Abstract
Oncolytic virotherapy is a promising antitumor therapeutic strategy. It is based on the ability of viruses to selectively kill cancer cells and induce host antitumor immune responses. However, the clinical outcomes of oncolytic viruses (OVs) vary widely. Therefore, we performed a meta-analysis to illustrate the efficacy and safety of oncolytic viruses. The Cochrane Library, PubMed, and EMBASE databases were searched for randomized controlled trials (RCTs) published up to 31 January 2020. The data for objective response rate (ORR), overall survival (OS), progression-free survival (PFS), and adverse events (AEs) were independently extracted by two investigators from 11 studies that met the inclusion criteria. In subgroup analyses, the objective response rate benefit was observed in patients treated with oncolytic DNA viruses (odds ratio (OR) = 4.05; 95% confidence interval (CI): 1.96–8.33; p = 0.0002), but not in those treated with oncolytic RNA viruses (OR = 1.00, 95% CI: 0.66–1.52, p = 0.99). Moreover, the intratumoral injection arm yielded a statistically significant improvement (OR = 4.05, 95% CI: 1.96–8.33, p = 0.0002), but no such improvement was observed for the intravenous injection arm (OR = 1.00, 95% CI: 0.66–1.52, p = 0.99). Among the five OVs investigated in RCTs, only talimogene laherparepvec (T-VEC) effectively prolonged the OS of patients (hazard ratio (HR), 0.79; 95% CI: 0.63–0.99; p = 0.04). None of the oncolytic virotherapies improved the PFS (HR = 1.00, 95% CI: 0.85–1.19, p = 0.96). Notably, the pooled rate of severe AEs (grade ≥3) was higher for the oncolytic virotherapy group (39%) compared with the control group (27%) (risk difference (RD), 12%; risk ratio (RR), 1.44; 95% CI: 1.17–1.78; p = 0.0006). This review offers a reference for fundamental research and clinical treatment of oncolytic viruses. Further randomized controlled trials are needed to verify these results.
Keywords: oncolytic viruses, oncolytic virotherapy, efficacy, adverse events, systematic review, meta-analysis
1. Introduction
Cancer is a common disease globally that seriously affects human health. The USA, for instance, projects to have 1,806,590 and 606,520 new cancer cases and cancer deaths, respectively, in 2020 [1]. Although traditional treatment methods such as radiotherapy, chemotherapy, and targeted drugs are preferred in cancer treatment, their disadvantages include severe adverse events, development of drug resistance, and cross-resistance [2,3]. Therefore, the development of more effective cancer treatment strategies is urgently needed. Oncolytic viruses (OVs) are natural or artificially modified viruses that selectively replicate in and destroy cancer cells; hence, they represent a promising approach for antitumor therapy [4,5]. Oncolytic viruses generally exert antitumor effects by two mechanisms, namely, the selective killing of tumor cells, and induction of antitumor immunity [6]. To achieve specificity for tumor cells, key proteins required by OVs to infect the host are first modified to reduce infection of normal tissues [7,8,9]. Besides, oncolytic viruses utilize signaling pathways such as p53, epidermal growth factor receptor (EGFR)/Ras, and protein kinase R (PKR) to target tumor cells for selective expansion [10,11,12,13]. OVs can also kill tumor cells by triggering the expression of the suicide gene [14,15]. The key steps employed by OVs to transform “cold tumors” into “hot tumors” and activate antitumor immune responses include targeted replication, the release of tumor-associated antigens through oncolysis, upregulation of chemokines and danger signals, recruitment of dendritic cells and lymphoid cells, and upregulation of immune checkpoint molecules [16,17,18].
Oncolytic viruses are either RNA or DNA viruses. RNA viruses such as reoviruses, paramyxoviruses, and picornaviruses, which encode only a few genes, often undergo rapid proliferation and lysis of tumor cells [5,18,19,20]. On the other hand, oncolytic DNA viruses such as herpes viruses, adenovirus, or poxviruses allow for the insertion of multiple foreign genes but are slower in replication and amplification [5,21,22]. The structure, gene components, expression strategies, and antineoplastic mechanisms are therefore different between the two types [23]. Talimogene laherparepvec (T-VEC), which is an oncolytic herpes virus type I, is presently the only oncolytic virus approved by the Food and Drug Administration. The success of T-VEC in the treatment of melanoma has further promoted the research of oncolytic viruses. With the increased number of clinical studies on oncolytic viruses, the efficacy and safety of oncolytic viruses have drawn much attention. Clinical trials of oncolytic viruses in combination with chemotherapeutic drugs, radiotherapy, and immune checkpoint inhibitors have shown massive progress in cancer treatment [5,16,24]. In particular, the combination of oncolytic virus and immune checkpoint inhibitors has yielded good results in melanoma [25]. Although many oncolytic viruses exist, a real champion among the oncolytic viruses has not yet emerged. In addition, no systematic review has been conducted on the efficacy and safety of oncolytic viruses in randomized controlled trials.
In this meta-analysis, we included the following viruses: T-VEC (herpes virus) [26,27], pelareorep (reovirus) [28,29,30,31,32,33], NTX-010 (seneca valley virus; picornavirus) [19], Ad5-yCD/mutTKSR39rep-ADP (adenovirus) [34], and pexastimogene devacirepvec (Pexa-Vec; poxvirus) [35]. We first evaluated the efficacy of oncolytic virus from objective response rate (ORR), overall survival (OS), and progression-free survival (PFS); then we analyzed severe adverse events (grade ≥3) and detailed adverse events (AEs). Overall, we conducted this meta-analysis to investigate the effectiveness and safety of oncolytic viruses in randomized controlled trials to provide insights for fundamental research and clinical treatment.
2. Methods
2.1. Literature Search Strategy
A systematic search was conducted in EMBASE, PubMed, and Cochrane databases for studies published up to 30/1/2020. The search terms included: “oncolytic viruses”, or “viruses, oncolytic”, or “oncolytic virus”, or “virus, oncolytic”, or “oncolytic virotherapy”, or “oncolytic virotherapies”, or “virotherapies, oncolytic”, or “virotherapy, oncolytic”, or “oncolytic virus therapy”, or “oncolytic virus therapies”, or “therapies, oncolytic virus”, or “therapy, oncolytic virus”, or “virus therapies, oncolytic”, or “virus therapy, oncolytic”. There was a language restriction of English in the search, and we followed the PRISMA guidelines for randomized controlled trials (RCTs) to conduct the meta-analysis [36].
2.2. Inclusion and Exclusion Criteria
We included studies in the meta-analysis if they met the following inclusion criteria: (1) the studies were randomized controlled trials in cancer patients treated with an oncolytic virus; (2) the articles had at least one of the following outcomes: objective response rate (ORR), overall survival (OS), progression-free survival (PFS), or adverse events (AEs); (3) cancer patients in the control group received the control regimen without oncolytic virus. However, articles were excluded if: (1) they were conference abstracts, case reports, letters, meta-analyses, cohort studies, single-arm studies, reviews, animal studies, or in vitro studies; (2) patients in the control group received oncolytic virotherapy; (3) they included literatures with overlapping patients. Two independent investigators screened the potentially eligible articles by reading the titles and abstracts. Thereafter, the full text of all remaining studies was read to determine if they met the set eligibility criteria. Disagreements on study selection were resolved by discussion with other investigators.
2.3. Data Extraction
Two investigators independently read full texts of the included literatures and extracted the data. Any divergence of opinions concerning the extracted data was resolved through consultation. The extracted data included first author, publication, year, country, treatment, injection mode of OVs, types of cancer, the total number of patients, and clinical endpoints. The primary endpoints were ORR, OS, and PFS, while secondary endpoints included adverse events, which were evaluated using the National Cancer Institute—Common Terminology Criteria for Adverse Events (version 3.0 or 4.0). In addition, we carefully read supplementary materials of the included literatures to prevent any loss of information.
