Abstract
Organellar gene expression (OGE) in chloroplasts and mitochondria is primarily modulated at post-transcriptional levels, including RNA processing, intron splicing, RNA stability, editing, and translational control. Nucleus-encoded Chloroplast or Mitochondrial RNA-Binding Proteins (nCMRBPs) are key regulatory factors that are crucial for the fine-tuned regulation of post-transcriptional RNA metabolism in organelles. Although the functional roles of nCMRBPs have been studied in plants, their cellular and physiological functions remain largely unknown. Nevertheless, existing studies that have characterized the functions of nCMRBP families, such as chloroplast ribosome maturation and splicing domain (CRM) proteins, pentatricopeptide repeat (PPR) proteins, DEAD-Box RNA helicase (DBRH) proteins, and S1-domain containing proteins (SDPs), have begun to shed light on the role of nCMRBPs in plant growth, development, and stress responses. Here, we review the latest research developments regarding the functional roles of organellar RBPs in RNA metabolism during growth, development, and abiotic stress responses in plants.
Keywords: organellar gene expression, chloroplast, mitochondria, RNA metabolism, RNA-binding proteins, abiotic stress
1. Introduction
Plant chloroplasts and mitochondria are thought to be derived from free-living cyanobacteria and α-proteobacteria, respectively [1,2]. During evolution, the organellar genes were largely transferred to the nucleus [2]. Current organellar genomes harbor only 15–209 proteins in the chloroplast, and 3–67 proteins in the mitochondrion [3], which are essential for photosynthetic apparatus, mitochondrial electron transport chain, and organellar gene expression (OGE) machinery [4]. OGE in plant organelles conserves both prokaryotic and eukaryotic properties [5]. However, the OGE mechanisms in plant organelles are much more complex than those of their bacterial ancestors [5,6] and require thousands of nucleus-encoded proteins for maintaining OGE machinery and organellar function. This indicates the importance of interactions between the organelles and nucleus in controlling fine-tuned OGE through a nucleus-to-organelle anterograde or an organelle-to nucleus retrograde signaling [7,8,9].
OGE is commonly regulated at the post-transcriptional level, including RNA processing, editing, stabilization, turnover, intron splicing, and translational control, all of which are crucial for a number of organellar processes [10,11,12,13]. The regulation of post-transcriptional RNA processing in organelles requires hundreds of nucleus-encoded chloroplast or mitochondrial RNA-binding proteins (nCMRBPs) during acclimation to environmental stress, as well as during plant growth and development [14,15]. Recent studies have uncovered that nCMRBPs play a critical role in plant growth and stress responses [15,16,17,18,19]. Moreover, analysis of the characteristics of nCMRBP has demonstrated that they possess multiple conserved motifs and domains, which include chloroplast RNA splicing and ribosome maturation (CRM), pentatricopeptide repeat (PPR), DEAD-box RNA helicase (DBRH), and S1 RNA-binding domain (SDP) [19,20,21,22]. Importantly, it is now known that the nCMRBPs function as either specific RNA-binding proteins or non-specific RNA-binding proteins (RNA chaperones), which facilitates the correct folding of the target RNA structure during plant growth and under environmental stress [15,23]. Chloroplast- or mitochondria-localized CRM, PPR, DBRH, and SDP proteins have been assessed in terms of their roles as RNA chaperones [21,24,25,26,27]. In this review, we will focus on the recent advances in research on the function and cellular mechanisms of CRM, PPR, DBRH, and SDP proteins in organellar RNA metabolism during plant growth, development, and abiotic stress responses.
2. Domain or Motif Features of CRM, PPR, DBRH, and SDP Proteins
2.1. CRM Proteins
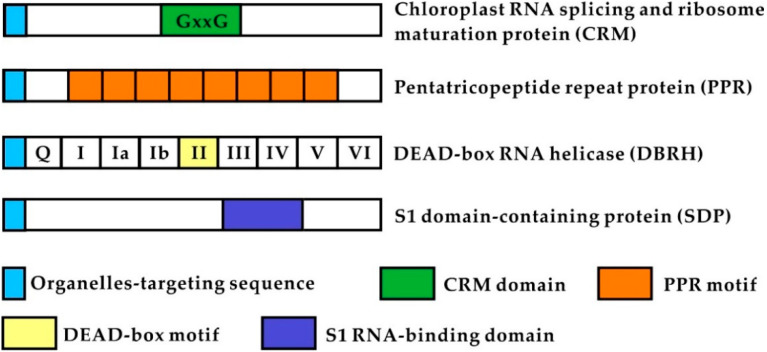
Single chloroplast RNA splicing and ribosome maturation (CRM) domain-containing proteins were first studied in archaea and bacteria [28,29], in which domain analysis revealed that CRM is orthologous to E. coli YhbY associated with pre-50S ribosomal subunits [30] (Figure 1). Land plants harbor single to multiple copies of CRM domains that can be classified into 4 subfamily groups, which include the CRS1 subfamily (for chloroplast RNA splicing), the CAF subfamily (for CRS2-associated factors), and CFM3 and 4 (for CRM family members), based on the proteome database of Arabidopsis and rice [20]. Furthermore, structural analysis has demonstrated that GxxG sequences conserved in the loop of the CRM domain contribute to RNA-binding capacity [20,30,31].
Figure 1.
Basic domains or motifs of chloroplast ribosome maturation and splicing domain (CRM), pentatricopeptide repeat (PPR), DEAD-Box RNA helicase (DBRH), and S1-domain containing proteins (SDP) proteins in plants.
2.2. PPR Proteins
Pentatricopeptide repeat proteins were first identified in the Arabidopsis genome and are comprised of tandem repeated motifs of 35-amino acid sequences, ranging from 2 to 30 tracts [23,32,33]. PPR motifs fold into a pair of antiparallel α helices and contribute to organellar RNA metabolism on the basis of modular one-repeat:one-nucleotide binding [23,34]. Plant PPR proteins are classified into two subfamilies; P- and PLS-class (P: 35 amino acids, L: 36 amino acids, and S: 31 amino acids, Figure 1). Most P-class proteins contain only PPR motifs, although some also harbor a PPR-small MutS-related (SMR) domain, which is important for organellar RNA stabilization, group II intron splicing, and intercistronic processing [23]. In contrast, the PLS-class proteins contain additional C-terminal domains of E, E+, and DYW, which are mainly involved in RNA C to U editing via recruiting additional proteins [33,35].
2.3. DEAD-Box RH Proteins (DBRH)
Helicase proteins are enzymes that catalyze the unwinding of double-stranded DNA or duplex RNA secondary structures in ATP-dependent rearrangements in both prokaryotic and eukaryotic cells [36,37]. They are divided into six superfamilies (SF1–SF6) based on the properties of the conserved motifs in their primary amino acid sequences [38,39]. The DBRH family belongs to the largest group, superfamily 2 (SF2), and harbors at least nine conserved motifs, such as Q, I, Ia, Ib, II, III, IV, V, and VI (Figure 1) [40,41,42]. The Q-motif, motif I (walker A motif), motif III, and motif VI of the DBRH family are essential for ATP binding and ATP hydrolysis [41,43,44]. Motif II (walker B motif), which contains residues of Asp-Glu-Ala-Asp (DEAD) is also crucial for ATP binding and ATP hydrolysis via the interaction of Mg2+ [39,41]. Only a few biochemical studies have been focused on the remaining (Ia, Ib, IV, and V) motifs. However, it has been suggested that they are also involved in RNA binding [41].
2.4. SDP Proteins
The S1 RNA domain-containing protein (SDP) was first observed in the ribosomal proteins S1 (RPS1) of E. coli. [45]. E. coli RPS1 is comprised of six copies of an S1 motif containing approximately 70 amino acids [46]. The structure of the S1 domain adopt a five-stranded antiparallel β barrel in which residues Phe-19, Phe-22, His-34, Asp-64, and Arg-68 are believed to infer its RNA-binding ability [46]. S1 domain repeats (Figure 1) vary from one to 15 in different species [47] and have been identified in RNase E endonuclease (RNase E), RNase II exonuclease (RNase II), transcription factor NusA, and C. elegans EMB-5 [46], which play a crucial role in mRNA turnover, rRNA processing, and translational initiation [48,49,50,51]. In addition, S1 domain repeats are also found in other RNA-associated proteins, such as bacterial polynucleotide phosphorylase (PNPase) [52,53], bacterial translation initiation factor 1 (IF1), eukaryotic eIF2a [54], and the RNA helicase-like protein PRP22 found in yeasts [55]. Furthermore, a recent study has demonstrated that the amino acid sequence homologies of S1 domains are approximately 43% in archaea, 51% in bacteria, and 46% in eukaryotes, and that the residues of Phe-28, Asp-66, and Arg-71 in archaea and Phe-25, Asp-68, and Arg-71 in eukaryotes are highly conserved [47]. These findings suggest that S1 domains are diverse with a low sequence identity among different species.
3. Functions of nCMRBPs in Plant Growth and Development
The latest studies have indicated the importance of nCMRBPs, including CRM, PPR, DBRH, and SDP, for organellar RNA metabolism during plant growth and development (Table 1). Fourteen and 16 CRM proteins are encoded in Arabidopsis and rice genomes, respectively [16,20]. Previous analysis has indicated that chloroplast-localized Arabidopsis AtCRS1 [56], AtCAF1 [56], AtCAF2 [56], AtCFM2 [28], and rice OsCFM3 [57], and dual-localized AtCFM3 [57] in both chloroplasts and mitochondria, are involved in the splicing of subsets of specific introns. The recent functional analysis of unknown CRM subfamilies has uncovered that Arabidopsis AtCFM4 [24] is involved in 16S and 23S rRNA processing, and that rice OsCAF1 [58] and OsCFM2 [59] is important for the splicing of chloroplast introns as the orthologues of AtCAF1 and AtCAF2. Furthermore, it has been demonstrated that mitochondria-localized Arabidopsis mCSF1 [60] and CFM9 [61] are involved in the splicing of multiple mitochondrial introns and can influence seed development and seedling growth, respectively, indicating that CRM proteins play a crucial role in plant growth and development.
Table 1.
