that showed the time of weaning was significantly lower in the off-pump group than in the on-pump group. However, in another study (20), no difference was found between the two groups in the time of weaning. Among the main reasons for the difference in the findings of these studies, we can point out the type of patients in the two groups; in studies that found significant differences between the two groups, patients were given vasoactive agent treatment after surgery. This can confirm that these patients had LCOS, which is similar to the criteria for inclusion in our study.
In our investigation, there were no significant differences in the CPB use and aortic cross-clamp time between the groups of study, which is parallel with the finding of similar studies (6, 18). However, the time of surgery in the off-pump group of our study had a significant relationship with the AKI incidence, as these patients had a lower grade of AKI with a lower time of surgery. This was a unique finding of this study. One of the most different results of this study is the lack of measurement of grades of AKI and the time of off-pump CABG, separately.
The final rare findings of our study were statistically significant differences between the urinary output volume at six, 12, and 24 h after CABG in the on-pump and off-pump surgery groups. Since now, no studies have reported the same finding. Similar studies (38, 39) have only evaluated the urinary output volume during CABG. The findings of these studies showed the prognostic role of the urinary output during surgery in the AKI incidence after surgery. On the other hand, Gravlee et al. (26) showed contrary results implying that the urinary output volume during surgery had no relationship with the incidence of AKI and kidney protection rate. Thus, it is suggested that the urinary output volume be examined after CABG as a clinical outcome of the patients because this factor may reflect the kidney function within 24 h after CABG.
This study had some limitations, such as the lack of urinary output calculation as mL/kg among patients and the probability of variable race in the population study. Other studies would be helpful to resolve the limitations of this study to obtain better instructions for managing the patients with LCOS after CABG.
5.1. Conclusions
The development of AKI after CABG, based on the KDIGO criteria, was correlated with the method of CABG surgery. Thus, to prevent the AKI incidence in patients after CABG, especially on-pump CABG, it is proposed to identify patients with the probability of LCOS after cardiac surgery in the preoperative period to receive special attention.
Acknowledgments
The authors would like to thank all patients who participated in this study. We would like to thank all of the perfusionists and ICU nurses at the Department of Cardiac Surgery, Imam Reza Hospital, Mashhad University of Medical Sciences, Mashhad, Iran.
Footnotes
Authors' Contribution: Mehdi Fathi contributed in the conception of the work, revising the draft, and approval of the final version of the manuscript and agreed for all aspects of the work. Morteza Valaei contributed in the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. Amene Ghanbari contributed in the conception of the work, approval of the final version of the manuscript, analysis and interpretation of data for the work, and agreed for all aspects of the work. Reza Ghasemi revising the draft, approval of the final version of the manuscript and agreed for all aspects of the work. Mohsen Yaghubi contributed in the conception of the work, drafting of manuscript, revising the draft, and agreed for all aspects of the work
Conflict of Interests: The authors declare no conflict of interest in this study.
Ethical Approval: This study was approved by the Ethics Committee of Mashhad University of Medical Sciences (no.: 960096) and it complies with the Declaration of Helsinki. Informed consent was obtained from the subjects.
Funding/Support: This article is the result of a thesis for the MSc degree in extra-corporeal circulation (ECC) technology at the Mashhad University of Medical Sciences with the code of 960096, sponsored by the Mashhad University of Medical Sciences, Mashhad, Iran.
Informed Consent: Informed consent was obtained from the patients.
Contributor Information
Mehdi Fathi, Email: fathim@mums.ac.ir.
Morteza Valaei, Email: valayimorteza@gmail.com.
Amene Ghanbari, Email: a.mghanbari@yahoo.com.
Reza Ghasemi, Email: rezaghasemi152@yahoo.com.
Mohsen Yaghubi, Email: n.m.yaghubi@gmail.com.