2.4. Quality Assessment
Quality assessment was done by two independent investigators using the Cochrane risk of bias tool. The risk of bias parameters included the random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias. Each entry was determined as high-risk, low-risk, or unclear. If an item could not be assessed due to lack of information, it was considered as having an unclear risk of bias. Disagreements on quality assessment were resolved by consensus.
2.5. Statistical Analysis
Statistical analyses were performed using Review Manager (RevMan) 5.3 software and STATA 12.0. Results were presented as hazard ratios (HRs), risk ratios (RRs), or odds ratios (ORs) with 95% CI (confidence interval). Heterogeneity among RCTs was assessed by the Chi-square test and index of heterogeneity (I2). A mixed-effects model was used when heterogeneity was not significant (I2 < 50% or p-value > 0.1); otherwise, the random-effects model was performed. Publication bias was evaluated statistically via funnel plots, Begg’s test, and Egger’s test. Statistical significance was set at p < 0.05.
3. Results
3.1. Systematic Review Process and Quality Assessment
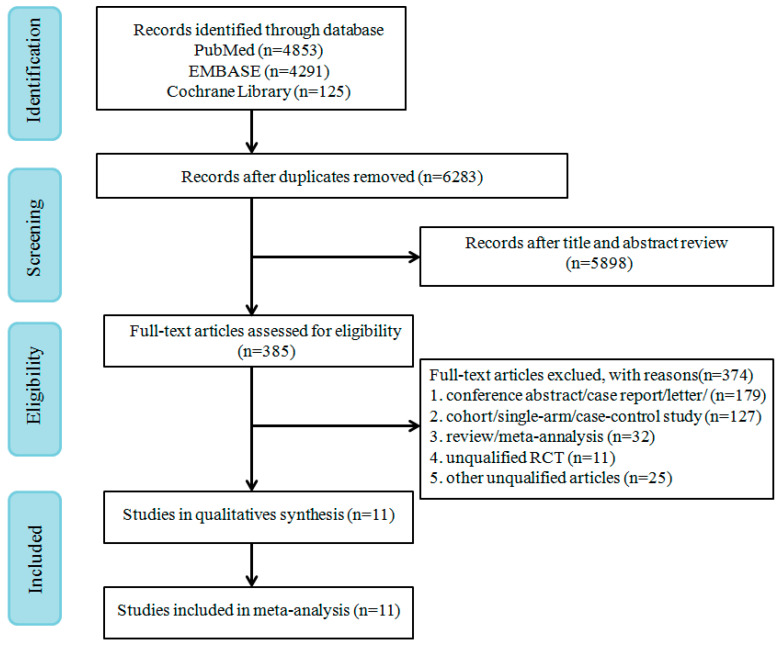
A total of 9269 records were retrieved from PubMed, EMBASE, and Cochrane Library. A flow chart of study screenings and the election process is shown in Figure 1. From the remaining 6283 references screened after removing duplicates, 385 potentially eligible references were identified. Eventually, 11 RCTs that met the inclusion criteria were selected for full-text review.
Figure 1.
PRISMA flow diagram of randomized controlled trials (RCTs) of patients treated with oncolytic virus.
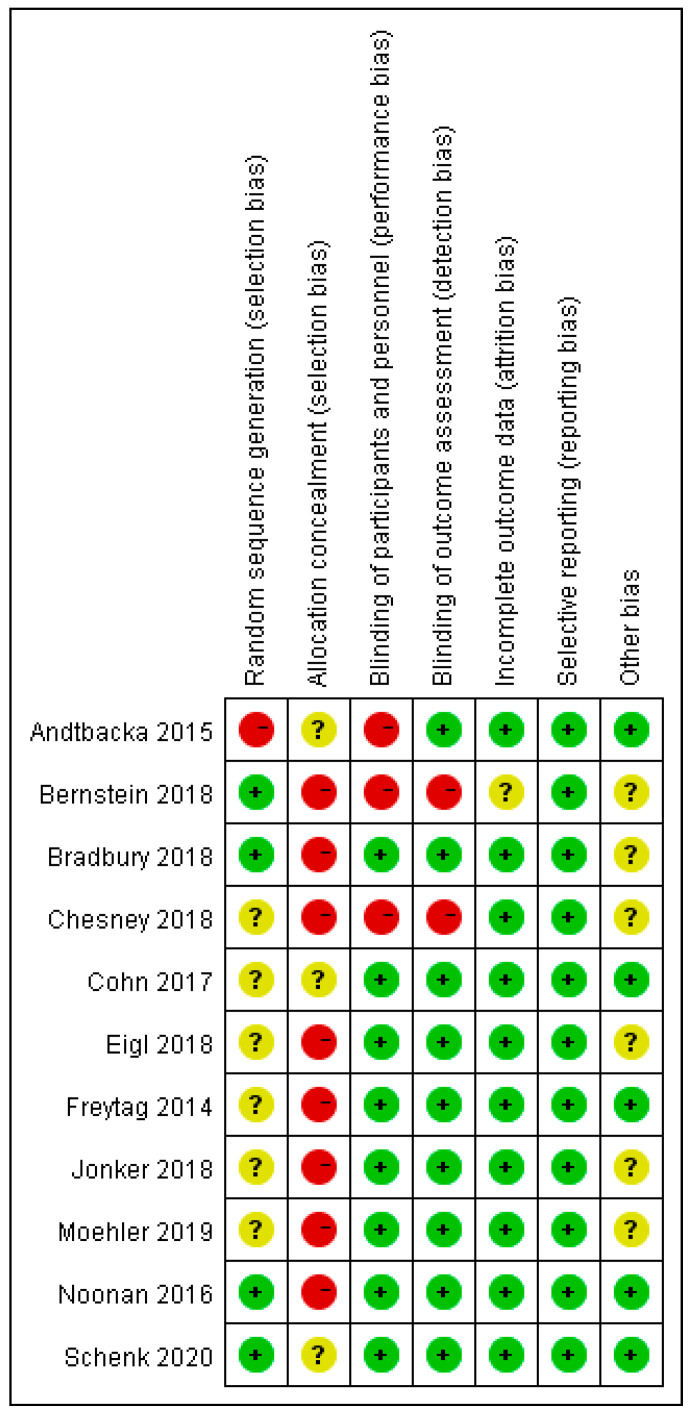
The risk of bias for the 11 included RCTs is shown in Figure 2. All the included RCTs were open-label trials. Most RCTs mentioned random allocation performed without using the random sequence generation method. Blinding was not performed because of the moral risk associated with the sham injection. In some RCTs [19,29,30,31,32,33,34,35], non-blinding had no significant effect on the efficacy or safety of oncolytic viruses; hence, they were judged as a low-risk factor.
Figure 2.
Assessment of risk of bias for 11 included randomized controlled trials.
3.2. Characteristics of Studies
We included eleven studies with a total of 1452 patients in this meta-analysis. The characteristics and outcomes of RCTs are presented in Table 1 and Table 2. The OVs used in the included trials were T-VEC (n = 2), pelareorep (n = 6), NTX-010 (n = 1), Ad5-yCD/mutTKSR39rep-ADP (n = 1), and Pexa-Vec (n = 1). The types of tumors included melanoma, breast cancer, lung cancer, prostate cancer, hepatocellular carcinoma, colorectal cancer, pancreatic adenocarcinoma, and ovarian, tubal, or peritoneal cancer. The injection methods were either intratumoral or intravenous. Eleven included clinical trials of oncolytic viruses were conducted in the United States and Canada.
Table 1.