Phenotypes and functions of CRM, PPR, DBRH, and SDP proteins in plant growth and development.
| Plant | Gene Name | Gene Number | Location | Molecular Function | Mutant Phenotype | Ref. |
|---|---|---|---|---|---|---|
| A. thaliana | CRM family | |||||
| AtCRS1 | At5g16180 | C | Splicing of group II intron (atpF) | Small and albino seedling | [28,56] | |
| AtCAF1 | At2g20020 | C | Splicing of group II introns (petD, rpl16, rps16, ndhA, rpoC1, ycf3-1, clpP-1, and trnG) | Albino seedling | [56] | |
| AtCAF2 | At1g23400 | C | Splicing of group II introns (ndhA, ndhB, petB, ycf3-1, and rps12-1) | Small and pale green seedling | [56] | |
| AtCFM2 | At3g01370 | C | Splicing of group I (trnL) and group II introns (ndhA, ycf3-1, and clpP-2) | Small and albino seedling | [28] | |
| AtCFM3a | At3g23070 | C/M | Splicing of group II intron (ndhB) | Stunted growth | [57] | |
| CFM4 | At4g39040 | C | 16S and 23S rRNA processing | Retarded growth | [24] | |
| mCSF1 | At4g31010 | M | Splicing of multiple mitochondrial introns | Embryo lethal Retarded growth |
[60] | |
| CFM9 | At3g27550 | M | Splicing of multiple mitochondrial introns | Retarded growth | [61] | |
| O. sativa | OsCAF1 | Os01g 0495900 |
C | Splicing of group II introns (atpF, rpl2, rps12, ndhA, ndhB, and ycf3) | Albino seedling | [58] |
| OsCFM2 | Os04g 0464800 |
C | Splicing of group I (trnL) and group II introns (atpF, rpl2, rps12, ndhA, and ycf3-1) | Albino seedling | [59] | |
| OsCFM3 | Os11g 37990 |
C | Splicing of group II introns (ndhB, petD, rpl16, rps16, trnG, and petB) | Albino seedling | [57] | |
| A. thaliana | PPR family | |||||
| OTP51 | At2g15820 | C | Splicing of ycf3 intron2 | Pale yellow seedling | [64] | |
| OTP70 | At4g25270 | C | Splicing of rpoC1 intron | Virescent seedling | [65] | |
| AtPPR4 | At5g04810 | C | Trans-splicing of rps12 intron1 | Embryo lethal, pale green, or albino seedling | [25] | |
| EMB2654 | At2g41720 | C | Trans-splicing of rps12 intron1 | Embryo lethal, pale green, or albino seedling | [66] | |
| PBF2 | At3g42630 | C | Splicing of ycf3 intron1 | Small and pale yellowish seedling | [67] | |
| SOT5/EMB2279 | At1g30610 | C | Splicing of rpl2 and trnK intron | Virescent seedling | [68] | |
| HCF152 | At3g09660 | C | Stabilization or processing of psbB-psbT-psbH-petB-petD | High chlorophyll fluorescence | [69] | |
| MRL1 | At4g34830 | C | Stabilization of rbcL | Pale green seedling | [70] | |
| PGR3 | At4g31850 | C | Stabilization of petL and probably ndhA | High chlorophyll fluorescence | [71] | |
| BFA2 | At4g30825 | C | Stabilization of atpH/F | Stunted growth | [72] | |
| AtPPR2 | At3g06430 | C | Chloroplast 23S rRNA processing | Embryo lethal or albino seedling | [75] | |
| SOT1 | At5g46580 | C | Chloroplast 23S-4.5 rRNA processing | Small and pale green seedling | [76] | |
| PPR287 | At4g59040 | C | Processing of chloroplast 16S, 23S, 4.5S, and 5S rRNAs | Yellowish seedling | [77] | |
| OTP43 | At1g74900 | M | Trans-splicing of nad1 intron1 | Small and delayed development | [78] | |
| BIR6 | At3g48250 | M | Splicing of nad7 intron1 | Small and retarded growth | [79] | |
|
TANG2
OTP439 |
At1g19290 At3g48810 |
M | Splicing of nad5 intron2 and 3 | Retarded growth | [80] | |
| SLO3 | At3g61360 | M | Splicing of nad7 intron2 | Delayed growth and development | [81] | |
|
MISF26
MISF68 MISF74 |
At1g66345 At3g16010 At4g01400 |
M | Splicing of nad2 intron3 (MISF26) Splicing of nad2 intron2, nad4 intron1, and nad5 intron4 (MISF68) Splicing of nad1 intron4 and nad2 intron4 (MISF74) |
Delayed growth | [82] | |
| EMB2794 | At2g02150 | M | Trans-splicing of nad2 intron2 | Retarded growth and developmental defect | [83] | |
| MTSF1 | At1g06710 | M | Stabilization of nad4 | Retarded growth | [84] | |
| PPR19 | At1g52620 | M | Stabilization of nad1 intron3 | Retarded growth and developmental defect | [85] | |
| Z. mays | PPR4 | Zm00001d026654 | C | Trans-splicing of rps12 intron1 | Seedling lethal pale green, or albino seedling |
[62] |
| THA8 | GRMZM2G466032 | C | Splicing of ycf3 intron2 and trnA intron | Pale green seedling | [63] | |
| ZmPPR5 | GRMZM2G025409 | C | Splicing of trnG intron | Seedling lethal or pale green seedling | [73] | |
| PPR10 | GRMZM2G177169 | C | Stabilization of atpH and psaJ | Seedling lethal or yellowish green seedling | [74] | |
| A. thaliana | DBRH family | |||||
| RH3 | At5g26742 | C | Splicing of group II introns (trnI, trnA, rps12-1, rps12-2, and rpl2) and chloroplast 23S rRNA processing | Embryo lethal or pale green seedling | [88] | |
| ISE2 | At1g70070 | C | Splicing of group II introns (rpl2, atpF, rps12, and clpP) | Chlorotic seedling | [92] | |
| PMH2 | At3g22330 | M | Splicing of nad2 introns | Similar to wild-type | [93] | |
| RH22 | At1g59990 | C | Chloroplast 23S-4.5S rRNA processing | Embryo lethal or virescent seedling | [94] | |
| RH39 | At4g09730 | C | Chloroplast 23S rRNA processing | Retarded growth | [95] | |
| RH50 | At3g06980 | C | Chloroplast 23S-4.5S rRNA maturation | Similar to wild-type | [89] | |
| A. thaliana | SDP family | |||||
| SDP | At1g12800 | C | Processing of chloroplast 16S, 23S, 4.5S, and 5S rRNAs | Pale green seedling | [21] | |
| RLSB | At1g71720 | C | Regulation of rbcL mRNA | Reduced seedling size | [96] | |
| N. benthamiana | STF | HM012811 | C | Regulation of plastid transcription | Yellowish leaves | [97] |
As the structural characteristics of PPR motifs were first determined in plants, the functions of a large number of plant PPR proteins have been reported over the last 20 years [23,26]. As it is not possible to consider all of these in this review, we will only discuss the P-type PPR proteins. Previous analysis of PPR proteins has demonstrated that chloroplast-localized maize PPR4 [62] and THA8 [63], and Arabidopsis OTP51 [64] and OTP70 [65], are essential for the splicing of chloroplast specific introns. Interestingly, recent studies of PPR4 [25], EMB2654 [66], PBF2 [67], and SOT5 [68] in Arabidopsis showed that these proteins play a role in the splicing of chloroplast introns through their role in the recognition of the specific RNA sequences. Arabidopsis HCF152 [69], MRL1 [70], PGR3 [71], BFA2 [72], and maize PPR5 [73] and PPR10 [74] were shown to be essential for the stabilization of chloroplast transcripts. AtPPR2 [75], SOT1 [76], and PPR287 [77] in Arabidopsis are involved in chloroplast rRNA processing. In addition, mitochondria-localized Arabidopsis OTP43 [78], BIR6 [79], TANG2/OTP439 [80], and SLO3 [81] are known to affect the splicing of mitochondrial introns. The latest studies of Arabidopsis MISF 26, 68, 74 [82], and EMB2794 [83] have also demonstrated their significance in mitochondrial intron splicing. Furthermore, Arabidopsis MTSF1 [84] and PPR19 [85] were found to be important for mitochondrial RNA stabilization as they bind to specific sequences, suggesting that organelle-localized PPR proteins perform versatile roles in organellar RNA metabolism.
Approximately 58 and 50 DBRH were annotated from the Arabidopsis and rice genome, respectively [86,87]. Although the functional roles of DBRH in plants have been investigated for several decades, the DBRH functions in the chloroplast and mitochondria are not as well understood as those in the nucleus. Nonetheless, the roles of DBRHs for organellar RNA metabolism have been emerged [26,88,89,90,91]. In Arabidopsis, chloroplast-localized RH3 [26] and ISE2 [92] and mitochondria-localized PMH2 [93] are involved in the splicing of diverse organellar introns, and RH22 [94], RH39 [95], and RH50 [89] are associated with chloroplast rRNA processing and ribosome biogenesis. In addition to this, chloroplast-localized SDP proteins, including Arabidopsis RLSB [96] and Nicotiana STF [97], play roles in plastid gene expression, supporting the notion that Arabidopsis SDP is crucial for chloroplast rRNA processing during plant growth and development.
Importantly, the aforementioned nCMRBPs are transported into chloroplasts and/or mitochondria, and mutations in these genes result in various phenotypes, including embryo lethality, albino, pale green, dwarfism, delayed growth, as well as impaired photosynthesis and mitochondrial respiration (Table 1). This indicates that nCMRBPs play central roles in a variety of cellular RNA metabolism processes in organelles during plant growth and development.
4. Physiological Functions of nCMRBPs in Abiotic Stress Responses
As sessile organisms, plants often face adverse environmental conditions, including extremes of temperature, high salinity, drought, and UV stresses, all of which can severely damage crop productivity and yield [98,99]. To survive these harsh conditions, plants need to adapt to these environmental challenges by reprogramming the expression of genes in their nucleus, chloroplasts, and mitochondria [18,100,101]. The organelles serve as a stress sensor, and the regulation of OGE [100,102] and organellar metabolic processes are essential for acclimatizing to abiotic stress responses [16,18]. A number of studies have determined the functional roles of nCMRBPs in organelles for environmental stress responses (Table 2).
Table 2.