References
- 1.Younessi Heravi MA, Yaghubi M, Joharinia S. Effect of change in patient's bed angles on pain after coronary angiography according to vital signals. J Res Med Sci. 2015;20(10):937–43. doi: 10.4103/1735-1995.172767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonow RO, Mann DL, Zipes DP, Libby P. Braunwald's heart disease e-book: A textbook of cardiovascular medicine. 10th ed. Philadelphia, PA: Elsevier Health Sciences; 2015. p. 1382. [Google Scholar]
- 3.Garg AX, Devereaux PJ, Yusuf S, Cuerden MS, Parikh CR, Coca SG, et al. Kidney function after off-pump or on-pump coronary artery bypass graft surgery: A randomized clinical trial. JAMA. 2014;311(21):2191–8. doi: 10.1001/jama.2014.4952. [DOI] [PubMed] [Google Scholar]
- 4.Lau G, Wald R, Sladen R, Mazer CD. Acute kidney injury in cardiac surgery and cardiac intensive care. Semin Cardiothorac Vasc Anesth. 2015;19(4):270–87. doi: 10.1177/1089253215593177. [DOI] [PubMed] [Google Scholar]
- 5.Chawla LS, Zhao Y, Lough FC, Schroeder E, Seneff MG, Brennan JM. Off-pump versus on-pump coronary artery bypass grafting outcomes stratified by preoperative renal function. J Am Soc Nephrol. 2012;23(8):1389–97. doi: 10.1681/ASN.2012020122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houlind K, Kjeldsen BJ, Madsen SN, Rasmussen BS, Holme SJ, Nielsen PH, et al. On-pump versus off-pump coronary artery bypass surgery in elderly patients: Results from the Danish on-pump versus off-pump randomization study. Circulation. 2012;125(20):2431–9. doi: 10.1161/CIRCULATIONAHA.111.052571. [DOI] [PubMed] [Google Scholar]
- 7.Puskas JD, Williams WH, Duke PG, Staples JR, Glas KE, Marshall JJ, et al. Off-pump coronary artery bypass grafting provides complete revascularization with reduced myocardial injury, transfusion requirements, and length of stay: A prospective randomized comparison of two hundred unselected patients undergoing off-pump versus conventional coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125(4):797–808. doi: 10.1067/mtc.2003.324. [DOI] [PubMed] [Google Scholar]
- 8.Shroyer AL, Grover FL, Hattler B, Collins JF, McDonald GO, Kozora E, et al. On-pump versus off-pump coronary-artery bypass surgery. N Engl J Med. 2009;361(19):1827–37. doi: 10.1056/NEJMoa0902905. [DOI] [PubMed] [Google Scholar]
- 9.Takagi H, Matsui M, Umemoto T. Off-pump coronary artery bypass may increase late mortality: A meta-analysis of randomized trials. Ann Thorac Surg. 2010;89(6):1881–8. doi: 10.1016/j.athoracsur.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Ding W, Ji Q, Shi Y, Ma R. Predictors of low cardiac output syndrome after isolated coronary artery bypass grafting. Int Heart J. 2015;56(2):144–9. doi: 10.1536/ihj.14-231. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Li X, Wu S, Ye W, Lou L. Metabolic syndrome and the incidence of hepatocellular carcinoma: A meta-analysis of cohort studies. Onco Targets Ther. 2018;11:6277–85. doi: 10.2147/OTT.S154848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardman RL, Jazaeri O, Yi J, Smith M, Gupta R. Overview of classification systems in peripheral artery disease. Semin Intervent Radiol. 2014;31(4):378–88. doi: 10.1055/s-0034-1393976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massoudy P, Wagner S, Thielmann M, Herold U, Kottenberg-Assenmacher E, Marggraf G, et al. Coronary artery bypass surgery and acute kidney injury--impact of the off-pump technique. Nephrol Dial Transplant. 2008;23(9):2853–60. doi: 10.1093/ndt/gfn153. [DOI] [PubMed] [Google Scholar]