Characteristics of the RCTs included in this meta-analysis.
| First Author (Year) | Tumor Type | Treatment Arm | Injection Mode | Age (Years) | Male, No. (%) |
|---|---|---|---|---|---|
| Andtbacka 2015 [26] | Melanoma | T-VEC vs. GM-CSF | IT | EG: median 63 (22–94) CG: median 64 (26–91) |
EG: 173/295 (59%) CG: 77/141 (55%) |
| Bernstein 2018 [28] | Breast cancer | Pelareorep + paclitaxel vs. paclitaxel | IV | EG: median 61 (44–78) CG: median 57 (36–73) |
EG: 0/36 (0%) CG: 0/38 (0%) |
| Bradbury 2018 [29] | Non-small cell lung cancer | Pelareorep + chemotherapy vs. chemotherapy | IV | EG-1: median 63 (43–78) EG-2: 64 (23–77) CG-1: median 65 (39–80) CG-2: 64 (41–84) |
EG: 36/77 (47%) CG: 41/75 (55%) |
| Chesney 2018 [27] | Melanoma | T-VEC + ipilimumab vs. ipilimumab | IT | EG: median 65 (23–93) CG: median 64 (23–90) |
EG: 62/98 (63%) CG: 55/100 (55%) |
| Cohn 2017 [30] | Ovarian, tubal, or peritoneal cancer | Pelareorep + paclitaxel vs. paclitaxel | IV | NR | EG: 0/54 (0%) CG: 0/54 (0%) |
| Eigl 2018 [31] | Prostate cancer | Pelareorep + docetaxel vs. docetaxel | IV | EG: median 69.1 (50.3–83.7) CG: median 68.6 (49.7–86.6) |
EG: 21/21 (100%) CG: 23/23 (100%) |
| Freytag 2014 [34] | Prostate cancer | Ad5-yCD/mutTKSR39rep-ADP + IMRT vs. IMRT | IT | EG: mean 68.0 (55–78) CG: mean 65.2 (51–79) |
EG: 41/41 (100%) CG: 44/44 (100%) |
| Jonker 2018 [32] | Colorectal cancer | Pelareorep + FOLFOX6/bevacizumab vs. FOLFOX6/bevacizumab | IV | EG: median 60 (34–79) CG: median 59 (31–78) |
EG: 19/51 (37%) CG: 21/52 (40%) |
| Moehler 2019 [35] | Hepatocellular carcinoma | Pexa-Vec + BSC vs. BSC | IV | EG: mean 60 ± 11 CG: mean 55 ± 12 |
EG: 72/86 (84%) CG: 33/43 (77%) |
| Noonan 2016 [33] | Pancreatic adenocarcinoma | Pelareorep + paclitaxel/carboplatin vs. paclitaxel/carboplatin | IV | EG: median 61.5 (39–84) CG: median 66 (45–81) |
EG: 22/36 (61.1%) CG: 19/37 (51.4%) |
| Schenk 2020 [19] | Small cell lung cancer | NTX-010 vs. placebo | IV | EG: median 67 (44–81) CG: median 60 (50–82) |
EG: 14/26 (53.9%) CG:10/24 (41.7%) |
EG, experimental group; CG, control group; NR, not reported; BSC, best supportive care; IMRT, intensity modulated radiation therapy; IT, intratumoral; IV, intravenous.
Table 2.
Summary of outcomes in the selected RCTs.
| First Author (Year) | Median OS (Months) | HR (95% CI) for OS | Median PFS (Months) |
HR (95% CI) for PFS | ORR | Severe Adverse Event |
|---|---|---|---|---|---|---|
| Andtbacka 2015 [26] | EG: 23.3 CG: 18.9 |
0.79 (0.62, 1.00) |
NR | NR | EG: 78 CG: 8 |
EG:105 CG: 27 |
| Bernstein 2018 [28] | EG: 17.4 CG: 10.4 |
0.61 (0.33, 1.12) |
EG: 3.78 CG: 3.38 |
1.11 (0.64, 1.92) |
EG: 9 CG: 9 |
EG: 18 CG: 18 |
| Bradbury 2018 [29] | EG: 7.8 CG: 7.4 |
0.98 (0.72, 1.34) |
EG: 3.0 CG: 2.8 |
0.90 (0.65, 1.25) |
EG: 11 CG: 11 |
NR |
| Chesney 2018 [27] | NR | 0.80 (0.44, 1.46) |
EG: 8.2 CG: 6.4 |
0.83 (0.56, 1.23) |
EG: 38 CG: 18 |
EG: 43 CG: 33 |
| Cohn 2017 [30] | EG: 12.6 CG: 13.1 |
1.01 (0.64, 1.58) |
EG: 4.4 CG: 4.3 |
1.11 (0.64, 1.91) |
EG: 8 CG: 9 |
NR |
| Eigl 2018 [31] | NR | 1.86 (0.97, 3.57) |
NR | NR | EG: 11 CG: 18 |
NR |
| Freytag 2014 [34] | No death | NR | No death | NR | NR | EG: 1 CG: 1 |
| Jonker 2018 [32] | EG: 19.2 CG: 20.1 |
1.18 (0.75, 1.87) |
EG: 7.33 CG: 9.13 |
1.65 (1.02, 2.67) |
EG: 27 CG: 18 |
NR |
| Moehler 2019 [35] | EG: 4.2 CG: 4.4 |
1.19 (0.77, 1.83) |
EG: 4.94 CG: 5.2 |
NR | EG: 0 CG: 0 |
EG: 45 CG: 7 |
| Noonan 2016 [33] | EG: 7.31 CG: 8.77 |
1.12 (0.66, 1.91) |
EG: 1.7 CG: 1.7 |
0.86 (0.52, 1.43) |
EG: 7 CG: 7 |
NR |
| Schenk 2020 [19] | EG: 6.6 CG: 13.2 |
1.49 (0.77, 2.87) |
NR | 1.03 (0.58, 1.83) |
EG: 1 CG: 4 |
EG: 9 CG: 5 |
EG, experimental group; CG, control group; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; NR, not reported; CI, confidence interval.
Oncolytic DNA viruses include T-VEC, Pexa-Vec, and Ad5-yCD/mutTKSR39rep-ADP, and they all carry transgenes. T-VEC is modified by deleting the ICP47 gene and ICP34.5 gene (the herpes virus neurovirulence factor) to reduce viral pathogenicity and enhance selective tumor replication [37,38]. In addition, T-VEC could elicit human granulocyte macrophage colony-stimulating factor (GM-CSF) to recruit and activate antigen-presenting cells with subsequent induction of tumor-specific T-cell responses [13]. Pexa-Vec (JX-594) is a thymidine kinase gene-inactivated vaccinia virus engineered by expressing the transgenes, including GM-CSF and β-galactosidase; it selectively targets tumor cells with activation of the Ras/MAPK signaling pathway [35,39]. Ad5-yCD/mutTKSR39rep-ADP is adenovirus carrying two cytotoxic gene systems, cytosine deaminase (cytosine deaminase (CD)/5-fluorocytosine (5-FC) and herpes simplex virus thymidine kinase (HSV-1 TK)/valganciclovir (vGCV), and it can enhance the sensitivity of tumor cells to specific drugs and radiation [34].
Oncolytic RNA viruses include pelareorep and NTX-010. Pelareorep is a human reovirus type 3 Dearing strain, which contains live, replication-competent reovirus, and has specific oncolysis with an activated Ras pathway [31,33]. Direct oncolysis of pelareorep led to release of “danger signals”, such as soluble tumor-associated antigens, viral pathogen-associated molecular patterns, and cell-derived damage-associated molecular patterns [16,40]. Therefore, direct oncolysis could result in generating innate and adaptive immune response to the tumor microenvironment and induces the antitumor immune response. Besides, NTX-010 (seneca valley virus) was a novel oncolytic picornavirus, which could target and lyse tumor cells [19,41].