Phenotypes and functions of CRM, PPR, DBRH, and SDP proteins in abiotic stress responses.
| Plant | Gene Name | Gene Number | Location | Molecular Function | Mutant Phenotype | Ref. |
|---|---|---|---|---|---|---|
| A. thaliana | CRM family | |||||
| CFM9 | At3g27550 | M | Splicing of multiple mitochondrial introns | Sensitive to salt, drought, or ABA | [61] | |
| CFM4 | At4g39040 | C | 16S and 23S rRNA processing | Sensitive to salt or cold stress | [24] | |
| A. thaliana | PPR family | |||||
| ABO5 | At1g51965 | M | Splicing of nad2 intron3 | Sensitive to ABA | [111] | |
| PPR40 | At3g16890 | M | Sensitive to salt, ABA, or oxidative stress Tolerant to salt stress in overexpression plants |
[108,109] | ||
| GUN1 | At2g31400 | C | Sensitive to sucrose or ABA | [103] | ||
| ABO8 | At4g11690 | M | Splicing of nad4 intron3 | Sensitive to ABA | [112] | |
| PPR96 | At2g03380 | M | Probably mitochondrial RNA editing | Tolerant to salt, ABA, or oxidative stress | [113] | |
| PGN | At1g56570 | M | Regulation of NAD1, RPL2, NAD9, and MATR genes | Sensitive to salt, glucose, or ABA | [110] | |
| O. sativa | OsV4 | Os04g39970 | C | Plastid gene expression associated with plastid translation machinery | Sensitive to cold stress | [105] |
| WSL | Os01g37870 | C | Splicing of chloroplast rpl2 intron | Sensitive to salt, sucrose, or ABA | [104] | |
| TCD10 | Os10g28600 | C | Regulation of OsV4, OsRpoTp, V1, V2, RNRL, RNRS, 16S rRNA, rpl21, and OsDG2 genes | Sensitive to cold stress | [106] | |
| WSL5 | Os04g0684500 | C | RNA editing of rpl2 and atpA, and splicing of rpl2 and rps12 intron2 | Sensitive to cold stress | [107] | |
| A. thaliana | DBRH family | |||||
| RH3 | At5g26742 | C | Splicing of ndhA and ndhB introns | Sensitive to salt or cold stress | [26] | |
| O. sativa | TCD33 | Os03g01830 | C | Probably chloroplast ribosome assembly | Sensitive to cold stress | [91] |
| OsRH58 | Os01g73900 | C | Translational control of chloroplast POR, rbcL, Clpb3, PsbA, and PetA transcripts | Tolerant to salt or drought stress | [90] | |
| B. rapa | BrRH22 | Bra035413 | C | Translational control of chloroplast rbcL, psbA, and ycf3 transcripts | Tolerant to salt or drought stress | [114] |
| A. thaliana | SDP family | |||||
| SRRP1 | At3g23700 | C | Splicing of chloroplast trnL intron and 5S rRNA processing | Sensitive to ABA | [115] | |
| RPS5 | At2g33800 | C | Chloroplast 16S rRNA processing | Tolerant to cold stress in overexpression plants | [116] | |
| SDP | At1g12800 | C | Processing of chloroplast 16S, 23S, 4.5S, and 5S rRNAs | Sensitive to UV, salt, heat, or freezing stress | [117] |
Chloroplast-localized Arabidopsis CRM-containing CFM4 (16S and 23S rRNA processing) has been determined as a positive effector in seed germination and seedling growth under low temperature and salt stress conditions [24]. Recent work to characterize mitochondria-localized Arabidopsis CFM9, which is involved in the splicing of multiple mitochondrial introns, has demonstrated its positive role in seed germination and seedling growth in the presence of the abscisic acid (ABA) and under high salinity or dehydration stress [61]. Although the cfm4 and the cfm9 mutants grew slowly under normal conditions, the mutant characteristics of growth retardation and delayed germination were much more severe under abiotic stress conditions compared to those of the wild type. This indicates that organelle-targeted CFM4 and CFM9 also play a crucial role in plant responses to abiotic stresses.
The diverse roles of organelle-localized PPR proteins have been demonstrated in the responses of plants to abiotic stresses. The loss-of-function mutant of chloroplast-localized Arabidopsis GUN1 was found to be hypersensitive to sucrose and ABA [103]. Chloroplast-localized rice WSL, which is involved in the splicing of chloroplast rpl2 introns, enhanced seed germination and seedling growth in response to multiple environmental factors, such as glucose, ABA, and salinity, owing to its reduced translation efficiency [104]. Chloroplast-localized rice OsV4 affects the gene expression of plastid translation machinery, TCD10 is important for the gene regulation of OsV4, OsRpoTp, V1, V2, RNRL, RNRS, 16S rRNA, rpl21, and OsDG2, and WSL5 are involved in the editing of rpl2 and atpA, as well as the splicing of rpl2 and rps12 intron2, are crucial for chloroplast biogenesis, the mutants of which lead to albino or pale yellowish phenotypes during cold stress [105,106,107]. The overexpression of mitochondria-localized Arabidopsis PPR40 has been shown to promote seed germination in the presence of salt or ABA and improve seedling growth under conditions of high salinity by reducing reactive oxygen species (ROS) damage in the mitochondria [108,109]. In addition, mitochondria-localized Arabidopsis PGN, which is involved in the expression of mitochondrial NAD1, RPL2, NAD9, and MATR genes, plays a role in both biotic and abiotic stress tolerance, and its loss-of-function mutants are susceptible to ABA, salt, and glucose, as well as necrotrophic fungal pathogens [110]. Arabidopsis ABO5 and ABO8, which are involved in the splicing of mitochondrial nad2 intron3 and nad4 intron3, respectively, have been shown to have enhanced sensitivity to ABA during post-germination and root growth phase due to the accumulation of ROS in the mitochondria [111,112]. Interestingly, Arabidopsis PPR96, which is thought to be involved in mitochondrial RNA editing, has a negative impact on seed germination and seedling growth [113].
The organelle-localized DBRHs are essential for the responses of plants to environmental stresses. The loss-of-function mutant of chloroplast-localized Arabidopsis RH3, which is involved in the splicing of ndhA and ndhB introns, displays hypersensitivity to salt and cold stress, and to ABA [26]. Recently, cold-inducible rice TCD33, which is thought to be involved in chloroplast ribosome assembly, has been shown to affect chloroplast biogenesis under cold stress [91]. Moreover, the ectopic expression of rice RH58, which is involved in the translation of chloroplast POR, RBCL, CLPB3, PSBA, and PETA transcripts, and cabbage RH22, which affects the translation of chloroplast RBCL, PSBA, and YCF3 genes, contributed to an enhanced tolerance to salt and drought stress in Arabidopsis by increasing the translational efficiency of chloroplast mRNAs [90,114].
Chloroplast-localized SRRP1 harboring two S1 domains was shown to decrease sensitivity to ABA by impairing the splicing of the chloroplast trnL intron and 5S rRNA processing in the presence of ABA [115]. Additionally, the overexpression of chloroplast RPS5, which is involved in 16S rRNA processing, enhanced seedling growth in response to cold stress [116]. A recent study has also demonstrated that chloroplast-localized SDP, which affects rRNA processing in chloroplasts under normal conditions, has positive effects on salt, heat, freezing, or UV stress tolerance, as it influences the stress-responsive genes in the nucleus [117]. Beyond organelles, it will be of great interest to investigate how nCMRBP-mediated organellar retrograde signaling (ORS) influences the reprogramming of the expression of stress-responsive nuclear genes, and the organellar and nuclear epigenetic modifications for stress priming and memory [101,118,119,120,121]. Although the study of organellar proteomics and metabolomics is far behind than those in the nucleus and cytoplasm, recent studies emphasize the importance of homeostasis between the nucleus and organelles in plant acclimation to environmental changes [122,123,124,125]. With these omics data, future tasks are to identify novel ORS molecules and pathways, which will widely expand our understanding of crosstalk between the nucleus and organelles.
5. Cellular Roles of nCMRBPs in Organellar RNA Metabolism
The mechanistic role of nCMRBPs in plant growth, development, and abiotic stress responses remains largely unknown. However, recent studies have revealed that nCMRBPs act as RNA chaperones in plant growth and development, as well as in stress adaptation processes.
RNA molecules must adopt correct structures in order to maintain functional RNAs. However, RNA molecules are often misfolded into non-functional secondary or tertiary structures in cells, due to intrinsic thermodynamic and kinetic folding problems [126,127]. As such, either specific RBPs or RNA chaperones are required to ensure correct folding. An RNA chaperone is defined as a non-specific RNA-binding protein that guides the folding of RNA molecules to ensure functionally active states are achieved through structural rearrangement [127,128]. RNA chaperones usually bind to a wide range of RNA species and are characterized as being non-specific [129,130]. Another of their typical features is that they do not require external energy input or ATP, and they generally adopt structurally disordered regions rendering RNA chaperone activity.
Research conducted over a number of decades has demonstrated that RNA chaperones are crucial for diverse cellular processes in prokaryotic and eukaryotic organisms [15]. It has been demonstrated that viral nucleocapsid proteins and E. coli Hfq and ProQ are important for stress responses because of their roles as RNA chaperones [131,132]. Studies characterizing multiple DBRHs in bacteria, animals, and yeast have demonstrated that CYT-19, DeaD, SrmBp, RhlE, and Mss116p are associated with the splicing of mitochondrial group I and II introns through their RNA chaperone activity [133,134,135]. In plants, it has also been demonstrated that U11/U12-31K, a minor spliceosomal protein of Arabidopsis and rice, is involved in the splicing of U12-type introns as an RNA chaperone, and it is essential for the correct folding of introns during normal growth and development [136,137]. Studies of RNA chaperones have also been expanded to a variety of CSDP, GRP, and RZ proteins in Arabidopsis, rice, cabbage (Brasscia rapa), and wheat (Triticum aestivum) under various environmental conditions [15,138,139,140,141,142,143,144,145,146,147]. In plant organelles, recent findings have illustrated that chloroplast-localized Arabidopsis CFM4, RH3, SDP, and SRRP1 have RNA chaperone properties that are crucial for maintaining the structures of the precursor-RNA molecules suitable for splicing or rRNA processing [21,24,115,147]. It has also been demonstrated that chloroplast-localized Arabidopsis and rice PPR4, containing both RRM and PPR motifs, possess RNA chaperone activity through its RRM motif, and thereby affect the trans-splicing of rps12 intron1 [25]. Moreover, chloroplast-localized rice OsRH58 and cabbage BrRH22 were shown to affect the translation of multiple chloroplast mRNAs through their RNA chaperone activities that aid in the structural rearrangement of target mRNAs for subsequent efficient translation control under environmental stresses [90,114]. Mitochondria-localized Arabidopsis CFM9 was shown to affect the splicing of multiple mitochondrial introns, and the cfm9 mutant was found to be sensitive to abiotic stresses [61]. As such, CFM9 is presumably important for mitochondrial intron splicing due to its RNA chaperon function. Taken together, these results clearly indicate that nCMRBPs, which carry out RNA chaperone activities, have significant roles in the regulation of organellar RNA metabolism during plant growth, development, and responses to abiotic stress.