- 14.Ghanei E, Hogat SA, OROUJI JT, Kolahi AA. Coronary artery bypass surgery and acute kidney injury: Impact of the off-pump technique. Iran Red Crescent Med J. 2012;14(2):65–9. [Google Scholar]
- 15.Moreira R, Jacinto T, Neves P, Vouga L, Baeta C. Predictors of acute kidney injury in the postoperative period of cardiac surgery associated with cardiopulmonary bypass. Rev Port Cir Cardiotorac Vasc. 2017;24(3-4):154. [PubMed] [Google Scholar]
- 16.Sajja LR, Mannam G, Chakravarthi RM, Sompalli S, Naidu SK, Somaraju B, et al. Coronary artery bypass grafting with or without cardiopulmonary bypass in patients with preoperative non-dialysis dependent renal insufficiency: A randomized study. J Thorac Cardiovasc Surg. 2007;133(2):378–88. doi: 10.1016/j.jtcvs.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 17.Singh RS, Thingnam SKS, Mishra AK, Verma I, Kumar V. Renal function after off-pump versus on-pump coronary artery bypass grafting. Asian Cardiovasc Thorac Ann. 2017;25(7-8):504–8. doi: 10.1177/0218492317730256. [DOI] [PubMed] [Google Scholar]
- 18.Amouzeshi A, Amouzeshi Z, Abbasi Teshnizi M, Moeinipour AA, Hosseinzadeh Maleki M. Off-pump versus on-pump coronary artery bypass graft surgery outcomes during 6 years: A prospective cohort study. Acta Med Iran. 2017;55(9):578–84. [PubMed] [Google Scholar]
- 19.Machado MN, Nakazone MA, Maia LN. Acute kidney injury based on KDIGO (kidney disease improving global outcomes) criteria in patients with elevated baseline serum creatinine undergoing cardiac surgery. Rev Bras Cir Cardiovasc. 2014;29(3):299–307. doi: 10.5935/1678-9741.20140049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jyrala A, Weiss RE, Jeffries RA, Kay GL. Effect of mild renal dysfunction (s-crea 1.2-2.2 mg/dl) on presentation characteristics and short- and long-term outcomes of on-pump cardiac surgery patients. Interact Cardiovasc Thorac Surg. 2010;10(5):777–82. doi: 10.1510/icvts.2009.231068. [DOI] [PubMed] [Google Scholar]
- 21.Devbhandari MP, Duncan AJ, Grayson AD, Fabri BM, Keenan DJ, Bridgewater B, et al. Effect of risk-adjusted, non-dialysis-dependent renal dysfunction on mortality and morbidity following coronary artery bypass surgery: A multi-centre study. Eur J Cardiothorac Surg. 2006;29(6):964–70. doi: 10.1016/j.ejcts.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 22.Zakkar M, Bruno VD, Guida G, Angelini GD, Chivasso P, Suleiman MS, et al. Postoperative acute kidney injury defined by RIFLE criteria predicts early health outcome and long-term survival in patients undergoing redo coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2016;152(1):235–42. doi: 10.1016/j.jtcvs.2016.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jamaati H, Najafi A, Kahe F, Karimi Z, Ahmadi Z, Bolursaz M, et al. Assessment of the EuroSCORE risk scoring system for patients undergoing coronary artery bypass graft surgery in a group of Iranian patients. Indian J Crit Care Med. 2015;19(10):576–9. doi: 10.4103/0972-5229.167033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mir Mohammad Sadeghi SM, Etesam Pour A, Mir Mohammad Sadeghi FS, Sadegh Pour M, Saeidi M, Nilforoush P, et al. Is ESModel an appropriate scale to estimate the mortality before the coronary bypass surgery in Iranian patients? J Isfahan Med Sch. 2011;28(117):1118–25. [Google Scholar]
- 25.Atashi A, Amini S, Tashnizi MA, Moeinipour AA, Aazami MH, Tohidnezhad F, et al. External validation of european system for cardiac operative risk evaluation ii (EuroSCORE II) for risk prioritization in an Iranian population. Braz J Cardiovasc Surg. 2018;33(1):40–6. doi: 10.21470/1678-9741-2017-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gravlee GP, Davis RF, Hammon JW, Kussman BD. Cardiopulmonary bypass, and mechanical support: Principles and practice. 4th ed. Canada: Wolters Kluwer; 2016. pp. 529–40. [Google Scholar]