3.3. Effectiveness
3.3.1. Objective Response Rate
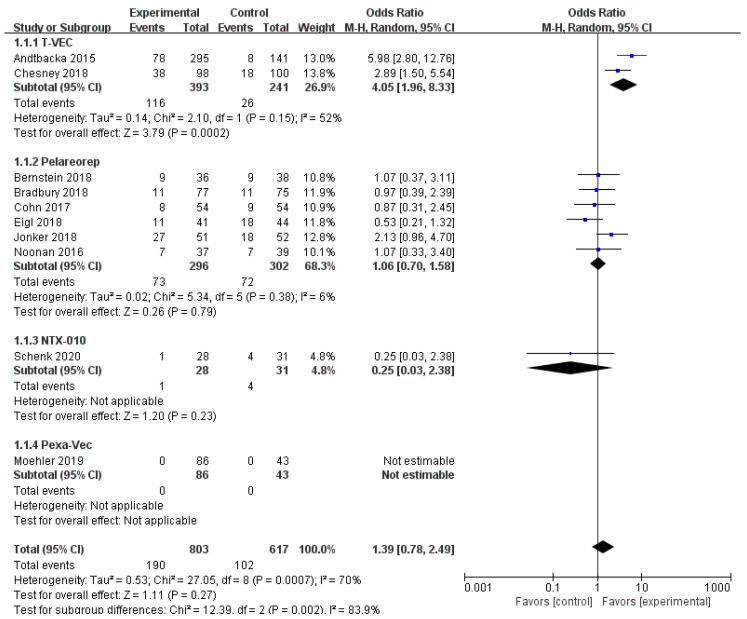
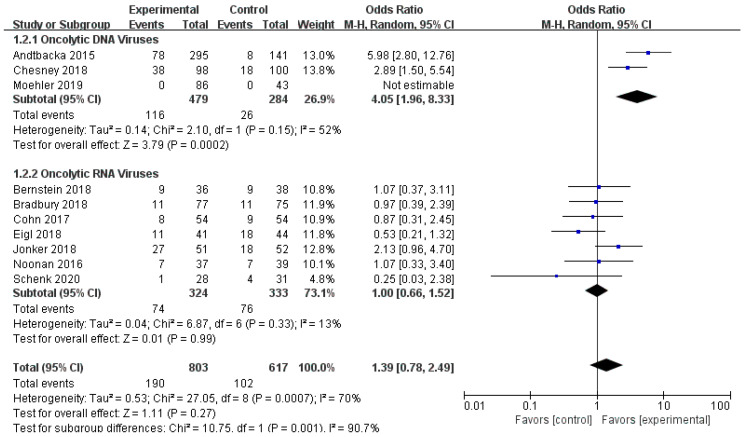
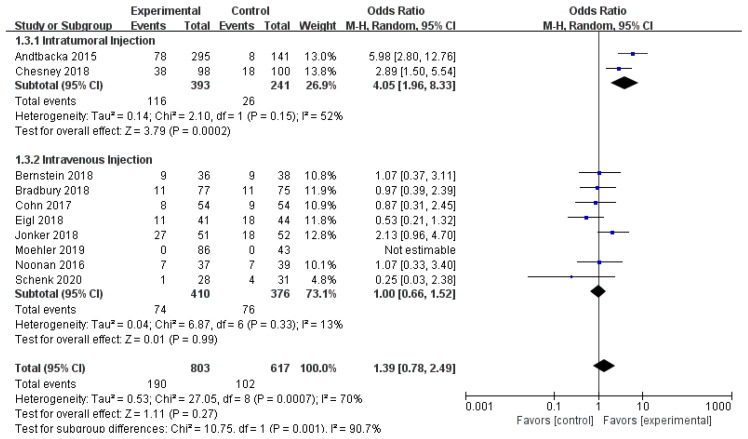
Ten RCTs reported objective response rate (ORR). Since differences were observed in efficacy among various OVs; we performed subgroup analysis on the ORR based on species, oncolytic DNA/RNA viruses, and injection mode. There was a statistically significant difference in ORRs between patients that received T-VEC (n = 2, OR = 4.05, 95% CI: 1.96–8.33, I2 = 52%, p = 0.0002). However, there was no significant difference in ORRs between patients treated with pelareorep (n = 6, OR = 1.06, 95% CI: 0.70–1.58, I2 = 6%, p = 0.79), NTX-010 (n = 1, OR = 0.25, 95% CI: 0.03–2.38, p = 0.23), and Pexa-Vec (n = 1, not estimable) (Figure 3). Objective response rate benefit was observed in patients that received oncolytic DNA viruses (n = 3, OR = 4.05, 95% CI: 1.96–8.33, I2 = 52%, p = 0.0002) but not in those treated with oncolytic RNA viruses (n = 7, OR = 1.00, 95% CI: 0.66–1.52, I2 = 13%, p = 0.99) (Figure 4). In the subgroup analysis for injection methods, results showed that the intratumoral injection arm produced significant improvement (n = 2, OR = 4.05, 95% CI: 1.96–8.33, I2 = 52%, p = 0.0002), but no significant improvement was found for the intravenous injection arm (n = 7, OR = 1.00, 95% CI: 0.66–1.52, I2 = 13%, p = 0.99) (Figure 5).
Figure 3.
Forest plot of the pooled odds ratios (ORs) for objective response rate (ORR) in different oncolytic virus species.
Figure 4.
Forest plot of the pooled odds ratios (ORs) for objective response rate (ORR) of oncolytic DNA viruses and oncolytic RNA viruses.
Figure 5.
Forest plot of the pooled odds ratios (ORs) for objective response rate (ORR) of intratumoral and intravenous injections.
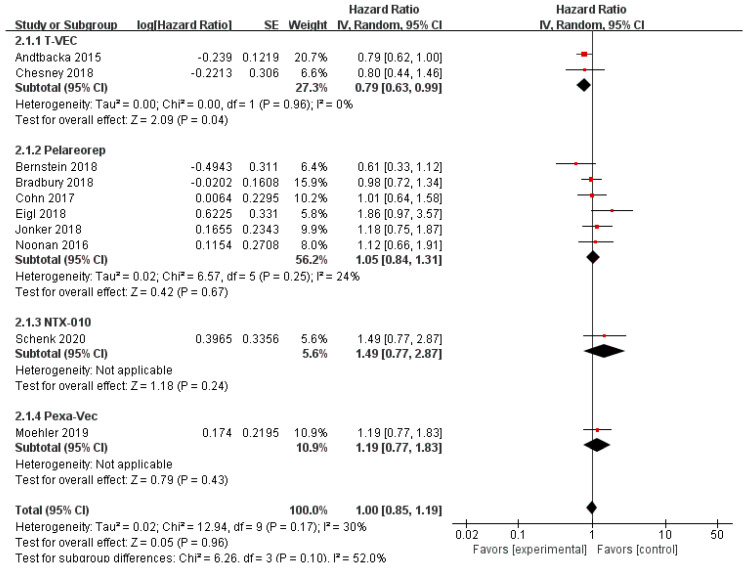
3.3.2. Overall Survival and Progression-Free Survival
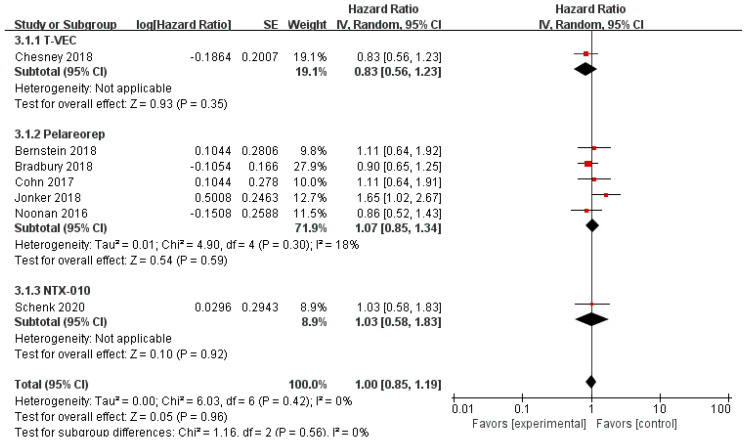
Data regarding overall survival (OS) were available in ten RCTs, seven of which provided data for progression-free survival (PFS). Compared with the control group, patients treated with T-VEC had better OS (n = 2, HR = 0.79, 95% CI: 0.63–0.99, p = 0.04). However, treatment with pelareorep (n = 6, HR = 1.05, 95% CI: 0.84–1.31, p = 0.67), Pexa-Vec (n = 1, HR = 1.19, 95% CI: 0.77–1.83, p = 0.43), and NTX-010 (n = 1, HR = 1.49, 95% CI: 0.77–2.87, p = 0.24) did not improve the OS significantly compared to the control group (Figure 6). In addition, none of the patients benefited from T-VEC (n = 1, HR = 0.83, 95% CI: 0.56–1.23, p = 0.35), pelareorep (n = 5, HR = 1.07, 95% CI: 0.85–1.34, p = 0.59), and NTX-010 treatment (n = 1, HR = 1.03, 95% CI: 0.58–1.83, p = 0.92) in terms of PFS (Figure 7).