6. Conclusions and Future Directions
Although the functional roles of nucleus-encoded organellar RBPs are still not fully understood, the latest studies of the cellular and physiological functions of nCMRBPs has shed some light on the significance of nCMRBPs for organellar RNA metabolism during plant growth, development, and environmental stress responses. It has been demonstrated that chloroplasts- or mitochondria-localized CRM, PPR, DBRH, and SDP proteins play pivotal roles in organellar post-transcriptional RNA metabolism, including intron splicing, rRNA processing, and translational control under normal and stressful conditions (Figure 2). Since different nCMRBPs often target same RNA for processing or splicing, it would be interesting to determine whether nCMRBPs interact together to mediate RNA metabolism. Moreover, given that the target organellar RNAs of many nCMRBPs are not known yet, determination of the sequence- and structure-dependent recognition of target RNAs by RBPs would be important for further understanding of the mechanistic roles of nCMRBPs. In particular, many nCMRBPs have been shown to play their roles as RNA chaperones that aid in the structural rearrangement of RNA molecules during plant growth and responses to environmental stimuli. However, further research is required to unravel the mechanisms underlying the RNA chaperone function and to identify any protein partners that may interact with nCMRBPs, which play indispensable roles in organellar RNA metabolism under both normal conditions and abiotic stress. In addition to this sequence- and structure-dependent RNA regulation, epitranscriptomic RNA methylation is recently emerging as a new form of post-transcriptional RNA regulation associated with plant development and stress responses [148,149,150]. However, to date, the significance of RNA methylation in the recognition of target RNAs by RBPs, and the importance of the interactions between RBPs and modified RNAs, have yet to be determined. With the recent advances in high-throughput methylated RNA immuneprecipitation-sequencing technology [151], transcriptome-wide m6A methylation patterns in the chloroplast and mitochondria RNAs have been reported [152]. It would be interesting to determine how the methylation in organellar RNAs influences the recognition and subsequent binding of nCMRBPs to target RNA. This knowledge will further our understanding of the regulation of RNA metabolism in organelles that are essential for stress adaptation, as well as plant growth and development.
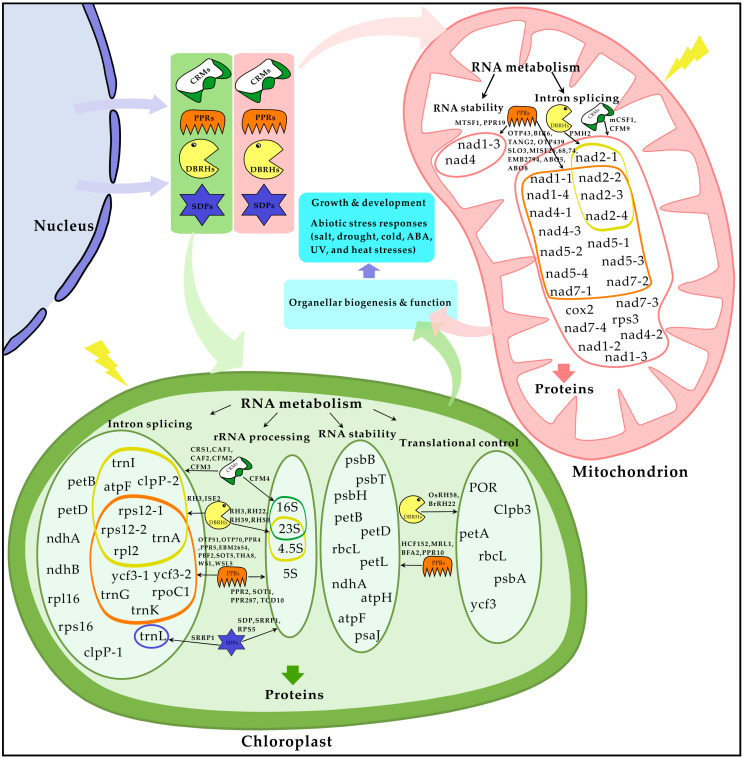
Figure 2.
Cellular function of nucleus-encoded Chloroplast or Mitochondrial RNA-Binding Proteins (nCMRBPs) in organellar RNA metabolism. Nucleus-encoded CRMs, PPRs, DBRHs, and SDPs are transported into chloroplasts and/or mitochondria and are involved in RNA metabolism, including intron splicing, RNA stability, rRNA processing, and translational control in organelles as described in Table 1 and Table 2. The nCMRBP-mediated RNA metabolism influences the homeostasis of organellar biogenesis and function, which plays an essential role in plant growth and development, as well as in abiotic stress responses. Yellow-colored thunder indicates environmental stimuli.
Acknowledgments
We thank a professional English service for English language editing.
Author Contributions
K.L. drafted the manuscript and H.K. revised it. H.K. and K.L. finalized the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a Humboldt Fellowship for Researchers, which was awarded to K.L., and by a grant from the Next-Generation BioGreen21 Program (PJ01314701), Rural Development Administration, Republic of Korea, which was awarded to H.K.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Andersson S.G.E., Karlberg O., Canback B., Kurland C.G. On the origin of mitochondria: A genomics perspective. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003;358:165–177. doi: 10.1098/rstb.2002.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Timmis J.N., Ayliffe M.A., Huang C.Y., Martin W. Endosymbiotic gene transfer: Organelle genomes forge eukaryotic chromosomes. Nat. Rev. Genet. 2004;5:123–135. doi: 10.1038/nrg1271. [DOI] [PubMed] [Google Scholar]
- 3.Kleine T., Maier U.G., Leister D. DNA transfer from organelles to the nucleus: The idiosyncratic genetics of endosymbiosis. Annu. Rev. Plant Biol. 2009;60:115–138. doi: 10.1146/annurev.arplant.043008.092119. [DOI] [PubMed] [Google Scholar]
- 4.Maier U.G., Zauner S., Woehle C., Bolte K., Hempel F., Allen J.F., Martin W.F. Massively convergent evolution for ribosomal protein gene content in plastid and mitochondrial genomes. Genome Biol. Evol. 2013;5:2318–2329. doi: 10.1093/gbe/evt181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liere K., Weihe A., Borner T. The transcription machineries of plant mitochondria and chloroplasts: Composition, function, and regulation. J. Plant Physiol. 2011;168:1345–1360. doi: 10.1016/j.jplph.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Barkan A. Expression of plastid genes: Organelle-specific elaborations on a prokaryotic scaffold. Plant Physiol. 2011;155:1520–1532. doi: 10.1104/pp.110.171231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Millar A.H., Heazlewood J.L., Kristensen B.K., Braun H.P., Moller I.M. The plant mitochondrial proteome. Trends Plant Sci. 2005;10:36–43. doi: 10.1016/j.tplants.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Millar A.H., Whelan J., Small I. Recent surprises in protein targeting to mitochondria and plastids. Curr. Opin. Plant Biol. 2006;9:610–615. doi: 10.1016/j.pbi.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Kleine T., Leister D. Retrograde signaling: Organelles go networking. Biochim. Biophy. Acta. 2016;1857:1313–1325. doi: 10.1016/j.bbabio.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Del Campo E.M. Post-transcriptional control of chloroplast gene expression. Gene Regul. Syst. Biol. 2009;3:31. doi: 10.4137/GRSB.S2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stern D.B., Goldschmidt-Clermont M., Hanson M.R. Chloroplast RNA metabolism. Annu. Rev. Plant Biol. 2010;61:125–155. doi: 10.1146/annurev-arplant-042809-112242. [DOI] [PubMed] [Google Scholar]
- 12.Hammani K., Giege P. RNA metabolism in plant mitochondria. Trends Plant Sci. 2014;19:380–389. doi: 10.1016/j.tplants.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Lorkovic Z.J. Role of plant RNA-binding proteins in development, stress response and genome organization. Trends Plant Sci. 2009;14:229–236. doi: 10.1016/j.tplants.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Quesada V. The roles of mitochondrial transcription termination factors (MTERFs) in plants. Physiol. Plant. 2016;157:389–399. doi: 10.1111/ppl.12416. [DOI] [PubMed] [Google Scholar]
- 15.Kang H., Park S.J., Kwak K.J. Plant RNA chaperones in stress response. Trends Plant Sci. 2013;18:100–106. doi: 10.1016/j.tplants.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Lee K., Kang H. Emerging roles of RNA-binding proteins in plant growth, development, and stress responses. Mol. Cells. 2016;39:179–185. doi: 10.14348/molcells.2016.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robles P., Quesada V. Transcriptional and post-transcriptional regulation of organellar gene expression (OGE) and its roles in plant salt tolerance. Int. J. Mol. Sci. 2019;20:1056. doi: 10.3390/ijms20051056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leister D., Wang L., Kleine T. Organellar gene expression and acclimation of plants to environmental stress. Front. Plant Sci. 2017;8:387. doi: 10.3389/fpls.2017.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nawaz G., Kang H. Chloroplast- or mitochondria-targeted DEAD-box RNA helicases play essential roles in organellar RNA metabolism and abiotic stress responses. Front. Plant Sci. 2017;8:871. doi: 10.3389/fpls.2017.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barkan A., Klipcan L., Ostersetzer O., Kawamura T., Asakura Y., Watkins K.P. The CRM domain: An RNA binding module derived from an ancient ribosome-associated protein. RNA. 2007;13:55–64. doi: 10.1261/rna.139607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han J.H., Lee K., Lee K.H., Jung S., Jeon Y., Pai H.S., Kang H. A nuclear-encoded chloroplast-targeted S1 RNA-binding domain protein affects chloroplast rRNA processing and is crucial for the normal growth of Arabidopsis thaliana. Plant J. 2015;83:277–289. doi: 10.1111/tpj.12889. [DOI] [PubMed] [Google Scholar]
- 22.Shi X., Bentolila S., Hanson M.R. Organelle RNA recognition motif-containing (ORRM) proteins are plastid and mitochondrial editing factors in Arabidopsis. Plant Signal. Behav. 2016;11:e1167299. doi: 10.1080/15592324.2016.1167299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barkan A., Small I. Pentatricopeptide repeat proteins in plants. Annu. Rev. Plant Biol. 2014;65:415–442. doi: 10.1146/annurev-arplant-050213-040159. [DOI] [PubMed] [Google Scholar]