- 27.Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1(1):19–32. doi: 10.2215/CJN.00240605. [DOI] [PubMed] [Google Scholar]
- 28.Kindzelski BA, Corcoran P, Siegenthaler MP, Horvath KA. Postoperative acute kidney injury following intraoperative blood product transfusions during cardiac surgery. Perfusion. 2018;33(1):62–70. doi: 10.1177/0267659117712405. [DOI] [PubMed] [Google Scholar]
- 29.Kaya A, Kurt M, Tanboga IH, Işık T, Günaydın ZY, Kaya Y, et al. Relation of neutrophil to lymphocyte ratio with the presence and severity of stable coronary artery disease. Clin Appl Thromb/Hemostasis. 2013;20(5):473–7. doi: 10.1177/1076029612473517. [DOI] [PubMed] [Google Scholar]
- 30.Muhmmed Suliman MA, Bahnacy Juma AA, Ali Almadhani AA, Pathare AV, Alkindi SS, Uwe Werner F. Predictive value of neutrophil to lymphocyte ratio in outcomes of patients with acute coronary syndrome. Arch Med Res. 2010;41(8):618–22. doi: 10.1016/j.arcmed.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Madjid M, Awan I, Willerson JT, Casscells SW. Leukocyte count and coronary heart disease: Implications for risk assessment. J Am Coll Cardiol. 2004;44(10):1945–56. doi: 10.1016/j.jacc.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 32.Eskandarian R, Ghorbani R, Toosi J, Malek M, Zahmatkesh M. Leukocyturia and coronary artery disease: Further evidence to the presence of inflammatory processes in acute coronary syndrome. ARYA Atherosclerosis. 2010;2(3) [Google Scholar]
- 33.Uysal HB, Dagli B, Akgullu C, Avcil M, Zencir C, Ayhan M, et al. Blood count parameters can predict the severity of coronary artery disease. Korean J Intern Med. 2016;31(6):1093–100. doi: 10.3904/kjim.2015.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montes FR, Maldonado JD, Paez S, Ariza F. Off-pump versus on-pump coronary artery bypass surgery and postoperative pulmonary dysfunction. J Cardiothorac Vasc Anesth. 2004;18(6):698–703. doi: 10.1053/j.jvca.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Abd-Allah AH, El-Rahman Mohamed AA, Amin SM, Selim S, Allam M. Immediate pulmonary dysfunction in ischemic heart disease patients undergoing off-pump versus on-pump CABG. J Egypt Soc Cardio Thorac Surg. 2016;24(1):15–20. doi: 10.1016/j.jescts.2016.04.005. [DOI] [Google Scholar]
- 36.Staton GW, Williams WH, Mahoney EM, Hu J, Chu H, Duke PG, et al. Pulmonary outcomes of off-pump vs on-pump coronary artery bypass surgery in a randomized trial. Chest. 2005;127(3):892–901. doi: 10.1378/chest.127.3.892. [DOI] [PubMed] [Google Scholar]
- 37.Chertow GM, Lazarus JM, Christiansen CL, Cook EF, Hammermeister KE, Grover F, et al. Preoperative renal risk stratification. Circulation. 1997;95(4):878–84. doi: 10.1161/01.cir.95.4.878. [DOI] [PubMed] [Google Scholar]
- 38.Song Y, Kim DW, Kwak YL, Kim BS, Joo HM, Ju JW, et al. Urine output during cardiopulmonary bypass predicts acute kidney injury after cardiac surgery: A single-center retrospective analysis. Medicine (Baltimore). 2016;95(22):e3757. doi: 10.1097/MD.0000000000003757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yilmaz M, Aksoy R, Kilic Yilmaz V, Balci C, Duzyol C, Tekeli Kunt A. Urine output during cardiopulmonary bypass predicts acute kidney injury after coronary artery bypass grafting. Heart Surg Forum. 2016;19(6):E289–93. doi: 10.1532/hsf.1495. [DOI] [PubMed] [Google Scholar]