Figure 6.
Forest plot of the pooled hazard ratios (HR) for overall survival (OS).
Figure 7.
Forest plot of the pooled hazard ratios (HR) for progression-free survival (PFS).
3.3.3. Safety
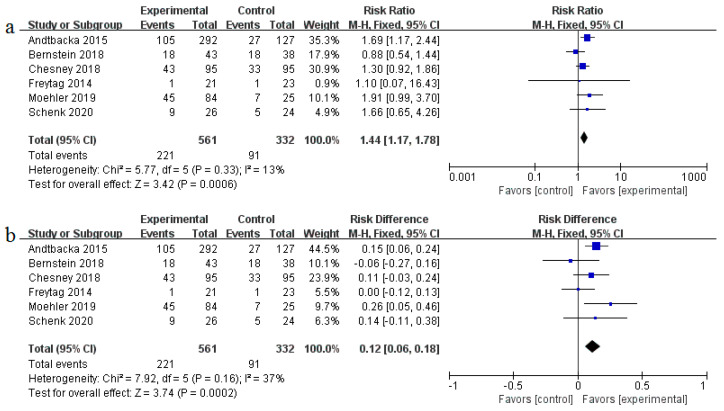
Safety of oncolytic viruses remains a concern and most trials evaluate the safety aspect. The pooled risk ratio (RR) of severe adverse events (grade ≥3) was 1.44 (95% CI: 1.17–1.78, p = 0.0006, I2 = 13%) as shown in Figure 8a. The incidence of severe adverse events (AEs) in the oncolytic virus treatment group was higher than the control group (39% vs. 27%), with a pooled risk difference (RD) of severe AEs recorded at 0.12 (95% CI: 0.06–0.18, p = 0.0002, I2 = 37%) (Figure 8b); RD represents the rate of severe AEs attributed to oncolytic virotherapy. Furthermore, we analyzed detailed adverse events that may be associated with oncolytic virus treatment (Table 3). Patients treated with OVs had a higher risk for all-grade AEs such as fever (RR = 3.87, 95% CI: 2.15–6.69, p < 0.00001), neutropenia (RR = 1.66, 95% CI:1.21–2.29, p = 0.002), diarrhea (RR = 1.56, 95% CI:1.26–1.95, p < 0.0001), nausea (RR = 1.49, 95% CI: 1.28–1.74, p < 0.00001), vomiting (RR = 1.65, 95% CI: 1.27–2.14, p = 0.0002), chills (RR = 7.04, 95% CI: 4.64–10.66, p < 0.00001), flu-like symptoms (RR = 4.13, 95% CI:2.15–7.94, p < 0.0001), arthralgia (RR = 1.51, 95% CI: 1.09–2.12, p = 0.01), myalgia (RR = 1.97, 95% CI: 1.32–2.96, p = 0.001), extreme pain (RR = 1.50, 95% CI: 1.06–2.11, p = 0.02), headache (RR = 1.90, 95% CI: 1.42–2.53, p < 0.0001), and thrombocytopenia (RR = 2.74, 95% CI: 1.65–4.57, p = 0.0001). However, only neutropenia treatment yielded statistically significant severe adverse events (RR = 1.36, 95% CI: 1.03–1.80, p = 0.03).
Figure 8.
Forest plot of severe adverse events (grade ≥3): (a) the pooled risk ratios (RR); (b) the pooled risk difference (RD).
Table 3.
Adverse events of interest.
| Adverse Event | All Grades | Grade ≥3 | ||||||
|---|---|---|---|---|---|---|---|---|
| I 2 | RR (95% CI) | p | Incidence of EG | I 2 | RR (95% CI) | p | Incidence of EG | |
| Fever | 73% | 3.87 (2.15, 6.69) |
<0.00001 * | 48.90% | 0% | 3.07 (0.62, 15.10) |
0.17 | 1.825% |
| Neutropenia | 67% | 1.66 (1.21, 2.29) |
0.002 * | 63.01% | 50% | 1.36 (1.03, 1.80) |
0.03 * | 40.36% |
| Febrile neutropenia | 66% | 1.76 (0.66,4.69) |
0.25 | 25.18% | 3% | 1.19 (0.77, 1.84) |
0.44 | 15.52% |
| Leukopenia | 36% | 1.21 (0.96, 1.51) |
0.11 | 71.23% | 90% | 1.84 (0.23, 14.36) |
0.56 | 26.61% |
| Diarrhea | 17% | 1.56 (1.26, 1.95) |
<0.0001 * | 28.78% | 13% | 1.12 (0.56, 2.22) |
0.75 | 2.178% |
| Nausea | 35% | 1.49 (1.28, 1.74) |
<0.00001 * | 45.24% | 0% | 1.05 (0.48, 2.29) |
0.89 | 1.754% |
| Vomiting | 36% | 1.65 (1.27, 2.14) |
0.0002 * | 27.84% | 5% | 0.68 (0.30, 1.52) |
0.35 | 1.983% |
| Chills | 32% | 7.04 (4.64, 10.66) |
<0.00001 * | 45.84% | NA | 0.92 (0.04, 21.85) |
0.96 | 0.1825% |
| Fatigue | 85% | 1.22 (0.95, 1.57) |
0.12 | 55.35% | 0% | 1.24 (0.83, 1.85) |
0.29 | 6.836% |
| Flu-like symptoms | 60% | 4.13 (2.15, 7.94) |
<0.0001 * | 31.29% | 0% | 4.41 (0.82, 23.81) |
0.08 | 1.23% |
| Decreased appetite/anorexi-a | 25% | 1.23 (0.98, 1.56) |
0.08 | 25.91% | 51% | 0.55 (0.17, 1.76) |
0.32 | 0.6048% |
| Arthralgia | 13% | 1.51 (1.09, 2.12) |
0.01 * | 19.01% | 0% | 0.94 (0.19, 4.67) |
0.94 | 0.6073% |
| Myalgia | 47% | 1.97 (1.32, 2.96) |
0.001 * | 18.42% | NA | 1.31 (0.05, 31.96) |
0.87 | 0.2208% |
| Pain in extremity | 0% | 1.50 (1.06, 2.11) |
0.02 * | 20.98% | 0% | 1.57 (0.40, 6.21) |
0.52 | 1.897% |
| Headache | 0% | 1.90 (1.42, 2.53) |
<0.0001 * | 24.11% | 0% | 1.86 (0.47, 7.34) |
0.38 | 1.095% |
| Cough | 17% | 0.85 (0.67, 1.07) |
0.17 | 21.66% | NA | 0.32 (0.01, 7.85) |
0.49 | 0 |
| Cellulitis | NA | 3.70 (0.87, 15.76) |
0.08 | 5.822% | NA | 2.64 (0.31, 22.18) |
0.37 | 2.055% |
| Thrombocytope-nia | 0% | 2.74 (1.65, 4.57) |
0.0001 * | 54.79% | 0% | 1.23 (0.58, 2.61) |
0.59 | 10.09% |
*, statistically significant value; 95% CI, 95% confidence interval; RR, risk ratio; NA, not available; I2, index of heterogeneity; EG, experimental group.