- 24.Lee K., Lee H.J., Kim D.H., Jeon Y., Pai H.S., Kang H. A nuclear-encoded chloroplast protein harboring a single CRM domain plays an important role in the Arabidopsis growth and stress response. BMC Plant Biol. 2014;14:98. doi: 10.1186/1471-2229-14-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee K., Park S.J., Colas des Francs-Small C., Whitby M., Small I., Kang H. The coordinated action of PPR4 and EMB2654 on each intron half mediates trans-splicing of rps12 transcripts in plant chloroplasts. Plant J. 2019;100:1193–1207. doi: 10.1111/tpj.14509. [DOI] [PubMed] [Google Scholar]
- 26.Gu L., Xu T., Lee K., Lee K.H., Kang H. A chloroplast-localized DEAD-box RNA helicase AtRH3 is essential for intron splicing and plays an important role in the growth and stress response in Arabidopsis thaliana. Plant Physiol. Biochem. 2014;82:309–318. doi: 10.1016/j.plaphy.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Xu T., Lee K., Gu L., Kim J.I., Kang H. Functional characterization of a plastid-specific ribosomal protein PSRP2 in Arabidopsis thaliana under abiotic stress conditions. Plant Physiol. Biochem. 2013;73:405–411. doi: 10.1016/j.plaphy.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 28.Asakura Y., Barkan A. A CRM domain protein functions dually in group I and group II intron splicing in land plant chloroplasts. Plant Cell. 2007;19:3864–3875. doi: 10.1105/tpc.107.055160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobs J., Kuck U. Function of chloroplast RNA-binding proteins. Cell. Mol. Life Sci. 2011;68:735–748. doi: 10.1007/s00018-010-0523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ostheimer G.J., Barkan A., Matthews B.W. Crystal structure of E. coli YhbY: A representative of a novel class of RNA binding proteins. Structure. 2002;10:1593–1601. doi: 10.1016/S0969-2126(02)00886-9. [DOI] [PubMed] [Google Scholar]
- 31.Keren I., Klipcan L., Bezawork-Geleta A., Kolton M., Shaya F., Ostersetzer-Biran O. Characterization of the molecular basis of group II intron RNA recognition by CRS1-CRM domains. J. Biol. Chem. 2008;283:2333–2342. doi: 10.1074/jbc.M710488200. [DOI] [PubMed] [Google Scholar]
- 32.Small I.D., Peeters N. The PPR motif–a TPR-related motif prevalent in plant organellar proteins. Trends Biochem. Sci. 2000;25:45–47. doi: 10.1016/S0968-0004(99)01520-0. [DOI] [PubMed] [Google Scholar]
- 33.Schmitz-Linneweber C., Small I. Pentatricopeptide repeat proteins: A socket set for organelle gene expression. Trends Plant Sci. 2008;13:663–670. doi: 10.1016/j.tplants.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Cheng S., Gutmann B., Zhong X., Ye Y., Fisher M.F., Bai F., Castleden I., Song Y., Song B., Huang J., et al. Redefining the structural motifs that determine RNA binding and RNA editing by pentatricopeptide repeat proteins in land plants. Plant J. 2016;85:532–547. doi: 10.1111/tpj.13121. [DOI] [PubMed] [Google Scholar]
- 35.Small I.D., Schallenberg-Rudinger M., Takenaka M., Mireau H., Ostersetzer-Biran O. Plant organellar RNA editing: What 30 years of research has revealed. Plant J. 2020;101:1040–1056. doi: 10.1111/tpj.14578. [DOI] [PubMed] [Google Scholar]
- 36.Gorbalenya A.E., Koonin E.V. Helicases: Amino acid sequence comparisons and structure-function relationships. Curr. Opin. Struct. Biol. 1993;3:419–429. doi: 10.1016/S0959-440X(05)80116-2. [DOI] [Google Scholar]
- 37.Bird L.E., Subramanya H.S., Wigley D.B. Helicases: A unifying structural theme? Curr. Opin. Struct. Biol. 1998;8:14–18. doi: 10.1016/S0959-440X(98)80004-3. [DOI] [PubMed] [Google Scholar]
- 38.Singleton M.R., Dillingham M.S., Wigley D.B. Structure and mechanism of helicases and nucleic acid translocases. Annu. Rev. Biochem. 2007;76:23–50. doi: 10.1146/annurev.biochem.76.052305.115300. [DOI] [PubMed] [Google Scholar]
- 39.Cordin O., Banroques J., Tanner N.K., Linder P. The DEAD-box protein family of RNA helicases. Gene. 2006;367:17–37. doi: 10.1016/j.gene.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 40.De la Cruz J., Kressler D., Linder P. Unwinding RNA in Saccharomyces cerevisiae: DEAD-box proteins and related families. Trends Biochem. Sci. 1999;24:192–198. doi: 10.1016/S0968-0004(99)01376-6. [DOI] [PubMed] [Google Scholar]
- 41.Rocak S., Linder P. Dead-box proteins: The driving forces behind RNA metabolism. Nat. Rev. Mol. Cell Biol. 2004;5:232–241. doi: 10.1038/nrm1335. [DOI] [PubMed] [Google Scholar]
- 42.Linder P., Jankowsky E. From unwinding to clamping—the DEAD box RNA helicase family. Nat. Rev. Mol. Cell Biol. 2011;12:505–516. doi: 10.1038/nrm3154. [DOI] [PubMed] [Google Scholar]
- 43.Tanner N.K., Linder P. DExD/H box RNA helicases: From generic motors to specific dissociation functions. Mol. Cell. 2001;8:251–262. doi: 10.1016/S1097-2765(01)00329-X. [DOI] [PubMed] [Google Scholar]
- 44.Jarmoskaite I., Russell R. RNA helicase proteins as chaperones and remodelers. Annu. Rev. Biochem. 2014;83:697–725. doi: 10.1146/annurev-biochem-060713-035546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Subramanian A.R. Structure and functions of ribosomal protein S1. Prog. Nucleic Acid Res. Mol. Biol. 1983;28:101–142. doi: 10.1016/s0079-6603(08)60085-9. [DOI] [PubMed] [Google Scholar]
- 46.Bycroft M., Hubbard T.J., Proctor M., Freund S.M., Murzin A.G. The solution structure of the S1 RNA binding domain: A member of an ancient nucleic acid–binding fold. Cell. 1997;88:235–242. doi: 10.1016/S0092-8674(00)81844-9. [DOI] [PubMed] [Google Scholar]
- 47.Deryusheva E.I., Machulin A.V., Matyunin M.A., Galzitskaya O.V. Investigation of the Relationship between the S1 Domain and Its Molecular Functions Derived from Studies of the Tertiary Structure. Molecules. 2019;24:3681. doi: 10.3390/molecules24203681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Draper D.E., Pratt C.W., Von Hippel P.H. Escherichia coli ribosomal protein S1 has two polynucleotide binding sites. Proc. Natl. Acad. Sci. USA. 1977;74:4786–4790. doi: 10.1073/pnas.74.11.4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacques N., Dreyfus M. Translation initiation in Escherichia coli: Old and new questions. Mol. Microbiol. 1990;4:1063–1067. doi: 10.1111/j.1365-2958.1990.tb00679.x. [DOI] [PubMed] [Google Scholar]
- 50.Aliprandi P., Sizun C., Perez J., Mareuil F., Caputo S., Leroy J.-L., Odaert B., Laalami S., Uzan M., Bontems F. S1 ribosomal protein functions in translation initiation and ribonuclease RegB activation are mediated by similar RNA-protein interactions: An NMR and SAXS analysis. J. Biol. Chem. 2008;283:13289–13301. doi: 10.1074/jbc.M707111200. [DOI] [PubMed] [Google Scholar]
- 51.Young C.L., Karbstein K. The roles of S1 RNA-binding domains in Rrp5’s interactions with pre-rRNA. RNA. 2011;17:512–521. doi: 10.1261/rna.2458811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Regnier P., Grunberg-Manago M., Portier C. Nucleotide sequence of the pnp gene of Escherichia coli encoding polynucleotide phosphorylase. Homology of the primary structure of the protein with the RNA-binding domain of ribosomal protein S1. J. Biol. Chem. 1987;262:63–68. [PubMed] [Google Scholar]
- 53.Yehudai-Resheff S., Portnoy V., Yogev S., Adir N., Schuster G. Domain analysis of the chloroplast polynucleotide phosphorylase reveals discrete functions in RNA degradation, polyadenylation, and sequence homology with exosome proteins. Plant Cell. 2003;15:2003–2019. doi: 10.1105/tpc.013326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gribskov M. Translational initiation factors IF-1 and eIF-2α share an RNA-binding motif with prokaryotic ribosomal protein S1 and polynucleotide phosphorylase. Gene. 1992;119:107–111. doi: 10.1016/0378-1119(92)90073-X. [DOI] [PubMed] [Google Scholar]
- 55.Company M., Arenas J., Abelson J. Requirement of the RNA helicase-like protein PRP22 for release of messenger RNA from spliceosomes. Nature. 1991;349:487–493. doi: 10.1038/349487a0. [DOI] [PubMed] [Google Scholar]
- 56.Asakura Y., Barkan A. Arabidopsis orthologs of maize chloroplast splicing factors promote splicing of orthologous and species-specific group II introns. Plant Physiol. 2006;142:1656–1663. doi: 10.1104/pp.106.088096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asakura Y., Bayraktar O.A., Barkan A. Two CRM protein subfamilies cooperate in the splicing of group IIB introns in chloroplasts. RNA. 2008;14:2319–2332. doi: 10.1261/rna.1223708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Q., Shen L., Wang Z., Hu G., Ren D., Hu J., Zhu L., Gao Z., Zhang G., Guo L., et al. OsCAF1, a CRM domain containing protein, influences chloroplast development. Int. J. Mol. Sci. 2019;20:4386. doi: 10.3390/ijms20184386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Q., Shen L., Ren D., Hu J., Zhu L., Gao Z., Zhang G., Guo L., Zeng D., Qian Q. Characterization of the CRM gene family and elucidating the function of OsCFM2 in rice. Biomolecules. 2020;10:327. doi: 10.3390/biom10020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zmudjak M., Colas des Francs-Small C., Keren I., Shaya F., Belausov E., Small I., Ostersetzer-Biran O. mCSF1, a nucleus-encoded CRM protein required for the processing of many mitochondrial introns, is involved in the biogenesis of respiratory complexes I and IV in Arabidopsis. New Phytol. 2013;199:379–394. doi: 10.1111/nph.12282. [DOI] [PubMed] [Google Scholar]