3.3.4. Publication Bias and Sensitivity Analysis
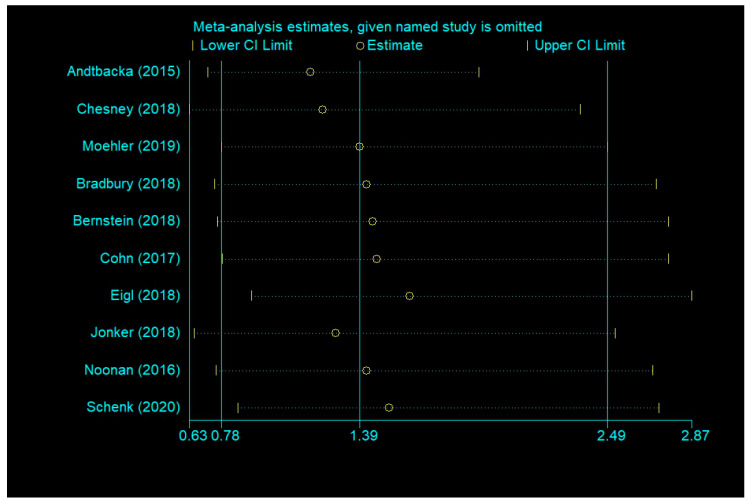
Publication bias was formally assessed using Begg’s test and Egger’s test. OS (Begg’s test, p = 0.283; Egger’s test, p = 0.126), PFS (Begg’s test, p = 0.548; Egger’s test, p = 0.307), and severe AEs (Begg’s test, p = 0.707; Egger’s test, p = 0.966) did not reveal any significant publication bias, but ORR (Begg’s test, p = 0.118; Egger’s test, p = 0.046 <0.1) had significant differences of publication bias. We made a sensitivity analysis by omitting a study to estimate meta-analysis of ORR. It suggested that omitting any one study had little effect on the overall result (each offset is minimal and between the upper CL limit and lower CL limits) (Figure 9). Therefore, the publication bias of ORR had limited impact on our conclusions.
Figure 9.
Sensitivity analysis of ORR.
4. Discussion
Oncolytic viruses possess the potential to kill cancerous cells (oncolysis); they also induce antitumor immune response through multiple mechanisms [42,43]. Such characteristics have made oncolytic virotherapy a promising immunotherapeutic approach for cancer patients. However, clinical trials have revealed that the presence of neutralizing antibodies in the blood prevents the oncolytic viruses (except reovirus) from replicating; activation of the immune system leads to rapid elimination of oncolytic viruses, and oncolytic viruses cannot target tumors due to physical parameters [5,44,45]. Furthermore, the best oncolytic virus, route of administration, prognosis of patients, and adverse reactions remain controversial.
In this study, we extracted data for objective response rate (ORR), overall survival (OS), and progression-free survival (PFS) for in-depth analysis of the effectiveness of oncolytic virotherapy. Generally, T-VEC (OR = 4.05, 95% CI: 1.96–8.33) showed remarkable clinical efficacy of ORR. Interestingly, the objective response rate benefit was observed in patients treated with oncolytic DNA viruses (OR = 4.05, 95% CI: 1.96–8.33) but not in those treated with oncolytic RNA viruses (OR = 1.00, 95% CI: 0.66–1.52). This may be because DNA viruses carry many external genes with important immunomodulatory effects. In addition, DNA viruses express high fidelity DNA polymerases, which maintain the integrity of the viral genome and sufficient amplification [16,43]. Increasing evidence suggests that the antitumor effect of oncolytic viruses is not only dependent on pure oncolysis but also virus-induced antitumor immunity [16,46,47]. The three mechanisms in which oncolytic virus breaks the immune tolerance include: (1) after the virus infects tumor cells, it induces antigen-presenting cells (APCs) to infiltrate the tumor infection site; (2) the tumor antigen released after the virus lyses tumor cells and enhances the antigen presentation ability of APCs, thereby generating a specific immune response against the tumor antigen, forming a long-term antitumor immune response; (3) while OVs replicate in the tumor, they also express immunomodulatory factors, and they jointly participate in further amplification of antitumor immunity [48,49]. Since RNA viruses often replicate quickly and only possess few foreign genes [16,23], their antitumor effect is mainly dependent on oncolysis than immune activation. In respect of injection mode, cancer patients gained a significant objective response rate benefit from intratumoral injection (OR = 4.05, 95% CI: 1.96–8.33). Due to physical parameters and virus dilution, the targeting and effect of intravenous injection were unsatisfactory [5]. Although intratumoral injection can circumvent the above-mentioned problems, it is also limited by tumor type.
From the survival data, only T-VEC (HR = 0.79, 95% CI: 0.63–0.99, p = 0.04) could effectively prolong overall survival (OS) of cancer patients. Pelareorep, Pexa-Vec, and NTX-010 were not statistically significant for OS. Moreover, no oncolytic virus affected progression-free survival (PFS) (HR = 1.00, 95% CI: 0.85–1.19). In patients with metastatic breast cancer, the median survival time of the experimental group (17.4 months) treated with pelareorep was remarkably longer than that of the control group (10.4 months). The HR of overall survival was 0.65 (80% CI: 0.46–0.91, p = 0.10). This suggests that pelareorep may be a new promising drug for metastatic breast cancer; more RCTs are, however, needed to validate it.
Oncolytic viruses are generally considered safe. However, the oncolytic virotherapies were associated with specific risks in this meta-analysis. The pooled risk ratios (RR) and risk difference (RD) of severe adverse events (AEs) were 1.44 (95% CI: 1.17–1.78, p = 0.0006) and 0.12 (95% CI: 0.06–0.18, p = 0.0002), respectively, indicating such therapies carry risks that should not be ignored. Any-grade AEs with an incidence greater than 10% included fever (48.90%), neutropenia (63.01%), febrile neutropenia (25.18%), leukopenia (71.23%), diarrhea (28.78%), nausea (45.24%), vomiting (27.84%), chills (45.84%), fatigue (55.35%), flu-like symptoms (31.29%), decreased appetite/anorexia (25.91%), arthralgia (19.01%), myalgia (18.42%), extreme pain (20.98%), headache (24.11%), cough (21.66%), and thrombocytopenia (54.79%). Severe AEs with an incidence greater than 5% included neutropenia (40.36%), febrile neutropenia (15.52%) leukopenia (26.61%), fatigue (6.836%), and thrombocytopenia (10.09%). In the one-sided test, statistically significance of high-grade flu-like symptoms (1.23%), cellulitis (5.822%) of any-grade, and decreased appetite/anorexia (25.91%) of any-grade were observed. Detailed severe AEs have not been reported yet, and may be due to the loss of follow up, leading to underestimation.
Our meta-analysis had the following limitations. First, we did not consider tumor types because of the insufficient number of RCTs to analyze same cancer. Secondly, in the subgroup analysis of objective response rate, there were few RCTs about oncolytic DNA viruses and intratumoral injection, and the conclusion needs more research to verify. Besides, the effective oncolytic virus was T-VEC. Therefore, the analysis results of the objective response rate may be affected by it. Finally, the heterogeneity of adverse events was biased upward since a wide range of oncolytic viruses was included. This review may provide new ideas for further research on oncolytic viruses to address the remaining challenges. We believe that oncolytic virotherapy will play an increasingly important role in cancer therapy with the increase of number of studies conducted.
5. Conclusions
In conclusion, the results of our meta-analysis showed that the objective response rate benefit was observed in oncolytic DNA viruses and intratumoral injections. Currently, only patients treated with T-VEC can prolong overall survival. Besides, our meta-analysis revealed that occurrence of severe adverse events associated with oncolytic virotherapy cannot be ignored. More qualitative RCTs are needed to test the efficacy and safety of oncolytic viruses.
Author Contributions
Study design: Z.L. and Q.L.; data extraction, quality assessment, and data analysis: Z.L., Z.J., Y.Z., and Q.L.; manuscript writing and edition: Z.L. and Y.Z., Q.L. and X.H. revised the manuscript for its integrity and accuracy. Q.L. and Z.L. approved the final version of this manuscript and take responsibility for its content. All authors have read and agree to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (31760261 and 31660035), the Science and Technology Research Project of Jiangxi Provincial Education Department (60224), the Key Research and Development Projects of Jiangxi Natural Science Foundation (20192BBG70067), National Innovation and Entrepreneurship Program for College Students (20190403070), and the Key Projects of Jiangxi Natural Science Foundation (20171ACB20003).
Conflicts of Interest
The authors declare that they have no conflict of interest
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Housman G., Byler S., Heerboth S., Lapinska K., Longacre M., Snyder N., Sarkar S. Drug resistance in cancer: An overview. Cancers. 2014;6:1769–1792. doi: 10.3390/cancers6031769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Ruysscher D., Niedermann G., Burnet N.G., Siva S., Lee A.W.M., Hegi-Johnson F. Radiotherapy toxicity. Nat. Rev. Dis. Primers. 2019;5:13. doi: 10.1038/s41572-019-0064-5. [DOI] [PubMed] [Google Scholar]
- 4.Gujar S., Bell J., Diallo J.S. SnapShot: Cancer Immunotherapy with Oncolytic Viruses. Cell. 2019;176:1240. doi: 10.1016/j.cell.2019.01.051. [DOI] [PubMed] [Google Scholar]
- 5.Harrington K., Freeman D.J., Kelly B., Harper J., Soria J.C. Optimizing oncolytic virotherapy in cancer treatment. Nat. Rev. Drug Discov. 2019;18:689–706. doi: 10.1038/s41573-019-0029-0. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy B.E., Sadek M., Gujar S.A. Targeted Metabolic Reprogramming to Improve the Efficacy of Oncolytic Virus Therapy. Mol. Ther. 2020 doi: 10.1016/j.ymthe.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y., Pong R.C., Bergelson J.M., Hall M.C., Sagalowsky A.I., Tseng C.P., Wang Z., Hsieh J.T. Loss of adenoviral receptor expression in human bladder cancer cells: A potential impact on the efficacy of gene therapy. Cancer Res. 1999;59:325–330. [PubMed] [Google Scholar]
- 8.Cripe T.P., Dunphy E.J., Holub A.D., Saini A., Vasi N.H., Mahller Y.Y., Collins M.H., Snyder J.D., Krasnykh V., Curiel D.T., et al. Fiber knob modifications overcome low, heterogeneous expression of the coxsackievirus-adenovirus receptor that limits adenovirus gene transfer and oncolysis for human rhabdomyosarcoma cells. Cancer Res. 2001;61:2953–2960. [PubMed] [Google Scholar]
- 9.van der Poel H.G., Molenaar B., van Beusechem V.W., Haisma H.J., Rodriguez R., Curiel D.T., Gerritsen W.R. Epidermal growth factor receptor targeting of replication competent adenovirus enhances cytotoxicity in bladder cancer. J. Urol. 2002;168:266–272. doi: 10.1016/S0022-5347(05)64905-1. [DOI] [PubMed] [Google Scholar]
- 10.Bischoff J.R., Kirn D.H., Williams A., Heise C., Horn S., Muna M., Ng L., Nye J.A., Sampson-Johannes A., Fattaey A., et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 11.Tazawa H., Hasei J., Yano S., Kagawa S., Ozaki T., Fujiwara T. Bone and Soft-Tissue Sarcoma: A New Target for Telomerase-Specific Oncolytic Virotherapy. Cancers. 2020;12:478. doi: 10.3390/cancers12020478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parato K.A., Breitbach C.J., Le Boeuf F., Wang J., Storbeck C., Ilkow C., Diallo J.S., Falls T., Burns J., Garcia V., et al. The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers. Mol. Ther. 2012;20:749–758. doi: 10.1038/mt.2011.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohlhapp F.J., Kaufman H.L. Molecular pathways: Mechanism of action for talimogene laherparepvec, a new oncolytic virus immunotherapy. Clin. Cancer Res. 2016;22:1048–1054. doi: 10.1158/1078-0432.CCR-15-2667. [DOI] [PubMed] [Google Scholar]
- 14.Ricordel M., Foloppe J., Antoine D., Findeli A., Kempf J., Cordier P., Gerbaud A., Grellier B., Lusky M., Quemeneur E., et al. Vaccinia Virus Shuffling: deVV5, a Novel Chimeric Poxvirus with Improved Oncolytic Potency. Cancers. 2018;10:231. doi: 10.3390/cancers10070231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer U., Steffens S., Frank S., Rainov N.G., Schulze-Osthoff K., Kramm C.M. Mechanisms of thymidine kinase/ganciclovir and cytosine deaminase/5-fluorocytosine suicide gene therapy-induced cell death in glioma cells. Oncogene. 2005;24:1231–1243. doi: 10.1038/sj.onc.1208290. [DOI] [PubMed] [Google Scholar]
- 16.Bommareddy P.K., Shettigar M., Kaufman H.L. Integrating oncolytic viruses in combination cancer immunotherapy. Nat. Rev. Immunol. 2018;18:498–513. doi: 10.1038/s41577-018-0014-6. [DOI] [PubMed] [Google Scholar]
- 17.Achard C., Surendran A., Wedge M.E., Ungerechts G., Bell J., Ilkow C.S. Lighting a Fire in the Tumor Microenvironment Using Oncolytic Immunotherapy. EBioMedicine. 2018;31:17–24. doi: 10.1016/j.ebiom.2018.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samson A., Scott K.J., Taggart D., West E.J., Wilson E., Nuovo G.J., Thomson S., Corns R., Mathew R.K., Fuller M.J., et al. Intravenous delivery of oncolytic reovirus to brain tumor patients immunologically primes for subsequent checkpoint blockade. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aam7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schenk E.L., Mandrekar S.J., Dy G.K., Aubry M.C., Tan A.D., Dakhil S.R., Sachs B.A., Nieva J.J., Bertino E., Lee Hann C., et al. A Randomized Double-Blind Phase II Study of the Seneca Valley Virus (NTX-010) versus Placebo for Patients with Extensive-Stage SCLC (ES SCLC) Who Were Stable or Responding after at Least Four Cycles of Platinum-Based Chemotherapy: North Central Cancer Treatment Group (Alliance) N0923 Study. J. Thorac. Oncol. 2020;15:110–119. doi: 10.1016/j.jtho.2019.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCarthy C., Jayawardena N., Burga L.N., Bostina M. Developing Picornaviruses for Cancer Therapy. Cancers. 2019;11:685. doi: 10.3390/cancers11050685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calton C.M., Kelly K.R., Anwer F., Carew J.S., Nawrocki S.T. Oncolytic Viruses for Multiple Myeloma Therapy. Cancers. 2018;10:198. doi: 10.3390/cancers10060198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo Z.S., Lu B., Guo Z., Giehl E., Feist M., Dai E., Liu W., Storkus W.J., He Y., Liu Z., et al. Vaccinia virus-mediated cancer immunotherapy: Cancer vaccines and oncolytics. J. Immunother. Cancer. 2019;7:6. doi: 10.1186/s40425-018-0495-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fountzilas C., Patel S., Mahalingam D. Review: Oncolytic virotherapy, updates and future directions. Oncotarget. 2017;8:102617–102639. doi: 10.18632/oncotarget.18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen A., Ho L., Wan Y. Chemotherapy and Oncolytic Virotherapy: Advanced Tactics in the War against Cancer. Front. Oncol. 2014;4:145. doi: 10.3389/fonc.2014.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bayan C.Y., Lopez A.T., Gartrell R.D., Komatsubara K.M., Bogardus M., Rao N., Chen C., Hart T.D., Enzler T., Rizk E.M., et al. The Role of Oncolytic Viruses in the Treatment of Melanoma. Curr. Oncol. Rep. 2018;20:80. doi: 10.1007/s11912-018-0729-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andtbacka R.H., Kaufman H.L., Collichio F., Amatruda T., Senzer N., Chesney J., Delman K.A., Spitler L.E., Puzanov I., Agarwala S.S., et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J. Clin. Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 27.Chesney J., Puzanov I., Collichio F., Singh P., Milhem M.M., Glaspy J., Hamid O., Ross M., Friedlander P., Garbe C., et al. Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J. Clin. Oncol. 2018;36:1658–1667. doi: 10.1200/JCO.2017.73.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernstein V., Ellard S.L., Dent S.F., Tu D., Mates M., Dhesy-Thind S.K., Panasci L., Gelmon K.A., Salim M., Song X., et al. A randomized phase II study of weekly paclitaxel with or without pelareorep in patients with metastatic breast cancer: Final analysis of Canadian Cancer Trials Group IND.213. Breast Cancer Res. Treat. 2018;167:485–493. doi: 10.1007/s10549-017-4538-4. [DOI] [PubMed] [Google Scholar]