- 61.Lee K., Park S.J., Park Y.I., Kang H. CFM9, a mitochondrial CRM protein, is crucial for mitochondrial intron splicing, mitochondria function and Arabidopsis growth and stress responses. Plant Cell Physiol. 2019;60:2538–2548. doi: 10.1093/pcp/pcz147. [DOI] [PubMed] [Google Scholar]
- 62.Schmitz-Linneweber C., Williams-Carrier R.E., Williams-Voelker P.M., Kroeger T.S., Vichas A., Barkan A. A pentatricopeptide repeat protein facilitates the trans-splicing of the maize chloroplast rps12 pre-mRNA. Plant Cell. 2006;18:2650–2663. doi: 10.1105/tpc.106.046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khrouchtchova A., Monde R.A., Barkan A. A short PPR protein required for the splicing of specific group II introns in angiosperm chloroplasts. RNA. 2012;18:1197–1209. doi: 10.1261/rna.032623.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Longevialle A.F., Hendrickson L., Taylor N.L., Delannoy E., Lurin C., Badger M., Millar A.H., Small I. The pentatricopeptide repeat gene OTP51 with two LAGLIDADG motifs is required for the cis-splicing of plastid ycf3 intron 2 in Arabidopsis thaliana. Plant J. 2008;56:157–168. doi: 10.1111/j.1365-313X.2008.03581.x. [DOI] [PubMed] [Google Scholar]
- 65.Chateigner-Boutin A.L., des Francs-Small C.C., Delannoy E., Kahlau S., Tanz S.K., de Longevialle A.F., Fujii S., Small I. OTP70 is a pentatricopeptide repeat protein of the E subgroup involved in splicing of the plastid transcript rpoC1. Plant J. 2011;65:532–542. doi: 10.1111/j.1365-313X.2010.04441.x. [DOI] [PubMed] [Google Scholar]
- 66.Aryamanesh N., Ruwe H., Sanglard L.V., Eshraghi L., Bussell J.D., Howell K.A., Small I., des Francs-Small C.C. The pentatricopeptide repeat protein EMB2654 is essential for trans-splicing of a chloroplast small ribosomal subunit transcript. Plant Physiol. 2017;173:1164–1176. doi: 10.1104/pp.16.01840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang X., Yang Z., Zhang Y., Zhou W., Zhang A., Lu C. Pentatricopeptide repeat protein PHOTOSYSTEM I BIOGENESIS FACTOR2 is required for splicing of ycf3. J. Integr. Plant Biol. 2020 doi: 10.1111/jipb.12936. (in press) [DOI] [PubMed] [Google Scholar]
- 68.Huang W., Zhu Y., Wu W., Li X., Zhang D., Yin P., Huang J. The pentatricopeptide repeat protein SOT5/EMB2279 is required for plastid rpl2 and trnK intron splicing. Plant Physiol. 2018;177:684–697. doi: 10.1104/pp.18.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meierhoff K., Felder S., Nakamura T., Bechtold N., Schuster G. HCF152, an Arabidopsis RNA binding pentatricopeptide repeat protein involved in the processing of chloroplast psbB-psbT-psbH-petB-petD RNAs. Plant Cell. 2003;15:1480–1495. doi: 10.1105/tpc.010397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johnson X., Wostrikoff K., Finazzi G., Kuras R., Schwarz C., Bujaldon S., Nickelsen J., Stern D.B., Wollman F.A., Vallon O. MRL1, a conserved pentatricopeptide repeat protein, is required for stabilization of rbcL mRNA in chlamydomonas and Arabidopsis. Plant Cell. 2010;22:234–248. doi: 10.1105/tpc.109.066266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cai W.H., Okuda K., Peng L.W., Shikanai T. PROTON GRADIENT REGULATION 3 recognizes multiple targets with limited similarity and mediates translation and RNA stabilization in plastids. Plant J. 2011;67:318–327. doi: 10.1111/j.1365-313X.2011.04593.x. [DOI] [PubMed] [Google Scholar]
- 72.Zhang L., Zhou W., Che L., Rochaix J.D., Lu C., Li W., Peng L. PPR protein BFA2 is essential for the accumulation of the atpH/F transcript in chloroplasts. Front. Plant Sci. 2019;10:446. doi: 10.3389/fpls.2019.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beick S., Schmitz-Linneweber C., Williams-Carrier R., Jensen B., Barkan A. The pentatricopeptide repeat protein PPR5 stabilizes a specific tRNA precursor in maize chloroplasts. Mol. Cell Biol. 2008;28:5337–5347. doi: 10.1128/MCB.00563-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pfalz J., Bayraktar O.A., Prikryl J., Barkan A. Site-specific binding of a PPR protein defines and stabilizes 5’ and 3’ mRNA termini in chloroplasts. EMBO J. 2009;28:2042–2052. doi: 10.1038/emboj.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu Y.Q., Li C., Wang H., Chen H., Berg H., Xia Y.J. AtPPR2, an Arabidopsis pentatricopeptide repeat protein, binds to plastid 23S rRNA and plays an important role in the first mitotic division during gametogenesis and in cell proliferation during embryogenesis. Plant J. 2011;67:13–25. doi: 10.1111/j.1365-313X.2011.04569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu W., Liu S., Ruwe H., Zhang D., Melonek J., Zhu Y., Hu X., Gusewski S., Yin P., Small I.D., et al. SOT1, a pentatricopeptide repeat protein with a small MutS-related domain, is required for correct processing of plastid 23S-4.5S rRNA precursors in Arabidopsis thaliana. Plant J. 2016;85:607–621. doi: 10.1111/tpj.13126. [DOI] [PubMed] [Google Scholar]
- 77.Lee K., Park S.J., Han J.H., Jeon Y., Pai H.S., Kang H. A chloroplast-targeted pentatricopeptide repeat protein PPR287 is crucial for chloroplast function and Arabidopsis development. BMC Plant Biol. 2019;19:244. doi: 10.1186/s12870-019-1857-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Longevialle A.F., Meyer E.H., Andres C., Taylor N.L., Lurin C., Millar A.H., Small I.D. The pentatricopeptide repeat gene OTP43 is required for trans-splicing of the mitochondrial nad1 intron 1 in Arabidopsis thaliana. Plant Cell. 2007;19:3256–3265. doi: 10.1105/tpc.107.054841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Koprivova A., des Francs-Small C.C., Calder G., Mugford S.T., Tanz S., Lee B.R., Zechmann B., Small I., Kopriva S. Identification of a pentatricopeptide repeat protein implicated in splicing of intron 1 of mitochondrial nad7 transcripts. J. Biol. Chem. 2010;285:32192–32199. doi: 10.1074/jbc.M110.147603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Des Francs-Small C.C., de Longevialle A.F., Li Y., Lowe E., Tanz S.K., Smith C., Bevan M.W., Small I. The pentatricopeptide repeat proteins TANG2 and ORGANELLE TRANSCRIPT PROCESSING439 are involved in the splicing of the multipartite nad5 transcript encoding a subunit of mitochondrial complex I. Plant Physiol. 2014;165:1409–1416. doi: 10.1104/pp.114.244616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hsieh W.Y., Liao J.C., Chang C.Y., Harrison T., Boucher C., Hsieh M.H. The SLOW GROWTH3 pentatricopeptide repeat protein is required for the splicing of mitochondrial NADH dehydrogenase subunit7 intron 2 in Arabidopsis. Plant Physiol. 2015;168:490–501. doi: 10.1104/pp.15.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang C., Aube F., Quadrado M., Dargel-Graffin C., Mireau H. Three new pentatricopeptide repeat proteins facilitate the splicing of mitochondrial transcripts and complex I biogenesis in Arabidopsis. J. Exp. Bot. 2018;69:5131–5140. doi: 10.1093/jxb/ery275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marchetti F., Cainzos M., Shevtsov S., Cordoba J.P., Sultan L.D., Brennicke A., Takenaka M., Pagnussat G., Ostersetzer-Biran O., Zabaleta E. Mitochondrial pentatricopeptide repeat protein, EMB2794, plays a pivotal role in NADH dehydrogenase subunit nad2 mRNA maturation in Arabidopsis thaliana. Plant Cell Physiol. 2020 doi: 10.1093/pcp/pcaa028. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haili N., Arnal N., Quadrado M., Amiar S., Tcherkez G., Dahan J., Briozzo P., Colas des Francs-Small C., Vrielynck N., Mireau H. The pentatricopeptide repeat MTSF1 protein stabilizes the nad4 mRNA in Arabidopsis mitochondria. Nucleic Acids Res. 2013;41:6650–6663. doi: 10.1093/nar/gkt337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee K., Han J.H., Park Y.I., Colas des Francs-Small C., Small I., Kang H. The mitochondrial pentatricopeptide repeat protein PPR19 is involved in the stabilization of NADH dehydrogenase 1 transcripts and is crucial for mitochondrial function and Arabidopsis thaliana development. New Phytol. 2017;215:202–216. doi: 10.1111/nph.14528. [DOI] [PubMed] [Google Scholar]
- 86.Mingam A., Toffano-Nioche C., Brunaud V., Boudet N., Kreis M., Lecharny A. DEAD-box RNA helicases in Arabidopsis thaliana: Establishing a link between quantitative expression, gene structure and evolution of a family of genes. Plant Biotechnol. J. 2004;2:401–415. doi: 10.1111/j.1467-7652.2004.00084.x. [DOI] [PubMed] [Google Scholar]
- 87.Umate P., Tuteja R., Tuteja N. Genome-wide analysis of helicase gene family from rice and Arabidopsis: A comparison with yeast and human. Plant Mol. Biol. 2010;73:449–465. doi: 10.1007/s11103-010-9632-5. [DOI] [PubMed] [Google Scholar]