- 29.Bradbury P.A., Morris D.G., Nicholas G., Tu D., Tehfe M., Goffin J.R., Shepherd F.A., Gregg R.W., Rothenstein J., Lee C., et al. Canadian Cancer Trials Group (CCTG) IND211: A randomized trial of pelareorep (Reolysin) in patients with previously treated advanced or metastatic non-small cell lung cancer receiving standard salvage therapy. Lung Cancer. 2018;120:142–148. doi: 10.1016/j.lungcan.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Cohn D.E., Sill M.W., Walker J.L., O’Malley D., Nagel C.I., Rutledge T.L., Bradley W., Richardson D.L., Moxley K.M., Aghajanian C. Randomized phase IIB evaluation of weekly paclitaxel versus weekly paclitaxel with oncolytic reovirus (Reolysin®) in recurrent ovarian, tubal, or peritoneal cancer: An NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2017;146:477–483. doi: 10.1016/j.ygyno.2017.07.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eigl B.J., Chi K., Tu D., Hotte S.J., Winquist E., Booth C.M., Canil C., Potvin K., Gregg R., North S., et al. A randomized phase II study of pelareorep and docetaxel or docetaxel alone in men with metastatic castration resistant prostate cancer: CCTG study IND 209. Oncotarget. 2018;9:8155–8164. doi: 10.18632/oncotarget.24263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jonker D.J., Tang P.A., Kennecke H., Welch S.A., Cripps M.C., Asmis T., Chalchal H., Tomiak A., Lim H., Ko Y.J., et al. A Randomized Phase II Study of FOLFOX6/Bevacizumab With or Without Pelareorep in Patients With Metastatic Colorectal Cancer: iND.210, a Canadian Cancer Trials Group Trial. Clin. Colorectal Cancer. 2018;17:231–239.e237. doi: 10.1016/j.clcc.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Noonan A.M., Farren M.R., Geyer S.M., Huang Y., Tahiri S., Ahn D., Mikhail S., Ciombor K.K., Pant S., Aparo S., et al. Randomized Phase 2 Trial of the Oncolytic Virus Pelareorep (Reolysin) in Upfront Treatment of Metastatic Pancreatic Adenocarcinoma. Mol. Ther. 2016;24:1150–1158. doi: 10.1038/mt.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freytag S.O., Stricker H., Lu M., Elshaikh M., Aref I., Pradhan D., Levin K., Kim J.H., Peabody J., Siddiqui F., et al. Prospective randomized phase 2 trial of intensity modulated radiation therapy with or without oncolytic adenovirus-mediated cytotoxic gene therapy in intermediate-risk prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014;89:268–276. doi: 10.1016/j.ijrobp.2014.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moehler M., Heo J., Lee H.C., Tak W.Y., Chao Y., Paik S.W., Yim H.J., Byun K.S., Baron A., Ungerechts G., et al. Vaccinia-based oncolytic immunotherapy Pexastimogene Devacirepvec in patients with advanced hepatocellular carcinoma after sorafenib failure: A randomized multicenter Phase IIb trial (TRAVERSE) Oncoimmunology. 2019 doi: 10.1080/2162402X.2019.1615817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shamseer L., Moher D., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 37.Goldsmith K., Chen W., Johnson D.C., Hendricks R.L. Infected cell protein (ICP)47 enhances herpes simplex virus neurovirulence by blocking the CD8+ T cell response. J. Exp. Med. 1998;187:341–348. doi: 10.1084/jem.187.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He B., Chou J., Brandimarti R., Mohr I., Gluzman Y., Roizman B. Suppression of the phenotype of gamma(1)34.5- herpes simplex virus 1: Failure of activated RNA-dependent protein kinase to shut off protein synthesis is associated with a deletion in the domain of the alpha47 gene. J. Virol. 1997;71:6049–6054. doi: 10.1128/JVI.71.8.6049-6054.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Breitbach C.J., Arulanandam R., De Silva N., Thorne S.H., Patt R., Daneshmand M., Moon A., Ilkow C., Burke J., Hwang T.H., et al. Oncolytic vaccinia virus disrupts tumor-associated vasculature in humans. Cancer Res. 2013;73:1265–1275. doi: 10.1158/0008-5472.CAN-12-2687. [DOI] [PubMed] [Google Scholar]
- 40.Chakrabarty R., Tran H., Selvaggi G., Hagerman A., Thompson B., Coffey M. The oncolytic virus, pelareorep, as a novel anticancer agent: A review. Investig. New Drugs. 2015 doi: 10.1007/s10637-015-0216-8. [DOI] [PubMed] [Google Scholar]
- 41.Reddy P.S., Burroughs K.D., Hales L.M., Ganesh S., Jones B.H., Idamakanti N., Hay C., Li S.S., Skele K.L., Vasko A.J., et al. Seneca Valley virus, a systemically deliverable oncolytic picornavirus, and the treatment of neuroendocrine cancers. J. Natl. Cancer Inst. 2007;99:1623–1633. doi: 10.1093/jnci/djm198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lemos de Matos A., Franco L.S., McFadden G. Oncolytic Viruses and the Immune System: The Dynamic Duo. Mol. Ther. Methods Clin. Dev. 2020;17:349–358. doi: 10.1016/j.omtm.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaufman H.L., Kohlhapp F.J., Zloza A. Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015;14:642–662. doi: 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng M., Huang J., Tong A., Yang H. Oncolytic Viruses for Cancer Therapy: Barriers and Recent Advances. Mol. Ther. Oncolytics. 2019;15:234–247. doi: 10.1016/j.omto.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berkeley R.A., Steele L.P., Mulder A.A., Van Den Wollenberg D.J.M., Kottke T.J., Thompson J., Coffey M., Hoeben R.C., Vile R.G., Melcher A., et al. Antibody-neutralized reovirus is effective in oncolytic virotherapy. Cancer Immunol. Res. 2018;6:1161–1173. doi: 10.1158/2326-6066.CIR-18-0309. [DOI] [PubMed] [Google Scholar]
- 46.Lichty B.D., Breitbach C.J., Stojdl D.F., Bell J.C. Going viral with cancer immunotherapy. Nat. Rev. Cancer. 2014;14:559–567. doi: 10.1038/nrc3770. [DOI] [PubMed] [Google Scholar]
- 47.Twumasi-Boateng K., Pettigrew J.L., Kwok Y.Y.E., Bell J.C., Nelson B.H. Oncolytic viruses as engineering platforms for combination immunotherapy. Nat. Rev. Cancer. 2018;18:419–432. doi: 10.1038/s41568-018-0009-4. [DOI] [PubMed] [Google Scholar]
- 48.Gujar S., Pol J.G., Kim Y., Lee P.W., Kroemer G. Antitumor Benefits of Antiviral Immunity: An Underappreciated Aspect of Oncolytic Virotherapies. Trends Immunol. 2018;39:209–221. doi: 10.1016/j.it.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Bommareddy P.K., Kaufman H.L. Unleashing the therapeutic potential of oncolytic viruses. J. Clin. Investig. 2018;128:1258–1260. doi: 10.1172/JCI120303. [DOI] [PMC free article] [PubMed] [Google Scholar]