- 88.Asakura Y., Galarneau E., Watkins K.P., Barkan A., van Wijk K.J. Chloroplast RH3 DEAD box RNA helicases in maize and Arabidopsis function in splicing of specific group II introns and affect chloroplast ribosome biogenesis. Plant Physiol. 2012;159:961–974. doi: 10.1104/pp.112.197525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Paieri F., Tadini L., Manavski N., Kleine T., Ferrari R., Morandini P., Pesaresi P., Meurer J., Leister D. The DEAD-box RNA helicase RH50 is a 23S-4.5S rRNA maturation factor that functionally overlaps with the plastid signaling factor GUN1. Plant Physiol. 2018;176:634–648. doi: 10.1104/pp.17.01545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nawaz G., Kang H. Rice OsRH58, a chloroplast DEAD-box RNA helicase, improves salt or drought stress tolerance in Arabidopsis by affecting chloroplast translation. BMC Plant Biol. 2019;19:17. doi: 10.1186/s12870-018-1623-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xiaomei W., Rongrong K., Ting Z., Yuanyuan G., Jianlong X., Zhongze P., Gangseob L., Dongzhi L., Yanjun D. A DEAD-box RNA helicase TCD33 that confers chloroplast development in rice at seedling stage under cold stress. J. Plant Physiol. 2020;248:153138. doi: 10.1016/j.jplph.2020.153138. [DOI] [PubMed] [Google Scholar]
- 92.Bobik K., Fernandez J.C., Hardin S.R., Ernest B., Ganusova E.E., Staton M.E., Burch-Smith T.M. The essential chloroplast ribosomal protein uL15c interacts with the chloroplast RNA helicase ISE2 and affects intercellular trafficking through plasmodesmata. New Phytol. 2019;221:850–865. doi: 10.1111/nph.15427. [DOI] [PubMed] [Google Scholar]
- 93.Kohler D., Schmidt-Gattung S., Binder S. The DEAD-box protein PMH2 is required for efficient group II intron splicing in mitochondria of Arabidopsis thaliana. Plant Mol. Biol. 2010;72:459–467. doi: 10.1007/s11103-009-9584-9. [DOI] [PubMed] [Google Scholar]
- 94.Chi W., He B., Mao J., Li Q., Ma J., Ji D., Zou M., Zhang L. The function of RH22, a DEAD RNA helicase, in the biogenesis of the 50S ribosomal subunits of Arabidopsis chloroplasts. Plant Physiol. 2012;158:693–707. doi: 10.1104/pp.111.186775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nishimura K., Ashida H., Ogawa T., Yokota A. A DEAD box protein is required for formation of a hidden break in Arabidopsis chloroplast 23S rRNA. Plant J. 2010;63:766–777. doi: 10.1111/j.1365-313X.2010.04276.x. [DOI] [PubMed] [Google Scholar]
- 96.Yerramsetty P., Stata M., Siford R., Sage T.L., Sage R.F., Wong G.K., Albert V.A., Berry J.O. Evolution of RLSB, a nuclear-encoded S1 domain RNA binding protein associated with post-transcriptional regulation of plastid-encoded rbcL mRNA in vascular plants. BMC Evol. Biol. 2016;16:141. doi: 10.1186/s12862-016-0713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jeon Y., Jung H.J., Kang H., Park Y.I., Lee S.H., Pai H.S. S1 domain-containing STF modulates plastid transcription and chloroplast biogenesis in Nicotiana benthamiana. New Phytol. 2012;193:349–363. doi: 10.1111/j.1469-8137.2011.03941.x. [DOI] [PubMed] [Google Scholar]
- 98.Nouri M.Z., Moumeni A., Komatsu S. Abiotic stresses: Insight into gene regulation and protein expression in photosynthetic pathways of plants. Int. J. Mol. Sci. 2015;16:20392–20416. doi: 10.3390/ijms160920392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kumar A.A., Mishra P., Kumari K., Panigrahi K.C. Environmental stress influencing plant development and flowering. Front. Biosci. 2012;4:1315–1324. doi: 10.2741/s333. [DOI] [PubMed] [Google Scholar]
- 100.Wang Y., Berkowitz O., Selinski J., Xu Y., Hartmann A., Whelan J. Stress responsive mitochondrial proteins in Arabidopsis thaliana. Free Radic. Biol. Med. 2018;122:28–39. doi: 10.1016/j.freeradbiomed.2018.03.031. [DOI] [PubMed] [Google Scholar]
- 101.Zhu J.K. Abiotic Stress Signaling and Responses in Plants. Cell. 2016;167:313–324. doi: 10.1016/j.cell.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Biswal B., Joshi P., Raval M., Biswal U. Photosynthesis, a global sensor of environmental stress in green plants: Stress signalling and adaptation. Curr. Sci. 2011:47–56. [Google Scholar]
- 103.Cottage A., Mott E.K., Kempster J.A., Gray J.C. The Arabidopsis plastid-signalling mutant gun1 (genomes uncoupled1) shows altered sensitivity to sucrose and abscisic acid and alterations in early seedling development. J. Exp. Bot. 2010;61:3773–3786. doi: 10.1093/jxb/erq186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tan J., Tan Z., Wu F., Sheng P., Heng Y., Wang X., Ren Y., Wang J., Guo X., Zhang X. A novel chloroplast-localized pentatricopeptide repeat protein involved in splicing affects chloroplast development and abiotic stress response in rice. Mol. Plant. 2014;7:1329–1349. doi: 10.1093/mp/ssu054. [DOI] [PubMed] [Google Scholar]
- 105.Gong X.D., Su Q.Q., Lin D.Z., Jiang Q., Xu J.L., Zhang J.H., Teng S., Dong Y.J. The rice OsV4 encoding a novel pentatricopeptide repeat protein is required for chloroplast development during the early leaf stage under cold stress. J. Integr. Plant Biol. 2014;56:400–410. doi: 10.1111/jipb.12138. [DOI] [PubMed] [Google Scholar]
- 106.Wu L., Wu J., Liu Y., Gong X., Xu J., Lin D., Dong Y. The rice pentatricopeptide repeat gene TCD10 is needed for chloroplast development under cold stress. Rice. 2016;9:67. doi: 10.1186/s12284-016-0134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu X., Lan J., Huang Y., Cao P., Zhou C., Ren Y., He N., Liu S., Tian Y., Nguyen T., et al. WSL5, a pentatricopeptide repeat protein, is essential for chloroplast biogenesis in rice under cold stress. J. Exp. Bot. 2018;69:3949–3961. doi: 10.1093/jxb/ery214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zsigmond L., Rigo G., Szarka A., Szekely G., Otvos K., Darula Z., Medzihradszky K.F., Koncz C., Koncz Z., Szabados L. Arabidopsis PPR40 connects abiotic stress responses to mitochondrial electron transport. Plant Physiol. 2008;146:1721–1737. doi: 10.1104/pp.107.111260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zsigmond L., Szepesi A., Tari I., Rigo G., Kiraly A., Szabados L. Overexpression of the mitochondrial PPR40 gene improves salt tolerance in Arabidopsis. Plant Sci. 2012;182:87–93. doi: 10.1016/j.plantsci.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 110.Laluk K., AbuQamar S., Mengiste T. The Arabidopsis mitochondria-localized pentatricopeptide repeat protein PGN functions in defense against necrotrophic fungi and abiotic stress tolerance. Plant Physiol. 2011;156:2053–2068. doi: 10.1104/pp.111.177501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu Y., He J., Chen Z., Ren X., Hong X., Gong Z. ABA overly-sensitive 5 (ABO5), encoding a pentatricopeptide repeat protein required for cis-splicing of mitochondrial nad2 intron 3, is involved in the abscisic acid response in Arabidopsis. Plant J. 2010;63:749–765. doi: 10.1111/j.1365-313X.2010.04280.x. [DOI] [PubMed] [Google Scholar]
- 112.Yang L., Zhang J., He J., Qin Y., Hua D., Duan Y., Chen Z., Gong Z. ABA-mediated ROS in mitochondria regulate root meristem activity by controlling PLETHORA expression in Arabidopsis. PLoS Genet. 2014;10:e1004791. doi: 10.1371/journal.pgen.1004791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu J.M., Zhao J.Y., Lu P.P., Chen M., Guo C.H., Xu Z.S., Ma Y.Z. The E-subgroup pentatricopeptide repeat protein family in Arabidopsis thaliana and confirmation of the responsiveness PPR96 to abiotic stresses. Front. Plant Sci. 2016;7:1825. doi: 10.3389/fpls.2016.01825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nawaz G., Lee K., Park S.J., Kim Y.O., Kang H. A chloroplast-targeted cabbage DEAD-box RNA helicase BrRH22 confers abiotic stress tolerance to transgenic Arabidopsis plants by affecting translation of chloroplast transcripts. Plant Physiol. Biochem. 2018;127:336–342. doi: 10.1016/j.plaphy.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 115.Gu L., Jung H.J., Kim B.M., Xu T., Lee K., Kim Y.O., Kang H. A chloroplast-localized S1 domain-containing protein SRRP1 plays a role in Arabidopsis seedling growth in the presence of ABA. J. Plant Physiol. 2015;189:34–41. doi: 10.1016/j.jplph.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 116.Zhang J., Yuan H., Yang Y., Fish T., Lyi S.M., Thannhauser T.W., Zhang L., Li L. Plastid ribosomal protein S5 is involved in photosynthesis, plant development, and cold stress tolerance in Arabidopsis. J. Exp. Bot. 2016;67:2731–2744. doi: 10.1093/jxb/erw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dinh S.N., Park S.J., Han J.H., Kang H. A chloroplast-targeted S1 RNA-binding domain protein plays a role in Arabidopsis response to diverse abiotic stresses. J. Plant Biol. 2019;62:74–81. doi: 10.1007/s12374-018-0325-y. [DOI] [Google Scholar]
- 118.Bigot S., Buges J., Gilly L., Jacques C., Le Boulch P., Berger M., Delcros P., Domergue J.B., Koehl A., Ley-Ngardigal B., et al. Pivotal roles of environmental sensing and signaling mechanisms in plant responses to climate change. Global Change Biol. 2018;24:5573–5589. doi: 10.1111/gcb.14433. [DOI] [PubMed] [Google Scholar]
- 119.Dourmap C., Roque S., Morin A., Caubriere D., Kerdiles M., Beguin K., Perdoux R., Reynoud N., Bourdet L., Audebert P.A., et al. Stress signalling dynamics of the mitochondrial electron transport chain and oxidative phosphorylation system in higher plants. Ann. Bot. 2020;125:721–736. doi: 10.1093/aob/mcz184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kawakatsu T., Huang S.C., Jupe F., Sasaki E., Schmitz R.J., Urich M.A., Castanon R., Nery J.R., Barragan C., He Y., et al. Epigenomic Diversity in a Global Collection of Arabidopsis thaliana Accessions. Cell. 2016;166:492–505. doi: 10.1016/j.cell.2016.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chang Y.N., Zhu C., Jiang J., Zhang H., Zhu J.K., Duan C.G. Epigenetic regulation in plant abiotic stress responses. J. Integr. Plant Biol. 2020;62:563–580. doi: 10.1111/jipb.12901. [DOI] [PubMed] [Google Scholar]
- 122.Furtauer L., Kustner L., Weckwerth W., Heyer A.G., Nagele T. Resolving subcellular plant metabolism. Plant J. 2019;100:438–455. doi: 10.1111/tpj.14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kustner L., Furtauer L., Weckwerth W., Nagele T., Heyer A.G. Subcellular dynamics of proteins and metabolites under abiotic stress reveal deferred response of the Arabidopsis thaliana hexokinase-1 mutant gin2-1 to high light. Plant J. 2019;100:456–472. doi: 10.1111/tpj.14491. [DOI] [PubMed] [Google Scholar]
- 124.Rao R.S., Salvato F., Thal B., Eubel H., Thelen J.J., Moller I.M. The proteome of higher plant mitochondria. Mitochondrion. 2017;33:22–37. doi: 10.1016/j.mito.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 125.Kleffmann T., Russenberger D., von Zychlinski A., Christopher W., Sjolander K., Gruissem W., Baginsky S. The Arabidopsis thaliana chloroplast proteome reveals pathway abundance and novel protein functions. Curr. Biol. 2004;14:354–362. doi: 10.1016/j.cub.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 126.Herschlag D. RNA chaperones and the RNA folding problem. J. Biol. Chem. 1995;270:20871–20874. doi: 10.1074/jbc.270.36.20871. [DOI] [PubMed] [Google Scholar]
- 127.Woodson S.A. Taming free energy landscapes with RNA chaperones. RNA Biol. 2010;7:677–686. doi: 10.4161/rna.7.6.13615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rajkowitsch L., Chen D., Stampfl S., Semrad K., Waldsich C., Mayer O., Jantsch M.F., Konrat R., Bläsi U., Schroeder R. RNA chaperones, RNA annealers and RNA helicases. RNA Biol. 2007;4:118–130. doi: 10.4161/rna.4.3.5445. [DOI] [PubMed] [Google Scholar]
- 129.Ivanyi-Nagy R., Davidovic L., Khandjian E., Darlix J.-L. Disordered RNA chaperone proteins: From functions to disease. Cell. Mol. Life Sci. 2005;62:1409–1417. doi: 10.1007/s00018-005-5100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ivanyi-Nagy R., Lavergne J.-P., Gabus C., Ficheux D., Darlix J.-L. RNA chaperoning and intrinsic disorder in the core proteins of Flaviviridae. Nucleic Acids Res. 2008;36:712–725. doi: 10.1093/nar/gkm1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chambers J.R., Bender K.S. The RNA chaperone Hfq is important for growth and stress tolerance in Francisella novicida. PLoS ONE. 2011;6:e19797. doi: 10.1371/journal.pone.0019797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chaulk S.G., Smith−Frieday M.N., Arthur D.C., Culham D.E., Edwards R.A., Soo P., Frost L.S., Keates R.A., Glover J.M., Wood J.M. ProQ is an RNA chaperone that controls ProP levels in Escherichia coli. Biochemistry. 2011;50:3095–3106. doi: 10.1021/bi101683a. [DOI] [PubMed] [Google Scholar]
- 133.Mohr S., Stryker J.M., Lambowitz A.M. A DEAD-box protein functions as an ATP-dependent RNA chaperone in group I intron splicing. Cell. 2002;109:769–779. doi: 10.1016/S0092-8674(02)00771-7. [DOI] [PubMed] [Google Scholar]
- 134.Mohr S., Matsuura M., Perlman P.S., Lambowitz A.M. A DEAD-box protein alone promotes group II intron splicing and reverse splicing by acting as an RNA chaperone. Proc. Natl. Acad. Sci. USA. 2006;103:3569–3574. doi: 10.1073/pnas.0600332103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huang H.-R., Rowe C.E., Mohr S., Jiang Y., Lambowitz A.M., Perlman P.S. The splicing of yeast mitochondrial group I and group II introns requires a DEAD-box protein with RNA chaperone function. Proc. Natl. Acad. Sci. USA. 2005;102:163–168. doi: 10.1073/pnas.0407896101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kim W.Y., Jung H.J., Kwak K.J., Kim M.K., Oh S.H., Han Y.S., Kang H. The Arabidopsis U12-type spliceosomal protein U11/U12-31K is involved in U12 intron splicing via RNA chaperone activity and affects plant development. Plant Cell. 2010;22:3951–3962. doi: 10.1105/tpc.110.079103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kwak K.J., Jung H.J., Lee K.H., Kim Y.S., Kim W.Y., Ahn S.J., Kang H. The minor spliceosomal protein U11/U12-31K is an RNA chaperone crucial for U12 intron splicing and the development of dicot and monocot plants. PLoS ONE. 2012;7:e43707. doi: 10.1371/journal.pone.0043707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Castiglioni P., Warner D., Bensen R.J., Anstrom D.C., Harrison J., Stoecker M., Abad M., Kumar G., Salvador S., D’Ordine R., et al. Bacterial RNA chaperones confer abiotic stress tolerance in plants and improved grain yield in maize under water-limited conditions. Plant Physiol. 2008;147:446–455. doi: 10.1104/pp.108.118828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chaikam V., Karlson D. Functional characterization of two cold shock domain proteins from Oryza sativa. Plant Cell Environ. 2008;31:995–1006. doi: 10.1111/j.1365-3040.2008.01811.x. [DOI] [PubMed] [Google Scholar]
- 140.Karlson D., Imai R. Conservation of the cold shock domain protein family in plants. Plant Physiol. 2003;131:12–15. doi: 10.1104/pp.014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Karlson D., Nakaminami K., Toyomasu T., Imai R. A cold-regulated nucleic acid-binding protein of winter wheat shares a domain with bacterial cold shock proteins. J. Biol. Chem. 2002;277:35248–35256. doi: 10.1074/jbc.M205774200. [DOI] [PubMed] [Google Scholar]
- 142.Kim Y.O., Kang H. The role of a zinc finger-containing glycine-rich RNA-binding protein during the cold adaptation process in Arabidopsis thaliana. Plant Cell Physiol. 2006;47:793–798. doi: 10.1093/pcp/pcj047. [DOI] [PubMed] [Google Scholar]
- 143.Kim Y.O., Kim J.S., Kang H. Cold-inducible zinc finger-containing glycine-rich RNA-binding protein contributes to the enhancement of freezing tolerance in Arabidopsis thaliana. Plant J. 2005;42:890–900. doi: 10.1111/j.1365-313X.2005.02420.x. [DOI] [PubMed] [Google Scholar]
- 144.Kim J.S., Jung H.J., Lee H.J., Kim K., Goh C.H., Woo Y., Oh S.H., Han Y.S., Kang H. Glycine-rich RNA-binding protein7 affects abiotic stress responses by regulating stomata opening and closing in Arabidopsis thaliana. Plant J. 2008;55:455–466. doi: 10.1111/j.1365-313X.2008.03518.x. [DOI] [PubMed] [Google Scholar]
- 145.Kim M.-H., Sasaki K., Imai R. Cold shock domain protein 3 regulates freezing tolerance in Arabidopsis thaliana. J. Biol. Chem. 2009;284:23454–23460. doi: 10.1074/jbc.M109.025791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kim J.Y., Kim W.Y., Kwak K.J., Oh S.H., Han Y.S., Kang H. Glycine-rich RNA-binding proteins are functionally conserved in Arabidopsis thaliana and Oryza sativa during cold adaptation process. J. Exp. Bot. 2010;61:2317–2325. doi: 10.1093/jxb/erq058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kim J.Y., Kim W.Y., Kwak K.J., Oh S.H., Han Y.S., Kang H. Zinc finger-containing glycine-rich RNA-binding protein in Oryza sativa has an RNA chaperone activity under cold stress conditions. Plant Cell Environ. 2010;33:759–768. doi: 10.1111/j.1365-3040.2009.02101.x. [DOI] [PubMed] [Google Scholar]
- 148.Hu J., Manduzio S., Kang H. Epitranscriptomic RNA methylation in plant development and abiotic stress responses. Front. Plant Sci. 2019;10:500. doi: 10.3389/fpls.2019.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yue H., Nie X., Yan Z., Weining S. N6-methyladenosine regulatory machinery in plants: Composition, function and evolution. Plant Biotech. J. 2019;17:1194–1208. doi: 10.1111/pbi.13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Arribas-Hernández L., Brodersen P. Occurrence and functions of m6A and other covalent modifications in plant mRNA. Plant Physiol. 2020;182:79–96. doi: 10.1104/pp.19.01156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shen L., Liang Z., Wong C.E., Yu H. Messenger RNA modifications in plants. Trends Plant Sci. 2019;24:328–341. doi: 10.1016/j.tplants.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 152.Wang Z., Tang K., Zhang D., Wan Y., Wen Y., Lu Q., Wang L. High-throughput m6A-seq reveals RNA m6A methylation patterns in the chloroplast and mitochondria transcriptomes of Arabidopsis thaliana. PLoS ONE. 2017;12:e0185612. doi: 10.1371/journal.pone.0185612. [DOI] [PMC free article] [PubMed] [Google Scholar]